Presence of Host ICAM-1 in Laboratory and Clinical Strains of Human Immunodeficiency Virus Type 1 Increases Virus Infectivity and CD4+-T-Cell Depletion in Human Lymphoid Tissue, a Major Site of Replication In Vivo (original) (raw)
Abstract
Human immunodeficiency virus type 1 (HIV-1) incorporates several host proteins. Earlier studies have indicated that such foreign constituents can modulate the virus life cycle, although the potential roles that these proteins might play in the viral pathology in vivo remain unclear. In an attempt to shed light on this issue, we first exposed explants of human lymphoid tissue to isogenic viruses except for the presence or absence of host-derived ICAM-1. Incorporation of ICAM-1 alone increased HIV-1 infectivity for human tonsillar tissue cultured ex vivo. This observation was made for viruses bearing distinct coreceptor utilization profiles. Conversion of LFA-1 to a high-affinity-high-avidity state for ICAM-1 further augmented the susceptibility of human tonsillar histocultures to infection by ICAM-1-bearing virions. A more massive depletion of CD4+ T lymphocytes was seen with X4 ICAM-1/POS viruses than with isogenic ICAM-1/NEG virions. Exposure of X4 and R5 primary isolates of HIV-1 to a blocking anti-ICAM-1 antibody resulted in a decrease of virus infection. Finally, X4 and R5 virions derived from a natural human lymphoid tissue microenvironment incorporated high levels of ICAM-1. Altogether, these results indicate that the incorporation of host ICAM-1 can significantly modulate the biology of HIV-1 in a cellular milieu recognized as the major site of replication in vivo and suggest that host proteins found in HIV-1 particles may participate in the pathogenesis of this disease.
Enveloped retrovirus particles are formed by extrusion (budding) through the host cell membrane, a process during which the newly formed viral entities become coated with a lipid bilayer derived from the cell membrane. At this stage, the virus-encoded envelope glycoproteins are inserted within the lipid envelope. The incorporation of surface glycoproteins into newly formed viral entities is not restricted to the homologous glycoproteins, as exemplified by the observation of viral pseudotyping. For example, pseudotyping of heterelogous viral glycoproteins has been reported to occur for Rous sarcoma virus that can carry influenza virus hemagglutinin protein (9) and for murine retrovirus and members of the lentivirus subfamily that can insert vesicular stomatitis virus G protein (45). The low selectivity of the process governing glycoprotein incorporation into retroviral particles is most likely responsible for the observation that retroviruses incorporate a plethora of cell-derived proteins in their envelopes. It is thus not surprising to find that propagation of human immunodeficiency virus type 1 (HIV-1) within its target cells results in the insertion of numerous foreign constituents into the virus envelope. The exterior surface of HIV-1 has been demonstrated to be constituted of numerous host cell membrane proteins such as major histocompatibility complex class I (MHC-I) and MHC-II (i.e., HLA-DR, HLA-DP, and HLA-DQ determinants), CD43, CD44, CD55, CD59, CD63, CD71, CD80, CD86, and adhesion receptors such as ICAM-1 and LFA-1 (1, 2, 5, 14, 15, 46, 52, 54).
Previous studies with established lymphoid cell lines, as well as primary peripheral blood mononuclear cells, have clearly shown that several virion-anchored host proteins are still functional and may thus participate in the pathogenesis of HIV-1 infection. For example, virion-associated foreign proteins can modulate several steps in the virus life cycle, including the attachment of virions to target cells, binding avidity between virus and cell, host cell range, and neutralization susceptibility of virions (reviewed in reference 62). Specifically, the presence of host cell membrane ICAM-1 on HIV-1 particles has been shown to lead to a marked enhancement of virus infectivity due to enhanced interaction between virion-bound host ICAM-1 with its physiological LFA-1 counterreceptor located on the surface of target cells (17, 49, 50). This direct contribution of host-encoded ICAM-1 proteins to enhancement of virus infectivity was unequivocally demonstrated when experiments were conducted in the presence of blocking agents of the ICAM-1-LFA-1 interaction. It was also shown that ICAM-1-containing virions were more resistant to antibody-mediated neutralization, and this decreased sensitivity to neutralization was dramatically increased when target cells expressed on their surface the activated form of LFA-1 (18, 19).
Human follicular dendritic cells (FDCs) can capture HIV-1 particles and render such viruses highly infectious to surrounding CD4-expressing T lymphocytes. It should be noted that ICAM-1 and LFA-1 adhesion molecules play a crucial role in the interaction between HIV-1 particles and FDCs (22, 34). However, the precise contribution of virally embedded host ICAM-1 protein in the replicative cycle of HIV-1 has never been fully investigated in a cellular microenvironment that contained both FDCs and CD4+ T cells. Previous reports have indicated that secondary lymphoid organs constitute preferred anatomical sites for HIV replication and propagation (12, 20, 21, 47, 48, 57, 60). Human lymphoid tissues contain germinal centers that are vital for the production of memory B and T cells. The germinal center microenvironment is composed of centroblasts, centrocytes, FDCs, macrophages, CD4+ and CD8+ T lymphocytes, and dendritic cells (4, 31, 35, 36, 39, 40). Glushakova et al. have previously developed an experimental cell system for the histoculture of human lymphoid tissue that supports productive infection with various HIV-1 isolates without any requirement for exogenous activation or stimulation (23, 24). This tissue culture system has been shown to preserve the general cytoarchitecture found in normal human lymphoid tissue, including a network of FDCs. In the present study, we used sections of human lymphoid tissue cultured ex vivo to define whether the increase in HIV-1 infectivity mediated by host-encoded ICAM-1 that is detected in continuous lymphoid and primary cells can still be seen in such a native microenvironment. Here we report that ICAM-1-bearing HIV-1 particles replicate to greater levels in human lymphoid tissue and mediate a more dramatic CD4+-T-cell depletion than isogenic virions devoid of host-derived ICAM-1. The physiological relevance of our data is provided by the observations that HIV-1 particles expanded in cultures of human lymphoid tissue incorporate host ICAM-1 and that replication of clinical and laboratory strains of HIV-1 in such tonsil tissue explants was inhibited by agents known to abrogate ICAM-1-LFA-1 interaction. These results suggest that the process of incorporation of host cell membrane molecules such as ICAM-1 into mature HIV-1 influenced the biological properties of the virus in lymphoid tissue ex vivo and that this factor should thus be considered an important determinant of HIV-1 pathogenesis.
(This work was performed by Salim Bounou in partial fulfillment of the requirements for a Ph.D. from the Microbiology-Immunology Program, Faculty of Medicine, Laval University, Ste-Foy, Quebec, Canada.)
MATERIALS AND METHODS
Production of virus stocks.
Isogenic virus particles differing only by the absence (ICAM-1/NEG) or the presence (ICAM-1/POS) of host-derived ICAM-1 proteins on their outer membranes were produced by calcium phosphate transfection in the 293T cells, as described previously (7, 17). Briefly, a typical transfection experiment was achieved by using vectors encoding fully infectious or recombinant luciferase-encoding viruses (i.e., pNL4-3, pJR-CSF, p89.6, pHXB-Luc, and pNL4-3-Luc−E−R+ in combination with pcDNA-1/Ada-M) in the presence or absence of an ICAM-1 expression vector (i.e., pCD1.8). Primary clinical HIV-1 isolates 92HT599 (X4) and 92TH026 (R5) were expanded in peripheral blood mononuclear cells obtained from healthy donors. All virus preparations underwent only one freeze-thaw cycle before initiation of infection studies. Virus stocks were normalized for virion content by using a p24 antibody capture assay developed in our laboratory (see below). The standardization on p24 contents is based on our previous observation indicating that virus preparations harvested from transfected 293T cells contain minimal amounts of p24 that are not associated with infectious virions (17).
p24 antibody capture assay.
The measurement of virus-encoded p24 protein was determined by an in-house enzymatic assay. Briefly, flat-bottom 96-well plates (Immulon 2; Dynatech, Ltd.) were initially coated with 183 H12-5C, a monoclonal anti-p24 antibody. After the wells were washed and blocked with 1% bovine serum albumin (Sigma, St. Louis, Mo.), viral lysates were added to the wells at various dilutions, along with samples of known p24 concentration (recombinant purified p25gag/SF2, kindly supplied by Chiron Corporation), in order to establish a standard curve. After a 60-min incubation at 37°C, the plates were washed, and a second biotinylated anti-p24 monoclonal antibody (i.e., clone 31-90-25) was then added. After a 1-h incubation at 37°C, the plates were washed, and a spreptavidin-peroxidase conjugate (Steptavidin-HRP-40; Research Diagnostics, Inc., Flanders, N.J.) was added; this was followed by the addition of the TMB-S substrate (Research Diagnostics, Inc.). After 30 min at room temperature, the reaction was terminated by adding 1 M H3PO4, and the absorbance was measured at 450 nm. Unknown p24 values were calculated on the basis of regression analysis of p24 standards over a linear range of 31.25 to 2,000 pg/ml.
HIV-1 infection of human lymphoid tissue ex vivo.
Human tonsillar tissue removed during routine tonsillectomy and not required for clinical purposes was processed within 4 h of excision. The tonsils were washed thoroughly with medium containing antibiotics and then sectioned into thin blocks of 2 to 3 mm2. These tissue sections were placed on top of collagen sponge gels in the culture medium at the air-liquid interface and infected with similar amounts of each virus stock (3 to 5 μl containing 2.5 ng of p24). Lymphoid tissue was either left untreated or treated with the anti-LFA-1 TS1/22.1 antibody (2 μg/ml) or the anti-CD3 OKT3 antibody (2 μg/ml) for 5 min at 37°C before virus inoculation. At 72 h after infection, cells were lysed and virus-encoded luciferase activity was monitored by using a microplate luminometer (MLX; Dynex Technologies, Chantilly, Va.). In some experiments viruses were pretreated for 30 min at 37°C with the anti-ICAM-1 RR1/1.1.1 antibody. To normalize the infection of histocultures, we inoculated two tissue blocks from the same donor in four separate wells of a 24-well plate with equal amounts of either ICAM-1/NEG or ICAM-1/POS viruses, based on the p24 content. The extent of virus production in cells infected with fully competent viruses was assessed by monitoring the level of p24 in culture medium bathing tissue blocks at different time points.
Antibodies and purified proteins.
The anti-ICAM-1 (anti-CD54) antibody RR1/1.1.1 was provided by Robert Rothlein (Boehringer Ingelheim, Ridgefield, Conn.) (51). The anti-LFA-1 α-chain antibody (anti-CD11a, clone TS1/22.1) was obtained from the American Type Culture Collection (Manassas, Va.). Fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G (IgG) was purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, Pa.). OKT3 hybridoma produces a murine monoclonal antibody specific for an epitope on the epsilon-subunit of human CD3 and was obtained from the American Type Culture Collection. Antibodies from this hybridoma were purified with mAbTrap protein G affinity columns according to the manufacturer’s instructions (Pharmacia LKB Biotechnology AB, Uppsala, Sweden).
Detection of virion-bound host ICAM-1 proteins by a virus capture assay.
The presence of virion-bound host ICAM-1 proteins was semiquantitatively estimated by using a modified version of a previously described virus capture assay (5). Briefly, 12.5 × 106 magnetic beads (BioMag, Fc specific; PerSeptive Diagnostics, Inc., Cambridge, Mass.), previously coated with the anti-ICAM-1 RR1/1.1.1 antibody, were incubated with similar amounts of studied virus preparations standardized in terms of the viral core p24 protein (2,500 pg of p24) in a final volume of 1 ml of binding medium (phosphate-buffered saline plus 0.1% bovine serum albumin). This mixture was incubated for 1 h at 4°C on a rotating plate. The beads were washed three times in binding medium with a magnetic separation unit and then resuspended in 100 μl of binding medium. The amount of immunocaptured HIV-1 particles was assessed by measuring the viral p24 protein content found associated with the immunomagnetic beads. Magnetic beads coated with an antibody specific for human CD45RO (clone UCHL-1) were used as a negative control because CD45 has been shown to be excluded from the HIV-1 envelope (14, 46).
Flow cytometry.
Flow cytometry analysis was performed on cells mechanically isolated from control and infected human tonsils. We chose mechanical tissue disruption for cell isolation from histocultures in order to avoid any possible enzymatic digestion of cell surface markers, which might affect the flow cytometry results. Briefly, cells were mechanically isolated from tissue blocks on day 10 after infection and stained with the Tritest kit (Becton Dickinson, San Jose, Calif.) comprising a mixture of anti-CD3-PerCP, anti-CD4-FITC, and anti-CD8-PE antibodies. Depletion of CD4+ T cells is expressed as a ratio of CD4+ to CD8+ T cells.
RESULTS
ICAM-1-bearing HIV-1 replicated to a higher level in human lymphoid tissue ex vivo than their isogenic ICAM-1/NEG counterpart.
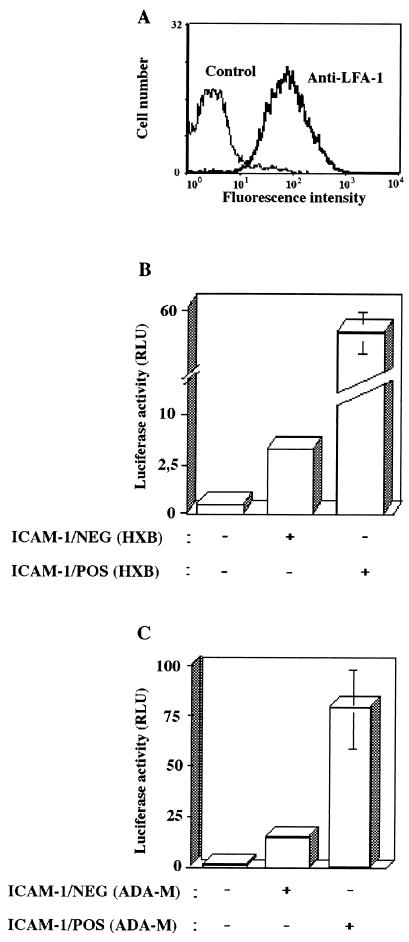
We have previously shown that incorporation of ICAM-1 alone increased HIV-1 infectivity for LFA-1-expressing cells by up to 10-fold (17, 49). We also described that the conformational state of LFA-1 through an influence on the affinity of LFA-1 for ICAM-1 represents another factor that can augment virus infectivity for peripheral mononuclear cells and lymphoid cell lines by an order of magnitude (18, 19). Given that some of the most critical events in HIV-1 disease occur in human lymphoid tissue and that blocks of human lymphoid tissue cultured ex vivo have been reported to support productive infection with HIV-1 without exogenous stimulation (23–25), we infected human lymphoid tissue ex vivo with pairs of isogenic viruses differing only in the acquisition of a single species of host adhesion molecule, i.e., ICAM-1. Flow cytometry was first used for a quantitative analysis of the frequency of LFA-1-expressing cells recovered from human tonsils that were mechanically dispersed into single-cell suspensions. Flow cytometry analysis revealed that the majority of cells found in histocultures of human tonsils do express high levels of LFA-1 on their surfaces (Fig. 1A). Next, the contribution of virion-anchored ICAM-1 adhesion molecule to HIV-1 infectivity for human lymphoid tissue was addressed by using single-cycle reporter virus. Similar amounts of isogenic ICAM-1/NEG and ICAM-1/POS HIV-1 particles (as standardized in terms of viral p24 protein) that are competent only for a single round of replication were used to infect histocultured blocks of human tonsils derived from several donors. The presence of host-encoded ICAM-1 protein on the HIV-1 envelope resulted in a 5- to 10-fold increase in virus infectivity after inoculation of histocultured tonsils with either T-tropic (Fig. 1B) or M-tropic isolates of HIV-1 (Fig. 1C). These experiments were performed with different donors giving reproducible results. This suggests that the marked enhancement of HIV-1 infectivity conferred by the incorporation process of a specific adhesion molecule such as ICAM-1 could be demonstrated in a natural microenvironment.
FIG. 1.
Virion-associated cellular ICAM-1 protein augments infectivity of both T-tropic and macrophage-tropic single-cycle reporter HIV-1 in human lymphoid tissue ex vivo. (A) Histocultures were mechanically disrupted to obtain cell suspensions that were processed for flow cytometric analyses. Single-cell suspensions were labeled with a monoclonal anti-LFA-1 antibody (clone TS22.1, bold curve) or an isotype-matched irrelevant commercial control antibody (thin curve), followed by a phycoerythrin-conjugated goat anti-mouse IgG antibody. (B and C) Human tonsillar tissues cultured ex vivo were inoculated with standardized amounts of T-tropic (i.e., HXB) or macrophage-tropic (i.e., Ada-M) luciferase reporter virus stocks (2.5 ng of p24) either bearing or not bearing host ICAM-1. Cells mechanically isolated from control and infected tissue explants were lysed 72 h after virus inoculation, and virus-encoded luciferase activity was monitored by using a microplate luminometer. Results shown are the means ± the standard deviation (SD) of two tissue blocks from the same donor in four separate wells (i.e., eight tissue blocks) and are representative of experiments conducted with human tonsillar tissue from three different donors.
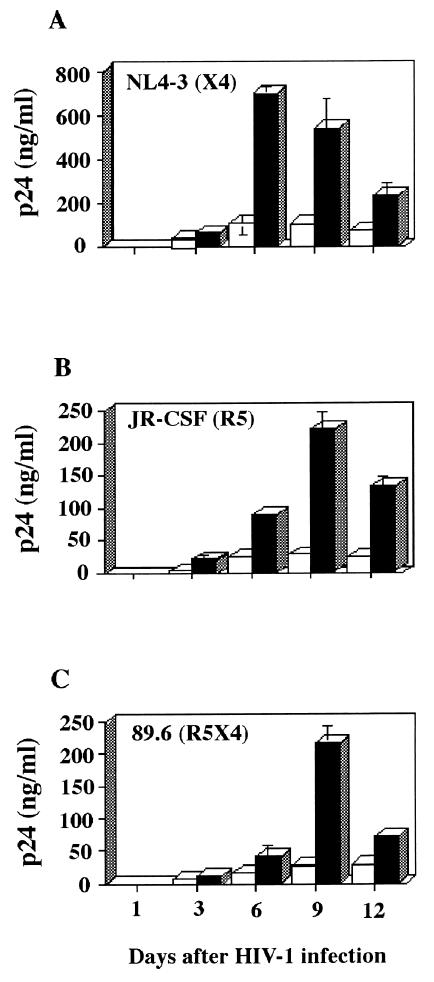
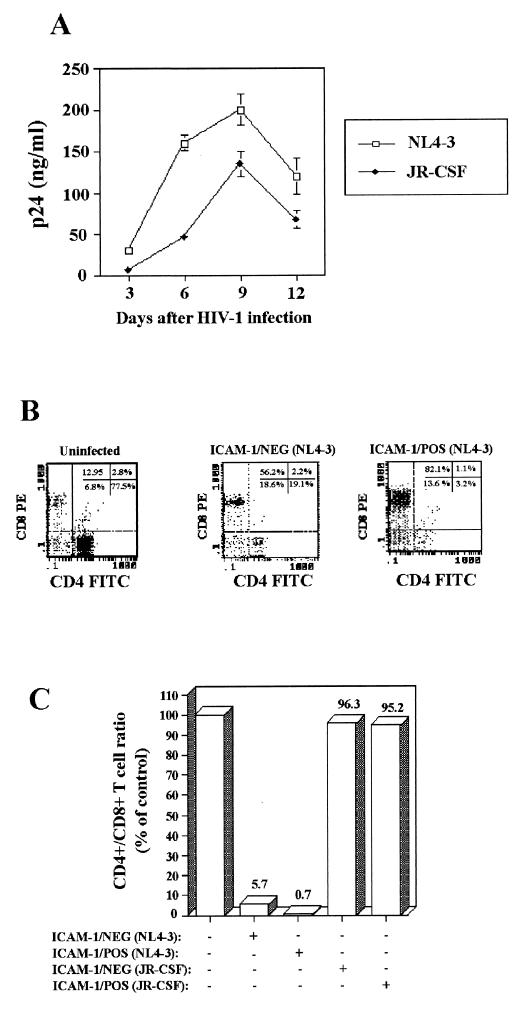
To further substantiate the relative contribution of virally embedded host ICAM-1 protein to virus infectivity, we infected lymphoid histocultures with fully competent T-cell/X4 (i.e., NL4-3), macrophage/R5 (i.e., JR-CSF), and dual/R5X4 (i.e., 89.6) HIV-1 isolates. The infectious viruses, as was the case for luciferase reporter viruses, were applied to the top of blocks of human tonsil tissue, and the concentration of p24 in the medium was measured at various times during infection. The increase of p24 level was first detected in the culture medium at day 3 after infection, and its concentration rose steadily, reaching a peak by 6 to 9 days after virus inoculation (Fig. 2). Fully infectious ICAM-1-bearing NL4-3 (X4), JR-CSF (R5), and 89.6 (R5X4) isolates of HIV-1 were all found to replicate at higher levels in human tonsil histocultures than were isogenic virions devoid of host-derived ICAM-1. These data indicate that virion-associated host cell ICAM-1 protein is still functional on the exterior of HIV-1 when infection occurs in lymphoid tissues cultured ex vivo.
FIG. 2.
Infectivity of replication-competent virus differing in envelope determinants for human lymphoid tissue is still enhanced by the acquisition of host ICAM-1 protein. Tonsillar tissue explants were infected with similar amounts of fully competent isogenic ICAM-1/NEG (open columns) and ICAM-1/POS (solid columns) HIV-1 particles bearing a distinct tropism and were analyzed for p24 concentration in the culture medium at the times indicated. Presented are the mean values ± the SD of two tissue blocks from the same donor in four separate wells (i.e., eight tissue blocks). These data are representative of experiments conducted with human tonsillar tissue from four different donors.
Enhancement of HIV-1 infectivity for cultured human lymphoid tissue is due to the interaction between virion-bound host ICAM-1 and cell surface LFA-1.
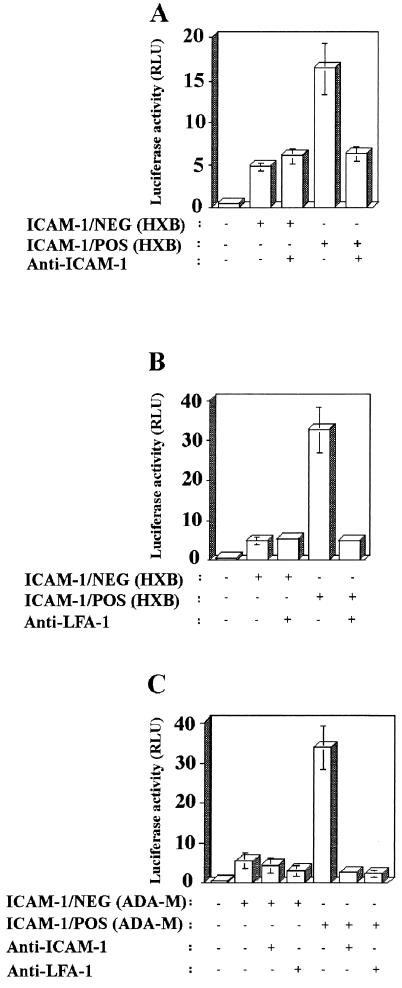
We next attempted to define the basis for the augmentation in virus infectivity conferred by the acquisition of ICAM-1, the physiological counterligand of LFA-1. Isogenic ICAM-1/NEG and ICAM-1/POS HXB-Luc particles were treated with RR1/1.1.1, an anti-ICAM-1 antibody known to abrogate the ICAM-1-LFA-1 interaction (43). The process of infection with viruses devoid of foreign ICAM-1 was unaffected by a pretreatment with the anti-ICAM-1 antibody, whereas infection with ICAM-1/POS X4 luciferase reporter viruses was significantly diminished by this treatment (Fig. 3A). Comparable results were obtained when explants of human lymphoid histocultures were pretreated with the anti-LFA-1 α-chain TS1/22.1 blocking antibody (Fig. 3B). Infection of human lymphoid tissue with macrophage-tropic HIV-1 pseudotypes that bear in their envelope host ICAM-1 protein was also similarly affected by either a pretreatment of virus with RR1/1.1.1 antibody or a pretreatment of target cells with TS1/22.1 antibody (Fig. 3C).
FIG. 3.
ICAM-1-LFA-1 interaction is responsible for the noticed increase in HIV-1 infectivity for histocultures of human lymphoid tissue that is conferred by cellular ICAM-1. Isogenic luciferase reporter ICAM-1/NEG and ICAM-1/POS viruses (panel A, T-tropic; panel C, M-tropic) were pretreated with an anti-ICAM-1 blocking antibody (i.e., RR1/1.1.1) before inoculation of human lymphoid tissue. In some instances, histocultures of human lymphoid tissue were pretreated with an anti-LFA-1 blocking antibody (i.e., TS1/22.1) prior to infection with reporter ICAM-1/NEG and ICAM-1/POS viruses (panel B, T-tropic; panel C, M-tropic). After 72 h, cells were mechanically isolated from control or infected tissue blocks, and infection was evaluated by measuring luciferase activity in the lysates. The results shown are the mean values ± the SD of two tissue blocks from the same donor in four separate wells (i.e., eight tissue blocks) and are representative of experiments with tissue from three donors.
Enhancement of HIV-1 infectivity is further augmented by activation of LFA-1 in lymphoid tissue explants.
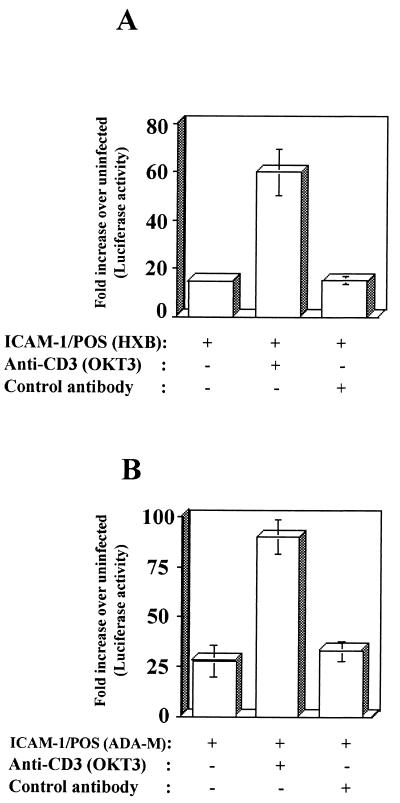
LFA-1 plays an important role in the initiation of immune responses by promoting cell-to-cell contacts. LFA-1-mediated cell adhesion requires its activation from a low- to a high-avidity-high-affinity state for its ligands that can be induced by the cross-linking of surface receptors such as the TCR/CD3 complex or upon treatment with phorbol esters (10, 51, 64). Thus, we set out to define whether the conformational state of LFA-1 could modulate the susceptibility of human lymphoid tissues cultured ex vivo to infection by ICAM-1-bearing virus. To achieve this goal, lymphoid tissue blocks were pretreated with anti-CD3 antibodies (i.e., OKT3) that induce the high-avidity-high-affinity LFA-1 state. Results from Fig. 4 demonstrate that infectivity of ICAM-1/POS X4 and R5 viruses was further accentuated when target cells present within human tonsils expressed an OKT3-mediated activated form of LFA-1. No such enhancing effect upon HIV-1 infectivity was noticed after pretreatment with an isotype-matched control antibody. Virus infectivity was also unaffected when infection was allowed to proceed with isogenic ICAM-1/NEG virus (data not shown). The process of HIV-1 infection was also enhanced when lymphoid tissue blocks were pretreated with NKI-L16, an anti-LFA-1 antibody known to activate LFA-1 (data not shown) (37).
FIG. 4.
Expression of an activated LFA-1 conformational state in human tonsillar tissue explants further augment infectivity of ICAM-1-bearing HIV-1 particles. Human lymphoid tissues cultured ex vivo were either left untreated or treated with an anti-CD3 antibody (i.e., OKT3) prior to infection with isogenic recombinant luciferase-encoding ICAM-1/NEG and ICAM-1/POS viruses (panel A, T-tropic; panel B, M-tropic). After 72 h, the cells were mechanically isolated from control or infected tissue blocks, and infection was evaluated by measuring the luciferase activity in the lysates. The results shown are the mean values ± the SD of two tissue blocks from the same donor in four separate wells (i.e., eight tissue blocks) and are representative of experiments with tissue from three donors. Data are expressed as the fold induction relative to the basal luciferase activity in uninfected control cells.
The acquisition of host ICAM-1 by X4 virions results in a more extensive CD4+-T-cell depletion.
CD4+-T-cell depletion is a hallmark of HIV-1 pathogenesis and since virally acquired host ICAM-1 protein enhances HIV-1 infectivity in human lymphoid tissue, it can be inferred that this enhancement may result in a greater cytopathic phenotype. To rigorously test the hypothesis that insertion of host ICAM-1 into the virus envelope causes a more massive T-cell depletion, we compared X4 (i.e., NL4-3) and R5 (i.e., JR-CSF) viruses bearing or not on their surface host ICAM-1 for their ability to deplete CD4+ T cells from ex vivo human tonsil histocultures. A quantitative analysis of CD4+ and CD8+ T lymphocytes recovered from HIV-1-infected and control blocks of human lymphoid tissue was performed by using flow cytometry. The number of cells per explant and the ratio of CD4+ to CD8+ T cells varied from experiment to experiment; therefore, the HIV-1-infected tissues were always compared with noninfected controls obtained from the same donor. In agreement with our previous results (Fig. 2), histocultures of lymphoid tissue supported productive viral infection with fully infectious X4 and R5 viruses (Fig. 5A). We found that the degree of depletion depends on the HIV-1 isolate tested. A significant decline in CD4+ T lymphocytes in histocultures was evident with the T-tropic isolate of HIV-1, while infection with the R5 molecular clone of HIV-1 resulted in a minimal depletion of tissue CD4+ lymphocytes (Fig. 5B). These findings are in support of previous results, indicating that infection with X4 but not R5 strains of HIV-1 results in CD4+-T-lymphocyte depletion in this experimental system (24). Infection with ICAM-1-bearing HIV-1NL4-3 particles resulted in a greater CD4+-T-cell depletion in human lymphoid tissue histocultures than did inoculation with isogenic virions negative for host-encoded ICAM-1 protein (ratios of CD4+/CD8+ T cells of 5.7 versus 0.7). No such enhancement of CD4+-T-cell depletion was noticed with the R5 strain JR-CSF. These data provide evidence that the presence of host-derived ICAM-1 protein increases the ability of infectious X4 HIV-1 to induce depletion of CD4+ T cells in human lymphoid tissues ex vivo.
FIG. 5.
Incorporation of foreign ICAM-1 protein in X4 HIV-1 variant results in a more dramatic depletion of CD4+ T cells in human lymphoid tissues. Histocultures of human tonsils were inoculated with similar concentrations of isogenic X4 (NL4-3) and R5 (JR-CSF) virions bearing or not on their surface host-derived ICAM-1. (A) Kinetics of viral p24 production is shown for ICAM-1/NEG NL4-3 and JR-CSF virions. (B) Tonsil explants were mechanically disrupted at 10 days after virus infection to obtain cell suspensions. Next, the cells were stained with the Tritest kit that allows measurements by flow cytometry of both CD4+ and CD8+ T lymphocytes. The numbers represent the frequency of events in each quadrant. (C) The ratio of CD4+ to CD8+ T cells as a percentage of the uninfected control cells from the same donor is indicated at the top of each bar for each virus preparation used. The results are given as pooled data on two tissue blocks from the same donor in four separate wells (i.e., eight tissue blocks) and are representative of experiments with tissue from three donors.
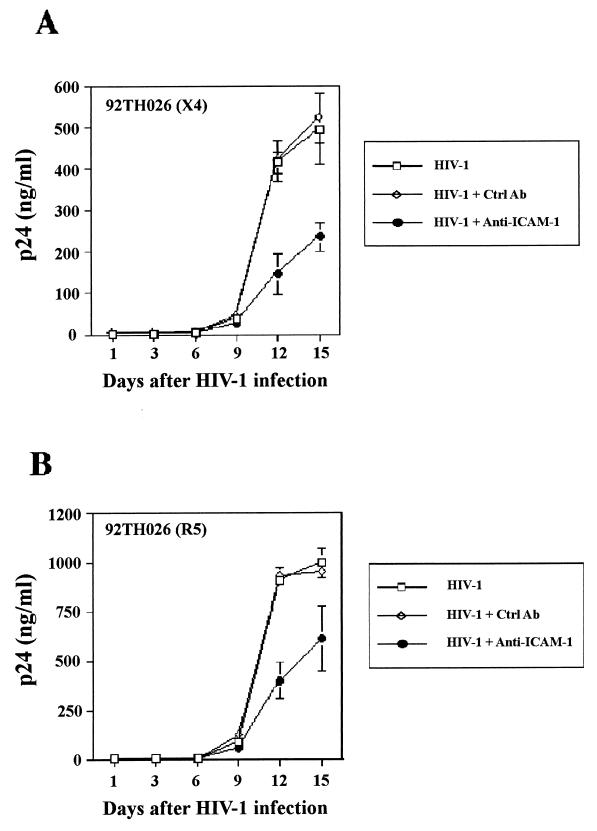
Productive infection of human lymphoid tissue with clinical strains of HIV-1 is inhibited by a blocker of ICAM-1-LFA-1 interaction.
Next, we attempted to establish the physiological significance of ICAM-1 incorporation. This goal was reached by determining whether antibodies to ICAM-1 impact productive infection of tonsillar tissue with clinical strains of HIV-1 produced in primary mononuclear cells. First, a virus capture assay revealed that the clinical X4 92HT599 and R5 92TH026 isolates of HIV-1 were found to carry in their envelope host-derived ICAM-1 protein (data not shown). Next, such virus preparations were pretreated with the anti-ICAM-1 RR1/1.1.1 antibody before inoculation of human tonsillar tissue maintained ex vivo. Productive infection of human tonsil tissue with both clinical isolates of HIV-1 was significantly diminished by an antibody-mediated blockade of ICAM-1-LFA-1 interaction (Fig. 6). Virus production was not affected by a pretreatment of virus stocks with an isotype-matched control antibody. In light of such findings, it can be concluded that the additional interaction between virion-anchored host ICAM-1 and cell surface LFA-1 represents an important event for productive infection of lymphoid tissue ex vivo by primary isolates of HIV-1.
FIG. 6.
Replication of X4 and R5 clinical HIV-1 isolates in human lymphoid tissue is reduced by a single pretreatment with a blocking anti-ICAM-1 antibody. Two primary isolates of HIV-1 that were expanded in human mononuclear cells were initially pretreated either with the anti-ICAM-1 RR1/1.1.1 blocking antibody or with an isotype-matched irrelevant control antibody. Next, the replication kinetics of such treated virus preparations in human lymphoid tissues was assessed by p24 measurements. The results shown are the mean values ± the SD of two tissue blocks from the same donor in four separate wells (i.e., eight tissue blocks) and are representative of experiments with tissue from three donors.
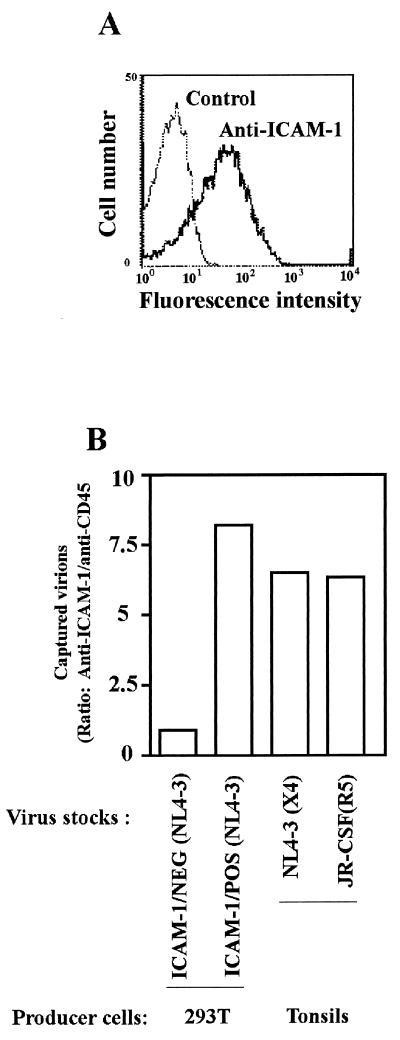
Comparable levels of host-derived ICAM-1 are found on virions produced either by transfected 293T cells or human lymphoid tissue.
ICAM-1 is an inducible cell surface constituent that is primarily expressed on lymphocytes, monocytes/macrophages, granulocytes, and certain epithelial cells, and its expression can be markedly increased (58). Since human tonsil histoculture is a system that preserves and maintains the mixed cell populations found in secondary lymphoid organs (i.e., CD4+ and CD8+ T cells, B cells, macrophages, and dendritic cells) (42), we therefore sought to assess and compare the degree of incorporation of ICAM-1 in viruses originating from transiently transfected 293T cells versus virions expanded in human tonsil histocultures. The incorporation of host ICAM-1 into the HIV-1 envelope was assessed by using a virus capture assay (3). First, flow cytometry was performed for a quantitative analysis of the percentage of ICAM-1-expressing cells in human tonsils that were mechanically dispersed into single-cell suspensions. As depicted in Fig. 7A, a high proportion of ICAM-1-positive cells was found in this tissue. Next, HIV-1NL4-3 and HIV-1JR-CSF were grown in human tonsil histocultures and were subjected to a virus precipitation assay. In this test, an anti-CD45 antibody was used as a control to estimate background levels of captured viruses since CD45 is not incorporated in HIV-1 (14). The two virus stocks made in 293T cells or expanded in human lymphoid tissue histocultures were similarly captured (Fig. 7B). These results support the idea that HIV-1 particles originating from a natural cellular reservoir such as lymphoid tissue do acquire levels of host-encoded ICAM-1 that are sufficient to modulate the virus life cycle.
FIG. 7.
T-tropic and M-tropic isolates of HIV-1 incorporate functionally relevant levels of cellular ICAM-1 when produced in histocultures of human tonsils. (A) Mechanically disrupted single-cell suspensions from tissue blocks were stained with a monoclonal anti-ICAM-1 antibody (clone RR1/1.1.1), followed by treatment with a phycoerythrin-conjugated goat anti-mouse IgG antibody. (B) The indicated virus preparations (2.5 ng of p24) were subjected to a capture virus assay by using magnetic beads coated with either an anti-ICAM-1 (clone RR1/1.1.1) or an isotype-matched anti-CD45RO antibody (clone UCHL-1). The amount of virus captured by each antibody was estimated with the use of a virus p24 antigen capture assay. Magnetic beads coated with the anti-CD45RO antibody served as controls to determine background levels of captured viruses. The data shown represent the ratio of captured virions with antibody reactive with human ICAM-1 and CD45RO, respectively.
DISCUSSION
It is currently well accepted that HIV-1 incorporates not only virally encoded proteins but also several membrane proteins derived from host cells during the budding process (reviewed in reference 62). Several reports have shown that the presence of host-derived proteins on the surface of the virion has several significant functional consequences for the biology of HIV-1, although the precise contribution of these host proteins in HIV-1 pathogenesis remains to be clarified. In an attempt to shed light on the physiological significance of finding host proteins in the envelope of HIV-1, the possible consequences of functional ICAM-1 proteins on HIV-1 particles were investigated in lymphoid organs. These tissues were deliberately chosen because they are considered to be the major reservoirs of HIV-1 and sites of intense viral replication. In this work, we made use of an experimental system based on the ex vivo culture of human tonsillar tissue. This cell culture model has been used to study various aspects of HIV-1 pathogenesis (23, 24, 26, 29, 41). Some of the most notable features of the ex vivo human tonsillar system are the lack of any requirement for exogenous activating stimuli to achieve productive HIV-1 infection and the preservation of their general cytoarchitecture, including a network of FDCs (23, 24).
Our results indicate that a high proportion of cells found in human lymphoid tissues cultured ex vivo expressed high levels of surface LFA-1 and that replication of HIV-1 particles bearing distinct tropism was significantly augmented by the acquisition of host ICAM-1 protein (Fig. 1 and 2). The significance of ICAM-1-LFA-1 interaction in the enhancement of virus infectivity was demonstrated by its abrogation when anti-ICAM-1 and anti-LFA-1 blocking antibodies were used (Fig. 3). The upregulating effect on the replicative capacity of ICAM-1/POS viruses was further accentuated by inducing conformational changes that increase both the avidity and the affinity of LFA-1 for its ICAM-1 ligand (Fig. 4). Incorporation of host-encoded ICAM-1 was associated with a more dramatic CD4+-T-cell depletion mediated by HIV-1 variants that use CXCR4 (i.e., T-tropic) (Fig. 5). The observation that infection of human lymphoid tissue with primary R5 and X4 isolates of HIV-1 was severely limited by blocking the host protein functions by an inhibitor of ICAM-1-LFA-1 interaction represents evidence that virion-anchored host ICAM-1 contributes to pathogenesis in vivo (Fig. 6). The physiological relevance of our findings is further strengthened by the fact that macrophage-tropic and T-tropic strains of HIV-1 do incorporate noticeable amounts of host ICAM-1 when expanded in a cellular setting that maintains the mixed cell populations and tissue cytoarchitecture that is normally present within human lymphoid tissue in vivo (Fig. 7).
The mechanism(s) by which virally embedded host ICAM-1 can contribute to the life cycle of HIV-1 in human lymphoid tissue remains unclear. However, it can be proposed that the attachment process to the surface of host cells is the most likely benefactor of the presence of ICAM-1 in the HIV-1 envelope. Indeed, although it is currently clear that the attachment process is dominated by binding of virion-associated gp120 to the cellular CD4-coreceptor complex, other secondary interactions between the viral entity and the cell surface have been proposed to positively influence HIV-1-cell binding (63). This postulate is strengthened when we consider that histocultures of human lymphoid tissue preserve a network of FDCs (24) and that the adhesion proteins ICAM-1 (CD54) and LFA-1 (CD11a) play an important role in the trapping of HIV-1 particles on the surface of FDCs (22). Since active viral replication is found in cells surrounding FDCs, which can trap large quantities of virus (estimated at 1010 to 1011 viruses) (11, 33, 48, 53, 57), the possible interaction between virion-bound host ICAM-1 and LFA-1 expressed on the surface of FDCs can contribute to an increase in the amount of virus trapped in the lymph nodes resulting in a quantitatively more important transfer of HIV-1 particles to target cells. Infection efficiency with ICAM-1-bearing virions can also be enhanced because a high proportion of CD4+ T cells are activated (25 to 50%) in the lymphoid tissues of HIV-1-infected patients (13), and such a T-cell activation event is known to result in surface expression of a high-affinity-high-avidity LFA-1 state.
AIDS is a multifaceted disease that starts with HIV-1 infection of CD4+ T cells and eventually leads to an inexorable deterioration of lymphoid tissue architecture (16). This is accompanied by a massive death of CD4+ T lymphocytes, along with destruction and/or dysfunction of various CD4+ cells that are not productively infected by the virus (i.e., bystander cells) (6, 28, 59). Studies with human peripheral blood mononuclear cells transplanted into immunodeficient mice have revealed complex relationships among viral replication rates, cellular tropism, and CD4+-T-cell depletion (32, 44, 55). Other experiments with human lymphoid tissue ex vivo have also demonstrated a strong link between T-cell tropism and CD4+-T-cell depletion (23, 24). In the present study, we provide evidence that the incorporation process of host ICAM-1 in the mature HIV-1 particle can be added to the list of killing mechanisms, both direct and indirect, that has been proposed to explain the massive depletion of CD4+ T lymphocytes seen in HIV-1-infected individuals (reviewed in reference 56). Our data are in agreement with previous reports showing that human HIV-1 coreceptor preferences determine target T-cell depletion and cellular tropism in human lymphoid tissue and that patients harboring X4 viruses progress to AIDS more rapidly than do similar cohorts where the majority of viruses bears a R5 phenotype (8, 27, 30, 38, 55, 61). It can thus be proposed that the acquisition of host-derived ICAM-1 protein by HIV-1 strains that utilize CXCR4 will accelerate the progression of the disease.
In summary, this series of investigations demonstrates that the incorporation of host ICAM-1 protein into HIV-1 bearing distinct tropism could have profound effects on the biology of this retrovirus when infection is allowed to proceed in human lymphoid tissues, which are considered major anatomical sites for the establishment and propagation of HIV-1 infection. This observation is highly relevant for in vivo situations since it reveals new potential pathological mechanisms for HIV-1.
Acknowledgments
We thank M. Dufour for technical assistance in flow cytometry studies and J. Corbeil for critical reading of the manuscript.
This study was supported by grants to M.J.T. from the Canadian Institutes of Health Research HIV/AIDS Research Program (grant HOP-14438) and the “Fonds de la Recherche en Santé du Québec” (Réseau FRSQ SIDA et Maladies Infectieuses). S.B. is the recipient of a Ph.D. fellowship from the Canadian Institutes of Health Research, and M.J.T. holds a Tier 1 Canada Research Chair in Human Immuno-Retrovirology.
REFERENCES
- 1.Arthur, L. O., J. W. J. Bess, R. C. Sowder II, R. E. Benveniste, D. L. Mann, J.-C. Cherman, and L. E. Henderson. 1992. Cellular proteins bound to immunodeficiency viruses: implication for pathogenesis and vaccines. Science 258**:**1935–1938. [DOI] [PubMed] [Google Scholar]
- 2.Benkirane, M., D. Blanc-Zouaoui, M. Hirn, and C. Deveaux. 1994. Involvement of human leukocyte antigen class I molecules in human immunodeficiency virus infection of CD4-positive cells. J. Virol. 68**:**6332–6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bounou, S., N. Dumais, and M. J. Tremblay. 2001. Attachment of human immunodeficiency virus-1 (HIV-1) particles bearing host-encoded B7-2 proteins leads to nuclear factor-κB- and nuclear factor of activated T cells-dependent activation of HIV-1 long terminal repeat transcription. J. Biol. Chem. 276**:**6359–6369. [DOI] [PubMed] [Google Scholar]
- 4.Butcher, E. C., and I. L. Weisman. 1989. Lymphoid tissues and organs, p.117–137. In Fundamental immunology, 2nd ed. Raven Press, New York, N.Y.
- 5.Cantin, R., J.-F. Fortin, and M. Tremblay. 1996. The amount of host HLA-DR proteins acquired by HIV-1 is virus strain- and cell type-specific. Virology 218**:**372–381. [DOI] [PubMed] [Google Scholar]
- 6.Casella, C. R., and T. H. Finkel. 1997. Mechanisms of lymphocyte killing by HIV. Curr. Opin. Hematol. 4**:**24–31. [DOI] [PubMed] [Google Scholar]
- 7.Chen, B. K., K. Saksela, R. Andino, and D. Baltimore. 1994. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. J. Virol. 68**:**654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185**:**621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong, J., M. G. Roth, and E. Hunter. 1992. A chimeric avian retrovirus containing the influenza virus hemagglutinin gene has an expanded host range. J. Virol. 66**:**7374–7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dustin, M. L., and T. A. Springer. 1989. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature 341**:**619–624. [DOI] [PubMed] [Google Scholar]
- 11.Embretson, J., M. Zupancic, J. L. Ribas, A. Burke, P. Racz, K. Tenner-Racz, and A. T. Haase. 1993. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature 362**:**359–362. [DOI] [PubMed] [Google Scholar]
- 12.Emilie, D., M. Peuchmaur, M. C. Maillot, M. C. Crevon, N. Brousse, J. F. Delfraissy, J. Dormont, and P. Galanaud. 1990. Production of interleukins in human immunodeficiency virus-1-replicating lymph nodes. J. Clin. Investig. 86**:**148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein, F. H. 1993. The immunopathogenesis of human immunodeficiency virus infection. N. Engl. J. Med. 328**:**327–335. [DOI] [PubMed] [Google Scholar]
- 14.Esser, M. T., D. R. Graham, L. V. Coren, C. M. Trubey, J. W. Bess, L. O. Arthur, D. E. Ott, and J. D. Lifson. 2001. Differential incorporation of CD45, CD80 (B7-1), CD86 (B7-2), and major histocompatibility complex class I and II molecules into human immunodeficiency virus type 1 virions and microvesicles: implications for viral pathogenesis and immune regulation. J. Virol. 75**:**6173–6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fais, S., M. R. Capobianchi, I. Abbate, C. Catilletti, M. Gentile, P. Cordiali Fei, F. Ameglio, and F. Dianzani. 1995. Unidirectional budding of HIV-1 at the site of cell-to-cell contact is associated with copolarization of intercellular adhesion molecules (ICAM-1) and HIV-1 viral matrix protein. AIDS 9**:**329–335. [PubMed] [Google Scholar]
- 16.Fauci, A. S. 1993. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science 262**:**1011–1017. [DOI] [PubMed] [Google Scholar]
- 17.Fortin, J.-F., R. Cantin, G. Lamontagne, and M. Tremblay. 1997. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 71**:**3588–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fortin, J.-F., R. Cantin, and M. Tremblay. 1998. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM-1. J. Virol. 72**:**2105–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fortin, J. F., B. Barbeau, H. Hedman, E. Lundgren, and M. J. Tremblay. 1999. Role of the leukocyte function antigen-1 conformational state in the process of human immunodeficiency virus type 1-mediated syncytium formation and virus infection. Virology 257**:**228–238. [DOI] [PubMed] [Google Scholar]
- 20.Fox, C. H., K. Tenner-Rácz, P. Rácz, A. Firpo, P. A. Pizzo, and A. S. Fauci. 1991. Lymphoid germinal centers are reservoirs of human immunodeficiency virus type 1 RNA. J. Infect. Dis. 164**:**1051–1057. [DOI] [PubMed] [Google Scholar]
- 21.Frankel, S. S., B. M. Wenig, A. P. Burke, P. Mannam, L. D. R. Thompson, S. L. Abbondanzo, A. M. Nelson, M. Pope, and R. M. Steinman. 1996. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science 272**:**115–117. [DOI] [PubMed] [Google Scholar]
- 22.Fujiwara, M., R. Tsunoda, S. Shigeta, T. Yokota, and M. Baba. 1999. Human follicular dendritic cells remain uninfected and capture human immunodeficiency virus type 1 through CD54-CD11a interaction. J. Virol. 73**:**3603–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glushakova, S., B. Baibakov, L. B. Margolis, and J. Zimmerberg. 1995. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat. Med. 1**:**1320–1322. [DOI] [PubMed] [Google Scholar]
- 24.Glushakova, S., B. Baibakov, J. Zimmerberg, and L. B. Margolis. 1997. Experimental HIV infection of human lymphoid tissue: correlation of CD4+ T cell depletion and virus syncytium-inducing/non-syncytium-inducing phenotype in histocultures inoculated with laboratory strains and patient isolates of HIV type 1. AIDS Res. Hum. Retrovir. 13**:**461–471. [DOI] [PubMed] [Google Scholar]
- 25.Glushakova, S., J. C. Grivel, W. Fitzgerald, A. Sylwester, J. Zimmerberg, and L. B. Margolis. 1998. Evidence for the HIV-1 phenotype switch as a causal factor in acquired immunodeficiency. Nat. Med. 4**:**346–349. [DOI] [PubMed] [Google Scholar]
- 26.Glushakova, S., Y. Yi, J. C. Grivel, A. Singh, D. Schols, E. De Clercq, R. G. Collman, and L. Margolis. 1999. Preferential coreceptor utilization and cytopathicity by dual-tropic HIV-1 in human lymphoid tissue ex vivo. J. Clin. Investig. 104**:**R7–R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goudsmit, J. 1995. The role of viral diversity in HIV pathogenesis. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10**:**S15–S19. [PubMed] [Google Scholar]
- 28.Gougeon, M. L., E. Ledru, H. Lecoeur, and S. Garcia. 1998. T cell apoptosis in HIV infection: mechanisms and relevance for AIDS pathogenesis. Results Probl. Cell Differ. 24**:**233–248. [DOI] [PubMed] [Google Scholar]
- 29.Grivel, J. C., and L. B. Margolis. 1999. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat. Med. 5**:**344–346. [DOI] [PubMed] [Google Scholar]
- 30.Grivel, J. C., M. L. Penn, D. A. Eckstein, B. Schramm, R. F. Speck, N. W. Abbey, B. Herndier, L. Margolis, and M. A. Goldsmith. 2000. Human immunodeficiency virus type 1 coreceptor preferences determine target T-cell depletion and cellular tropism in human lymphoid tissue. J. Virol. 74**:**5347–5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grouard, G., I. Durand, L. Filgueira, J. Banchereau, and Y. J. Liu. 1996. Dendritic cells capable of stimulating T cells in germinal centres. Nature 384**:**364–367. [DOI] [PubMed] [Google Scholar]
- 32.Gulizia, R. J., J. A. Levy, and D. E. Mosier. 1996. The envelope gp120 gene of human immunodeficiency virus type 1 determines the rate of CD4-positive T-cell depletion in SCID mice engrafted with human peripheral blood leukocytes. J. Virol. 70**:**4184–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haase, A. T., K. Henry, M. Zupancic, G. Sedgewick, R. A. Faust, H. Melroe, W. Cavert, K. Gebhard, K. Staskus, Z. Q. Zhang, P. J. Dailey, H. H. Balfour, Jr., A. Erice, and A. S. Perelson. 1996. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science 274**:**985–989. [DOI] [PubMed] [Google Scholar]
- 34.Heath, S. L., J. G. Tew, J. G. Tew, A. K. Szakal, and G. F. Burton. 1995. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature 377**:**740–744. [DOI] [PubMed] [Google Scholar]
- 35.Heinen, E., M. Braun, E. Louis, N. Cormann, R. Tsunoda, C. Kinet-Denoel, F. Lesage, and L. J. Simar. 1988. Interactions between follicular dendritic cells and lymphoid cells. Adv. Exp. Med. Biol. 237**:**181–184. [DOI] [PubMed] [Google Scholar]
- 36.Imal, Y., and M. Yamakawa. 1996. Morphology, function and pathology of follicular dendritic cells. Pathol. Int. 46**:**807–833. [DOI] [PubMed] [Google Scholar]
- 37.Keizer, G. D., W. Visser, M. Vliem, and C. C. Figdor. 1988. A monoclonal antibody (NKI-L16) directed against a unique epitope on the α-chain of human leukocyte function-associated antigen 1 induces homotypic cell-cell interactions. J. Immunol. 140**:**1393–1400. [PubMed] [Google Scholar]
- 38.Koot, M., I. P. Keet, A. H. Vos, R. E. de Goede, M. T. Roos, R. A. Coutinho, F. Miedema, P. T. Schellekens, and M. Tersmette. 1993. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann. Intern. Med. 118**:**681–688. [DOI] [PubMed] [Google Scholar]
- 39.Liu, Y., and P. S. Linsley. 1992. Costimulation of T-cell growth. Curr. Opin. Immunol. 4**:**265–270. [DOI] [PubMed] [Google Scholar]
- 40.MacLennan, I. C. M. 1994. Germinal centers. Annu. Rev. Immunol. 12**:**117–139. [DOI] [PubMed] [Google Scholar]
- 41.Margolis, L. B., W. Fitzgerald, S. Glushakova, S. Hatfill, N. Amichay, B. Baibakov, and J. Zimmerberg. 1997. Lymphocyte trafficking and HIV infection of human lymphoid tissue in a rotating wall vessel bioreactor. AIDS Res. Hum. Retrovir. 13**:**1411–1420. [DOI] [PubMed] [Google Scholar]
- 42.Margolis, L. B., S. Glushakova, J. C. Grivel, and P. M. Murphy. 1998. Blockade of CC chemokine receptor 5 (CCR5)-tropic human immunodeficiency virus-1 replication in human lymphoid tissue by CC chemokines. J. Clin. Investig. 101**:**1876–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marlin, S. D., and T. A. Springer. 1987. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell 51**:**813–819. [DOI] [PubMed] [Google Scholar]
- 44.Mosier, D. E., R. J. Gulizia, P. D. MacIsaac, B. E. Torbett, and J. A. Levy. 1993. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science 260**:**689–692. [DOI] [PubMed] [Google Scholar]
- 45.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272**:**263–267. [DOI] [PubMed] [Google Scholar]
- 46.Orentas, R. J., and J. E. K. Hildreth. 1993. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res. Hum. Retrovir. 9**:**1157–1165. [DOI] [PubMed] [Google Scholar]
- 47.Pantaleo, G., C. Graziosi, L. Butini, P. A. Pizzo, S. M. Schnittman, D. P. Kotler, and A. S. Fauci. 1991. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 88**:**9838–9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pantaleo, G., C. Graziosi, J. F. Demarest, L. Butini, M. Montroni, C. H. Fox, J. M. Orenstein, D. P. Kotler, and A. S. Fauci. 1993. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 362**:**355–358. [DOI] [PubMed] [Google Scholar]
- 49.Paquette, J. S., J. F. Fortin, L. Blanchard, and M. J. Tremblay. 1998. Level of ICAM-1 surface expression on virus producer cells influences both the amount of virion-bound host ICAM-1 and human immunodeficiency virus type 1 infectivity. J. Virol. 72**:**9329–9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rizzuto, C. D., and J. G. Sodroski. 1997. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J. Virol. 71**:**4847–4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rothlein, R., M. L. Dustin, S. D. Marlin, and T. A. Springer. 1986. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J. Immunol. 137**:**1270–1274. [PubMed] [Google Scholar]
- 52.Saifuddin, M., C. J. Parker, M. E. Peeples, M. K. Gorny, S. Zolla-Pazner, M. Ghassemi, I. A. Rooney, J. P. Atkinson, and G. T. Spear. 1995. Role of virion-associated glycosylphosphatidylinositol-linked proteins CD55 and CD59 in complement resistance of cell line-derived and primary isolates of HIV-1. J. Exp. Med. 182**:**501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schacker, T., S. Little, E. Connick, K. Gebhard-Mitchell, Z. Q. Zhang, J. Krieger, J. Pryor, D. Havlir, J. K. Wong, D. Richman, L. Corey, and A. T. Haase. 2000. Rapid accumulation of human immunodeficiency virus (HIV) in lymphatic tissue reservoirs during acute and early HIV infection: implications for timing of antiretroviral therapy. J. Infect. Dis. 181**:**347–354. [DOI] [PubMed] [Google Scholar]
- 54.Schols, D., R. Pauwels, J. Desmyter, and E. De Clerk. 1992. Presence of class II histocompatibility DR proteins on the envelope of human immunodeficiency virus demonstrated by FACS analysis. Virology 189**:**374–376. [DOI] [PubMed] [Google Scholar]
- 55.Schuitemaker, H., M. Koot, N. A. Kootstra, M. W. Dercksen, R. E. de Goede, R. P. van Steenwijk, J. M. Lange, J. K. Schattenkerk, F. Miedema, and M. Tersmette. 1992. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J. Virol. 66**:**1354–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shearer, G. M. 1998. HIV-induced immunopathogenesis. Cell 9**:**587–593. [DOI] [PubMed] [Google Scholar]
- 57.Spiegel, H., H. Herbst, G. Niedobitek, H. D. Foss, and H. Stein. 1992. Follicular dendritic cells are a major reservoir for human immunodeficiency virus type 1 in lymphoid tissues facilitating infection of CD4+ T-helper cells. Am. J. Pathol. 140**:**15–22. [PMC free article] [PubMed] [Google Scholar]
- 58.Springer, T. A. 1990. Adhesion receptors of the immune system. Nature 346**:**425–434. [DOI] [PubMed] [Google Scholar]
- 59.Stewart, S. A., B. Poon, J. Y. Song, and I. S. Y. Chen. 2000. Human immunodeficiency virus type 1 Vpr induces apoptosis through caspase activation. J. Virol. 74**:**3105–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tenner-Racz, K., P. Racz, M. Dietrich, and P. Karin. 1985. Altered dendritic follicular cells and virus-like particles in AIDS and AIDS-related lymphadenopathy. Lancet i**:**105–106. [DOI] [PubMed] [Google Scholar]
- 61.Tersmette, M., R. A. Gruters, F. de Wolf, R. E. de Goede, J. M. Lange, P. T. Schellekens, J. Goudsmit, H. G. Huisman, and F. Miedema. 1989. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J. Virol. 63**:**2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tremblay, M. J., J.-F. Fortin, and R. Cantin. 1998. The acquisition of host-encoded proteins by nascent HIV-1. Immunol. Today 19**:**346–351. [DOI] [PubMed] [Google Scholar]
- 63.Ugolini, S., I. Mondor, and Q. J. Sattentau. 1999. HIV-1 attachment: another look. Trends Microbiol. 7**:**144–149. [DOI] [PubMed] [Google Scholar]
- 64.van Kooyk, Y., P. van de Wiel-van Kemenade, P. Weder, T. W. Kuijpers, and C. G. Figdor. 1989. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature 342**:**811–813. [DOI] [PubMed] [Google Scholar]