Divergent Role of Gamma Interferon in a Murine Model of Pulmonary versus Systemic Klebsiella pneumoniae Infection (original) (raw)
Abstract
Klebsiella pneumoniae is a leading cause of both community-acquired and nosocomial gram-negative-bacterial pneumonia. A further clinical complication of pulmonary K. pneumoniae infection is dissemination of bacteria from the lung into the peripheral blood, resulting in bacteremia concurrent with the localized pulmonary infection. Here, we report studies detailing the divergent role of gamma interferon (IFN-γ) in pulmonary versus systemic K. pneumoniae infection. Intratracheal inoculation of IFN-γ knockout mice resulted in significantly increased mortality compared to that observed for wild-type infected animals. Increased mortality correlated with a 100-fold increase in pulmonary bacteria within 2 days postinfection and upregulation of lung-associated interleukin-10 (IL-10) mRNA. Interestingly, IFN-γ knockout mice had a twofold reduction in plasma aminospartate transferase activity, indicating diminished liver injury following peripheral blood bacterial dissemination. To study the host response towards blood-borne bacteria in the absence of the ongoing pulmonary infection, intravenous inoculation studies were initiated. IFN-γ knockout mice were no more susceptible to intravenous infection than their wild-type counterparts. The consistent observation in IFN-γ knockout mice was for improved survival correlating with increased clearance of blood- and liver-associated bacteria. Intravenous inoculation resulted in a two- to threefold increase in hepatic IL-10 production 24 and 48 h postinfection. Liver injury was also significantly reduced in IFN-γ knockout mice. These data indicate that IFN-γ secretion is a critical mediator in the resolution of localized gram-negative pulmonary pneumonia. Surprisingly, host responses towards systemic infection with the same bacteria appear to be IFN-γ independent.
Klebsiella pneumoniae is a leading cause of nosocomial and community-acquired gram-negative bacterial pneumonia, resulting in a severe pyrogenic infection with high mortality rates without therapeutic intervention (3, 34, 37). A significant clinical complication of Klebsiella pneumonia is dissemination of bacteria from within the pulmonary airspace into the bloodstream, resulting in bacteremia concurrent with the localized pulmonary infection (16, 54). Inability to clear bacteria from the bloodstream can lead to a state of overwhelming bacteremia, resulting in exposure to high levels of bacterial endotoxin, which can culminate in multiple organ dysfunction syndrome and rapid death (35).
The primary host defense mechanism in bacterial pneumonia is the rapid clearance of the invading bacteria from the respiratory tract (reviewed in reference 27). In addition to direct bacterial phagocytosis and killing, alveolar macrophages secrete a variety of cytokines and chemokines capable of recruiting and activating blood neutrophils and monocytes into the pulmonary microenvironment (29). In contrast, blood-borne infections are cleared primarily by the innate immune responses within the liver (reviewed in references 7 and 21). Kupffer cells efficiently phagocytize bacteria from peripheral blood. In addition to Kupffer cells, phagocytic killing of bacteria within the liver is accomplished by rapidly recruited neutrophils (12, 13).
Gamma interferon (IFN-γ) has been shown to be an important signal in cell-mediated immunity against a broad array of infectious agents (2, 30, 56). While extensively studied in models of T-cell-mediated immunity against intracellular pathogens, the role of IFN-γ in acute extracellular bacterial infections is less well understood. IFN-γ has been shown to be important in the clearance of pulmonary infections with Streptococcus pneumoniae or Pseudomonas aeruginosa (36, 40). In contrast, models of systemic Staphylococcus aureus and Escherichia coli infections indicate a detrimental role of IFN-γ (22, 39, 43). Additionally, a significant percentage of liver specific IFN-γ transgenic mice die within 1 year due to enteric bacteremia (48), suggesting that excessive IFN-γ production decreases host resistance to gram-negative bacteria.
To address the importance of IFN-γ in localized pulmonary versus disseminated blood-borne Klebsiella infection, IFN-γ knockout (KO) mice were intratracheally or intravenously inoculated with K. pneumoniae. These studies indicate that IFN-γ is a critical mediator for the resolution of localized, pulmonary gram-negative pneumonia, whereas resolution of systemic, blood-borne gram-negative bacterial infections is independent of IFN-γ secretion. Furthermore, IFN-γ production during systemic bacterial infection may actually be detrimental, as liver cellular injury was significantly reduced in the absence of IFN-γ along with modestly improved bacterial clearance and survival.
MATERIALS AND METHODS
Animals.
B6.129S7-Ifngtm1Ts (IFN-γ KO) and C57BL/6J wild-type mice were purchased from The Jackson Laboratory and housed in specific pathogen-free conditions within the animal care facility at the University of Michigan until the day of sacrifice. All experimental animal procedures were approved by the University Committee On Use And Care Of Animals at the University of Michigan.
K. pneumoniae inoculation.
K. pneumoniae strain 43816 serotype 2 (American Type Culture Collection, Manassas, Va.) was grown in tryptic soy broth (Difco, Detroit, Mich.) overnight at 37°C. Bacterial concentration was determined by measuring the amount of absorbance at 600 nm and compared to a predetermined standard curve. Bacteria were then diluted to the desired concentration for inoculation. For pulmonary infection, mice were anesthetized with pentobarbital (diluted 1:7 in saline). The trachea was exposed, and 30 μl of bacterial inoculum or saline was administered via a sterile 26-gauge needle. For intravenous infection, mice were warmed under a heat lamp for an appropriate time to allow vasodilation of the tail vein. Bacteria, diluted in pyrogen-free saline, were injected in a 0.5-ml volume through a 27-gauge needle. For all experiments, an aliquot of the inoculated K. pneumoniae suspension was serially diluted onto blood agar plates to determine the actual dose of injected bacteria.
Whole-lung or -liver homogenization for CFU and cytokine analyses.
At designated time points, mice were euthanized by inhalation of CO2. The lungs or liver were perfused with 2 to 3 ml of phosphate-buffered saline (PBS)-5 mM EDTA and removed for analyses as previously described (15, 28). Briefly, organs were homogenized using a tissue homogenizer (Biospec Products, Bartlesville, Okla.) in 1 ml of PBS-complete protease inhibitor cocktail (Boehringer Mannheim Biochemical, Chicago, Ill.). For determination of CFU in organs, a small aliquot of tissue homogenate was serially diluted, moved to blood agar plates, and incubated at 37°C, and colonies were counted.
For total lung or liver cytokine enzyme-linked immunosorbent assay (ELISA) analyses, tissue homogenates were sonicated briefly to ensure complete cellular disruption and then centrifuged at 1,500 × g for 10 min. The supernatants were collected and assessed for cytokine levels by ELISA. Murine interleukin-10 (IL-10), IL-12, and TNF-α were quantitated using a modification of a sandwich ELISA method. Assays have been shown to be specific for the indicated murine cytokine and show no cross-reactivity with any other murine cytokines tested (11).
Peripheral blood CFU analyses.
For determination of peripheral blood bacterial numbers, mice were euthanized and heparinized blood was collected by cardiac puncture at the indicated time points. Serial dilutions were moved onto blood agar plates, incubated at 37°C and colonies counted.
Plasma aspartate aminotransferase (AST) analyses.
Levels of AST in plasma, as an indication of hepatic cellular injury, were determined on peripheral blood samples collected 1 or 2 days post-K. pneumoniae inoculation. Heparinized blood samples were spun at 10,000 × g for 15 min, and then plasma samples were collected and frozen at −70°C until AST activities were analyzed. AST activity was quantitated by the Clinical Chemistry Laboratory at the University of Michigan Medical Center using an automated spectrophotometric assay.
Tissue harvesting for histologic examination and TUNEL staining.
Lungs for histochemistry were perfused with 4% paraformaldehyde in PBS and then inflated with 4% paraformaldehyde to improve resolution of anatomic relationships. For liver histology, two lobes were excised following perfusion and placed in 4% paraformaldehyde for 24 h prior to sectioning. Tissue sections were then stained with hematoxylin and eosin (H&E) to determine inflammatory responses during infection. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining on liver sections was performed using the ApopTag detection kit following manufactures' recommended procedure (Intergen, Purchase, N.Y.).
Isolation and RT-PCR amplification of lung and liver mRNA.
Whole lung or liver (two lobes) was harvested at the indicated time points, immediately snap frozen in liquid nitrogen, and then stored at −70°C for further analyses. Total cellular RNA from the frozen tissue was isolated by homogenizing in 3 ml of TRIzol reagent (Gibco BRL, Gaithersburg, Md.) following the TRIzol protocol. Total RNA was determined by spectrometric analysis at 260 nm. Expression of mRNA was determined by reverse transcription (RT)-PCR using the Access RT-PCR system kit from Promega (Madison, Wis.) following the manufactures' protocol. The following primer pairs (all primers 5′→3′) were used for specific mRNA amplification: mIFN-γ sense, CCT CAG ACT CTT TGA AGT CT; mIFN-γ antisense, CAG CGA CTC CTT TTC CGC TT; mTNF-α sense, CCT GTA GCC CAC GTC GTA GC; mTNF-α antisense, AGC AAT GAC TCC AAA GTA GAC C; mIL-10 sense, GCA GAA AAG AGA GCT CCA TCA TG; mIL-10 antisense, CCT GGA GTC CAG CAG ACT CAA TAC; mβ-actin sense: CTT CTA CAA TGA GCT GCG TGT G; mβ-actin antisense, GAT TCC ATA CCC AAG AAG GAA GG. cDNA products were detected on a 2% agarose gel containing ethidium bromide and bands visualized and photographed using UV transillumination.
Statistical analyses.
Statistical significance was determined using the unpaired, two-tailed student t test and analysis of variance for multiple group comparisons using the Student-Newman-Keuls posttest. Calculations were performed using InStat 3 for Macintosh (GraphPad Software, San Diego, Calif.). Statistical analyses of survival curves were performed by the Logrank test using the Prism 3 For Macintosh software program (GraphPad Software).
RESULTS
Increased mortality in IFN-γ-deficient mice following pulmonary K. pneumoniae infection.
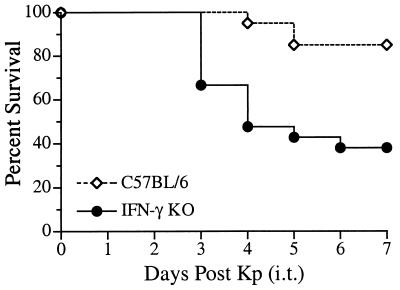
To examine the role for IFN-γ during a localized pulmonary infection, IFN-γ KO mice were intratracheally inoculated with K. pneumoniae. A dose of 2 × 103 bacteria induced minimal mortality over the course of seven days in C57BL/6 control mice (Fig. 1). In contrast, IFN-γ KO mice were significantly more susceptible to intratracheally instilled K. pneumoniae (P < 0.005). Increased rates of mortality were observed at both early (day 3) and late time points (day 7) following infection. Mortality in mice administered an IFN-γ neutralizing monoclonal antibody was similar to that seen in IFN-γ KO mice (data not shown). Together, these data indicate a critical role for IFN-γ during gram-negative bacterial pneumonia.
FIG. 1.
Increased mortality following intratracheal K. pneumoniae inoculation in IFN-γ KO mice. IFN-γ KO and C57BL/6 wild-type control mice were intratracheally inoculated with 2 × 103 CFU of K. pneumoniae, and survival was observed over the course of 7 days. At this dose of bacteria, 85% of control mice survive through day 7, compared to only 38% of IFN-γ KO mice. Survival curves were generated from two independent experiments with a total of 20 animals per group and were statistically different (P < 0.005) by log-rank analysis.
Impaired pulmonary clearance of K. pneumoniae in the absence of IFN-γ.
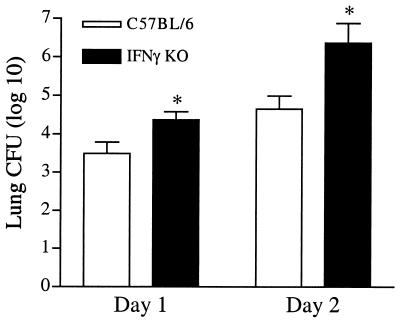
The most creditable explanation for the increased mortality seen in IFN-γ KO mice following intratracheal inoculation of K. pneumoniae would be impaired clearance of bacteria from the pulmonary airspace. To address this, lung bacterial burden in IFN-γ KO mice and their wild-type controls was examined 1 and 2 days post-intratracheal infection (Fig. 2). Within 1 day, IFN-γ-deficient mice displayed an ∼10-fold increase in lung bacterial burden when to control infected mice (P < 0.05). This impaired ability to clear the pulmonary infection was even more pronounced by day 2, when IFN-γ KO mice contained a nearly 100-fold increase in pulmonary bacterial numbers versus the infected control group (P < 0.01).
FIG. 2.
Impaired pulmonary bacterial clearance in IFN-γ KO mice following intratracheal K. pneumoniae infection. Infected IFN-γ KO and wild-type control mice were analyzed on days 1 and 2 postinfection for bacterial burden in total lung homogenates. Total lung CFU were significantly higher in mice lacking IFN-γ on day 1 (P < 0.05) and day 2 (P < 0.01) postinfection. CFU data are expressed as mean + standard error of the mean (error bars) from two independent experiments representing 15 to 18 total animals.
Histological examination of lung sections 2 days postinfection confirmed the heightened pulmonary infection in the absence of IFN-γ. Significant inflammatory cell infiltration was observed in IFN-γ KO mice, consisting predominantly of neutrophils. Additionally, extracellular bacteria residing in airspaces were observed in mice lacking IFN-γ which were rarely seen in wild-type infected mice (data not shown).
Lung TNF-α mRNA induction was unaffected while IL-10 mRNA was increased in IFN-γ-deficient mice following intratracheal K. pneumoniae infection.
Induction of TNF-α has been shown to be critical during pulmonary bacterial infections (25, 45, 50). To determine whether altered production of this mediator could be responsible for the impaired bacterial clearance and increased mortality in IFN-γ KO mice, lung TNF-α mRNA induction was examined by RT-PCR 2 days postinfection (Fig. 3). Interestingly, no overt impairment in TNF-α mRNA induction was observed in IFN-γ KO mice following infection. Message for IFN-γ was readily observed 48 h following infection in wild-type infected animals, confirming induction of this cytokine during acute pneumonia. As expected, induction of IFN-γ mRNA was not observed in IFN-γ KO mice. Production of IL-10 during pulmonary Klebsiella infection has been shown to dampen the host response, resulting in impaired bacterial clearance and subsequent survival (11). To determine if enhanced IL-10 production in IFN-γ KO mice could, in part, explain the impaired bacterial clearance, IL-10 mRNA induction was determined following intratracheal infection. Interestingly, IL-10 message was clearly upregulated in IFN-γ KO mice compared to infected wild-type control animals.
FIG. 3.
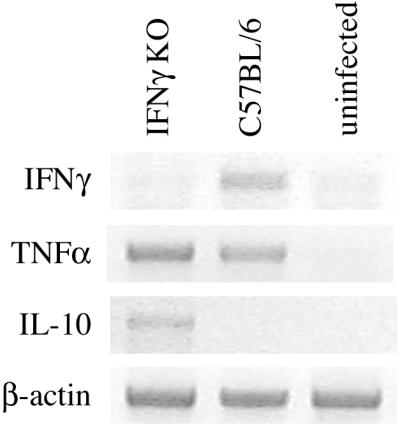
Lung RT-PCR analyses of IFN-γ KO mice 2 days post-intratracheal K. pneumoniae inoculation. Total lung RNA was isolated and RT-PCR was performed for the indicated cytokine mRNA. IFN-γ KO and C57BL/6 control mice expressed similar levels of TNF-α mRNA following pulmonary infection. Interestingly, lung IL-10 message was increased in the absence of IFN-γ. As expected, IFN-γ message was upregulated in control animals while being absent in the genetically deficient mice.
Decreased liver injury in IFN-γ-deficient mice following peripheral blood dissemination of the primary pulmonary infection.
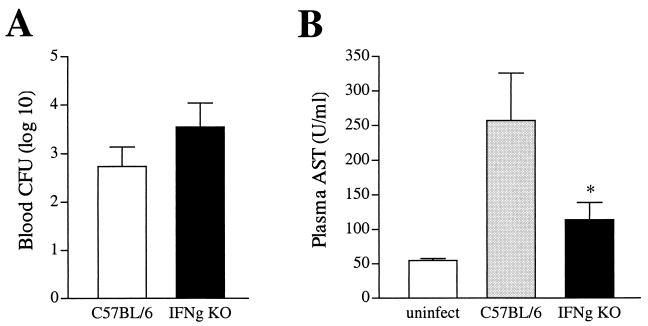
Peripheral blood dissemination is a consequence of K. pneumonia, resulting in systemic infection concurrent with the localized pulmonary infection. Minimal blood dissemination was observed in wild-type or IFN-γ KO mice 1 day postinfection (data not shown). Correlating with the increased lung bacterial burden seen on day 2 was a trend for increased peripheral blood bacterial dissemination in IFN-γ KO animals. (Fig. 4A). Bacteria present in the systemic circulation following pulmonary infection can induce liver cellular injury. To determine the extent of hepatic injury following peripheral blood dissemination of the pulmonary infection, plasma AST levels were measured two days postinfection. Wild-type infected mice displayed elevated plasma AST levels compared to uninfected animals (Fig. 4B). Notably, IFN-γ KO mice had significantly decreased liver injury (P < 0.05) in spite of similar bacterial numbers in the peripheral circulation.
FIG. 4.
IFN-γ KO mice display decreased liver cellular injury in spite of similar bacterial numbers in the peripheral circulation following dissemination of pulmonary infection. (A) Peripheral blood (per milliliter of blood) was collected from animals 2 days post-intratracheal infection. Bacterial numbers were similar in both groups of mice, with a consistent trend for higher numbers in IFN-γ KO mice. CFU data are expressed as means + standard errors of the means (error bars) from two independent experiments representing 15 to 18 total mice. (B) Peripheral blood was collected as described in Materials and Methods, and plasma AST levels were determined on day 2 postinfection. Plasma AST levels were significantly lower (P < 0.05) in IFN-γ KO animals.
Survival following intravenous K. pneumoniae infection is independent of IFN-γ production.
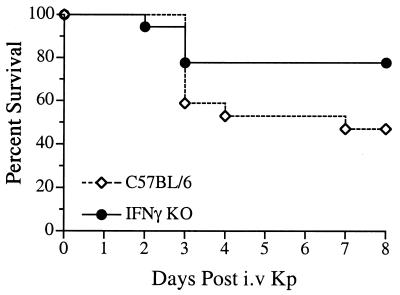
Data thus far indicated that IFN-γ is critical for the resolution of localized, pulmonary gram-negative pneumonia. Decreased liver tissue injury following peripheral blood bacterial dissemination in the absence of IFN-γ suggested that resolution of a systemic gram-negative bacterial infection may occur independently of IFN-γ production. To more rigorously examine the role of IFN-γ during blood-borne infection without the complication of an ongoing localized pulmonary infection, an intravenous infection model was established. Intravenous injection of wild-type mice with 5 × 104 CFU K. pneumoniae resulted in an overall survival rate of 50% (Fig. 5). Surprisingly, intravenous inoculation of IFN-γ KO mice had no detrimental effect on survival. In fact, while not reaching the level of statistical significance (P = 0.09), the trend in all experiments was for modestly improved survival. Neutralizing anti-IFN-γ monoclonal antibody treatment resulted in similar rates of mortality as IFN-γ KO mice (data not shown).
FIG. 5.
Survival following intravenous K. pneumoniae infection is independent of IFN-γ production. IFN-γ KO and C57BL/6 wild-type control mice were intravenously inoculated with 5 × 104 bacteria and survival was observed over the course of 8 days. While not reaching the level of statistical significance (P = 0.09), the consistent trend in all experiments was for improved survival in IFN-γ KO mice compared to C57BL/6 controls. Survival curves were generated from two independent experiments with a total of 18 animals per group.
Unimpaired bacterial clearance in IFN-γ-deficient mice following intravenous K. pneumoniae infection.
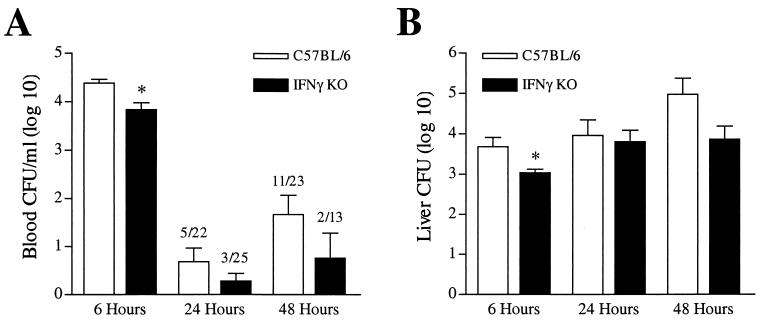
To determine bacterial clearance kinetics from the bloodstream, C57BL/6 and IFN-γ KO mice were analyzed at defined time points following intravenous inoculation. Both groups of animals contained significant numbers of blood-borne bacteria 6 h following infection. Surprisingly, IFN-γ KO mice had a modest, but statistically significant (P < 0.01) reduction in bacterial numbers in blood 6 h postinfection (Fig. 6A). By 24 h, bacteria could be found in the blood of only a small percentage of wild-type and IFN-γ KO mice (23 and 12%, respectively). However, by 48 h a significant percentage (48%) of wild-type mice contained detectable bacteria. Interestingly, the percentage of IFN-γ KO mice with detectable blood-borne bacteria only marginally increased from 24 to 48 h (12 to 15% at 48 h). This trend of improved bacterial clearance in IFN-γ KO mice at 48 h postinfection compared to wild-type animals was a consistent observation in all experiments (P = 0.07 [Fisher's exact test]).
FIG. 6.
Unimpaired bacterial clearance in IFN-γ KO mice following intravenous K. pneumoniae inoculation. Bacterial clearance from blood (A) and liver (B) of intravenously infected IFN-γ KO and C57BL/6 mice was determined as described. Six hours postinfection, IFN-γ KO mice had a modest but statistically significant reduction in bacterial burden in blood and liver (P < 0.05). By 48 h, there was a consistent trend towards improved clearance of peripheral blood bacteria in IFN-γ KO mice compared to wild-type animals in all experiments (P = 0.07 [Fisher's exact test]). Liver bacterial clearances by 24 and 48 h postinfection were similar in both IFN-γ KO and wild-type infected animals. CFU data were generated from two to three independent experiments with a total of 14 to 25 animals per group.
In contrast to the markedly impaired pulmonary clearance of K. pneumoniae in the absence of IFN-γ following intratracheal inoculation (Fig. 2), clearance of bacteria within the liver following intravenous infection was independent of IFN-γ production (Fig. 6B). As with numbers of blood-borne bacteria 6 h postinfection, the liver bacterial burden in IFN-γ KO mice was statistically decreased (P < 0.05) compared to that in wild-type mice. By 24 and 48 h postinfection, numbers of bacteria in liver were similar in both IFN-γ KO and wild-type infected animals.
Decreased liver injury in IFN-γ-deficient mice following intravenous K. pneumoniae infection.
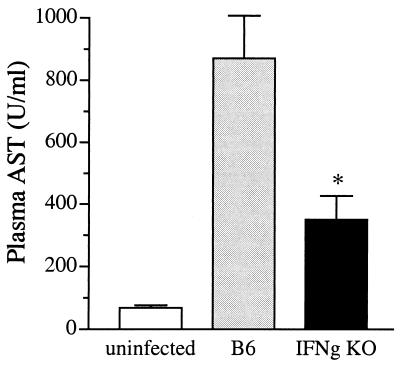
IFN-γ KO mice displayed decreased liver injury following blood dissemination of the primary pulmonary infection (Fig. 4B). To determine the extent of hepatocyte injury following intravenous inoculation of K. pneumonia, plasma AST levels were measured 24 h postinfection (Fig. 7). Mice lacking IFN-γ had significantly reduced levels of plasma AST compared to those observed for control mice (351 versus 871 U/ml, respectively; P < 0.05). This reduction in liver injury is independent from bacterial burden as both groups of mice (IFN-γ KO and wild type) contained equivalent numbers of bacteria in the liver 24 h postinfection (Fig. 6B).
FIG. 7.
Diminished liver cellular injury in IFN-γ KO mice following intravenous K. pneumoniae infection. Plasma AST levels were determined 1 day postinfection from IFN-γ KO and wild-type mice. Mice lacking IFN-γ had significantly reduced levels of plasma AST compared to control mice (351 versus 871 U/ml, respectively; P < 0.05). It is worth noting that both groups of mice had equivalent bacterial numbers in liver at this time point. AST levels were determined from two independent experiments with a total of 15 animals per group.
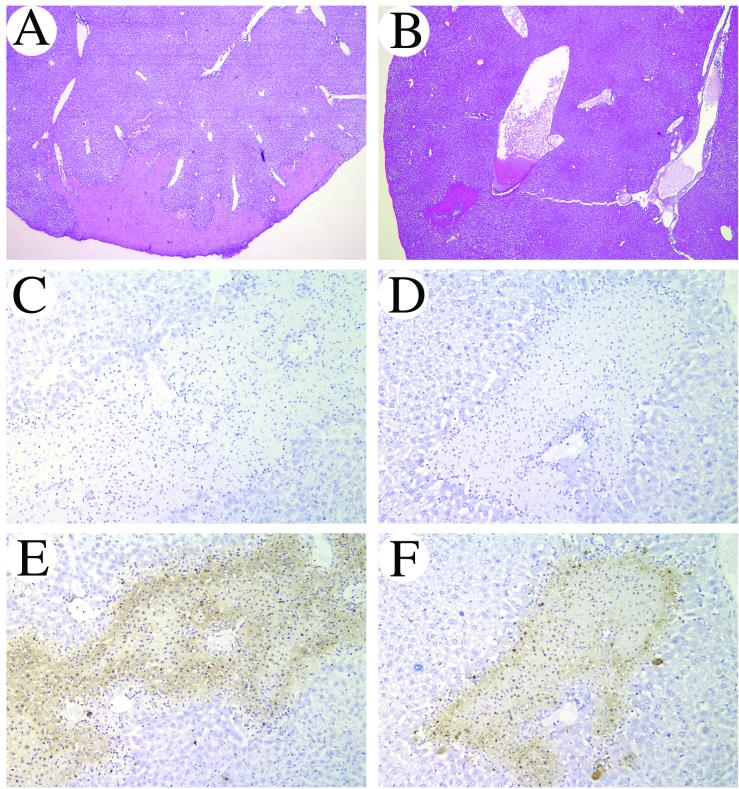
Liver histology confirmed decreased liver injury in IFN-γ KO mice 24 h postinfection. Sites of focal hepatocyte cellular injury were both fewer in number and smaller in size when compared to wild-type animals (Fig. 8A and B). Additionally, high-power examination revealed increased neutrophil and mononuclear cell infiltration in necrotic lesions of wild-type mice compared to that observed for IFN-γ KO mice (data not shown). To confirm the extent of cellular injury, TUNEL staining on liver sections was performed. Without exception, all sites of overt tissue injury seen on H&E-stained sections also stained TUNEL positive (Fig. 8 E and F). TUNEL staining, however, was more intense in wild-type liver sections than in IFN-γ KO mice.
FIG. 8.
Improved liver pathology in IFN-γ KO mice 1 day post-intravenous K. pneumoniae infection. Liver sections from C57BL/6 (A, C, and E) and IFN-γ KO (B, D, and F) mice were prepared and stained with H&E (A and B) or TUNEL (C to F) and then examined at low-power (40×) (A and B) and intermediate-power (200×) (C to F) magnifications. Lesions of hepatocyte cellular injury and necrosis were smaller in number and individual size in IFN-γ KO mice when compared to wild-type animals (compare panels A and B). Without exception, all sites of overt tissue injury seen on the H&E-stained sections also stained TUNEL positive (E and F). TUNEL staining, however, was more intense in wild-type liver sections compared to IFN-γ KO mice. TUNEL staining in the absence of terminal deoxynucleotidyl transferase (C and D) confirmed the specificity of the TUNEL reaction.
Elevated liver IL-10 production in IFN-γ-deficient mice following intravenous K. pneumoniae infection.
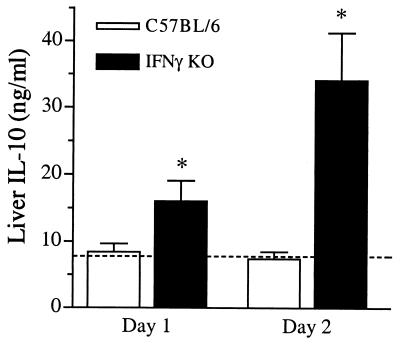
Since lung-associated IL-10 was upregulated in IFN-γ KO mice following intratracheal infection, we determined the production of liver-associated IL-10 following intravenous inoculation. IL-10 was not induced 24 and 48 h following systemic infection in wild-type mice. IFN-γ KO mice, however, displayed significantly elevated liver IL-10 levels at both time points (Fig. 9). Induction of the proinflammatory cytokines TNF-α and IL-12 occurred to a similar degree in both wild-type and IFN-γ KO mice (data not shown).
FIG. 9.
Elevated liver IL-10 production in IFN-γ KO mice following intravenous bacterial infection. Total liver homogenates were prepared from IFN-γ KO and C57BL/6 infected animals at the indicated time points following intravenous inoculation, and levels of IL-10 were determined by ELISA. IL-10 levels were significantly elevated in IFN-γ KO mice compared to wild-type infected animals and uninfected, baseline levels (dotted line). Data are expressed as means + standard errors of the means (error bars) from two independent experiments with a total of 10 to 12 animals per group.
DISCUSSION
Host innate antibacterial responses consist of a balancing act between clearance of the invading organism on one hand and avoidance of tissue injury due to the inflammatory response on the other. In this context, the role of a particular cytokine may vary significantly depending on the site of infection. Previously published data have suggested that the role of IFN-γ varies between organ-specific and systemic infections. A complicating factor in these studies has been the use of a variety of different pathogens or their products administered in a variety of different ways (intratracheal, intraperitoneal, intravenous). Here, we detail the divergent role of IFN-γ in pulmonary versus systemic antibacterial host responses against the same gram-negative bacteria. Intratracheal inoculation of IFN-γ KO mice with K. pneumoniae resulted in significantly increased mortality compared to that observed in wild-type infected animals. This increased mortality correlated with a 100-fold increase in the numbers of pulmonary bacteria within 2 days postinfection. Of interest was the decreased liver tissue injury subsequent to peripheral blood dissemination seen in mice lacking IFN-γ. To study the systemic response to blood-borne bacteria in the absence of the ongoing pulmonary infection, intravenous inoculation studies were initiated. Unlike intratracheal infection, intravenously infected IFN-γ KO mice were no more susceptible than their wild-type counterparts. In fact, the consistent observation was for modestly improved survival correlating with the trend for increased clearance of blood and liver-associated bacteria. As seen in the intratracheal studies, liver cellular injury following intravenous inoculation was significantly decreased in IFN-γ KO mice. Additionally, increased IL-10 production was noted in both lung and liver tissue from IFN-γ KO mice following either localized or systemic infection. These data indicate that IFN-γ is a critical component in the clearance and resolution of a localized, pulmonary infection with K. pneumoniae. Surprisingly, host responses towards blood-borne, systemic infection with the same bacteria appear to be IFN-γ independent.
The importance of IFN-γ in the context of cell-mediated immunity against a broad array of infectious agents has been well established (2, 30, 56). However, the majority of these studies have utilized models of T cell immunity against intracellular organisms. The role of IFN-γ in acute extracellular bacterial infections is less well understood. Pulmonary inoculation of IFN-γ KO mice with S. pneumoniae resulted in significantly increased mortality (36). Pulmonary administration of IFN-γ in P. aeruginosa infected rats also improved lung clearance of the organism (24). Additionally, the pulmonary immunosuppression seen in alcohol treated rats could be reversed by adenoviral vector mediated overexpression of IFN-γ (23). In contrast, it has been reported that IFN-γ-receptor KO mice displayed enhanced clearance of pulmonary inoculated P. aeruginosa (41). These authors concluded that endogenous IFN-γ was detrimental for host defense against pulmonary P. aeruginosa infections. Recently, IFN-γ KO mice have been shown to be more susceptible to intranasally inoculated K. pneumoniae (55). Impaired lung and blood bacterial clearance was also noted in this study. Our studies confirm and extend these recent observations to include decreased hepatocyte cellular injury following blood dissemination coincident with unimpaired clearance of liver-associated bacteria (data not shown). Combined, the preponderance of data suggest a requisite role for IFN-γ during extracellular bacterial lung infections.
In contrast to localized bacterial infections, production of proinflammatory cytokines such as IFN-γ during systemic infections has been linked to increased mortality due to complications of sepsis (32). Systemic infections with S. aureus and E. coli have indicated that IFN-γ plays a detrimental role in the resolution of these pathogens (22, 39, 43). In these studies, neutralization of IFN-γ activity resulted in increased survival. Of note, improved survival did not correlate with increased bacterial clearance in two independent studies of E. coli infection (22, 43). In contrast, exogenous administration of IFN-γ has been shown to be detrimental for clearance of E. coli (22). Furthermore, LPS-induced mortality and liver injury have been shown to be significantly reduced in IFN-γ KO mice (4, 49). Additionally, a significant percentage of liver specific IFN-γ transgenic mice die within 1 year due to enteric bacteremia, suggesting that excessive localized, liver IFN-γ production decreases host resistance to gram-negative bacteria (48). In contrast to studies indicating a detrimental role for IFN-γ during systemic infections, exogenous treatment with liposome-encapsulated IFN-γ significantly increased survival, while anti-IFN-γ antibody treatment decreased survival an intraperitoneal model of Klebsiella septicemia (46, 47). Also, clearance of the intracellular bacillus Salmonella enterica serovar Typhimurium has been shown to be dependent upon IFN-γ production (31). Our results are in general agreement with the observation that IFN-γ production in the context of systemic K. pneumoniae infection is detrimental. While survival benefits were modest, the consistent trend was for improved survival and increased bacterial clearance. We have extended these observations to include the reduction in liver cellular injury in the absence of IFN-γ.
We observed increased production of IL-10 in IFN-γ KO mice following infection with K. pneumoniae. IL-10, in contrast to IFN-γ, inhibits the generation of T1 cellular immunity while promoting T2 cellular responses (6). Additionally, IL-10 has been shown to inhibit macrophage and neutrophil microbicidal activities and to suppress the production of proinflammatory cytokines (1, 5, 19, 33). Thus, enhanced IL-10 production could, in part, explain the decreased survival and bacterial clearance following localized, pulmonary infection with K. pneumoniae. In the context of Klebsiella pneumonia, we have previously shown that anti-IL-10 immunotherapy resulted in decreased mortality, improved bacterial clearance, and increased production of lung-associated TNF-α and IFN-γ (11). In a murine model of pneumococcal pneumonia, exogenous administration of IL-10 resulted in increased mortality, increased bacterial burden, and decreased production of TNF-α and IFN-γ (52). When IL-10 bioactivity was neutralized in this model, increased survival was observed which correlated with increased bacterial clearance and proinflammatory cytokine production. Collectively, these data support the concept that IL-10 downregulates the immune response in the setting of pulmonary bacterial infections, most likely by inhibiting phagocytic and bactericidal activities. Thus, elevated IL-10 production in IFN-γ KO mice during K. pneumoniae pulmonary infection could contribute to the worsened outcome in these mice.
In contrast to playing a role in localized pulmonary infections, IL-10 has been shown to play a beneficial role in endotoxemia and sepsis models. Neutralization of IL-10 during endotoxemia results in uncontrolled production of proinflammatory cytokines and increased mortality. Conversely, exogenous administration of IL-10 decreased proinflammatory cytokines and mortality (10, 14, 38). Results from mice undergoing cecal ligation puncture (a commonly used model of abdominal sepsis syndrome) are similar to those of LPS-induced endotoxemia in that exogenous IL-10 administration results in enhanced survival (20, 51, 53). Therefore, in our model of intravenous K. pneumoniae infection, increased production of liver-derived IL-10 in IFN-γ KO mice may contribute the modest beneficial effects seen on survival and bacterial clearance.
We were intrigued by the reduced hepatic injury observed in IFN-γ KO mice following infection. IFN-γ has been shown to directly induce hepatocyte apoptosis in a Fas/FasL-independent manner (17). Treatment of hepatocytes in vitro with IFN-γ has been shown to induce apoptosis (18, 42). Additionally, hepatocyte cellular respiration and protein synthesis are suppressed by IFN-γ treatment (26). Of relevance to _K. pneumoniae-_induced liver injury, LPS potentiates the IFN-γ mediated hepatocyte apoptosis in vitro via activation of caspase 3 (44). Our in vivo data confirm and extend these in vitro studies and suggest that the decreased liver injury observed during gram-negative bacterial infections in the absence of IFN-γ may be due to decreased hepatocyte apoptosis. TUNEL staining analyses confirm decreased cellular death in IFN-γ KO versus wild-type animals following infection. It is worth noting, however, that the TUNEL staining pattern observed in both wild-type and IFN-γ KO liver sections is more indicative of necrotic rather than apoptotic cell death. The nonspecific detection of necrotic cells by the TUNEL technique has been reported previously (8, 9). Regardless, analyses of both H&E- and TUNEL-stained liver sections clearly indicate decreased hepatocyte cellular injury in mice deficient in production of IFN-γ.
These studies are the first to directly compare the localized, pulmonary antibacterial host response to the systemic, blood-borne response against the same gram-negative bacterial pathogen. The data clearly point to a divergent requirement for IFN-γ in these two infection models. IFN-γ secretion is a critical mediator in the resolution of gram-negative pulmonary pneumonia, as IFN-γ KO mice are significantly impaired in bacterial clearance and overall survival when compared to wild-type mice. In contrast, the absence of IFN-γ has no detrimental effect on antibacterial responses to an intravenous challenge of K. pneumoniae. Furthermore, IFN-γ KO mice display decreased liver cellular injury following systemic bacterial infection. Elevated IL-10 production in response to infection may, in part, contribute to the detrimental or beneficial effects of the absence of IFN-γ production in pulmonary versus systemic K. pneumoniae infections.
Acknowledgments
This research was supported in part by a Research Grant from the American Lung Association (T.A.M.) and grant AI49448 (T.A.M.) and grants HL57243, HL58200, and P50HL60289 (T.J.S.) from the National Institutes of Health. T.A.M. is the recipient of a Parker B. Francis Fellowship in Pulmonary Research and an Edward Livingston Trudeau Fellowship of the American Lung Association.
REFERENCES
- 1.Armstrong, L., N. Jordan, and A. Millar. 1996. Interleukin 10 (IL-10) regulation of tumour necrosis factor alpha (TNF-alpha) from human alveolar macrophages and peripheral blood monocytes. Thorax 51**:**143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15**:**749-795. [DOI] [PubMed] [Google Scholar]
- 3.Burwen, D. R., S. N. Banerjee, and R. P. Gaynes. 1994. Ceftazidime resistance among selected nosocomial gram-negative bacilli in the United States. National Nosocomial Infections Surveillance System. J. Infect. Dis. 170**:**1622-1625. [DOI] [PubMed] [Google Scholar]
- 4.Car, B. D., V. M. Eng, B. Schnyder, L. Ozmen, S. Huang, P. Gallay, D. Heumann, M. Aguet, and B. Ryffel. 1994. Interferon gamma receptor deficient mice are resistant to endotoxic shock. J. Exp. Med. 179**:**1437-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Andrea, A., M. Aste-Amezaga, N. M. Valiante, X. Ma, M. Kubin, and G. Trinchieri. 1993. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 178**:**1041-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vries, J. E. 1995. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann. Med. 27**:**537-541. [DOI] [PubMed] [Google Scholar]
- 7.Doherty, D. G., and C. O'Farrelly. 2000. Innate and adaptive lymphoid cells in the human liver. Immunol. Rev. 174**:**5-20. [DOI] [PubMed] [Google Scholar]
- 8.Frankfurt, O. S., and A. Krishan. 2001. Enzyme-linked immunosorbent assay (ELISA) for the specific detection of apoptotic cells and its application to rapid drug screening. J. Immunol. Methods 253**:**133-144. [DOI] [PubMed] [Google Scholar]
- 9.Frankfurt, O. S., and A. Krishan. 2001. Identification of apoptotic cells by formamide-induced dna denaturation in condensed chromatin. J. Histochem. Cytochem. 49**:**369-378. [DOI] [PubMed] [Google Scholar]
- 10.Gerard, C., C. Bruyns, A. Marchant, D. Abramowicz, P. Vandenabeele, A. Delvaux, W. Fiers, M. Goldman, and T. Velu. 1993. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J. Exp. Med. 177**:**547-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberger, M. J., R. M. Strieter, S. L. Kunkel, J. M. Danforth, R. E. Goodman, and T. J. Standiford. 1995. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J. Immunol. 155**:**722-729. [PubMed] [Google Scholar]
- 12.Gregory, S. H., L. K. Barczynski, and E. J. Wing. 1992. Effector function of hepatocytes and Kupffer cells in the resolution of systemic bacterial infections. J. Leukoc. Biol. 51**:**421-424. [DOI] [PubMed] [Google Scholar]
- 13.Gregory, S. H., A. J. Sagnimeni, and E. J. Wing. 1996. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J. Immunol. 157**:**2514-2520. [PubMed] [Google Scholar]
- 14.Howard, M., T. Muchamuel, S. Andrade, and S. Menon. 1993. Interleukin 10 protects mice from lethal endotoxemia. J. Exp. Med. 177**:**1205-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huffnagle, G. B., M. F. Lipscomb, J. A. Lovchik, K. A. Hoag, and N. E. Street. 1994. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J. Leukoc. Biol. 55**:**35-42. [DOI] [PubMed] [Google Scholar]
- 16.Jong, G. M., T. R. Hsiue, C. R. Chen, H. Y. Chang, and C. W. Chen. 1995. Rapidly fatal outcome of bacteremic Klebsiella pneumoniae pneumonia in alcoholics. Chest 107**:**214-217. [DOI] [PubMed] [Google Scholar]
- 17.Kano, A., T. Haruyama, T. Akaike, and Y. Watanabe. 1999. IRF-1 is an essential mediator in IFN-gamma-induced cell cycle arrest and apoptosis of primary cultured hepatocytes. Biochem. Biophys. Res. Commun. 257**:**672-677. [DOI] [PubMed] [Google Scholar]
- 18.Kano, A., Y. Watanabe, N. Takeda, S. Aizawa, and T. Akaike. 1997. Analysis of IFN-gamma-induced cell cycle arrest and cell death in hepatocytes. J. Biochem. (Tokyo) 121**:**677-683. [DOI] [PubMed] [Google Scholar]
- 19.Kasama, T., R. M. Strieter, N. W. Lukacs, M. D. Burdick, and S. L. Kunkel. 1994. Regulation of neutrophil-derived chemokine expression by IL-10. J. Immunol. 152**:**3559-3569. [PubMed] [Google Scholar]
- 20.Kato, T., A. Murata, H. Ishida, H. Toda, N. Tanaka, H. Hayashida, M. Monden, and N. Matsuura. 1995. Interleukin 10 reduces mortality from severe peritonitis in mice. Antimicrob. Agents Chemother. 39**:**1336-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knolle, P. A., and G. Gerken. 2000. Local control of the immune response in the liver. Immunol. Rev. 174**:**21-34. [DOI] [PubMed] [Google Scholar]
- 22.Kohler, J., D. Heumann, G. Garotta, D. LeRoy, S. Bailat, C. Barras, J. D. Baumgartner, and M. P. Glauser. 1993. IFN-gamma involvement in the severity of gram-negative infections in mice. J. Immunol. 151**:**916-921. [PubMed] [Google Scholar]
- 23.Kolls, J. K., D. Lei, D. Stoltz, P. Zhang, P. O. Schwarzenberger, P. Ye, G. Bagby, W. R. Summer, J. E. Shellito, and S. Nelson. 1998. Adenoviral-mediated interferon-gamma gene therapy augments pulmonary host defense of ethanol-treated rats. Alcohol. Clin. Exp. Res. 22**:**157-162. [PubMed] [Google Scholar]
- 24.Kolls, J. K., S. Nelson, and W. R. Summer. 1993. Recombinant cytokines and pulmonary host defense. Am. J. Med. Sci. 306**:**330-335. [DOI] [PubMed] [Google Scholar]
- 25.Laichalk, L. L., S. L. Kunkel, R. M. Strieter, J. M. Danforth, M. B. Bailie, and T. J. Standiford. 1996. Tumor necrosis factor mediates lung antibacterial host defense in murine Klebsiella pneumonia. Infect. Immun. 64**:**5211-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang, J. F., and T. Akaike. 1998. Protective effect of linoleic acid on IFN gamma-induced cellular injury in primary culture hepatocytes. J. Biochem. (Tokyo) 123**:**213-218. [DOI] [PubMed] [Google Scholar]
- 27.Lipscomb, M. F., D. E. Bice, C. R. Lyons, M. R. Schuyler, and D. Wilkes. 1995. The regulation of pulmonary immunity. Adv. Immunol. 59**:**369-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore, T. A., B. B. Moore, M. W. Newstead, and T. J. Standiford. 2000. Gamma delta-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J. Immunol. 165**:**2643-2650. [DOI] [PubMed] [Google Scholar]
- 29.Moore, T. A., and T. J. Standiford. 2001. Cytokine immunotherapy during bacterial pneumonia: from benchtop to bedside. Semin. Respir. Infect. 16**:**27-37. [DOI] [PubMed] [Google Scholar]
- 30.Murray, H. W. 1996. Current and future clinical applications of interferon-gamma in host antimicrobial defense. Intensive Care Med. 22**:**S456-S461. [DOI] [PubMed] [Google Scholar]
- 31.Nauciel, C., and F. Espinasse-Maes. 1992. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect. Immun. 60**:**450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oberholzer, A., C. Oberholzer, and L. L. Moldawer. 2001. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock 16**:**83-96. [DOI] [PubMed] [Google Scholar]
- 33.Oswald, I. P., T. A. Wynn, A. Sher, and S. L. James. 1992. Interleukin 10 inhibits macrophage microbicidal activity by blocking the endogenous production of tumor necrosis factor alpha required as a costimulatory factor for interferon gamma-induced activation. Proc. Natl. Acad. Sci. USA 89**:**8676-8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Podschun, R., and U. Ullmann. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 11**:**589-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raetz, C. R. 1990. Biochemistry of endotoxins. Annu. Rev. Biochem. 59**:**129-170. [DOI] [PubMed] [Google Scholar]
- 36.Rubins, J. B., and C. Pomeroy. 1997. Role of gamma interferon in the pathogenesis of bacteremic pneumococcal pneumonia. Infect. Immun. 65**:**2975-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahm, D. F., M. K. Marsilio, and G. Piazza. 1999. Antimicrobial resistance in key bloodstream bacterial isolates: electronic surveillance with the Surveillance Network Database-USA. Clin. Infect. Dis. 29**:**259-263. [DOI] [PubMed] [Google Scholar]
- 38.Santucci, L., S. Fiorucci, M. Chiorean, P. M. Brunori, F. M. Di Matteo, A. Sidoni, G. Migliorati, and A. Morelli. 1996. Interleukin 10 reduces lethality and hepatic injury induced by lipopolysaccharide in galactosamine-sensitized mice. Gastroenterology 111**:**736-744. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki, S., S. Nishikawa, T. Miura, M. Mizuki, K. Yamada, H. Madarame, Y. I. Tagawa, Y. Iwakura, and A. Nakane. 2000. Interleukin-4 and interleukin-10 are involved in host resistance to Staphylococcus aureus infection through regulation of gamma interferon. Infect. Immun. 68**:**2424-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawa, T., D. B. Corry, M. A. Gropper, M. Ohara, K. Kurahashi, and J. P. Wiener-Kronish. 1997. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J. Immunol. 159**:**2858-2866. [PubMed] [Google Scholar]
- 41.Schultz, M. J., A. W. Rijneveld, P. Speelman, S. J. van Deventer, and T. van der Poll. 2001. Endogenous interferon-gamma impairs bacterial clearance from lungs during Pseudomonas aeruginosa pneumonia. Eur. Cytokine Netw. 12**:**39-44. [PubMed] [Google Scholar]
- 42.Shinagawa, T., K. Yoshioka, S. Kakumu, T. Wakita, T. Ishikawa, Y. Itoh, and M. Takayanagi. 1991. Apoptosis in cultured rat hepatocytes: the effects of tumour necrosis factor alpha and interferon gamma. J. Pathol. 165**:**247-253. [DOI] [PubMed] [Google Scholar]
- 43.Silva, A. T., and J. Cohen. 1992. Role of interferon-gamma in experimental gram-negative sepsis. J. Infect. Dis. 166**:**331-335. [DOI] [PubMed] [Google Scholar]
- 44.So, H. S., B. H. Jung, S. S. Yeum, J. S. Park, M. S. Kim, J. H. Lee, S. Y. Chung, S. Choi, H. J. Chae, H. R. Kim, C. B. Ko, H. T. Chung, and R. Park. 2000. LPS induces direct death of IFN-gamma primed murine embryonic hepatocyte, BNL CL2 cells in a TNF-alpha independent manner. Immunol. Investig. 29**:**383-396. [PubMed] [Google Scholar]
- 45.Standiford, T. J., J. M. Wilkowski, T. H. Sisson, N. Hattori, B. Mehrad, K. A. Bucknell, and T. A. Moore. 1999. Intrapulmonary tumor necrosis factor gene therapy increases bacterial clearance and survival in murine gram-negative pneumonia. Hum. Gene Ther. 10**:**899-909. [DOI] [PubMed] [Google Scholar]
- 46.ten Hagen, T. L., W. van Vianen, and I. A. Bakker-Woudenberg. 1995. Modulation of nonspecific antimicrobial resistance of mice to Klebsiella pneumoniae septicemia by liposome-encapsulated muramyl tripeptide phosphatidylethanolamine and interferon-gamma alone or combined. J. Infect. Dis. 171**:**385-392. [DOI] [PubMed] [Google Scholar]
- 47.ten Hagen, T. L., W. van Vianen, H. F. Savelkoul, H. Heremans, W. A. Buurman, and I. A. Bakker-Woudenberg. 1998. Involvement of T cells in enhanced resistance to Klebsiella pneumoniae septicemia in mice treated with liposome-encapsulated muramyl tripeptide phosphatidylethanolamine or gamma interferon. Infect. Immun. 66**:**1962-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toyonaga, T., O. Hino, S. Sugai, S. Wakasugi, K. Abe, M. Shichiri, and K. Yamamura. 1994. Chronic active hepatitis in transgenic mice expressing interferon-gamma in the liver. Proc. Natl. Acad. Sci. USA 91**:**614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuji, H., N. Mukaida, A. Harada, S. Kaneko, E. Matsushita, Y. Nakanuma, H. Tsutsui, H. Okamura, K. Nakanishi, Y. Tagawa, Y. Iwakura, K. Kobayashi, and K. Matsushima. 1999. Alleviation of lipopolysaccharide-induced acute liver injury in Propionibacterium acnes-primed IFN-gamma-deficient mice by a concomitant reduction of TNF-alpha, IL-12, and IL-18 production. J. Immunol. 162**:**1049-1055. [PubMed] [Google Scholar]
- 50.van der Poll, T., C. V. Keogh, W. A. Buurman, and S. F. Lowry. 1997. Passive immunization against tumor necrosis factor-alpha impairs host defense during pneumococcal pneumonia in mice. Am. J. Respir. Crit. Care Med. 155**:**603-608. [DOI] [PubMed] [Google Scholar]
- 51.van der Poll, T., A. Marchant, W. A. Buurman, L. Berman, C. V. Keogh, D. D. Lazarus, L. Nguyen, M. Goldman, L. L. Moldawer, and S. F. Lowry. 1995. Endogenous IL-10 protects mice from death during septic peritonitis. J. Immunol. 155**:**5397-5401. [PubMed] [Google Scholar]
- 52.van der Poll, T., A. Marchant, C. V. Keogh, M. Goldman, and S. F. Lowry. 1996. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J. Infect. Dis. 174**:**994-1000. [DOI] [PubMed] [Google Scholar]
- 53.Walley, K. R., N. W. Lukacs, T. J. Standiford, R. M. Strieter, and S. L. Kunkel. 1996. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect. Immun. 64**:**4733-4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yinnon, A. M., A. Butnaru, D. Raveh, Z. Jerassy, and B. Rudensky. 1996. Klebsiella bacteraemia: community versus nosocomial infection. QJM 89**:**933-941. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida, K., T. Matsumoto, K. Tateda, K. Uchida, S. Tsujimoto, Y. Iwakurai, and K. Yamaguchi. 2001. Protection against pulmonary infection with Klebsiella pneumoniae in mice by interferon-gamma through activation of phagocytic cells and stimulation of production of other cytokines. J. Med. Microbiol. 50**:**959-964. [DOI] [PubMed] [Google Scholar]
- 56.Young, H. A., and K. J. Hardy. 1995. Role of interferon-gamma in immune cell regulation. J. Leukoc. Biol. 58**:**373-381. [PubMed] [Google Scholar]