Differential oxidative damage and expression of stress defence regulons in culturable and non-culturable Escherichia coli cells (original) (raw)
Abstract
Potentially pathogenic bacteria, such as Escherichia coli and Vibrio cholerae, become non-culturable during stasis. The analysis of such cells has been hampered by difficulties in studying bacterial population heterogeneity. Using in situ detection of protein oxidation in single E. coli cells, and using a density-gradient centrifugation technique to separate culturable and non-culturable cells, we show that the proteins in non-culturable cells show increased and irreversible oxidative damage, which affects various bacterial compartments and proteins. The levels of expression of specific stress regulons are higher in non-culturable cells, confirming increased defects relating to oxidative damage and the occurrence of aberrant, such as by amino-acid misincorporation, proteins. Our data suggest that non-culturable cells are produced due to stochastic deterioration, rather than an adaptive programme, and pinpoint oxidation management as the 'Achilles heel' of these cells.
Introduction
The existence of a viable but non-culturable (VBNC) state, in which cells are apparently intact, but have lost the ability to form colonies on standard plate-count media, led Roszak & Colwell (1987) to propose that the formation of VBNC cells is analogous to spore formation in differentiating bacteria. VBNC bacteria are a major concern in public-health risk assessments because many pathogenic Gram-negative bacteria, such as Vibrio cholerae, Vibrio vulnificus and Escherichia coli, have been reported to enter a VBNC state, from which they are able to return to the infectious state after passaging in animal hosts (Colwell, 2000; Huq et al., 2000; Olivier, 2000). However, the concept of VBNC formation as a programmed and adaptive phenomenon during nutrient starvation is controversial (for example, see Kell et al., 1998) and another model suggests that cells become non-culturable due to cellular deterioration, and that these cells are moribund (Dukan & Nyström, 1999; Bogosian & Bourneuf, 2001). Experimental data that could settle this controversy would have an obvious impact on the evaluation of the public health hazard posed by non-culturable bacteria. However, such studies have been hampered by a lack of techniques that would allow experiments to be carried out on single cells and on subpopulations of cells.
In this study, in situ detection of protein oxidation, and a density-gradient centrifugation technique, allowed us to analyse cell-specific oxidative deterioration and differential gene expression in culturable and non-culturable cells from the same population.
RESULTS
Stasis-induced carbonylation affects only a subpopulation
We used an immunological assay for the detection of protein carbonyls, and developed this method for use at the single-cell level (see Methods; Fig. 1A,D). To confirm that the signal obtained using this detection assay reflected the oxidative damage to proteins, we exposed cells to hydrogen peroxide and found that the in situ signal was significantly increased in parallel with the signal obtained from slot-blot analysis (Fig. 1B,E,H). Treatment of cells with proteinase K (Fig. 1C,F,I), but not with RNase, DNase, or lipase (not shown), abolished the signal. Next, we analysed growing and stationary-phase (48-h) E. coli populations. The in situ detection of protein carbonyls showed that the stationary-phase-induced increase in oxidative modification (Dukan & Nyström, 1998) affects only a subpopulation of cells (Fig. 2A,B). At this time during stationary phase, all cells have an intact membrane (Fig. 2F,G), and about 60% of the cells in the population have become non-culturable (see next section).
Figure 1.
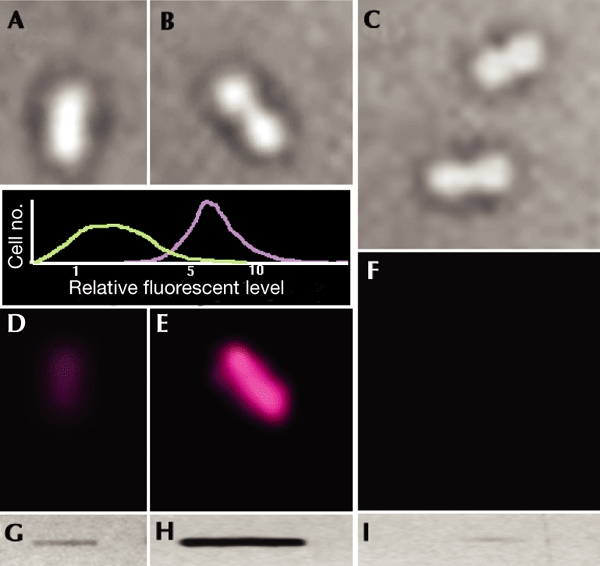
Detection of in situ carbonylation in single Escherichia coli cells. Cells were grown in Luria–Bertani medium at 37 °C with no hydrogen peroxide (A,D,G) or with 200 mM hydrogen peroxide (B,E,H). Transmission images of cells (A–C) and the fluorescence emission from fluorescently labelled antibodies (D–F) are shown. Cell samples from hydrogen-peroxide-stressed cells were treated with proteinase K (C,F,I). Slot-blot analysis of carbonyl levels in protein extracts (G–I) was carried out in parallel with carbonyl imaging to ensure that the carbonyl signal intensities corresponded to the expected signal from the total-protein extracts. The graph (inset) shows the results of a statistical analysis of the carbonyl-intensity signal in 200 untreated (green line) and 200 hydrogen-peroxide-treated (purple line) cells. Cell no., number of cells.
Figure 2.
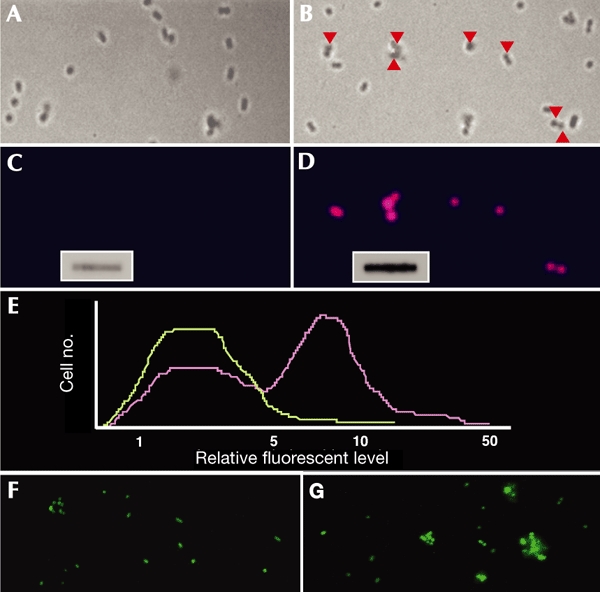
Protein carbonylation levels in single cells. The levels of protein carbonylation in growing (A,C) and starved (B,D) populations of Escherichia coli were analysed. Transmission images of cells (A,B) and the fluorescence emission from fluorescently labelled antibodies (C,D) are shown. The red arrowheads in (B) indicate cells that show an increased carbonyl signal, as shown in (D). The insets in (C) and (D) show the total carbonyl levels (determined by slot-blot analysis) in protein extracts from growing (C) and starved (D) populations. (E) Carbonyl-intensity analysis of ∼200 growing (green line) and ∼300 starved (purple line) cells. The integrity of growing (F) and starving (G) cells was analysed using the fluorescent LIVE/DEAD BacLight bacterial viability kit (Molecular Probes), as described in Haugland (1998).
Non-culturable cells are specifically carbonylated
To determine whether the increased differential carbonylation of cells is correlated to the VBNC phenomenon, we used a gradient centrifugation technique that has been reported to separate culturable and non-culturable subpopulations on the basis of a slight difference in cell densities (Siegele et al., 1993). Two density fractions were clearly visible at the time in stationary phase when part of the population had become non-culturable (Fig. 3A,B). The isolation of cells from the two fractions showed that only a small percentage of the cells in the high-density fraction were able to form colonies, whereas more than 90% of the cells in the low-density fraction were culturable (Fig. 3C). Moreover, using the LIVE/DEAD BactLight kit (Molecular Probes) on separate populations, we found that more than 90% of the cells were intact in both subpopulations (Fig. 3C). As shown in Fig. 3, oxidative damage is mainly found in the fraction containing non-culturable cells.
Figure 3.
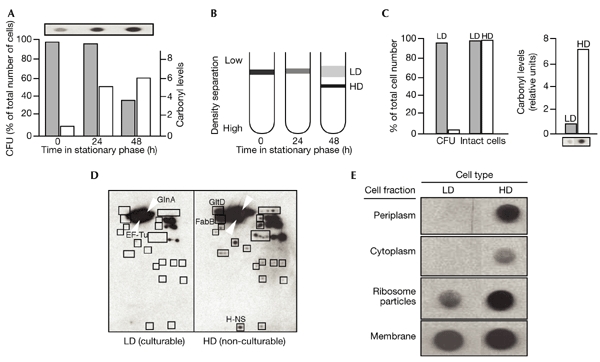
Separation and analysis of culturable and non-culturable cells. (A) Reproductive ability (colony-forming units (CFU), expressed as a percentage of the total number of cells; shaded bars) and protein carbonyl levels (unshaded bars) in growing (0 h) and stationary-phase (24 h and 48 h) cultures of Escherichia coli. The slot-blot (above the graph) shows the raw data from the carbonylation detection experiments. (B) Scheme for the separation of high-density (HD) and low-density (LD) cell populations at stationary phase after 48 h. (C) Determination of reproductive ability (CFU), cell integrity and carbonyl levels in the HD (unshaded bars) and LD (shaded bars) cell populations from a 48-h stationary-phase culture. (D) Pattern of protein carbonylation in the culturable (LD) and non-culturable (HD) fractions, analysed by a combined proteomic/immunochemical approach. (E) Analysis of carbonyl levels in cellular compartments of culturable (LD) and non-culturable (HD) cells.
Specific protein carbonylation in non-culturable populations
The pattern of protein oxidation in culturable and non-culturable cells was similar, but several proteins were oxidized only in the non-culturable cell fraction (Fig. 3D). Some of these proteins were identified, and included the histone-like DNA-binding protein H-NS, glutamate synthase (GltD) and β-ketoacyl (acyl carrier protein) synthetase (FabB) (Fig. 3D). Other qualitative differences between oxidation in non-culturable and culturable cells included the significant oxidative damage in the ribosome particle and periplasmic proteins of non-culturable cells (Fig. 3E).
Upregulating stress regulons in non-culturable populations
We investigated whether the increased level of oxidative damage was accompanied by a change in the levels of expression of stress regulons. The levels of expression of indicator genes controlled by RpoS, SoxRS, RpoH, RpoE and CpxR were elevated in non-culturable cells (Fig. 4A). The levels of σS (Hengge-Aronis, 2000), but not β-galactosidase from the transcriptional Φ(rpoS_–_lacZ) fusion, were higher in non-culturable cells, indicating that σS levels are increased in non-culturable cells by a post-transcriptional mechanism (Fig. 4A,C). The increase in σS levels in non-culturable cells was confirmed by measuring the activities of catalase and glutathione reductase (which are RpoS-dependent; Fig. 4B) and of σS-dependent promoters (sodCp, katEp and uspBp; Fig. 4A). In addition, the activities of the soxS promoter (Fig. 4A) and glucose-6-phosphate dehydrogenase (Fig. 4B) were higher in non-culturable cells, indicating the activation of the SoxRS regulon in this subpopulation. Indicator promoters, PdnaK, regulated by the of the heatshock regulon, and P3rpoH and PcpxP regulated by σE of the extra-cytoplasmatic stress response, were also upregulated in non-culturable cells (Fig. 4A,C). The elevated activites of the rpoH3 and cpxP promoters suggests that non-culturable cells have defects in the management of proteins in the extra-cytoplasmatic compartments (Raivio & Silhavy, 2000; Fig. 4A). This is consistent with the data showing elevated carbonylation damage to the periplasmic proteins of non-culturable cells (Fig. 3E).
Figure 4.
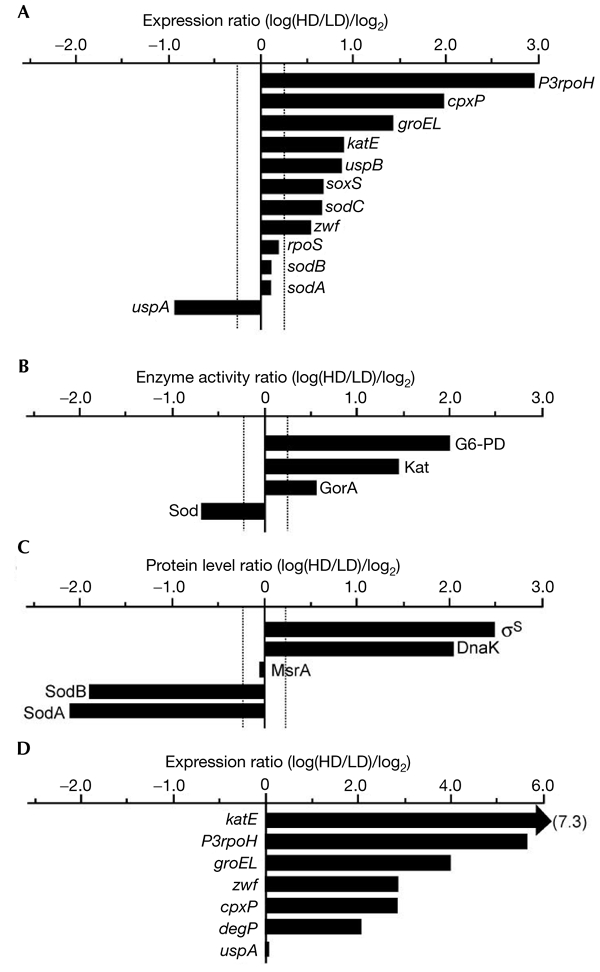
Expression of stress regulons in the culturable and non-culturable cell populations. Levels of selected transcripts (A), proteins (B) and enzyme activities (C) in culturable and non-culturable subpopulations from a 48-h culture in stationary phase. Transcript levels were measured (in Miller units) by using fusions of lacZ to the selected promoters. Protein levels were measured by western blot analysis using specific antibodies. Superoxide dismutase, glutathione reductase and catalase activities were also measured. Comparisons were made by dividing the values from the high-density (HD) cell population by the values from the low-density (LD) cell population. The data are expressed as log(HD/LD)/log2, such that a value of 1 indicates a twofold increase in absolute terms and a value of −1 indicates a twofold decrease in the HD (non-culturable) subpopulation. (D) Levels of selected transcripts in exponential-phase sodA mutant and wild-type cells. The data are expressed as described for (A–C). G6-PD, glucose-6-phosphate dehydrogenase (encoded by zwf).
In contrast to most of the stress genes analysed, expression of Φ(sodA_–_lacZ) and Φ(sodB_–_lacZ) was similar in culturable and non-culturable cells (Fig. 4A). However, the levels and activities of the corresponding proteins were significantly lower in non-culturable cells (Fig. 4B,C). Moreover, the gene encoding universal stress-protein A (UspA) (Nyström & Neidhardt, 1994) was expressed at lower levels in non-culturable cells. This is interesting because mutants devoid of uspA and sodA sodB have been shown to lose culturability at an increased rate during stasis (Nyström & Neidhardt, 1994; Benov & Fridovich, 1995).
sodA mutants mimic non-culturable cells
We investigated whether the low abundance and activity of superoxide dismutases (Sods) in non-culturable cells is a cause of increased stress-regulon expression. As shown in Fig. 4D, all of the stress genes analysed, except uspA, showed higher levels of expression in sodA mutants compared with wild-type cells. Thus, the RpoS, SoxRS, RpoH, RpoE and CpxR regulons seem to be activated in response to diminished Sod activity. By contrast, the inactivation of uspA had no such effects on the expression of stress-regulon genes (data not shown).
DISCUSSION
The data presented in this paper support the theory that starving E. coli cells lose their reproductive ability due to deterioration, rather than due to a programmed and adaptive entry into a VBNC state, and that this sterility is associated with increased oxidative damage. Indeed, previously observed symptoms of bacterial damage in the global bacterial population (Dukan & Nystrom, 1998) might be associated specifically with sterility. It is possible that sterility might be reversible, depending on the amount of cellular damage. Nevertheless, we propose that, if starvation and oxidation damage are allowed to proceed for an extended period, the non-culturable cells become moribund and irreversibly lose their lifesupporting mechanisms (see also Ericsson et al., 2000). We infer that the increased production of stress-defence proteins in non-culturable cells is a response to increased cellular damage, rather than a programmed developmental pathway that leads to VBNC formation. The increased oxidation damage in the non-culturable cell population, which is of a highly detrimental and irreversible nature, supports this interpretation. This is also consistent with data showing that starving E. coli cells remain culturable for much longer periods of time in the absence of oxygen (Dukan & Nyström, 1999), and that V. vulnificus cells that were previously thought to be VBNC are, in fact, a subpopulation of the culture that fails to reproduce due to starvation-induced damage and hydrogen-peroxide sensitivity (Bogosian & Bourneuf, 2001).
One interesting question is how asymmetry in population damage is generated. Recent reports have shown that more genes than expected show changes in their expression levels during progression through the bacterial division cycle (Bechtloff et al., 1999; Laub et al., 2000; Weitao et al., 2000). It is possible that sudden starvation and growth arrest at a time in the cycle when specific gene products (for example, SodA, SodB and UspA) are present at low levels could generate a subpopulation of cells that undergo increased damage during prolonged stasis. There is no direct evidence for this, but it is interesting to note that the amount of Sod is much lower in non-culturable cells, and that the pattern of protein carbonylation is similar in non-culturable cells and cells that lack cytoplasmic Sod activity. For example, self-inflicted oxidation of proteins is increased both in sod mutants (Dukan & Nyström, 1999) and in non-culturable cells (this study). Moreover, the oxidation of specific proteins, such as H-NS, GltD and FabB, is similar in _sod_-deficient cells (Dukan & Nyström, 1999) and in non-culturable wild-type cells (Fig. 3). In addition, the elevated expression of specific stress regulons (RpoS, SoxRS, RpoH, RpoE and CpxR) in non-culturable cells might be mimicked by decreased Sod activity. Last, sodA sodB mutants have been shown to lose culturability at an increased rate during stasis (Liochev & Fridovich, 1992; Benov & Fridovich, 1995; Dukan & Nyström, 1999). Thus, it is possible that the loss of reproductive ability of some cells as they enter stationary phase is linked to the abundance of Sod in these individual cells. If so, the longevity of stationary-phase E. coli cells might, as in ageing fruitflies (Sun & Tower, 1999), be limited by the cellular availability of Sod.
METHODS
Chemicals and reagents.
Anti-DnaK mouse monoclonal antibodies were from StressGen Biotechnologies and anti-RpoS mouse monoclonal antibodies were from Neoclone. Anti-SodA and anti-SodB rabbit polyclonal antibodies were gifts from D. Touati. Anti-mouse and anti-rabbit IgG peroxidase conjugates were from Sigma. The chemiluminescence blotting substrate (ECL+) and Hybond-P polyvinylidene difluoride membrane (Amersham) were used in accordance with the manufacturers' instructions. Radioselectan (76%) was from Schering.
Bacterial strains and media.
All strains used are E. coli K-12 derivatives of strain MG1655. Cultures were grown aerobically at 37 °C in liquid Luria–Bertani (LB) medium, using Erlenmeyer flasks, in a rotary shaker.
General methods.
Crude cell extracts were obtained using an SLM-Aminco French Pressure Cell. Culture samples were processed to produce extracts for resolution on two-dimensional polyacrylamide gels using the method of O'Farrell (1975) with the modifications described in VanBoegelen & Neidhardt (1990). β-galactosidase levels were measured as described by Miller (1972), with the modifications described by Albertson & Nyström (1994). Fractionation of cellular compartments was carried out as described previously (Imlay & Fridovich, 1991).
Carbonylation and antioxidant measurements.
Detection of carbonylated proteins was carried out as described previously (Dukan & Nyström, 1998). Superoxide dismutase activity was assayed as described by Imlay & Fridovich (1991). Catalase, glucose-6-phosphate dehydrogenase and glutathione reductase activities were measured as described by Gonzalez-Flecha et al. (1993), Fraenkel & Levisohn (1967) and Lopez-Barea & Lee (1979).
Single-cell analysis.
Cells were fixed using 50% ethanol in PBS, and were then permeabilized with lysozyme (5 mg ml−1) in 100 mM Tris-HCl, 50 mM EDTA, pH 8.0 for 15 min at 20 °C. The cells were then washed twice in buffer 1 (1.2 M sorbitol, 0.1 M potassium phosphate (pH 6.5), 1% 2-mercaptoethanol) and resuspended in buffer 2 (1.2 M sorbitol, 0.1 M potassium phosphate (pH 6.5)). Cellular carbonyl groups were derivatized as described in Dukan & Nyström (1998). Cells were then placed on slides that had been pre-treated with poly-lysine, and were rinsed with PBS and immersed in cold methanol (−20 °C) for 6 min, followed by immersion in cold acetone (−20 °C) for 30 s. After drying, slides were incubated in blocking buffer (1% BSA in PBS–Tween) for 15 min at 20 °C. Samples were incubated with primary antibody for 2 h and then immersed for 5 min in PBS containing 0.05% Igepal CA-360. The slides were then incubated with biotinylated secondary antibody (45 min) and, after immersion in PBS, 0.05% Igepal CA-360 (5 min), were incubated with strepavidin–fluorescein-isothiocyanate (2 min). Transmission images of cells were obtained, and fluorescence emission from fluorescently labelled antibodies was analysed using Radiance 2000-MP confocal/multiphoton equipment (BioRad). All samples were laserscanned in three dimensions to ensure that any low signals obtained were not the result of the cells being out of focus.
Radioselectan equilibrium density-gradient centrifugation.
Cells were grown in LB medium at 37 °C and collected after 48 h. Cells were washed in cold (4 °C) phosphate buffer (0.05 M, pH 7) and concentrated tenfold in cold (4 °C) 26.45% Radioselectan. Gradients were made as described previously (Siegele et al., 1993) using 10 ml of radioselectan in polycarbonate centrifuge tubes (25 mm × 89 mm). The gradients can be layered with 1 ml of bacterial solution (5 × 1010 cells) without loss of resolution. Gradients were spun at 55,000 r.p.m. in a Ti60 rotor in a Beckman tabletop ultracentrifuge at 4 °C for 4 h. After collection, the cells were pelleted, rinsed and resuspended in sterile MilliQ water.
Acknowledgments
This work was funded by a grant from the Swedish Natural Science Research Council and the Foundation for Strategic Research (to T.N.) and by the CNRS–INSU, ATI 2001 (to S.D.).
References
- Albertson N.H. & Nyström T. (1994) Effects of starvation for exogenous carbon on functional mRNA stability and rate of peptide chain elongation in Escherichia coli. FEMS Microbiol. Lett., 117, 181–187. [DOI] [PubMed] [Google Scholar]
- Bechtloff D., Grunenfelder B., Åkerlund T. & Nordström K. (1999) Analysis of protein synthesis rates after initiation of chromosome replication in Escherichia coli. J. Bacteriol., 181, 6292–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benov L. & Fridovich I. (1995) A superoxide dismutase mimic protects sodA sodB Escherichia coli against aerobic heating and stationary-phase death. Arch. Biochem. Biophys., 322, 291–294. [DOI] [PubMed] [Google Scholar]
- Bogosian G. & Bourneuf E.V. (2001) A matter of bacterial life and death. EMBO Rep., 2, 770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell R.R. (2000) in Non-culturable Microorganisms in the Environment (eds Colwell, R.R. & Grimes, D.J.), 325–342. American Society for Microbiology, Washington DC, USA. [Google Scholar]
- Dukan S. & Nyström T. (1998) Bacterial senescence: stasis results in increased and differential oxidation of cytoplasmic proteins leading to developmental induction of the heat shock regulon. Genes Dev., 12, 3431–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukan S. & Nyström T. (1999) Oxidative stress defense and deterioration of growth-arrested Escherichia coli cells. J. Biol. Chem., 274, 26027–26032. [DOI] [PubMed] [Google Scholar]
- Ericsson M., Hanstorp D., Hagberg P., Enger J. & Nyström T. (2000) Sorting out bacterial viability with optical tweezers. J. Bacteriol., 182, 5551–5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D.G. & Levisohn S.R. (1967) Glucose and gluconate metabolism in a mutant of Escherichia coli lacking gluconate-6-phosphate dehydrase. J. Bacteriol., 93, 1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Flecha B., Cutrin J.C. & Boveris A. (1993) Time course and mechanism of oxidative stress and tissue damage in rat liver subjected to in vivo ischemia-reperfusion. J. Clin. Invest., 91, 456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland R.P. (1998) in Handbook of Fluorescent Probes and Research Chemicals, 163–173, 381. Molecular Probes, Eugene, Oregon, USA. [Google Scholar]
- Hengge-Aronis R. (2000) in Bacterial Stress Responses (eds Storz, G. & Hengge-Aronis, R.), 161–178. American Society for Microbiology, Washington DC, USA. [Google Scholar]
- Huq A., Rivera I.N.G. & Colwell R.R. (2000) in Non-culturable Microorganisms in the Environment (eds Colwell R.R. & Grimes, D.J.), 301–324. American Society for Microbiology, Washington DC, USA. [Google Scholar]
- Imlay J.A. & Fridovich I. (1991) Assay of metabolic superoxide production in Escherichia coli. J. Biol. Chem., 266, 6957–6965. [PubMed] [Google Scholar]
- Kell D.B., Keprelyants A.S., Weichart D.H., Harwood C.R. & Barer M.R. (1998) Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie van Leeuwenhoek, 73, 169–187. [DOI] [PubMed] [Google Scholar]
- Laub M.T., McAdams H.H., Feldblyum T., Fraser C.M. & Shapiro L. (2000) Global analysis of the genetic network controlling a bacterial cell cycle. Science, 290, 2144–2148. [DOI] [PubMed] [Google Scholar]
- Liochev S.I. & Fridovich I. (1992) Effects of overproduction of superoxide dismutases in Escherichia coli on inhibition of growth and on induction of glucose-6-phosphate dehydrogenase by paraquat. Arch. Biochem. Biophys., 294, 138–143. [DOI] [PubMed] [Google Scholar]
- Lopez-Barea J. & Lee C.Y. (1979) Mouse-liver glutathione reductase. Purification, kinetics, and regulation. Eur. J. Biochem., 98, 487–499. [DOI] [PubMed] [Google Scholar]
- Miller J. (1972) Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, USA. [Google Scholar]
- Nyström T. & Neidhardt F.C. (1994) Expression and role of the universal stress protein, UspA, of Escherichia coli during growth arrest. Mol. Microbiol. 11, 537–544. [DOI] [PubMed] [Google Scholar]
- O'Farrell P.H. (1975) High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem., 250, 4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oliver J.D. (2000) in Non-culturable Microorganisms in the Environment (eds Colwell, R.R. & Grimes, D.J.), 277–300. American Society for Microbiology, Washington DC, USA. [Google Scholar]
- Raivio T.L. & Silhavy T.J. (2000) in Bacterial Stress Responses (eds Storz, G. & Hengge-Aronis, R.), 19–32. American Society for Microbiology, Washington DC, USA. [Google Scholar]
- Roszak D.B. & Colwell R.R. (1987) Survival strategies of bacteria in the natural environment. Microbiol. Rev., 51, 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegele R.D.A., Almiron M. & Kolter R. (1993) in Starvation in Bacteria (ed. Kjelleberg, S.), 151–170. Plenum, New York, New York, USA. [Google Scholar]
- Sun J. & Tower J. (1999) FLP recombinase-mediated induction of Cu/Znsuperoxide dismutase transgene expression can extend the lifespan of adult Drosophila melanogaster flies. Mol. Cell. Biol., 19, 216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBoegelen R.A. & Neidhardt F.C. (1990) Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc. Natl Acad. Sci. USA, 87, 5589–5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitao T., Nordström K. & Dasgupta S. (2000) Escherichia coli cell cycle control genes affect chromosome superhelicity. EMBO Rep., 1, 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]