Herpes Simplex Virus ICP0 and ICP34.5 Counteract Distinct Interferon-Induced Barriers to Virus Replication (original) (raw)
Abstract
Interferon inhibits virus replication through multiple mechanisms. Here we show that herpes simplex virus proteins ICP0 and ICP34.5 overcome interferon-induced barriers to viral transcription and translation, respectively. These cytokine-induced antiviral mechanisms are differentially expressed in established cell lines: U2OS cells do not mount the IFN-induced mechanism targeted by ICP0, and Vero cells may be defective for the mechanism targeted by ICP34.5.
The interferon (IFN)-induced cellular antiviral response is the primary defense mechanism against viral infection in the intact mammalian host (15, 16, 19). Many viruses have therefore evolved strategies to evade the effects of IFN by blocking IFN production or its antiviral actions (15, 20, 21). IFN induces the synthesis of many proteins capable of inhibiting viral replication, with the double-stranded RNA (dsRNA)-dependent protein kinase PKR being among the most effective (14). Latent PKR resides within the cytoplasm and is activated by dsRNA, a common by-product of viral infection. Activated PKR phosphorylates the alpha subunit of eukaryotic initiation factor 2 (eIF-2α), thereby inhibiting translation. It is thus not surprising that each step of the PKR pathway is subject to negative regulation by diverse RNA and DNA viruses (20).
IFN only marginally reduces the replication of herpes simplex virus type 1 (HSV-1) in cultured cells, yet it plays a major role in limiting acute infection in intact animals (22, 24, 25, 32). At least three HSV-1 gene products appear to modulate IFN-related pathways. We previously demonstrated that viral mutants deficient in the immediate-early (IE) protein ICP0 are hypersensitive to IFN and fail to accumulate viral mRNAs in Vero cells pretreated with the cytokine (29). In addition, the late proteins ICP34.5 and US11 have been implicated in the PKR pathway: ICP34.5 serves as a regulatory subunit of protein phosphatase 1 and acts to reverse PKR-induced phosphorylation of eIF-2α while US11 is an RNA binding protein that prevents PKR activation (4, 6, 18, 34).
Although ICP34.5 interacts with a component of the PKR pathway, its contribution to the relative resistance of HSV-1 to IFN in tissue culture has not been investigated. We therefore asked if the ICP34.5-deficient chain termination mutant termA (2) is hypersensitive to IFN by using a plaque reduction assay. Monolayers of otherwise permissive Vero and U2OS cells were pretreated for 16 h with 1,000 U of IFN-α per ml and then infected with serial dilutions of termA, its wild-type marker rescue product termAR, wild-type herpes simplex virus type 1 (HSV-1) KOS, and the KOS ICP0 null mutant _n_212 (3). Plaques were counted 2 days later, and the results were expressed as the ratio of the titers observed on treated versus untreated monolayers (Table 1). As previously reported, IFN had only a modest effect on wild-type HSV (KOS and termAR) in both cell types, while _n_212 was severely inhibited on Vero but not U2OS cells (29). In contrast, the ICP34.5 mutant termA displayed the converse pattern: plaquing was severely reduced on U2OS cells (>200-fold), but little effect was observed on Vero cells. These data indicate that IFN induces an antiviral mechanism in U2OS cells that is targeted by ICP34.5. Inasmuch as multiple ICP0 mutants are resistant to the cytokine in this cell type (29), the data indicate that ICP34.5 and ICP0 overcome distinct antiviral pathways and suggest that U2OS cells may lack the pathway targeted by ICP0; alternatively, these cells may express a cellular ICP0-like activity (37) capable of disarming a subset of IFN-induced mechanisms. The data also raise the possibility that Vero cells lack the pathway targeted by ICP34.5; alternatively, these cells may support low-level suppression of the chain-termination mutation in termA, as previously inferred for analogous ICP34.5 mutants in human foreskin fibroblasts (7). Further experiments are required to distinguish between these alternatives.
TABLE 1.
Effects of IFN-α and cell type on plaque formation by ICP0 and ICP34.5 mutantsa
| Virus | Gene inactivated | Titer ratio (untreated/treated) | |
|---|---|---|---|
| Vero cells | U2OS cells | ||
| KOS | None | 2.3 | 2.9 |
| _n_212 | ICP0 | >200b | 3.5 |
| termAR | None | 2.1 | 1.9 |
| 4.0 | 8.7 | ||
| 3.7 | 3.6 | ||
| 2.8 | 2.3 | ||
| termA | ICP34.5 | 3.1 | >200b |
| 10 | >1,000b | ||
| 8.5 | >1,000b | ||
| 4.8 | >1,000b |
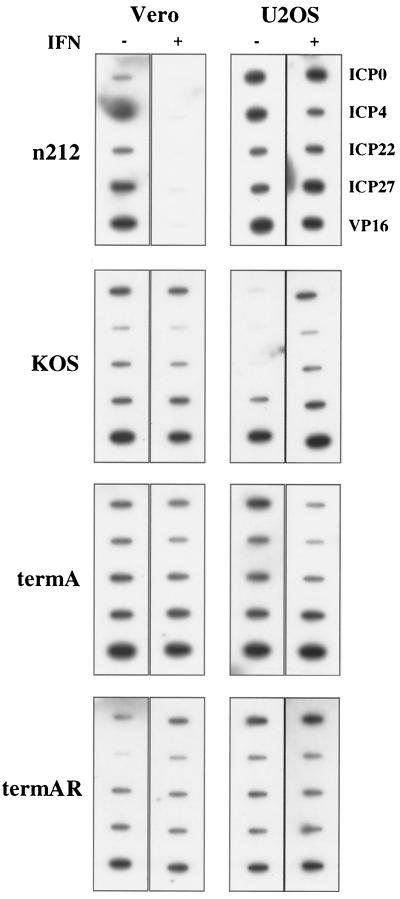
ICP34.5 mutants display a host-range phenotype in the absence of exogenous IFN, being unable to replicate in a subset of cell lines. The block to viral replication in such constitutively restrictive cell lines occurs at the level of translation (8, 9) and is accompanied by the accumulation of phosphorylated eIF-2α (6). In contrast, ICP0 mutants fail to accumulate viral mRNA in IFN-treated Vero cells, consistent with a defect in transcription, pre-mRNA processing, or mRNA stability (29). To begin to define how ICP34.5 and ICP0 act to promote viral replication in the presence of IFN, we performed nuclear run-on transcription assays. Nuclei were harvested from control and IFN-treated Vero and U2OS cells 6 h postinfection, and 20 PFU of _n_212 per cell were used in contrast to 5 PFU of KOS, termA, and termAR per cell in order to increase the run-on signal in Vero cells. Transcription in isolated nuclei was done in the presence of [32P]UTP and a buffer containing 150 mM KCl (36). Each reaction mixture contained 107 nuclei, and incorporation ranged from 5 × 106 to 1 × 107 cpm/reaction mixture between experiments; however, within each experiment, incorporation differed by no more than 20% between reactions. The RNA product from 107 nuclei was purified and hybridized to a membrane containing single-stranded probes designed to detect sense transcription of the IE genes encoding ICP-0, -4, -22, and -27 and the late gene encoding VP16. IFN had relatively little inhibitory effect on viral transcription in either cell type during infection with KOS, termA, or termAR; indeed, we consistently observed a marginal increase in the run-on signal for the IE genes with KOS in U2OS cell in the presence of IFN. Although the basis for this latter effect remains unclear, it may reflect an IFN-induced defect in the repression of IE gene transcription that normally occurs in the early phase of infection. In contrast, transcription of the _n_212 genome was almost completely blocked in Vero cells pretreated with IFN (Fig. 1). However, this effect was not observed in U2OS cells which complement ICP0 null mutants (37). As shown previously (10, 28, 33), IFN did not block delivery of the viral genome to the nucleus (data not shown). Taken in combination, these data demonstrate that ICP0 is required to overcome an IFN-induced intranuclear block to viral transcription in Vero cells, whereas ICP34.5 promotes virus replication in IFN-treated U2OS cells at a posttranscriptional level.
FIG. 1.
ICP0 functions to overcome an IFN-induced block to viral transcription. Transcription levels were measured by nuclear run on analysis in Vero and U2OS cells either mock treated or pretreated with IFN-α prior to infection with wild type-HSV-1 (KOS and termAR) or mutant _n_212 (ICP0−) or termA (ICP34.5−). Nuclei were harvested 6 h postinfection, and transcription was allowed to proceed in the presence of radiolabeled UTP. RNA products were purified and hybridized to membranes bearing single-stranded DNA probes designed to detect sense transcription of the indicated viral genes.
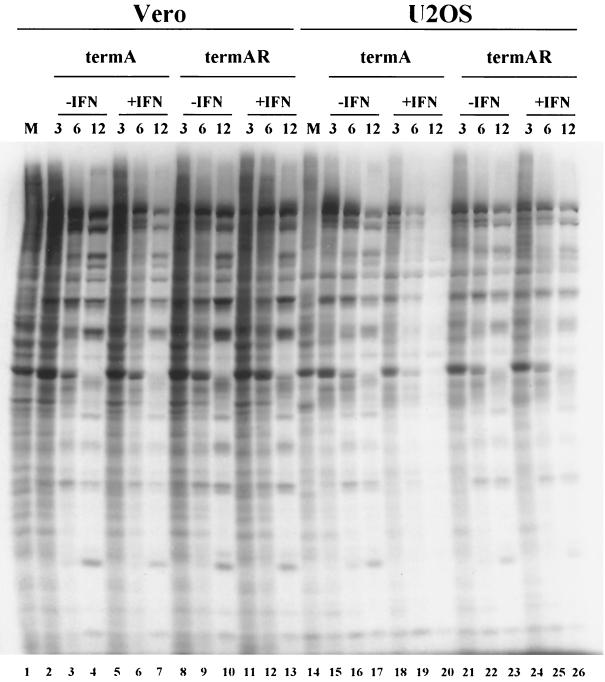
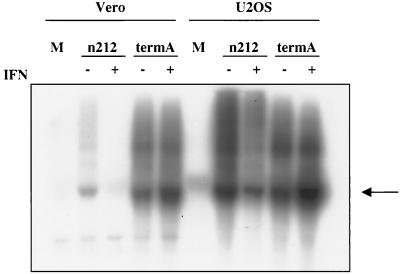
ICP34.5 mutants display complete shutoff of viral protein synthesis at intermediate times postinfection in constitutively restrictive cell lines. We asked if IFN induces a similar phenotype in otherwise permissive U2OS cells. Control and IFN-treated monolayers of Vero and U2OS cells were infected with 5 PFU of termA or termAR per cell for 3, 6, or 12 h and then labeled with [35S]methionine for 1 h as previously described (30) (Fig. 2). IFN had no significant effect on the pattern of protein synthesis by either virus in Vero cells, with the possible exception of a marginal reduction with termA at 12 h postinfection. In marked contrast, IFN selectively inhibited protein synthesis by termA in U2OS cells: protein synthesis was reduced at 6 h postinfection and virtually abolished by 12 h, while translation was sustained for 12 h in control cells. Inasmuch as IFN had no effect on termAR in these cells (Fig. 2), these data indicate that ICP34.5 overcomes an IFN-induced barrier to viral protein synthesis in U2OS cells. Northern blot analysis showed that high levels of ICP27 mRNA were maintained at 6 h postinfection with termA in IFN-treated U2OS cells (Fig. 3), demonstrating that the translational arrest observed in the absence of ICP34.5 was not accompanied by the loss of viral mRNA. In contrast, as shown previously (29), IFN prevented accumulation of ICP27 mRNA in Vero cells infected with _n_212 (Fig. 3). Taken in combination, these data demonstrate that IFN induces an ICP34.5 null phenotype in U2OS cells that is reminiscent of that observed in constitutively restrictive cell lines in the absence of IFN. By far, the simplest interpretation is that the antiviral system targeted by ICP34.5 is induced by IFN in some cell lines and expressed constitutively in others.
FIG. 2.
ICP34.5 functions to overcome an IFN-induced block to translation. Vero and U2OS cells either mock treated or pretreated with IFN-α were infected with 5 PFU/cell termA or termAR, incubated for the indicated times (in hours), and then labeled with [35S]cysteine-methionine for 1 h. Lysates were subjected to SDS-PAGE analysis and visualized by autoradiography.
FIG. 3.
Effect of IFN-α pretreatment on accumulation of viral IE mRNA. Vero and U2OS cells were mock treated or pretreated with IFN-α prior to infection with 5 PFU of either _n_212 (ICP0−) or termA (ICP34.5−) per cell. Total RNA was harvested 6 h postinfection, and ICP27 transcript levels were analyzed by Northern blot analysis. Arrow indicates ICP27 mRNA.
Our data demonstrate that ICP0 and ICP34.5 each contribute to the relatively IFN-resistant phenotype of HSV in tissue culture. It is interesting and perhaps significant that the genes encoding these proteins are immediately adjacent in the viral genome and lie within the domain that is transcribed from the opposite DNA strand during latency (35), raising the possibility that they may be coregulated in certain circumstances.
ICP34.5 acts as a regulatory subunit of protein phosphatase 1 that directs the enzyme to dephosphorylate eIF-2α (6, 18). Inamuch as eIF-2α is phosphorylated by PKR and PKR plays a key role in limiting the replication of many viruses, the simplest interpretation is that PKR activity limits the growth of ICP34.5 mutants under restricting conditions. Indeed, strong evidence that this is the case in intact mice has been presented (23), and our data are consistent with this hypothesis. However, it is worth noting that PKR is not the only eIF-2α kinase capable of arresting translation in mammalian cells (1, 17). Therefore, further experiments are required to definitively identify the IFN-inducible target of ICP34.5 in U2OS cells.
ICP0 was first characterized as a promiscuous activator capable of stimulating gene expression controlled by viral and cellular promoters in a sequence-independent fashion (13). More recently, evidence has emerged that ICP0 plays a fundamental role in launching the viral lytic cycle by targeting several cellular proteins believed to be involved in an innate cellular repression mechanism that silences infecting viral genomes (11). Possibly key to this repression system are discrete nuclear domains called ND10, which are considered sites of DNA virus transcription and regulation (26). ICP0 localizes to and subsequently disrupts ND10 (27), in part through the proteasome-dependent degradation of several ND10 constituents, some of which are highly inducible by IFN (5, 12, 31). Thus, our observation that ICP0 reverses an IFN-induced block to viral transcription is intriguing. To our knowledge, this is the first report of a viral protein that overcomes an IFN-induced nuclear block to viral transcription by RNA polymerase II. We believe that deciphering the interplay between IFN and ICP0 will provide further insight into the antiviral role of IFN and illuminate how ICP0 reverses the cellular repression mechanism that targets incoming viral genomes for quiescence or latency.
Acknowledgments
We thank P. Schaffer and R. L. Thompson for providing viral mutants.
This work was supported by a grant from the Medical Research Council of Canada/Canadian Institutes for Health Research. K.L.M. was funded by fellowships from the MRC and the Alberta Heritage Foundation for Medical Research.
REFERENCES
- 1.Berlanga, J. J., J. Santoyo, and C. De Haro. 1999. Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2alpha kinase. Eur. J. Biochem. 265**:**754-762. [DOI] [PubMed] [Google Scholar]
- 2.Bolovan, C. A., N. M. Sawtell, and R. L. Thompson. 1994. ICP34.5 mutants of herpes simplex virus type 1 strain 17syn+ are attenuated for neurovirulence in mice and for replication in confluent primary mouse embryo cell culture. J. Virol. 68**:**48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai, W., and P. A. Schaffer. 1989. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J. Virol. 63**:**4570-4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassady, K. A., M. Gross, and B. Roizman. 1998. The herpes simplex virus US11 protein effectively compensates for the gamma1(34.5) gene if present before activation of protein kinase R by precluding its phosphorylation and that of the alpha subunit of eukaryotic translation initiation factor 2. J. Virol. 72**:**8620-8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chelbi-Alix, M. K., and H. deThe. 1999. Herpes virus induced proteosome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18**:**935-941. [DOI] [PubMed] [Google Scholar]
- 6.Chou, J., J. J. Chen, M. Gross, and B. Roizman. 1995. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation facor eIF-2 alpha and premature shutoff of protein synthesis after infection with gamma 1 34.5 mutants of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 92**:**10516-10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou, J., A. P. Poon, J. Johnson, and B. Roizman. 1994. Differential response of human cells to deletions and stop codons in the gamma(1)34.5 gene of herpes simplex virus. J. Virol. 68**:**8304-8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou, J., and B. Roizman. 1992. The gamma 1(34.5) gene of herpes simplex virus1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programmed cell death in neuronal cells. Proc. Natl. Acad. Sci. USA 89**:**3266-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou, J., and B. Roizman. 1994. Herpes simplex virus 1 gamma(1)34.5 gene function, which blocks the host response to infection, maps in the homologous domain of the genes expressed during growth arrest and DNA damage. Proc. Natl. Acad. Sci. USA 91**:**5247-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Stasio, P. R., and M. W. Taylor. 1990. Specific effect of interferon on the herpes simplex virus type 1 transactivation event. J. Virol. 64**:**2588-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. BioEssays 22**:**761-770. [DOI] [PubMed] [Google Scholar]
- 12.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson. 1998. The disruption of ND10 during herpes simplex virus infection with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72**:**6581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everett, R. D., C. M. Preston, and N. D. Stow. 1991. Functional and genetic analysis of the role of Vmw110 in herpes simplex virus replication, p. 49-76. In E. K. Wagner (ed.), The control of herpes virus gene expression. CRC Press Inc., Boca Raton, Fla.
- 14.Gale, M., and M. G. Katze. 1998. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol. Ther. 78**:**29-46. [DOI] [PubMed] [Google Scholar]
- 15.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral responses and virus countermeasures. J. Gen. Virol. 81**:**2341-2364. [DOI] [PubMed] [Google Scholar]
- 16.Gresser, I. 1997. Wherefore interferon? J. Leukoc. Biol. 61**:**567-574. [DOI] [PubMed] [Google Scholar]
- 17.Harding, H. P., Y. Zhang, and D. Ron. 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397**:**271-274. [DOI] [PubMed] [Google Scholar]
- 18.He, B., M. Gross, and B. Roizman. 1997. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1 α to dephosphorylate the α subunit of the eukaryotic translation inititation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94**:**843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isaacs, A., and J. Lindenmann. 1957. Virus interference. I. The interferon. Proc. R. Soc. Lond. Ser. B 147**:**258-267. [PubMed] [Google Scholar]
- 20.Jacobs, B. L., and J. O. Langland. 1997. Viral inhibitors of interferon action: inhibitors of the PKR and 2"5" oligoadenulate synthetase/RNaseL pathways, p. 155-173. In G. Karupiah (ed.), Gamma interferon in antiviral defense. R. G. Landes Company, Austin, Tex.
- 21.Kotwal, G. J. 1997. Microorganisms and their interaction with the immune system. J. Leukoc. Biol. 62**:**415-429. [DOI] [PubMed] [Google Scholar]
- 22.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotypes of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189**:**663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leib, D. A., M. A. Machalek, B. R. Williams, R. H. Silverman, and H. W. Virgin. 2000. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc. Natl. Acad. Sci. USA 97**:**6097-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipp, M., and G. Brandner. 1985. Herpes simplex virus gene expression in interferon-treated cells, p. 355-360. In H. Kirchner and H. Schellekens (ed.), The biology of the interferon system. Elsevier, Amsterdam, The Netherlands.
- 25.Lipp, M., and G. Brandner. 1980. Inhibition of herpes simplex virus type 1 specific translation in cells treated with poly(rI) · poly(rC). J. Gen. Virol. 47**:**97-111. [DOI] [PubMed] [Google Scholar]
- 26.Maul, G. G. 1998. Nuclear domain 10, the site of DNA virus transcription and replication. BioEssays 20**:**660-667. [DOI] [PubMed] [Google Scholar]
- 27.Maul, G. G., H. H. Guldner, and J. G. Spivack. 1993. Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene 1 product (ICP0). J. Gen. Virol. 74**:**2679-2690. [DOI] [PubMed] [Google Scholar]
- 28.Mittnacht, S., P. Straub, H. Kirchner, and H. Jacobsen. 1988. Interferon treatment inhibits onset of herpes simplex virus immediate-early transcription. Virology 164**:**201-210. [DOI] [PubMed] [Google Scholar]
- 29.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 74**:**2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mossman, K. L., R. Sherburne, C. Lavery, J. Duncan, and J. R. Smiley. 2000. Evidence that herpes simplex virus VP16 is required for viral egress downstream of the initial envelopment event. J. Virol. 74**:**6287-6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller, S., and A. Dejean. 1999. Viral immediate-early proteins abrogate the modification by SUMO-1 of PML and Sp100 proteins, correlating with nuclear body disruption. J. Virol. 73**:**5137-5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholl, M. J., and C. M. Preston. 1996. Inhibition of herpes simplex virus type 1 immediate-early gene expression by alpha interferon is not VP16 specific. J. Virol. 70**:**6336-6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oberman, F., and A. Panet. 1989. Characterization of the early steps of herpes simplex virus replication in interferon-treated human cells. J. Interferon Res. 9**:**563-571. [DOI] [PubMed] [Google Scholar]
- 34.Poppers, J., M. Mulvey, D. Khoo, and I. Mohr. 2000. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J. Virol. 74**:**11215-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roizman, B., and A. E. Sears. 1996. Herpes simplex viruses and their replication, p. 1043-1107. In B. N. Fields, D. M. Knipe, P. M. Howley, et al. (ed.), Fundamental virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 36.Spencer, C. A., M. E. Dahmus, and S. A. Rice. 1997. Repression of host RNA polymerase II transcription by herpes simplex virus type 1. J. Virol. 71**:**2031-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao, F., and P. A. Schaffer. 1995. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J. Virol. 69**:**6249-6258. [DOI] [PMC free article] [PubMed] [Google Scholar]