Rescue Surgery for Unresectable Colorectal Liver Metastases Downstaged by Chemotherapy: A Model to Predict Long-term Survival (original) (raw)
Abstract
Objective:
To evaluate the long-term survival of patients resected for primarily unresectable colorectal liver metastases (CRLM) downstaged by systemic chemotherapy and to use prognostic factors of outcome for a model predictive of survival on a preoperative setting.
Summary Background Data:
Surgery of primarily unresectable CRLM after downstaging chemotherapy is still questioned, and prognostic factors of outcome are lacking.
Methods:
From a consecutive series of 1439 patients with CRLM managed in a single institution during an 11-year period (1988–1999), 1104 (77%) initially unresectable (NR) patients were treated by chemotherapy and 335 (23%) resectable were treated by primary liver resection. Chemotherapy mainly consisted of 5-fluorouracil and leucovorin combined to oxaliplatin (70%), irinotecan (7%), or both (4%) given as chronomodulated infusion (87%). NR patients were routinely reassessed every 4 courses. Surgery was reconsidered every time a documented response to chemotherapy was observed. Among 1104 NR patients, 138 “good responders” (12.5%) underwent secondary hepatic resection after an average of 10 courses of chemotherapy. At time of diagnosis, mean number of metastases was 4.4 (1–14) and mean maximum size was 5.2 cm (1–25). Extrahepatic tumor was present in 52 patients (38%). Multinodularity or extrahepatic tumor was the main cause of initial unresectability. All factors likely to be predictive of survival after liver resection were evaluated by uni- and multivariate analysis. Estimation of survival was adjusted on risk factors available preoperatively.
Results:
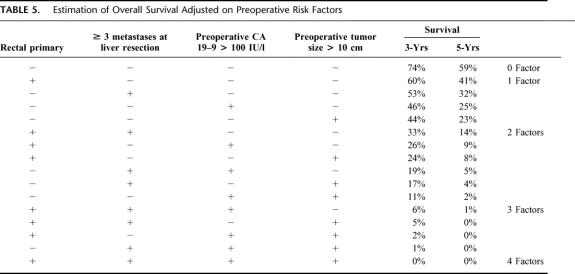
Seventy-five percent of procedures were major hepatectomies (≥3 segments) and 93% were potentially curative. Liver surgery was combined to portal embolization, to ablative treatment, or to a second-stage hepatectomy in 42 patients (30%) and to resection of extrahepatic tumor in 41 patients (30%). Operative mortality within 2 months was 0.7%, and postoperative morbidity was 28%. After a mean follow-up of 48.7 months, 111 of the 138 patients (80%) developed tumor recurrence, 40 of which were hepatic (29%), 12 extrahepatic (9%), and 59 both hepatic and extrahepatic (43%). Recurrence was treated in 52 patients by repeat hepatectomy (71 procedures) and in 42 patients by extrahepatic resection (77 procedures). Survival was 33% and 23% at 5 and 10 years with a disease-free survival of 22% and 17%, respectively. It was decreased as compared with that of patients primarily resected within the same period (48% and 30% respectively, P = 0.01). At the last follow-up, 99 patients had died (72%) and 39 (28%) were alive; 25 were disease free (18%) and 14 had recurrence (10%). At multivariate analysis, 4 preoperative factors were independently associated to decreased survival: rectal primary, ≥3 metastases, maximum tumor size >10 cm, and CA 19–9 >100 UI/L. Mean adjusted 5-year survival according to the presence of 0, 1, 2, 3, or 4 factors was 59%, 30%, 7%, 0%, and 0%.
Conclusions:
Modern chemotherapy allows 12.5% of patients with unresectable CRLM to be rescued by liver surgery. Despite a high rate of recurrence, 5-year survival is 33% overall, with a wide use of repeat hepatectomies and extrahepatic resections. Four preoperative risk factors could select the patients most likely to benefit from this strategy.
Modern chemotherapy allows 12.5% of patients with unresectable colorectal liver metastases to be rescued by liver surgery. This strategy offers a possibility of long-term survival (33% at 5 years and 22% at 10 years) with a low operative risk. It involves a wide use of repeat hepatectomies and extrahepatic resections. Four preoperative risk factors are able to select the patients more likely to benefit from this strategy.
Liver resection is the only available treatment with an option of long-term survival in patients with metastases of colorectal cancer.1–5 However, the resectability rate of metastases at the time of diagnosis is low, accounting for the low proportion of patients who may benefit from a surgical approach. Until recently, patients initially considered as unresectable were definitively treated by palliative chemotherapy, with obviously little chance of 5-year survival.5,6 Chemotherapy as a first-line treatment of metastatic colorectal cancer has greatly improved within the last decade. Response rates achieved with 5-fluorouracil and leucovorin have been significantly increased by combination with oxaliplatin, irinotecan, and changes in delivery regimens.7–9 This improved efficacy has not only allowed increased patient survival in a palliative setting but has also offered a possibility of cure to previously unresectable patients with liver surgery after tumor downstaging.10–12 By reconsidering the initial unresectability of patients who strongly respond to chemotherapy, we have shown that survival could be achieved by liver resection in a significant proportion of patients otherwise promised a poor outcome.10–12
Similar experience has been further reported by others either after systemic13–17 or intra-arterial hepatic chemotherapy.18–21 However, the benefit of this “rescue surgery” is still debated, and prognostic factors are lacking for the outcome of these patients. The aim of the study was to review the results of our 11-year experience to assess the long-term survival of patients treated by a combination of downstaging chemotherapy and surgery. The objective was to determine the survival benefit of such a strategy as compared with that of initially resectable patients, to define the critical factors influencing the outcome, and to propose a model likely to be used by oncologists and surgeons to estimate the survival on a preoperative setting.
PATIENTS AND METHODS
From April 1988 to July 1999, 1439 consecutive patients with CLRM were managed at our institution. From these, only 335 (23%) were primarily resected from their CRLM and 1104 (77%) were initially considered as unresectable. These latter patients were treated by systemic chemotherapy and were prospectively reviewed every 4 courses of treatment by the same multidisciplinary team. Liver surgery was reconsidered every time a documented response to chemotherapy was observed.
Selection of the Study Population
Among the 1104 initially unresectable patients, 138 (12.5%) whose metastases were significantly downstaged by chemotherapy, underwent hepatic resection and formed the population of the study (Fig. 1). When unresectability had been stated by an other team prior to the referral to our unit, the patient chart was routinely reviewed by one of us (R.A.) to confirm or not initial unresectability. Only patients with unresectability assessed with our own criteria of selection were included in the study group. These yet defined criteria10,11 mainly relied either on the impossibility to perform a curative hepatectomy leaving at least 30% of nontumoral liver parenchyma or to the presence of concomitant extrahepatic disease. The decision to perform surgery after downstaging of the metastatic process was taken only when the overall strategy could potentially achieve a complete treatment of the tumors.
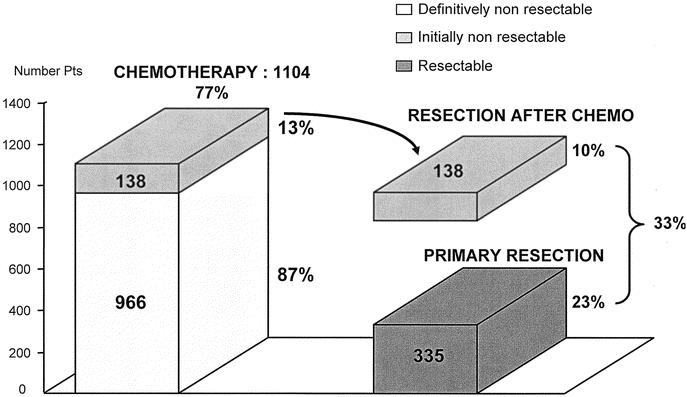
FIGURE 1. Paul Brousse Experience (1988–1999) in the management of colorectal liver metastases.
Patient Demographics
There were 77 men and 61 women with a mean age of 57.3 years (33–85) (Table 1). Liver metastases were unresectable either because too large (n = 10-7%), ill-located (n = 21-15%), multinodular (n = 77-56%), or associated to extrahepatic tumor (n = 30-22%). In some patients, however, several causes of nonresectability were encountered, the more prominent cause being considered.
TABLE 1. Demographics of Patients, of Primary Tumor, of Metastases and of Chemotherapy
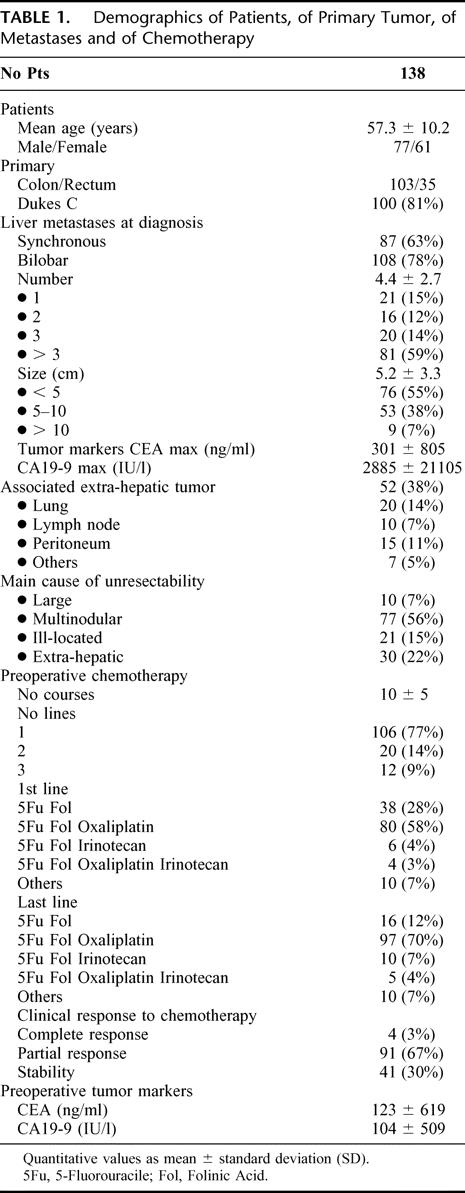
At the time of diagnosis, the mean number of metastases was 4.4 (1-14) with a mean maximum size of 5.2 cm (1-25). Extrahepatic tumor was present in 52 of the 138 patients (38%) with by decreasing frequency: lungs (14%), peritoneum (11%), lymph nodes (7%), and other locations (5%). These extrahepatic metastases were not always the main cause of unresectability.
Preoperative Chemotherapy
Patients received an average of 10 courses of chemotherapy (3–39). The response that allowed surgery was obtained with the first line of treatment in most cases (77%) but 14% of patients needed 2 lines and 9%, 3 lines of chemotherapy before liver resection (Table 1). The last preoperative protocol used 5-fluorouracil-leucovorin either alone (12%) or combined with oxaliplatin (70%), irinotecan (7%) or both (4%). Chronomodulated infusion was used as previously described6,7 in 120 of the 138 patients (87%). Response to chemotherapy was evaluated from serial imaging studies (computed tomography [CT] scan of abdomen, pelvis, and chest and abdominal ultrasound), based on tumor diameter changes according to the World Health Organization criteria.22 A partial response (defined as 50% or more decrease in total tumor size) was observed in 95 patients (69%; Fig. 2), while a minor response (<50% decrease in total tumor size of the lesions) graded as “stabilization” was sufficient to indicate surgery in the remnant patients (31%). All patients were periodically reviewed by oncologists and surgeons up to the point that surgical resection became possible. While our initial policy was to perform liver surgery when the reduction in tumor size and tumor markers had reached a plateau,10 more recently we performed surgery as soon as resectability was technically possible. Also, the date of operation was chosen to avoid a long time interval between the last chemotherapy and surgery. A 2- or 3-week interval from the last course was our preferred timing.
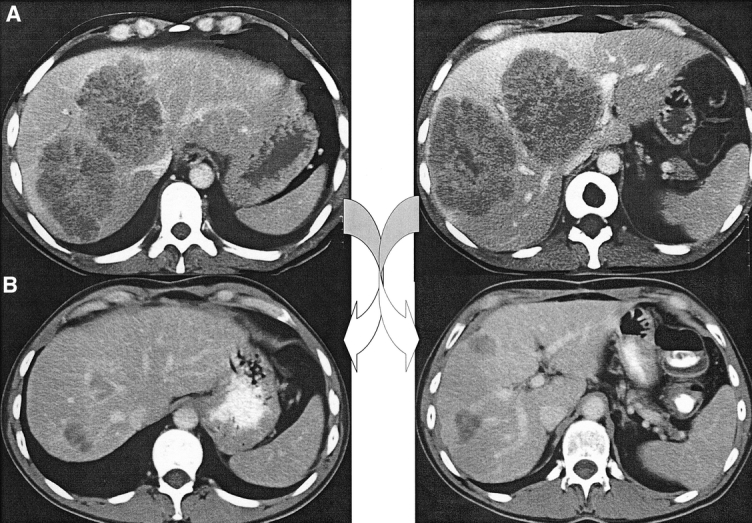
FIGURE 2. a, A 40-year-old man presenting huge liver metastases not amenable to primary resection because of too large liver involvement and close contact with portal bifurcation, hepatic veins, and vena cava. (b) Tumor downstaging by chemotherapy allowed secondary liver resection by right hepatectomy. The patient is currently alive without recurrence at 5.5 years.
Liver Resection
Operative technique was as previously described.10,11 The objective was based on attempt at a radical resection sparing the highest amount of liver parenchyma as possible but providing a tumor-free margin of ≥1 cm whenever possible. When free margins could not be obtained owing to the vascular relationship of tumors or because the need for economical resection in relation to the multiple number of nodules, resection was nonetheless performed when complete and potentially curative. All previous sites of metastases were cautiously examined at operation particularly when they had apparently disappeared on preoperative imaging with the aim to resect all the potentially tumoral tissue. However when no lesion was seen, was palpated, or detected at intraoperative ultrasound, no resection was made “de principe.”
Specific Techniques to Achieve Resectability
Portal vein embolization (PVE) was performed when the estimated volume of remnant functional liver parenchyma assessed by CT scan volumetry after the projected hepatectomy was below 30%. The technique of percutaneous PVE was reported in detail elsewhere.23 It could be performed mainly before liver resection or less frequently at the first stage of a 2-stage hepatectomy.
Radiofrequency or cryosurgery was exclusively used for multiple bilateral metastases in combination to liver resection in patients otherwise unresectable owing to the extent of liver resection. They were usually reserved to remnant metastases, up to 3 cm in size, contralateral to the site of hemihepatectomy.
Two-stage hepatectomy was performed in patients for whom a complete resection of all tumors could not be achieved with a single procedure even when combined to a local treatment.24 It exclusively concerned multinodular bilateral metastases for which the less invaded hemiliver contained either more than 3 lesions or at least 1 lesion exceeding 3 cm in diameter.
Postoperative Management
Patients had planned follow-up at 1 month and then every 4 months, with evaluation of tumor markers (CEA and CA 19–9), liver function tests, hepatic ultrasonography. Abdominal and chest CT scan were performed every 8 months. In case of associated extrahepatic metastases, resection of the extrahepatic site was usually performed 2 to 3 months after hepatic surgery, with systemic chemotherapy in between, to prevent tumor progression.
Owing to the estimated high risk of recurrence, systemic chemotherapy was continued postoperatively for 6 to 8 courses. Discontinuation of the treatment was decided in the absence of any persistent or recurrent tumor.
Statistical Analysis
All factors likely to be predictive of survival after liver resection were evaluated. They concerned patient's data (age, gender), the colorectal primary (location, stage, invasion of lymph nodes), the characteristics of liver metastases at time of diagnosis (size, number, bilaterality, timing from the primary, initial main cause of unresectability, tumor markers), the preoperative chemotherapy (protocol, number of courses, type of drug delivery, response), the operative procedure (anatomic versus wedge resection, number of resected segments, curative pattern, blood transfused units), the use if any adjuvant treatment (portal embolization, cryotherapy or radiofrequency, 2-stage hepatectomy), the number of hepatectomies and the pathology of the resected specimen (number, size of metastases, resection margins, percentage of necrosis). All these factors were evaluated by uni- and multivariate analysis. For quantitative items, evaluation of cutoff values was performed to determine the more discriminant value with regard to survival. Overall and disease-free survival probabilities were calculated using the Kaplan-Meier method and data were compared by the log-rank test. A P value <0.05 was considered significant. Multivariate analysis using a Cox model was completed for all factors with a P value ≤0.15 at univariate analysis.
Estimation of survival was adjusted on risk factors.25,26 The model was focused on the sole items available preoperatively with the aim to allow medical oncologists or surgeons to predict the expected survival of any individual patient, turned resectable, before the decision of liver surgery.
RESULTS
Liver Resection
The majority of procedures were first hepatectomies (n = 128, 93%) (Table 2). There were 103 major (75%) and 35 limited (25%) hepatectomies. Mean perioperative blood transfusion was 2.6 units (0–30). Most liver resections were nonanatomic or mixed—anatomic and nonanatomic—procedures (69%). The majority of resections were potentially curative either after 1- or 2-stage procedures (93%, 129/138).
TABLE 2. Main Data Concerning Liver Resection, Associated Procedures and Outcome
Combined Techniques to Achieve Resectability
Preoperative portal embolization was used in 12 patients (9%). Radiofrequency or cryosurgery was combined to liver resection in 20 patients (14%). Two-stage hepatectomy was needed in 15 patients (11%). Overall, 42 patients (30%) needed at least 1 of these specific techniques, sometimes combined to each other to allow surgery to be feasible and potentially curative (Fig. 3).
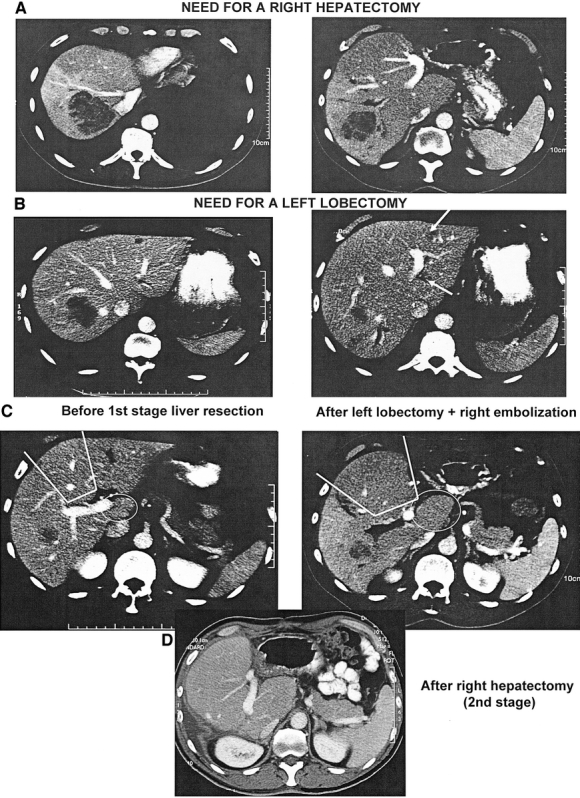
FIGURE 3. A 50-year-old patient with 7 liver metastases requiring both resection of the right liver (segments: 5, 6, 7, 8) (a), and of the left lobe (Segment 2 and 3) (b) impossible to perform in a single procedure. After tumor downstaging by chemotherapy, performance of bisegmentectomy 2, 3 with concomitant right portal branch embolization. Further hypertrophy of segment 4 and segment 1 with right liver atrophy permitted a second-stage right hepatectomy (c). Remnant liver consisted of only segment 4 and segment 1 (d). The patient was alive without recurrence 4.5 years after resection.
Surgery of Previous or Concomitant Extrahepatic Metastases
Resection was achieved in 41 of the 52 patients with previous or concomitant extrahepatic tumor (79%). Extrahepatic tumor spread was resected prior to liver surgery in 16 patients (12%). The procedures concerned resection of peritoneal nodules (n = 5), lymphadenectomy of the hepatic pedicle (n = 2), pulmonary resection (n = 3), ovariectomy (n = 2), bone resection (n = 2), and resection of colorectal tumor recurrence (n = 2). At the time of hepatectomy, 11 patients (8%) had concomitant surgery of peritoneal nodules (n = 6), lymphadenectomy of the hepatic pedicle (n = 4), or both (n = 1). After liver resection, 14 patients (10%) underwent pulmonary resection (n = 10), colectomy (n = 1), nephrectomy (n = 1), bone resection (n = 1), and lymphadenectomy combined to peritoneal resection (n = l). Among the 11 patients for whom a curative approach of extrahepatic disease could not be achieved, 2 had also a noncurative hepatectomy. There were 18 patients overall (13%) who did not have complete treatment of their metastases.
Operative Mortality
Operative mortality within 2 months was 0.7% (1/138). One patient died 1.5 month after liver resection from unexpected cardiac arrest caused by cardiac arrhythmia during the realization of the first postoperative course of chemotherapy.
Postoperative Complications
Postoperative morbidity rate was 28% (39/138 patients). Fifteen patients had local complications only (11%), 13 had general complications only (9%), and 11 had both local and general complications (8%). Twenty-six patients (19%) suffered from 35 local complications: 7 transient postoperative liver failure (5%); 8 biliary leaks (6%); 3 reoperations (2%) for either hepatic bleeding, parietal hemorrhage or bowel perforation; 3 postoperative bleeding not requiring reoperation (2%); and 6 infected (4%) and 8 noninfected (6%) fluid collections, all treated with percutaneous drainage.
General complications occurred in 24 patients (17%), 11 of whom (8%) had also hepatic complications. There were 11 pleural effusions (8%), 4 pulmonary infections (3%), 3 cardiac arrhythmias (2%), of which 1 led to patient death at day 36, and 6 miscellaneous complications (4%; anaphylactic shock, caval thrombosis, renal insufficiency, urinary tract infection, deep vein thrombosis, infected port). Mean duration of hospital stay was 13.6 days (6–39).
Pathologic Findings
Among the 138 specimens of liver resection, a complete necrosis of metastases was found in 10 cases, representing 7.2% of complete pathologic response. In the nontumoral liver, fatty infiltration was found in 53 cases (38%), 43 of which mild (<30% of hepatocytes) and 10 important (7%; >30% of hepatocytes). Sinusoidal dilatation and congestion was assessed in 11 cases (8%) and fibrosis in 32 of 123 cases (26%), mainly mild (strictly portal, n = 29), rarely important (porto-portal, n = 2; septal, n = 1). In 15 resection specimens, no sufficient liver tissue could be examined at distance from the tumor to assess the presence of fibrosis. Regenerative nodular hyperplasia was found in 6 cases (4%).
Outcome
After a mean follow-up of 48.7 months (1.2–179), 111 of the 138 operated patients (80%) developed tumor recurrence, 40 (29%) of whom were merely hepatic, 12 (9%) merely extrahepatic (Fig. 4), and 59 (43%) both hepatic and extrahepatic (Table 2). Of the 40 isolated hepatic recurrences, 22 (55%) could be treated by a repeat hepatectomy. Four of the 12 isolated extrahepatic recurrences (33%) were submitted to further surgical resection. Of the 59 patients with both hepatic and extrahepatic recurrences, 30 (51%) underwent a repeat hepatectomy, and 24 (41%) could be resected of their extrahepatic recurrence. At the last update, the 138 patients totaled 342 surgical procedures, including 223 hepatectomies (14 hepatectomies before the 138 of the study and 71 repeat hepatectomies), 42 specific procedures to allow resectability and 77 extrahepatic surgical procedures.
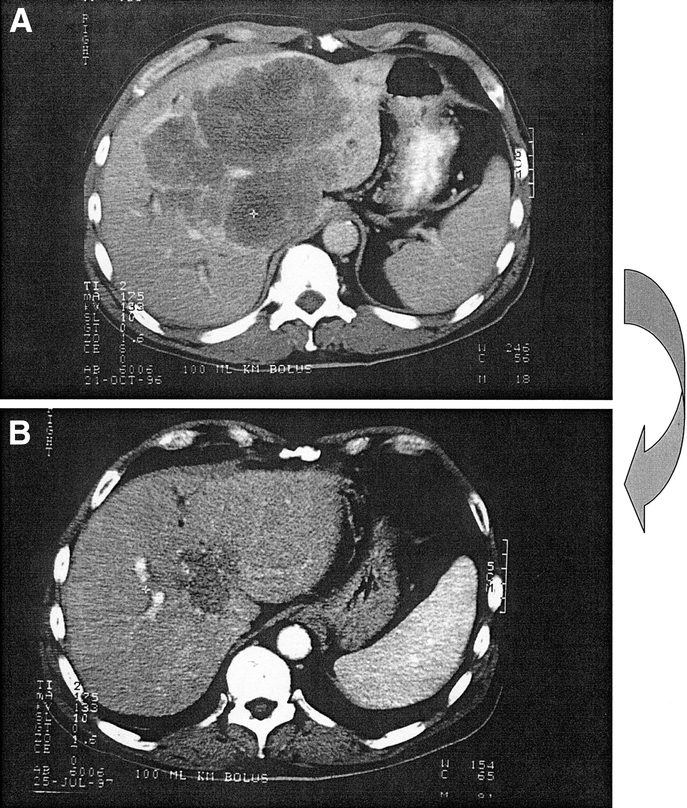
FIGURE 4. a, A 56-year-old patient with a 16-cm central synchronous metastasis progressing despite 3 lines of chemotherapy and considered as unresectable. (b) Reduction in size (16 to 6 cm) after chronomodulated chemotherapy with 5-fluorouracil, folinic acid, and oxaliplatin allowed potentially curative left hepatectomy (segments 2, 3, 4) extended to segment 1. No hepatic recurrence but pelvic recurrence of the rectal primary was responsible for the patient's death at 3.2 years after liver resection.
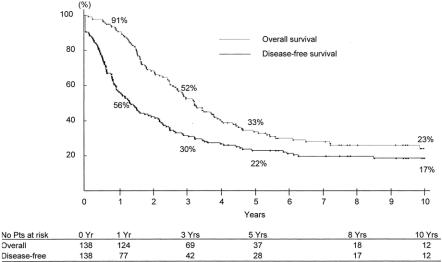
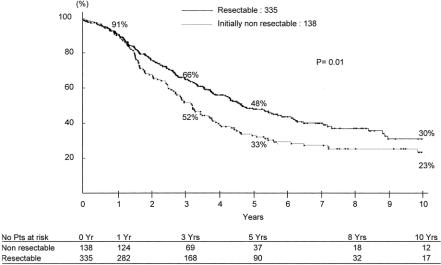
Survival was 52%, 33%, and 23% at 3, 5, and 10 years, with a disease-free survival of 30%, 22%, and 17%, respec tively (Fig. 5). Overall survival was decreased as compared with that of patients primarily resected within the same period (66%, 48% and 30% respectively, P = 0.01) (Fig. 6). At the last follow-up, 99 patients had died (72%) and 39 (28%) were alive, of whom 25 were disease-free (18%) and 14 had recurrence (10%) (Table 2).
FIGURE 5. Overall and disease-free survival of 138 patients resected for initially unresectable colorectal liver metastases, turned resectable by systemic chemotherapy.
FIGURE 6. Compared survival of liver resection for primarily resectable (n = 335 patients) versus primarily unresectable metastases downstaged by chemotherapy (n = 138 patients).
Predictive Factors of Survival: Univariate Analysis
Overall Survival
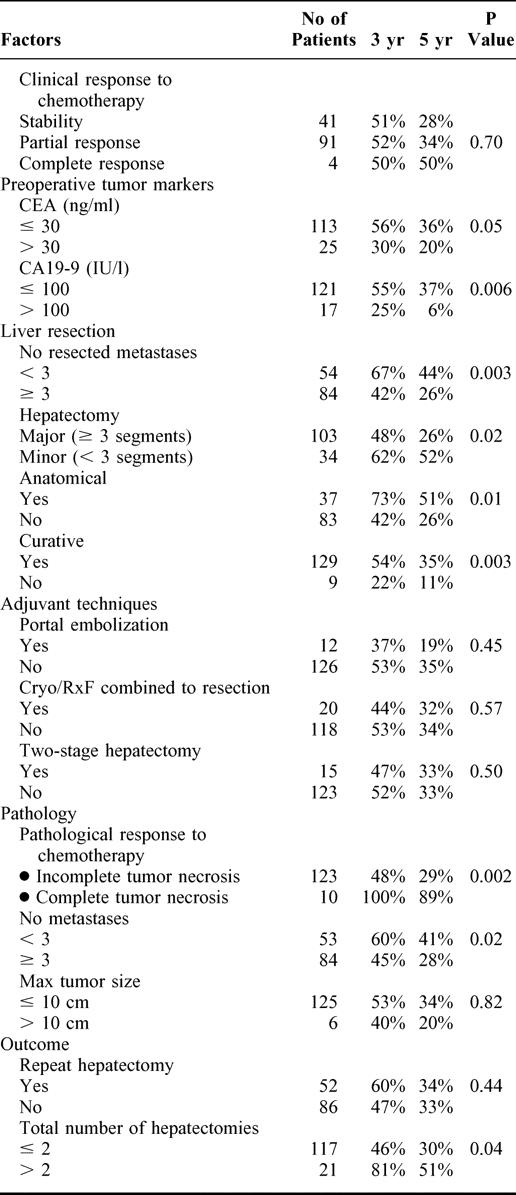
Among the 31 factors likely to influence survival after liver surgery, 10 were significantly associated to outcome at univariate analysis (Table 3): the preoperative value of serum CA 19–9 (P = 0.006), the preoperative level of CEA (P = 0.05), the number of liver metastases either at operation (P = 0.003) or in the resection specimen (P = 0.02), their bilobar distribution (P = 0.05), a number ≥3 of resected segments (P = 0.02), the nonanatomic and noncurative pattern of liver resection (P = 0.01 and P = 0.003, respectively), a history of less than 3 hepatectomies (P = 0.04), and an incomplete necrosis of tumors on the resected specimen (P = 0.002).
TABLE 3. Impact of Prognostic Factors on Survival (Univariate Analysis)
TABLE 3. continued.
Disease-Free Survival
In addition to factors yet demonstrated to influence overall survival (except bilobar distribution and nonanatomic liver resection), tumoral invasion of hepatic and celiac lymph nodes (P = 0.02) was significantly associated to decreased disease-free survival.
Multivariate Analysis
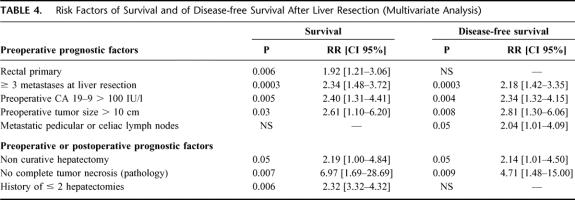
Seven factors were independently associated to decreased overall survival: a rectal primary, a number ≥3 metastases at liver resection, a preoperative CA 19–9 >100 UI/L, a preoperative tumor size >10 cm, a noncurative hepatectomy, an incomplete necrosis of the tumor on the specimen, and a history of ≤2 hepatectomies (Table 4). Six were associated to decreased disease-free survival (Table 4). While the rectal primary was a risk factor only for overall survival, the presence of metastatic hepatic or celiac lymph nodes emerged as a risk factor for disease-free survival.
TABLE 4. Risk Factors of Survival and of Disease-free Survival After Liver Resection (Multivariate Analysis)
Preoperative Model to Predict Survival
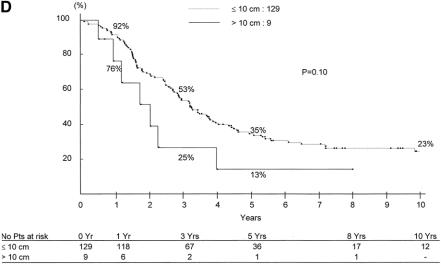
As the objective was to estimate survival on the preoperative setting, only risk factors that were available preoperatively were considered in the model. Therefore 4 factors (rectal primary, ≥3 metastases, CA 19–9 >100 UI/L, maximum tumor diameter >10 cm) (Fig. 7a-d), were used to adjust overall survival. Whereas the survival expectancy at 5 years was 59% for patients without any risk factors, the range was 23% to 41% for 1 factor (mean:30%), 2% to 14% for 2 factors (mean 7%), and 0% to 1% for 3 and 4 factors (Table 5).
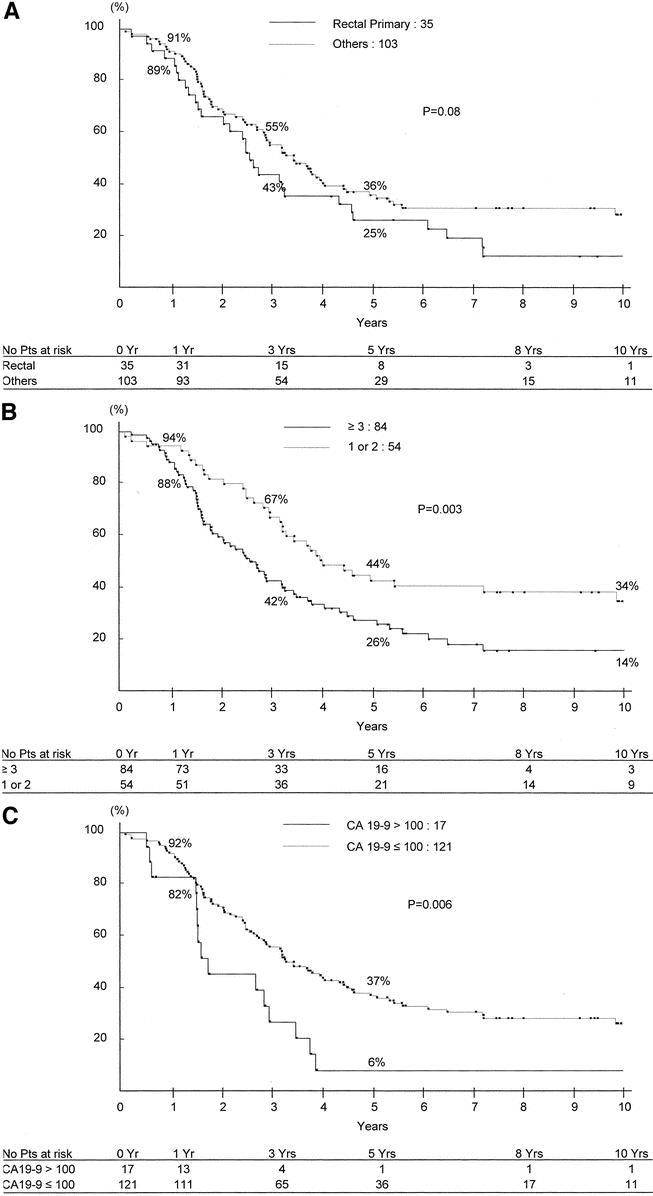
FIGURE 7. Survival after liver resection in relation to preoperative risk factors: a, rectal primary versus colon primary; b, number of metastases ≥3; c, CA 19–9 >100 UI/L; and d, maximum tumor size >10 cm.
FIGURE 7. Continued.
TABLE 5. Estimation of Overall Survival Adjusted on Preoperative Risk Factors
With regard to disease-free survival, the 5-year survival expectancy was 42% in the absence of any risk factors, 10% to 16% for 1 factor, 0% to 1% for 2 risk factors, and 0% for 3 and 4 risk factors.
DISCUSSION
The results of the present study demonstrate that liver resection could offer a possibility of long-term survival to patients with primarily unresectable metastases downstaged by chemotherapy. This potential benefit may be obtained with low postoperative mortality (less than 1%) and acceptable morbidity. To predict 5-year survival is possible in relation to 4 risk factors, available preoperatively.
The strategy to resect unresectable patients after effective chemotherapy has been a major breakthrough in the recent history of metastatic colorectal cancer. It allows patients under palliative chemotherapy otherwise promised poor outcome within 3 years to have a chance of long-term survival. Initiated by our group some years ago,10–12 this oncosurgical strategy is now increasingly adopted worldwide, whichever the chemotherapy regimen and the mode of drug delivery.27 However, owing to the recent adoption of such a strategy, the series are limited, and the follow-up of patients resected after neoadjuvant chemotherapy did not exceed 4 years.13–21 In our preliminary experience, we reported an actuarial 5-year survival of 35% to 40%.11–12 With a much longer follow-up, survival is currently 33% at 5 years and 22% at 10 years, with a median survival of 39 months. These results strongly support the policy to propose liver surgery to all patients whose metastases are sufficiently downstaged by chemotherapy to envisage their complete resection. By comparison, chemotherapy alone is now able to provide a median survival of around 20 months, but even with the use of new drugs, 5-year survival is almost impossible.5,6 Accordingly, a controlled study comparing chemotherapy alone to chemotherapy followed by surgery would be unethical. On the opposite side, surgery alone is associated with 5-year survival rates of 30% to 40%1–5 but is obviously addressed to a selected group of patients with limited liver involvement (10%-20% of the overall population). Otherwise, for patients with widespread disease, incomplete tumor resection has little impact on long-term outcome.28
Our patients treated by the combination of chemotherapy and surgery had initially highly advanced metastatic disease as assessed by the proportion of 80% of bilobar metastases, 60% exceeding 3 lesions and the presence of extrahepatic tumor in 38% of cases. Such patients would not have been operated some years ago owing to the dogma that unresectable metastases should definitively be treated by palliative chemotherapy. The decision of surgery was taken with the hypothesis that provided a complete treatment; a hope of long-term survival could be obtained. Although appreciable, the survival rate obtained was lower than that of primarily resected patients (33% versus 48% at 5 years), and this relied on the more extensive tumor spread of patients that were initially unresectable.
The policy adopted was that of an attempt of eradication of the tumoral process using prolonged chemotherapy (as neoadjuvant and then adjuvant therapy) and aggressive surgery not only for the initial metastases but also for any recurrence who could be resected with an option of radicalness. Accordingly, the 138 patients of the study totalized 342 surgical procedures, including 42 specific procedures to allow resectability, 223 hepatectomies, and 77 extrahepatic surgical procedures.
Recurrence was frequent, particularly in the liver, either isolated (29%) or associated to extrahepatic tumor (43%), reflecting both the severity of initial metastatic disease and the possible remanence of tumors, downstaged to such a point that they could have apparently disappeared. However, some patients suffered postoperative reappearance of lesions considered as “ablated” by chemotherapy, a fact that should be connected to the evidence that only 7% of patients had complete necrosis of their metastases after chemotherapy, at pathology of the resection specimen. Therefore, active tumor persisted in previous tumor sites in most cases. For this reason, we presently advocate medical oncologists to refer patients to surgeons before obtaining a “complete response.” Otherwise, surgery is totally blinded and highly risky to miss “dormant” metastases that will progress as soon as chemotherapy is withdrawn or become less effective.
Another important issue was that despite the heaviness of therapy, the operative risk was low, with an operative mortality of less than 1% and a morbidity of 28%, similar to that previously reported for primary liver resection.1–4,29 These patients had received prolonged chemotherapy known to produce histologic lesions on the liver. Accordingly, fatty infiltration, vascular congestion, and some extent of fibrosis were observed in the nontumoral liver, but no clear impact on mortality and morbidity was observed compared with primary surgery of liver metastases.
The Proportion of Patients Turned Resectable With Chemotherapy
The initial assessment of unresectability of the patients of this series was a critical point. Obviously, if resectable liver metastases would have been declared erroneously inoperable at first assessment, the study could easily turn meaningless.30 Fortunately, all cases of initial unresectability were reviewed according to well-established criteria, and unresectability was defined either by the impossibility to resect all tumors while leaving at least 30% of the total liver volume or by the concomitant presence of extrahepatic metastases. The resectability rate of 12.5% after effective chemotherapy was in agreement with our previous results.10,11 However, criteria of unresectability may vary from one team to another, and higher proportions of 30% to 43% of converted resectability have been reported by others,15,16 reflecting probably different selection policies. Nonetheless, the important fact was that whatever the group considered and its adopted selection criteria for resectability, a proportion of patients initially nonoperable was secondarily considered by the same team as amenable to liver resection. Owing to the high number of patients with colorectal cancer worldwide, half of them developing liver metastases, this strategy could concern thousands of patients for whom a hope of long-term survival is currently offered.
Prognostic Factors of Survival Following Surgery
Another important issue was the benefit provided by such a strategy. To date, our policy was to perform liver resection in all patients whose tumors were sufficiently downstaged as to allow radical surgery. The first objective of curative surgery could be achieved in 93% of liver metastases but in only 79% of extrahepatic metastases, leading to 87% of patients successfully treated by a curative approach for both hepatic and extrahepatic tumor. The second objective was long-term survival. It was not achieved for all patients. This prompted us to determine the prognostic factors of long-term survival to predict, on an individual basis, the expected benefit of surgery. A rectal primary (versus colon cancer) was independently associated to decreased survival. This could be related to the higher difficulty of radical surgery in rectal cancers and to their increased risk of local recurrence as compared with colon cancers. Similarly, a negative impact of rectal site of primary tumor on survival after liver resection of metastases was recently reported.31 In addition, the context of initially unresectable liver metastases made that the radicalness of surgery of the primary was often neglected in view of the usual poor prognosis of these patients, as illustrated by the case of Figure 4. The advent of effective chemotherapy should currently lead colorectal surgeons to achieve radical resection of the primary even in the cases of initially unresectable liver metastases.
Not surprisingly, the noncurative pattern of hepatectomy was identified as a prognostic factor, as well as a number ≥3 metastases and a tumor size exceeding 10 cm. These are factors commonly reported in main series1,2,4,5 or prognostic scores.3,32,33 Tumor markers were also predictive of survival, but in our study CA 19–1 obviously performed better than CEA. CEA is usually regarded as a better indicator of poor prognosis and recurrence of colorectal cancer.34 However, in advanced colorectal carcinoma, a study suggested serum CA 19–1 as one of the most important prognostic factors.35 Both markers were recommended in patients with colorectal liver metastases.36 Of clinical importance was the fact that concomitant extrahepatic metastases, present in 38% of our patients, had no significant impact on survival except metastatic lymph nodes of the hepatic pedicle. Extrahepatic disease has long been considered as an absolute contraindication to surgery of colorectal liver metastases. Since some years, this has been challenged by some groups, including ours.1,3,10,11,29,37,38 However, extrahepatic tumor has been commonly reported as a factor of poor prognosis.1,2,3,32 The absence of significant effect on overall survival in our study suggests that effective chemotherapy combined to sequential surgery of extrahepatic metastases could have erased their negative effect on prognosis.
Individual Assessment of the Expected Survival After Surgery
Four risk factors were retained for predicting survival of patients whose liver metastases were downstaged by chemotherapy and for whom surgery should be indicated. Interestingly, the expected survival benefit at 5 years is appreciable in the presence of up to 2 risk factors but is almost nil for more than 2 factors. This could be of utmost clinical importance, allowing appropriate selection of patients and adequate timing for surgery. Of course, these results should be further validated on a prospective basis, but they may at once help oncologists and surgeons to make the “good decision” for patients whose indication for liver resection is highly questioned.
In summary, modern chemotherapy allows 12.5% of patients with unresectable CRLM to be rescued by liver surgery. This strategy offers a possibility of long-term survival (33% at 5 years and 22% at 10 years) with a low operative risk. It involves a wide use of repeat hepatectomies and extrahepatic resections. Four preoperative risk factors are able to select the patients more likely to benefit from this strategy.
Discussions
Dr. Harold J. Wanebo (Providence, Rhode Island): Dr. Adam and members of the Bismuth group are continuing to push the envelope for neoadjuvant therapy of high stage liver metastases from colorectal cancer. Their focus is on the responders who were made amenable to resection following ten courses of adjuvant therapy. Of interest, 22% of these had extrahepatic metastases. The thrust of my questions will focus on this group.
The presence of lymph node metastases is classically thought to be a reason to exclude patients from resection. And the question is, what would the authors recommend to the surgeon who encounters lymph node metastases at the time of planned resection? Should one just proceed with the resection and remove the lymph node metastases or should you back out and give adjuvant therapy? I also noticed that they had a group of patients with peritoneal metastases, some of whom I believe may have survived. And my question, what should be the optimum management for that group if encountered in the operating room?
A second line of questioning focuses on the very interesting utilization of the CA 19–9 in comparison to the CEA. I think most of us have been brought up on the CEA as a useful tumor marker to identify patients with recurrence or metastases from colorectal cancer. The potential prognostic relationships of CEA are well defined in colorectal cancer but the CA 19–1 is not commonly used, so we are intrigued at the author's use of this tumor marker.
And lastly, there still is a fairly substantial failure rate, approaching 80%, in the liver. Do the authors have any plans to alter that? They are able to do re-resections in some of the patients, but the question remains whether some other approach might be considered such as hepatic artery infusion.
I enjoyed the presentation. Thank you very much.
Dr. Rene Adam (Villejuif, France): Thank you, Dr. Wanebo, for your interesting questions.
With regard to the first point concerning lymph node involvement by the tumor, I fully agree with you that this is probably the most negative prognostic factor with regard to survival. All the patients of the present series had been downstaged by chemotherapy and our policy is indeed to operate only patients who have responded to chemotherapy. In practice, whether we know preoperatively that metastatic lymph nodes are present and we submit the patient to neoadjuvant chemotherapy. Response to chemotherapy will help us to select those patients to operate. Whether metastatic lymph nodes are discovered during liver resection. In this case, we proceed to hepatectomy and lymphadenectomy when all the tumoral tissue could be resected and when hepatectomy is not highly risky. Otherwise, we also prefer delayed liver surgery after chemotherapy. In both cases, postoperative chemotherapy is done systematically. The same attitude is adopted for peritoneal carcinomatosis.
With regard to CA 19–9, it was also a surprise for us that this tumor marker shown better prognostic value than CEA. However, we have yet observed this for pulmonary metastases from colorectal cancer. CA 19–9 had better prognostic value for survival after resection than CEA.
Your third question concerns repeat hepatectomy. Indeed, all the patients of this series were exposed to a high risk of recurrence and repeat hepatectomy was used in all the cases for which it could be potentially curative. Accordingly, 71 repeat hepatectomies were performed in 52 patients with hepatic recurrence. The same aggressive policy was adopted for extrahepatic recurrence explaining that a total of 342 surgical procedures were performed in the 138 patients of the series.
Dr. Robert J. Fitzgibbons, Jr. (Omaha, Nebraska): It seems to me the logical extrapolation from this study is the use of a less morbid therapy for these patients that are converted to a resectable stage, such as radiofrequency ablation. My question is, do you have any experience with such an approach rather than resection?
Dr. Rene Adam (Villejuif, France): In our unit, we use radiofrequency as well as surgery in the treatment of liver metastases. However, we restrict the use of local treatments to non-resectable lesions when limited in number (< 3) and in size (< 4 cm). This treatment could, of course, be combined to liver resection but never preferred to resection for resectable metastases since long-term results of surgery are well known, not those of radiofrequency.
Dr. Steven M. Strasberg (St. Louis, Missouri): Professor Adam, I enjoyed your paper. Congratulations on the continuing contributions of your group to this area, important contributions.
I have 2 questions. One, are you using FDG-PET scanning? And if you are, how does that fit into the algorithm that you use for these patients?
Secondly, it just seems to me that the percent of patients that are being down-staged is high compared to the overall group of patients that you are treating. And you have shown us examples of unresectable patients and we would all agree that the patients that you showed us are unresectable. Is there a tendency to use chemotherapy now in your group in those patients who are perhaps barely resectable? Do you tend to say, when the lesions are large and perhaps close to the veins, let us use chemotherapy and see if we can make these patients perhaps more resectable than they are at the time you see them for the first time?
Dr. Rene Adam (Villejuif, France): Thank you, Dr. Strasberg, for your kind comments and pertinent questions.
PET scan has been rarely used in this series. Our common policy relied on the combination of abdomino-pelvic ultrasound with CT scan of chest, abdomen and pelvis. However, when there was a doubt on the tumoral nature of a lesion or on the presence of extrahepatic disease, undetectable by conventional means, we used the PET scan. With regard to the increasing response rate to chemotherapy and its impact on liver surgery, it is true that current protocols allow 70–80% of patients to be downstaged or at least stabilized by chemotherapy. I fully agree with you that a reduction in tumor size may help the surgeon to perform a better curative operation for patients who are a little bit “marginal” for the indication of resection. Also, for multinodular metastases, it is now our policy to treat all patients with more than 3 nodules by neoadjuvant chemotherapy, even when they are initially resectable, in a way to control the tumor disease before surgery. One factor is for us critical on this setting: not to operate a patient whose tumor progresses while on chemotherapy.
Dr. David W. Easter (San Diego, California): Dr. Adam, that was a great paper, well presented. I am going to struggle to help you with control of your selection bias.
I wonder if there are any patients who declined your aggressive approach and said “no thanks” to the hepatic artery infusion, or “even though I might be resectable, I am going to take my adjuvant chemotherapy alone.” Do you have a subset analysis of that group that might have refused your approach?
Dr. Rene Adam (Villejuif, France): Thank you for this question. You point out a crucial issue. Obviously, the program of treatment of all these patients was a heavy one, including sometimes repeat operations with chemotherapy in between. For this reason, the first thing I explain to such patients is that the treatment will be long and sometimes difficult to face but that they should be convinced about the possibility to obtain a long-term remission and why not of cure. With this shared objective, I have been almost never faced with a patient saying, “No, Doctor, thank you, I don't want to enter into this program. I continue on chemotherapy.” This is really very, very rare and anyway much less frequent than the reverse situation of a majority of patients on prolonged chemotherapy, who ask for an operation unfortunately impossible to perform because of insufficient downstaging.
Footnotes
Reprints: René Adam, MD, PhD, Centre Hépato-Biliaire, Hopital Paul Brousse, 14 Av PV, Couturier 94800, Villejuif, France. E-mail: rene.adam@pbr.ap-hop-paris.fr.
REFERENCES
- 1.Scheele J, Stangl R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. [DOI] [PubMed] [Google Scholar]
- 2.Jaeck D, Bachellier P, Guiguet M, et al. Long-term survival following resection of colorectal hepatic metastases: Association Francaise de Chirurgie. Br J Surg. 1997;84:977–980. [DOI] [PubMed] [Google Scholar]
- 3.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam R, Pascal G, Azoulay D, et al. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg. 2003;238:871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stangl R, Altendorf-Hofmann A, Charnley RM, et al. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–1410. [DOI] [PubMed] [Google Scholar]
- 6.Giacchetti S, Itzhaki M, Gruia G, et al. Long-term survival of patients with unresectable colorectal cancer liver metastases following infusional chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin and surgery. Ann Oncol. 1999;10:663–669. [DOI] [PubMed] [Google Scholar]
- 7.Levi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer: International Organization for Cancer Chronotherapy. Lancet. 1997;350:681–686. [DOI] [PubMed] [Google Scholar]
- 8.De Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. [DOI] [PubMed] [Google Scholar]
- 9.Douillard J-Y, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomized trial. Lancet. 2000;355:1041–1047. [DOI] [PubMed] [Google Scholar]
- 10.Bismuth H, Adam R, Levi F, et al. Resection of unresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adam R, Avisar E, Ariche A, et al. Five-year survival following hepatic resection after neoadjuvant therapy for unresectable colorectal. Ann Surg Oncol. 2001;8:347–353. [DOI] [PubMed] [Google Scholar]
- 12.Levi F, Misset JL, Brienza S, et al. A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump: high antitumor effectiveness against metastatic colorectal cancer. Cancer. 1992;69:893–900. [DOI] [PubMed] [Google Scholar]
- 13.Fowler WC, Eisenberg BL, Hoffman JP. Hepatic resection following systemic chemotherapy for metastatic colorectal carcinoma. J Surg Oncol. 1992;51:122–125. [DOI] [PubMed] [Google Scholar]
- 14.Wein A, Riedel C, Kockerling F, et al. Impact of surgery on survival in palliative patients with metastatic colorectal cancer after first line treatment with weekly 24-hour infusion of high-dose 5-fluorouracil and folinic acid. Ann Oncol. 2001;12:1721–1727. [DOI] [PubMed] [Google Scholar]
- 15.Alberts SR, Horwarth W, Donohue JH, et al. Oxaliplatin, 5-fluorouracil and leucovorin for patients with liver only metastases from colorectal cancer: a North Central Cancer Treatment Group Phase II Study [abstract]. Proc ASCO. 2001,20:2. [Google Scholar]
- 16.Rivoire M, De Cian F, Meeus P, et al. Combination of neoadjuvant chemotherapy with cryotherapy and surgical resection for the treatment of unresectable liver metastases from colorectal carcinoma. Cancer. 2002;95:2283–2292. [DOI] [PubMed] [Google Scholar]
- 17.Adam R. Chemotherapy and surgery: new perspectives on the treatment of unresectable liver metastases. Ann Oncol. 2003;14:ii13–ii16. [DOI] [PubMed] [Google Scholar]
- 18.Elias D, Lasser P, Rougier P, et al. Frequency, technical aspects, results, and indications of major hepatectomy after prolonged intra-arterial hepatic chemotherapy for initially unresectable hepatic tumors. J Am Coll Surg. 1995;180:213–219. [PubMed] [Google Scholar]
- 19.Link KH, Pillasch J, Formentini A, et al. Downstaging by regional chemotherapy of non-resectable isolated colorectal liver metastases. Eur J Surg Oncol. 1999;25:381–388. [DOI] [PubMed] [Google Scholar]
- 20.Clavien PA, Selzner N, Morse M, et al. Downstaging of hepatocellular carcinoma and liver metastases from colorectal cancer by selective intra-arterial chemotherapy. Surgery. 2002;131:433–442. [DOI] [PubMed] [Google Scholar]
- 21.Shankar A, Leonard P, Renaut AJ, et al. Neo-adjuvant therapy improves resectability rates for colorectal liver metastases. Ann R Coll Surg Engl. 2001;83:85–88. [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Handbook for Reporting Results of Cancer Treatment. Geneva: World Health Organization; 1979. WHO offset publication no. 48.
- 23.Azoulay D, Castaing D, Krissat J, et al. Percutaneous portal vein embolization increases the feasibility and safety of major resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adam R, Laurent A, Azoulay D, et al. Two-stage hepatectomy: a planned strategy to treat unresectable liver tumors. Ann Surg. 2000;232:777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breslow NE, et al. Covariance analysis of censored survival data. Biometrics. 1974;30:89–99. [PubMed] [Google Scholar]
- 26.Cox DR, et al. Regression models and life tables (with discussion). J R Stat Soc B. 1972;34:187–202. [Google Scholar]
- 27.Topham C, Adam R. Oncosurgery: a new reality in metastatic colorectal carcinoma. Semin Oncol. 2002;29(suppl 15):3–10. [DOI] [PubMed] [Google Scholar]
- 28.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. [DOI] [PubMed] [Google Scholar]
- 29.Adam R. The importance of visceral metastasectomy in colorectal cancer. Ann Oncol. 2000;11:29–36. [DOI] [PubMed] [Google Scholar]
- 30.Borner MM. Neoadjuvant chemotherapy for unresectable liver metastases of colorectal cancer: too good to be true? Ann Oncol. 1999;10:623–626. [DOI] [PubMed] [Google Scholar]
- 31.Laurent C, Sa Cunha A, Couderc P, et al. Influence of postoperative morbidity on long-term survival following liver resection for colorectal metastases. Br J Surg. 2003;90:1131–1136. [DOI] [PubMed] [Google Scholar]
- 32.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection based on 1588 patients: Association Française de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 33.Iwatsuki S, Dvorchik I, Madariaga JR, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morita S, Nomura T, Fukushima Y, et al. Does serum CA 19–9 play a practical role in the management of patients with colorectal cancer? Dis Colon Rectum. 2004;47:227–232. [DOI] [PubMed] [Google Scholar]
- 35.Kouri M, Nordling S, Kuusela P, et al. Poor prognosis associated with elevated serum CA 19–9 levels in advanced colorectal carcinoma, independent of DNA ploidy and SPF. Eur J Cancer. 1993;29A:1691–1696. [DOI] [PubMed] [Google Scholar]
- 36.Ishizuka D, Shirai Y, Sakai Y, et al. Colorectal carcinoma liver metastases: clinical significance of preoperative measurement of serum carcinoembryonic antigen and carbohydrate antigen 19–9 levels. Int J Colorectal Dis. 2001;16:32–37. [DOI] [PubMed] [Google Scholar]
- 37.Minagawa M, Makuuchi M, Torzilli G, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elias D, Ouellet JF, Bellon N, et al. Extrahepatic disease does not contraindicate hepatectomy for colorectal liver metastases. Br J Surg. 2003;90:367–374. [DOI] [PubMed] [Google Scholar]