Distinct Cellular Functions of MK2 (original) (raw)
Abstract
Mitogen-activated protein kinase (MAPK)-activated protein kinase 2 (MK2) is activated upon stress by p38 MAPKα and -β, which bind to a basic docking motif in the C terminus of MK2 and which subsequently phosphorylate its regulatory sites. As a result of activation MK2 is exported from the nucleus to the cytoplasm and cotransports active p38 MAPK to this compartment. Here we show that the amount of p38 MAPK is significantly reduced in cells and tissues lacking MK2, indicating a stabilizing effect of MK2 for p38. Using a murine knockout model, we have previously shown that elimination of MK2 leads to a dramatic reduction of tumor necrosis factor (TNF) production in response to lipopolysaccharide. To further elucidate the role of MK2 in p38 MAPK stabilization and in TNF biosynthesis, we analyzed the ability of two MK2 isoforms and several MK2 mutants to restore both p38 MAPK protein levels and TNF biosynthesis in macrophages. We show that MK2 stabilizes p38 MAPK through its C terminus and that MK2 catalytic activity does not contribute to this stabilization. Importantly, we demonstrate that stabilizing p38 MAPK does not restore TNF biosynthesis. TNF biosynthesis is only restored with MK2 catalytic activity. We further show that, in MK2-deficient macrophages, formation of filopodia in response to extracellular stimuli is reduced. In addition, migration of MK2-deficient mouse embryonic fibroblasts (MEFs) and smooth muscle cells on fibronectin is dramatically reduced. Interestingly, reintroducing catalytic MK2 activity into MEFs alone is not sufficient to revert the migratory phenotype of these cells. In addition to catalytic activity, the proline-rich N-terminal region is necessary for rescuing the migratory phenotype. These data indicate that catalytic activity of MK2 is required for both cytokine production and cell migration. However, the proline-rich MK2 N terminus provides a distinct role restricted to cell migration.
The stress-activated kinase mitogen-activated protein kinase (MAPK)-activated protein kinase 2 (MK2) is a direct substrate of p38 MAPKα and -β (also designated stress-activated protein kinase 2a and 2b). Phosphorylation of MK2 by p38 MAPK serves a dual function. First, it results in the activation of MK2 kinase activity, which in turn leads to the phosphorylation of substrates of MK2 such as small heat shock protein Hsp25/27, tyrosine hydroxylase, and leukocyte-specific protein 1. In addition, MK2 determines the subcellular localization of p38 MAPK. Phosphorylation of MK2 by p38 MAPK unmasks a nuclear export signal (NES) in the C-terminal part of the molecule (4). This unmasking is a prerequisite for nucleocytoplasmic transport of MK2, which in turn coexports activated p38 MAPK from the nucleus to the cytoplasm. Notably, MK2 catalytic activity is not required for cotransport of p38 (1). Using a targeted deletion of MK2, we have shown that MK2 is posttranscriptionally required for the lipopolysaccharide (LPS)-induced production of several cytokines including tumor necrosis factor (TNF) and interleukin-6 (IL-6) in mouse macrophages (9). Hence, the phenotype of the MK2 knockout (KO) mouse resembles at least in part the immunosuppressive effects of small molecules, such as SB-203580, that inhibit the activity of p38 MAPK (12). However, until now, it had not been clearly elucidated whether the role of MK2 in TNF biosynthesis is exclusively restricted to transport of p38 MAPK or whether the catalytic activity provided by MK2 is required as well.
Recently, a role for the p38 MAPK cascade in regulation of cell migration was described (6, 7). Inhibition of p38 MAPK by SB-203580 leads to a block of platelet-derived growth factor (PDGF)- and epidermal growth factor-induced migration. Interestingly, Hsp27 phosphorylation mutants also inhibit smooth muscle cell migration. These data suggest that one of the Hsp27 kinases downstream of p38 MAPK, such as MK2, could be involved in this regulation (6).
In this paper we report a significant reduction in the level of p38 MAPK in cells lacking MK2. We tested the ability of two MK2 isoforms and several MK2 mutants to rescue p38 MAPK expression levels and LPS-induced TNF production in MK2-deficient macrophages. In addition, we describe a new migratory phenotype for MK2-deficient cells. By reintroducing different MK2 mutants into MK2-deficient cells, we show that there are distinct protein domains within this kinase, which rescue these distinct MK2 phenotypes.
MATERIALS AND METHODS
Materials and animals.
Recombinant murine macrophage colony-stimulating factor (M-CSF) was from R&D Systems (Minneapolis, Minn.), SB-203580 and SB-202474 were from Calbiochem (La Jolla, Calif.), Complete protease inhibitor cocktail was from Roche Molecular Biochemicals, and tetramethyl rhodamine isocyanate (TRITC)-labeled phalloidin was from Molecular Probes.
Antibodies and their sources were as follows. Anti-p38 MAPK and anti-phosphospecific p38 MAPK were from New England Biolabs, Inc., anti-MK2 antiserum was derived as described previously (17), the anti-Myc antibody (9E10) was from Santa Cruz Biotechnology, the anti-hemagglutinin (HA) antibody (12CA5) was from Boehringer Mannheim, and GammaBind-Sepharose was from Amersham Pharmacia Biotech. All other chemicals were purchased from Sigma.
All mice used in this study were maintained under specific-pathogen-free conditions. MK2 KO mice were generated as described previously (9). MK2 KO and wild-type mice were littermates from heterozygote crossings and had a mixed C57BL/6J × 129/Ola genetic background.
Genotyping.
One microgram of tail DNA was used for each PCR. For genotyping the MK2 gene, three primers, MK2-1, MK2-2, and Neo-1, were used. The sequences of the primers were as follows: MK2-1, 5′-CGT GGG GGT GGG GTG ACA TGC TGG TTG AC-3′; MK2-2, 5′-GGT GTC ACC TTG ACA TCC CGG TGA G-3′; Neo-1, 5′-TGC TCG CTC GAT GCG ATG TTT CGC-3′. Primer pair MK2-1 and MK2-2 were used to amplify the wild-type MK2 allele to yield a 500-bp DNA fragment. Primer pair MK2-1 and Neo-1 were used to amplify the mutated MK2 allele to produce an 800-bp DNA fragment. The PCR was carried out in a Trio-Thermoblock (Biometra) by using the following program: 94°C for 15 min; 35 cycles of 94°C for 45 s, 55°C for 1 min, and 72°C for 1 min 20 s; and 72°C for 7 min.
Primary cell culture.
Resident peritoneal macrophages were collected after intraperitoneal injection of 5 ml of Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum, washed once with phosphate-buffered saline (PBS), resuspended in complete medium, and plated at 5 × 103 cells on chamber slides (Nunc, Naperville, Ill.). After 2 h at 37°C in a 95% air-5% CO2 incubator, the macrophages were washed twice with DMEM to remove nonadherent cells and cultivated for a further 16 h prior to stimulation.
Bone marrow-derived macrophages (BMDM) were obtained from marrow plugs flushed from femurs with 2.5 ml of ice-cold complete medium. After harvest, cells (11 × 106 cells/dish) were cultured for 5 days in DMEM supplemented with 10% heat-inactivated, low-LPS fetal calf serum (FCS), 10 ng of M-CSF/ml, 100 μg of streptomycin/ml, and 100 U of penicillin/ml at 37°C. After nonadherent cells were removed, adherent macrophages were aspirated from the surface, plated at 2.5 × 104 cells/well in 24-well tissue culture plates (Nunc), and rested for 24 h in M-CSF-free medium before they were washed and infected with adenovirus at a multiplicity of infection of 2 × 104 viral particles per cell. As an infection control, green fluorescent protein (GFP) expression after infection with GFP-coding adenovirus was analyzed by fluorescence microscopy. After 30 h cells were stimulated for 6 h with LPS (final concentration, 2 μg/ml).
Tracheal smooth muscle cells were dispersed with collagenase (0.6 mg/ml) and grown to confluence in DMEM culture medium (Life Technologies, Inc.) containing 10% fetal bovine serum. More than 90% of these cells from each donor mouse were smooth muscle cells, as determined by immunohistochemistry performed with an antibody raised against smooth muscle α-actin (actin clone 1A4 fluorescein isothiocyanate conjugate; Sigma). Cells were placed in serum-free DMEM for 24 h prior to migration experiments.
Western blot detection of p38 MAPK in mouse tissues.
Immediately after cervical dislocation mouse tissues were harvested and homogenized with a glass-Teflon homogenizer in ice-chilled buffer consisting of 20 mM Tris-acetate, pH 7.0, 0.1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 10 mM β-glycerophosphate, 50 mM NaF, 5 mM pyrophosphate, 1% Triton X-100, 1 mM benzamidine, 0.1% β-mercaptoethanol, 0.27 M sucrose, and 0.2 mM phenylmethylsulfonyl fluoride and supplemented with a protease inhibitor cocktail for mammalian cells (3.3%; Sigma). The protein concentration was measured, and 5× Laemmli's sodium dodecyl sulfate (SDS) sample buffer was added. Samples were boiled, briefly centrifuged, and resolved by SDS-polyacrylamide gel electrophoresis (PAGE; 5 to 12.5% acrylamide), and proteins were transferred from the gels to Hybond ECL membranes (Amersham Pharmacia Biotech). Blots were incubated for 2 h in PBS-0.2% Tween 20 (PBST) containing 5% powdered skim milk. After three washes with PBST, membranes were incubated for 16 h with the primary antibody (pan-p38; New England Biolabs; 1,000-fold diluted in PBST) and for 1 h with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (2,000-fold diluted). Bound antibodies were detected with an ECL detection kit (Santa Cruz Biotechnology). Semiquantitative estimation of the concentration of p38 MAPK was obtained by using serial dilutions of recombinant glutathione _S_-transferase-p38 MAPK as the standard.
Cotransfections of epitope-tagged MK2 and p38 MAPK in HEK293 cells and immunodetection.
The expression vector for HA-p38 is pcDNA3 and was a gift from Roger Davis. The expression vector for myc-MK2-CT1 and myc-MK2-CT2 is also pcDNA3. Human embryonic kidney 293 (HEK293) cells were cultured in DMEM containing 10% fetal bovine serum. These cells were maintained in 5% CO2 at 37°C. Cells at ∼60% confluence were transfected by the Lipofectamine method (Life Technologies, Inc.). After incubation for 12 h, the transfected cells were washed with DMEM and incubated in fresh growth medium for 48 h. The cells were harvested and lysed in a solution consisting of 20 mM Tris, pH 7.5, 1 mM MgCl2, 125 mM NaCl, and 1% Triton X-100 supplemented with Complete protease inhibitor cocktail (Roche Molecular Biochemicals). Tagged proteins were immunoprecipitated from cell lysates (6 × 106 cells in each samples) with 5 μg of anti-Myc antibody (9E10) (Santa Cruz Biotechnology) or 1 μg of anti-HA antibody (12CA5) (Boehringer Ingelheim) and 20 μl of GammaBind Sepharose (Amersham Pharmacia Biotech). The proteins were separated by SDS-PAGE and analyzed by immunoblotting.
Adenovirus transductions.
cDNAs for the MK2 isoforms (MK2-CT1 and MK2-CT2) and mutants (MK2-CT1-KR and MK2-CAT) and enhanced GFP were inserted into the pAdori expression vector under the control of the cytomegalovirus promoter. Replication-defective, recombinant type 5 (del327) adenovirus with E1 and E3 deleted was generated by homologous recombination in HEK293 cells (American Type Culture Collection, Manassas, Va.). Recombinant adenovirus was isolated and propagated on HEK293 cells under endotoxin-free conditions. Virus was released from infected HEK293 cells by three cycles of freezing and thawing and purified by two cesium chloride centrifugation gradients and dialyzed against PBS, pH 7.2, at 4°C. Following dialysis, glycerol was added to a concentration of 10% and the virus was stored at −80°C or stored in PBS-100 mM NaCl-0.1% bovine serum albumin-50% glycerol at 4oC until use. Virus titer (particles per milliliter) was determined by measuring the optical density at 260 nm. Virus stocks were free of endotoxin, as determined by measurement with a Limulus amebocyte lysate kit (BioWhittaker, Walkersville, Md.).
MK2 Western blotting.
HEK293 cells were plated at 3 × 106 cells per 100-mm-diameter plate and were infected the following day at a multiplicity of infection of 100 viral particles per cell. After 24 h cells were stimulated with 0.4 M sorbitol for 20 min, rinsed with PBS, and lysed in a solution consisting of 20 mM Tris, pH 7.5, 125 mM NaCl, 1 mM MgCl2, 1% Triton X-100, 10 mM NaF, 2.5 mM β-glycerol, and 1 mM sodium orthovanadate with protease inhibitor cocktail (Roche Molecular Biochemicals) added. Western blots were probed with MK2-specific antibody 06602, diluted 1:500, from Upstate Biotechnology.
For semiquantitative estimation of the concentration of MK2 in spleen, serial dilutions of recombinant His-tagged MK2 were used as the standard. In this experiment, a rabbit polyclonal antiserum against recombinant glutathione _S_-transferase-MK2 (17) was used for immunodetection.
MK2 kinase assay.
Kinase activity was measured as described previously (5). Briefly, cells were lysed in lysis buffer consisting of 20 mM Tris-acetate, pH 7.0, 0.1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 10 mM β-glycerophosphate, 50 mM NaF, 5 mM pyrophosphate, 1% Triton X-100, 1 mM benzamidine, 0.1% β-mercaptoethanol, 0.27 M sucrose, and 0.2 mM phenylmethylsulfonyl fluoride. The kinase was measured by incubation of equal amounts of lysate protein with the reaction mixture (50 mM β-glycerophosphate [pH 7.4], 0.1 mM EDTA, 10 μg of Hsp25, 100 μM [γ-32P]ATP, 10 mM magnesium acetate) for 15 min at 30°C. The reaction mixture was resolved by SDS-PAGE, and the labeling of Hsp25 was visualized by phosphorimager (Cyclon imaging system) and quantified by the use of OptiQuant software (Packard Instruments).
TNF measurement.
TNF-α was measured in macrophage supernatant by enzyme-linked immunosorbent assay (Quantikine M mouse TNF-α immunoassay kit; R&D Systems).
Fluorescence microscopy of macrophages.
Cells were fixed with 4% formaldehyde in PBS for 20 min at room temperature and were permeabilized with 0.2% Triton X-100 in PBS for 5 min. For localization of F-actin filaments, cells were incubated with 0.1 μg of TRITC-labeled phalloidin (Molecular Probes)/ml for 1 h. Images of cells were obtained with an Axiovert 100 fluorescence microscope (Zeiss) with Visitron Systems and Metamorph software.
Migration assay.
The cell migration assay was performed with a 48-well microchemotaxis chamber (Neuroprobe, Plaesanton, Calif.). Polyvinylpyrrolidone-free polycarbonate filters (Nuclepore, Corning Costar Corp., Cambridge, Mass.) with a pore size of 8 μm were coated with 0.01% collagen type I or fibronectin. The lower compartment of a Boyden chamber was filled with DMEM containing chemotactic factors at various concentrations (IL-1, 3 and 6 ng/ml; PDGF, 5 and 10 ng/ml). Subconfluent cultures, which had been starved for 24 h in serum-free DMEM, were harvested and resuspended in DMEM at a final concentration of 2 × 105 cells/ml. After the filter was placed between lower and upper chambers, 50 μl of the cell suspension was seeded in the upper compartment. Cells were allowed to migrate for 6 h at 37°C in a humidified atmosphere with 5% CO2. The filter was then removed, and cells on the upper side were scraped off with a rubber policeman. Migrated cells were fixed in methanol, stained with Giemsa solution (Diff-Quick; Baxter Diagnostics, Rome, Italy), and counted from five randomly chosen fields (magnification, ×100) for each well. Each experimental point was studied in triplicate.
Quantitative cell migration assay collagen I and cell migration assay fibronectin (Chemicon) were used for the transfected cells according to the manufacturer's instructions.
Transfection of MEFs and HEK cells.
Mouse embryonic fibroblasts (MEFs; MK2+/+ and MK2−/−) (9) were cultured in DMEM supplemented with 10% FCS, 100 U of penicillin per ml, and 100 μg of streptomycin per ml (complete medium). Transfection of MEFs was performed with 0.4 μg of DNA per well in 24-well tissue culture plates with Lipofectamine Plus (Life Technologies, Inc.). Transfection efficiency was monitored with GFP expression vector pEGFP-C1 (Clontech); that ∼50% of the transfected cells expressed GFP was confirmed by fluorescence microscopy. The cells were incubated in serum-free medium at 37°C for 5 h. Subsequently, complete medium was added, and cells were grown for an additional 24 h before analysis.
HEK293 were cultured in DMEM containing 10% FCS. These cells were maintained in 5% CO2 at 37°C. Cells at ∼60% confluence were transfected by the Lipofectamine method (Life Technologies, Inc.). After incubation for 12 h, the transfected cells were washed with DMEM and incubated in fresh growth medium for 48 h.
Site-directed mutagenesis.
Mutant MK2-CT1-FP-AA and deletion mutant MK2-CT1-ΔP were generated by PCR with the QuickChange site-directed mutagenesis kit (Stratagene) using the following primer pairs, respectively 5′-CTC CGC CGG CGC CTG CCG CCA GCC CTC CAC CGC C-3′ and 5′-GGC GGT GGA GGG CTG GCG GCA GGC GCC GGC GGA G-3′; 5′-GGC TCT CCG GGC CAG ACT CAG TTC CAC GTC AAG TCG G-3′ and 5′-CCG ACT TGA CGT GGA ACT GAG TCT GGC CCG GAG AGC C-3′.
RESULTS
Reduced p38 MAPK level in MK2−/− tissues.
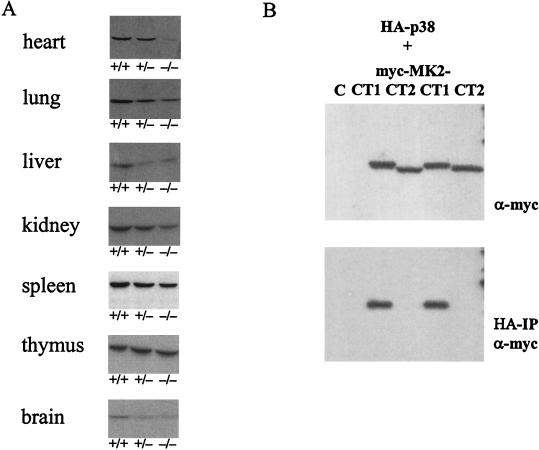
There are at least two docking sites for MK2 in p38 MAPK. The common docking (CD) site, located at the C terminus, which binds to a basic sequence in the nuclear localization signal (NLS) of MK2, and the Glu-Asp (ED) site (20, 21), located in the kinase domain. The association between MK2 and p38 led us to investigate whether p38 remains stable in the absence of MK2. A pan-p38 antibody was used to detect p38 MAPK in Western blots prepared from different tissues that lacked one or two alleles for MK2 and from control, wild-type littermates. As shown in Fig. 1A, reduced levels or the absence of MK2 leads to a significant reduction of p38 MAPK in heart, lung, liver, kidney, brain, and, to a certain degree, spleen. Thymus alone showed no significant reduction in p38 MAPK.
FIG. 1.
Reduced expression of p38 MAPK in MK2-deficient tissues (A) and complex formation between p38 MAPK and MK2 (B). (A) Western blot detection of p38 MAPK in lysates from different tissues of wild-type mice (+/+) and animals lacking one (+/−) or both alleles (−/−) of the MK2 gene. (B) p38 MAPK-MK2 complex formation in transfected HEK293 cells. Plasmids encoding HA-tagged p38 MAPK and myc-tagged isoforms of MK2 (myc-MK2-CT1 and myc-MK2-CT2) were transfected to HEK293 cells, and interaction of the proteins expressed was analyzed by using combined anti-HA immunoprecipitation (HA-IP) and anti-Myc Western blot detection (α-myc; lower blot). As an expression control, the cell lysates were subjected to anti-myc Western blotting without immunoprecipitation (upper blot). As a negative control (C) cells were transfected with the HA-p38 MAPK construct only.
Only the MK2 isoform carrying the NLS-containing C terminus (MK2-CT1) interacts with p38 MAPK.
Two different isoforms of MK2 have been detected in mice and humans. Since in the MK2−/− mouse both isoforms are absent (9), it is likely that these isoforms result from differential splicing of the same mouse gene or from posttranslational modification of the MK2 protein. In humans, two different cDNAs for MK2 coding for proteins with different C termini (MK2-CT1 and MK2-CT2) have been isolated (18, 22), strengthening the notion that the isoforms may result from differential splicing. Against this notion stands the fact that expressed sequence tags for MK2-CT2 are extremely underrepresented in the data bases (A. Kotlyarov and M. Gaestel, unpublished data) although the two isoforms show similar levels of protein and similar levels of activity as judged by Western blotting or gel kinase assay (9). In MK2-CT1 the C terminus contains the NLS with the binding site for the p38 MAPK CD motif and a NES. MK2-CT2 does not contain these sites. To test whether the MK2-CT1 C terminus is obligatory for p38 MAPK-MK2 complex formation we expressed both cDNAs of human MK2 in HEK293 cells as myc-tagged proteins together with HA-tagged p38 MAPK. After cell lysis and immunoprecipitation using a HA-specific antibody, the binding of MK2 to p38 MAPK was analyzed with an anti-myc Western blot of the immunoprecipitate (Fig. 1B). An effective binding between MK2 and p38 MAPK can be detected for MK2-CT1 but not for MK2-CT2. These data indicate that the interaction between the p38 CD domain and amino acids in the NLS of MK2-CT1 is essential for complex formation and that this interaction is a likely prerequisite for stabilization of p38.
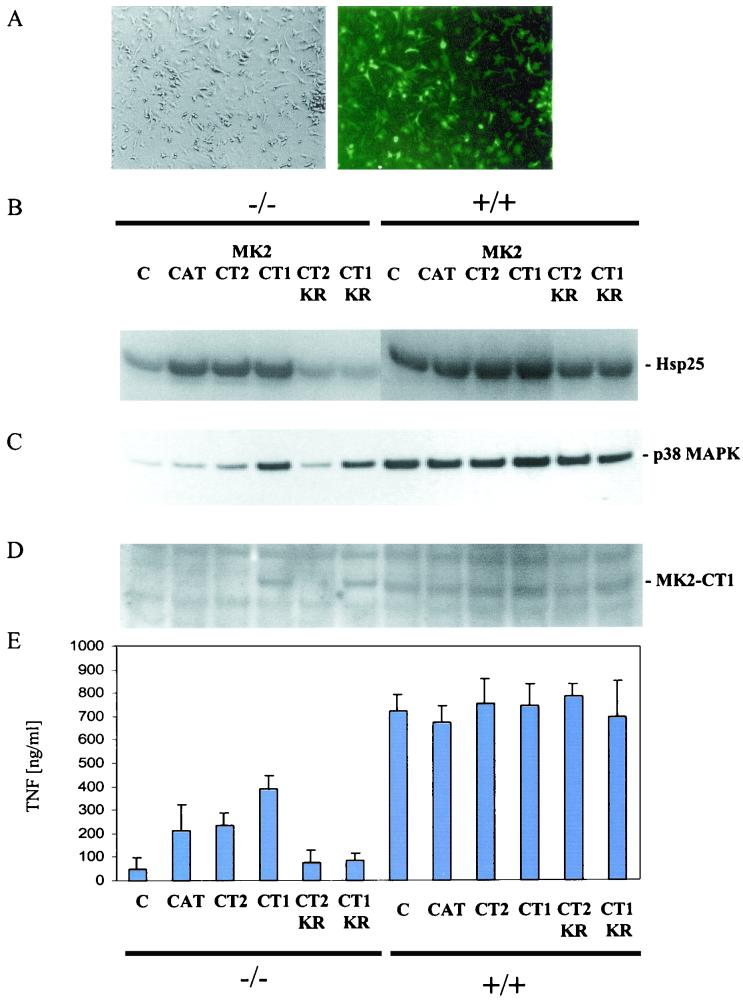
Adenovirus constructs express high levels of MK2 in mouse BMDMs.
To determine the mechanism of action of MK2, we expressed both isoforms of MK2 and several MK2 mutants in macrophages, the main producer of proinflammatory cytokine TNF, isolated from MK2−/− mice and assayed their ability to restore TNF biosynthesis. To express the MK2 proteins at high levels in BMDMs cultivated in vitro, we used a highly efficient adenovirus expression system. In our experiments about 77% of the infected-cell population express protein as judged by control infections using an adenovirus encoding GFP (Fig. 2A). Constructs for both isoforms of MK2 (MK2-CT1 and -CT2) and a mutant (MK2-CAT) which encodes only the catalytic domain of the kinase (amino acids 48 to 338) were introduced into MK2−/− BMDMs. Kinase assays using BMDM lysates and MK2 substrate Hsp25 showed that these three proteins had catalytic activity comparable to or higher than the MK2 activity seen in wild-type macrophages (Fig. 2B).
FIG. 2.
Rescue of p38 MAPK protein level and TNF production by transduction of macrophages with constructs expressing different human MK2 isoforms (MK2-CT1 and MK2-CT2) and mutants (MK2-CAT, MK2-CT1-KR, and MK2-CT2-KR). (A) High efficiency of macrophage transduction by the control GFP-encoding construct. About 77% of the cells seen in phase contrast (left) show GFP expression (right). (B) Assay of MK2 kinase activity in lysates of transduced MK2-deficient macrophages (−/−) and, as a control, of wild-type (+/+) macrophages. The basal Hsp25 phosphorylation in MK2−/− macrophages probably results from 3pK/MK3 and PRAK/MK5 activity in the lysate. (C) Rescue of the p38 MAPK protein level in transduced MK2-deficient macrophages. The order of the lanes is the same as in panel B. (D) Western blot detection of the MK2-CT1 in lysates from macrophages. The antibody used is directed against the C-terminal region of MK2-CT1 and is specific for this isoform. Mouse MK2-CT1, with a slightly lower molecular mass, is detected in wild-type (+/+) macrophages. The order of the lanes is the same as in panel B. (E) TNF production after LPS treatment of transduced macrophages. The TNF in the supernatant was measured by enzyme-linked immunosorbent assay. Data shown are means of three independent experiments.
p38 MAPK levels are rescued by MK2-CT1 and a catalytically inactive MK2-CT1 mutant (MK2-CT1-KR) but not by MK2-CT2.
We then asked whether two MK2 isoforms and kinase-dead MK2 mutants, in which lysine 93 in catalytic subdomain I is replaced with arginine (MK2-CT1-KR and MK2-CT2-KR, Fig. 2B), are able to rescue p38 MAPK levels when introduced into macrophages isolated from MK2−/− mice. Western blot analysis revealed that the MK2-CT1 isoform, which has the NES- and NLS-containing C terminus, is able to effectively restore p38 MAPK levels even without catalytic activity (Fig. 2C). A control Western blot with an MK2-CT1-specific antibody shows that MK2-CT1 and MK2-CT1-KR are expressed at levels comparable to those for the endogenous mouse enzyme in wild-type macrophages (Fig. 2D). In contrast, neither MK2-CT2 nor the catalytic domain alone (MK2-CAT) is able to increase p38 MAPK levels. Since the catalytic activity of MK2-CT2 and MK2-CAT was comparable to that of MK2-CT1 (Fig. 2B), this result suggests that p38 MAPK levels are increased or stabilized through a direct interaction between the NLS-containing C terminus of MK2 and p38 MAPK. This mechanism is independent of MK2 catalytic activity.
Increased levels of catalytically active MK2, but not p38, rescue TNF production in MK2−/− macrophages.
In MK2-deficient mice and macrophages, LPS-induced biosynthesis of TNF is dramatically reduced to about 10% of that observed in wild-type cells (9). To determine whether MK2 can rescue this proinflammatory phenotype, macrophages were infected with MK2-expressing adenoviruses and LPS-induced TNF production was measured. When introduced into BMDMs, MK2-CAT, MK2-CT1, and MK2-CT2 were able to significantly increase LPS-induced TNF synthesis. However, MK2 catalytically inactive mutants are not able to rescue TNF biosynthesis (Fig. 2E). Hence, the rescue of TNF production does not correspond to the rescue of the p38 MAPK level (Fig. 2C). Instead, MK2 catalytic activity (Fig. 2B) is necessary for TNF production. Interestingly, the p38 MAPK-stabilizing isoform MK2-CT1 is responsible for a somewhat higher TNF production than MK2-CT2 or MK2-CAT. This observation suggests that p38 MAPK stabilization and/or MK2/p38 MAPK nucleocytoplasmic translocation could be required to maximally restore TNF biosynthesis. However, MK2/p38 MAPK translocation and p38 MAPK stabilization in the absence of MK2 catalytic activity (MK2-CT1-KR) are clearly not sufficient for stimulation of TNF synthesis. The inability of increased p38 level to rescue TNF production is not due to a dominant-negative or negative-interfering effect of MK2-CT1-KR in this experimental system, since expression of MK2-CT1-KR to a similar level in MK2+/+ macrophages does not inhibit MK2 activity and TNF production (Fig. 2B and E). Hence, it becomes clear that the restoration of p38 MAPK level alone is not able to compensate for MK2 deficiency.
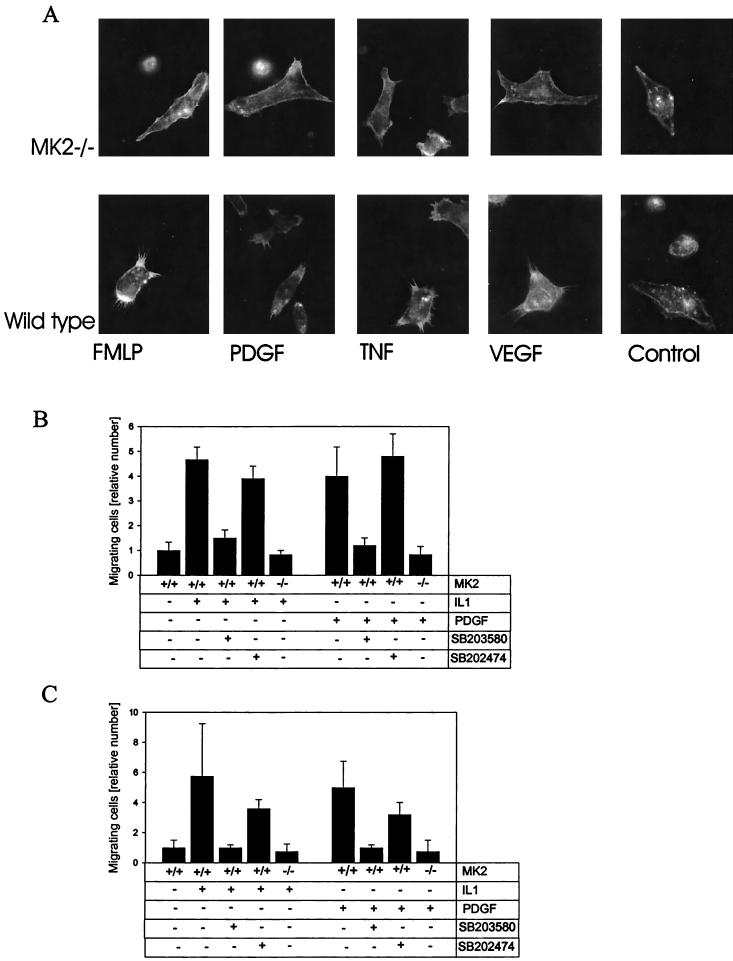
Reduced filopodium formation in MK2−/− macrophages and reduced migration of MK2-deficient embryonic fibroblasts and smooth muscle cells.
We were interested in knowing whether MK2+/+ and MK2−/− BMDMs have morphological differences. Stimulation of macrophages by diverse factors such as formyl methionine-leucine-phenylalanine, PDGF, TNF, and vascular/endothelial growth factor leads to an increase in formation of actin-rich membrane protrusions such as filopodia and microspikes. A comparison of wild-type and MK2-deficient cells revealed that formation of these membrane protrusions is reduced in MK2-deficient cells (Fig. 3A). Since it is known that filopodia and microspikes are necessary for cell motility (13) and since inhibitors of p38 MAPK affect cell migration (6), we decided to analyze the migration of MK2-deficient cells. Both immortalized MEFs (Fig. 3B) and tracheal mouse smooth muscle cells (Fig. 3C) were assayed for migration through fibronectin-treated membranes in a gradient of PDGF or IL-1. Wild-type cells were treated with p38 MAPK inhibitor SB-203580 (10 μM) and structurally related inactive substance SB-202474 as positive and negative controls, respectively. Similar reductions in IL-1- and PDGF-induced migration for MK2-deficient and SB-203580-treated cells were detected. No change in migration for SB-202474-treated cells was detected. This finding suggests that p38 MAPK signals primarily through MK2 to Hsp27 to control cell migration. We conclude that other p38 MAPK substrates which also phosphorylate Hsp27, such as 3pK/MK3 (14) and PRAK/MK5 (15), do not have a major role in regulating cellular migration in this system.
FIG. 3.
Filopodium formation and migration of MK2-deficient cells. (A) Reduced filopodium formation of MK2−/− macrophages in response to formyl Met-Leu-Phe (FMLP) peptide, PDGF, TNF, and vascular/endothelial growth factor (VEGF). Actin filaments are stained by TRITC-labeled phalloidin. Immortalized MEFs (B) and tracheal mouse smooth muscle cells (C) were assayed for migration through fibronectin-treated membranes in a gradient of PDGF or IL-1. Wild-type cells were treated with p38 MAPK inhibitor SB-203580 (10 μM) and structurally related inactive substance SB-202474 as positive and negative controls, respectively.
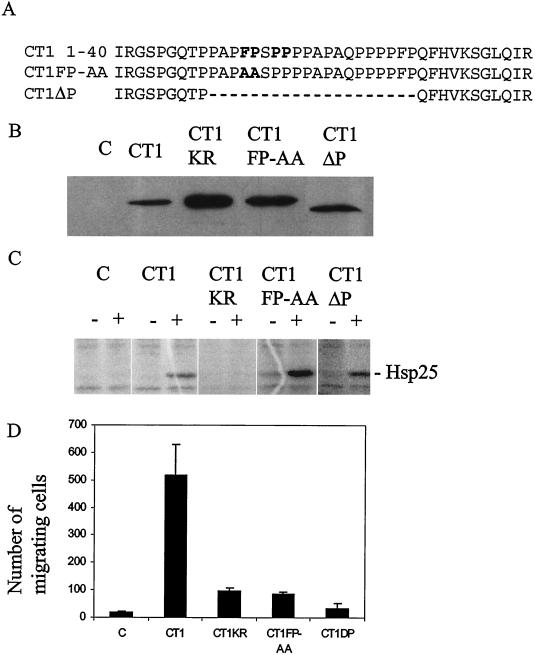
Rescue of cellular migration requires both catalytic activity and the N-terminal proline-rich type 2 motif of MK2.
To determine the molecular mechanisms underlying the migratory phenotype of MEF−/− cells, we assayed several MK2 mutants for their ability to reverse the migration defect. MK2-deficient MEFs were transfected with expression constructs coding for myc-epitope tagged wild-type MK2 (myc-MK2-CT1) and full-length catalytically inactive mutant myc-MK2-CT1-KR. In addition, two N-terminal mutants were constructed: one with point mutations F13A and P14A (myc-MK2-CT1-FP-AA) and one with the proline-rich N-terminal region deleted (myc-MK2-CT1-ΔP) (Fig. 4A). Expression of these constructs was monitored in parallel transfections of HEK293 cells and anti-myc Western blotting (Fig. 4B). Different mutants were expressed at levels comparable to that of wild-type MK2 (Fig. 4B, CT1). Catalytic activity in transfected MK2-deficient MEFs, resting and stress stimulated, was determined by kinase assays with recombinant Hsp25 as the substrate. As expected, CT1-KR did not exhibit catalytic activity. In contrast, similar levels of stress-inducible catalytic activity for both the N-terminal mutants and the wild-type construct were detected (Fig. 4C). Remarkably, only the full-length catalytically active kinase was able to rescue the migration defect (Fig. 4D). This is in contrast to the LPS-induced production of TNF in macrophages, where catalytic activity provided by the catalytic domain alone is sufficient to restore TNF production. These results show that catalytic activity in combination with the MK2 N terminus is required for cellular migration.
FIG. 4.
Catalytic activity and the proline-rich N-terminal region of MK2 are necessary for rescue of cell migration. (A) Different N-terminal proline-rich motif mutants (myc-MK2-CT1-AA and myc-MK2-CT1-ΔP) used in the migration rescue experiment. The core of the proline-rich motif and the mutuated residues are shown in boldface. (B) Control of Myc-tagged protein expression from the different constructs transfected into HEK293 cells by Western blotting. (C) MK2 kinase assay of lysates from transfected MK2-deficient MEFs before (−) and after (+) stress stimulation using recombinant Hsp25 as the substrate. (D) Migration of the transfected MEFs through fibronectin-coated membranes (absolute values of migrated cells when 30,000 cells were assayed in a well of 8 mm2). Error bars indicate the standard deviations from three independent experiments.
DISCUSSION
Elimination of the stress-activated protein kinase MK2 results in a complex phenotype represented by decreased p38 MAPK levels in most tissues analyzed, especially in heart and liver, and by a decrease in the biosynthesis of several proinflammatory cytokines at the posttranscriptional level in mouse macrophages (9). In addition, impaired filopodium formation in growth factor-stimulated macrophages and altered migration of embryonic fibroblasts and smooth muscle cells were observed. By introducing full-length and mutant forms of MK2 into MK2−/− cells, we were able to assign distinct MK2 domains, which provide distinct cellular functions.
Stabilization and nucleocytoplasmic transport of p38 MAPK by MK2.
The reduced level of p38 MAPK in MK2-deficient cells and tissues is probably due to absence of a specific protein-protein interaction between activated p38 MAPK and MK2. After activation, p38 MAPK enters the nucleus (16), binds to MK2, and phosphorylates MK2 at different regulatory sites (2, 5). The CD domain of p38 MAPK binds a domain localized in the NLS of MK2, thereby blocking the NLS of MK2 (21). Further, this binding or the phosphorylation of MK2 or both expose the adjacent nuclear export sequence, thus promoting the translocation of MK2 from nucleus to cytoplasm (1, 4). Importantly, the enzyme-substrate complex remains stable even after MK2 is activated as a result of its phosphorylation. Hence, p38 MAPK is coexported with MK2 to the cytoplasm (1). Our experiments show that, in addition to providing for p38 MAPK nucleocytoplasmic transport, MK2 has an additional role in stabilizing p38. The exact mechanism by which this stabilization occurs is not clear at this point. p38 MAPK could be more stable in the cytoplasm than in the nucleus or more stable in the signaling complex than in its unbound form. Since the concentration of MK2 in spleen determined by semiquantitative Western blotting (6 nmol/g of total protein) is about threefold lower than the concentration of p38 MAPKα (17 nmol/g of total protein), stabilization of p38 MAPK by an equimolar permanent signaling complex between p38 MAPK and MK2 becomes unlikely and stabilization as a result of a transporter function of MK2 seems more realistic.
Since protein levels and possibly the cellular localization of p38 MAPK are affected in MK2-deficient animals, it had yet to be determined whether the TNF phenotype in MK2-deficient cells results from decreased p38 MAPK levels or p38 MAPK dislocation or from loss of MK2 catalytic activity. We have shown that, even when catalytically inactive MK2 stabilizes p38 MAPK, cells do not produce TNF. Since it is known that the catalytically inactive mutants are still phosphorylated by p38 MAPK and still translocate to the cytoplasm (1), it is clear that restoration of nuclear export of p38 MAPK is not sufficient for rescuing TNF production in MK2−/− cells. Remarkably, the catalytic domain of MK2 alone is sufficient for TNF production. Taken together, these data clearly demonstrate that the catalytic activity of MK2 is necessary for LPS-induced TNF biosynthesis and that therefore its lack is responsible for the TNF deficiency in MK2 KO mice. However, since p38 MAPK-interacting isoform MK2-CT1 is significantly more efficient in restoring TNF production than MK2-CT2 and MK-CAT, which lack the p38 MAPK docking site, the NLS, and the NES, it is possible that stabilization and/or cotransport of p38 MAPK together with the kinase activity of MK2 can further stimulate TNF synthesis. Alternatively, the C-terminal NES and NLS in MK2-CT1 might be required to maximize restoration of TNF biosynthesis by providing for optimal subcellular localization of catalytically active MK2 itself.
MK2 regulates formation of the actin cytoskeleton.
The defect in filopodium and microspike formation and the migratory phenotype for MK2-deficient cells described here support a role for MK2 in the regulation of actin remodeling in the cytoskeleton of the cell. Interestingly, the major known substrate of MK2, small heat shock protein Hsp25/27 (19), is also involved in regulation of actin polymerization. It has been shown that the nonphosphorylated form of Hsp25 inhibits actin polymerization at the barbed ends of filaments and that Hsp25 phosphorylation blocks this inhibition (3). Furthermore, a nonphosphorylatable Hsp27 mutant acts as a dominant-negative mutant on mitogenic stimulation of submembranous actin filament formation (11). It is interesting that the N-terminal proline-rich region of MK2 together with MK2 catalytic activity is necessary for the rescue of the MK2−/− migratory phenotype. One may speculate that the proline-rich region of MK2 targets the enzyme to the actin cytoskeleton, where phosphorylation of Hsp25/27 and its subsequent release from these filaments could be a mechanism of derepression of actin polymerization.
Distinct cellular functions of MK2.
Studies have illustrated that many protein kinases have multiple substrates and that some enzymes have evolved to provide more than one cellular function. Our results indicate that this is likely to be the case for MK2. Since evolutionarily more ancient MK2 molecules from sea urchins (8) and Drosophila melanogaster (10) do not contain the proline-rich N-terminal region, we predict that stabilization and translational control of AU-rich element-containing mRNAs were the primary functions of this enzyme prior to the emergence of its secondary function in modulation of actin remodeling. As we demonstrated here, the N-terminal proline-rich region is not necessary for reestablishing TNF biosynthesis in MK2-deficient cells but is obligatorily involved in actin-based cell migration in the cellular system studied. Our present work establishes the essential role of MK2 catalytic activity in TNF biosynthesis and cellular migration of mouse macrophages. Since Hsp25 binds to the barbed ends of the actin fibers (3) and has been implicated in regulating cell migration (6), it is very likely to be the relevant MK2 substrate involved in actin remodeling. The relevant substrate(s) of MK2 in controlling TNF biosynthesis is still unknown. Future work aimed at identifying such a substrate(s) is critical for understanding how MK2 regulates the biosynthesis of TNF as well as of many important cytokines which contribute to the inflammatory response.
REFERENCES
- 1.Ben-Levy, R., S. Hooper, R. Wilson, H. F. Paterson, and C. J. Marshall. 1998. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr. Biol. 8**:**1049-1057. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Levy, R., I. A. Leighton, Y. N. Doza, P. Attwood, N. Morrice, C. J. Marshall, and P. Cohen. 1995. Identification of novel phosphorylation sites required for activation of MAPKAP kinase-2. EMBO J. 14**:**5920-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benndorf, R., K. Hayess, S. Ryazantsev, M. Wieske, J. Behlke, and G. Lutsch. 1994. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J. Biol. Chem. 269**:**20780-20784. [PubMed] [Google Scholar]
- 4.Engel, K., A. Kotlyarov, and M. Gaestel. 1998. Leptomycin B-sensitive nuclear export of MAPKAP kinase 2 is regulated by phosphorylation. EMBO J. 17**:**3363-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engel, K., H. Schultz, F. Martin, A. Kotlyarov, K. Plath, M. Hahn, U. Heinemann, and M. Gaestel. 1995. Constitutive activation of mitogen-activated protein kinase-activated protein kinase 2 by mutation of phosphorylation sites and an A-helix motif. J. Biol. Chem. 270**:**27213-27221. [DOI] [PubMed] [Google Scholar]
- 6.Hedges, J. C., M. A. Dechert, I. A. Yamboliev, J. L. Martin, E. Hickey, L. A. Weber, and W. T. Gerthoffer. 1999. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J. Biol. Chem. 274**:**24211-24219. [DOI] [PubMed] [Google Scholar]
- 7.Klekotka, P. A., S. A. Santoro, and M. M. Zutter. 2001. Alpha 2 integrin subunit cytoplasmic domain-dependent cellular migration requires p38 MAPK. J. Biol. Chem. 276**:**9503-9511. [DOI] [PubMed] [Google Scholar]
- 8.Komatsu, S., N. Murai, G. Totsukawa, M. Abe, K. Akasaka, H. Shimada, and H. Hosoya. 1997. Identification of MAPKAPK homolog (MAPKAPK-4) as a myosin II regulatory light-chain kinase in sea urchin egg extracts. Arch. Biochem. Biophys. 343**:**55-62. [DOI] [PubMed] [Google Scholar]
- 9.Kotlyarov, A., A. Neininger, C. Schubert, R. Eckert, C. Birchmeier, H. D. Volk, and M. Gaestel. 1999. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat. Cell Biol 1**:**94-97. [DOI] [PubMed] [Google Scholar]
- 10.Larochelle, S., and B. Suter. 1995. The Drosophila melanogaster homolog of the mammalian MAPK-activated protein kinase-2 (MAPKAPK-2) lacks a proline-rich N-terminus. Gene 163**:**209-214. [DOI] [PubMed] [Google Scholar]
- 11.Lavoie, J. N., E. Hickey, L. A. Weber, and J. Landry. 1993. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J. Biol. Chem. 268**:**24210-24214. [PubMed] [Google Scholar]
- 12.Lee, J. C., J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. J. Blumenthal, J. R. Heys, S. W. Landvatter, et al. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372**:**739-746. [DOI] [PubMed] [Google Scholar]
- 13.McClay, D. R. 1999. The role of thin filopodia in motility and morphogenesis. Exp. Cell Res. 253**:**296-301. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin, M. M., S. Kumar, P. C. McDonnell, S. Van Horn, J. C. Lee, G. P. Livi, and P. R. Young. 1996. Identification of mitogen-activated protein (MAP) kinase-activated protein kinase-3, a novel substrate of CSBP p38 MAP kinase. J. Biol. Chem. 271**:**8488-8492. [DOI] [PubMed] [Google Scholar]
- 15.New, L., Y. Jiang, M. Zhao, K. Liu, W. Zhu, L. J. Flood, Y. Kato, G. C. Parry, and J. Han. 1998. PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO J. 17**:**3372-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raingeaud, J., S. Gupta, J. S. Rogers, M. Dickens, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270**:**7420-7426. [DOI] [PubMed] [Google Scholar]
- 17.Schultz, H., K. Engel, and M. Gaestel. 1997. PMA-induced activation of the p42/44ERK- and p38RK-MAP kinase cascades in HL-60 cells is PKC dependent but not essential for differentiation to the macrophage-like phenotype. J. Cell. Physiol. 173**:**310-318. [DOI] [PubMed] [Google Scholar]
- 18.Stokoe, D., B. Caudwell, P. T. Cohen, and P. Cohen. 1993. The substrate specificity and structure of mitogen-activated protein (MAP) kinase-activated protein kinase-2. Biochem. J. 296**:**843-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stokoe, D., K. Engel, D. G. Campbell, P. Cohen, and M. Gaestel. 1992. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett. 313**:**307-313. [DOI] [PubMed] [Google Scholar]
- 20.Tanoue, T., M. Adachi, T. Moriguchi, and E. Nishida. 2000. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat. Cell Biol. 2**:**110-116. [DOI] [PubMed] [Google Scholar]
- 21.Tanoue, T., R. Maeda, M. Adachi, and E. Nishida. 2001. Identification of a docking groove on ERK and p38 MAP kinases that regulates the specificity of docking interactions. EMBO J. 20**:**466-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zu, Y. L., F. Wu, A. Gilchrist, Y. Ai, M. E. Labadia, and C. K. Huang. 1994. The primary structure of a human MAP kinase activated protein kinase 2. Biochem. Biophys. Res. Commun. 200**:**1118-1124. [DOI] [PubMed] [Google Scholar]