Notch Signaling Induces Rapid Degradation of Achaete-Scute Homolog 1 (original) (raw)
Abstract
In neural development, Notch signaling plays a key role in restricting neuronal differentiation, promoting the maintenance of progenitor cells. Classically, Notch signaling causes transactivation of Hairy-enhancer of Split (HES) genes which leads to transcriptional repression of neural determination and differentiation genes. We now report that in addition to its known transcriptional mechanism, Notch signaling also leads to rapid degradation of the basic helix-loop-helix (bHLH) transcription factor human achaete-scute homolog 1 (hASH1). Using recombinant adenoviruses expressing active Notch1 in small-cell lung cancer cells, we showed that the initial appearance of Notch1 coincided with the loss of hASH1 protein, preceding the full decay of hASH1 mRNA. Overexpression of HES1 alone was capable of down-regulating hASH1 mRNA but could not replicate the acute reduction of hASH1 protein induced by Notch1. When adenoviral hASH1 was coinfected with Notch1, we still observed a dramatic and abrupt loss of the exogenous hASH1 protein, despite high levels of ongoing hASH1 RNA expression. Notch1 treatment decreased the apparent half-life of the adenoviral hASH1 protein and increased the fraction of hASH1 which was polyubiquitinylated. The proteasome inhibitor MG132 reversed the Notch1-induced degradation. The Notch RAM domain was dispensable but a lack of the OPA and PEST domains inactivated this Notch1 action. Overexpression of the hASH1-dimerizing partner E12 could protect hASH1 from degradation. This novel function of activated Notch to rapidly degrade a class II bHLH protein may prove to be important in many contexts in development and in cancer.
Multicellular organisms require a precise coordination of gene functions in order to generate a diverse array of tissue types from undifferentiated precursors. Multipotential cells must integrate extracellular cues to control the timing and rate of commitment and maturation within specific lineages. Notch signaling plays a critical role in the restriction of many vertebrate cellular lineages, such as neurons versus glia (43), T cells versus B cells (52), and neuroendocrine (NE) versus epithelial cells in the lung (29). Typically, loss of Notch signaling function leads to premature differentiation and depletion of multipotential, uncommitted cellular precursors (14, 22). Gain of Notch function via chromosomal translocation or virally mediated insertional mutagenesis is associated with T-cell acute lymphoblastic leukemia and with mouse mammary tumorigenesis (16, 53).
Vertebrate Notch family genes (37, 38, 63, 65) encode transmembrane receptors which are capable of receiving signals from neighboring cells expressing ligands, including Delta, Jagged1, and Jagged2 (5, 39, 54). Ligand binding to the extracellular epidermal growth factor-like repeats of Notch results in activation of the Notch receptor by a complex cleavage process, liberating a carboxyl-terminal intracellular fragment from the cell membrane (8, 44, 57, 62). Despite intensive studies, the downstream steps of Notch signaling are only partially understood. In the most widely accepted model for Notch action, an intracellular Notch fragment translocates to the nucleus and heterodimerizes with CBF1/RBPjκ, creating a complex capable of transactivating target genes, including the mammalian Hairy-enhancer of Split (HES) family (24, 30, 31). The best characterized Notch target, HES1, is a transcriptional repressor which has been shown to down-regulate basic helix-loop-helix (bHLH) neurogenic and myogenic transcription factors such as mammalian achaete-scute homolog 1 (MASH1)/human achaete-scute homolog 1 (hASH1) (12), and MyoD (36). Alternative CBF1- and HES1-independent actions of Notch also have been described. In C2C12 myoblast differentiation, overexpression of a cytoplasmic form of Notch1, lacking the amino-terminal CBF1-interacting domain, inhibits myogenic differentiation, whereas overexpression of HES1 alone is insufficient (48, 55). In T-cell development, HES1 can account for some, but not all, activities of Notch in the negative selection of CD4-reactive T cells (34). To date, the mechanisms and targets of these alternative, HES1-independent, Notch pathways remain largely unknown.
Genetic studies of mammalian nervous system development have shown that MASH1 is an important target of negative regulation by the Notch-HES pathway (14, 26). In lung development, MASH1 is absolutely essential for the differentiation of normal pulmonary NE cells (6, 29). hASH1 appears to have a comparably important role in maintaining neural and NE properties in small-cell lung cancer (SCLC) cells (6). In addition, hASH1 also can confer NE properties and promote tumorigenesis when targeted to non-NE airway epithelial cells in transgenic mice (40). Our interest in the control of hASH1 in lung development and cancer led us to explore the role of Notch in regulating this bHLH factor. As in nervous system development, elements of the Notch signaling pathway, especially HES1, appear to be critical negative regulators of achaete-scute homolog 1 expression in normal lungs and in lung cancer. For example, HES1 transgenic knockout mice exhibit substantial hyperplasia and premature differentiation of lung NE cells associated with an increase in MASH1-expressing pulmonary epithelium (29). We have shown that overexpression of HES1 in SCLC cells leads to repression of hASH1 expression via a transcriptional mechanism (12). In the present study, we have compared hASH1 regulation in SCLC cells by HES1 and by an activated form of Notch1. As anticipated, overexpression of Notch1 caused a reduction of hASH1 mRNA. Remarkably, Notch1, in addition to its known transcriptional effects, also exerted a dramatic posttranslational effect on hASH1 protein abundance. Our data show that a constitutively active form of Notch1 mediated rapid, proteasome-dependent proteolysis of the bHLH protein hASH1. This novel mechanism potentially may be important in Notch regulation of cell fates in normal development and cancer contexts.
MATERIALS AND METHODS
Cell culture.
DMS53 cells were cultured in Waymouth's medium supplemented with 16% fetal bovine serum (FBS), 100 U of penicillin per ml, and 100 μg of streptomycin per ml. 911 cells were maintained in Dulbecco's minimal essential medium (Life Technologies) with 10% FBS. Low-passage 293 cells were cultured in minimal essential medium (Life Technologies) with 10% FBS. Proteasome inhibitors MG132, lactacystin, and Proteasome Inhibitor I (Calbiochem) were used at 50, 10, and 10 μM concentrations, respectively.
Recombinant adenovirus generation and infection procedure.
The production of high-titer recombinant adenovirus with the AdEasy system has been described previously (21). Briefly, human intracellular Notch1, HES1, hASH1, E12, and mouse MyoD coding sequences were generated by PCR and subcloned into a pAdTrackCMV shuttle plasmid, which allows bicistronic expression of green fluorescent protein (GFP) and the inserted gene under cytomegalovirus (CMV) promoters. All intracellular Notch1 fragments were amplified from human Notch1 plasmid (gift of S. Artavanis-Tsakonas, Massachusetts General Hospital Cancer Center, Charlestown, Mass.). The Notch1 fragments contained the following amino acids: AdNotch1 or AdRANOP, amino acids 1759 to 2556; AdRANO, amino acids 1759 to 2444; AdRAN, amino acids 1759 to 2358; AdRA, amino acids 1759 to 2095; AdRAt, amino acids 1759 to 1991; AdANOP, amino acids 1848 to 2556; and AdAN, amino acids 1848 to 2358. The HES1 fragment was amplified from human lung cancer cDNA. The hASH1 fragment was amplified from a hASH1 expression vector. The E12 cDNA was amplified from human E12 plasmid (gift from G. Kato, Johns Hopkins University, Baltimore, Md.) and the cDNAs in both HES1 (AdHES1) and hASH1 (AdHAhASH1) adenoviruses were tagged with a hemagglutinin (HA) epitope fused to their amino termini. High-titer viral stocks were produced in 911 cells. The control virus expresses the Escherichia coli beta-galactosidase gene (AdβGal). The titers of each viral stock were determined by plaque assay in low-passage 293 cells. A series of preliminary infections were performed in each cell line to determine the optimal dose of viruses, allowing at least 70% GFP-expressing cells with minimal cytotoxicity, determined by growth rate compared to the mock-infected cells. The final doses were 2.0 to 5.0 PFU/cell. In each experiment, the level of GFP expression at 48 h postinfection was assessed to confirm the efficiency of infection.
RPA.
RNase protection assays (RPAs) were performed by using the MAXISCRIPT and RPAIII kits (Ambion) according to the manufacturer's protocol. Probes for hASH1 and glyceraldehyde-3-phosphate dehydrogenase (G3PDH) have been previously described (11). The HES1 probe was generated by PCR from normal human placental genomic DNA spanning exon 5 (17). Total RNAs were isolated by using the Trizol reagent (Life Technologies) and following the manufacturer's instruction. Ten micrograms of total RNA was used for each sample.
Indirect immunofluorescence.
DMS53 cells were seeded on glass coverslips. One day after infection, cells were washed once with phosphate-buffered saline (PBS), fixed in 3% paraformaldehyde for 20 min, and permeabilized with 0.5% Triton X-100 solution for 10 min. Coverslips were blocked with 3% horse serum for 30 min, incubated with anti-Notch1 antibody (Santa Cruz Biotechnology) at a 1:500 dilution in blocking buffer for 1 h, and washed with PBS. Secondary rhodamine-conjugated anti-mouse immunoglobulin G (Jackson Immunoresearch) 1:100 in blocking buffer was added and incubated for 1 h. Coverslips were washed with PBS, mounted onto glass microscope slides with Prolong antifade reagent (Molecular Probes), and examined under fluorescence microscopy.
Immunoblotting.
Adenovirus-infected cells were harvested at the times indicated in each experiment. Cells were washed with PBS and lysed in sodium dodecyl sulfate (SDS) sample buffer (62.5 mM Tris [pH 7.5], 2% SDS, 10% glycerol) with aprotinin, leupeptin, pepstatin, and 4-(2-aminoethyl)-benzenesulfonyl fluoride. Cell suspensions were briefly sonicated, and the protein concentration for each cell lysate was determined by using the DC Protein assay (Bio-Rad) according to the manufacturer's instructions. Fifty to 100 μg of total protein from the whole-cell lysate was loaded onto each lane during gel electrophoresis. Equivalent loading and transfer were verified by filter staining with Fast Green (Fisher Scientific). Western blot analysis was performed by using 0.1 M Tris [pH 7.5], 0.9% NaCl, 0.05% Tween 20 with 5% nonfat dry milk as a blocking and antibody dilution buffer. Working concentrations of antisera were as follows: Notch1, 1:50 (anti-bTAN20; gift from S. Artavanis-Tsakonas); Notch1, 1:1,000 (Santa Cruz Biotechnology); Notch1, 1:1,000 (anti-T6; gift from J. Aster, Brigham and Women's Hospital, Boston, Mass.); HES1, 1:10,000 (gift from T. Sudo, Toray Industries, Inc., Kanagawa, Japan); MASH1, 1:1,000 (BD Pharmingen); HA, 1:1,000 (Santa Cruz Biotechnology); ubiquitin, 1:3,000 (gift from C. Pickart, Johns Hopkins University); E12, 1:500 (Santa Cruz Biotechnology); and G3PDH, 1:5,000 (Trevigen). A Supersignal West Pico chemiluminescence kit (Pierce) was used for all antibodies except for antisera to MASH1, for which the Supersignal West Femto kit (Pierce) was used.
Immunoprecipitation and pulse-chase analysis.
DMS53 cells were seeded in 100-mm-diameter dishes or 75-cm2 flasks at 3 × 106 cells per dish or flask. AdHAhASH1 and/or AdE12 was added at 2 PFU/cell and followed by AdβGal or AdNotch1 at 5 PFU/cell 24 h later. For pulse-chase analysis, cells were harvested at 20 h after AdβGal or AdNotch1 infection with SDS lysis buffer (0.5% SDS, 50 mM Tris [pH 7.5], 1 mM EDTA, 1 mM dithiothreitol) supplemented with protease inhibitors as described in “Immunoblotting” above. Cell suspensions were heated in a boiling water bath for 10 min and centrifuged at 12,000 × g for 10 min at 4°C to collect the supernatant. For coimmunoprecipitation experiments, cells were harvested at 24 h after infection with Triton X-100 lysis buffer (0.1% Triton X-100, 50 mM Tris [pH 7.5], 15 mM EDTA, 100 mM NaCl, 1 mM dithiothretol) supplemented with protease inhibitors. Cell suspensions were briefly sonicated and centrifuged to collect the supernatant. Five hundred micrograms of total protein was used for immunoprecipitation for each sample. Cell lysates were precleared with protein G-agarose and normal mouse immunoglobulin G (Santa Cruz Biotechnology) and then incubated overnight at 4°C with anti-HA (Santa Cruz Biotechnology). After binding of antibodies to protein G beads for 2 h, the beads were washed 3 times with IP buffer (0.1% Triton X-100, 2 mM EDTA, 50 mM Tris) and resuspended in SDS sample buffer. Samples were electrophoresed through SDS-11 or 12% polyacrylamide gels, transferred to polyvinylidene difluoride membranes, and probed by anti-ubiquitin, anti-Notch1 (Santa Cruz Biotechnology), or anti-E12 as described above. For the pulse-chase study, 20 h after Notch1 or control virus infection, cells were washed twice with PBS and incubated with methionine-free RPMI 1640 medium with 10% FBS for 30 min. Translabel [35S]methionine (ICN) was then added at 100 μCi/ml, and the cells were incubated at 37°C for 1 h. Cells were then washed, the medium was changed to Waymouth's medium with 16% FBS, and the cells were harvested at 15, 30, 45, and 60 min after the chase. Cell lysates were subjected to the same immunoprecipitation procedure as described above. After electrophoresis, gels were treated with the fluorographic agent NAMP (Amersham) according to the manufacturer's instructions and dried before exposure to film.
RESULTS
Expression profile of Notch1, HES1, and hASH1 in SCLC cells.
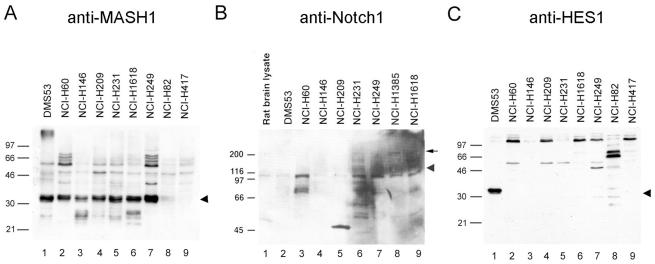
To investigate the mechanism of regulation of hASH1 expression by the Notch signaling pathway, we first determined expression patterns for hASH1, Notch1, and HES1 by immunoblotting in SCLC cell lines. It was previously shown that SCLC cells exhibit an NE phenotype in tight association with hASH1 mRNA expression (6). We confirmed a high level of hASH1 protein expression in all classic SCLC cell lines in a fashion closely paralleling published data on hASH1 mRNA (Fig. 1A, lanes 1 to 7) (3). We observed a tightly grouped protein triplet of 31 to 33 kDa in most lines. Fainter immunoreactive products of approximately 26 kDa were also observed. As described in the studies that follow, these may represent hASH1 proteolytic cleavage fragments. NCI-H82 and NCI-H417 are variant SCLC cell lines, which have detectable hASH1 mRNA only at the level of reverse transcription-PCR; both showed minimally detectable to undetectable hASH1 protein (Fig. 1A, lanes 8 to 9). Typical non-SCLC lines show no hASH1 reactivity by immunoblotting (data not shown). Notch1 expression was variable in the tested SCLC cells (Fig. 1B). Low-level expression of the 116-kDa Notch1 cleaved transmembrane fragment was ubiquitously present, whereas full-length 300-kDa proteins were detectable in a few of the tested lines (Fig. 1B, lanes 6, 8, and 9). In contrast, the Notch signaling target HES1 was undetectable at the protein level in all SCLC cell lines except DMS53 (Fig. 1C).
FIG. 1.
hASH1-expressing lung cancer cells express minimal HES1 in spite of detectable Notch1. Lysates from cultured SCLC cell lines were subjected to immunoblotting to determine the expression profiles of hASH1, Notch1, and HES1. (A) hASH1 was strongly expressed in all classic SCLC lines (lanes 1 to 7), whereas it was minimally expressed or undetectable in variant SCLC lines (lanes 8 and 9). The 31- to 33-kDa triplet is identified by the arrowhead. (B) A 116-kDa processed species of Notch1 (arrowhead) was variably abundant in SCLC cells. In NCI-H1385 and NCI-H1618 (lanes 8 and 9), the 300-kDa full-length form of Notch1 (arrow) was detectable. Rat brain lysate was used as a positive control. NCI-H1385 (lane 8) is a non-SCLC line with NE features. All other lanes are classified as classic SCLC. (C) An approximately 31-kDa HES1-reactive species was observed only in DMS53 cells (arrowhead). NCI-H82 and NCI-H417 (lanes 8 and 9) are variant SCLC lines. The others are classic SCLC lines.
Activated Notch1 causes a profound reduction of hASH1 expression.
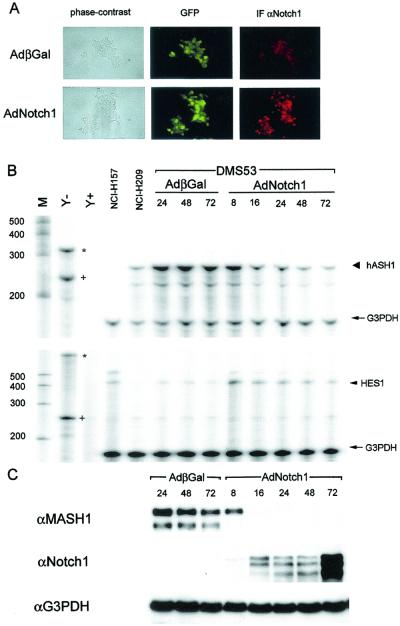
As described above, Notch1 negatively regulates MASH1 in nervous system development associated with up-regulation of the HES family of transcriptional repressors. We have suggested that the Notch signaling pathway may regulate hASH1 in human cancer cells in a similar manner. Supporting this hypothesis, earlier studies had shown that an inducible HES1 construct can down-regulate the activity of the hASH1 promoter (11, 12). The abundance of hASH1 and the low level of HES1 expression that we observe in most cultured SCLC cell lines are also consistent with the pathway by which Notch regulates MASH1 in neurogenesis. This model would suggest that Notch signaling activity is low in these SCLC cell lines and would predict that increased Notch signaling would result in increased expression of HES1 and down-regulation of hASH1. To examine this possibility, we studied the effect of overexpressing a constitutively active form of Notch1 in DMS53 SCLC cells by using recombinant adenoviruses to acutely and efficiently induce Notch activity. Expression of the constitutively active form of Notch1 was mainly localized in the nuclei of DMS53 cells, as shown by indirect immunofluorescence with antisera to Notch1 (Fig. 2A). We first explored the transcriptional regulation of HES1 and hASH1 by Notch1. Adenoviral transfer of active Notch1 induced an increase in HES1 mRNA as early as 8 h postinfection. HES1 mRNA was maximally increased at 8 h and then decreased to a stable, but higher than the basal, level through 72 h of observation (Fig. 2B, bottom panel), although Notch1 expression progressively increased (Fig. 2C, middle panel). Similar expression data were observed for the HES1 protein (data not shown). These findings are consistent with an autoinhibitory transcriptional feedback mechanism of HES1 as previously described (36). Active Notch1 caused a progressive reduction of hASH1 mRNA throughout the 72 h duration of the experiment, reciprocal to the increase of Notch1 (Fig. 2B, top panel). Significantly, hASH1 protein abruptly disappeared between 8 and 16 h postinfection and remained undetectable through 72 h (Fig. 2C, top panel). This rapid disappearance of the hASH1 protein could not be fully explained by the more gradual decrement of hASH1 mRNA. We then compared the effects of HES1 and Notch1 on hASH1 mRNA and protein disappearance. Overexpression of HES1 caused a progressive decrease of hASH1 mRNA, though more gradual and incomplete than the decline induced by Notch1 (Fig. 3A). In contrast to the abrupt loss of hASH1 protein caused by Notch1, HES1 induced a gradual and incomplete loss of hASH1 protein comparable to the rate of mRNA decline (Fig. 3A, lanes 8 to 10, and B, lanes 7 to 10). These data suggest that Notch signaling inhibits hASH1 gene transcription in SCLC cells via a mechanism(s) in addition to transactivation of the HES1 gene. The rapid decrease of hASH1 protein induced by Notch1 also suggested the distinct possibility of posttranscriptional control of hASH1 expression by Notch1 in this system.
FIG. 2.
Activated Notch1 down-regulates hASH1 mRNA and protein and up-regulates HES1. DMS53 cells were infected with control virus or the Notch1 virus and analyzed for protein expression by immunofluorescence and immunoblotting. (A) Comparison of GFP fluorescence (middle panel) and phase contrast (left panel) indicated highly efficient adenovirus infection in DMS53 cells. Indirect immunofluorescence (IF) (right panel) with anti-Notch1 (αNotch1) (red staining) confirmed predominantly nuclear localization of the activated Notch form. Cells were fixed and stained 24 h postinfection. (B) RPA of hASH1, HES1, and G3PDH. The predicted 280-bp protected hASH1 fragment (large arrowhead) was down-regulated between 8 and 16 h and then progressively declined over 72 h after AdNotch1 infection (top panel). The 375-bp protected HES1 fragment (small arrowhead) rose abruptly at 8 h with gradual tapering over 72 h. G3PDH (arrow) was used as a control for sample loading (lower panel). Undigested hASH1 (asterisk in the top panel), HES1 (asterisk in the lower panel), and G3PDH (plus sign in both panels) are indicated with the RNA size standards (lane M) and yeast tRNAs (lanes Y− [without RNase] and Y+ [with RNase]). NCI-H157, a non-SCLC line, was used as a positive control for HES1 and as a negative control for hASH1. NCI-H209 RNAs were used as a positive control for hASH1 and as a negative control for HES1. (C) Immunoblotting with anti-MASH1 (αMASH1) showed abrupt down-regulation of hASH1 between 8 and 16 h after infection with AdNotch1 (top panel), corresponding to the expression of cytoplasmic forms of Notch1 (middle panel). From the same cell lysate, G3PDH expression was used as a control for sample loading (lower panel). αG3PDH, anti-G3PDH; βGal, beta-galactosidase.
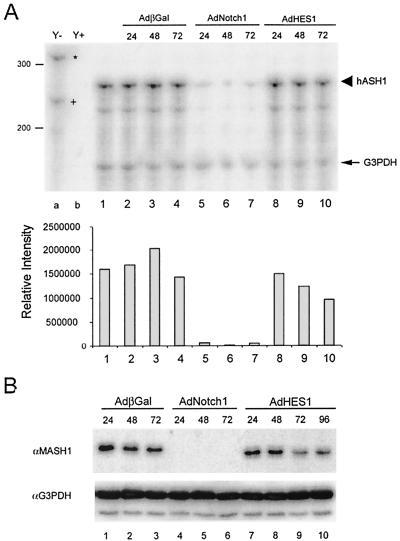
FIG. 3.
HES1 causes gradual and incomplete hASH1 down-regulation compared to Notch1. DMS53 cells were treated with identical doses of the indicated adenoviruses and harvested for total RNAs and cell lysates. (A) RPA of hASH1 and G3PDH. The upper panel illustrates the down-regulation of hASH1 transcripts by adenoviral Notch1 (lanes 5 to 7) or HES1 (lane 8 to 10). In contrast to Notch1, HES1 caused gradual and incomplete decline of hASH1 transcripts. The lower panel shows hASH1 mRNA quantitation. Protected hASH1 (large arrowhead) and G3PDH fragments (arrow), the undigested probe of hASH1 (∗), and G3PDH (+) are indicated. (B) Immunoblotting with anti-MASH1 (αMASH1) (upper panel) showing HES1 overexpression was sufficient to cause gradual reduction of hASH1 protein (lanes 7 to 10) corresponding to the decline of hASH1 transcripts in panel A, whereas Notch1 caused a profound reduction of hASH1 (lanes 4 to 6). G3PDH was used as a loading control (lower panel). αG3PDH, anti-G3PDH; βGal, beta-galactosidase.
Posttranscriptional regulation of hASH1 expression by active Notch1 but not HES1.
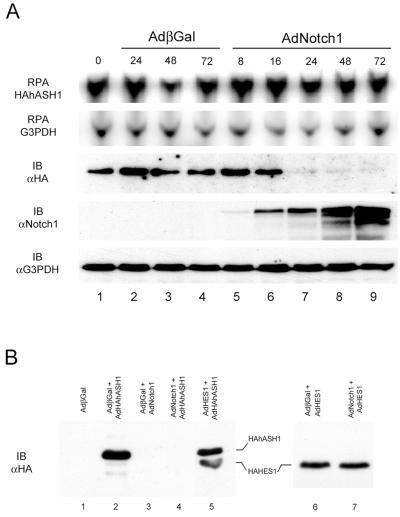
To further characterize the presence of a posttranscriptional regulation of hASH1 by Notch, we used an exogenous hASH1 adenoviral construct under the control of a CMV promoter rather than its native HES1-sensitive promoter. Using adenoviral hASH1, we were able to achieve robust expression of the exogenous hASH1 mRNA and protein in SCLC cells (Fig. 4A, lanes 1 to 4). As expected, the exogenous hASH1 mRNA remained clamped at a stable level, even in the presence of activated Notch1. Remarkably, active Notch1 caused a dramatic reduction of the exogenous epitope-tagged hASH1 protein between 16 and 24 h after Notch treatment, despite the stable levels of exogenous hASH1 mRNA (Fig. 4A, compare lanes 6 to 9 in the top and middle panels). Overexpression of HES1 alone had no obvious effect on exogenous hASH1 protein expression (Fig. 4B, compare lanes 2 and 5). The absence of a HES1 effect on exogenous hASH1 protein abundance is consistent with HES1 working via a transcriptional mechanism. The effect of Notch1 in the reduction of the exogenous hASH1 protein was specific, since we did not observe Notch-mediated down-regulation of exogenous HES1, a class I bHLH protein (Fig. 4B, lanes 6 to 7). Similar down-regulation of exogenous hASH1 protein by Notch1 could be observed in a second SCLC cell line, NCI-H209, and in the gastrointestinal carcinoid cell line, BON (V. Sriuranpong and E. K. Nakakura, unpublished data). Together, these data indicate hASH1 posttranscriptional control by activated Notch1 in a process which HES1 alone is insufficient to mediate. The ability of Notch to greatly reduce the level of the abundant adenovirus-encoded exogenous hASH1 in this experiment underscores the efficiency and high capacity of the Notch1-mediated down-regulation of hASH1.
FIG. 4.
Notch1, but not HES1, causes loss of exogenous hASH1 protein. DMS53 cells were infected with AdHAhASH1 and then, 24 h later, treated with either no virus, AdβGal (adenovirus beta-galactosidase) control, or AdNotch1 and harvested for total RNA and cell lysates. (A) The upper two panels show protected RNA species of the amino-terminal HA-tagged hASH1 and G3PDH. The lower three panels show immunoblotting (IB) with antisera to HA (αHA), Notch1 (αNotch1), and G3PDH (αG3PDH) in cell lysates corresponding to the samples analyzed by RPA. Despite ongoing high levels of exogenous hASH1 mRNA (lanes 5 to 9), Notch was sufficient to rapidly deplete exogenous hASH1 protein over 16 to 24 h (lanes 6 to 9). (B) Immunoblotting with anti-HA to detect either exogenous hASH1 or HES1 showed that exogenous hASH1 was sensitive to active Notch1 (lane 4) but not to HES1 (lane 5). Exogenous HES1 was slightly smaller than exogenous hASH1 (lane 5) and was not down-regulated when exposed to active Notch1 (lanes 6 to 7). Each lane was subjected to an equivalent final dose of adenovirus at a total of 10 PFU/cell. Lysates were prepared 48 h postinfection.
Activated Notch1 enhanced hASH1 proteolysis through a proteasome-dependent mechanism.
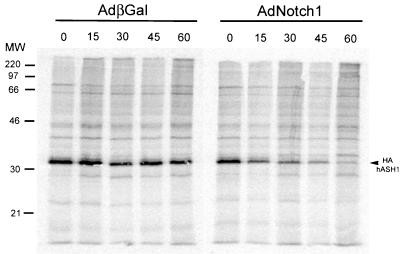
The dramatic reduction of exogenous hASH1 could be due to either translational control or enhanced protein turnover. To address this question, we determined the half-life of exogenous hASH1 protein under the influence of active Notch1 in a pulse-chase study in SCLC cells. Adenoviral Notch1 resulted in a markedly reduced half-life of exogenous hASH1, 14 min, compared to 50 min in the DMS53 cells infected with the control virus (Fig. 5). The initial incorporation of the radioactive methionine pulse into immunoprecipitable hASH1 protein was similar in both AdNotch1-infected and control cells (Fig. 5). A similar Notch-induced decline in the apparent half-life of the exogenous hASH1 protein was observed in immunoblotting when protein synthesis was blocked with cycloheximide (data not shown). These results suggest that active Notch1 induces a rapid proteolysis of hASH1 in SCLC cells.
FIG. 5.
Increased exogenous hASH1 protein turnover induced by active Notch1. For pulse-chase studies, DMS53 cells were infected with HAhASH1 adenovirus followed by Notch1 or control adenovirus 24 h later. (A) After 20 h of exposure to AdβGal (adenovirus beta-galactosidase) or AdNotch1, cells were labeled with [35S]methionine and harvested at the indicated time points (in minutes) after the onset of the cold chase. Immediately after the 1-h labeling interval, immunoprecipitable exogenous hASH1 proteins were equally detectable in both AdβGal- and AdNotch1-treated cells. During the cold chase, labeled hASH1 levels in Notch1-treated cells declined rapidly compared to levels seen in control virus-treated cells. Using PhosphorImager quantitation and graphical plotting, the calculated half-life for HAhASH1 was 14 min in the presence of Notch1 and 50 min in control-infected cells. MW, molecular mass in kilodaltons.
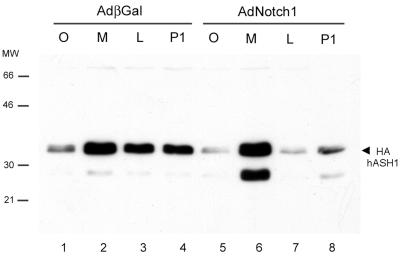
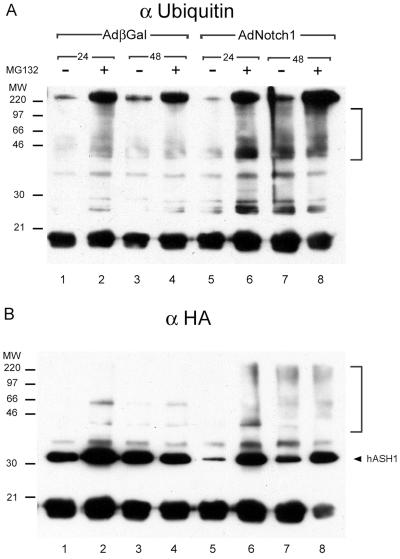
Cellular degradation of many proteins occurs in the proteasome. The rapid hASH1 proteolysis induced by Notch1 prompted us to investigate whether this process is proteasome-dependent. We first used proteasome inhibitors to address this possibility. Proteasome inhibitors MG132, lactacystin, and Proteasome Inhibitor I increased exogenous hASH1 protein abundance in control virus-infected cells, indicating that basal hASH1 turnover in SCLC cells is partly dependent on proteasome function (Fig. 6, lanes 1 to 4). MG132 effectively restored hASH1 from Notch1-induced degradation, and Proteasome Inhibitor I was modestly effective (Fig. 6, lanes 5 to 8). Lactacystin was ineffective in our experimental conditions. In addition, proteasome inhibitor treatment, especially with MG132, resulted in an increased abundance of an approximately 27-kDa HA-tagged species. The combination of Notch expression and MG132 treatment superinduced this apparent proteolytic fragment. It is conceivable that the proteasome inhibitors may indirectly stabilize hASH1 by affecting protein(s) that regulate hASH1 abundance. To further investigate whether direct proteasome degradation of hASH1 was influenced by Notch signaling, we determined the relative abundance of polyubiquitinylated hASH1 as a function of Notch1. In this coexpression experiment, we immunoprecipitated exogenous epitope-tagged hASH1 and immunoblotted with antisera to either ubiquitin or HA. The HA immunoblotting confirmed the substantial reduction of hASH1 abundance by Notch1 and restoration of hASH1 abundance upon MG132 treatment (Fig 7B). Immunoblotting with anti-ubiquitin (Fig. 7A) showed an enhancement of higher molecular mass forms in response to MG132, corresponding to polyubiquitinylated HAhASH1. Notch1-treated cells had a significantly greater abundance of polyubiquitinylated HAhASH1 than did control virus-treated cells. Enhanced HAhASH1 ubiquitinylation could be observed 48 h after Notch1 exposure, even in the absence of MG132 (Fig. 7A, compare lanes 7 and 3). Similar patterns of higher-molecular- mass HAhASH1 were also observed in the anti-HA immunoblot (Fig. 7B). Taken together, these data indicate that active Notch1 induces hASH1 proteolysis in a proteasome pathway-dependent manner.
FIG. 6.
Notch1 enhances hASH1 proteasomal degradation. DMS53 cells were infected with AdHAhASH1 followed 24 h later by AdNotch1 or the control virus AdβGal (adenovirus beta-galactosidase). Cells were incubated for 2 h with proteasome inhibitors MG132 (M), lactacystin (L), Proteasome Inhibitor I (P1), or carrier (O) at 48 h after infection by AdNotch1 or AdβGal. The arrowhead indicates the predicted 32- to 34-kDa HAhASH1 product (see the text for discussion of the 27-kDa forms). Without proteasome inhibitor, active Notch1 induced exogenous hASH1 protein degradation (lane 5). MG132 caused a marked accumulation of hASH1 protein abundance even in the presence of Notch1 (lane 6), whereas Proteasome Inhibitor I partially restored hASH1 from Notch1-induced proteolysis (lane 8). Lactacystin had no rescuing effect (lane 7). In AdβGal-treated cells, all proteasome inhibitors increased exogenous hASH1 protein abundance above the basal level (lanes 1 to 4). MW, molecular mass in kilodaltons.
FIG. 7.
Active Notch1 increases polyubiquitinylated forms of exogenous hASH1. In a coexpression experiment similar to that described in the legend to Fig. 6, cell lysates were harvested at the indicated time points (24 or 48 h) with or without MG132 treatment and subjected to immunoprecipitation with anti-HA (αHA) and immunoblotting with anti-ubiquitin (αUbiquitin) or anti-HA. (A) Anti-ubiquitin immunoblotting showed an increased abundance of high-molecular-mass species in response to both Notch1 and MG132 (lanes 2, 4, 6, 7, and 8) consistent with polyubiquitinylated exogenous hASH1. Notch1 and MG132 treatment caused more abundant high-molecular-mass immunoreactivity than the AdβGal (adenovirus beta-galactosidase)- and MG132-treated cells (compare lanes 6 and 8 to lanes 2 and 4). (B) The same filter was stripped and reprobed with anti-HA, revealing high-molecular-mass reactivity predominantly in the Notch1 lanes (bracket) (lanes 6 to 8). The arrowhead indicates the expected size of exogenous hASH1. MW, molecular mass in kilodaltons.
The Notch RAM domain is dispensable but both the OPA and PEST domains are essential for hASH1 degradation.
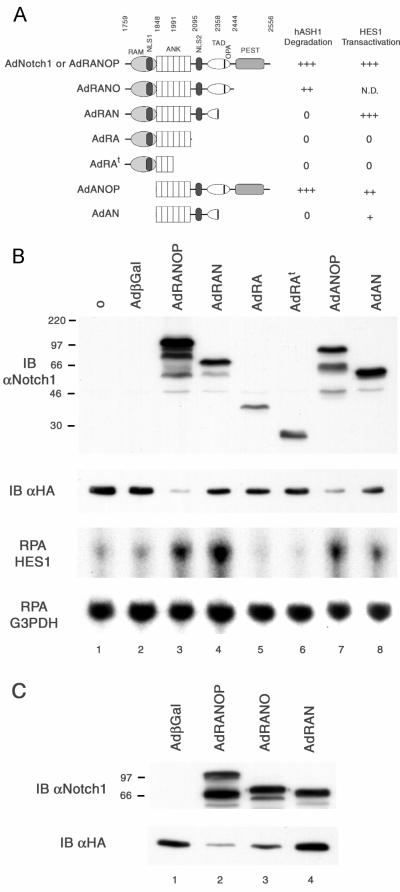
The cytoplasmic portion of Notch1 contains multiple functional domains (3, 32), some of which are illustrated schematically in Fig. 8A. To gain further insight in the mechanism by which Notch1 induces rapid hASH1 proteolysis, we attempted to define the minimal domains of Notch1 that were sufficient for this function. A series of Notch1 deletion fragments were constructed in recombinant adenovirus expression vectors. With antisera recognizing the Notch1 ankyrin-linked repeat region (2), we observed effective expression of Notch1 mutant proteins (Fig. 8B and C, top panels). We observed that a Notch1 mutant lacking the RAM domain was as effective as full-length cytoplasmic Notch1 in decreasing exogenous hASH1 protein abundance (Fig. 8B, compare lanes 3 and 7). Carboxyl terminus deletion of both the OPA and PEST domains appeared to markedly attenuate this Notch1 action regardless of the presence of the RAM domain (Fig. 8B, lanes 3, 4, 7, and 8). A previous study has shown that the OPA domain is a component of a Notch1 carboxyl terminus transactivating domain (3). We then questioned which part of the carboxyl terminus of Notch1 is essential in hASH1 degradation. A cytoplasmic Notch1 fragment containing the OPA domain but lacking the PEST domain was still capable of decreasing exogenous hASH1 protein (Fig. 8C, lane 3). However, we consistently observed a slight reduction of hASH1 degradative potential with the PEST domain deletion compared to full-length cytoplasmic Notch1 (Fig. 8C, compare lanes 2 and 3 in the lower panel). These results indicate that the minimal effective region of cytoplasmic Notch1 in inducing hASH1 proteolysis may localize to the ankyrin repeats and the OPA domain.
FIG. 8.
Analysis of Notch1 mutant proteins in hASH1 degradation. In a coexpression experiment similar to the one described in the legend to Fig. 4, DMS53 cells expressing exogenous hASH1 were exposed to mutant forms of cytoplasmic Notch1. Cell lysates were harvested 48 h after exposure to AdβGal (adenovirus beta-galactosidase) or AdNotch1 mutants. (A) Schematic representation of Notch1 deletion proteins used in these experiments is correlated with hASH1 degradative potential and HES1-transactivating potential assessed by immunoblotting and RPA, respectively. Notch1 amino acid numbering is from GenBank accession number AAG33848. N.D., not done; ANK, ankyrin-linked repeat region. (B) The upper two panels show immunoblotting (IB) with antisera to the Notch1 (αNotch1) ankyrin repeats and to HA (αHA). The lower two panels show protected RNA species of HES1 and G3PDH corresponding to the samples analyzed by immunoblotting. Full-length cytoplasmic Notch1 and a construct lackingthe RAM domain exhibited comparable effectiveness in decreasing the hASH1 protein. Deletion of the RAM domain attenuates HES1 mRNA abundance. (C) A second set of Notch mutants was created with an epitope-tagged carboxyl terminus to allow detection by immunofluorescence. All Notch1 mutants in this panel were expressed predominantly in the nuclei of DMS53 cells (data not shown). Carboxyl-terminal deletion of the PEST sequence led to a modest reduction in hASH1 degradative potential, but an additional OPA deletion completely abrogated the effect. βGal, beta-galactosidase.
We further tested the ability of these Notch1 mutants to increase HES1 expression. Lack of the RAM domain resulted in a slight reduction of HES1 transactivation potential (Fig. 8B, compare lanes 3 and 7 and lanes 4 and 8) consistent with the known role of RAM as a CBF1-interacting domain. Deletion of the OPA and PEST domains seemed to be less critical to HES1 induction (Fig. 8B, compare lanes 3 and 4 and lanes 7 and 8). The discrepancies between the hASH1 degradative potential and the ability to transactivate HES1 corroborated the earlier findings that HES1 alone was not sufficient to induce hASH1 proteolysis. These data suggest that the degradation of hASH1 by Notch1 may utilize the C-terminal transactivating domain and requires a distinct set of interacting proteins compared to the HES1 induction function of Notch1.
Coexpression of E12, a hASH1-dimerizing protein, protects hASH1 from degradation by active Notch1.
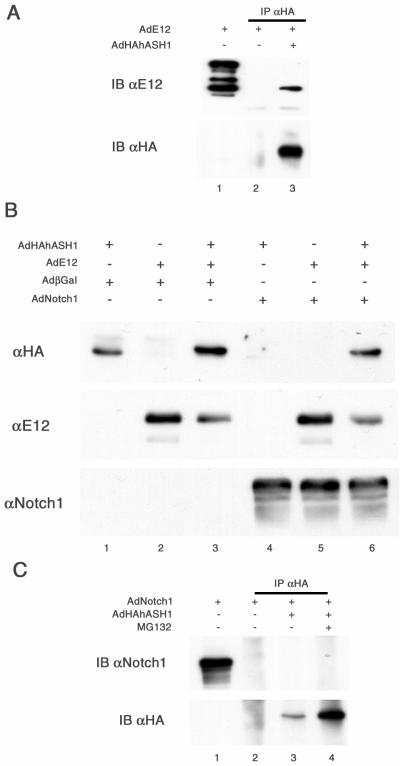
In order to bind and transactivate the E box element, MASH1 requires a heterodimerizing interaction to a class I bHLH protein, including E12, E47, E2-2, or HEB (33, 46, 51). MASH1 alone cannot form a homodimerized complex or efficiently transactivate target genes via E box promoters (33). A similar function also has been described for the human ortholog, hASH1 (51). We then examined the possibility that the hASH1 binding partner may also play a role in the regulation of hASH1 protein degradation. In coimmunoprecipitation experiments, we demonstrated, as expected, a direct interaction between exogenous hASH1 and a coinfected exogenous E12 protein (Fig. 9A). Under the sensitivity of our experimental conditions, the expression of endogenous E12 protein was undetectable in these SCLC cells. Interestingly, coexpression of adenoviral E12 with hASH1 resulted in an increase in the steady-state abundance of hASH1 compared to cells infected with adenoviral hASH1 alone (Fig. 9B, top panel, compare lanes 1 and 3). In the presence of active Notch1, adenoviral E12 proteins significantly restored the level of hASH1 protein from degradation (Fig. 9B, top panel, compare lanes 4 and 6). Since the expression level of adenoviral Notch1 remained stable regardless of the expression level of E12, these findings did not appear to result from a squelching artifact (Fig. 9B, lower panel). Additionally, the E12 protein abundance remained stable despite the expression of Notch1. It appears likely that the positive effect of E12 on hASH1 abundance both in the presence and in the absence of activated Notch1 is based on dimerization between E12 and hASH1 (Fig. 9B, middle panel). One potential interpretation is that hASH1 monomers are intrinsically prone to proteasomal degradation and that Notch1 signaling is capable of disrupting protective complexes with E proteins (see Discussion).
FIG. 9.
Coexpression of E12 can rescue hASH1 from degradation by Notch1. (A) hASH1 associates with E12. DMS53 cells coexpressing hASH1 and E12 were harvested 48 h postinfection and subjected to coimmunoprecipitation (IP) with anti-HA (αHA). The filter was probed with anti-E12 (αE12) (upper panel) and anti-HA (bottom panel). One-tenth of the input was used as a positive control for anti-E12 without coimmunoprecipitation (lane 1). E12 proteins were immunoprecipitable together with the exogenous hASH1. (B) DMS53 cells were infected with AdHAhASH1 followed 24 h later by AdβGal (adenovirus beta-galactosidase), AdNotch1, or AdE12 as indicated. Control viruses were added to equalize the final dose of virus in each sample. Cell lysates were harvested at 48 h post-second virus addition. In the top panel, immunoblotting (IB) with anti-HA shows that the addition of E12 protects exogenous hASH1 from Notch1-induced degradation (compare lanes 4 and 6). Notch1 and E12 expression levels remained relatively consistent (middle and lower strip). (C) Notch1 does not directly associate with hASH1. MG132 was used to enrich hASH1 protein and increase the sensitivity of detection. Despite immunoprecipitation of abundant HAhASH1 (lower panel, lane 4), Notch1 could not be detected in the immunoprecipitated material (upper panel, lane 4). αNotch1, anti-Notch1.
Finally, we performed coimmunoprecipitation experiments to address the question of whether Notch1-induced hASH1 degradation could require a direct association between Notch1 and hASH1 proteins. With MG132 treatment, the exogenous hASH1 proteins were effectively immunoprecipitable in the presence of activated Notch1 (Fig. 9C, lower panel). However, we could not observe active Notch1 protein coimmunoprecipitation with hASH1 (Fig. 9C, upper panel). Thus, it is less likely that the hASH1 degradative effect of Notch1 requires a direct complex between the two proteins.
DISCUSSION
In the present study, we explored the regulation of hASH1 gene expression by the Notch pathway in cultured SCLC cells. In these neuroendocrine lung cancer cells, which generally exhibit a relatively low basal level of Notch activity in culture, introduction of an active form of Notch1 rapidly down-regulated hASH1 by two distinct pathways. First, through a known transcriptional regulatory cascade, Notch1 transactivated the downstream effector gene, HES1, associated with hASH1 transcriptional repression. The hASH1 transcriptional repression by activated Notch1 was more profound than that induced by HES1 itself, suggesting the involvement of additional HES1-independent Notch signaling targets in this process. Second, through a novel pathway, Notch1 signaling enhanced the basal level of hASH1 ubiquitinylation, targeting hASH1 to a degradative process in a proteasome-dependent pathway. Overexpression of HES1 alone was insufficient to accelerate hASH1 degradation but still capable of reducing hASH1 mRNA abundance. These data suggest a tight, rapid control of hASH1 gene expression by the Notch signaling pathway, utilizing both HES1-dependent and -independent mechanisms.
In Drosophila neurogenesis, down-regulation of bHLH proneural transcription factors of the achaete-scute complex is critical to the selection of a neuronal fate among equipotent neuroectodermal precursor cells (9, 13, 59). Genetic and transactivation assay studies in this model system have focused on Notch activity in the transcriptional control of achaete-scute complex gene expression. Vertebrate studies of Notch regulation of achaete-scute homolog 1, conducted largely in transgenic knockout mice (14, 26), do not easily distinguish between potential transcriptional and posttranscriptional effects of Notch signaling. Our findings provide direct biochemical evidence for an additional regulatory mechanism for Notch down-regulation of hASH1. In these SCLC cells, transcriptional repression, which appears to be partly HES1 dependent, provides a slow, progressive decline in hASH1 mRNA. In striking contrast, the posttranslational control allowed a faster and more abrupt reduction of hASH1 protein, which may serve as an immediate response to Notch activation. This dual regulation by Notch signaling possibly may allow an effective short-term control by enhanced hASH1 protein turnover and longer-term regulation by transcriptional repression. Down-regulation of MASH1 protein, potentially via degradation, has been associated with inhibition of neurogenesis in olfactory epithelial cultures treated with bone morphogenetic proteins (58). We suggest that these two synergistic mechanisms of hASH1 regulation by Notch may help to render a precise regulation of cell fate commitment in the rapidly changing environment of nervous system development. Additionally, the Notch1-induced degradation appeared to have some specificity on class II bHLH proteins. We showed that class II bHLH protein hASH1 was susceptible to Notch1-induced degradation, whereas E12 and HES1, belonging to classes I and VI (41), were unaffected. When substituting another class II bHLH protein, MyoD, in place of hASH1 under the same experimental conditions, activated Notch1 also could down-regulate MyoD protein abundance (data not shown). These results suggest that this Notch1 function may be generally important and applicable to other cellular contexts as well.
Several lines of investigation have also suggested that transcriptional effects, via HES1, may not account for many critical Notch functions (34, 48, 50, 55). For example, activation of Notch signaling causes down-regulation of MyoD mRNA and inhibition of myotube formation in a C2C12 myoblast differentiation assay. Overexpression of the cytoplasmic form of Notch1 silences CD4 gene expression, mediated through multiple transcriptional control elements in developing T cells. In both systems, overexpression of HES1 protein cannot replicate all Notch effects. In our experimental system, HES1 alone could down-regulate endogenous hASH1 mRNA but had no effect on exogenous hASH1 protein abundance. Interestingly, activation of Notch also caused a greater transcriptional repression of hASH1 than overexpressed HES1 in SCLC cells. The limited capacity of HES1 to act as a transcriptional repressor may stem from a relative lack of critical corepressors or dimerizing partners such as the Groucho-like TLE proteins and HES-related HERP proteins (18, 28, 61) or the presence of the newly described HES1 inhibitor, HES6 (35). Consistent with the above findings, our Notch1 mutant studies also indicate that the RAM domain, a CBF1-interacting domain, is not essential for the hASH1 degradative function. Moreover, this Notch1 action may utilize a carboxyl-terminal transcriptional activation region overlapping with the OPA domain, which has been shown to possess a separable transactivating action other than HES gene transactivation (3). These findings support the existence of Notch effector(s) other than HES1 participating in control of hASH1 transcription and degradation. The family of potential Notch-interacting proteins, in addition to CBF1, is large, including Numb, Deltex, CIR, and SKIP, as well as components of the Wnt pathways (4, 20, 25, 42, 66). Furthermore, Notch can transactivate other mammalian HES family members such as HES5 or HES-like genes such as HERP1 and HERP2 (10, 27, 47, 49). To further understand the scope and importance of Notch-induced destabilization of bHLH proteins, it will be critical to characterize which Notch signaling targets are involved.
Turnover of many short-lived proteins, including transcription factors and signal transduction molecules, is regulated by proteasome degradation which frequently utilizes ubiquitinylation as a targeting mechanism (23). Although proteasome inhibitors may have an indirect effect on protein abundance and all ubiquitinylated proteins may not be targeted for degradation (56), our results strongly indicate that Notch signaling enhances hASH1 degradation through the 26S proteasome pathway. We showed a shortened hASH1 half-life in conjunction with an increase in polyubiquitinylated forms of hASH1, suggesting an active proteolytic process. A combination of sensitivity to two different proteasome inhibitors, MG132 and Proteasome Inhibitor I, supports the view that hASH1 proteolysis occurs mainly in the 26S proteasome. We cannot clearly explain why lactacystin was relatively ineffective in our experimental conditions. Poor cell penetration (15) is less likely in these experiments since lactacystin seemed to have activity in the control beta-galactosidase-expressing cells. We further explored the possibility that Notch may target hASH1 via the N-end rule (64), whereby destabilization of the amino terminus targets the protein for degradation. Substituting a carboxyl-terminal HA-tagged hASH1 resulted in similar degradation efficiency compared to the amino-tagged protein (data not shown), suggesting that the identity of the amino-terminal amino acids is irrelevant to the proteolytic process.
Posttranslational modification and proteolysis have been shown to play crucial roles in the regulation of the abundance of the class II bHLH protein MyoD (7, 19, 60). A heterodimerizing complex of MyoD and E protein exhibits a greater binding affinity to the consensus binding motif E box than the homodimerizing complex of either protein (33, 45). Binding to the target DNA, MyoD is then stabilized and has a longer protein half-life (1). Similar to MyoD, hASH1 also shares the ability to form a complex with class I bHLH proteins to transactivate the downstream targets (41). Our results show that E12 can function to stabilize hASH1 protein from Notch1-induced degradation in addition to its role in activating transcription. Consistent with a protective function of E proteins, we observed a modest increase of steady-state exogenous hASH1 expression when coexpressing E12 (Fig. 9, compare lanes 1 and 3). Several possible mechanisms could account for E12 stabilization of hASH1 in the presence of Notch1. Dimerization of hASH1 to E12 and subsequent DNA binding could change the conformation of the hASH1 protein and obscure a targeting epitope of hASH1 for the proteolytic process. Alternatively, E12 binding and/or binding to DNA might trigger further posttranslational modification of hASH1 that protects it from a default fate of degradation. In light of this model, it is interesting that coexpression of E12 with hASH1 resulted in increased recovery of 32- to 33-kDa forms, rather than the 31-kDa form (Fig. 9B, compare lanes 1 and 3). Taken together, our data suggest that transcriptional targets of Notch1 (distinct from HES1) enhance hASH1 degradation by interfering with the formation of protective hASH1-E protein complexes. Our adenovirus gene delivery system clearly resulted in supraphysiologic levels of Notch signaling. An unresolved question is whether typical Notch activation in the context of a developing tissue also is associated with enhanced bHLH protein degradation. In future studies, it will be important to determine whether this novel regulatory pathway is applicable to other cell contexts and other bHLH proteins known to be regulated by Notch, to attempt to reproduce these effects with Notch ligands, and to understand the detailed mechanisms underlying Notch-regulated bHLH protein destabilization.
Acknowledgments
We are greatly indebted to S. Baylin (Johns Hopkins Oncology Center) for discussion and support, S. Artavanis-Tsakonas (Massachusetts General Hospital Cancer Center, Harvard Medical School) for helpful discussion and gifts of the Notch1 vectors and the Notch1 antisera, C. Pickart (Johns Hopkins University School of Hygiene and Public Health) for discussion and the ubiquitin antisera, J. Aster (Brigham and Women's Hospital) for the T6 antisera, G. Kato (Johns Hopkins University School of Medicine) for the E12 plasmid, and T. He (B. Vogelstein lab, Johns Hopkins Oncology Center) for gifts of the adenovirus vector and cell lines.
This work was supported by NIH-National Cancer Institute grant RO1CA70244 to D.W.B. and NIH-National Cancer Institute grant RO1CA47480 to B.D.N. V.S. is a recipient of the Royal Thai Government Scholarship, Chulalongkorn University, Bangkok, Thailand.
REFERENCES
- 1.Abu Hatoum, O., S. Gross-Mesilaty, K. Breitschopf, A. Hoffman, H. Gonen, A. Ciechanover, and E. Bengal. 1998. Degradation of myogenic transcription factor MyoD by the ubiquitin pathway in vivo and in vitro: regulation by specific DNA binding. Mol. Cell. Biol. 18**:**5670-5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aster, J. C., E. S. Robertson, R. P. Hasserjian, J. R. Turner, E. Kieff, and J. Sklar. 1997. Oncogenic forms of NOTCH1 lacking either the primary binding site for RBP-Jkappa or nuclear localization sequences retain the ability to associate with RBP-Jkappa and activate transcription. J. Biol. Chem. 272**:**11336-11343. [DOI] [PubMed] [Google Scholar]
- 3.Aster, J. C., L. Xu, F. G. Karnell, V. Patriub, J. C. Pui, and W. S. Pear. 2000. Essential roles for ankyrin repeat and transactivation domains in induction of T-cell leukemia by Notch1. Mol. Cell. Biol. 20**:**7505-7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelrod, J. D., K. Matsuno, S. Artavanis-Tsakonas, and N. Perrimon. 1996. Interaction between Wingless and Notch signaling pathways mediated by dishevelled. Science 271**:**1826-1832. [DOI] [PubMed] [Google Scholar]
- 5.Bettenhausen, B., M. Hrabe de Angelis, D. Simon, J. L. Guenet, and A. Gossler. 1995. Transient and restricted expression during mouse embryogenesis of Dll1, a murine gene closely related to Drosophila Delta. Development 121**:**2407-2418. [DOI] [PubMed] [Google Scholar]
- 6.Borges, M., R. I. Linnoila, H. J. van de Velde, H. Chen, B. D. Nelkin, M. Mabry, S. B. Baylin, and D. W. Ball. 1997. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature 386**:**852-855. [DOI] [PubMed] [Google Scholar]
- 7.Breitschopf, K., E. Bengal, T. Ziv, A. Admon, and A. Ciechanover. 1998. A novel site for ubiquitination: the N-terminal residue, and not internal lysines of MyoD, is essential for conjugation and degradation of the protein. EMBO J. 17**:**5964-5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brou, C., F. Logeat, N. Gupta, C. Bessia, O. LeBail, J. R. Doedens, A. Cumano, P. Roux, R. A. Black, and A. Israel. 2000. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol. Cell 5**:**207-216. [DOI] [PubMed] [Google Scholar]
- 9.Cabrera, C. V. 1990. Lateral inhibition and cell fate during neurogenesis in Drosophila: the interactions between scute, Notch and Delta. Development 109**:**733-742. (Author's correction, **110:**733-742.) [PubMed] [Google Scholar]
- 10.Cau, E., G. Gradwohl, S. Casarosa, R. Kageyama, and F. Guillemot. 2000. Hes genes regulate sequential stages of neurogenesis in the olfactory epithelium. Development 127**:**2323-2332. [DOI] [PubMed] [Google Scholar]
- 11.Chen, H., M. A. Biel, M. W. Borges, A. Thiagalingam, B. D. Nelkin, S. B. Baylin, and D. W. Ball. 1997. Tissue-specific expression of human achaete-scute homologue-1 in neuroendocrine tumors: transcriptional regulation by dual inhibitory regions. Cell Growth Differ. 8**:**677-686. [PubMed] [Google Scholar]
- 12.Chen, H., A. Thiagalingam, H. Chopra, M. W. Borges, J. N. Feder, B. D. Nelkin, S. B. Baylin, and D. W. Ball. 1997. Conservation of the Drosophila lateral inhibition pathway in human lung cancer: a hairy-related protein (HES-1) directly represses achaete-scute homolog-1 expression. Proc. Natl. Acad. Sci. USA 94**:**5355-5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cubas, P., J. F. de Celis, S. Campuzano, and J. Modolell. 1991. Proneural clusters of achaete-scute expression and the generation of sensory organs in the Drosophila imaginal wing disc. Genes Dev. 5**:**996-1008. [DOI] [PubMed] [Google Scholar]
- 14.de la Pompa, J. L., A. Wakeham, K. M. Correia, E. Samper, S. Brown, R. J. Aguilera, T. Nakano, T. Honjo, T. W. Mak, J. Rossant, and R. A. Conlon. 1997. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development 124**:**1139-1148. [DOI] [PubMed] [Google Scholar]
- 15.Dick, L. R., A. A. Cruikshank, A. T. Destree, L. Grenier, T. A. McCormack, F. D. Melandri, S. L. Nunes, V. J. Palombella, L. A. Parent, L. Plamondon, and R. L. Stein. 1997. Mechanistic studies on the inactivation of the proteasome by lactacystin in cultured cells. J. Biol. Chem. 272**:**182-188. [DOI] [PubMed] [Google Scholar]
- 16.Ellisen, L. W., J. Bird, D. C. West, A. L. Soreng, T. C. Reynolds, S. D. Smith, and J. Sklar. 1991. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 66**:**649-661. [DOI] [PubMed] [Google Scholar]
- 17.Feder, J. N., L. Li, L. Y. Jan, and Y. N. Jan. 1994. Genomic cloning and chromosomal localization of HRY, the human homolog to the Drosophila segmentation gene, hairy. Genomics 20**:**56-61. [DOI] [PubMed] [Google Scholar]
- 18.Fisher, A. L., S. Ohsako, and M. Caudy. 1996. The WRPW motif of the Hairy-related basic helix-loop-helix repressor proteins acts as a 4-amino-acid transcription repression and protein-protein interaction domain. Mol. Cell. Biol. 16**:**2670-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Floyd, Z. E., J. S. Trausch-Azar, E. Reinstein, A. Ciechanover, and A. L. Schwartz. 2001. The nuclear ubiquitin-proteasome system degrades MyoD. J. Biol. Chem. 276**:**22468-22475. [DOI] [PubMed] [Google Scholar]
- 20.Guo, M., L. Y. Jan, and Y. N. Jan. 1996. Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron 17**:**27-41. [DOI] [PubMed] [Google Scholar]
- 21.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95**:**2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heitzler, P., and P. Simpson. 1991. The choice of cell fate in the epidermis of Drosophila. Cell 64**:**1083-1092. [DOI] [PubMed] [Google Scholar]
- 23.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67**:**425-479. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh, J. J.-D., T. Henkel, P. Salmon, E. Robey, M. G. Peterson, and S. D. Hayward. 1996. Truncated mammalian Notch1 activates CBF1/RBPJk-repressed genes by a mechanism resembling that of Epstein-Barr virus EBNA2. Mol. Cell. Biol. 16**:**952-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsieh, J. J., S. Zhou, L. Chen, D. B. Young, and S. D. Hayward. 1999. CIR, a corepressor linking the DNA binding factor CBF1 to the histone deacetylase complex. Proc. Natl. Acad. Sci. USA 96**:**23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishibashi, M., S. L. Ang, K. Shiota, S. Nakanishi, R. Kageyama, and F. Guillemot. 1995. Targeted disruption of mammalian hairy and enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 9**:**3136-3148. [DOI] [PubMed] [Google Scholar]
- 27.Iso, T., V. Sartorelli, G. Chung, T. Shichinohe, L. Kedes, and Y. Hamamori. 2001. HERP, a new primary target of Notch regulated by ligand binding. Mol. Cell. Biol. 21**:**6071-6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iso, T., V. Sartorelli, C. Poizat, S. Iezzi, H. Y. Wu, G. Chung, L. Kedes, and Y. Hamamori. 2001. HERP, a novel heterodimer partner of HES/E(spl) in Notch signaling. Mol. Cell. Biol. 21**:**6080-6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito, T., N. Udaka, T. Yazawa, K. Okudela, H. Hayashi, T. Sudo, F. Guillemot, R. Kageyama, and H. Kitamura. 2000. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development 127**:**3913-3921. [DOI] [PubMed] [Google Scholar]
- 30.Jarriault, S., C. Brou, F. Logeat, E. H. Schroeter, R. Kopan, and A. Israel. 1995. Signalling downstream of activated mammalian Notch. Nature 377**:**355-358. [DOI] [PubMed] [Google Scholar]
- 31.Jarriault, S., O. Le Bail, E. Hirsinger, O. Pourquié, F. Logeat, C. F. Strong, C. Brou, N. G. Seidah, and A. Israël. 1998. Delta-1 activation of Notch-1 signaling results in HES-1 transactivation. Mol. Cell. Biol. 18**:**7423-7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeffries, S., and A. J. Capobianco. 2000. Neoplastic transformation by Notch requires nuclear localization. Mol. Cell. Biol. 20**:**3928-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson, J. E., S. J. Birren, T. Saito, and D. J. Anderson. 1992. DNA binding and transcriptional regulatory activity of mammalian achaete-scute homologous (MASH) proteins revealed by interaction with a muscle-specific enhancer. Proc. Natl. Acad. Sci. USA 89**:**3596-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim, H. K., and G. Siu. 1998. The Notch pathway intermediate HES-1 silences CD4 gene expression. Mol. Cell. Biol. 18**:**7166-7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koyano-Nakagawa, N., J. Kim, D. Anderson, and C. Kintner. 2000. Hes6 acts in a positive feedback loop with the neurogenins to promote neuronal differentiation. Development 127**:**4203-4216. [DOI] [PubMed] [Google Scholar]
- 36.Kuroda, K., S. Tani, K. Tamura, S. Minoguchi, H. Kurooka, and T. Honjo. 1999. Delta-induced Notch signaling mediated by RBP-J inhibits MyoD expression and myogenesis. J. Biol. Chem. 274**:**7238-7244. [DOI] [PubMed] [Google Scholar]
- 37.Lardelli, M., J. Dahlstrand, and U. Lendahl. 1994. The novel Notch homologue mouse Notch 3 lacks specific epidermal growth factor-repeats and is expressed in proliferating neuroepithelium. Mech. Dev. 46**:**123-136. [DOI] [PubMed] [Google Scholar]
- 38.Lardelli, M., and U. Lendahl. 1993. Motch A and motch B—two mouse Notch homologues coexpressed in a wide variety of tissues. Exp. Cell Res. 204**:**364-372. [DOI] [PubMed] [Google Scholar]
- 39.Lindsell, C. E., C. J. Shawber, J. Boulter, and G. Weinmaster. 1995. Jagged: a mammalian ligand that activates Notch1. Cell 80**:**909-917. [DOI] [PubMed] [Google Scholar]
- 40.Linnoila, R. I., B. Zhao, J. L. DeMayo, B. D. Nelkin, S. B. Baylin, F. J. DeMayo, and D. W. Ball. 2000. Constitutive achaete-scute homologue-1 promotes airway dysplasia and lung neuroendocrine tumors in transgenic mice. Cancer Res. 60**:**4005-4009. [PubMed] [Google Scholar]
- 41.Massari, M. E., and C. Murre. 2000. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol. Cell. Biol. 20**:**429-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuno, K., D. Eastman, T. Mitsiades, A. M. Quinn, M. L. Carcanciu, P. Ordentlich, T. Kadesch, and S. Artavanis-Tsakonas. 1998. Human deltex is a conserved regulator of Notch signalling. Nat. Genet. 19**:**74-78. [DOI] [PubMed] [Google Scholar]
- 43.Morrison, S. J., S. E. Perez, Z. Qiao, J. M. Verdi, C. Hicks, G. Weinmaster, and D. J. Anderson. 2000. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 101**:**499-510. [DOI] [PubMed] [Google Scholar]
- 44.Mumm, J. S., E. H. Schroeter, M. T. Saxena, A. Griesemer, X. Tian, D. J. Pan, W. J. Ray, and R. Kopan. 2000. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol. Cell 5**:**197-206. [DOI] [PubMed] [Google Scholar]
- 45.Murre, C., P. S. McCaw, and D. Baltimore. 1989. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell 56**:**777-783. [DOI] [PubMed] [Google Scholar]
- 46.Murre, C., P. S. McCaw, H. Vaessin, M. Caudy, L. Y. Jan, Y. N. Jan, C. V. Cabrera, J. N. Buskin, S. D. Hauschka, A. B. Lassar, et al. 1989. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell 58**:**537-544. [DOI] [PubMed] [Google Scholar]
- 47.Nishimura, M., F. Isaka, M. Ishibashi, K. Tomita, H. Tsuda, S. Nakanishi, and R. Kageyama. 1998. Structure, chromosomal locus, and promoter of mouse Hes2 gene, a homologue of Drosophila hairy and Enhancer of split. Genomics 49**:**69-75. [DOI] [PubMed] [Google Scholar]
- 48.Nofziger, D., A. Miyamoto, K. M. Lyons, and G. Weinmaster. 1999. Notch signaling imposes two distinct blocks in the differentiation of C2C12 myoblasts. Development 126**:**1689-1702. [DOI] [PubMed] [Google Scholar]
- 49.Ohtsuka, T., M. Ishibashi, G. Gradwohl, S. Nakanishi, F. Guillemot, and R. Kageyama. 1999. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 18**:**2196-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ordentlich, P., A. Lin, C. P. Shen, C. Blaumueller, K. Matsuno, S. Artavanis-Tsakonas, and T. Kadesch. 1998. Notch inhibition of E47 supports the existence of a novel signaling pathway. Mol. Cell. Biol. 18**:**2230-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Persson, P., A. Jogi, A. Grynfeld, S. Pahlman, and H. Axelson. 2000. HASH-1 and E2-2 are expressed in human neuroblastoma cells and form a functional complex. Biochem. Biophys. Res. Commun. 274**:**22-31. [DOI] [PubMed] [Google Scholar]
- 52.Pui, J. C., D. Allman, L. Xu, S. DeRocco, F. G. Karnell, S. Bakkour, J. Y. Lee, T. Kadesch, R. R. Hardy, J. C. Aster, and W. S. Pear. 1999. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity 11**:**299-308. [DOI] [PubMed] [Google Scholar]
- 53.Robbins, J., B. J. Blondel, D. Gallahan, and R. Callahan. 1992. Mouse mammary tumor gene _int_-3: a member of the notch gene family transforms mammary epithelial cells. J. Virol. 66**:**2594-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shawber, C., J. Boulter, C. E. Lindsell, and G. Weinmaster. 1996. Jagged2: a serrate-like gene expressed during rat embryogenesis. Dev. Biol. 180**:**370-376. [DOI] [PubMed] [Google Scholar]
- 55.Shawber, C., D. Nofziger, J. J. Hsieh, C. Lindsell, O. Bogler, D. Hayward, and G. Weinmaster. 1996. Notch signaling inhibits muscle cell differentiation through a CBF1-independent pathway. Development 122**:**3765-3773. [DOI] [PubMed] [Google Scholar]
- 56.Sheaff, R. J., J. D. Singer, J. Swanger, M. Smitherman, J. M. Roberts, and B. E. Clurman. 2000. Proteasomal turnover of p21Cip1 does not require p21Cip1 ubiquitination. Mol. Cell 5**:**403-410. [DOI] [PubMed] [Google Scholar]
- 57.Shimizu, K., S. Chiba, N. Hosoya, K. Kumano, T. Saito, M. Kurokawa, Y. Kanda, Y. Hamada, and H. Hirai. 2000. Binding of Delta1, Jagged1, and Jagged2 to Notch2 rapidly induces cleavage, nuclear translocation, and hyperphosphorylation of Notch2. Mol. Cell. Biol. 20**:**6913-6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shou, J., P. C. Rim, and A. L. Calof. 1999. BMPs inhibit neurogenesis by a mechanism involving degradation of a transcription factor. Nat. Neurosci. 2**:**339-345. [DOI] [PubMed] [Google Scholar]
- 59.Skeath, J. B., and S. B. Carroll. 1991. Regulation of achaete-scute gene expression and sensory organ pattern formation in the Drosophila wing. Genes Dev. 5**:**984-995. [DOI] [PubMed] [Google Scholar]
- 60.Song, A., Q. Wang, M. G. Goebl, and M. A. Harrington. 1998. Phosphorylation of nuclear MyoD is required for its rapid degradation. Mol. Cell. Biol. 18**:**4994-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stifani, S., C. M. Blaumueller, N. J. Redhead, R. E. Hill, and S. Artavanis-Tsakonas. 1992. Human homologs of a Drosophila Enhancer of split gene product define a novel family of nuclear proteins. Nat. Genet. 2**:**119-127. (Erratum, **2:**343.) [DOI] [PubMed] [Google Scholar]
- 62.Struhl, G., and A. Adachi. 1998. Nuclear access and action of notch in vivo. Cell 93**:**649-660. [DOI] [PubMed] [Google Scholar]
- 63.Uyttendaele, H., G. Marazzi, G. Wu, Q. Yan, D. Sassoon, and J. Kitajewski. 1996. Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific mammalian Notch gene. Development 122**:**2251-2259. [DOI] [PubMed] [Google Scholar]
- 64.Varshavsky, A. 1996. The N-end rule: functions, mysteries, uses. Proc. Natl. Acad. Sci. USA 93**:**12142-12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinmaster, G., V. J. Roberts, and G. Lemke. 1992. Notch2: a second mammalian Notch gene. Development 116**:**931-941. [DOI] [PubMed] [Google Scholar]
- 66.Zhou, S., M. Fujimuro, J. J. Hsieh, L. Chen, A. Miyamoto, G. Weinmaster, and S. D. Hayward. 2000. SKIP, a CBF1-associated protein, interacts with the ankyrin repeat domain of NotchIC to facilitate NotchIC function. Mol. Cell. Biol. 20**:**2400-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]