A New Prognostic Staging System for Rectal Cancer (original) (raw)
Abstract
Objective:
To clarify the appropriateness of tumor “budding,” a quantifiable histologic variable, as 1 parameter in the construction of a new prognostic grading system for rectal cancer.
Summary Background Data:
Patient division according to an accurate prognostic prediction could enhance the effectiveness of postoperative adjuvant therapy and follow-up.
Patients and Methods:
Tumor budding was defined as an isolated cancer cell or a cluster composed of fewer than 5 cells in the invasive frontal region, and was divided into 2 grades based on its number within a microscopic field of ×250. We analyzed 2 discrete cohorts comprising 638 and 476 patients undergoing potentially curative surgery.
Results:
In the first cohort, high-grade budding (10 or more foci in a field) was observed in 30% of patients and was significantly associated with a lower 5-year survival rate (41%) than low-grade budding (84%). Similarly, in the second cohort, the 5-year survival rate was 43% in high-grade budding patients and 83% in low-grade budding patients. In both cohorts, multivariate analyses verified budding to be an independent prognosticator, together with nodal involvement and extramural spread. These 3 variables were given weighted scores, and the score range was divided to provide 5 prognostic groups (97%; 86%; 61%; 39%; 17% 5-year survival). The model was tested on the second cohort, and similar prognostic results were obtained.
Conclusions:
We propose that because of its relevance to prognosis and its reproducibility, budding is an excellent parameter for use in a grading system to provide a confident prediction of clinical outcome.
Based on 2 discrete cohorts of rectal cancer patients, tumor “budding” was identified as a reproducible independent prognostic indicator that can improve the erroneous staging of advanced disease as early-stage disease. A grading system using the 3 parameters (tumor depth, nodal involvement, and budding) provided a wider spectrum of 5-year survival rates (18–98%).
Dukes's original classification was proposed more than 70 years ago.1,2 Since then, this classification for rectal cancer, composed of the 2 parameters, tumor penetration depth and nodal involvement, has been the most widely employed prognostic classification for colorectal cancer, even after some modifications3–6 such as the Astler-Coller's classification7 and the UICC classification.8 However, the multiple attempts to refine this system attest to its imperfection.9–12 One major flaw is that only a small proportion of patients can be classified into either the excellent prognostic group (Dukes A) or the poor prognostic group (Dukes C2). Furthermore, the Dukes B group, or the UICC stage II group, has proved to be a perplexingly broad category with respect to patient-survival outcomes, primarily because it is difficult for current routine techniques to exclude patients with occult lymph node metastases from this category. Patient division according to a more accurate prognostic prediction could enhance the effectiveness of postoperative adjuvant therapy and follow-up. Several alternative prognostic grading systems consisting of both clinical and pathologic prognostic parameters, in addition to tumor depth and the nodal involvement, have been proposed with the idea that the prediction of clinical endpoints is improved by the use of multiple independent variables.9,12–14 However, these grading systems have not come into wide use, primarily because they include parameters that fail to show satisfactory reproducibility, a fundamental requirement for prognostic markers in routine practice.15
Tumor “budding” is a pathologic characteristic that is thought to correspond to the initial phase of tumor invasion11 and has been reported to be relevant to metastatic activity16–19 and prognostic outcome.11,20,21 Based on the premise that this cancer-related feature could be quantifiable by a method similar to that used with mitotic counts, an important feature in distinguishing malignant from benign connective tissue tumors,22 we devised criteria for its assessment. The aims of the present study were to analyze a set of clinical and pathologic variables in rectal cancer patients to identify the variables having an independent effect upon cancer-associated mortality, and to estimate the appropriateness of tumor budding as one variable in the construction of a new prognostic classification. To verify the results, we performed the same analytical procedures in 2 separate patient data sets at St. Mark's Hospital. Both sets comprised patients who underwent potentially curative surgery and had been followed up for a long period.
MATERIALS AND METHODS
Patients
A total of 638 rectal cancer patients who underwent complete surgical resection of their tumors between 1960 and 1969 at St. Mark's Hospital formed the first data set. This data set was that used for the previous prognostic study from which Jass's prognostic classification was established.9 These tumors were located in the lower rectum in 174 cases, in the middle rectum in 173 cases, in the upper rectum in 241 cases, and in the rectosigmoid in 50 cases. These patients did not include those with synchronous cancers or cancers complicating familial adenomatous polyposis or inflammatory bowel disease. There were 401 males and 237 females with an average age at the time of surgery of 61.1 (range: 26–89). A second data set consisted of 476 rectal cancer patients who were operated on between 1970 and 1980 at St. Mark's Hospital. The tumor locations of these patients were as follows: the lower rectum in 138 cases, the middle rectum in 142 cases, the upper rectum in 155 cases, and the rectosigmoid in 41 cases. The average age at operation was 62.9 (range: 23–97). The collection of survival data in the second data set was performed by one of the authors (KW) after the pathologic review.
For these tumors, the morphologic features of known prognostic importance such as the tumor diameter, tumor type, differentiation, extent of local spread, and extramural venous invasion had previously been recorded in the pathologic reports. All patients selected were followed up for at least 5 years (average: 140 months; range: 61–322 months) or until death. It was exceptionally rare for adjuvant therapy to be used at St. Mark's in the 1960s and 1970s. It was only used for patients with very advanced and barely operable tumors, such cases not being among those in the present study. Only deaths attributable to recurrent cancer were counted as events in the process of establishing the prognostic grading system.
Definition of Tumor “Budding”
An isolated single cancer cell or a cluster composed of fewer than 5 cancer cells, which were observed in the stroma of the actively invasive region, was defined as a “budding” focus (Fig. 1). Tumor budding was not counted in the field where the tumor was fragmented because of the aggregation of inflammatory cells or because of a technical artifact. After overviewing all the slides containing tumors from each case, a field where budding was the most intense was selected. The number of budding foci was counted using a ×25 microscope objective, giving a final magnification of ×250 (field diameter 700 μm). A count of 0 to 9 per field was considered to be low grade, and a count of 10 or more was regarded as high grade based on the results of a previous study.21 A judgment of budding in each case was made by one of the authors (HU), whose intraobserver variation has been reported previously,21 with no information about patient prognostic outcome.
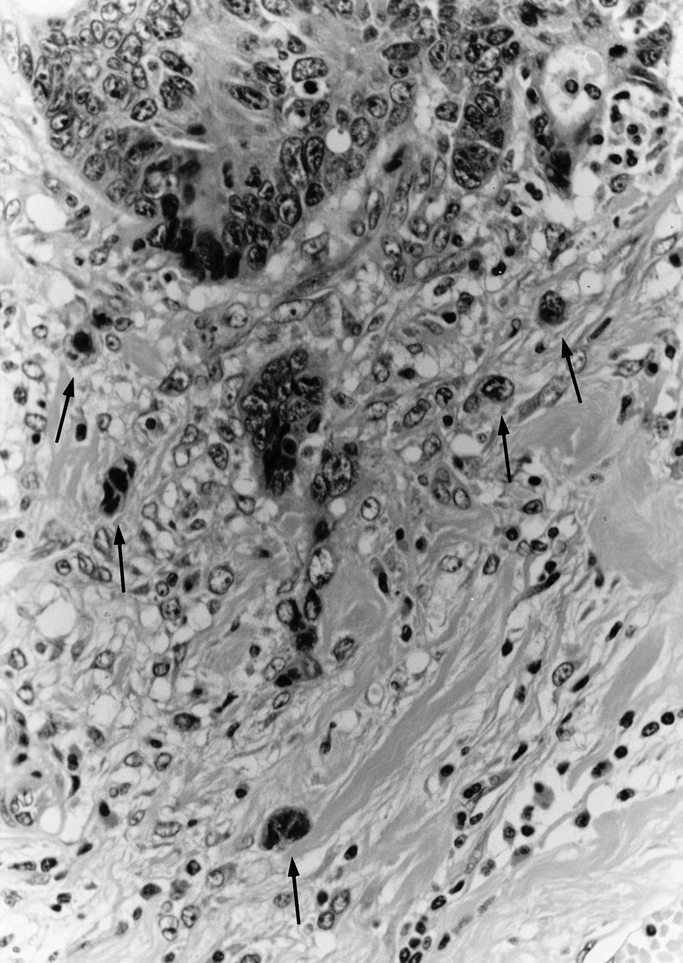
FIGURE 1. Histologic finding of tumor budding. An isolated cancer cell or a small cluster composed of fewer than 5 cancer cells (arrows) in the actively invasive region was defined as a budding focus. Original magnification: ×100
Statistical Analysis
The χ2 test was used to check for the association between categorical variables. Survival curves were drawn by the Kaplan-Meier method, and their comparisons were analyzed by the log-rank test. After the process of categorization, each variable was entered into a multivariate analysis by the Cox stepwise regression model to determine which factors had an independent effect on long-term survival.
RESULTS
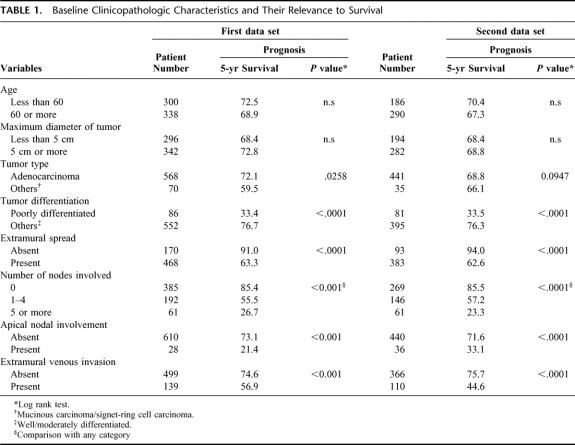
Among the baseline clinicopathological characteristics estimated (Table 1), tumor differentiation, extramural spread, the number of nodes involved, apical nodal involvement, and extramural venous invasion were shown to be significant prognostic features in both data sets.
TABLE 1. Baseline Clinicopathologic Characteristics and Their Relevance to Survival
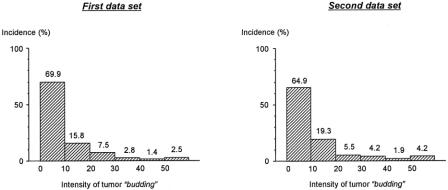
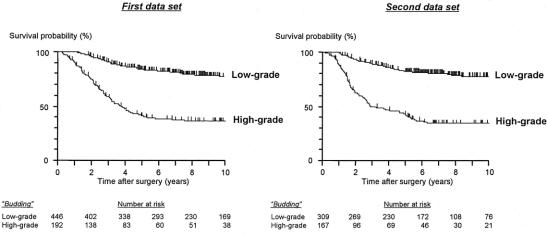
The patient number decreased in proportion to the intensity of tumor budding (Fig. 2). Tumor budding divided patients into 2 groups with significantly different survival outcomes; ie, 5-year survival rates of patients with low-grade budding were 84.0% in the first data set and 82.5% in the second, whereas those of patients with high-grade budding were 40.7% in the first set and 42.8% in the second, respectively (Fig. 3).
FIGURE 2. Patient distribution according to the intensity of tumor “budding.” Intensity of budding_:_ number of budding foci in a microscopic field of ×250 (field diameter of 700 μm) where budding was the most intensive
FIGURE 3. Kaplan-Meier estimates of cancer-specific survival based on the intensity of tumor “budding.” P value < 0.0001 (both data sets)
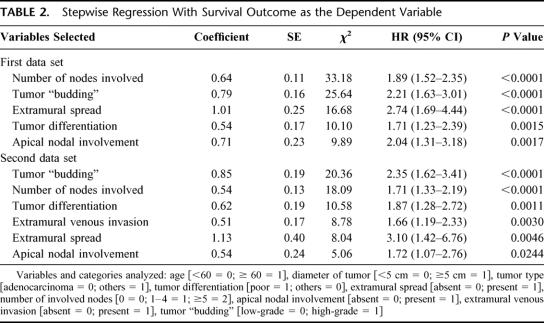
According to the multivariate analysis in the first data set, tumor budding was selected as a significant prognostic parameter together with the number of nodes involved, extramural spread, apical nodal involvement, and tumor differentiation (Table 2). In the second data set, tumor budding was an independent prognosticator, and other prognostic factors that influenced long-term survival were, in order of P value, the number of nodes involved, tumor differentiation, extramural venous invasion, extramural spread, and apical nodal involvement (Table 2).
TABLE 2. Stepwise Regression With Survival Outcome as the Dependent Variable
Among the parameters selected as independent in both multivariate analyses, the number of patients classified into an unfavorable group was relatively few in terms of tumor differentiation (the rate of poor differentiation: 13.5% in the first data set and 17.1% in the second) and apical nodal involvement (4.4% in the first data set and 7.6% in the second). In contrast, the high-grade budding group included 30.1% and 35.1% of patients in the first and second data sets, respectively. When dividing patients into 2 groups based on the intensity of tumor budding, a significant survival difference was observed according to whether the patients had a tumor confined to the rectal wall or whether it penetrated the muscularis propria (Fig. 4). In the same way, tumor budding influenced the long-term survival outcome regardless of the number of nodes involved (Fig. 4). No significant survival difference was observed between UICC stage II patients (5-year survival rate, 80.2%) and UICC stage I patients with high-grade budding (80.0%). In addition, high-grade budding reduced the survival probability of UICC stage II patients (5-year survival rate, 59.4%) to the same level as that of UICC stage III(N1) patients (58.0%) (Fig. 5).
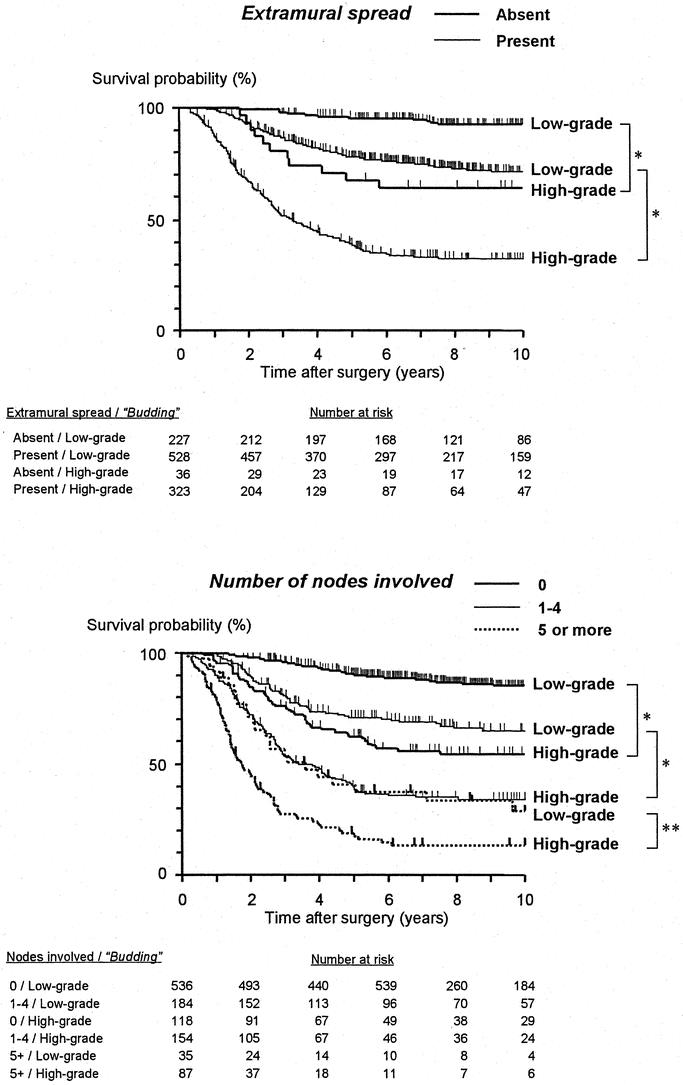
FIGURE 4. Correlation between tumor budding and extramural spread and nodal involvement in terms of their influence on postoperative survival (both data sets combined). * P < 0.0001, **P = 0.0058
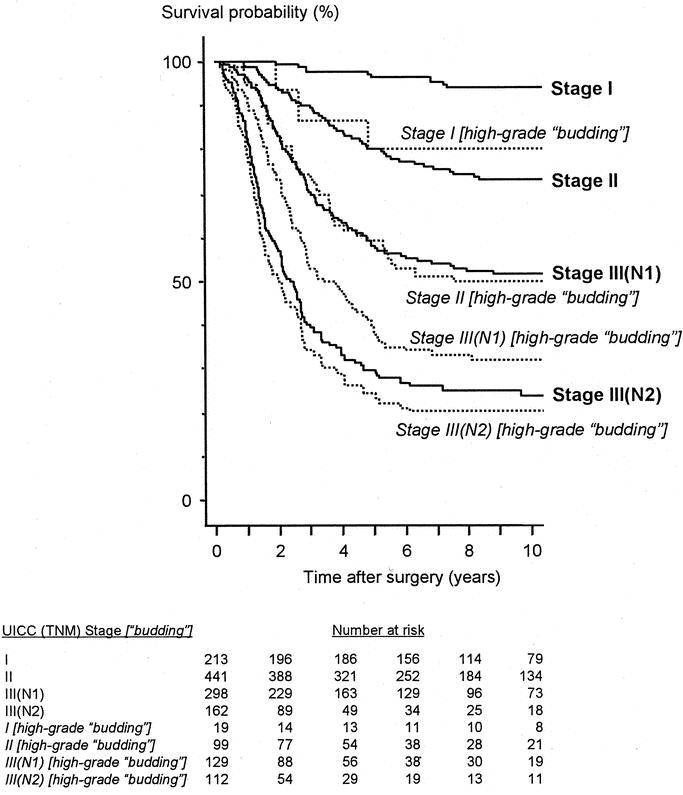
FIGURE 5. Survival impact of high-grade budding on the UICC (TNM) classification (both data sets combined). Stage II versus Stage I [high-grade budding]: P = 0.94; Stage III(N1) versus Stage II [high-grade budding]: P = 0.98; Stage III(N2) versus Stage III(N1) [high-grade budding]: P = 0.018
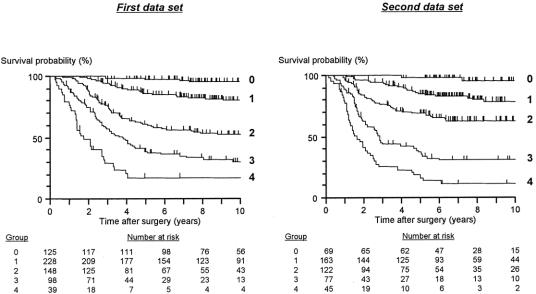
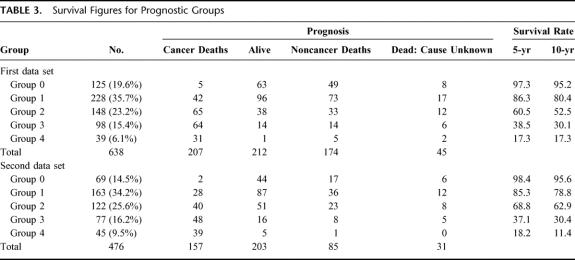
Based on these results, 3 pathologic variables, ie, tumor budding, the number of nodes involved, and extramural spread, were the most accurate parameters of prognosis. The simplified scoring system for these 3 variables that was developed based on the results of above multivariate analyses,9 with the derived prognostic groups (group 0 to group 4), is illustrated in Figure 6. Scores were assigned to categories consistent with their regression coefficients in 2 patient cohorts. In the first data set, group 0 comprised 19.6% of the patients with a 97.3% 5-year survival rate. Group 1 comprised a further 35.7% of patients with an 86.3% 5-year survival rate; 23.2% of patients belonged to group 2 and had a 60.5% 5-year survival rate; and 15.4% of patients were in group 3 with a 38.5% 5-year survival rate. The remaining 6.1% of patients were placed in group 4 with only a 17.3% survival rate at 5 years (Fig. 7). When the scoring system was applied to the second data set, similar survival results were obtained (Fig. 7). Details of the survival figures for the 2 data sets are presented in Table 3.
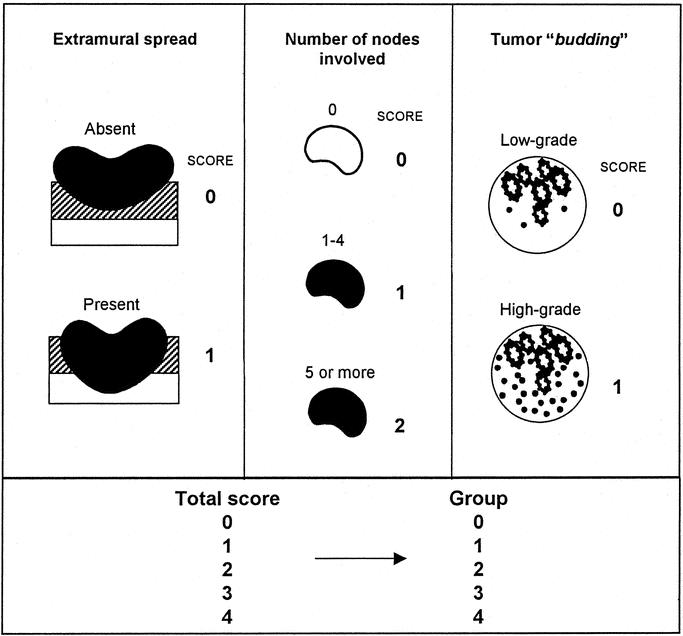
FIGURE 6. Scoring system for pathologic variables to make a prognostic grading system.
FIGURE 7. Kaplan-Meier estimates of cancer-specific survival with the prognostic grading system. First data set: gr.0 versus gr.1: P = 0.0001; gr.1 versus gr.2: P < 0.0001; gr.2 versus gr.3: P = 0.0005; gr.3 versus gr.4: P = 0.0021. Second: gr.0 versus gr.1: P = 0.0027; gr.1 versus gr.2: P = 0.0013; gr.2 versus gr. 3: P < 0.0001; gr.3 versus gr.4: P = 0.0023
TABLE 3. Survival Figures for Prognostic Groups
DISCUSSION
The present results reconfirm that both the depth of tumor penetration and lymph node involvement are important prognostic indicators, as has been demonstrated previously.9,13,23 Additionally, tumor budding was identified as another independent prognostic indicator that can improve the erroneous staging of advanced disease as early-stage disease, possibly because of occult micrometastasis; ie, 9% of UICC stage I patients and 22% of stage II patients were demonstrated to be upstaged by estimating tumor budding by the present standards.
De-differentiation and dissociation of cancer cells has been reported to be the first event of invasion and metastasis in experimental studies.24 Tumor budding is a morphologic expression of this event,11 detectable in both surgical specimens and biopsy specimens.16,19 With regard to molecular background, budding has recently been demonstrated to be relevant to cell-to-cell or cell-to-extracellular matrix interactions through regulation of Laminin-5,25,26 CD44,27 and syndecan-1 protein,28 and has also been demonstrated to be associated with carbonic anhydrase, a marker of mucosal differentiation.29 There is a strong connection between tumor budding and extramesorectal spread of rectal cancer,19 and furthermore, budding is highly relevant to the process of metastasis, as confirmed by its impact on survival not only in rectal cancer patients, but also in colon cancer patients11 or in patients with colorectal liver metastases.20 Venous invasion, which is an essential event in the process of metastasis, has often been reported to be a valuable prognostic indicator,14,30 although this study has shown that tumor budding exceeds extramural vessel invasion as an independent prognosticator. It may be that venous invasion becomes evident later than the events of de-differentiation and dissociation.
Using a computer-assisted multivariate statistical analysis, Jass et al have established a prognostic staging system utilizing 4 pathologic parameters: tumor depth, nodal involvement, peritumoral lymphocytic infiltration, and tumor growth pattern.9 Compared with the Dukes’ classification, Jass's staging system is superior in that it can place twice as many patients into groups that provide a confident prediction of clinical outcome (ie, the most favorable and the worst prognostic categories).9 However, it has been pointed out that the prognostic value of Jass's staging system is not well reproduced in other patient cohorts,12,31 and the judgment reproducibility of observed lymphocytic infiltration and growth patterns is less than optimal in routine practice.12,31,32 On the other hand, the impact of tumor budding intensity, and furthermore, the staging system using tumor depth, nodal involvement, and budding with regard to postoperative long-term survival observed in the first data set of the present study was closely reproduced in the second. Our preliminary study to assess the degree of interobserver variations using 50 randomly selected cases (the average number of slides examined was 4.2 per each case) by 3 observers (HU, ICT, and ABP; for the latter 2 observers, this was the first occasion to estimate budding, and prior to the study, the concept of budding was briefly explained) showed kappa values of 0.58–0.71 (observed agreement, 0.82–0.88). These figures are better than reported analogous figures on growth pattern (0.41–0.61) and lymphocyte infiltration (0.05–0.57),31,33 although there is apparently room for improvement through the provision of appropriate guidelines, more opportunities for discussion of common concepts, the process of identifying where disagreements arise, or modifications of criteria (eg, should judgment be based on 1 field where budding is the most intense21 or be based on the average count using several fields16). Because tumor budding could be a semiquantified parameter, similar to the mitotic index, it is thought to be promising that this parameter would improve on the observer variations inherent in current grading schemes using subjective components.
We used neither apical nodal involvement nor tumor differentiation as parameters of the grading system, even though these features were selected as independent prognosticators by multivariate analysis. One reason for this exclusion was that the proportion of cases with apical nodal involvement or with poor differentiation is not generally large (6% and 15% in our combined data sets), and that these parameters would be unlikely to significantly improve the prognostic model. Another reason was that apical nodal involvement is affected by the surgical method, ie, the extent of dissection. A treatment-related parameter such as apical nodal involvement provides useful prognostic information more as a measure of the adequacy of resection than as an assessment of the biologic nature of the tumor. Apical nodal involvement is rare when high ligation of the inferior mesenteric artery has been achieved. This kind of argument might also be applied to other surgery-related parameters, including involvement of the circumferential surgical margin, which has been reported to be an important factor in local recurrence.34–36 With regard to tumor differentiation, a long-established pathologic characteristic,37 the problem of observer reproducibility remains.31,38
Theoretically, as the number of independent parameters in a prognostic classification increases, the prediction of survival improves in precision. However, assuming that the simplicity of assessment and the reproducibility of outcome are important, it is clear that the depth of penetration and nodal involvement remain gold standards as prognostic parameters. We propose that tumor budding be added to these 2 parameters in routine practice. A grading system using the 3 parameters provides a wider spectrum of 5-year survival rates (18–98%) compared with conventional systems such as Dukes (28–96%), Astler-Coller (45–95%), and the UICC classification (30–96%) in the 1114 patients from the combined data sets. Therefore, patient selection can be performed efficiently both for postoperative adjuvant therapy and intensive follow-up examinations. A “phase III” study,39 ie, prospective evaluation of the prognostic criteria using a large number of cases, in the form of multicenter trials, would confirm the clinical utility of tumor budding and the new prognostic classification that is now based entirely on patient cohorts at St. Mark's Hospital.
Footnotes
Presented in part at the 182nd Meeting of the Pathological Society of Great Britain and Ireland (Joint Meeting with the Dutch Pathological Society), Maastricht, Netherlands, January 3–5, 2001.
Reprints: Hideki Ueno, MD, Department of Surgery I, National Defense Medical College 3-2, Namiki, Tokorozawa, Saitama 359-8513, Japan. E-mail: ueno@me.ndmc.ac.jp.
REFERENCES
- 1.Dukes C. The classification of cancer of the rectum. J Pathol Bacteriol. 1932;35:323–332. [Google Scholar]
- 2.Gabriel WB, Dukes C, Bussey HJ. Lymphatic spread in cancer of the rectum. Br J Surg. 1935;23:395–413. [Google Scholar]
- 3.Kirklin JW, Dockerty MB, Waugh JM. The role of the peritoneal reflection in the prognosis of carcinoma of the rectum and sigmoid colon. Surg Gynecol Obstet. 1949;88:326–331. [PubMed] [Google Scholar]
- 4.Gunderson LL, Sosin H. Areas of failure found at reoperation (second or symptomatic look) following “curative surgery” for adenocarcinoma of the rectum: clinicopathologic correlation and implications for adjuvant therapy. Cancer. 1974;34:1278–1292. [DOI] [PubMed] [Google Scholar]
- 5.Whittaker M, Goligher JC. The prognosis after surgical treatment for carcinoma of the rectum. Br J Surg. 1976;63:384–388. [DOI] [PubMed] [Google Scholar]
- 6.Pihl E, Hughes ES, McDermott FT, et al. Carcinoma of the rectum and rectosigmoid: cancer specific long-term survival: a series of 1061 cases treated by one surgeon. Cancer. 1980;45:2902–2907. [DOI] [PubMed] [Google Scholar]
- 7.Astler VB, Coller FA. The prognostic significance of direct extension of carcinoma of colon and rectum. Ann Surg. 1954;139:846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming ID, American Joint Committee on Cancer, American Cancer Society, American College of Surgeons. AJCC Cancer Staging Manual. 5th Edition. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 9.Jass JR, Love SB, Northover JMA. A new prognostic classification of rectal cancer. Lancet 1987;June 6:1303–1306. [DOI] [PubMed]
- 10.Fitzgerald RH. What is the Dukes’ system for carcinoma of the rectum? Dis Colon Rectum. 1982;25:474–477. [DOI] [PubMed] [Google Scholar]
- 11.Hase K, Shatney C, Johnson D, et al. Prognostic value of tumor “budding” in patients with colorectal cancer. Dis Colon Rectum. 1993;36:627–635. [DOI] [PubMed] [Google Scholar]
- 12.Harrison JC, Dean PJ, El-Zeky F, et al. From Dukes through Jass: pathological prognostic indicators in rectal cancer. Hum Pathol. 1994;25:498–505. [DOI] [PubMed] [Google Scholar]
- 13.Fielding LP, Phillips RKS, Fry JS, et al. Prediction of outcome after curative resection for large bowel cancer. Lancet 1986; October 18:904–906. [DOI] [PubMed]
- 14.Michelassi F, Ayala JJ, Balestracci T, et al. Verification of a new clinicopathologic staging system for colorectal adenocarcinoma. Ann Surg. 1991;214:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon R, Altman DG. Statistical aspects of prognostic factor studies in oncology. Br J Cancer. 1994;69:979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morodomi T, Isomoto H, Shirouzu K, et al. An index for estimating the probability of lymph node metastasis in rectal cancers. Lymph node metastasis and the histopathology of actively invasive regions of cancer. Cancer. 1989;63:539–543. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein NS, Hart J. Histologic features associated with lymph node metastasis in stage T1 and superficial T2 rectal adenocarcinomas in abdominoperineal resection specimens. Anat Pathol. 1999;111:51–58. [DOI] [PubMed] [Google Scholar]
- 18.Okuyama T, Oya M, Ishikawa H. Budding as a risk factor for lymph node metastasis in pT1 or pT2 well-differentiated colorectal adenocarcinoma. Dis Colon Rectum. 2002;45:628–634. [DOI] [PubMed] [Google Scholar]
- 19.Ueno H, Mochizuki H, Shinto E, et al. Histological indices in biopsy specimens for estimating the probability of extended local spread in rectal cancer. Cancer. 2002;94:2882–2891. [DOI] [PubMed] [Google Scholar]
- 20.Ueno H, Mochizuki H, Hatsuse K, et al. Indicators for treatment strategies of colorectal liver metastases. Ann Surg. 2000;231:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueno H, Murphy J, Jass JR, et al. Tumour “budding” as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology. 2002;40:127–132. [DOI] [PubMed] [Google Scholar]
- 22.Morson BC, Dawson IMP, Day DW, et al. Morson & Dawson's Gastrointestinal Pathology—third edition. Oxford and London: Blackwell Scientific Publications; 1990. [Google Scholar]
- 23.Dukes C. The spread of rectal cancer and its effect on prognosis. Br J Cancer. 1959;12:309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabbert H. Mechanism of tumor invasion: evidence from in vivo observations. Cancer Metastasis Rev. 1985;4:293–309. [DOI] [PubMed] [Google Scholar]
- 25.Pyke C, Salo S, Ralfkiær E, et al. Laminin-5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase plasminogen activator in budding cancer cells in colon adenocarcinomas. Cancer Res. 1995;55:4132–4139. [PubMed] [Google Scholar]
- 26.Sordat I, Rousselle P, Chaubert P, et al. Tumor cell budding and laminin-5 expression in colorectal carcinoma can be modulated by the tissue micro-environment. Int J Cancer. 2000;88:708–717. [DOI] [PubMed] [Google Scholar]
- 27.Masaki T, Goto A, Sugiyama M, et al. Possible contribution of CD44 variant 6 and nuclear β-catenin expression to the formation of budding tumor cells in patients with T1 colorectal carcinoma. Cancer. 2001;92:2539–2546. [DOI] [PubMed] [Google Scholar]
- 28.Fujiya M, Watari J, Ashida T, et al. Reduced expression of syndecan-1 affects metastatic potential and clinical outcome in patients with colorectal cancer. Jpn J Cancer Res. 2001;92:1074–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bekku S, Mochizuki H, Yamamoto T, et al. Expression of carbonic anhydrase I or II and correlation to clinical aspects of colorectal cancer. Hepato-Gastroenterol. 2000;47:998–1001. [PubMed] [Google Scholar]
- 30.Talbot IC, Ritchie S, Leighton M, et al. Invasion of veins by carcinoma of rectum: method of detection, histological features and significance. Histopathology. 1981;5:141–163. [DOI] [PubMed] [Google Scholar]
- 31.Deans GT, Heatley M, Anderson N, et al. Jass’ classification revisited. J Am Coll Surg. 1994;179:11–17. [PubMed] [Google Scholar]
- 32.Jass JR, Ajioka Y, Allen JP, et al. Assessment of invasive growth pattern and lymphocytic infiltration in colorectal cancer. Histopathology. 1996;28:543–548. [DOI] [PubMed] [Google Scholar]
- 33.Shepherd NA, Saraga EP, Love SB, et al. Prognostic factors in colonic cancer. Histopathology. 1989;14:613–620. [DOI] [PubMed] [Google Scholar]
- 34.Quirke P, Durdey P, Dixon MF, et al. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;November 1:996–998. [DOI] [PubMed]
- 35.Adam IJ, Mohamdee MO, Martin IG, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–711. [DOI] [PubMed] [Google Scholar]
- 36.Heald RJ, Ryall RDH. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;i:1479–1482. [DOI] [PubMed] [Google Scholar]
- 37.Rankin FW, Broders AC. Factors influencing prognosis in carcinoma of the rectum. Surg Gynecol Obstet. 1928;46:660–667. [Google Scholar]
- 38.Jass JR, Atkin WS, Cuzick J, et al. The grading of rectal cancer: histological perspectives and a multivariate analysis of 447 cases. Histopathology. 1986;10:437–459. [DOI] [PubMed] [Google Scholar]
- 39.Hall PA, Going JJ. Predicting the future: a critical appraisal of cancer prognosis studies. Histopathology. 1999;35:489–494. [DOI] [PubMed] [Google Scholar]