FGF-2 promotes neurogenesis and neuroprotection and prolongs survival in a transgenic mouse model of Huntington's disease (original) (raw)
Abstract
There is no satisfactory treatment for Huntington's disease (HD), a hereditary neurodegenerative disorder that produces chorea, dementia, and death. One potential treatment strategy involves the replacement of dead neurons by stimulating the proliferation of endogenous neuronal precursors (neurogenesis) and their migration into damaged regions of the brain. Because growth factors are neuroprotective in some settings and can also stimulate neurogenesis, we treated HD transgenic R6/2 mice from 8 weeks of age until death by s.c. administration of FGF-2. FGF-2 increased the number of proliferating cells in the subventricular zone by ≈30% in wild-type mice, and by ≈150% in HD transgenic R6/2 mice. FGF-2 also induced the recruitment of new neurons from the subventricular zone into the neostriatum and cerebral cortex of HD transgenic R6/2 mice. In the striatum, these neurons were DARPP-32-expressing medium spiny neurons, consistent with the phenotype of neurons lost in HD. FGF-2 was neuroprotective as well, because it blocked cell death induced by mutant expanded Htt in primary striatal cultures. FGF-2 also reduced polyglutamine aggregates, improved motor performance, and extended lifespan by ≈20%. We conclude that FGF-2 improves neurological deficits and longevity in a transgenic mouse model of HD, and that its neuroprotective and neuroproliferative effects may contribute to this improvement.
Keywords: trophic factor, polyglutamine
Huntington's disease (HD) is a progressive and fatal neurological disorder caused by a polyglutamine expansion in the N terminus of the protein huntingtin (Htt). Expansions of >36 glutamines cause the disease. There is currently no treatment to delay the appearance or progression of the disease. HD is characterized by a dramatic loss of neurons in the striatum and cerebral cortex, resulting in chorea, dementia, and early death. Multiple molecular pathways are involved in the pathophysiology of HD. Disease initiation and progression are thought to involve a conformational change in the Htt protein due to the polyglutamine expansion (1), altered protein-protein interactions (2), abnormal protein aggregation (3) and proteolysis, leading to transcriptional dysregulation (4-6), excitotoxicity (7), and mitochondrial dysfunction (8), and culminating in extensive loss of neurons in the striatum and cerebral cortex (9). The precise cause of neuronal cell death and the relative contributions of the various above-mentioned abnormalities to this process are not known.
Possible approaches to the treatment of HD include blocking neuronal dysfunction and death and replacing lost cells. Several growth factors have been implicated in neuroprotection and neurogenesis, suggesting that they might have salutary effects in HD. We chose to investigate FGF-2 for a variety of reasons. FGF-2 knockout (FGF-2-/-) mice exhibit impaired neurogenesis, suggesting a requirement for FGF-2 in this process (10). FGF-2 also protects striatal neurons in toxin-induced models of HD (11), exerts trophic effects on striatal neurons (12), stimulates proliferation (13) of striatal neural stem cells, and regulates Htt expression by cultured striatal neurons (14). FGF-2 may also stabilize cellular calcium homeostasis and enhance antioxidant defense systems that are impaired in HD. Finally, FGF-2 can be administered systemically and cross the blood-brain barrier to produce cerebral effects (15, 16), obviating the requirement for more complex or invasive modes of delivery.
Therefore, we examined whether FGF-2 treatment can reduce neuronal loss, increase neurogenesis, and improve functional outcome in HD transgenic R6/2 mice, which express exon-1 of Htt with 145 polyglutamine repeats. This is the most extensively characterized genetic model of HD and has a reproducible phenotype manifested by early onset (5-6 weeks) of tremor, irregular gait, and abnormal movements, rapid disease progression, and death at 12-15 weeks (17). We show that FGF-2 stimulates neurogenesis, induces migration of newborn cells into the striatum and cortex, is neuroprotective in vitro, and significantly extends the lifespan of HD transgenic R6/2 mice.
Methods
Tissue Culture, Western Blot Analysis, and Immunohistochemistry. The methods are found in refs. 3, 16, and 17 and Supporting Text, which is published as supporting information on the PNAS web site.
R6/2 Transgenic and Wild-Type Mice. All animal experiments were performed according to procedures approved by the Institutional Animal Care and Use Committee. Heterozygous Htt exon-1-trangenic mice of strain R6/2 (145 CAG repeats) were obtained from The Jackson Laboratory. Animals were geno-typed by PCR of tail-tip DNA, and CAG repeat size was determined. Wild-type (n = 20) and R6/2 (n = 20) were separated into equal FGF-2 (Chemicon; GF003) or vehicle (PBS) treatment groups according to ref. 17. The treatment groups contained the same number of females and males. FGF-2 (250 ng per animal) was administered s.c. twice daily, 3 days per week, until animals were used for immunohistochemistry or survival studies starting at 59 days of age. The BrdUrd+ counts and survival studies were carried out in a double blind manner.
Behavorial Analysis. Disease progression and survival status were monitored daily; the first day on which limb tremors were detected was designated the day of disease onset. Rotarod performance (accelerating regime) and lifespan were analyzed as described (17).
Statistical Analysis. Statistical comparisons of rotarod, weight data, and histology data are compared by ANOVA. Survival data were analyzed by using Kaplan-Meier survival curves (n = 10 per treatment group).
BrdUrd Administration. FGF-2 and vehicle was administered for three weeks before BrdUrd treatment starting at 59 days. BrdUrd (Sigma) was dissolved in saline and given as two i.p. doses of 50 mg/kg each, spaced 8 h apart per day, for 3 days, and then mice were killed 24 h or 7 days later.
Results
Basal and FGF-2-Stimulated Neurogenesis Is Increased in Subventricular Zone (SVZ) of HD Transgenic R6/2 Mice. Growth factors can stimulate neurogenesis in some settings, but whether they can do so in genetic HD mouse models is not known. Compared to age-matched controls, brain from HD patients expresses increased levels of proliferating cell nuclear antigen, a mitotic marker protein (18), and Htt itself may be required for neurogenesis (19). To measure basal and FGF-2-stimulated neurogenesis, vehicle or FGF-2 was injected s.c. in 8-week-old HD transgenic R6/2 mice and wild-type littermate controls for 3 weeks. BrdUrd, used to detect proliferating cells, was injected i.p. for 3 days, and animals were killed 24 h later for BrdUrd immunohistochemistry (Fig. 1_a_). Neurogenesis was measured by counting BrdUrd-labeled cells in the two principal neuroproliferative regions of the adult rodent brain: the SVZ and the subgranular zone of the hippocampal dentate gyrus (DG). Like HD patients, untreated HD transgenic R6/2 mice showed a statistically significant increase in the number of proliferating cells in the SVZ compared to controls, but the magnitude of increase was modest (Fig. 1_b_; *, P < 0.05). However, FGF-2 increased BrdUrd labeling in SVZ of HD transgenic R6/2 mice by ≈150%, whereas in control mice the magnitude of increase was only ≈30% (Fig. 1 a and b). In contrast to SVZ, there was no increase in BrdUrd labeling in DG (data not shown) upon FGF-2 treatment in control and HD transgenic R6/2 mice. Untreated 8-week-old HD transgenic R6/2 mice and wild-type littermate controls had similar BrdUrd labeling in the DG as has been reported.
Fig. 1.
FGF-2 treatment enhances neurogenesis in HD transgenic R6/2. (a and b) HD transgenic R6/2 and wild-type control mice were given i.p. BrdUrd for 3 days, treated with s.c. vehicle (PBS) or FGF-2 (3 weeks), and killed 24 h later. Immunocytochemistry showed a modest increase in the number of BrdUrd-labeled cells (red) in SVZ of PBS-treated HD transgenic R6/2 compared to wild-type mice. FGF-2 enhanced BrdUrd labeling slightly in wild-type and markedly in HD transgenic R6/2 mice. (Scale bar, 150 μm.) Data are representative fields from at least three experiments per panel or mean ± SEM, n = 3. *, P < 0.05; **, P < 0.01 relative to PBS-treated mice. (c and d) Immunocytochemistry with anti-DCX antibody showed an increase in the number of DCX-expressing (new) neurons in FGF-compared to PBS-treated HD transgenic R6/2 mice. (c Inset) DCX migrating cell. (Scale bars, 200 μmin c and 50 μmin d.)
Staining for doublecortin (DCX), a marker for newborn and migrating neurons, was also increased in the SVZ of the HD transgenic R6/2 mice (198% ± 20% compared to control), suggesting migration of these cells from the SVZ into affected areas of the brain (Fig. 1 c and d) in the presence of FGF-2. As observed in young adult mice (20), FGF-2 did not increase proliferating cells in the hippocampus (data not shown). These results demonstrate that neurogenesis is increased in SVZ of HD transgenic R6/2 mice, and that FGF-2 stimulates neurogenesis to a greater extent in these mice than in controls.
New Neurons in FGF-2-Treated HD Transgenic R6/2 Mice Express Phenotypic Features of Medium Spiny Neurons. Virally induced expression of brain-derived neurotrophic factor (BDNF) together with noggin, which inhibits glial differentiation, can stimulate the production of striatal neurons expressing markers of medium spiny neurons (the principal cell type lost in HD), including calbindin, glutamic acid decarboxylase (GAD67), and DARPP-32, a dopamine-regulated phosphoprotein (21). To determine whether FGF-2 has a similar effect, we double-labeled brain sections taken from HD transgenic R6/2 mice treated with FGF-2 and killed at 11 weeks, with antibodies against DCX and DARPP-32 (Fig. 2_a_). Cells migrating into the striatum expressed DARPP-32 (Fig. 2_a_), suggesting maturation along a lineage appropriate for medium spiny neurons. In cerebral cortex, some DCX-expressing cells also expressed the neuronal marker NeuN, consistent with continuing differentiation toward a mature neuronal phenotype (Fig. 2_b_).
Fig. 2.
FGF-2 treatment generates DARPP-32-expressing striatal and NeuN-expressing cortical neurons. (a and b) DARPP-32 (green) and DCX (red) were coexpressed in striatal (Str) neurons (a) and NeuN (green) and DCX (red) show co-expression in cortical (Ctx) neurons (b) from FGF-2-treated HD transgenic R6/2 mice. Littermate control mice treated with FGF-2 did not have DCX and DARPP-32 coexpressed. (Scale bar, 10 μm.) (c) Mice received stereotaxic injection (anterior posterior -0.3 mm, lateral 1.7 mm, depth 3.5 mm) into the globus pallidus with retrograde tracer Alexa Fluor 488 (Left). Retrograde tracer Alexa Fluor 488 (Right) could be detected in the caudate via retrograde transport. No diffusion of Alexa Fluor 488 was detected from the globus pallidus. (d) Alexa Fluor 488 (green), DCX (red), and NeuN (blue) were coexpressed in FGF-2-treated mice, demonstrating extending fibers into the pallidal targets. (Scale bar, 10 μm.) (e) Confocal images of Alexa Fluor 488 (green) and BrdUrd (red) in the striata of FGF-2-treated HD transgenic R6/2 mice are shown as both single optical sections and orthogonal views in the xz and yz planes, to confirm that some BrdUrd cells were positive for retrograde tracer (Left). Confocal images of DARPP-32 (green) and BrdUrd (red) in the striata of FGF-2 treated HD transgenic R6/2 mice to confirm that some BrdUrd cells were positive for DARPP-32 (Right).
Newly Generated Neurons Develop Projections to the Globus Pallidus. Next, we tested whether the new neurons in HD transgenic R6/2 mice extend processes to their normal target, the globus pallidus. To address this question, we injected the retrograde tracer Alexa Fluor 488 into the globus pallidus of HD transgenic R6/2 mice treated with FGF-2 (Fig. 2_c_ Left) and given BrdUrd. One week after FGF-2 treatment and 72 h after injection of the tracer, the mice were killed, and striata were examined for the presence of DCX, NeuN, Alexa Fluor 488, and BrdUrd. The coexpression of DCX, NeuN, and Alexa Fluor 488 (Fig. 2_d_), and of BrdUrd with Alexa Fluor 488 (Fig. 2_e_ Left), indicates that newly produced striatal neurons form projections to globus pallidus. DARPP-32, a marker of mature striatal neurons, was also expressed in BrdUrd-labeled striatal cells (Fig. 2_e_ Right). FGF-2 treatment appears to be responsible for the presence of newborn neurons in the striatum of HD transgenic R6/2 mice, because no DCX/BrdUrd immunopositive striatal cells were found in mice given PBS (data not shown).
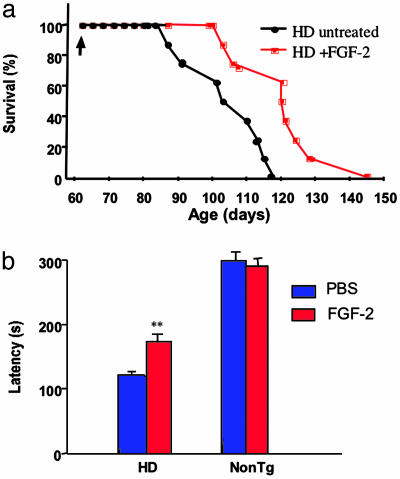
FGF-2 Treatment Ameliorates Motor Symptoms and Increases Lifespan in HD Transgenic R6/2 Mice. To evaluate the functional consequences of FGF-2 treatment, we measured lifespan and motor function in HD transgenic R6/2 mice, as described (22). FGF-2 or PBS was administered by s.c. injection twice daily for 3 days per week, starting at 59 days of age (Fig. 3_a_). The rotarod latency and weight of FGF-2-treated and untreated mice were the same before treatment, and each treatment group had the same composition of females and males. FGF-2 delayed the onset of mortality in HD transgenic R6/2 mice from 84 to 100 days, the average survival from 102 to 123 days, and the maximum survival from 118 to 145 days (P < 0.05). FGF-2 also reduced tremor (data not shown) and improved rotarod performance measured at 11 and 13 weeks of age (Fig. 3_b_, P < 0.01). Finally, FGF-2 treatment produced a modest decrement in weight loss in HD transgenic R6/2 mice, which reached statistical significance at 13 weeks of age, when mean weights were 17.6 ± 1.2 g for FGF-2-treated and 15.4 ± 0.8 g for PBS-treated mice (P < 0.05).
Fig. 3.
FGF-2 prolongs survival and improves rotarod performance in HD transgenic R6/2 mice. (a) HD transgenic R6/2 mice were given PBS (HD untreated) or FGF-2 (HD + FGF-2) beginning at 59 days of age (arrow), and survival was plotted. FGF-2 increased survival, as described in Results (n = 10; *, P < 0.05). (b) Motor performance was evaluated with a rotarod apparatus in 11- and 13-week-old littermate controls (NonTg) and in HD transgenic R6/2 mice (HD) treated with PBS or FGF-2. The total time spent on the rod during a 5-min period (latency) was recorded. Values are the means ± SEM (n = 10 per group); **, P < 0.01 compared to PBS.
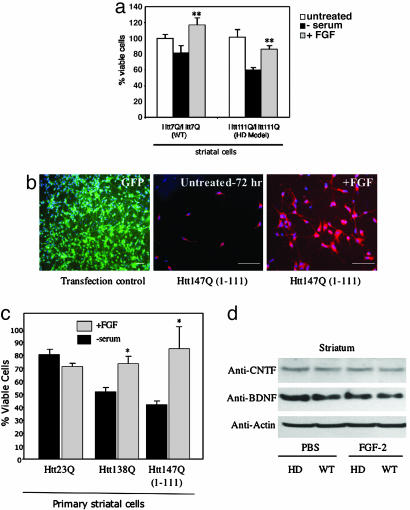
FGF-2 Is Neuroprotective in Cell Culture Models of HD. Growth factors such as BDNF, ciliary neurotrophic factor (CNTF), and IGF-1 inhibit mutant Htt-induced cell death in primary striatal cultures (23) or toxin-induced HD mouse models (24), but FGF-2 has not been evaluated in genetic models of HD. To test whether FGF-2 is neuroprotective in vitro, we used immortalized striatal wild-type _STHtt_Q7/Q7 and mutant _STHtt_Q111/Q111 cells derived from HD knock-in mice expressing Htt with expanded, 111-polyglutamine repeats (25). Withdrawal of serum from _STHtt_Q111/Q111 cell cultures caused death of 44% of cells after 24 h (Fig. 4_a_). This cell death had features of apoptosis, including nuclear fragmentation and activation of caspase-3-like activity (data not shown). Treatment of _STHtt_Q7/Q7 and mutant _STHtt_Q111/Q111 cells with FGF-2 (10 ng/ml) significantly increased cell survival (Fig. 4_a_). In contrast, FGF-2 did not increase cell proliferation, as measured by cell number and BrdUrd incorporation (data not shown).
Fig. 4.
FGF-2 is neuroprotective in HD striatal neuron cultures and does not increase BDNF or CNTF levels in HD transgenic R6/2 mice. (a) Immortalized striatal neurons expressing wild-type Htt (_STHtt_Q7/Q7) or a knocked-in HD mutation with 111 polyglutamine repeats (_STHtt_Q111/Q111) were subjected to serum withdrawal, and cellular viability was assessed with WST-1 assay. **, P < 0.01 compared to untreated cultures (ANOVA, _n_ = 3). (_b_) Electroporation with a GFP-expressing vector resulted in >50% transfection efficiency in primary cultures of striatal neurons, as shown by immunostaining for GFP (Left). Striatal neurons transfected with a mutant Htt147Q(1-111) construct showed extensive cell death (72 h, Center), which was rescued by treatment with FGF-2 (Right). Cultures shown at Center and Right were immunostained with monoclonal anti-Htt 2170 (Chemicon; 1:100). Nuclei were counterstained with DAPI (blue). (c) Striatal neurons were transfected with Htt23Q(1-111), Htt138Q-GFP, or Htt147Q(1-111) and cell viability was assessed by using calcein-AM and ethidium homodimer-1 (LIVE/DEAD kit, Molecular Probes) at 48 h. Data shown are mean values ± SEM (n = 3-5). *, P < 0.05 compared to untreated cultures (ANOVA, n = 3). (d) Western blot analysis of BDNF and CNTF levels of striatal lysates from 11-week-old littermate controls (WT) and HD transgenic R6/2 mice (HD) treated with PBS or FGF-2 for 3 weeks; anti-actin was used as a control for differences in protein loading. It should be noted that the absence of BDNF expression changes by Western blot does not rule out involvement of BDNF in specific cells in response to FGF-2.
Next, we evaluated whether cell death induced by expression of mutant Htt (full-length Htt or N-terminal polyQ Htt fragments) in primary striatal cultures was rescued by FGF-2 treatment. Using GFP as a positive control, we found we could transfect primary striatal neurons by electroporation with >50% transfection efficiency (Fig. 4_b_). Both full-length Htt138Q-GFP and an N-terminal fragment [Htt147Q(1-111)] produced cell death, affecting 38% and 48% of cells, respectively, by 48 h. Treatment with FGF-2 (10 ng/ml) resulted in a significant decrease in mutant Htt-induced neuronal death (Fig. 4_c_). Again, FGF-2 did not increase cell proliferation as measured by cell number and BrdUrd incorporation (data not shown).
The mechanisms by which FGF-2 stimulates neurogenesis and affords neuroprotection are not known. Because BDNF stimulates striatal neurogenesis (21) and may be depleted in HD striatum (26), we evaluated whether FGF-2 alters BDNF expression in HD transgenic R6/2 mouse striatum. Western blot analysis of striatal lysates derived from FGF-2-treated HD transgenic R6/2 and control mice (Fig. 4_d_) showed no FGF-2-induced increase in the expression of either BDNF or CNTF, another trophic factor that is protective in some models of HD (27).
FGF-2 Reduces Aggregate Formation and Corrects Signaling Defects in HD Transgenic R6/2 Mice. Because alterations in cellular signaling pathways and the hallmark formation of nuclear and cytoplasmic aggregates are well characterized in HD transgenic R6/2 mice, we evaluated whether FGF-2 treatment corrected any of these cellular changes.
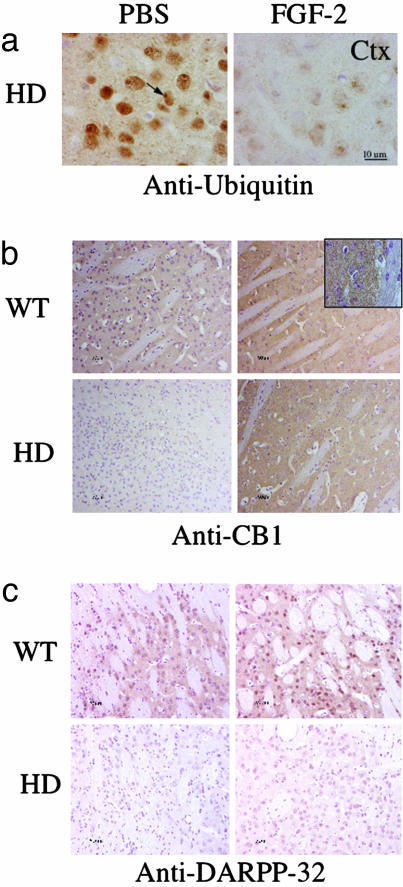
There is an early and dramatic increase in Htt-immunoreactive intraneuronal aggregates in R6/2 mice. Treatment with FGF-2 resulted in a significant reduction in Htt-positive striatal, cortical, nuclear, and perinuclear aggregates, detected by antibodies against ubiquitin-positive nuclear inclusions (Fig. 5_a_) or against the polyglutamine tract of Htt (data not shown), at 11 weeks of age. Quantification of the ubiquitin positive nuclear inclusions demonstrated FGF-2 treatment reduced inclusions by 17% in the striatum (74 ± 4% to 57 ± 4%) and 16% in the cortex (82 ± 4% to 66 ± 1%).
Fig. 5.
Histopathological evidence of in vivo neuroprotection by FGF-2 in 11-week old HD transgenic R6/2 mice. (a) Ubiquitin immunohistochemistry (brown) in PBS- and FGF-2-treated HD transgenic R6/2 mice showed ubiquitin immunoreactivity in PBS-treated HD mice, but not in wild-type (not shown) or FGF-2-treated HD mice. Htt immunohistochemistry (brown) in neostriatum (Str, data not shown) and cortex (Ctx) showed Htt-immunoreactive aggregates (arrows) in PBS-treated, but not FGF-2-treated, HD transgenic R6/2 mice (bottom two rows). (b and c) CB1 cannabinoid receptor (b) and DARPP-32 immunoreactivity (c) in PBS- and FGF-2-treated littermate control (WT) and HD transgenic R6/2 (HD) mice showed that both CB1 and DARPP-32 were depleted from the affected striatum and restored by FGF-2 treatment. The counterstain is hematoxylin.
CB1 cannabinoid receptors are down-regulated at the mRNA and protein levels in postmortem HD brain tissue and HD transgenic R6/2 mice (28, 29). CB1 receptors have been implicated in FGF-2-induced axonal growth (30), and also regulate adult neurogenesis (31). Moreover, cannabinoids are neuroprotective in a variety of cerebral injury models. Therefore, we evaluated whether FGF-2 treatment modified expression of CB1 receptors in HD transgenic R6/2 mouse striatum. CB1 receptor levels were reduced significantly in the striatum of HD transgenic R6/2 mice (Fig. 5_b_), but FGF-2 treatment restored CB1 receptor expression (Fig. 5_b_).
The mutation in HD transgenic R6/2 mice also down-regulates the dopamine- and cAMP-regulated 32-kDa phosphoprotein, DARPP-32 (32). DARPP-32 is normally enriched in prefrontal cortex and striatum (32), where it participates in dopamine and serotonin signaling. We found that DARPP-32 levels were reduced by 50% in the striatum of HD transgenic R6/2 mice (Fig. 5_c_). Western blotting (data not shown) and immunostaining also showed that FGF-2 increased DARPP-32 expression in 11-week-old HD transgenic R6/2 mice (Fig. 5_c_).
Discussion
The major findings we report are that FGF-2 stimulates neurogenesis, provides neuroprotection, and extends lifespan in a transgenic mouse model of HD. Neurogenesis, detected by BrdUrd labeling and DCX expression, was increased under basal conditions, and stimulated to a greater extent by FGF-2, in SVZ but not DG of HD transgenic R6/2 mice. The increase in neurogenesis was associated with migration of nascent neurons into the affected striatum, where they assumed phenotypic features of medium spiny neurons, the principal striatal cell type lost in HD, and extended processes to the globus pallidus, where medium spiny neurons normally project. Neuroprotection was observed both in vitro and in vivo, and was manifested by reductions in neuronal death and protein aggregate formation, restoration toward normal of CB1 cannabinoid receptor and DARPP-32 protein expression, improved neurological function and prolonged survival. Whether neurogenesis, direct neuroprotection, or both contribute to the improvement in neurological function and longevity that we observed cannot be resolved based on the present data. FGF-2 may also have peripheral (nonbrain) effects that contribute to longevity. However, the effect of FGF-2 in HD transgenic R6/2 mice raises the possibility that FGF-2 or a drug that recapitulates one or more of its effects may provide a prototype for the treatment of HD.
Despite recent advances in understanding the molecular pathogenesis of HD, clinical measures to slow or arrest disease progression are lacking. However, growth factors have received considerable attention in preclinical studies, and several, including nerve growth factor, BDNF, neurotrophins 3 and 4, glial cell-derived neurotrophic factor, neurturin, and CNTF, have shown some benefit in excitotoxic models of HD when administered directly or by gene or cell therapy (reviewed in ref. 27). Genetic models of HD have been studied less extensively in this regard, but BDNF expression is decreased in transgenic murine (26) and human (33) HD, and both BDNF and CNTF rescue cultured striatal neurons from death induced by transfection with mutant Htt. FGF-2 treatment in our studies did not alter BDNF levels in the striatum, suggesting that FGF-2 may promote neurogenesis and survival independent of BDNF levels. Prolonged survival has been reported in HD transgenic mice treated with a dominant-negative inhibitor of caspase-1, creatine, minocycline, dichloroacetate, α-lipoic acid, cystamine, coezyme Q10, remacemide, the antioxidant BN82451, histone deacetylase inhibitors, rapamycin, or the disaccharide trehalose (reviewed in ref. 34). In these studies, the increase in mean survival has ranged from 10 to 20%. The mechanisms through which some of these treatments may operate have been inferred from their actions in other systems, but in other cases are unclear. Additional measures that prolong survival in HD transgenic mice include environmental enrichment, the anti-excitotoxin riluzole, and the antidepressant paroxetine, which also have in common the ability to stimulate neurogenesis (reviewed in ref. 34), although this has not been shown to be the basis for their protective effect in HD.
FGF-2 is neuroprotective in a variety of neurological disease models (reviewed in ref. 35), including global and focal cerebral ischemia, kainate-induced seizures, and MPTP-mediated parkinsonism. FGF-2 is expressed in substantia nigra, striatum, and globus pallidus of human brain, and FGF receptor expression is increased in HD (36). In the quinolinic acid model of HD in rats, FGF-2 attenuates changes in cytochrome c oxidase (37) and nitric oxide synthase (24) activity, but there is little other prior evidence to connect FGF-2 with HD. The mechanisms through which FGF-2 produced neuroprotection in our HD transgenic R6/2 mice may relate to the Akt signaling pathway. FGF-2 activates a range of signal transduction pathways (reviewed in ref. 38), among which the phosphatidylinositol 3′-kinase/Akt pathway may be especially prominent in promoting cell survival. Notably, Akt signaling has also been implicated in the protective effect of IGF-1 in cultured cells expressing mutant Htt (23).
The ability of FGF-2 to stimulate neurogenesis in the adult brain is well established. FGF-2 increases the proliferation of neuronal precursors in vitro (39), and intraventricular infusion of FGF-2 enhances the proliferation and migration of neuronal precursors in the SVZ (40). Moreover, injury-induced neurogenesis is impaired in FGF-2 knockout mice, and is restored by replacement of FGF-2 using a herpes virus amplicon vector (10). As mentioned above in connection with FGF-2-mediated neuroprotection, the molecular basis for FGF-2-induced neurogenesis remains speculative, although cytoproliferative actions of FGF-2 in other systems have been ascribed to activation of mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK/ERK) pathways (38), and MEK/ERK signaling has also been implicated in neurogenesis induced by NT-3 and BDNF (41).
The increased neurogenesis that we observed in HD transgenic R6/2 mice represents another illustration of the emerging theme that neurogenesis is stimulated in neurological diseases, possibly as an adaptive response directed toward neuronal replacement. Examples include HD (18) and Alzheimer's disease (AD) (42) in humans, as well as animal models of Parkinson's disease (43), global (44) and focal (45) cerebral ischemia, and epilepsy (46). Injury-induced neurogenesis shows similarities and differences across disease models, but the examples cited above demonstrate that it can be precipitated by either an acute or chronic and by focal or diffuse brain pathology. There appears to be some degree of regional specificity in the propensity of brain lesions to evoke neurproliferative responses in DG or SVZ. Thus, we observed increased BrdUrd labeling in the juxtastriatal SVZ but not in the DG of our HD transgenic R6/2 mice, whereas neuronal precursors in DG appear to be mobilized preferentially in disorders that prominently affect the hippocampus, such as global cerebral ischemia (44) and AD (42). Where injury occurs at a distance from the brain's principal neuroproliferative zones, as best exemplified in focal ischemia, but also true for animal models of HD and AD, newborn neurons migrate from their sites of origin into affected brain areas. In ischemia affecting the striatum (47) and in HD transgenic R6/2 mice, new neurons migrating into the striatum differentiate toward a phenotype resembling that of the dead or injured cells. Whether these neurons integrate into the surviving brain circuitry and function normally is still unknown, notwithstanding that in our HD transgenic R6/2 mice, they extend projections to anatomically appropriate targets. The contribution of neurogenesis to functional recovery from brain injury is difficult to ascertain because, as in the present study, treatments that stimulate neurogenesis may have additional, potentially beneficial effects on cell function. However, one recent report showed that blocking neurogenesis by cerebral x-irradiation in gerbils impaired recovery from global cerebral ischemia (48), suggesting that neurogenesis contributes to recovery.
Our findings leave several questions unanswered regarding a possible role for measures that stimulate FGF-2 signaling in the therapeutic approach to HD. These include whether the beneficial effect of FGF-2 might be optimized by, for example, earlier onset of administration, alterations in dosage or route of delivery, or combining FGF-2 with one or more of the numerous growth factors or drugs (discussed above) that yield benefit in animal models of HD, enhance neurogenesis, or both. Other, more fundamental, questions relate to the molecular mechanisms through which FGF-2 modifies the pathophysiology of HD. For example, how does FGF-2 prevent or clear abnormal protein aggregates, and what is the relationship between reduction of protein aggregates and improved neuronal function? It may not be overly optimistic to speculate that new insights into HD might emerge from pursuing these lines of inquiry.
Supplementary Material
Supporting Text
Acknowledgments
We thank Gerette Kerby and Jessica Young for technical assistance on some of the experiments. This work was supported by National Institutes of Health Grant NS40251, grants from the Huntington's Disease Society of America, the Hereditary Disease Foundation, and the Multiple Dystrophy Association (to L.M.E.); National Research Service Award (NSRA) Postdoctoral Fellowships F32 NS43823 (to M.L.-B.) and F32 NS043937 (to J.G.); and National Institutes of Health Grants NS44921 (to D.A.G.) and AG21980 (to K.J.).
Author contributions: K.J., D.A.G., and L.M.E. designed research; K.J., M.L.-B., Y.S., S.C., J.G., D.C., A.L., and L.M.E. performed research; C.A.R. contributed new reagents/analytic tools; K.J. and L.M.E. analyzed data; and L.M.E. and D.A.G. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HD, Huntington's disease; SVZ, subventricular zone; DG, dentate gyrus; BDNF, brain-derived neurotrophic factor; CNTF, ciliary neurotrophic factor.
See Commentary on page 17889.
References
- 1.Perutz, M. F. (1999) Trends Biochem. Sci. 24**,** 58-63. [DOI] [PubMed] [Google Scholar]
- 2.Wanker, E. E., Rovira, C., Scherzinger, E., Hasenbank, R., Walter, S., Tait, D., Colicelli, J. & Lehrach, H. (1997) Hum. Mol. Genet. 6**,** 487-495. [DOI] [PubMed] [Google Scholar]
- 3.Davies, S. W., Turmaine, M., Cozens, B. A., DiFiglia, M., Sharp, A. H., Ross, C. A., Scherzinger, E., Wanker, E. E., Mangiarini, L. & Bates, G. P. (1997) Cell 90**,** 537-548. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg, Y. P., Nicholson, D. W., Rasper, D. M., Kalchman, M. A., Koide, H. B., Graham, R. K., Bromm, M., Kazemi-Esfarjani, P., Thornberry, N. A., Vaillancourt, J. P. & Hayden, M. R. (1996) Nat. Genet. 13**,** 442-449. [DOI] [PubMed] [Google Scholar]
- 5.Nucifora, F. C., Jr., Sasaki, M., Peters, M. F., Huang, H., Cooper, J. K., Yamada, M., Takahashi, H., Tsuji, S., Troncoso, J., Dawson, V. L., et al. (2001) Science 291**,** 2423-2428. [DOI] [PubMed] [Google Scholar]
- 6.Zuccato, C., Tartari, M., Crotti, A., Goffredo, D., Valenza, M., Conti, L., Cataudella, T., Leavitt, B. R., Hayden, M. R., Timmusk, T., et al. (2003) Nat. Genet. 35**,** 76-83. [DOI] [PubMed] [Google Scholar]
- 7.Zeron, M. M., Chen, N., Moshaver, A., Lee, A. T., Wellington, C. L., Hayden, M. R. & Raymond, L. A. (2001) Mol. Cell Neurosci. 17**,** 41-53. [DOI] [PubMed] [Google Scholar]
- 8.Panov, A. V., Gutekunst, C. A., Leavitt, B. R., Hayden, M. R., Burke, J. R., Strittmatter, W. J. & Greenamyre, J. T. (2002) Nat. Neurosci. 5**,** 731-736. [DOI] [PubMed] [Google Scholar]
- 9.Ferrante, R. J., Kowall, N. W., Beal, M. F., Richardson, E. P., Jr., Bird, E. D. & Martin, J. B. (1985) Science 230**,** 561-563. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura, S., Takagi, Y., Harada, J., Teramoto, T., Thomas, S. S., Waeber, C., Bakowska, J. C., Breakefield, X. O. & Moskowitz, M. A. (2001) Proc. Natl. Acad. Sci. USA 98**,** 5874-5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjugstad, K. B., Zawada, W. M., Goodman, S. & Free, C. R. (2001) J. Inherit. Metab. Dis. 24**,** 631-647. [DOI] [PubMed] [Google Scholar]
- 12.Zhou, D. & DiFiglia, M. (1993) Exp. Neurol. 122**,** 171-188. [DOI] [PubMed] [Google Scholar]
- 13.Palmer, T. D., Ray, J. & Gage, F. H. (1995) Mol. Cell Neurosci. 6**,** 474-486. [DOI] [PubMed] [Google Scholar]
- 14.Haque, N. S. & Isacson, O. (2000) Cell Transplant 9**,** 623-627. [DOI] [PubMed] [Google Scholar]
- 15.Wagner, J. P., Black, I. B. & DiCicco-Bloom, E. (1999) J. Neurosci. 19**,** 6006-6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin, K., Xie, L., Childs, J., Sun, Y., Mao, X., Logvinova, A. & Greenberg, D. (2003) Ann. Neurol. 53**,** 405-409. [DOI] [PubMed] [Google Scholar]
- 17.Hockly, E., Richon, V. M., Woodman, B., Smith, D. L., Zhou, X., Rosa, E., Sathasivam, K., Ghazi-Noori, S., Mahal, A., Lowden, P. A., et al. (2003) Proc. Natl. Acad. Sci. USA 100**,** 2041-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtis, M. A., Penney, E. B., Pearson, A. G., van Roon-Mom, W. M., Butterworth, N. J., Dragunow, M., Connor, B. & Faull, R. L. (2003) Proc. Natl. Acad. Sci. USA 100**,** 9023-9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White, J. K., Auerbach, W., Duyao, M. P., Vonsattel, J. P., Gusella, J. F., Joyner, A. L. & MacDonald, M. E. (1997) Nat. Genet. 17**,** 404-410. [DOI] [PubMed] [Google Scholar]
- 20.Jin, K., Sun, Y., Xie, L., Batteur, S., Mao, X. O., Smelick, C., Logvinova, A. & Greenberg, D. A. (2003) Aging Cell 2**,** 175-183. [DOI] [PubMed] [Google Scholar]
- 21.Chmielnicki, E., Benraiss, A., Economides, A. N. & Goldman, S. A. (2004) J. Neurosci. 24**,** 2133-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hockly, E., Cordery, P. M., Woodman, B., Mahal, A., van Dellen, A., Blakemore, C., Lewis, C. M., Hannan, A. J. & Bates, G. P. (2002) Ann. Neurol. 51**,** 235-242. [DOI] [PubMed] [Google Scholar]
- 23.Humbert, S., Bryson, E. A., Cordelieres, F. P., Connors, N. C., Datta, S. R., Finkbeiner, S., Greenberg, M. E. & Saudou, F. (2002) Dev. Cell 2**,** 831-837. [DOI] [PubMed] [Google Scholar]
- 24.Maksimovic, I. D., Jovanovic, M. D., Malicevic, Z., Colic, M. & Ninkovic, M. (2002) Vojnosanit. Pregl. 59**,** 119-123. [DOI] [PubMed] [Google Scholar]
- 25.Trettel, F., Rigamonti, D., Hilditch-Maguire, P., Wheeler, V. C., Sharp, A. H., Persichetti, F., Cattaneo, E. & MacDonald, M. E. (2000) Hum. Mol. Genet. 9**,** 2799-2809. [DOI] [PubMed] [Google Scholar]
- 26.Zuccato, C., Ciammola, A., Rigamonti, D., Leavitt, B. R., Goffredo, D., Conti, L., MacDonald, M. E., Friedlander, R. M., Silani, V., Hayden, M. R., et al. (2001) Science 293**,** 493-498. [DOI] [PubMed] [Google Scholar]
- 27.Alberch, J., Perez-Navarro, E. & Canals, J. M. (2004) Prog. Brain Res. 146**,** 195-229. [DOI] [PubMed] [Google Scholar]
- 28.Glass, M., van Dellen, A., Blakemore, C., Hannan, A. J. & Faull, R. L. (2004) Neuroscience 123**,** 207-212. [DOI] [PubMed] [Google Scholar]
- 29.Lastres-Becker, I., De Miguel, R. & Fernandez-Ruiz, J. J. (2003) Curr. Drug Target CNS Neurol. Disord. 2**,** 335-347. [DOI] [PubMed] [Google Scholar]
- 30.Williams, E. J., Walsh, F. S. & Doherty, P. (2003) J. Cell Biol. 160**,** 481-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin, K., Xie, L., Hee, K. S., Parmentier-Batteur, S., Sun, Y., Mao, X. O., Childs, J. T. & Greenberg, D. A. (2004) Mol. Pharmacol. 66**,** 204-208. [DOI] [PubMed] [Google Scholar]
- 32.Bibb, J. A., Yan, Z., Svenningsson, P., Snyder, G. L., Pieribone, V. A., Horiuchi, A., Nairn, A. C., Messer, A. & Greengard, P. (2000) Proc. Natl. Acad. Sci. USA 97**,** 6809-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrer, I., Goutan, E., Marin, C., Rey, M. J. & Ribalta, T. (2000) Brain Res. 866**,** 257-261. [DOI] [PubMed] [Google Scholar]
- 34.Hersch, S. M. & Ferrante, R. J. (2004) NeuroRx 1**,** 298-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reuss, B. & von Bohlen und Halbach, O. (2003) Cell Tissue Res. 313**,** 139-157. [DOI] [PubMed] [Google Scholar]
- 36.Tooyama, I., Kremer, H. P., Hayden, M. R., Kimura, H., McGeer, E. G. & McGeer, P. L. (1993) Brain Res. 610**,** 1-7. [DOI] [PubMed] [Google Scholar]
- 37.Maksimovic, I. D., Jovanovic, M. D., Colic, M., Mihajlovic, R., Micic, D., Selakovic, V., Ninkovic, M., Malicevic, Z., Rusic-Stojiljkovic, M. & Jovicic, A. (2001) Vojnosanit. Pregl. 58**,** 237-242. [PubMed] [Google Scholar]
- 38.Ensoli, B., Sgadari, C., Barillari, G. & Monini, P. (2003) in The Cytokine Handbook, eds. Thomson, A. W. & Lotze, M. T. (Elsevier, London), Vol. 2, pp. 747-781. [Google Scholar]
- 39.Gensburger, C., Labourdette, G. & Sensenbrenner, M. (1987) FEBS Lett. 217**,** 1-5. [DOI] [PubMed] [Google Scholar]
- 40.Kuhn, H. G., Winkler, J., Kempermann, G., Thal, L. J. & Gage, F. H. (1997) J. Neurosci. 17**,** 5820-5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnabe-Heider, F. & Miller, F. D. (2003) J. Neurosci. 23**,** 5149-5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin, K., Peel, A., Mao, X. O., Xie, L., Cottrell, B. & Greenberg, D. A. (2004) Proc. Natl. Acad. Sci. USA 101**,** 343-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao, M., Momma, S., Delfani, K., Carlen, M., Cassidy, R. M., Johansson, C. B., Brismar, H., Shupliakov, O., Frisen, J. & Janson, A. M. (2003) Proc. Natl. Acad. Sci. USA 100**,** 7925-7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu, J., Solway, K., Messing, R. O. & Sharp, F. R. (1998) J. Neurosci. 18**,** 7768-7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin, K., Minami, M., Lan, J. Q., Mao, X. O., Batteur, S., Simon, R. P. & Greenberg, D. A. (2001) Proc. Natl. Acad. Sci. USA 98**,** 4710-4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parent, J. M., Yu, T. W., Leibowitz, R. T., Geschwind, D. H., Sloviter, R. S. & Lowenstein, D. H. (1997) J. Neurosci. 17**,** 3727-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parent, J. M., Vexler, Z. S., Gong, C., Derugin, N. & Ferriero, D. M. (2002) Ann. Neurol. 52**,** 802-813. [DOI] [PubMed] [Google Scholar]
- 48.Raber, J., Fan, Y., Matsumori, Y., Liu, Z., Weinstein, P. R., Fike, J. R. & Liu, J. (2004) Ann. Neurol. 55**,** 381-389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Text