Inhibition of Protease-Resistant Prion Protein Formation in a Transformed Deer Cell Line Infected with Chronic Wasting Disease (original) (raw)
Abstract
Chronic wasting disease (CWD) is an emerging transmissible spongiform encephalopathy (prion disease) of North American cervids, i.e., mule deer, white-tailed deer, and elk (wapiti). To facilitate in vitro studies of CWD, we have developed a transformed deer cell line that is persistently infected with CWD. Primary cultures derived from uninfected mule deer brain tissue were transformed by transfection with a plasmid containing the simian virus 40 genome. A transformed cell line (MDB) was exposed to microsomes prepared from the brainstem of a CWD-affected mule deer. CWD-associated, protease-resistant prion protein (PrPCWD) was used as an indicator of CWD infection. Although no PrPCWD was detected in any of these cultures after two passes, dilution cloning of cells yielded one PrPCWD-positive clone out of 51. This clone, designated MDBCWD, has maintained stable PrPCWD production through 32 serial passes thus far. A second round of dilution cloning yielded 20 PrPCWD-positive subclones out of 30, one of which was designated MDBCWD2. The MDBCWD2 cell line was positive for fibronectin and negative for microtubule-associated protein 2 (a neuronal marker) and glial fibrillary acidic protein (an activated astrocyte marker), consistent with derivation from brain fibroblasts (e.g., meningeal fibroblasts). Two inhibitors of rodent scrapie protease-resistant PrP accumulation, pentosan polysulfate and a porphyrin compound, indium (III) meso-tetra(4-sulfonatophenyl)porphine chloride, potently blocked PrPCWD accumulation in MDBCWD cells. This demonstrates the utility of these cells in a rapid in vitro screening assay for PrPCWD inhibitors and suggests that these compounds have potential to be active against CWD in vivo.
Chronic wasting disease (CWD) is a transmissible spongiform encephalopathy (TSE) or prion disease similar to scrapie of sheep and goats, bovine spongiform encephalopathy (BSE) of cattle, and Creutzfeldt-Jakob disease (CJD) of humans. In North America, CWD is contagious among mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus), and Rocky Mountain elk (wapiti, Cervus elaphus nelsoni) (42). CWD can be transmitted via environmental contamination (27), although the natural mechanisms of spread are not well understood.
As is true for TSEs generally, CWD is characterized by the conversion of the host's normal protease-sensitive prion protein (PrPC or PrP-sen) to a partially protease-resistant form (generically “PrP-res” or specifically “PrPCWD”). In the wake of the BSE epidemic and the transmission of BSE to humans, CWD is of concern due to its apparent spread among free-ranging and farmed cervids in the United States and Canada. Indeed, CWD has the distinction of being the only TSE that is known to be endemic to locations with wild, free-ranging animal populations. It is not clear whether CWD poses a threat to humans or other species with potential exposure to CWD infectivity. Direct experimental transmissions to ferrets (2), cattle (17), and “cervidized” transgenic mice (5) have been reported. Cross-species cell-free prion protein (PrP) conversion assays have suggested that the rank order of susceptibilities to CWD is cervids > sheep > cattle > humans (33).
One important experimental model that has been lacking in CWD research is a CWD-infected cell line. Several scrapie-infected cell lines have been established, including the SMB (13), N2a (7, 32), GT1 (36), Rov9 (35), and fibroblast (41) cell lines. A CJD-infected human cell line was reported (24), but apparently this cell line was unstable and has been lost (M. Pocchiari, personal communication). Otherwise, we know of no cell lines chronically infected with BSE, CWD, or any human TSE not previously adapted to rodents. Such cell lines would be critical not only for basic studies of the cellular and molecular biology of these TSE strains but also for the screening of potential drugs and treatments. Numerous inhibitors of PrP-res accumulation have been identified initially using scrapie-infected cell lines, and many of these inhibitors have proven to have at least prophylactic activity against experimental scrapie in rodents. Nonetheless, striking TSE strain and species dependence has been observed with some antiscrapie compounds, and thus, it cannot be assumed that a compound that works against one TSE strain will be effective against others, such as CWD (10, 19, 23).
To help refine the search for possible treatments of CWD and to facilitate other aspects of CWD research, we have developed a cell line that is chronically infected with CWD. Using this cell line, we have identified the first two inhibitors of PrPCWD formation, pentosan polysulfate (PPS) and indium (III) meso-tetra (4-sulfonatophenyl)porphine chloride (In-TSP). PPS is a well known anti-TSE compound in other experimental models and is currently being tested to treat human CJD patients (39). In-TSP is a newly identified inhibitor and a member of the well-established cyclic tetrapyrrole class of anti-TSE compounds (11, 30, 31).
MATERIALS AND METHODS
Primary cultures from mule deer brain.
Primary cultures were derived from a hunter-harvested mule deer brain that was determined to be negative for CWD using an immunohistochemical assay (26). All of the following steps were done aseptically. Within 8 h of harvest, the thalamus and cerebellum of the brain were removed, and excess meninges and other extraneous tissues were discarded. Approximately 5 g of tissue was put into 100 ml medium 199 with Hank's salts (Sigma) supplemented with 10% fetal bovine serum (FBS), 200 U/ml penicillin, 200 μg/ml streptomycin, and 0.5 μg/ml amphotericin B (Sigma) and processed following the method of Cole and deVellis (15). Briefly, the tissue was rinsed with sterile calcium- and magnesium-free saline, dissociated mechanically by mincing, and pressed through tissue sieves first using a no. 60 mesh screen followed by a no. 100 mesh screen. The sieve was rinsed, and the filtrate was centrifuged for 8 min in a Beckman JS 5.2 rotor at 1 000 rpm (250 × g). The pellet was resuspended in 45 ml of high-glucose Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% gamma-irradiated FBS (DF) growth medium (Sigma), with 100 U/ml penicillin G, 100 μg/ml streptomycin sulfate, and 0.5 μg/ml amphotericin B. The cells were plated into two 75-cm2 Primaria flasks (BD Biosciences) at approximately 2 × 107 cells per flask and incubated at 37°C in a humidified 5% CO2 incubator. A day later, nonadherent cells were removed (>90% of the cells) and the growth medium was changed. At weekly intervals thereafter, half the medium was exchanged with fresh DF without antibiotics. Actively growing and surviving cells were grown using standard techniques (16) until there were ∼2 × 107 cells per flask; ∼5 × 106 of these cells, suspended in 7.5% dimethyl sulfoxide in DF, were frozen per vial in liquid nitrogen. For all cell passes in this study, flasks were rinsed once with 3 to 5 ml growth medium without FBS, followed by one rinse of 3 to 5 ml 1× trypsin-EDTA (Invitrogen), and then cells were dissociated by incubation for 5 to 10 min at 37°C in the residual trypsin-EDTA liquid and seeded into new flasks as specified.
Transformation of brain cells.
For transformation, cells were thawed rapidly at 37°C, diluted into 10 ml of 45% Dulbecco's modified Eagle's medium-45% OptiMEM (Invitrogen)-10% FBS (DOF), centrifuged at 500 × g, resuspended in the DOF growth medium, and plated into six-well plates at ∼7.5 × 105 cells/well. After 24 h, the cells were rinsed and the medium was replaced with OptiMEM without FBS. The cells were then transfected with an expression plasmid carrying the simian virus 40 genome (pBRSV, ATCC 45019) using Lipofectamine 2000 reagent (Invitrogen) per the manufacturer's instructions. The medium was changed 48 h later to OptiMEM and 10% FBS (OF), and the cells were grown for an additional 1 to 2 weeks, when clusters of cells showing loss of contact inhibition and an increased rate of cell division were selected using cloning cylinders. The selected transformed cell line (MDB) was expanded and frozen as described above.
Preparation of CWD brain microsomes.
The microsomes were prepared aseptically. A section of the medulla oblongata at the level of the obex from an experimentally CWD-infected and clinically affected mule deer was dissected using new tools. The tissue was immediately frozen, except for a portion that was used for histopathological examination. The latter was formalin fixed and subsequently determined to be CWD positive (26). For the microsomes used in the cell infections, 1.1 g of the frozen tissue was prepared using a previously described method (1), except that the low-speed pellet was not reextracted. The final volume of the microsome preparation was 0.55 ml in phosphate-buffered saline (PBS).
Infection of transformed brain cells with CWD microsomes.
A frozen vial of the MDB cells was thawed rapidly at 37°C, diluted in OF, centrifuged at 250 × g, resuspended in OF growth medium and seeded into a 25-cm2 flask. The specific lots of OptiMEM and FBS (certified grade; Invitrogen) used were independently pretested for the ability to sustain RML scrapie infection in mouse N2a cells for five passes as measured by detection of PrP-res by immunoblotting (data not shown). This pretesting procedure may be critical because, for unknown reasons, RML scrapie infections in mouse N2a cells can be rapidly lost in some lots of OptiMEM and occasional lots of Invitrogen-certified FBS (data not shown). For adaptation to the pretested OF, the cells were passed serially when near confluent at 1:10 dilution seven times. At pass 8, the cells were passed into 24-well plates at 1:8 and grown for 2 days prior to infection, when the cultures were ∼60% confluent. The medium was removed, and the cells were washed twice with prewarmed OptiMEM without FBS. Immediately before addition to the cells, the CWD microsome preparation was sonicated in a cup horn at maximum power for 1.5 min and then diluted in additional PBS to a total volume of 100 μl containing either 750 or 2,500 ng PrPCWD. Each of these microsome suspensions was mixed with 100 μl OptiMEM, added to triplicate wells of cells, and incubated at 37°C for 4 h, at which time 0.5 ml of OF with 100 units/ml penicillin and 100 μg/ml streptomycin was added. After 40 h, the cells were trypsinized and passed, as described above, into 25-cm2 flasks (designated pass 1) in OF. After 3 serial passes at ∼1:10, cultures originally exposed to both concentrations of CWD microsomes were cloned using dilution. Wells containing one or two colonies of cells were expanded, serially passed at ∼1:10, and analyzed for PrPCWD by immunoblot assay at the eighth pass, resulting in a screen of 51 colonies. The single PrPCWD-positive clone (MDBCWD) originated from a well with a single colony. Additional rounds of dilution subcloning increased the probability of clonality and generated the subclone MDBCWD2 (see Results).
DNA sequencing and determination of amino acid sequences.
The sequences of the PrP genes from samples of mule deer brain and brain-derived cell lines were determined from PCR-amplified DNA of open reading frames from genomic DNA as described using primer set 1 (33). DNA sequencing was done using an ABI 3700 DNA sequencer and the same primer set.
Antibody generation.
Mouse monoclonal antibody 12B2 was produced from PrP-knockout mice, generously provided by Charles Weissmann (6), by immunization with a synthetic peptide corresponding to ovine PrP amino acid residues 89 to 107 that was conjugated to Keyhole limpet hemocyanin as described previously (40). The R521 polyclonal antibody was raised against an ovine PrP peptide, residues 94 to 105 (40), the sequence of which is conserved in cervid PrP (33).
To detect the linear epitope specificities of 12B2, Pepscan analysis of solid-phase synthetic peptides bound as described previously was performed in an enzyme-linked immunosorbent assay-like system as previously described (38). A set of overlapping 15-mer peptides covering the complete amino acid sequence of ovine PrP were synthesized (GenBank accession number AJ000739). The epitope of 12B2 was found to require at least the residues 93WGQGG97, which are conserved in the mule deer and Syrian hamster PrP molecules analyzed in these studies.
Immunoblot assays for PrP-sen and PrPCWD in cell cultures.
For detection of PrP-sen, cells in a nearly confluent 25-cm2 flask were lysed with 1 ml 0.5% (wt/vol) Triton X-100, 0.5% (wt/vol) sodium deoxycholate, 50 mM Tris-HCl, pH 8.0 at 4°C, 5 mM EDTA, and 150 mM NaCl (LB) and centrifuged at 5,000 rpm in a microcentrifuge for 5 min to remove nuclei. Lysate supernatant proteins were methanol precipitated and solubilized in a detergent-phospholipid solution by sonication (3, 8). PrP was immunoprecipitated from the solution by incubation with 2 μl of R521 at 4°C overnight, precipitation of antibody-PrP complexes with 25 μl of a 50% vol/vol slurry of protein A-Sepharose CL-4B beads (Amersham-Pharmacia) in LB for 1 to 2 h at 4°C, and elution of the PrP from the beads by boiling for 5 min in 15 μl of 2× loading buffer containing 25 mM dithiothreitol (8). Samples were separated on 10% Bis-Tris NuPAGE sodium dodecyl sulfate (SDS) gels (Invitrogen), electroblotted onto a polyvinylidene difluoride membrane (Immobilon-P; Millipore), and immunostained with 12B2 at 0.34 μg/ml. The secondary antibody was an alkaline phosphatase-conjugated anti-mouse immunoglobulin G (Zymed) diluted 1:5,000. The immunoblot was developed with AttoPhos solution (Promega), air dried, and scanned on a STORM fluorescent detection system (Amersham). Relative band intensities were quantitated using ImageQuant software (Amersham).
For detection of PrPCWD, lysates were prepared as described above for the detection of PrP-sen and frozen at −20°C. After thawing rapidly at 37°C, 0.5-ml aliquots were treated with 10 μg/ml Proteinase K (PK; Calbiochem) at 37°C for 30 min, and then 2.5 μl of 0.1 M Pefabloc (Roche Diagnostics) was added to stop PK activity. After 5 min on ice, the samples were ultracentrifuged in a Beckman TL120.1 at 90,000 rpm (350,000 × g) for 60 min at 4°C. After thorough removal of the supernatants, pellets were air-dried for 5 min, and then 10 μl of SDS-polyacrylamide gel electrophoresis (PAGE)-dithiothreitol loading buffer was added, followed by sonication in a cup horn for 2 min at maximum power and then boiling for 5 min to solubilize.
Immunofluorescence of MDBCWD2 cell line.
MDBCWD2 cells were seeded at 1:10 in 35-mm glass-bottom culture dishes (MatTek Corp.) and grown to ∼50 to 60% confluence. All of the following steps were done at room temperature. Cells were fixed with 4% paraformaldehyde in PBS for 10 min and washed twice with PBS. Then the cells were permeabilized with 0.01% saponin in PBS for 5 min. To block nonspecific antibody binding, cells were incubated with PBS containing 10% normal goat serum and 0.01% saponin for 10 min. The following antibodies were diluted in blocking solution and added to separate dishes of cells: rabbit polyclonal antibody against human fibronectin (1:200; Dakocytomation), rabbit polyclonal anti-bovine glial fibrillary acidic protein (GFAP, 1:2,000; Dakocytomation), chicken polyclonal anti-bovine microtubule-associated protein 2 (MAP2, 1:5,000; EnCor Biotechnology). After 1 h of incubation, the cells were washed three times with PBS containing 0.01% saponin and incubated with an appropriate secondary antibody conjugated with Alexa Fluor 488 (1:1,000) for 1 h. Cells were washed three times with PBS containing 0.01% saponin and observed by confocal microscopy.
To immunostain PrP-res with 12B2, the same procedure was performed, except that the cells were incubated with 3 M guanidinium thiocyanate (GdnSCN) for 5 min between the permeabilization and blocking steps and the antibody was used at 5 μg/ml.
Assay of compounds for inhibition of PrPCWD.
Sodium PPS (Sigma) and In-TSP (Mid-Century, Inc.) were tested for their ability to block PrPCWD accumulation in MDBCWD2 cells. Cells were passed 1:10 in replicate 25-cm2 flasks with OF growth medium containing various concentrations of the test compounds. When nearly confluent, half of the cell lysate from each flask was assayed for PrPCWD by immunoblotting using the 12B2 antibody. Total proteins in inhibitor-treated and untreated cell lysates (without PK digestion) were compared by SDS-PAGE on 10% Bis-Tris NuPAGE SDS gels stained with GelCode blue (Pierce).
RESULTS
Transformed mule deer brain cells.
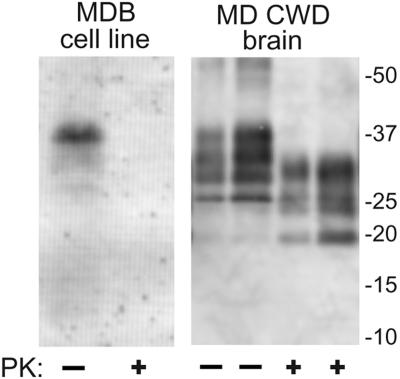
Primary brain cell cultures were derived from a wild, CWD-negative mule deer homozygous for the PrP genotype encoding residues G96M132S138S225Q226. The cells were transformed with a plasmid carrying the simian virus 40 genome. Clusters of cells exhibiting phenotypes of transformation, e.g., rapid cell division and a lack of contact inhibition, were selected and combined to give a transformed brain-derived cell line (MDB for _m_ule _d_eer _b_rain). Because PrP-sen expression is required for susceptibility to TSE infection, we analyzed the MDB cell cultures for the presence of PrP-sen. Immunoblot analysis of MDB cell lysates using monoclonal antibody 12B2 showed PrP-immunoreactive molecules migrating between 25- and 37-kDa markers that are typical of the multiple PrP-sen glycoforms (Fig. 1). As expected, these bands were fully sensitive to digestion with PK, and when not PK treated, migrated with a higher apparent molecular mass than bands from PK-treated PrPCWD isolated from CWD-infected mule deer (MD CWD) brain tissue (33).
FIG. 1.
Expression of PrP-sen by the transformed cell line derived from mule deer brain (MDB). The image is an immunoblot of PK-treated (+) and untreated (−) cell extracts using antibody 12B2 to detect PrP. PK-treated and untreated PrPCWD purified (34) from CWD-affected MD brain (20- and 60-ng samples of each) is shown for comparison. The migration of molecular mass standards in kilodaltons is shown on the right.
CWD infection of immortalized brain cell culture.
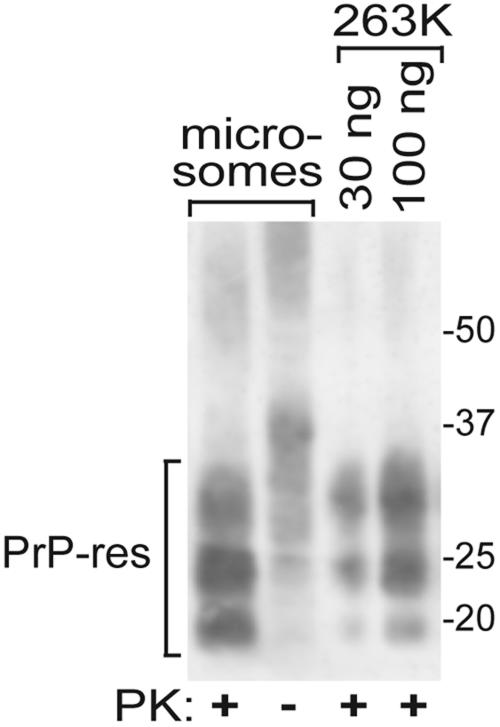
To obtain a source of CWD infectivity that was potentially more concentrated and less cytotoxic than crude brain homogenates, microsomes were prepared using tissue isolated from a section of the medulla oblongata (at the level of the obex) of an experimentally infected mule deer with clinical symptoms of CWD. Like the donor of the uninfected brain cells, this deer was homozygous for the PrP genotype encoding amino acid residues G96M132S138S225Q226. This deer was confirmed to be CWD positive by immunohistochemical staining of the brain tissue (26; data not shown) and immunoblotting of the microsome preparation for the detection of PrPCWD (Fig. 2). Treatment of the CWD microsomes with PK resulted in partial truncation of the PrPCWD molecules, which is typical of PrPCWD from animals infected with CWD (33) and other TSEs. The amount of PrPCWD in the microsome preparation was estimated by comparison to 263K hamster scrapie PrP-res standards.
FIG. 2.
PrPCWD in microsomes isolated from the CWD-affected MD brain used to infect the MDB cell line. Aliquots of the microsome preparations were incubated with PK (+) or without PK (−) and analyzed on an immunoblot using antibody 12B2 to detect PrP molecules. In this blot, the upper glycoform bands in the PK-treated microsome and 263K lanes were underrepresented relative to the other bands compared to similar samples used in experiments for which results are shown in other figures; this is likely artifactual due to differences in sample protein content or electroblotting conditions. The designated amounts of purified hamster (263K) PrP-res (34) were used to estimate the amounts of PrPCWD in the microsome preparations. The migration of molecular mass standards in kilodaltons is shown on the right.
The transformed mule deer brain cell culture was exposed to CWD brain microsomes containing approximately 25, 75, 250, 750, or 2,500 ng of PrPCWD and a buffer-only negative control and then passed serially. Immunoblot analyses of cells from each of these CWD-treated bulk cultures at the first pass did not reveal any detectable PrPCWD (data not shown). Considering that only a small subset of cells may have become infected, cells exposed to microsome preparations containing 750 or 2,500 ng PrPCWD were cloned by dilution to isolate and expand possible infected cells. Out of 51 clones analyzed, only one, designated MDBCWD, produced detectable PrPCWD after expansion from a single colony and seven serial passages (Fig. 3). Figure 3A shows a subset of eight of these primary clones, one of which was PrPCWD positive. The positive MDBCWD clone was isolated from the cell culture exposed to microsomes containing 2,500 ng of PrPCWD. As expected, in the MDBCWD clone, the characteristic PrP glycoforms were reduced in size when treated with PK due to the truncation from the amino termini of the PrP molecules (Fig. 3B). The PK-treated PrP glycoform pattern from the MDBCWD cells was clearly distinct from those of MD CWD brain and scrapie-infected N2a cells (RML), with higher molecular masses, most notably for the upper diglycosylated form. These bands were also recognized on immunoblots by R505, a distinct polyclonal anti-PrP antiserum (33; data not shown). Since glycan biosynthesis can vary significantly between cell types, the higher molecular masses of the upper glycosylated bands in both the PK-treated and untreated MDBCWD samples likely reflects differences in the size and nature of the glycans added to PrP molecules in these cells relative to MD brain tissue and N2a cells. The unique PrPCWD glycoform pattern provides evidence that MDBCWD cells were not derived from inadvertent contamination of MDB cultures with scrapie-infected N2a cells or any other scrapie-infected cell line in our facility. Furthermore, genotyping of the MDBCWD cell line confirmed that like their mule deer source, their PrP genes encode the G96M132S138S225Q226 cervid PrP sequence. MDBCWD cells from multiple passes between 7 and 32 (the latest pass tested as of this writing) were clearly positive for PrPCWD, demonstrating persistent CWD infection in this cell line.
FIG. 3.
Immunoblot analysis of mule deer brain cell clones after exposure to CWD microsomes. The MDB-transformed cell line was incubated with microsomes prepared from a CWD-positive mule deer brain. At the third pass, selected lines (see Materials and Methods) were dilution cloned and expanded. At the eighth pass after cloning, cell lysates were analyzed for PrPCWD. (A) PK-treated lysates of a PrPCWD-positive clone (MDBCWD) and 7 representative negative clones. Also shown are PK-treated (+) samples of PrP-res purified from scrapie-infected hamster brain (263K br), CWD mule deer brain (MD CWD br), and a mouse N2a cell line infected with the RML scrapie strain (RML) to compare the glycoform patterns and mass differences among these different TSE strains. (B) Comparison of PK sensitivities of PrP present in lysates of MDBCWD cells versus mock-infected cultures of the transformed MDB cells from which MDBCWD was derived. For comparison, RML cell extracts (PK treated) are shown. The immunoblots shown in panels A and B were developed using the 12B2 antibody to detect PrP. The migration of molecular mass standards in kilodaltons is shown on the sides. −, not PK treated.
Clonal analysis of the MDBCWD cell line.
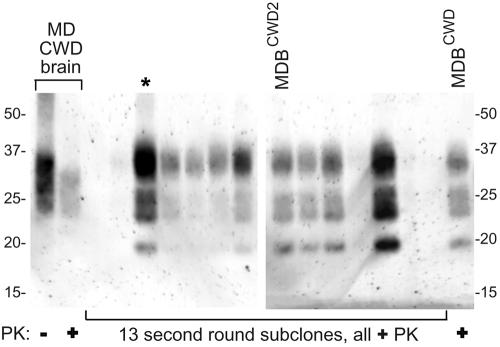
To increase the likelihood that the MDBCWD cell line was clonal, a second round of dilution subcloning was done at the ninth pass after the first round. Immunoblot analyses revealed that 20 of 30 of these MDBCWD subclones were positive for PrPCWD and that the amount of PrP-res produced in the PrPCWD-positive subclones was variable (Fig. 4). One of the PrPCWD-positive subclones, designated MDBCWD2, was selected for cell lineage and inhibitor studies. In addition, a third round of dilution subcloning was done to a PrPCWD-positive subclone at the fifth pass after the second round. From this subcloning, 11 viable clones were obtained, 8 of which were PrPCWD positive (data not shown). The PrPCWD signal among positive subclones of the third round was less variable compared to second-round subclones. These results provided evidence that PrPCWD levels varied between individual cells in apparently clonal MDBCWD cell lines, even though these lines maintained relatively consistent PrPCWD production overall through many in vitro passes.
FIG. 4.
Immunoblot analysis of MDBCWD subclones from the second-round dilution subcloning. Subclone lysates were PK treated for detection of PrPCWD. The original MDBCWD clone is shown in the last lane on the right. This blot shows 13 representatives of the 30 total second-round subclones that were analyzed. PrPCWD samples in the lanes labeled “MD CWD brain” were purified from a CWD-affected mule deer brain and were PK digested (+) or not (−) (34). The lane labeled “MDBCWD2” represents the subclone used in the inhibitor and cell lineage studies. The lane marked with an asterisk contained the subclone used for the third round of dilution subcloning. The left and right panels are from different blots, so it is unclear whether the subtle differences in the relative glycoform band intensities between the clones in these two panels are real or artifactual. The antibody 12B2 was used to detect PrP. The migration of molecular mass standards in kilodaltons is shown on the sides of both panels.
Analysis of PrPCWD by immunofluorescence.
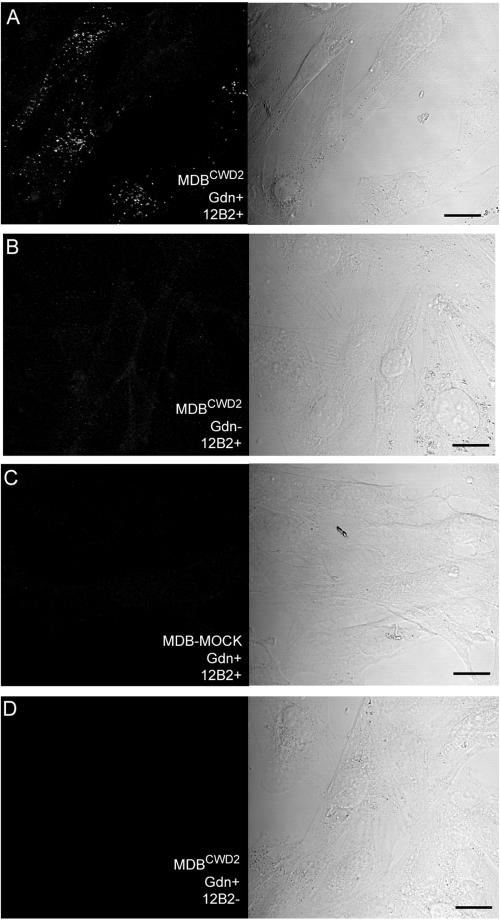
The PrPCWD produced by the MDBCWD2 cell line was analyzed using immunofluorescence staining (Fig. 5). For in situ staining of PrPCWD, MDBCWD2 cells were fixed and treated with GdnSCN; this denaturing treatment was necessary to expose the 12B2 epitope (residues 93 to 97), as has been observed with other conformationally occluded epitopes located in this region of PrP-res molecules (25, 29, 37). About half of the cells had extensive punctate intracellular structures that were immunostained with 12B2 (Fig. 5A). Several observations were consistent with the punctate staining being due to the presence of PrPCWD. When the GdnSCN treatment was omitted, only a few faintly stained punctate structures were observed (Fig. 5B). No punctate staining was observed in mock-infected transformed mule deer brain cell cultures (Fig. 5C, MDB-MOCK). When the primary antibody was omitted, no staining was observed in the MDBCWD2 cell line (Fig. 5D). The PrPCWD accumulation pattern observed in MDBCWD2 cells suggested that PrP-res accumulates in intracellular compartments like those observed in other types of TSE-infected cell lines (25, 37).
FIG. 5.
Immunofluorescence staining of PrPCWD in MDBCWD2 cells. MDBCWD2 cells were stained with anti-PrP antibody 12B2 after treatment with GdnSCN (Gdn+, panel A, left). The extensive punctate structures are typical of PrP-res staining. When GdnSCN treatment was omitted (Gdn−), only a few faint punctate structures were observed (panel B, left). Only very faint fluorescence was apparent in mock-infected cells (MDB-MOCK, panel C, left). The MDBCWD2 cells stained only with secondary antibody (panel D, left) had no apparent fluorescence. All images are reconstructions of Z-series acquired by confocal microscopy with an interval of 0.54 μm. The panels on the right are corresponding differential interference contrast images to show the cell positions. Bar, 20 μm.
Lineage characterization of the MDBCWD2 cell line.
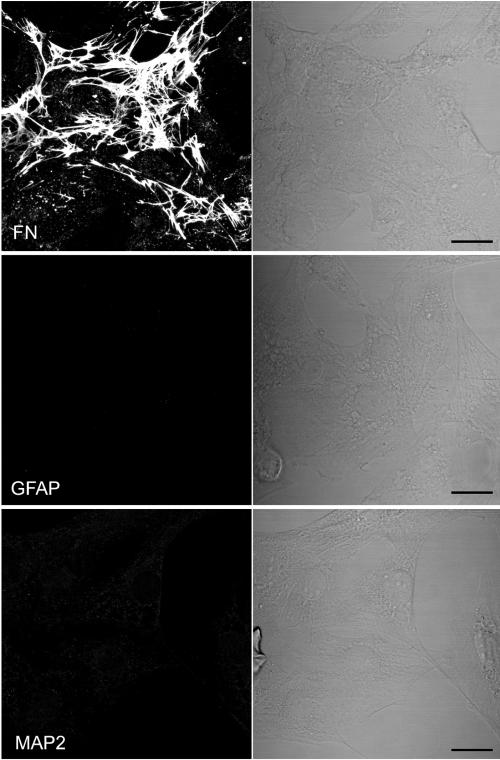
To assess the lineage of the MDBCWD2 cell line, fixed and permeabilized cells were stained with antibodies to specific cell type marker proteins. Due to the lack of antibodies raised specifically against mule deer cell marker proteins, antibodies against proteins of other species were used. Antibodies raised against human fibronectin showed extensive immunofluorescent staining of extracellular fibrils and, to a lesser extent, intracellular punctate deposits (Fig. 6). These staining data are consistent with a fibroblast-like origin for the MDBCWD; however, other brain cells such as astrocytes can also express fibronectin (18). However, no staining was seen with antibodies against bovine GFAP, an activated astrocyte marker. In addition, little staining was seen with antibodies against bovine MAP2, a neuronal marker. The reactivity of these human and bovine antibodies to their corresponding mule deer antigens was confirmed with formalin-fixed and paraffin-embedded sections of mule deer brain tissue and similarly prepared MDBCWD2 cell pellets (data not shown). Therefore, these data support the conclusion that the MDBCWD2 is a fibroblast-like cell line.
FIG. 6.
Immunostaining of MDBCWD2 cells. MDBCWD2 cells were stained with antibodies against human fibronectin (FN), GFAP, and MAP2, which are markers for fibroblast cells, astrocytes, and neuronal cells, respectively. Reconstructed confocal microscopic images of Z-series acquired with an interval of 0.54 μm are shown in the left panel, and differential interference contrast images are shown in the right panel. Bar, 20 μm.
Inhibition of PrPCWD accumulation in MDBCWD2 cells.
To investigate the utility of MDBCWD2 cells for screening anti-CWD compounds, we tested the ability of two inhibitors of rodent PrP-res accumulation, sodium PPS (9) and In-TSP (W. S. Caughey, E. Olsen, D. A. Kocisko, B. Caughey, unpublished data) to block PrPCWD accumulation in these CWD-infected cells. PPS and In-TSP blocked PrPCWD accumulation by >90% with IC50s (concentrations giving half-maximal inhibition) of ∼10 ng/ml (∼3 nM, based on an average, but heterogeneous, molecular mass of ∼3,800 Da) and <0.3 μM, respectively (Fig. 7A and B). This IC50 for PPS is similar to the low nanomolar IC50 observed with RML-infected N2a cells (9). At effective inhibitory concentrations, these inhibitors appeared to be largely selective for PrPCWD formation because they did not substantially alter the overall profile of cellular proteins (Fig. 7C) or PrP-sen levels detected by immunoblotting (Fig. 7D). When either 1 μg/ml PPS or 1 μM In-TSP was added directly to untreated MDBCWD2 lysates immediately before the PK digestion step, the PrP-res detected was similar to that seen from untreated control lysates (data not shown); thus, these compounds did not artifactually alter the recovery or detection of PrPCWD from cell lysates. No evidence of cytotoxicity as reflected by the rate of cell division and the gross morphology of cells was seen at ≤3 μM for In-TSP. PPS started to show minor cytotoxicity at 1 μg/ml, i.e., ∼100-fold higher than the IC50 for PrPCWD inhibition. These results showed that PPS and In-TSP can potently block PrPCWD accumulation in MDBCWD cells at concentrations that are far below those required to affect cell growth or PrP-sen biosynthesis.
FIG. 7.
Effects of PPS and In-TSP on PrPCWD biosynthesis in MDBCWD2 cells. Upon plating at 1:10 dilution, cells were treated with the designated amounts of the compounds and grown until near confluence (∼4 days). (A) Cell lysates were analyzed for PrPCWD by immunoblotting using antibody 12B2. The migration of molecular mass standards in kilodaltons is shown on the sides. (B) Mean values and standard deviations of relative PrPCWD band intensities (as proportions of untreated controls) from multiple experiments, like those shown in panel A. Three to six replicates of each concentration of inhibitor were tested. (C) GelCode blue-stained SDS-PAGE gels of equivalent aliquots of lysates (prior to PK treatment) from PPS-treated, In-TSP-treated, or untreated (Cont) cells. (D) Immunoblot of PrP immunoprecipitated from lysates (without PK treatment) of control (Cont), PPS-treated, and In-TSP-treated cells.
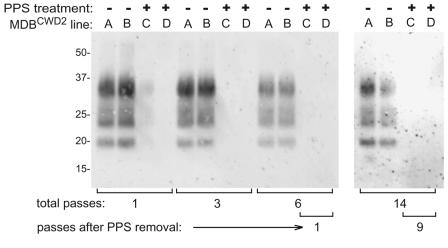
To test whether the PPS effect on PrPCWD was reversible and also to attempt to cure the MDBCWD cell line of the CWD infection, duplicate lines of MDBCWD2 cells were serially passed 1:10 five times in the presence of 0.3 μg/ml PPS and then subsequently passed without PPS (Fig. 8). After the first pass in PPS, the immunoblot-detectable PrPCWD was decreased to <10% of untreated duplicate parallel flasks, and after three passes, PrPCWD was no longer detectable. The PPS-treated lines were tested for PrPCWD at the first and ninth passes after removal of the PPS, and no signal was detected. Parallel untreated flasks of MDBCWD2 cells showed no observable loss of PrPCWD content throughout the series of passages.
FIG. 8.
Lack of recovery of PrPCWD after PPS treatment of MDBCWD2 cells. Duplicate lines of MDBCWD2 cells were passed at ∼1:10 dilution in medium for the designated number of passes (A, B) or in medium containing 0.3 μg/ml PPS for 5 passes and unsupplemented medium thereafter (C, D). One-half nearly confluent T-25 flask equivalents of PK-treated cell lysates were analyzed for PrPCWD content by immunoblotting as described in Materials and Methods. +, PPS treated; −, not PPS treated.
DISCUSSION
Expansion of the known geographic distribution of CWD, whether due to the spread of the disease or increased surveillance, makes it important to develop screens for compounds that might prevent CWD spread among cervid populations and, potentially, the transmission from cervids to other species. As exemplified by the results of experiments with PPS and In-TSP shown in Fig. 7, the MDBCWD cell line should be useful in the search for anti-CWD compounds. When administered prophylactically, pentosan polysulfate and certain porphyrins have been especially effective against intraperitoneal infections of rodent-adapted scrapie (30, 31). Those previous results and our observations that pentosan polysulfate and In-TSP are effective blockers of PrPCWD accumulation in the MDBCWD cell line provide evidence that these or related compounds might have activity against CWD in vivo. Thus, it is tempting to speculate that PPS or In-TSP may help prevent the spread of CWD on game farms and in the wild, where most transmissions would be expected to occur via peripheral routes of infection. In addition, our findings attest to the broad inhibitory activities of both sulfated glycans and porphyrins, which differ from some other inhibitors that have strain and/or species specificities (10, 22, 23).
The CWD infection in MDBCWD cells appears to be persistent because PrPCWD production has been stable and robust through 32 serial passes despite the fact that, in the first dilution cloning, 33% of the subclones were apparently negative for PrPCWD. The reason for the generation of PrPCWD-negative subclones from the original MDBCWD culture is unclear, although similar observations of cell-to-cell differences in levels of PrP-res formation have been noted in other cell lines (21, 28, 41). It is possible that the cell line obtained in the first dilution cloning was not derived from a single-cell clone. This potential for lack of clonality should have been reduced in subsequent dilution cloning steps. Even if the initial MDBCWD line was in fact clonal, it is possible that a certain percentage of daughter cells became less able to maintain the infection and produce PrPCWD, as was apparent in the variable PrPCWD band intensities from the secondary clones (Fig. 4). This might be due to genetic instability (a common feature in transformed cell lines), to unequal distribution of PrPCWD between daughter cells after division, to destabilizing effects of the dilution cloning itself (in which cells are forced to survive and proliferate at extremely low densities), or perhaps, to toxicity and death if cells accumulate too much PrP-res.
The probable fibroblast-like origin of MDBCWD cells is not surprising because fibroblast-like cells have been shown to be capable of maintaining chronic scrapie infections (12, 14, 41). In brain-derived cultures, fibroblast-like cells are often derived from the meninges. This is interesting to consider in the context of iatrogenic transmissions of CJD that have occurred via dura mater transplants. Dura mater contains fibroblasts, and in this study, we have shown that fibroblast-like cells derived from brain tissue are susceptible to infection by CWD. If these cells are susceptible to TSE infection in vivo, they could represent a direct and integral source of CJD contamination of dura mater taken from CJD-infected humans.
The MDBCWD cell line is the first to be persistently infected with CWD. The G96M132S138S225Q226 PrP genotype of the mule deer donors of both the MDB cell line and the CWD infectivity is by far the most common in both mule deer and white-tailed deer (∼95% in wild populations analyzed) (4, 20). Moreover, this allelic type is probably the most susceptible to natural CWD infection (M. Miller, unpublished observations). Thus, MDBCWD cells appear to be an apt experimental model of CWD infection in Odocoileus spp. and should facilitate in vitro experimentation into the cell biology, molecular biology, biochemistry, and strain- and species-dependent characteristics of this TSE disease.
Acknowledgments
This research was partially supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases (NIAID), the US DOD Prion Interagency Transfer NP020114, the Colorado Division of Wildlife, and the University of Wyoming. The production of monoclonal antibody 12B2 was funded by the Dutch Ministry of Agriculture, Nature Management, and Food Quality.
We thank Bruce Chesebro and Valerie Sim for critical reading of the manuscript. We thank C. T. Larsen and P. Jaeger for laboratory assistance at the Colorado Division of Wildlife, Kent Barbian of the NIAID/RML Genomics Core Facility for DNA sequencing, and Neil Anderson and the Montana Division of Fish, Wildlife, and Parks for generously supplying mule deer brain samples used for the analysis of cell lineage. Karel Riepema, Esther de Jong, and Jorg Jacobs are acknowledged for skillful generation and characterization of antibody 12B2.
Footnotes
‡
We dedicate this paper to the memory of Elizabeth S. Williams, a pioneer of CWD research.
REFERENCES
- 1.Baron, G. S., K. Wehrly, D. W. Dorward, B. Chesebro, and B. Caughey. 2002. Conversion of raft associated prion protein to the protease-resistant state requires insertion of PrP-res (PrP(Sc)) into contiguous membranes. EMBO J. 21**:**1031-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartz, J. C., R. F. Marsh, D. I. McKenzie, and J. M. Aiken. 1998. The host range of chronic wasting disease is altered on passage in ferrets. Virology 251**:**297-301. [DOI] [PubMed] [Google Scholar]
- 3.Borchelt, D. R., M. Scott, A. Taraboulos, N. Stahl, and S. B. Prusiner. 1990. Scrapie and cellular prion proteins differ in the kinetics of synthesis and topology in cultured cells. J. Cell Biol. 110**:**743-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brayton, K. A., K. I. O'Rourke, A. K. Lyda, M. W. Miller, and D. P. Knowles. 2004. A processed pseudogene contributes to apparent mule deer prion gene heterogeneity. Gene 326**:**167-173. [DOI] [PubMed] [Google Scholar]
- 5.Browning, S. R., G. L. Mason, T. Seward, M. Green, G. A. Eliason, C. Mathiason, M. W. Miller, E. S. Williams, E. Hoover, and G. C. Telling. 2004. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J. Virol. 78**:**13345-13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bueler, H., M. Fischer, Y. Lang, H. Bluethmann, H.-P. Lipp, S. J. DeArmond, S. B. Prusiner, M. Aguet, and C. Weissmann. 1992. Normal development and behavior of mice lacking the neuronal cell-surface PrP protein. Nature 356**:**577-582. [DOI] [PubMed] [Google Scholar]
- 7.Butler, D. A., M. R. D. Scott, J. M. Bockman, D. R. Borchelt, A. Taraboulos, K. K. Hsiao, D. T. Kingsbury, and S. B. Prusiner. 1988. Scrapie-infected murine neuroblastoma cells produce protease-resistant prion proteins. J. Virol. 62**:**1558-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caughey, B., and G. J. Raymond. 1991. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J. Biol. Chem. 266**:**18217-18223. [PubMed] [Google Scholar]
- 9.Caughey, B., and G. J. Raymond. 1993. Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J. Virol. 67**:**643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caughey, B., L. D. Raymond, G. J. Raymond, L. Maxson, J. Silveira, and G. S. Baron. 2003. Inhibition of protease-resistant prion protein accumulation in vitro by curcumin. J. Virol. 77**:**5499-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caughey, W. S., L. D. Raymond, M. Horiuchi, and B. Caughey. 1998. Inhibition of protease-resistant prion protein formation by porphyrins and phthalocyanines. Proc. Natl. Acad. Sci. USA 95**:**12117-12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherednichenko, Y. N., L. L. Fadeeva, and V. K. Sologub. 1985. Antigenic changes in the cells latently infected with the scrapie agent. Acta Virol. 29**:**515. [PubMed] [Google Scholar]
- 13.Clarke, M. C., and D. A. Haig. 1970. Evidence for the multiplication of scrapie agent in cell culture. Nature 225**:**100-101. [DOI] [PubMed] [Google Scholar]
- 14.Clarke, M. C., and G. C. Millson. 1976. Infection of a cell line of mouse L fibroblasts with scrapie agent. Nature 261**:**144-145. [DOI] [PubMed] [Google Scholar]
- 15.Cole, R., and J. deVellis. 1989. A dissection and tissue culture manual of the nervous system. Alan Liss, New York, N.Y.
- 16.Freshney, R. I. 2005. Culture of animal cells. John Wiley & Sons, Hoboken, N.J.
- 17.Hamir, A. N., R. C. Cutlip, J. M. Miller, E. S. Williams, M. J. Stack, M. W. Miller, K. I. O'Rourke, and M. J. Chaplin. 2001. Preliminary findings on the experimental transmission of chronic wasting disease agent of mule deer to cattle. J. Vet. Diagn. Investig. 13**:**91-96. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch, S., and M. Bahr. 1999. Immunocytochemical characterization of reactive optic nerve astrocytes and meningeal cells. Glia 26**:**36-46. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa, K., K. Doh-ura, Y. Kudo, N. Nishida, I. Murakami-Kubo, Y. Ando, T. Sawada, and T. Iwaki. 2004. Amyloid imaging probes are useful for detection of prion plaques and treatment of transmissible spongiform encephalopathies. J. Gen. Virol. 85**:**1785-1790. [DOI] [PubMed] [Google Scholar]
- 20.Jewell, J. E., M. M. Conner, L. L. Wolfe, M. W. Miller, and E. S. Williams. 2005. Low frequency of PrP genotype 225SF among free-ranging mule deer (Odocoileus hemionus) with chronic wasting disease. J. Gen. Virol. 86**:**2127-2134. [DOI] [PubMed] [Google Scholar]
- 21.Klohn, P. C., L. Stoltze, E. Flechsig, M. Enari, and C. Weissmann. 2003. A quantitative, highly sensitive cell-based infectivity assay for mouse scrapie prions. Proc. Natl. Acad. Sci. USA 100**:**11666-11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kocisko, D. A., G. S. Baron, R. Rubenstein, J. Chen, S. Kuizon, and B. Caughey. 2003. New inhibitors of scrapie-associated prion protein formation in a library of 2000 drugs and natural products. J. Virol. 77**:**10288-10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kocisko, D. A., A. L. Engel, K. Harbuck, K. M. Arnold, E. A. Olsen, L. D. Raymond, D. Vilette, and B. Caughey. 2005. Comparison of protease-resistant prion protein inhibitors in cell cultures infected with two strains of mouse and sheep scrapie. Neurosci. Lett. 388**:**106-111. [DOI] [PubMed] [Google Scholar]
- 24.Ladogana, A., Q. Liu, Y. G. Xi, and M. Pocchiari. 1995. Proteinase-resistant protein in human neuroblastoma cells infected with brain material from Creutzfeldt-Jakob patient. Lancet 345**:**594-595. [DOI] [PubMed] [Google Scholar]
- 25.Magalhaes, A. C., G. S. Baron, K. S. Lee, O. Steele-Mortimer, D. Dorward, M. A. Prado, and B. Caughey. 2005. Uptake and neuritic transport of scrapie prion protein coincident with infection of neuronal cells. J. Neurosci. 25**:**5207-5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, M. W., and E. S. Williams. 2002. Detection of PrP(CWD) in mule deer by immunohistochemistry of lymphoid tissues. Vet. Rec. 151**:**610-612. [DOI] [PubMed] [Google Scholar]
- 27.Miller, M. W., and E. S. Williams. 2003. Prion disease: horizontal prion transmission in mule deer. Nature 425**:**35-36. [DOI] [PubMed] [Google Scholar]
- 28.Nishida, N., D. A. Harris, D. Vilette, H. Laude, Y. Frobert, J. Grassi, D. Casanova, O. Milhavet, and S. Lehmann. 2000. Successful transmission of three mouse-adapted scrapie strains to murine neuroblastoma cell lines overexpressing wild-type mouse prion protein. J. Virol. 74**:**320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peretz, D., R. A. Williamson, Y. Matsunaga, H. Serban, C. Pinilla, R. B. Bastidas, R. Rozenshteyn, T. L. James, R. A. Houghten, F. E. Cohen, S. B. Prusiner, and D. R. Burton. 1997. A conformational transition at the N terminus of the prion protein features in formation of the scrapie isoform. J. Mol. Biol. 273**:**614-622. [DOI] [PubMed] [Google Scholar]
- 30.Priola, S. A., A. Raines, and W. Caughey. 2003. Prophylactic and therapeutic effects of phthalocyanine tetrasulfonate in scrapie-infected mice. J. Infect. Dis. 188**:**699-705. [DOI] [PubMed] [Google Scholar]
- 31.Priola, S. A., A. Raines, and W. S. Caughey. 2000. Porphyrin and phthalocyanine anti-scrapie compounds. Science 287**:**1503-1506. [DOI] [PubMed] [Google Scholar]
- 32.Race, R. E., L. H. Fadness, and B. Chesebro. 1987. Characterization of scrapie infection in mouse neuroblastoma cells. J. Gen. Virol. 68**:**1391-1399. [DOI] [PubMed] [Google Scholar]
- 33.Raymond, G. J., A. Bossers, L. D. Raymond, K. I. O'Rourke, L. E. McHolland, P. K. Bryant III, M. W. Miller, E. S. Williams, M. Smits, and B. Caughey. 2000. Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO J. 19**:**4425-4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raymond, G. J., and J. Chabry. 2004. Purification of the pathological isoform of prion protein (PrPSc or PrPres) from transmissible spongiform encephalopathy-affected brain tissue, p. 16-26. In S. Lehmann and J. Grassi (ed.), Techniques in prion research. Birkhauser Verlag, Basel, Switzerland.
- 35.Sabuncu, E., S. Petit, A. Le Dur, L. T. Lan, J. L. Vilotte, H. Laude, and D. Vilette. 2003. PrP polymorphisms tightly control sheep prion replication in cultured cells. J. Virol. 77**:**2696-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schatzl, H. M., L. Laszlo, D. M. Holtzman, J. Tatzelt, S. J. DeArmond, R. I. Weiner, W. C. Mobley, and S. B. Prusiner. 1997. A hypothalamic neuronal cell line persistently infected with scrapie prions exhibits apoptosis. J. Virol. 71**:**8821-8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taraboulos, A., D. Serban, and S. B. Prusiner. 1990. Scrapie prion proteins accumulate in the cytoplasm of persistently infected cultured cells. J. Cell Biol. 110**:**2117-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thuring, C. M., L. J. van Keulen, J. P. Langeveld, M. E. Vromans, F. G. van Zijderveld, and T. Sweeney. 2005. Immunohistochemical distinction between preclinical bovine spongiform encephalopathy and scrapie infection in sheep. J. Comp. Pathol. 132**:**59-69. [DOI] [PubMed] [Google Scholar]
- 39.Todd, N. V., J. Morrow, K. Doh-ura, S. Dealler, S. O'Hare, P. Farling, M. Duddy, and N. G. Rainov. 2005. Cerebroventricular infusion of pentosan polysulphate in human variant Creutzfeldt-Jakob disease. J. Infect. 50**:**394-396. [DOI] [PubMed] [Google Scholar]
- 40.van Keulen, L. J. M., B. E. C. Schreuder, R. H. Meloen, M. Poelen-Van Den Berg, G. Mooij-Harkes, M. E. W. Vromans, and J. P. M. Langeveld. 1995. Immunohistochemical detection and localization of prion protein in brain tissue of sheep with natural scrapie. Vet. Pathol. 32**:**299-308. [DOI] [PubMed] [Google Scholar]
- 41.Vorberg, I., A. Raines, B. Story, and S. A. Priola. 2004. Susceptibility of common fibroblast cell lines to transmissible spongiform encephalopathy agents. J. Infect. Dis. 189**:**431-439. [DOI] [PubMed] [Google Scholar]
- 42.Williams, E. S., and S. Young. 1980. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J. Wildl. Dis. 16**:**89-98. [DOI] [PubMed] [Google Scholar]