Apolipoprotein (apo) E4 enhances amyloid β peptide production in cultured neuronal cells: ApoE structure as a potential therapeutic target (original) (raw)
Abstract
Apolipoprotein (apo) E4 is a major risk factor for Alzheimer's disease, and many studies have suggested that apoE has isoform-specific effects on the deposition or clearance of amyloid β (Aβ) peptides. We examined the effects of apoE isoforms on the processing of amyloid precursor protein (APP) and on Aβ production in rat neuroblastoma B103 cells stably transfected with human wild-type APP695 (B103-APP). Lipid-poor apoE4 increased Aβ production in B103-APP cells to a greater extent than lipid-poor apoE3 (60% vs. 30%) due to more pronounced stimulation of APP recycling by apoE4 than apoE3. The difference in Aβ production was abolished by preincubating the cells with the receptor-associated protein (25 nM), which blocks the low-density lipoprotein receptor-related protein (LRP) pathway, or by reducing LRP expression by small interference RNA. The differences were also attenuated by replacing Arg-61 with threonine in apoE4 or pretreating apoE4 with small molecules, both of which abolish apoE4 intramolecular domain interaction. Thus, apoE4 appears to modulate APP processing and Aβ production through both the LRP pathway and domain interaction. These findings provide insights into why apoE4 is associated with increased risk for Alzheimer's disease and may represent a potential target for drug development.
Keywords: Alzheimer's disease, neurodegeneration
Human apolipoprotein (apo) E, a major component of lipoproteins, plays a central role in the metabolism and redistribution of lipids such as cholesterol (1-3). Synthesized primarily in the liver, apoE is also produced in abundance in the brain and has significant functions in central nervous system integrity and remodeling (4-10). Human apoE exists in three major isoforms, apoE2, apoE3, and apoE4, which differ in primary structure (2, 5, 11). ApoE4 is a major risk factor for Alzheimer's disease (AD) (12-15), but the underlying mechanism is poorly understood.
One of the neuropathological hallmarks of AD is senile plaques of primarily aggregated forms of amyloid β (Aβ) peptides (16, 17). Aβ is a proteolytic cleavage product of amyloid precursor protein (APP) (18), a transmembrane protein that undergoes two types of proteolytic processing during intracellular transport (17). In a nonamyloidogenic pathway, cleavage by α-secretase generates a secreted form of APP (sAPPα). In an amyloidogenic pathway, cleavage by β- and γ-secretases generates Aβ. Although cleavage by β- and γ-secretases occurs in the rough endoplasmic reticulum and transGolgi network (17), the majority of secreted Aβ may actually be generated along the endosomal pathway as mature APP recycles back from the cell surface (19).
ApoE colocalizes with extracellular amyloid deposits (12, 20). Considerable evidence suggests that apoE isoforms interact differently with Aβ, resulting in isoform-specific effects on Aβ deposition or clearance (21-25). Studies in transgenic animals have suggested that, compared with no apoE, mouse apoE enhances and human apoE reduces Aβ deposition (26). However, in older mice, apoE4 enhances Aβ deposition compared with apoE3 (26).
Few studies have focused on whether apoE also influences APP processing and Aβ production (27, 28). ApoE is a major cholesterol transport protein, and cholesterol increases Aβ production (29) by affecting α-, β-, or γ-secretase activity (30-34). Furthermore, statins, which reduce cholesterol levels, decrease Aβ levels in an animal model (35) and may lower the risk for AD in humans (36, 37).
In the present study, we investigated whether apoE affects APP processing and Aβ production directly by modulating secretase activities or indirectly by altering cholesterol metabolism.
Materials and Methods
Reagents. Purified recombinant human apoE3, apoE4, the Thr-61 mutant of apoE4 (apoE4-Thr-61), and the receptor-related protein (RAP) were produced as described (38-40). The mAb 6E10 against residues 1-17 of Aβ (detecting sAPPα) and the mAb 4G8 against residues 17-24 of Aβ were from Signet Laboratories (Dedham, MA). mAbs 266 and 3D6, which recognize residues 1-5 and 13-28 of Aβ, respectively, were from Elan Pharmaceuticals (South San Francisco, CA). mAb 1G7, which recognizes the extracellular domain of APP (residues 380-665), was kindly provided by Edward H. Koo (University of California at San Diego, La Jolla). Lovastatin was from Merck Sharp & Dohme. GIND-25 (azocarmine G), mevalonate, and methyl-β-cyclodextrin were from Sigma. GIND-105 (3-butyl-1-ethyl-5-[2-(3-sulfobutyl-benzo[1,3]oxazolin-2-ylidene)-ethylidene]-2-thioxo-imidazolidin-4-one potassium salt) was from Synthon (Wolfen, Germany).
Preparation of Lipoproteins. Rabbit β-migrating very low-density lipoproteins (β-VLDL) were prepared from rabbits fed a high-cholesterol diet as described (41). ApoE-enriched β-VLDL were prepared by incubating the particles with human apoE isoforms at 37°C for 1 h.
Cell Culture. Rat neuroblastoma B103 cells stably expressing human wild-type APP (hAPP695wt) (42, 43) were generated in Lennart Mucke's laboratory at the Gladstone Institute of Neurological Disease and maintained in DMEM (Gibco) containing 400 μg/ml G418, 10% FBS, and 5% horse serum at 37°C. Twenty-four hours after plating into 48-well plates (1 × 105 cells per well), cells were washed twice with serum-free DMEM and cultured for 24 h in DMEM containing 1% N-2 supplement (Gibco) to induce differentiation. The cells were treated with β-VLDL (25 μg/ml cholesterol), recombinant human apoE isoform-enriched β-VLDL (7.5 μg/ml apoE and 25 μg/ml cholesterol), or recombinant human apoE (7.5 μg/ml apoE) in fresh DMEM containing 1% N-2 supplement for 24 h. In some experiments, RAP (25 nM or 1 μM) was added to the cells 1 h before apoE treatment.
In some experiments, cells were treated with lovastatin as described but with a minor modification (34). Briefly, cells were maintained in differentiation medium containing 4 μM lovastatin and 0.25 mM mevalonate for 24 h. After treatment for 5 min with 5 mM methyl-β-cyclodextrin to deplete cell membrane cholesterol (32, 33), the cells were incubated in fresh differentiation medium containing lovastatin and mevalonate for 24 h. The conditioned medium was collected; cellular cholesterol was extracted with chloroform/methanol (44) and quantitated with a kit from Abbott.
Detection of sAPPα. Media conditioned for 24 h were normalized by protein content and subjected to SDS/PAGE. Proteins were transferred onto nitrocellulose membranes. sAPPα was detected with mAb 6E10 and visualized with an enhanced chemiluminescence system (Pierce).
Aβ Assay. Aβ secreted into the medium was detected with a sandwich ELISA by using mAb 266 as a capture antibody and 3D6 as a detection antibody, as described (45). Aβ was quantified from a standard curve (freshly prepared Aβ42; Bachem) and normalized to total cellular protein.
Cell Association and Degradation of 125I-Aβ40. B103 cells stably transfected with a neomycin-resistance gene (B103-neo) were incubated with a 125I-labeled 40-aa form of Aβ (125I-Aβ40) [225 pg/ml/0.1 μCi/ml (1 Ci = 37 GBq)], prepared as described (46), at 37°C in the presence of apoE3 or apoE4 (7.5 μg/ml). Culture medium was collected after 24 h, and the degradation products of 125I-Aβ40 in the medium were assayed as described (47). The cells were washed five times on ice with PBS containing 0.2% BSA and once with PBS and lysed with 0.1 M NaOH. Cell-associated 125I-Aβ40 was determined by counting the radioactivity in the cell lysate.
β-Secretase Assay. β-Secretase activity in lysates of cells treated with or without apoE isoforms was assayed as described (33) with a fluorogenic substrate (10 μM, MCA-Glu-Val-Lys-Met-Asp-Ala-Glu-Phe-Lys-DNP-NH2) (Calbiochem). Fluorescence was recorded on a spectrofluorimeter for 10 min with excitation and emission wavelengths of 325 and 393 nm, respectively. β-Secretase activity was calculated as the increase in fluorescence per min per mg of cellular protein.
APP Internalization Assay. The internalization of cell-surface APP was measured as described with mAb 1G7, which recognizes the N-terminal domain of APP (19, 48).
Search for Compounds to Disrupt ApoE4 Domain Interaction. The Available Chemicals Directory of >200,000 compounds (Molecular Design Limited, San Leandro, CA) was screened computationally by using dock 4.0 (49, 50) and the x-ray structures of the N-terminal domains of human apoE4 and apoE3 (51, 52). To identify molecules that could disrupt the domain interaction interface of apoE4, a docking site centered on residues 109, 112, and 61 was defined. The negative image of this site was defined by a set of overlapping spheres that collectively filled the docking site. The centers of the spheres were then treated as possible ligand atom positions, and each molecule from the Available Chemicals Directory was combinatorially placed in the site in hundreds to thousands of positions by a combinatorial strategy. Simple scoring functions, one reflecting shape complementarity and another consisting of a Lennard-Jones van der Waals term and a Coulombic electrostatic term, were used to evaluate the plausibility of each docked conformation. Precalculated grids allowed rapid scoring (53). For each molecule, the best position according to each scoring function was saved. At the end of the process, several hundred of the best-scoring molecules were examined graphically by using the shape- and energy-based functions.
Over 2,000 molecules that scored well when docked to apoE4 were obtained from the dock search. In most cases, molecules that also appeared on the corresponding list for apoE3 were removed from consideration to gain selectivity. Compounds were further screened visually by using the graphics program midas (54) for electrostatic and shape complementarity with the target site (55). This process yielded 115 compounds, with 65 initial recommendations (one per set of close analogs).
Preparation of Emulsion Particles and VLDL-Binding Affinity Assay. VLDL-like emulsion particles were prepared by using triolein (160 mg) and l-α-phosphatidylcholine (40 mg), and the binding of 125I-labeled apoE3 and apoE4 to the particles was determined as described (38, 52), with and without small-molecule compounds.
Small Interference RNA (siRNA) Preparation and Transfection. Double-stranded siRNAs specific for the rat LDL receptor-related protein (LRP) gene were chemically synthesized by Dharmacon (Lafayette, CO) according to the following sequences: siLRP6600 sense, 5′-UGGCAUCUCAGUAGACUAUUU-3′, and antisense, 5′-AUAGUCUACUGAGAUGCCAUU-3′; siLRP12348 sense, 5′-UGUGUACUGGACCGAUUCAUU-3′, and antisense, 5′-UGAAUCGGUCCAGUACACAUU-3′. B103-APP cells grown in 48-well plates (1.0 × 105 cells/well) for 24 h were transfected with both siRNAs (2 μg/ml each) with Lipofectamine (Invitrogen). The transfection complex was diluted in a final volume of 250 μl of Opti-MEM and was replaced 3 h later with DMEM supplemented with 10% FBS and 5% horse serum. Seventy-two hours after transfection, cells were treated with apoE3 or apoE4, and Aβ production was assayed 24 h later, as described above.
Statistical Analysis. Results are reported as mean ± SD. Differences were evaluated by t test or ANOVA.
Results
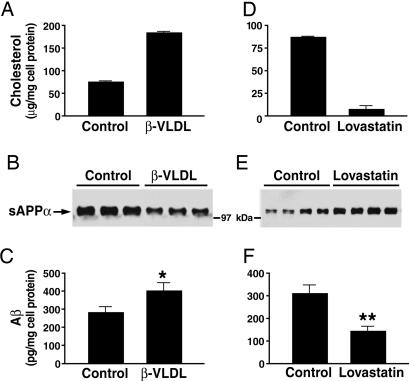
Aβ Production Is Inversely Related to Cellular Cholesterol Content. In stably transfected rat neuroblastoma B103 cells expressing human APP at levels similar to those in mouse brains (43), incubation with cholesterol-rich β-VLDL increased the cholesterol content (Fig. 1_A_), decreased sAPPα secretion (Fig. 1_B_), and increased Aβ production (Fig. 1_C_). When cellular cholesterol was lowered with lovastatin (Fig. 1_D_), sAPPα secretion increased (Fig. 1_E_), and Aβ production decreased (Fig. 1_F_).
Fig. 1.
Effects of cellular cholesterol content and apoE isoforms on the secretion of sAPPα and Aβ. B103-APP cells were treated with β-VLDL (25 μg/ml cholesterol), lovastatin (4 μM), or medium alone (control), as described. Cellular cholesterol content was determined after treatment with β-VLDL (A) or lovastatin (D) treatment. sAPPα levels in 24-h conditioned medium were determined by using mAb 6E10 (1 μg/ml) after treatment with β-VLDL (B, lanes 1-3 and 4-6 are replicates of the same treatment) or lovastatin (E, lanes 1-4 and 5-8 are replicates of the same treatment). (C and F) Total Aβ in 24-h conditioned medium was detected by ELISA after treatment with β-VLDL (C) or lovastatin (F). Mean ± SD of two experiments, each repeated four to six times. *, P < 0.05 vs. control; **, P < 0.01 vs. control.
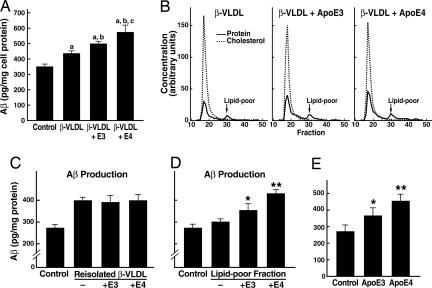
Lipid-Poor Human ApoE Isoforms Differ in Effects on APP Processing and Aβ Production. Incubation of cultured B103-APP cells with apoE-enriched rabbit β-VLDL stimulated Aβ production compared with cells incubated without lipoproteins or with β-VLDL alone (Fig. 2_A_). However, when β-VLDL enriched with human apoE was fractionated by fast-performance liquid chromatography into apoE-containing β-VLDL and a smaller fraction of lipid-poor apoE (Fig. 2_B_), the reisolated β-VLDL enriched with either apoE3 or apoE4 stimulated Aβ production to the same extent (Fig. 2_C_). In addition, the apoE-containing β-VLDL gave results identical to those of reisolated β-VLDL that were not incubated with human apoE (Fig. 2_C_). Interestingly, there was no difference in the cholesterol content of any of the treated cells (data not shown).
Fig. 2.
Lipid-poor apoE fractions or lipid-free apoE increase Aβ production in an isoform-specific manner. (A) ApoE3- or apoE4-enriched β-VLDL was prepared by incubating apoE isoforms with β-VLDL at 37°C for 1 h. Cells were then treated with either medium alone (control), β-VLDL (25 μg/ml cholesterol), or apoE-enriched β-VLDL (7.5 μg/ml apoE and 25 μg/ml cholesterol). Conditioned media were collected after 24 h and assayed for total Aβ by ELISA. Values are the mean ± SD of two experiments, each repeated four times for each condition. (a) P < 0.05 vs. control; (b) P < 0.05 vs. β-VLDL; (c) P < 0.05 vs. β-VLDL + apoE3. (B) ApoE isoforms were incubated with β-VLDL at 37°C for 1 h. The apoE3- or apoE4-enriched β-VLDL and β-VLDL alone were then fractionated by fast-performance liquid chromatography as described. The elution profiles, which were monitored by quantitation of cholesterol and protein, showed two distinct fractions: a major β-VLDL or apoE-containing β-VLDL fraction and a smaller, lipid-poor apoE-containing fraction. (C and D) Samples from the major β-VLDL or apoE-containing β-VLDL fractions (C) were normalized by cholesterol content and incubated with B103-APP cells at 25 μg/ml cholesterol. Samples from the smaller, lipid-poor apoE-containing fractions (D) were normalized by protein content and incubated with the cells at 7.5 μg/ml protein. The 24-h conditioned media were assayed for total Aβ by ELISA. Values are the mean ± SD of two experiments, each repeated four to six times for each condition. *, P < 0.05 vs. control (medium only). **, P < 0.05 vs. lipid-poor fraction of apoE3 or free apoE3. (E) Recombinant human apoE3 or apoE4 (7.5 μg/ml) was incubated with B103-APP cells for 24 h. The conditioned media were assayed for total Aβ by ELISA. Values are the mean ± SD of three experiments, each repeated four to six times for each condition. *, P < 0.05 vs. control (medium only). **, P < 0.05 vs. apoE3.
However, the lipid-poor apoE fraction (Fig. 2_B_) increased Aβ production in an isoform-specific manner: apoE4 was more active than apoE3 (Fig. 2_D_). Treating the cells with lipid-free apoE4 also increased Aβ production more than lipid-free apoE3 (≈60% vs. 30%) (Fig. 2_E_). Because the cellular cholesterol content was not changed by lipid-free apoE (Fig. 6, which is published as supporting information on the PNAS web site), these data suggest that apoE and cholesterol may regulate Aβ production by different mechanisms.
To explore the mechanisms for the isoform-specific effects of apoE on Aβ production, we determined whether the apoE isoforms interact with Aβ and prevent its reuptake and degradation differentially, thereby retaining different amounts of Aβ in the medium. 125I-labeled Aβ (350 pg/ml) was incubated with B103-neo cells with or without apoE3 or apoE4. The cell association and degradation of Aβ were not significantly different after incubation for 24 h (Table 1, which is published as supporting information on the PNAS web site). Furthermore, apoE3 and apoE4 had no significant effect on sAPPα secretion (Fig. 6_B_) or α-secretase activity (data not shown). Similarly, apoE3 and apoE4 did not significantly affect β-secretase enzyme activity in whole-cell lysates (Fig. 6_C_).
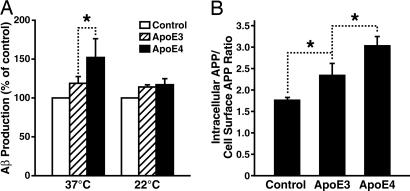
ApoE3 and ApoE4 Differ in Effects on APP Recycling. Because the majority of secreted Aβ is generated within the endosomal pathway when mature APP recycles back from the cell surface to endosomes (19, 48, 56-59), it is possible that differences in the effect of apoE3 and apoE4 on Aβ production reflect differences in APP recycling. In support of this hypothesis, inhibition of endocytosis by growing cells at 22°C completely abolished the isoform-specific effects of apoE on Aβ production (Fig. 3_A_).
Fig. 3.
ApoE3 and apoE4 exert isoform-specific effects on Aβ production through their differential effects on intracellular APP recycling. (A) Blockage of APP recycling by culturing cells at low temperature abolished the apoE4-enhanced Aβ production. Recombinant human apoE3 or apoE4 (7.5 μg/ml) was incubated with B103-APP cells at either 22°C or 37°C for 24 h. The conditioned media were assayed for total Aβ by ELISA. Values are the mean ± SD of two experiments, each repeated four to six times for each condition. *, P < 0.05. (B) ApoE4 increased the internalization of cell-surface APP to a greater extent than apoE3. Internalization of cell-surface APP after apoE treatment was determined by measuring the uptake of radioiodinated 1G7 antibody, as described in Materials and Methods. The results are expressed as a ratio of the radioactivity associated with the internalized vs. cell-surface pools of APP. Values are the mean ± SD of two experiments, each repeated three times for each condition. *, P < 0.05.
To assess further the effects of apoE on APP recycling, we used the internalization assay established by Koo and associates (19, 48). ApoE increased the internalization (or recycling) of APP in an isoform-specific manner, with apoE4 being more effective than apoE3 (Fig. 3_B_).
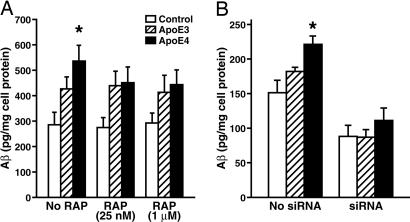
LRP Mediates Enhancement of Aβ Production by ApoE4. To identify the receptor that mediates the stimulation of Aβ production by apoE4, B103-APP cells were preincubated without or with RAP at a low concentration (25 nM), which blocks the LRP pathway (60), or at a high concentration (1 μM), which blocks both the LRP and the LDL receptor pathway (61), at 37°C for 1 h and then apoE3 or apoE4 (7.5 μg/ml) was added and incubation continued for 24 h. At both concentrations, RAP abolished the apoE4-induced enhancement of Aβ production (Fig. 4_A_), suggesting that the LRP pathway, but not the LDL receptor pathway, was involved in apoE's effects on Aβ production. However, we cannot exclude the possibility that the LRP and the LDL receptor cooperate in this process. Knockdown (70-80%) of LRP expression with a specific siRNA also abolished the enhancement of Aβ production by apoE4 (Fig. 4_B_), confirming a critical role for the LRP. Interestingly, knockdown of the LRP also significantly decreased Aβ production in control cells, suggesting involvement of the LRP in baseline production of Aβ.
Fig. 4.
The LRP mediates the enhancement of Aβ production by apoE4. (A) B103-APP cells were preincubated without or with RAP at a low concentration (25 nM), which blocks the LRP pathway, or a high concentration (1 μM), which blocks both the LRP and the LDL receptor pathway, at 37°C for 1 h and were then incubated with apoE3 or apoE4 (7.5 μg/ml) for 24 h. The conditioned media were assayed for total Aβ by ELISA. *, P < 0.05 vs. apoE3. (B) B103-APP cells were treated for 3 days with siRNA (2 μg of nucleotides per well) specific for the rat LRP gene and were then incubated with apoE3 or apoE4 (7.5 μg/ml) for 24 h. The conditioned media were collected 24 h after apoE treatment and assayed for total Aβ by ELISA. Values are the mean ± SD of percent of control B103 cells without apoE treatment (n = 4). *, P < 0.05 vs. apoE3.
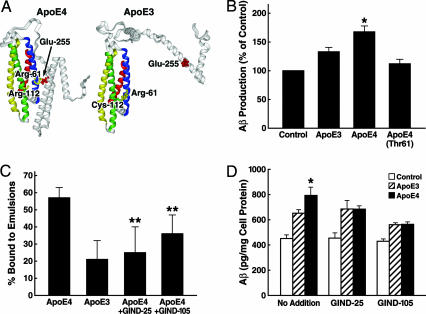
ApoE4 Domain Interaction Enhances Aβ Production by ApoE4. Next, we assessed the role of domain interaction (Fig. 5_A_), a unique property of apoE4, in the stimulation of Aβ production by apoE4. B103-APP cells were incubated at 37°C for 24 h with apoE4-Thr-61, which lacks domain interaction (38, 52), apoE3, or apoE4 (7.5 μg/ml each). ApoE4-Thr-61 abolished the enhancement of Aβ production, suggesting that apoE4 domain interaction is involved in stimulating Aβ production (Fig. 5_B_).
Fig. 5.
ApoE4 domain interaction is responsible for the enhancement of Aβ production by apoE4. (A) A model of apoE4 domain interaction as a target for drug development. (B) B103-APP cells were incubated with apoE3, apoE4, or apoE4-Thr-61 (7.5 μg/ml) at 37°C for 24 h. The conditioned media were collected and assayed for total Aβ by ELISA. Values are the mean ± SD of three experiments, each repeated four times for each condition. *, P < 0.05 vs. apoE3 or apoE4-Thr-61. (C) Both GIND-25 (disulfonate) and GIND-105 (monosulfoalkyl) are capable of blocking apoE4 domain interaction as determined by a VLDL-like emulsion binding assay. Values are the mean ± SD of five to eight assays. **, P < 0.01 for both compounds vs. apoE4 alone. (D) Compounds GIND-25 and -105 abolish the enhancement of Aβ production by apoE4. Recombinant human apoE3 or apoE4 (7.5 μg/ml) was preincubated with or without GIND-25 or -105 (5 μM) at 37°C for 30 min and then further incubated with B103-APP cells for 24 h. The conditioned media were collected and assayed for total Aβ by ELISA. Values are the mean ± SD of three experiments, each repeated three to five times for each condition. *, P < 0.05 vs. apoE3.
Disrupting Domain Interaction with Small Molecules Abolishes the Enhancement of Aβ Production by ApoE4. The preferential binding of apoE4 to VLDL is mediated by domain interaction (38). Of the 65 small-molecule compounds identified by dock screening (49, 50), eight significantly inhibited the binding of apoE4 to VLDL-like emulsion particles (data not shown), suggesting that they disrupt domain interaction. Most of the compounds had little or no effect on the binding of apoE3 to the particles (data not shown). Two compounds (GIND-25, a disulfonate, and GIND-105, a monosulfoalkyl) that significantly inhibited the binding of apoE4 (Fig. 5_C_), but not of apoE3, were selected for further analysis. Both are water-soluble and have no significant toxicity to B103 cells at the micromolar level (data not shown). Both decreased Aβ production induced by apoE4 to levels similar to those induced by apoE3 (Fig. 5_D_).
Discussion
This study shows that apoE3 and apoE4 increased Aβ production in B103 cells stably transfected with human wild-type APP695. However, apoE4 stimulated Aβ production more effectively than apoE3. This isoform-specific effect on Aβ production was mediated by the LRP pathway and results from an alteration in APP recycling but not in cellular cholesterol. Furthermore, the stimulatory effect of apoE4 on Aβ production has been linked to an intramolecular interaction between the two structural domains of apoE4. This biophysical property, “domain interaction,” distinguishes apoE4 from apoE3.
The observation that apoE4 enhances Aβ production more than apoE3 is consistent with some in vivo findings and may help explain why apoE4 is associated with greater Aβ deposition than apoE3 in AD brains and in transgenic mice expressing mutant APP (62-64). For instance, cortical levels of Aβ40 are higher in apoE4- than apoE3-positive AD patients (63). In addition, in mutant APP transgenic mice (line 2576), elevation of mouse apoE correlated with increasing Aβ levels (64), whereas the lack of mouse apoE dramatically reduced Aβ deposition (62). Furthermore, expression of human apoE3 or apoE4 reduced Aβ deposition in young mutant APP transgenic mice on the mouse apoE-null background. In older mice, more Aβ deposits were observed in mutant APP transgenic mice expressing apoE4 than in those expressing apoE3 (26). Although these results may reflect the greater ability of apoE3 to stimulate Aβ clearance (26), our data provide an alternative explanation for the isoform-specific effects of apoE on APP processing and Aβ production. At a young age, a robust Aβ clearance machinery may compensate for the overproduction of Aβ caused by apoE4. During aging, the Aβ clearance machinery may become less effective and be slowly overwhelmed by the stimulatory effect of apoE4, leading to increased Aβ deposits.
ApoE does not have isoform-specific effects on APP processing in glioma cells expressing both apoE and APP (65). We also found that endogenous expression of apoE3 or apoE4 in B103-APP cells did not stimulate Aβ production (data not shown). Thus, the isoform-specific effects of apoE on APP processing and Aβ production might depend on the source of apoE. The isoform-specific effect of exogenous apoE on Aβ production demonstrated here is supported by other studies (27, 28). One study suggests that apoE secreted by astrocytes stimulates Aβ production in neurons (28). We speculate that differences in the lipid composition of apoE-containing particles from different sources may result in different effects on APP processing and Aβ production. Although incubation with recombinant apoE for 24 h increased Aβ production, it is unclear whether apoE formed lipid-poor particles during the incubation by sequestering lipid from cell membranes. Further studies will resolve this issue.
Because cholesterol affects APP processing (30-33, 35-37), we speculated that cholesterol might mediate the effects of apoE on Aβ production. However, our data do not support this idea. Cellular cholesterol levels were similar before and after treatment with either apoE3 or apoE4 (Fig. 4_A_), and neither apoE3 nor apoE4 altered α-secretase activity (Fig. 4_B_). Thus, apoE regulates Aβ production through a mechanism that does not involve cholesterol.
Our data demonstrate that the isoform-specific effects of apoE on Aβ production result from an alteration in APP recycling. ApoE4 increased the intracellular recycling of APP, which could be associated with increased Aβ production compared with apoE3. Several lines of evidence support the concept that enhanced endocytic APP recycling is linked to Aβ production (19, 48). Aβ can be generated in both the endocytic and secretory pathways (17, 19), but the majority of secreted Aβ appears to be generated in the endocytic pathway when mature APP recycles from the plasma membrane (19, 48). In addition, the brains of patients with sporadic forms of AD contain cells (neurons) with enlarged early endosomes and increased expression of rabaptins 4 and 5, findings that are consistent with enhanced endocytic recycling (66, 67). Furthermore, the brains of apoE4-positive AD patients contain neurons with pronounced endocytic pathway markers (68).
Our data also indicate that the LRP, a member of the LDL receptor family (69), may mediate the isoform-specific effects of apoE on Aβ production. Low concentrations of RAP, which interferes with LRP-mediated ligand binding and uptake (60), and LRP-specific siRNA abolished the apoE4-enhanced Aβ production in B103 neurons expressing human APP. Interestingly, RAP only abolished apoE4-enhanced Aβ production, but LRP-specific siRNA reduced Aβ production in both apoE4 and apoE3 cells. This difference might reflect different effects of the two approaches. RAP treatment affects only cell-surface LRP, but siRNA affects both cell-surface and intracellular LRP. Thus, in neurons, it appears that the lipid-poor apoE4 interacts directly with the LRP to alter APP recycling. The LRP mediates the endocytosis and processing of cell-surface APP containing a Kunitz proteinase inhibitor domain (70, 71). In our studies, human APP expressed in B103 cells lacked the Kunitz proteinase inhibitor domain, excluding an extracellular interaction between APP and the LRP through this mechanism. Alternatively, the LRP may interact with APP through an intracellular interaction of the cytoplasmic domains of these molecules and adaptor proteins (72-74). Importantly, the adaptor protein Fe65 can mediate such an interaction (72, 75). We speculate that the binding of apoE4 to the LRP stimulates an interaction between the LRP and APP, which accelerates APP recycling and thereby enhances Aβ production. However, it remains to be determined whether there is a significant difference between the binding affinities of apoE3 and apoE4 to the LRP on neuronal cells.
The different effects of apoE3 and apoE4 on Aβ production have been linked to a biophysical property that distinguishes the isoforms. ApoE possesses N- and C-terminal domains (76, 77). In apoE4, these domains interact (38, 52), and the interaction is mediated through a salt bridge between Arg-61 and Glu-255 (38). Analysis of the 3D structure of the N-terminal region of apoE4 reveals that Arg-112, which distinguishes apoE4 from apoE3 (apoE3 has cysteine at this residue), appears to reorient the side chain of Arg-61 away from the surface of the four-helix bundle, allowing it to interact with residues in the C-terminal domain (52). In apoE3, Arg-61 is not as exposed, and domain interaction does not occur to the same extent that it does in apoE4. The substitution of threonine for Arg-61 or treatment of apoE4 with compounds GIND-25 and -105, both of which abolish domain interaction and disrupt apoE4's functional activity (38, 78) (current study), reduced the apoE4-enhanced Aβ production to the level obtained with apoE3. This finding suggests that a small molecule designed to block apoE4 domain interaction by binding to the interface between the two domains in the vicinity of residue 61 and altering the conformation of apoE4 could reduce its detrimental effects in neurodegenerative disorders by converting it to an apoE3-like molecule. The current study provides a theoretical basis and a proof of concept for developing a novel therapeutic intervention for the treatment of AD.
Supplementary Material
Supporting Information
Acknowledgments
We thank Lennart Mucke and Gui-Qiu Yu for their review of and helpful comments on our manuscript, Karina Fantillo and Sylvia Richmond for manuscript preparation, Gary Howard and Stephen Ordway for editorial assistance, John C. W. Carroll and Jack Hull for graphics, and Chris Goodfellow for photography. This work was supported in part by National Institutes of Health Grants AG22074, NS46465, HL37063, and HL64162.
Author contributions: S.Y., Y.H., K.H.W., and R.W.M. designed research; S.Y., K.M., L.D., G.G., and E.C.M. performed research; K.M., L.D., G.G., and E.C.M. contributed new reagents/analytic tools; S.Y., Y.H., L.D., E.C.M., F.E.C., I.D.K., K.H.W., and R.W.M. analyzed data; and S.Y., Y.H., E.C.M., F.E.C., I.D.K., K.H.W., and R.W.M. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: Aβ, amyloid β; AD, Alzheimer's disease; apo, apolipoprotein; APP, amyloid precursor protein; LDL, low-density lipoprotein; β-VLDL, β-migrating very low-density lipoprotein; LRP, LDL receptor-related protein; RAP, receptor-related protein; sAPPα, a secreted form of APP; siRNA, small interference RNA.
References
- 1.Mahley, R. W. (1988) Science 240**,** 622-630. [DOI] [PubMed] [Google Scholar]
- 2.Mahley, R. W. & Rall, S. C., Jr. (2001) in The Metabolic and Molecular Bases of Inherited Disease, eds. Scriver, C. R., Beaudet, A. L., Sly, W. S., Valle, D., Childs, B., Kinzler, K. W. & Vogelstein, B. (McGraw-Hill, New York), Vol. 2, pp. 2835-2862. [Google Scholar]
- 3.Huang, Y. & Mahley, R. W. (1999) in Plasma Lipids and Their Role in Disease, eds. Barter, P. J. & Rye, K.-A. (Harwood, Amsterdam), pp. 257-284.
- 4.Weisgraber, K. H., Roses, A. D. & Strittmatter, W. J. (1994) Curr. Opin. Lipidol. 5**,** 110-116. [DOI] [PubMed] [Google Scholar]
- 5.Mahley, R. W. & Rall, S. C., Jr. (2000) Annu. Rev. Genomics Hum. Genet. 1**,** 507-537. [DOI] [PubMed] [Google Scholar]
- 6.Mahley, R. W. & Huang, Y. (1999) Curr. Opin. Lipidol. 10**,** 207-217. [DOI] [PubMed] [Google Scholar]
- 7.Nathan, B. P., Bellosta, S., Sanan, D. A., Weisgraber, K. H., Mahley, R. W. & Pitas, R. E. (1994) Science 264**,** 850-852. [DOI] [PubMed] [Google Scholar]
- 8.Pitas, R. E., Boyles, J. K., Lee, S. H., Foss, D. & Mahley, R. W. (1987) Biochim. Biophys. Acta 917**,** 148-161. [DOI] [PubMed] [Google Scholar]
- 9.Elshourbagy, N. A., Liao, W. S., Mahley, R. W. & Taylor, J. M. (1985) Proc. Natl. Acad. Sci. USA 82**,** 203-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyles, J. K., Pitas, R. E., Wilson, E., Mahley, R. W. & Taylor, J. M. (1985) J. Clin. Invest. 76**,** 1501-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rall, S. C., Jr., Weisgraber, K. H. & Mahley, R. W. (1982) J. Biol. Chem. 257**,** 4171-4178. [PubMed] [Google Scholar]
- 12.Strittmatter, W. J., Saunders, A. M., Schmechel, D., Pericak-Vance, M., Enghild, J., Salvesen, G. S. & Roses, A. D. (1993) Proc. Natl. Acad. Sci. USA 90**,** 1977-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Small, G. W., Roses, A. D., Haines, J. L. & Pericak-Vance, M. A. (1993) Science 261**,** 921-923. [DOI] [PubMed] [Google Scholar]
- 14.Saunders, A. M., Strittmatter, W. J., Schmechel, D., St George-Hyslop, P. H., Pericak-Vance, M. A., Joo, S. H., Rosi, B. L., Gusella, J. F., Crapper-MacLachlan, D. R., Alberts, M. J., et al. (1993) Neurology 43**,** 1467-1472. [DOI] [PubMed] [Google Scholar]
- 15.Farrer, L. A., Cupples, L. A., Haines, J. L., Hyman, B., Kukull, W. A., Mayeux, R., Myers, R. H., Pericak-Vance, M. A., Risch, N. & Van Duijn, C. M. (1997) J. Am. Med. Assoc. 278**,** 1349-1356. [PubMed] [Google Scholar]
- 16.Glenner, G. G. & Wong, C. W. (1984) Biochem. Biophys. Res. Commun. 120**,** 885-890. [DOI] [PubMed] [Google Scholar]
- 17.Selkoe, D. J. (1991) Neuron 6**,** 487-498. [DOI] [PubMed] [Google Scholar]
- 18.Tanzi, R. E., Gusella, J. F., Watkins, P. C., Bruns, G. A. P., St George-Hyslop, P., Van Keuren, M. L., Patterson, D., Pagan, S., Kurnit, D. M. & Neve, R. L. (1987) Science 235**,** 880-884. [DOI] [PubMed] [Google Scholar]
- 19.Koo, E. H. & Squazzo, S. L. (1994) J. Biol. Chem. 269**,** 17386-17389. [PubMed] [Google Scholar]
- 20.Namba, Y., Tomonaga, M., Kawasaki, H., Otomo, E. & Ikeda, K. (1991) Brain Res. 541**,** 163-166. [DOI] [PubMed] [Google Scholar]
- 21.Kounnas, M. Z., Moir, R. D., Rebeck, G. W., Bush, A. I., Argraves, W. S., Tanzi, R. E., Hyman, B. T. & Strickland, D. K. (1995) Cell 82**,** 331-340. [DOI] [PubMed] [Google Scholar]
- 22.LaDu, M. J., Falduto, M. T., Manelli, A. M., Reardon, C. A., Getz, G. S. & Frail, D. E. (1994) J. Biol. Chem. 269**,** 23403-23406. [PubMed] [Google Scholar]
- 23.Ma, J., Yee, A., Brewer, H. B., Jr., Das, S. & Potter, H. (1994) Nature 372**,** 92-94. [DOI] [PubMed] [Google Scholar]
- 24.Sanan, D. A., Weisgraber, K. H., Russell, S. J., Mahley, R. W., Huang, D., Saunders, A., Schmechel, D., Wisniewski, T., Frangione, B., Roses, A. D., et al. (1994) J. Clin. Invest. 94**,** 860-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strittmatter, W. J., Weisgraber, K. H., Huang, D. Y., Dong, L.-M., Salvesen, G. S., Pericak-Vance, M., Schmechel, D., Saunders, A. M., Goldgaber, D. & Roses, A. D. (1993) Proc. Natl. Acad. Sci. USA 90**,** 8098-8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holtzman, D. M., Bales, K. R., Tenkova, T., Fagan, A. M., Parsadanian, M., Sartorius, L. J., Mackey, B., Olney, J., McKeel, D., Wozniak, D., et al. (2000) Proc. Natl. Acad. Sci. USA 97**,** 2892-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masinovsky, B., Urdal, D. & Gallatin, W. M. (1990) J. Immunol. 145**,** 2886-2895. [PubMed] [Google Scholar]
- 28.Vincent, B. & Smith, J. D. (2001) Eur. J. Neurosci. 14**,** 256-266. [DOI] [PubMed] [Google Scholar]
- 29.Howland, D. S., Trusko, S. P., Savage, M. J., Reaume, A. G., Lang, D. M., Hirsch, J. D., Maeda, N., Siman, R., Greenberg, B. D., Scott, R. W., et al. (1998) J. Biol. Chem. 273**,** 16576-16582. [DOI] [PubMed] [Google Scholar]
- 30.Wrenn, S. M., Jr., Parks, J. S., Immermann, F. W. & Rudel, L. L. (1995) J. Lipid Res. 36**,** 1199-1210. [PubMed] [Google Scholar]
- 31.Wain-Hobson, S. (1996) Nature 384**,** 117-118. [DOI] [PubMed] [Google Scholar]
- 32.Bodovitz, S. & Klein, W. L. (1996) J. Biol. Chem. 271**,** 4436-4440. [DOI] [PubMed] [Google Scholar]
- 33.Kojro, E., Gimpl, G., Lammich, S., März, W. & Fahrenholz, F. (2001) Proc. Natl. Acad. Sci. USA 98**,** 5815-5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simons, M., Keller, P., De Strooper, B., Beyreuther, K., Dotti, C. G. & Simons, K. (1998) Proc. Natl. Acad. Sci. USA 95**,** 6460-6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fassbender, K., Simons, M., Bergmann, C., Stroick, M., Lütjohann, D., Keller, P., Runz, H., Kühl, S., Bertsch, T., von Bergmann, K., et al. (2001) Proc. Natl. Acad. Sci. USA 98**,** 5856-5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jick, H., Zornberg, G. L., Jick, S. S., Seshadri, S. & Drachman, D. A. (2000) Lancet 356**,** 1627-1631. [DOI] [PubMed] [Google Scholar]
- 37.Wolozin, B., Kellman, W., Ruosseau, P., Celesia, G. G. & Siegel, G. (2000) Arch. Neurol. 57**,** 1439-1443. [DOI] [PubMed] [Google Scholar]
- 38.Dong, L.-M. & Weisgraber, K. H. (1996) J. Biol. Chem. 271**,** 19053-19057. [DOI] [PubMed] [Google Scholar]
- 39.Morrow, J. A., Hatters, D. M., Lu, B., Höchtl, P., Oberg, K. A., Rupp, B. & Weisgraber, K. H. (2002) J. Biol. Chem. 277**,** 50380-50385. [DOI] [PubMed] [Google Scholar]
- 40.Morrow, J. A., Arnold, K. S. & Weisgraber, K. H. (1999) Protein Expr. Purif. 16**,** 224-230. [DOI] [PubMed] [Google Scholar]
- 41.Ji, Z.-S., Brecht, W. J., Miranda, R. D., Hussain, M. M., Innerarity, T. L. & Mahley, R. W. (1993) J. Biol. Chem. 268**,** 10160-10167. [PubMed] [Google Scholar]
- 42.Xu, X., Yang, D., Wyss-Coray, T., Yan, J., Gan, L., Sun, Y. & Mucke, L. (1999) Proc. Natl. Acad. Sci. USA 96**,** 7547-7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esposito, L., Gan, L., Yu, G.-Q., Essrich, C. & Mucke, L. (2004) J. Neurochem. 91**,** 1260-1274. [DOI] [PubMed] [Google Scholar]
- 44.Huang, Y., von Eckardstein, A., Wu, S., Maeda, N. & Assmann, G. (1994) Proc. Natl. Acad. Sci. USA 91**,** 1834-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson-Wood, K., Lee, M., Motter, R., Hu, K., Gordon, G., Barbour, R., Khan, K., Gordon, M., Tan, H., Games, D., et al. (1997) Proc. Natl. Acad. Sci. USA 94**,** 1550-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolton, A. E. & Hunter, W. M. (1973) Biochem. J. 133**,** 529-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldstein, J. L., Basu, S. K. & Brown, M. S. (1983) Methods Enzymol. 98**,** 241-260. [DOI] [PubMed] [Google Scholar]
- 48.Perez, R. G., Soriano, S., Hayes, J. D., Ostaszewski, B., Xia, W., Selkoe, D. J., Chen, X., Stokin, G. B. & Koo, E. H. (1999) J. Biol. Chem. 274**,** 18851-18856. [DOI] [PubMed] [Google Scholar]
- 49.Kuntz, I. D. (1992) Science 257**,** 1078-1082. [DOI] [PubMed] [Google Scholar]
- 50.Ewing, T. J. A. & Kuntz, I. D. (1997) J. Comput. Chem. 18**,** 1175-1189. [Google Scholar]
- 51.Wilson, C., Wardell, M. R., Weisgraber, K. H., Mahley, R. W. & Agard, D. A. (1991) Science 252**,** 1817-1822. [DOI] [PubMed] [Google Scholar]
- 52.Dong, L.-M., Wilson, C., Wardell, M. R., Simmons, T., Mahley, R. W., Weisgraber, K. H. & Agard, D. A. (1994) J. Biol. Chem. 269**,** 22358-22365. [PubMed] [Google Scholar]
- 53.Meng, E. C., Shoichet, B. K. & Kuntz, I. D. (1992) J. Comput. Chem. 13**,** 505-524. [Google Scholar]
- 54.Ferrin, T. E., Huang, C. C., Jarvis, L. E. & Langridge, R. (1988) J. Mol. Graphics 6**,** 13-27. [Google Scholar]
- 55.Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. (1997) Adv. Drug Deliv. Rev. 23**,** 3-25. [DOI] [PubMed] [Google Scholar]
- 56.Koo, E. H., Squazzo, S. L., Selkoe, D. J. & Koo, C. H. (1996) J. Cell Sci. 109**,** 991-998. [DOI] [PubMed] [Google Scholar]
- 57.Marquez-Sterling, N. R., Lo, A. C. Y., Sisodia, S. S. & Koo, E. H. (1997) J. Neurosci. 17**,** 140-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Selkoe, D. J., Yamazaki, T., Citron, M., Podlisny, M. B., Koo, E. H., Teplow, D. B. & Haass, C. (1996) Ann. N.Y. Acad. Sci. 777**,** 57-64. [DOI] [PubMed] [Google Scholar]
- 59.Yamazaki, T., Koo, E. H. & Selkoe, D. J. (1996) J. Cell Sci. 109**,** 999-1008. [DOI] [PubMed] [Google Scholar]
- 60.Iadonato, S. P., Bu, G., Maksymovitch, E. A. & Schwartz, A. L. (1993) Biochem. J. 296**,** 867-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Medh, J. D., Fry, G. L., Bowen, S. L., Pladet, M. W., Strickland, D. K. & Chappell, D. A. (1995) J. Biol. Chem. 270**,** 536-540. [DOI] [PubMed] [Google Scholar]
- 62.Bales, K. R., Verina, T., Dodel, R. C., Du, Y., Altstiel, L., Bender, M., Hyslop, P., Johnstone, E. M., Little, S. P., Cummins, D. J., et al. (1997) Nat. Genet. 17**,** 263-264. [DOI] [PubMed] [Google Scholar]
- 63.Ishii, K., Tamaoka, A., Mizusawa, H., Shoji, S.-I., Ohtake, T., Fraser, P. E., Takahashi, H., Tsuji, S., Gearing, M., Mizutani, T., et al. (1997) Brain Res. 748**,** 250-252. [DOI] [PubMed] [Google Scholar]
- 64.Kuo, Y.-M., Crawford, F., Mullan, M., Kokjohn, T. A., Emmerling, M. R., Weller, R. O. & Roher, A. E. (2000) Mol. Med. 6**,** 430-439. [PMC free article] [PubMed] [Google Scholar]
- 65.Biere, A. L., Ostaszewski, B., Zhao, H., Gillespie, S., Younkin, S. G. & Selkoe, D. J. (1995) Neurobiol. Dis. 2**,** 177-187. [DOI] [PubMed] [Google Scholar]
- 66.Bucci, C., Parton, R. G., Mather, I. H., Stunnenberg, H., Simons, K., Hoflack, B. & Zerial, M. (1992) Cell 70**,** 715-728. [DOI] [PubMed] [Google Scholar]
- 67.Cuschieri, A. & Bannister, L. H. (1975) J. Anat. 119**,** 277-286. [PMC free article] [PubMed] [Google Scholar]
- 68.Cataldo, A., Rebeck, G. W., Ghetti, B., Hulette, C., Lippa, C., Van Broeckhoven, C., van Duijn, C., Cras, P., Bogdanovic, N., Bird, T., et al. (2001) Ann. Neurol. 50**,** 661-665. [PubMed] [Google Scholar]
- 69.Herz, J. & Beffert, U. (2000) Nat. Rev. Neurosci. 1**,** 51-58. [DOI] [PubMed] [Google Scholar]
- 70.Knauer, M. F., Orlando, R. A. & Glabe, C. G. (1996) Brain Res. 740**,** 6-14. [DOI] [PubMed] [Google Scholar]
- 71.Ulery, P. G., Beers, J., Mikhailenko, I., Tanzi, R. E., Rebeck, G. W., Hyman, B. T. & Strickland, D. K. (2000) J. Biol. Chem. 275**,** 7410-7415. [DOI] [PubMed] [Google Scholar]
- 72.Trommsdorff, M., Borg, J.-P., Margolis, B. & Herz, J. (1998) J. Biol. Chem. 273**,** 33556-33560. [DOI] [PubMed] [Google Scholar]
- 73.Sabo, S. L., Lanier, L. M., Ikin, A. F., Khorkova, O., Sahasrabudhe, S., Greengard, P. & Buxbaum, J. D. (1999) J. Biol. Chem. 274**,** 7952-7957. [DOI] [PubMed] [Google Scholar]
- 74.Zambrano, N., Buxbaum, J. D., Minopoli, G., Fiore, F., De Candia, P., De Renzis, S., Faraonio, R., Sabo, S., Cheetham, J., Sudol, M., et al.. (1997) J. Biol. Chem. 272**,** 6399-6405. [DOI] [PubMed] [Google Scholar]
- 75.Pietrzik, C. U., Yoon, I.-S., Jaeger, S., Busse, T., Weggen, S. & Koo, E. H. (2004) J. Neurosci. 24**,** 4259-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aggerbeck, L. P., Wetterau, J. R., Weisgraber, K. H., Wu, C.-S. C. & Lindgren, F. T. (1988) J. Biol. Chem. 263**,** 6249-6258. [PubMed] [Google Scholar]
- 77.Wetterau, J. R., Aggerbeck, L. P., Rall, S. C., Jr. & Weisgraber, K. H. (1988) J. Biol. Chem. 263**,** 6240-6248. [PubMed] [Google Scholar]
- 78.Weisgraber, K. H. (1994) Adv. Protein Chem. 45**,** 249-302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information