Hrs mediates downregulation of multiple signalling receptors in Drosophila (original) (raw)
Abstract
Endocytosis and subsequent lysosomal degradation of activated signalling receptors can attenuate signalling. Endocytosis may also promote signalling by targeting receptors to specific compartments. A key step regulating the degradation of receptors is their ubiquitination. Hrs/Vps27p, an endosome-associated, ubiquitin-binding protein, affects sorting and degradation of receptors. Drosophila embryos mutant for hrs show elevated receptor tyrosine kinase (RTK) signalling. Hrs has also been proposed to act as a positive mediator of TGF-β signalling. We find that Drosophila epithelial cells devoid of Hrs accumulate multiple signalling receptors in an endosomal compartment with high levels of ubiquitinated proteins: not only RTKs (EGFR and PVR) but also Notch and receptors for Hedgehog and Dpp (TGF-β related). Hrs is not required for Dpp signalling. Instead, loss of Hrs increases Dpp signalling and the level of the type-I receptor Thickveins (Tkv). Finally, most _hrs_-dependent receptor turnover appears to be ligand independent. Thus, both active and inactive signalling receptors are targeted for degradation in vivo and Hrs is required for their removal.
Introduction
Monoubiquitination of membrane proteins has an important role in regulating their internalization and sorting to lysosomal degradation (Polo et al., 2002; Haglund et al., 2003). The ubiquitin tag is recognized by proteins containing a ubiquitin interaction motif (UIM), such as epsins (Shih et al., 2002), Hse1p/STAM (Bilodeau et al., 2002) and Eps15 (Polo et al., 2002). Hrs and its budding yeast homologue, Vps27p, also have a UIM and bind to ubiquitin (Bilodeau et al., 2002; Lloyd et al., 2002; Polo et al., 2002;Raiborg et al., 2002). The ubiquitin-binding ability of Hrs and Vps27p is required for the efficient sorting of ubiquitinated transferrin receptors in mammalian cells and Fth1p in yeast (Bilodeau et al., 2002;Raiborg et al., 2002).
Results and discussion
Ubiquitinated proteins accumulate in hrs mutant cells
To determine whether Hrs was generally required for sorting and degradation of ubiquitinated proteins in Drosophila tissues, we generated clones of cells mutant for hrs within an epithelium using somatic recombination. We looked at the follicle cells of the Drosophila ovary and wing imaginal disc cells from third instar larvae. Follicular cells form a simple monolayer epithelium surrounding the germline cells and are large enough to detect subcellular localization of protein. The imaginal disc cells are smaller and form a pseudo-stratified epithelium. The mosaic tissues were stained with an antibody that recognizes mono- and polyubiquitinated proteins. Both follicle cells and wing disc cells lacking Hrs showed a dramatic accumulation of ubiquitinated proteins (Fig. 1B–D). Most of the signal localizes to intracellular structures. In some cases we could also observe accumulation at the cell cortex (arrows in Fig. 1C). Thus, Hrs is required for the efficient removal of ubiquitinated proteins from the cell.
Figure 1.
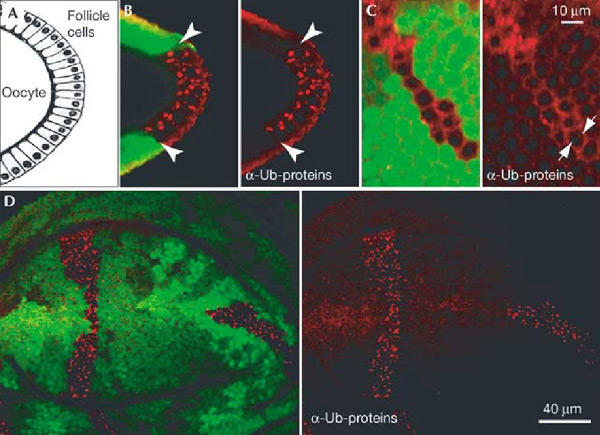
Accumulation of ubiquitinated proteins in hrs mutant cells. Ubiquitinated proteins accumulate intracellularly in hrs mutant cells. Mosaic egg chambers and wing discs were stained with an antibody against ubiquitinated proteins (red). (A) Schematic diagram of a transverse section through the oocyte and the follicle cells, as shown in (B). (C) Optical section in the plane of the follicular epithelium.hrs mutant cells are marked by the absence of GFP (green) in both ovarian follicle cells (B, C) and wing discs (D). Some ubiquitinated proteins appear to be at the cell cortex (arrows in (C)). Arrowheads in (B) indicate the boundary between mutant and wild-type cells.
An enlarged vesicular structure, the ‘class E' compartment, has been observed in yeast cells mutant for VPS27 (Piper et al., 1995). Genetic studies in mice and_Drosophila_ have also shown that cells mutant for hrs have enlarged endosomes (Komada et al., 1997;Lloyd et al., 2002), possibly due to impaired membrane invagination and multivesicular body (MVB) formation (Lloyd et al., 2002). To determine whether ubiquitinated proteins accumulate in the endosomal compartment in hrs mutant cells, we expressed GFP-Rab5 or GFP-2xFYVE fusion proteins in hrs mutant cells (Wucherpfennig et al., 2003). Rab5, a small GTPase regulating endosome fusion, is a marker of early endosomes. FYVE domains bind to phosphatidylinositol-3-phosphate, which is enriched in endosomal membranes (Gaullier et al., 1998; Christoforidis et al., 1999), and can also be used to specifically label endosomes (Wucherpfennig et al., 2003). The ubiquitinated protein signal and the GFP-2xFYVE signal showed extensive overlap in hrs mutant follicle cells (Fig. 2A,C). GFP-Rab5 and ubiquitinated proteins also showed significant, although not complete, overlap (Fig. 2B). These data indicate that nondegraded ubiquitinated proteins accumulate in the endosomal compartment. Additionally, when we compared the GFP-2xFYVE signal in hrs mutant and nonmutant cells, we could observe an enlargement of FYVE-positive structures in mutant cells, consistent with an enlargment of the endosomal compartment (Fig. 2C).
Figure 2.
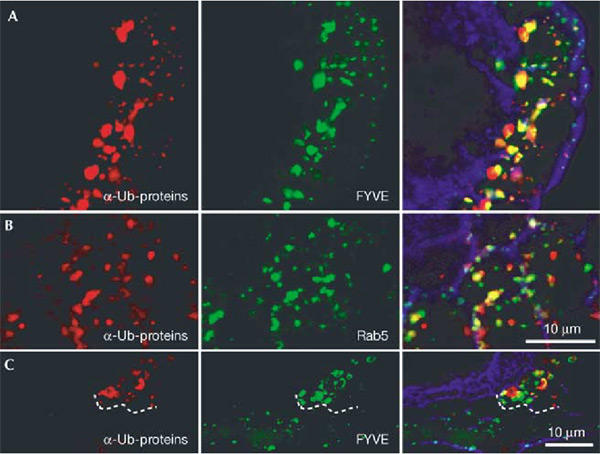
Ubiquitinated proteins accumulate in enlarged endosomes in_hrs_ mutant cells. Egg chambers expressing GFP-2xFYVE (A, C) or GFP-Rab5 (B) (green) and carrying patches of hrs mutant follicle cells were stained with an antibody against ubiquitinated proteins (red). In (A) and (B) all cells shown are hrs mutant, and in (C) the boundary between mutant and wild-type cells is indicated with a dashed line. hrs mutant cells can be detected by distinctive staining with the ubiquitinated protein antibody. Note the increased staining with the endosomal marker (FYVE) in mutant cells relative to neighbouring cells. Phalloidin-stained F-actin (blue) outlines cells in the overlay to the right.
Hrs affects multiple signalling receptors
Hrs was already known to affect degradation of receptor tyrosine kinases (RTKs). Indeed the two RTKs that we analysed in follicle cells, EGFR and PVR (PDGF/VEGF receptor), accumulated within hrs mutant cells, mostly in intracellular structures. These structures were also positive for the ubiquitinated protein signal, indicating that the receptors accumulate in endosomes (Fig. 3A,B).
Figure 3.
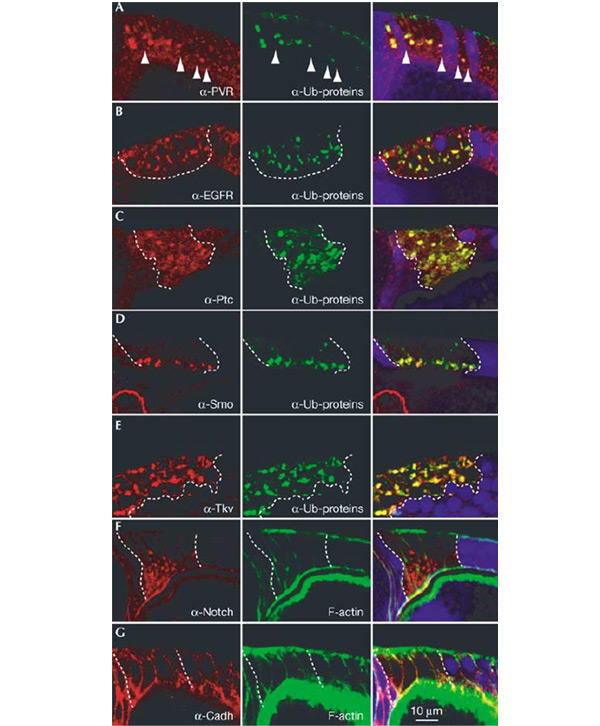
Colocalization of signalling receptors and ubiquitinated proteins in_hrs_ mutant cells. Egg chambers with patches of hrs mutant follicle cells were stained with an antibody against ubiquitinated protein (green) and antibodies against the following specific proteins (red): PVR (A), EGFR (B), Ptc (C), Smo (D), Tkv (E), Notch (F) and DE-cadherin (G). Notch could not be analysed for colocalization with the ubiquitinated protein due to antibody incompatibility. Instead, labelled phalloidin (green) is used to mark cell outlines in (F) and (G). The overlap between the signals is yellow in the merged images (right). The boundary between hrs mutant and wild-type cells is indicated with arrowheads (A) or with dashed lines (B–G). Mutant cells are marked by the absence of GFP (blue) in the merged images. (A, D, F, G) Similar transverse sections through the egg chamber, with the apical side of the follicle cells towards the oocyte (bottom of image). (B, C,E) More oblique sections through the follicular epithelium.
To test whether the requirement for Hrs was limited to RTKs, other types of signalling receptors were analysed. The Hedgehog receptor Patched and the Hedgehog signal transducer Smoothened are multi- and seven-pass transmembrane proteins, respectively. Thickveins (Tkv) is a type-I serine–threonine kinase receptor for the TGF-β family ligand Dpp. Notch is a single-pass transmembrane protein that undergoes specific proteolytic cleavage upon activation. Interestingly, hrs mutant follicle cells showed a marked accumulation of each of these receptors (Fig. 3C–F). As for RTKs, most of the receptor molecules accumulated intracellularly and showed significant colocalization with the ubiquitinated protein signal (Fig. 3 and enlargement insupplementary figure 1 online). Thus, Hrs has a general role in regulating the sorting and degradation of diverse classes of signalling receptors. The homotypic adhesion molecule DE-cadherin was not affected visibly in hrs mutant cells (Fig. 3G). The latter observation is in agreement with previous observations that nonsignalling transmembrane proteins were not upregulated in hrs mutant animals (Lloyd et al., 2002). Either the trafficking of these proteins is independent of Hrs function or they have a low turnover rate in the examined tissues.
The high degree of overlap between the signal for each of the receptors and the signal for ubiquitinated proteins means that the receptors accumulate in roughly the same endosomal compartment. This, together with the increase in receptor levels in hrs mutant cells, suggests that these receptors are degraded through the same Hrs-dependent pathway. Ubiquitination of the inhibitory Smad7 by the E3 ubiquitin ligase Smurf2 has been shown to target the Smad7-TGF-β receptor complex for lysosomal degradation (Kavsak et al., 2000). In follicle cells, a similar complex may be sorted for degradation in an Hrs-dependent manner. It has been argued that the turnover of Hedgehog receptors is strongly regulated and may be critical for signalling (Denef et al., 2000; Incardona et al., 2000), but a role of ubiquitination in this event has not been reported. The observation that Patched and Smoothened accumulate in compartments highly enriched in ubiquitinated proteins in hrs mutant cells suggests that trafficking of Patched and Smoothened is also regulated by ubiquitination.
When analysing hrs mutant clones, we occasionally noticed an increase of ubiquitinated proteins at the cell cortex in addition to the intracellular accumulation (Fig. 1C). Some cortical accumulation could also be observed directly for the signalling receptors, in particular for Tkv (Fig. 4G,H). This accumulation could be due to inefficient endocytosis from the plasma membrane or increased recycling of endocytosed proteins. Hrs does not appear to be required directly for endocytosis (Lloyd et al., 2002), but downstream defects may ‘clog up' the endocytosis machinery. Hrs can also affect receptor recycling. Overexpression of Hrs in tissue culture cells increases the retention of ubiquitinated transferrin receptors (Raiborg et al., 2002). The strong intracellular accumulation of receptors in hrs mutant cells could therefore either be due to defective sorting towards lysosomal degradation or due to defective post-endocytic retention, a concomitant general increase in the steady-state levels of the receptors at the plasma membrane, and therefore in endosomes. We favour the first explanation because we often did not detect increased surface levels of receptors or ubiquitinated proteins even when strong intracellular accumulation was evident (see for example Figs 1B,D and3B,D). Receptors therefore seem to be retained intracellularly, rather than recycled, in hrs mutant cells. Hrs is most likely not the only factor responsible for the post-endocytic retention of receptors. Redundancy in sorting to the vacuole has been reported for the yeast α-factor receptor Ste3p. In this case, Vps27p and Hse1 have overlapping roles to sort Ste3p to the vacuolar lumen (Bilodeau et al., 2002).
Figure 4.
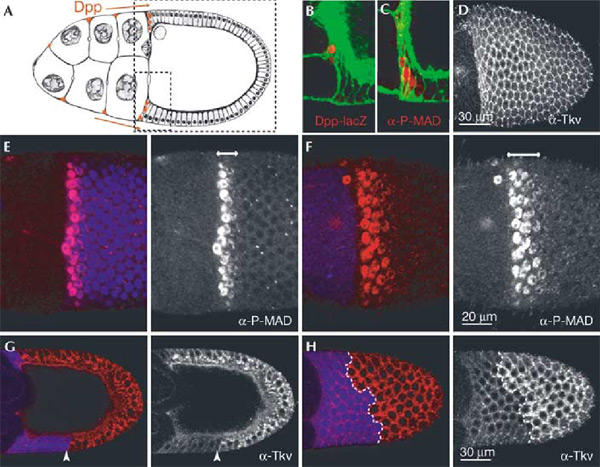
Role of Hrs in Dpp signalling and Tkv trafficking in follicle cells. (A) Schematic cross-section of stage 10 egg chamber. A small and a large stippled box indicate areas shown in (B, C) and (D,G, H), respectively. (D, H) Surface views of the epithelium, as are (E) and (F). Anterior follicle cells (red in (A)) express Dpp and Dpp-lacZ (see also (B)). P-MAD staining visualizes Dpp signalling activity in the cells expressing Dpp and in adjacent cells (C). The Dpp receptor Tkv is detected in all follicle cells**(D). Wild-type (C, D) and hrs mosaic (E–H**) stage 10 egg chambers stained with antibodies (red in double-staining) against β-galactosidase (B), P-MAD (C,E, F) or Tkv (D, G, H). (B) and (C) are also stained with phalloidin (green). Mutant cells are marked by the absence of GFP (blue) in (E–H). The boundary between_hrs_ mutant and wild-type cells is also indicated with an arrowhead (G) or a dashed line (H). In (E) no follicle cells are mutant, and in (F) all follicle cells are mutant for hrs. The double-headed arrow (E, F) indicates the P-MAD positive domain, which is expanded when cells are mutant for hrs.
Hrs is not required for Dpp signalling
Hrs has been suggested to play a critical positive role in TGF-β signal transduction in mice by stimulating the recruitment of Smad2 to the receptor (Miura et al., 2000). We decided to test whether Hrs is required for TGF-β/Dpp signalling in_Drosophila_ and thus might serve a conserved role in this pathway. In the egg chamber, Dpp is expressed in the anterior follicle cells and contributes to the patterning of the follicular epithelium (Twombly et al., 1996; Peri & Roth, 2000) (Fig. 4A,B). The receptor Tkv is expressed uniformly in the epithelium (Fig. 4D). Active signalling downstream of the receptor can be monitored by the presence of the phosphorylated form of MAD (P-MAD) in the nucleus (anti-P-MAD; Persson et al., 1998). In wild-type stage 10 egg chambers, P-MAD was detected in the Dpp-producing anterior follicle cells and 1–2 rows of follicle cells immediately adjacent to the source (Fig. 4C,E). In_hrs_ mutant follicle cells close to the Dpp ligand source, MAD phosphorylation and nuclear translocation still occurred efficiently (Fig. 4F). Thus, Hrs is not required for Dpp signalling in this context.
The P-MAD expression domain was expanded to 3–4 rows of follicle cells if the epithelium was mutant for hrs (compareFig. 4E,F). The P-MAD signal was graded, indicating that signalling was still dependent on the Dpp ligand gradient. Apparently, the_hrs_ mutant follicle cells have increased sensitivity to Dpp. Since a higher level of Tkv protein is known to sensitize cells to low levels of Dpp (Lecuit & Cohen, 1998), the expansion of the P-MAD domain can most simply be explained by the increased amount of Tkv at the surface of hrs mutant cells (Fig. 4G).
We next determined the effect of Hrs on Dpp target gene activation. The wing disc was used for this analysis as Dpp signalling and target gene activation are well characterized. Dpp is expressed in the middle of the wing disc (at the anterior–posterior (A–P) boundary) and forms a morphogen gradient. Spalt, a target of Dpp signalling, is expressed in a characteristic band at both sides of the A–P boundary (Fig. 5A). hrs mutant patches within the endogenous Splat domain showed a slight increase in Spalt levels (Fig. 5B). When hrs clones were located at the edge of the Spalt domain, a modest expansion of the expression was observed (Fig. 5C). Thus, Hrs is not required for Dpp target gene activation in the wing disc. Instead, Hrs has a slightly negative effect on the pathway. As for the P-MAD signal in follicle cells, the Spalt signal was still graded in_hrs_ mutant clones and we did not observe ectopic Spalt expression in_hrs_ mutant cells far from the Dpp source. This indicates that Spalt activation was still dependent on endogenous Dpp. The effects of hrs appear to be cell autonomous and positive in all parts of the Spalt expression domain, suggesting that hrs mutant wing disc cells are simply more sensitive to Dpp.
Figure 5.
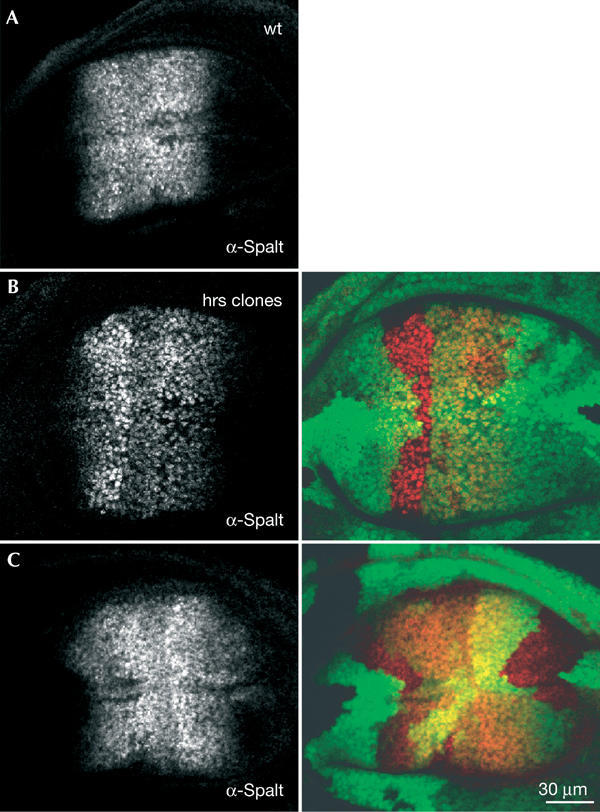
Dpp target gene expression is increased in hrs mutant wing disc cells. Wild-type (A) and hrs mosaic (B, C) wing discs were stained with an antibody against Spalt (red in the merged images). hrs mutant cells are marked by the absence of GFP (green). Dpp is expressed in the middle of the disc and induces Spalt expression in a broad domain. Note the slight increase in Spalt expression in hrs mutant cells within the normal expression domain (B) and the expansion of the Spalt expression domain when hrs mutant cells are at the edge of this domain ((C), most clear in the middle of the disc, to the right).
Hrs mutant mouse embryonic cells show dramatically decreased responses to TGF-β stimulation (Miura et al., 2000). We found that Hrs was not required for Dpp signalling in_Drosophila_ wing disc cells and ovarian follicle cells. The difference between these results may reflect an acquired aspect of TGF-β pathway regulation in mammals or a specific regulation in mouse embryonic stem cells. However, it is clear that Hrs does not play a conserved general role in this otherwise quite conserved signalling pathway.
Constitutive, ligand-independent receptor turnover
To analyse the importance of ligand stimulation for Hrs-dependent downregulation of receptors, we first compared Tkv accumulation in hrs mutant cells close to, and far from, the endogenous Dpp source. We looked at follicle cells that allow a clear detection of intracellular as well as cortical Tkv staining. There could be other TGF-β-related ligands in the ovary, but the P-MAD staining indicates that the only significant source of signal stimulating this pathway comes from the anterior. Interestingly, high levels of Tkv accumulation were observed even in those hrs mutant follicle cells that were farthest from the Dpp source (100–120 μm away), experiencing no or very little Dpp ligand and signalling (no MAD phosphorylation) (Fig. 4G,H). Tkv accumulation was apparently uniform in all hrs mutant follicle cells, that is, cells closest and farthest from the Dpp source accumulated similar amounts of the receptor (Fig. 4G,H). These observations indicate that the bulk of Hrs-dependent downregulation of Tkv is constitutive in these cells, independent of ligand. This does not rule out the possibility that ligand-induced endocytosis also occurs. In the follicular epithelium, the spread of Dpp may be limited to a few cell diameters by high levels of receptor. It is possible that only a small fraction of the receptor molecules bind Dpp ligand. In this case, given the high rate of constitutive receptor turnover, stimulation would not affect visibly the bulk of receptor trafficking.
The bulk of Hrs-dependent downregulation of other signalling receptors appeared to be constitutive as well. Hedgehog acts very early in egg chamber development (Forbes et al., 1996), and patched-lacZ, which reflects Hedgehog activity, has very restricted expression. Downregulation of Patched in the stage 10 egg chamber should therefore be ligand independent (Fig. 3C). Smoothened protein is, in turn, controlled by Patched (Denef et al., 2000; Incardona et al., 2000). EGFR ligands are highly enriched and active at the dorsal side of the egg chamber (Neumansilberberg & Schüpbach, 1996), whereas a PVR ligand is present throughout the oocyte (Duchek et al., 2001). However, for both receptors, the level of receptor accumulation in hrs mutant cells was similar throughout the follicular epithelium. Signal-induced endocytosis is well established for acute stimulation of signalling receptors, in particular RTKs. Yet signalling does not appear to control the bulk of receptor turnover in follicle cells. The physiological levels of stimulatory ligands may be relatively low compared to the levels used for acute stimulation experiments.
Precise regulation of signalling strength is essential for interpreting morphogen gradients and thus for correct patterning during development. The control of signalling receptor levels at the cell membrane is an important aspect of this regulation. It is therefore of interest to know how receptor levels are regulated under physiological conditions. The results presented here indicate that diverse classes of signalling receptors undergo constitutive (ligand-independent) ubiquitination, endocytosis and Hrs-dependent degradation. The efficiency of this traffic affects the responsiveness of cells to patterning signals: blockage of trafficking in hrs mutants can sensitize cells to a low level of signalling molecules, whether RTK ligands (Lloyd et al., 2002) or Dpp (this study). However, it does not lead to ligand-independent signalling, supporting the conclusion that most endocytosed receptor molecules are not activated. Ligand-induced endocytosis may also occur, but affects only a minority of receptor molecules in this in vivo context. Constitutive turnover of receptors may serve as quality control by removing damaged receptors or receptors in partially formed signalling complexes. A constant flux of all receptor molecules may also facilitate the efficient clearance of activated receptors.
Methods
A fragment encoding the carboxy-terminal 227 amino acids of_Drosophila_ EGFR was PCR amplified and cloned into pGEX4 (Promega). The GST fusion protein was expressed in Escherichia coli, purified on glutathione beads, and used as antigens for polyclonal antibody production in rats. Specificity of the antibody was confirmed by loss of immunofluorescence staining in clones of EGFR null mutant cells (_top_CO). All other antibodies have been described previously: antibody against mono- and polyubiquitinated proteins (Affiniti), anti-Notch (Developmental Studies Hybridoma Bank, C17.9C6), anti-PVR (Duchek et al., 2001), rat anti-Ptc (Capdevila et al., 1994), rat anti-Smo (Denef et al., 2000), rat anti-Tkv and rat anti-Spalt (Teleman & Cohen, 2000) and rat monoclonal anti-DE-cadherin (Oda et al., 1994).
In all experiments, the hrs D28 allele was used. It contains a nonsense mutation at the beginning of the UIM at amino acid Q270 (Lloyd et al., 2002). To generate_hrs_ mutant clones, larvae of the genotype hs-FLP/+; hrs FRT40A/Ubi-GFP FRT40A were heatshocked for 1 h at 37 °C. To generate_hrs_ mutant follicle cells expressing GFP-Rab5 or GFP-2xFYVE, the genotype hs-FLP/+; hrs FRT40A/Ubi-GFP FRT40A; slbo-Gal4/UAS-GFP-Rab5 (or UAS-GFP-2xFYVE) and larval heatshock were used. The nuclear GFP clonal marker is weak relative to the GFP-2xFYVE signal and clearly distinguishable from it. Wing discs from third instar larvae or ovaries from adult females were dissected 4–5 or 7–10 days later, respectively, fixed in 4% paraformaldehyde and stained using standard techniques. All images are confocal sections.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/4-7400019-s1.pdf).
Supplementary Material
Supplementary Figure
Acknowledgments
We thank H. Bellen for the hrs mutant flies, P. ten Dijk for P-MAD antibody, M. González-Gaitán for the GFP-Rab5 and GFP-2xFYVE flies, and S.M. Cohen for antibodies and for helpful discussions.
References
- Bilodeau P.S., Urbanowski J.L., Winistorfer S.C. & Piper R.C. ( 2002) The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat. Cell Biol., 4, 534–539. [DOI] [PubMed] [Google Scholar]
- Capdevila J., Estrada M.P., Sanchez-Herrero E. & Guerrero I. ( 1994) The Drosophila segment polarity gene patched interacts with decapentaplegic in wing development. EMBO J., 13, 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S., Miaczynska M., Ashman K., Wilm M., Zhao L., Yip S.C., Waterfield M.D., Backer J.M. & Zerial M. ( 1999) Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat. Cell Biol., 1, 249–252. [DOI] [PubMed] [Google Scholar]
- Denef N., Neubuser D., Perez L. & Cohen S.M. ( 2000) Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell, 102, 521–531. [DOI] [PubMed] [Google Scholar]
- Duchek P., Somogyi K., Jékely G., Beccari S. & Rørth P. ( 2001) Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell, 107, 17–26. [DOI] [PubMed] [Google Scholar]
- Forbes A.J., Lin H., Ingham P.W. & Spradling A.C. ( 1996) hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in_Drosophila_. Development, 122, 1125–1135. [DOI] [PubMed] [Google Scholar]
- Gaullier J.M., Simonsen A., D'Arrigo A., Bremnes B., Stenmark H. & Aasland R. ( 1998) FYVE fingers bind PtdIns(3)P. Nature, 394, 432–433. [DOI] [PubMed] [Google Scholar]
- Haglund K., Sigismund S., Polo S., Szymkiewicz I., Di Fiore P.P. & Dikic I. ( 2003) Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nature Cell Biol., 5, 461–466. [DOI] [PubMed] [Google Scholar]
- Incardona J.P., Lee J.H., Robertson C.P., Enga K., Kapur R.P. & Roelink H. ( 2000) Receptor-mediated endocytosis of soluble and membrane-tethered Sonic hedgehog by Patched-1. Proc. Natl Acad. Sci. USA, 97, 12044–12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavsak P., Rasmussen R.K., Causing C.G., Bonni S., Zhu H., Thomsen G.H. & Wrana J.L. ( 2000) Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol. Cell, 6, 1365–1375. [DOI] [PubMed] [Google Scholar]
- Komada M., Masaki R., Yamamoto A. & Kitamura N. ( 1997) Hrs, a tyrosine kinase substrate with a conserved double zinc finger domain, is localized to the cytoplasmic surface of early endosomes. J. Biol. Chem., 272, 20538–20544. [DOI] [PubMed] [Google Scholar]
- Lecuit T. & Cohen S.M. ( 1998) Dpp receptor levels contribute to shaping the Dpp morphogen gradient in the Drosophila wing imaginal disc. Development, 125, 4901–4907. [DOI] [PubMed] [Google Scholar]
- Lloyd T.E., Atkinson R., Wu M.N., Zhou Y., Pennetta G. & Bellen H.J. ( 2002) Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell, 108, 261–269. [DOI] [PubMed] [Google Scholar]
- Miura S. et al. ( 2000) Hgs (Hrs), a FYVE domain protein, is involved in Smad signaling through cooperation with SARA. Mol. Cell. Biol., 20, 9346–9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman-Silberberg F.S. & Schüpbach T. ( 1996) The Drosophila TGF-_α_-like protein Gurken: expression and cellular localization during Drosophila oogenesis. Mech. Dev., 59, 105–113. [DOI] [PubMed] [Google Scholar]
- Oda H., Uemura T., Harada Y., Iwai Y. & Takeichi M. ( 1994) A Drosophila homolog of cadherin associated with armadillo and essential for embryonic cell–cell adhesion. Dev. Biol., 165, 716–726. [DOI] [PubMed] [Google Scholar]
- Peri F. & Roth S. ( 2000) Combined activities of Gurken and decapentaplegic specify dorsal chorion structures of the Drosophila egg. Development, 127, 841–850. [DOI] [PubMed] [Google Scholar]
- Persson U., Izumi H., Souchelnytskyi S., Itoh S., Grimsby S., Engstrom U., Heldin C.H., Funa K. & ten Dijke P. ( 1998) The L45 loop in type I receptors for TGF-beta family members is a critical determinant in specifying Smad isoform activation. FEBS Lett., 434, 83–87. [DOI] [PubMed] [Google Scholar]
- Piper R.C., Cooper A.A., Yang H. & Stevens T.H. ( 1995) VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J. Cell Biol., 131, 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo S., Sigismund S., Faretta M., Guidi M., Capua M.R., Bossi G., Chen H., De Camilli P. & Di Fiore P.P. ( 2002) A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature, 416, 51–55. [DOI] [PubMed] [Google Scholar]
- Raiborg C., Bache K.G., Gillooly D.J., Madshus I.H., Stang E. & Stenmark H. ( 2002) Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nature Cell Biol., 4, 394–398. [DOI] [PubMed] [Google Scholar]
- Shih S.C., Katzmann D.J., Schnell J.D., Sutanto M., Emr S.D. & Hicke L. ( 2002) Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nature Cell Biol., 4, 389–393. [DOI] [PubMed] [Google Scholar]
- Teleman A.A. & Cohen S.M. ( 2000) Dpp gradient formation in the Drosophila wing imaginal disc. Cell, 103, 971–980. [DOI] [PubMed] [Google Scholar]
- Twombly V., Blackman R.K., Jin H., Graff J.M., Padgett R.W. & Gelbart W.M. ( 1996) The TGF-beta signaling pathway is essential for_Drosophila_ oogenesis. Development, 122, 1555–1565. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig T., Wilsch-Brauninger M. & Gonzalez-Gaitan M. ( 2003) Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J. Cell Biol., 161, 609–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure