Tollip Regulates Proinflammatory Responses to Interleukin-1 and Lipopolysaccharide (original) (raw)
Abstract
Activation of interleukin-1 (IL-1) receptor (IL-1R), Toll-like receptor 2 (TLR2), and TLR4 triggers NF-κB and mitogen-activated protein kinase (MAPK)-dependent signaling, thereby initiating immune responses. Tollip has been implicated as a negative regulator of NF-κB signaling triggered by these receptors in in vitro studies. Here, deficient mice were used to determine the physiological contribution of Tollip to immunity. NF-κB, as well as MAPK, signaling appeared normal in Tollip-deficient cells stimulated with IL-1β or the TLR4 ligand lipopolysaccharide (LPS). Similarly, IL-1β- and TLR-driven activation of dendritic cells and lymphocytes was indistinguishable from wild-type cells. In contrast, the production of the proinflammatory cytokines, IL-6 and tumor necrosis factor alpha was significantly reduced after IL-1β and LPS treatment at low doses but not at lethal doses of LPS. Tollip therefore controls the magnitude of inflammatory cytokine production in response to IL-1β and LPS.
Toll-like receptor 2 (TLR2), TLR4, and interleukin-1 (IL-1) receptor type I (IL-1RI) are key components of the innate immune system. Their activation in response to microbial infection and inflammation triggers NF-κB and mitogen-activated protein kinase (MAPK) signaling and culminates in the induction of host defense genes, including the production of numerous cytokines, chemokines, adhesion molecules, and enzymes (e.g., cycloxygenase and nitric oxide synthase). Stimulation through TLRs is essential for the early identification and eradication of infectious microorganisms. However, excessive and prolonged activation of innate immunity is harmful to the host and may even be fatal (17). Signaling must therefore be tightly controlled to ensure a beneficial response with the appropriate magnitude and duration. Multiple mechanisms exist (for recent reviews see references 1 and 18) to halt IL-1RI and TLR signaling pathways, including the activity of several inducible signaling suppressors (i.e., MyD88s and IRAK-M).
Overexpression of Tollip impairs IL-1RI-, TLR2-, and TLR4-triggered NF-κB and JNK signaling pathways (2, 3, 9, 21). Tollip is a small protein containing an internal C2 domain and a C-terminal CUE domain that was identified in a two-hybrid screen using the cytoplasmic tail of IL-1R accessory protein (IL-1RAcP) as bait (4). Tollip binds the activated IL-1RI complex, as well as TLR2 and TLR4 complexes (2, 21). Tollip also interacts with IL-1R-associated kinase 1 (IRAK-1) prior to stimulation and suppresses IRAK-1's kinase activity (4, 21). This has led to the concept that a Tollip-mediated pathway is required to maintain immune cells in a quiescent state in the absence of infection and facilitate the termination of IL-1R- and TLR-induced signaling during inflammation via suppression of IRAK-1's activity (3, 21).
In this regard Tollip resembles the negative regulators MyD88s and IRAK-M, which also bind to IRAK-1 and halt signaling by blocking recruitment of IRAK-4 and thereby the phosphorylation and/or activation of IRAK-1 (4, 7). IRAK-M-null mice have been generated showing that IRAK-M is important for suppression of IL-1β and various TLR ligands (7). This suggests that Tollip (or MyD88s) does not act interchangeably with IRAK-M but that Tollip may function as a suppressor in a cooperative manner and/or in specific cellular contexts (i.e., specific cell types or under specific conditions of stimulation). Unlike IRAK-M/MyD88s or indeed many other inflammation suppressors (reference 18 and references therein), Tollip is constitutively expressed (4, 11, 12). Its presence in unstimulated cells, together with several newly identified binding partners (discussed below) also suggests the possibility that Tollip's function is of a more multifaceted nature. To examine these possibilities, we generated Tollip-deficient mice by gene-targeting. Here we report the initial characterization of Tollip-deficient cells and mice and, importantly, that Tollip deficiency results in attenuated responses to the proinflammatory cytokines, IL-6 and tumor necrosis factor alpha (TNF-α).
MATERIALS AND METHODS
Tollip mice generation and screening.
Genomic DNA containing the Tollip gene was isolated from a bacterial artificial chromosome (BAC) clone containing 129J genomic DNA. The targeting vector was constructed by replacing a 11.5-kb genomic fragment encoding the amino-terminal region of Tollip with a neomycin resistance cassette (neo). The targeting vector was given to Anteq (Switzerland) for the generation of Tollip-deficient mice. Briefly, homologous recombinant G418-resistant embryonic stem cells were verified by Southern blotting, and two different clones were microinjected into blastocytes of SV129 mice. Chimeric mice were mated to obtain heterozygous F1 progenies, which were intercrossed to obtain Tollip−/− mice. The Tollip−/− homozygous progenies were then backcrossed four times into C57BL/6, and all experiments were performed on age-matched littermates. Tollip−/− mice were screened by Western blot analysis with anti-Tollip antibodies or by PCR on genomic DNA with the primers 5′-GGATTTGGGATTCATCAGAGGC-3′ and 5′-ACAAGAGTGGGACGGAAACTTC-3′ for the Tollip gene and the primer GGAGAGGCTATTCGGCTATG for the neomycin gene. MyD88−/− mice were kindly provided by S. Akira (Japan).
Reagents and antibodies.
Antibodies to mouse Tollip were made by immunizing a rabbit with GST-Tollip fusion protein (Apotech, Switzerland**)**. Antibodies to IκBα and IRAK1 were purchased from Santa Cruz. Phospho-Erk1/2 or tubulin comes from Sigma. Anti-p38, anti-JNK, anti-phospho-IκBα were purchased from Cell Signaling. Anti-phospho-specific JNK and p38 were obtained from Biosource. Anti-ISG15 antibody was kindly provided by D. Lenschow (St. Louis, Missouri). Murine IL-1β, lipopolysaccharide (LPS; Escherichia coli O111:B4 or O55:B5), CpG-ODN (5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′), PGN, and poly(I-C) were purchased from Sigma. Murine IL-18, IL-2, and IL-12 come from MBL. Measurements of cytokines or RANTES were performed on serially diluted samples of cell supernatants or sera in triplicate by using enzyme-linked immunosorbent assay (ELISA) kits for murine IL-6, TNF-α, RANTES (R&D Systems), IL-12p40, and IFN-γ (BD Pharmingen) according to the manufacturers' instructions. Statistical analysis for cytokine values were performed by using the Student t test.
Primary cell generation and cell culture.
Mouse embryonic fibroblasts (MEF) were prepared from embryonic day 14 (E14) embryos. Briefly, minced embryonic issues were treated with trypsin and then cultured in Dulbecco modified Eagle medium (Gibco) with 10% fetal calf serum (FCS) for 1 to 2 weeks. Bone marrow (BM) macrophages or dendritic cells (DC) were derived from femoral and tibial bone marrow cells. The BM macrophages were cultured for 6 to 7 days in Dulbecco modified Eagle medium supplemented with 20% FCS and 30% supernatant from L929 cells. For BM-derived DC, cells depleted of red blood cells were cultured for 6 days at 106/ml in RPMI 1640 medium (Invitrogen Life Technologies) supplemented with 10% FCS, 2 mM l-glutamine, 50 μM 2-mercaptoethanol, 10 mM HEPES buffer (pH 7.4), 50 μg of gentamicin/ml, and granulocyte-macrophage colony-stimulating factor (10). The activation of DC was performed at day 5 of the culture by the addition of 0.25 or 250 ng of LPS/ml for 20 h. Splenic DC were isolated as described in reference 5, and peritoneal macrophages were collected by peritoneal washes of thioglycolate-injected mice. Spleen or thymus cell suspensions were depleted of red blood cells and cultured with indicated stimuli in RPMI supplemented with 10% FCS, 0.05 mM β-mercaptoethanol, and penicillin-streptomycin (Gibco).
Western blotting.
Cell extracts were prepared from unstimulated or stimulated MEFs or BM-derived macrophages (BMDM) by lysis on ice with radioimmunoprecipitation assay buffer containing 250 mM NaCl2, 20 mM Tris-HCl (pH 7.4), 0.1% sodium dodecyl sulfate, 1% NP-40, 0.5% deoxycholic acid, and Complete protease inhibitor (Roche). Cell extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Amersham). The membranes were immunoblotted with the indicated antibodies, and the bound antibodies were visualized with horseradish peroxidase-conjugated antibodies to rabbit or mouse immunoglobulin G by using the enhanced chemiluminescence Western blotting system (Amersham).
Fluorescence-activated cell sorting analysis.
The following monoclonal antibodies conjugated to fluorescein isothiocyanate, phycoerythrin, CyChrome, or biotin were used: CD11c (HL3), major histocompatibility complex (MHC) class II (HB11.54.3), B220 (RA3.6B2), CD86 (GL-1), CD40 (3/23), F4/80 (F4/80), all from BD Pharmingen. Biotinylated antibodies were revealed with streptavidin conjugated to CyChrome (BD Pharmingen). For all stainings, Fc receptors were blocked by incubating cells with anti-CD16/32 antibody (2.4G2). Flow cytometry was performed by using FACSCalibur, and the data were analyzed by using CellQuest software.
Proliferation assay.
For ex vivo functional assays, B cells were purified from the spleen by using anti-B220-biotin and streptavidin-coated MACS beads (Miltenyi Biotech; purity, >95%) and were stimulated in RPMI with 5% serum for 48 h at 5 × 105 cells/well. Proliferation was monitored after 48 h stimulation in 96-well plate (105 cells/well) by pulsing cells for 6 h with [3H]thymidine. For proliferation assays with total splenocytes or thymocytes (105 cells per well in a 96-well plate) were treated or not treated with various stimuli as indicated in the figure legends. During the final 18 h, we pulsed cells with 1 mCi of [3H]thymidine. Plates were collected and analyzed for [3H]thymidine incorporation by using a Filtermate harvester and Top Count NXT reader (Packard). Radioactive incorporation was counted by scintillation in a β-counter.
Luciferase assay.
Primary MEFs were cotransfected by using Lipofectamine 2000 (Invitrogen) with 500 ng of a NF-κB-dependent luciferase (pNFκBluc) reporter plasmid from V. Jongeneel (Lausanne, Switzerland) and 20 ng of Renilla luciferase transfection efficiency vector (phRLTK; Promega). Cells were stimulated for 6 h with 20 ng of murine IL-1β or 1 mg of LPS/ml prior to lysis in passive lysis buffer (Promega). Luciferase activity was measured in a TD-20/20 luminometer (Turner Designs) by using the dual-luciferase reporter assay system (Promega), according to the manufacturer's directions.
Real-time PCR analysis.
Total RNA was prepared from MEFs by using TRIzol (Invitrogen) according to the manufacturer's directions. PCR amplification was performed on a MyiQ iCycler (Bio-Rad, Hercules, CA) using 96-well microtiter plates (Bio-Rad). For each sample, the PCR was performed in duplicate with the iQ SYBR Green Supermix (Bio-Rad). Melting curves of the amplified products were performed to identify the amplicon. The primers used were as follows: GAPDH (900 nM, 4 mM MgCl2; 5′-GCTAAGCAGTTGGTGGTGCA-3′ and 5′-TCACCACCATGGAGAAGGC-3′), IL-6 (200 nM, 3 mM MgCl2; 5′-AAGTGCATCATCGTTGTTCATACA-3′ and 5′-CAGAATTGCCATCGTACAACTCTTTTCTCA-3′), and TNF-α (900 nM, 3 mM MgCl2; 5′-TGGGAGTAGACAAGGTACAACCC-3′ and 5′-CATCTTCTCAAAATTCGAGTGACAA-3′). Quantification of input cDNA from the unknown samples was performed by including a standard curve. To construct the standard DNA curve, amplicons generated by reverse transcription-PCR (RT-PCR) using the primers described above were purified on silica columns (QiAquick PCR Purification; QIAGEN) and cloned into pGEM-Teasy (Promega, Madison, WI). DNA plasmid concentrations were measured by optical density spectrophotometry, and the corresponding copy numbers were calculated by using the equation 1 μg of 1,000 bp DNA = 9.1 × 1011 molecules. Serial 10-fold dilutions of plasmids ranging from 107 to 102 DNA copies were used as a standard curve in each PCR run. To minimize interassay variability, all samples analyzed from a single experiment were performed in the same 96-well microtiter plate. The results are expressed as ratios between mRNA copy numbers of IL-6 or TNF-α and mRNA copy numbers of the housekeeping gene GAPDH.
RESULTS
Targeted disruption of Tollip.
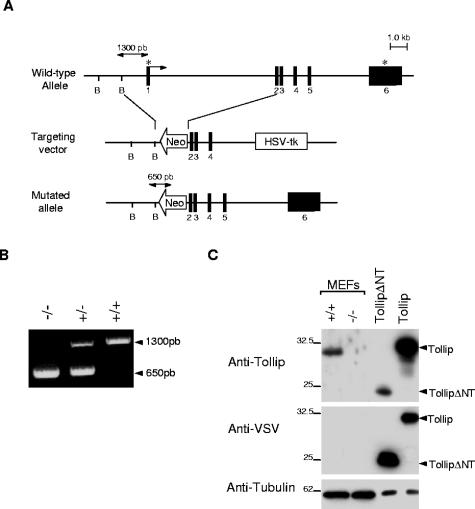
Tollip-deficient mice were generated with a targeting vector constructed by replacing exon 1 (containing the start codon) with a neomycin resistance cassette (Fig. 1A). Two independent and correctly targeted embryonic stem cell clones were used to generate Tollip−/+ mice. These heterozygotes were intercrossed to produce Tollip−/− progeny, as confirmed by Southern blotting (data not shown) or PCR (Fig. 1B). Homozygous mutant mice were born at the expected Mendelian ratio, and mice derived from both embryonic stem cell lines had identical phenotypes. Mice lacking Tollip were healthy and fertile. Western blot analysis with a specific polyclonal antibody to Tollip demonstrated that neither intact nor truncated Tollip proteins were present in Tollip−/− fibroblasts, peripheral blood leukocytes, splenocytes, and thymocytes (Fig. 1C and data not shown), confirming the null nature of the mutation.
FIG. 1.
Generation of Tollip−/− mice. (A) Organization of the mouse Tollip gene, targeting vector, and mutated allele. Filled boxes denote exons (1-6). The arrow and asterisks denote the translational initiator ATG and terminator TAA sequences, respectively. (B) Analysis of genomic DNA by PCR using primers spanning the regions indicated in panel A. (C) Western blot analysis of cellular extracts from Tollip+/+ and Tollip−/− MEFs or 293T cells transfected with VSV TollipΔN (amino acids 47 to 274) or full-length VSV Tollip using antibodies to Tollip (upper panel) or VSV (middle panel). To confirm equal loading the same blot was reprobed with antitubulin antibodies (lower panel).
Normal IκB degradation and phosphorylation of MAPK by IL-1R, TLR-2, and TLR-4 in Tollip−/− cells.
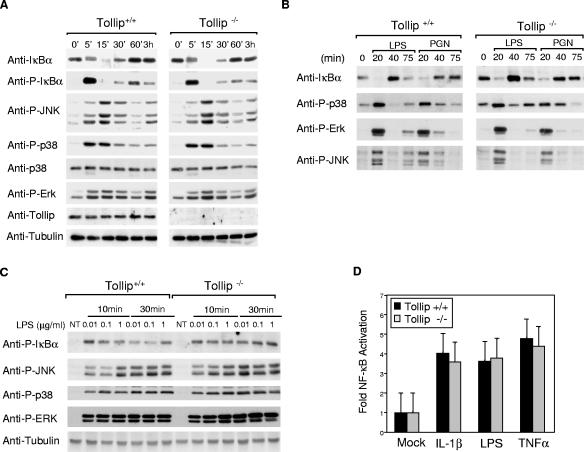
To facilitate characterization of signaling cascades potentially modulated by Tollip, BMDM, or MEFs were isolated from Tollip−/− and wild-type mice. The NF-κB signaling pathway downstream of IL-1RI, TLR2, or TLR4 was initially examined by looking at whether IκBα was phosphorylated and then degraded after stimulation. Western blot analysis revealed no consistent differences either in transient phosphorylation of IκBα or overall levels of IκBα in MEFs treated with IL-1β, in BMDM treated with LPS or PGN, or in peritoneal macrophages treated with LPS (Fig. 2A, B, and C). Similarly, IL-1β- and LPS-induced activation of an NF-κB-dependent luciferase reporter was indistinguishable in Tollip−/− and Tollip+/+ MEFs (Fig. 2D), suggesting that Tollip is dispensable for IL-1β-, LPS-, and PGN-induced activation of NF-κB. Further, the findings that the levels of IκBα, an NF-κB-inducible gene, returned to basal levels after 60 min of stimulation suggest that Tollip is also dispensable for switching off of NF-κB-dependent gene transcription. To access Tollip's ability to regulate various MAPK signaling pathways phosphorylation of c-Jun N-terminal kinase (JNK), p38 or extracellular signal-regulated kinase (ERK) was monitored by Western analysis with phospho-specific antibodies to the respective kinases. After stimulation of MEFs, peritoneal macrophages, or BMDM with IL-1β, LPS, or PGN, again no significant differences between Tollip−/− and Tollip+/+ mice were detectable in the phosphorylation of these MAPKs (Fig. 2A, B, and C). Even at low doses of LPS (Fig. 2C), where defects in signaling are detected in IRAK-1-deficient mice (16), signaling appeared to be normal in the absence of Tollip. Thus, Tollip also appears not to be crucial for regulation of IL-1β-, LPS-, and PGN-induced phosphorylation of JNK, p38, and ERK1/2.
FIG. 2.
Analysis of IL-1β-, LPS-, and PGN-triggered signaling cascades. (A) MEFs (A) or bone marrow-derived macrophages (B) from wild-type (+/+) and Tollip-deficient (−/−) mice were stimulated with LPS (1 μg/ml), PGN (10 μg/ml), or IL-1β (50 ng/ml) for the indicated times. Cell lysates were prepared and immunoblotted with various antibodies as indicated. Shown are representative experiments, repeated at least three times with similar results. (C) Peritoneal macrophages from wild-type (+/+) and Tollip-deficient (−/−) mice were stimulated with LPS at the indicated concentrations. Cell lysates were prepared and immunoblotted with the indicated antibodies. (D) MEFs were transfected with 500 ng of a pLuc (a NF-κB-dependent reporter plasmid) and 20 ng of phRLTK (Renilla luciferase). The cells were stimulated for 6 h with IL-1β (20 ng/ml), LPS (1 μg/ml), or TNF-α (100 ng/ml) and lysed, and the luciferase activities determined by using the dual-luciferase reporter assay system. Values shown are from data obtained in two transfection experiments. NT, nontreated.
IRAK is degraded in Tollip−/− cells.
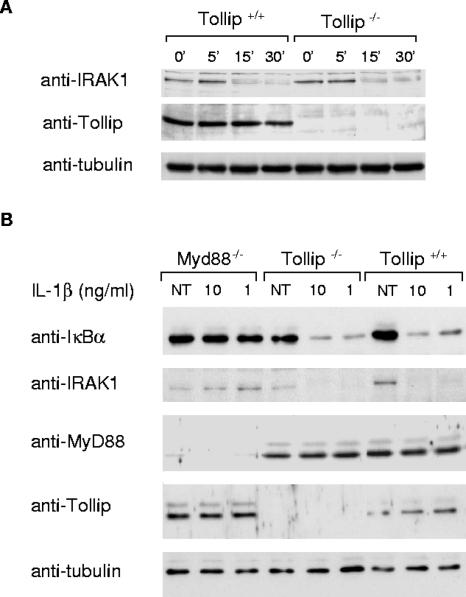
Tollip binds IRAK-1 in unstimulated cells and is recruited with similar kinetics to the IL-1RI complex (3). The Tollip-IRAK-1 interaction also suppresses the kinase activity of IRAK-1 (21), suggesting that Tollip may be important for recruitment of IRAK-1 to the receptor complex while maintaining IRAK in an “off state.” The kinase activity of IRAK-1, which is rapidly activated after recruitment to a receptor complex, drives its hyperphosphorylation, release from Tollip, MyD88, and an interaction with TRAF6 (8). Hyperphosphorylation of IRAK-1 also tags it for ubiquitination and proteasome-dependent degradation (20). Therefore, if Tollip were crucial for suppression of IRAK-1's kinase activity, the cellular levels of IRAK-1 and/or its ability to be degraded would predictably be affected. Comparable levels of IRAK-1 were detected in unstimulated wild-type and deficient cells (Fig. 3A) by Western blot analysis. Also, IRAK-1 was degraded with similar efficiency and kinetics in both cell types after stimulation with IL-1β (Fig. 3A). Further, even at suboptimal concentrations of IL-1β (i.e., where degradation of IκBα is not yet complete at 15 min), degradation of IRAK occurred in both +/+ and −/− cells. As a control, under the same conditions the degradation of IRAK was not detected in MyD88-deficient MEFs (Fig. 3B). In conclusion, these experiments indicate that the Tollip-IRAK-1 interaction is not essential for recruitment to the IL-1RI complex nor for the activation and/or degradation of IRAK. That Tollip is phosphorylated by IRAK-1 (21) suggests the contrary: that IRAK-1 has a role in regulating Tollip's activity.
FIG. 3.
IRAK degradation occurs normally in Tollip−/− cells. (A) Tollip-deficient (−/−) or wild-type (+/+) MEFs were stimulated with IL-1β (20 ng/ml) for the indicated times. Cell lysates were prepared and immunoblotted with anti-IRAK-1 or anti-IκBα antibodies. (B) Tollip-deficient (−/−), wild-type (+/+), or MyD88-deficient (−/−) MEFs were stimulated with the indicated concentrations of IL-1β for 15 min. Cell lysates were prepared and immunoblotted with various antibodies as indicated. As a loading control in these experiments, the same membrane was reprobed with antibodies against tubulin. NT, not treated.
Activation of DC and lymphocytes are normal in Tollip−/− mice.
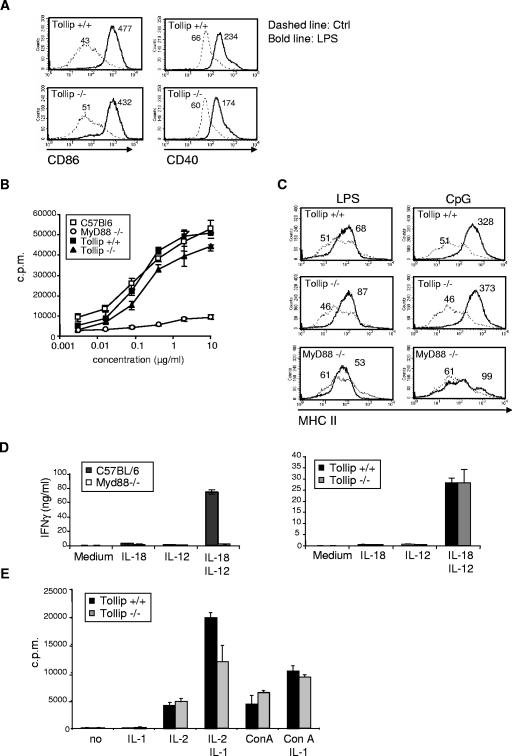
IL-1RI and TLRs are known to induce the activation and proliferation of immune cells such as lymphocytes and antigen presenting cells. Since deregulated signaling may affect the cellular composition of immune organs, the cellular composition of the thymus, spleen, lymph nodes, and bone marrow was evaluated and found to be comparable between Tollip−/− and Tollip+/+ mice (data not shown). TLR-induced DC activation is essential for the priming of antigen-specific T cells. We therefore assessed whether the lack of Tollip would influence DC activation by monitoring the upregulation of costimulatory molecules at their surface after TLR engagement in vivo. As shown in Fig. 4A, CD86 and CD40 levels were similarly increased on CD11c+ splenic DC after LPS stimulation in the presence or absence of Tollip. Similar results were obtained after stimulation with other TLR ligands, including polyinosinic-poly(C) [poly(I-C)], CpG, flagellin, TLR3, TLR9, and TLR5 agonists (data not shown). TLR signaling also induces the proliferation of splenocytes, in particular B lymphocytes. Proliferation triggered by TLR2, TLR4, and TLR9 agonists was comparable in Tollip−/− and Tollip+/+ splenocytes (Fig. 4B and data not shown). In contrast to MyD88, Tollip deficiency did not affect TLR-mediated activation (MHC II upregulation) of isolated naive B cells (Fig. 4C). Since Tollip also interacts with the cytoplasmic tail of IL-18R in coexpression experiments, the ability of splenocytes to proliferate and to produce IFN-γ in response to stimulation with IL-18 was monitored. Although the response was completely abrogated in MyD88−/− cells, no significant difference in IFN-γ production (Fig. 4D) or proliferation (data not shown) was seen between Tollip−/− and Tollip+/+ cells. IL-1β is known to be a costimulant for T-cell proliferation. Both wild-type and Tollip−/− thymocytes showed similar enhanced proliferation when cultured with concanavalin A or IL-2 in the presence of IL-1β (Fig. 4E). In summary, TLR- and IL-1β-dependent activation of dendritic cells and lymphocytes appears independent of Tollip.
FIG. 4.
TLR- and IL-1β-dependent activation of immune cells is not impaired in the absence of Tollip. (A) Mice were injected intravenously with 1 μg of LPS, and 6 h later splenic DCs were analyzed by flow cytometry for the indicated activation markers. Histograms represent data for F4/80−/low CD11c+ gated cells. (B) Splenocytes were incubated with LPS at the indicated concentrations, and proliferation was monitored 48 h later by pulsing the cells overnight with [3H]thymidine. (C) Splenic B cells were isolated by using B220+ MACS beads and incubated with 5 μg of LPS/ml or 10 μg of CpG/ml for 48 h. Surface expression of MHC II was then monitored by flow cytometry. (D) Splenocytes were plated in triplicate in medium containing 10 ng of IL-12/ml, 20 ng of IL-18/ml, or both. The production of IFN-γ in the supernatant was measured by ELISA 72 h later. (E) Thymocytes were incubated in medium containing 20 U of IL-2/ml, 10 ng of IL-1β/ml, and 2.5 μg of ConA/ml as indicated for 3 days. The cell proliferation was measured by pulsing the cells overnight with [3H]thymidine. The data are averages of quadruplicates and are representative of three independent experiments.
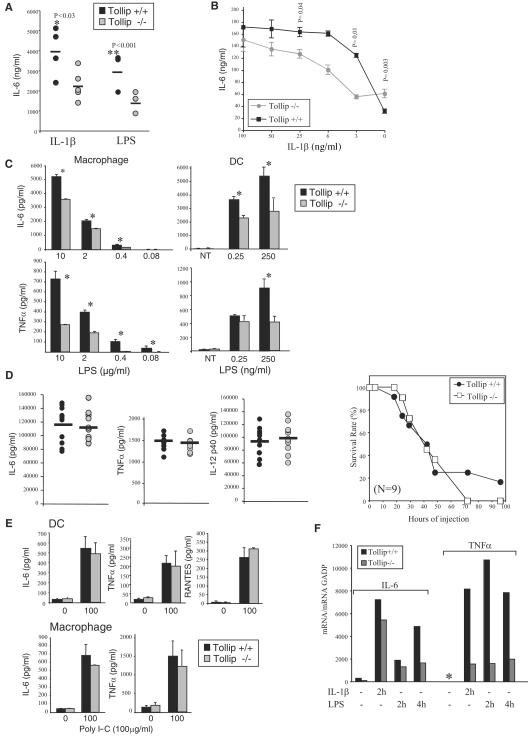
IL-6 and TNF-α production is decreased in Tollip-deficient mice after IL-1β or LPS stimulation.
To determine the in vivo contribution of Tollip, we then focused on the early phase of immune responses to inflammatory signals. For this purpose, proinflammatory cytokine production (i.e., IL-6 and TNF-α) was measured in the sera of mice after intravenous (i.v.) injection IL-1β or LPS. Strikingly, Tollip−/− mice produced significantly less IL-6 than their wild-type littermates after i.v. injection of IL-1β (1 μg) or LPS (1 μg) (Fig. 5A). Similarly macrophages stimulated with IL-1β secreted less IL-6 than wild-type macrophages (Fig. 5B). This effect was more pronounced when cells were stimulated with low doses of IL-1β. Likewise, IL-6 and TNF-α production was attenuated in Tollip-deficient macrophages and BM DC stimulated with LPS (Fig. 5C). However, after administration of a lethal dose of LPS (40 mg/kg), the production of IL-6, TNF-α, as well as IL-12p40 was similar in wild-type and deficient mice (Fig. 5E), indicating that Tollip is not required at high ligand concentrations for cytokine production. Correspondingly, no differences in susceptibility to LPS-induced septic shock were detected: Tollip-deficient mice died as rapidly as their wild-type littermates.
FIG. 5.
Tollip-deficient mice produce less IL-6 and TNF-α mRNA and protein upon LPS and IL-1β stimulation. (A) Tollip−/− or wild-type mice were injected intravenously with 1 μg of IL-1β (five mice/group) or 1 μg of LPS (three mice/group), and the production of IL-6 in the serum was measured at 2 h by ELISA. (B) BMDM were treated with 100 ng of IFN-γ/ml and stimulated with the indicated concentrations of IL-1β, and the production of IL-6 in the supernatant was measured by ELISA 24 h later. (C) BMDM or BMDC were stimulated with the indicated concentrations of LPS, and the production of IL-6 and TNF-α in the supernatant was measured by ELISA 24 h later. The experiments in panels B and C were repeated at least twice in triplicates with similar results. Significant differences are indicated by an asterisks (✽, P < 0.04). (D) Tollip-deficient (−/−) or wild-type mice (+/+) were injected intraperitoneally with a lethal dose of LPS (E. coli O111:B4) at a dose of 40 mg/kg (body weight) to induce septic shock. The serum concentrations of IL-6, TNF-α, and IL-12p40 for all mice/group (n = 9) were measured by ELISA 3 h after injection, and the data shown are averages for each group. Animals were then monitored every 12 h for moribund state and then sacrificed. (E) Peritoneal macrophages or DC were stimulated with 10 or 100 μg of poly(I-C)/ml, and the production of IL-6, TNF-α, and RANTES in the supernatant was measured by ELISA 24 h later. DC from wild-type (+/+) and Tollip-deficient (−/−) mice were stimulated with LPS (1 μg/ml) or poly(I-C) (100 μg/ml) for 24 h. Cell lysates were prepared and immunoblotted as indicated. (F) Wild-type and Tollip−/− MEFs were stimulated with IL-1β (5 ng/ml) or LPS (100 ng/ml) for the indicated times, and the IL-6 or TNF-α mRNA levels were determined by quantitative real-time RT-PCR analysis. ✽, not detected.
IL-1RI or TLRs (i.e., TLR2, -5, and -9) signaling requires the adapter protein MyD88, whereas the stimulation of TLR4 facilitates the activation of two pathways: one dependent on MyD88 leading to the production of inflammatory cytokines and a second dependent on TRIF leading to the production of IFN-β and the expression of IFN-inducible genes (1). To determine whether Tollip also has a role in modulation of MyD88-independent pathways, wild-type and deficient cells were stimulated with poly(I-C), thereby activating TLR3 signaling and a TRIF-dependent, MyD88-independent pathway. As shown in Fig. 5E, IL-6 or TNF-α production was similar in wild-type and Tollip-deficient peritoneal macrophages or BMDC treated with poly(I-C), suggesting that Tollip is more fundamental to MyD88-dependent pathways. Further no differences in the production of the IFN-inducible genes RANTES or the IFN-inducible gene ISG15 were detected after poly(I-C) or LPS stimulation in Tollip-deficient mice (Fig. 4E and data not shown). Therefore, Tollip appears to control the magnitude of inflammatory cytokine production in response to IL-1RI and TLR pathways dependent on MyD88.
To determine whether reduced cytokine production is due to a defect at the transcriptional level, the amount of IL-6 and TNF-α message was measured by RT and quantitative PCR (RT-PCR). After stimulation with IL-1β or LPS, MEFs from Tollip-deficient animals produced significantly less IL-6 and TNF-α mRNA than wild-type cells (Fig. 5F). Tollip deficiency therefore results in attenuated immune responses to IL-1β and LPS, which are likely the result of impaired induction of IL-6 and TNF-α at the mRNA level.
DISCUSSION
In summary, Tollip regulates the magnitude and kinetics of IL-6 and TNF-α production upon stimulation with IL-1β and low doses or physiological doses of LPS. We did not observe any effect of Tollip deficiency in response to lethal doses of LPS, suggesting that Tollip acts in “fine tuning” or coordinating optimal signaling through IL-1RI and TLR4. In this way, Tollip resembles a scaffolding protein that is required for optimal responses. As such, high concentrations of Tollip may titrate out critical signaling proteins in IL-1R and TLR4 pathways, thereby blocking signaling. This would accommodate the apparently conflicting observations (i.e., repressor versus activator) and predicts that the cellular concentration of Tollip may be crucial for its capacity to regulate signaling. Further it could explain why overexpression or upregulation of Tollip in intestinal epithelial cells correlates with a hyporesponsive state, after a second challenge with a TLR ligand (12).
Quantitative PCR suggests that Tollip deficiency affects IL-6 and TNF-α production at the transcriptional level. NF-κB, JNK, and p38 signaling cascades are crucial both for the transcription and translation of IL-6 and TNF-α (1). However, surprisingly, activation of NF-κB, JNK, and p38 signaling appeared to be normal in Tollip−/− cells. This could easily be explained if Tollip deficiency resulted in subtle defects in the activation of the membrane proximal signaling complex (Tollip is rapidly and transiently recruited to this complex) and thereby ultimately in downstream activation of these signaling cascades. Interestingly, IRAK-1-deficient macrophages have a similar phenotype in that TNF-α secretion is only impaired upon low doses of LPS (16). The Tollip-IRAK-1 interaction may thus be important for optimal responses to these stimuli.
Although we cannot exclude that Tollip does not contribute entirely or partly to the initial activation of NF-κB, JNK, and p38 signaling, it is also possible that Tollip modulates signaling via alternative means. Several recent observations suggest that Tollip may participate in the recognition and trafficking of ubiquitinated proteins within endocytic/biosynthetic pathways. Tollip partially localizes to late endosomes or lysosomes (B. Brissoni, L. Agostini, M. Kropf, H. Everett, N. Aebi, V. Swoboda, S. Janssens, E. Meylan, F. Martinen, M. Felderbaum-Corti, H. Hirling, J. Gruenberg, J. Tschopp, and K. Burns, submitted for publication), binds Tom1 and Tom1L1 (molecules likely to have a role in endocytic trafficking of proteins to the lysosome) (6, 13, 19), interacts with phosphatidylinositol-3-phosphate [PtdIns(3)P] and phosphatidylinositol-3,4,5-phosphate (phospholipids abundant on endocytic vesicles) (9), and binds ubiquitin, an internalization and sorting tag on cargo proteins of the endocytic or biosynthetic pathways (14).
Endosomes, via associated proteins, can function as platforms for the formation and activation of specific signaling complexes (15). Interestingly, overexpression of a Tollip mutant, which does not bind PtdIns(3)P and is presumably not endosomally localized, does not block LPS signaling (9). Several TLRs are known to be endosomally localized (reviewed in reference 1). We have recently shown that IL-1RI traffics via the late endosome to lysosomes for degradation (Brissoni et al., submitted). It thus conceivable that Tollip acts in the recruitment of a signalosome at the late endosome that is required for optimal responses to IL-1β, LPS, and other TIR ligands that signal via MyD88-dependent pathways. Future studies are now required to address the precise molecular mechanisms by which Tollip modulates cytokine production and/or secretion. Given that the physiological concentrations of IL-1β or LPS at the sites of infection/inflammation most likely do not exceed the low nanomolar range, Tollip is undoubtedly an important mediator of these responses.
Acknowledgments
We thank Fabio Martinon and Etienne Meylan for critical reading of the manuscript and Percy Sumariwalla for help with statistical analysis. We also thank all of the members of the Tschopp laboratory.
This study was supported by grants of the Swiss National Science Foundation, CTI, and Roche Foundation to J.T.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4**:**499-511. [DOI] [PubMed] [Google Scholar]
- 2.Bulut, Y., E. Faure, L. Thomas, O. Equils, and M. Arditi. 2001. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J. Immunol. 167**:**987-994. [DOI] [PubMed] [Google Scholar]
- 3.Burns, K., J. Clatworthy, L. Martin, F. Martinon, C. Plumpton, B. Maschera, A. Lewis, K. Ray, J. Tschopp, and F. Volpe. 2000. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat. Cell Biol. 2**:**346-351. [DOI] [PubMed] [Google Scholar]
- 4.Burns, K., S. Janssens, B. Brissoni, N. Olivos, R. Beyaert, and J. Tschopp. 2003. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J. Exp. Med. 197**:**263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Didierlaurent, A., I. Ferrero, L. A. Otten, B. Dubois, M. Reinhardt, H. Carlsen, R. Blomhoff, S. Akira, J. P. Kraehenbuhl, and J. C. Sirard. 2004. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. J. Immunol. 172**:**6922-6930. [DOI] [PubMed] [Google Scholar]
- 6.Katoh, Y., Y. Shiba, H. Mitsuhashi, Y. Yanagida, H. Takatsu, and K. Nakayama. 2004. Tollip and Tom1 form a complex and recruit ubiquitin-conjugated proteins onto early endosomes. J. Biol. Chem. 279**:**24435-24443. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi, K., L. D. Hernandez, J. E. Galan, C. A. Janeway, Jr., R. Medzhitov, and R. A. Flavell. 2002. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110**:**191-202. [DOI] [PubMed] [Google Scholar]
- 8.Kollewe, C., A. C. Mackensen, D. Neumann, J. Knop, P. Cao, S. Li, H. Wesche, and M. U. Martin. 2004. Sequential autophosphorylation steps in the interleukin-1 receptor-associated kinase-1 regulate its availability as an adapter in interleukin-1 signaling. J. Biol. Chem. 279**:**5227-5236. [DOI] [PubMed] [Google Scholar]
- 9.Li, T., J. Hu, and L. Li. 2004. Characterization of Tollip protein upon lipopolysaccharide challenge. Mol. Immunol. 41**:**85-92. [DOI] [PubMed] [Google Scholar]
- 10.Lutz, M. B., N. Kukutsch, A. L. Ogilvie, S. Rossner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223**:**77-92. [DOI] [PubMed] [Google Scholar]
- 11.Melmed, G., L. S. Thomas, N. Lee, S. Y. Tesfay, K. Lukasek, K. S. Michelsen, Y. Zhou, B. Hu, M. Arditi, and M. T. Abreu. 2003. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J. Immunol. 170**:**1406-1415. [DOI] [PubMed] [Google Scholar]
- 12.Otte, J. M., E. Cario, and D. K. Podolsky. 2004. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology 126**:**1054-1070. [DOI] [PubMed] [Google Scholar]
- 13.Puertollano, R. 2004. Interactions of TOM1L1 with the multivesicular body sorting machinery. J. Biol. Chem. 280**:**9258-9264. [DOI] [PubMed]
- 14.Shih, S. C., G. Prag, S. A. Francis, M. A. Sutanto, J. H. Hurley, and L. Hicke. 2003. A ubiquitin-binding motif required for intramolecular monoubiquitylation, the CUE domain. EMBO J. 22**:**1273-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorkin, A., and M. Von Zastrow. 2002. Signal transduction and endocytosis: close encounters of many kinds. Nat. Rev. Mol. Cell. Biol. 3**:**600-614. [DOI] [PubMed] [Google Scholar]
- 16.Swantek, J. L., M. F. Tsen, M. H. Cobb, and J. A. Thomas. 2000. IL-1 receptor-associated kinase modulates host responsiveness to endotoxin. J. Immunol. 164**:**4301-4306. [DOI] [PubMed] [Google Scholar]
- 17.Ulevitch, R. J., and P. S. Tobias. 1995. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 13**:**437-457. [DOI] [PubMed] [Google Scholar]
- 18.Wells, C. A., T. Ravasi, and D. A. Hume. 2005. Inflammation suppressor genes: please switch out all the lights. J. Leukoc. Biol. 78**:**9-13. [DOI] [PubMed]
- 19.Yamakami, M., T. Yoshimori, and H. Yokosawa. 2003. Tom1, a VHS domain-containing protein, interacts with tollip, ubiquitin, and clathrin. J. Biol. Chem. 278**:**52865-52872. [DOI] [PubMed] [Google Scholar]
- 20.Yamin, T. T., and D. K. Miller. 1997. The interleukin-1 receptor-associated kinase is degraded by proteasomes following its phosphorylation. J. Biol. Chem. 272**:**21540-21547. [DOI] [PubMed] [Google Scholar]
- 21.Zhang, G., and S. Ghosh. 2002. Negative regulation of Toll-like receptor-mediated signaling by Tollip. J. Biol. Chem. 277**:**7059-7065. [DOI] [PubMed] [Google Scholar]