Adenovirus Binding to the Coxsackievirus and Adenovirus Receptor or Integrins Is Not Required To Elicit Brain Inflammation but Is Necessary To Transduce Specific Neural Cell Types (original) (raw)
Abstract
Intracranial administration of adenovirus vectors elicits rapid, capsid-mediated dose-dependent brain inflammation. The mechanisms through which adenovirus capsids trigger inflammation in the brain remain unknown. We determined whether adenovirus interaction with the primary and secondary cell surface receptors for infection (CAR and αv integrins) was necessary to trigger acute adenovirus-mediated brain inflammation, and, furthermore, whether capsid mutations that abrogated CAR and integrin binding altered vector tropism in the brain. Vectors ablated for CAR binding, but retaining integrin binding function, transduced equivalent areas of brain compared to vectors with wild-type capsids; however, vector tropsim was dramatically altered. Vectors with wild-type capsids predominantly transduced oligodendrocytes, whereas mutation of the fiber protein to ablate CAR binding resulted in a loss of oligodendrocyte transduction and a consequent redirection of transduction to neurons and other types of glial cells. Combined mutations of fiber and penton base that ablate both CAR and integrin binding almost abolished brain transduction. Thus, doubly-ablated capsids engineered to express new ligands should allow complete vector retargeting in the central nervous system. Although transduction by the doubly-ablated vectors was reduced by greater than 95%, inflammation was not reduced compared to wild-type vectors, demonstrating that brain inflammation occurs independently of adenovirus binding and infection of cells via CAR and integrin receptors.
Recombinant adenoviruses are one of the most efficient vector systems available for delivering genes into nondividing cells, such as neurons and glia. Although likely to find clinical utility for the treatment of many diseases, research has highlighted important limitations of these vector systems: namely, their intrinsic inflammatory potential and their lack of restricted tropism.
Administration of adenovirus vectors to the central nervous system (CNS) elicits a rapid, capsid-mediated inflammatory response, which is dose dependent, independent of viral gene expression, and characterized by rapid astrocyte and microglial cell activation, infiltration of mononuclear inflammatory cells across the blood-brain barrier to the site of vector injection, and febrile responses at high vector doses (5, 6, 23, 24). In the presence of preexisting systemic immunity to adenovirus, the early inflammatory response in the brain is followed by a T-lymphocyte response directed against transduced neural cells presenting capsid protein-derived epitopes and de novo-expressed viral proteins (11, 16, 25). Novel high-capacity adenovirus vectors, which are deleted of all viral genes, elicit reduced anti-adenovirus T-lymphocyte responses in the brain (23, 25) and, in peripheral organs, have been shown to result in decreased toxicity associated with expression of viral genes (18, 21). Altering the genome of adenovirus vectors does not, however, reduce the early capsid-mediated brain inflammation (23).
Exactly which components of the adenovirus capsid are responsible for eliciting acute brain inflammation or, indeed, the cellular elements that initiate the inflammatory response to adenovirus in the brain remain unknown. Various groups have demonstrated that adenovirus infection of cells triggers intracellular signaling cascades that lead to the release of inflammatory cytokines by the infected cell (3, 4, 7, 13, 14, 17). Infection of cells by subgroup C adenoviruses is mediated via two cell surface receptors. The fiber protein of subgroup C adenoviruses first binds to the cell surface coxsackievirus and adenovirus receptor (CAR) (1, 2). Virus internalization is then mediated through an interaction between an arginine-glycine-aspartate (RGD) sequence on the adenovirus penton base protein and cell surface αvβ3 or αvβ5 integrins (26). When CAR is not expressed on cells, a less-efficient integrin-dependent infection pathway has been observed (10).
We hypothesized that if adenovirus infection of cells through the CAR-integrin pathway is necessary to trigger cytokine release in the CNS, capsid modifications that abrogate the adenovirus-CAR or -integrin interactions should reduce acute capsid-mediated brain inflammation. We investigated whether the capacity of adenovirus vectors to induce acute brain inflammation was correlated with their ability to bind and transduce neural cells via CAR and integrin receptors. The adenovirus vectors injected into the brain were of four classes: (i) capsids retaining wild-type CAR and integrin receptor binding; (ii) capsids defective for CAR binding, but retaining the wild-type capacity to bind integrins; (iii) caspids defective for both CAR binding and integrin binding; and (iv) capsids retaining wild-type CAR and integrin binding functions and incorporating a polylysine insertion into the fiber H1 loop to promote capsid binding to cell surface heparan sulfate (27).
Ablation of CAR binding altered adenovirus vector tropsim in the CNS, without decreasing the total area of brain transduction. Wild-type vectors transduced oligodendrocytes, astrocytes, microglial cells, and neurons, whereas CAR-ablated vectors showed a dramatic loss of oligodendrocyte transduction. Ablation of both CAR and integrin binding almost completely eliminated vector-mediated brain transduction. Despite a failure to transduce the brain, inflammation elicited by the doubly-ablated vector was indistinguishable from that induced by wild-type vectors. This indicates that brain inflammation can be triggered by the adenovirus capsid through mechanisms that do not involve binding and entry into cells via the classical adenovirus infection pathway. Our work has important implications for neurological gene therapy with retargeted adenovirus vectors: CAR and integrin binding-deficient doubly-ablated adenovirus capsids should be effective backbones for introducing alternative ligands to confer complete vector retargeting to specific neural cell types and to elucidate the molecular mechanisms by which adenoviruses elicit an acute inflammatory response in the brain.
MATERIALS AND METHODS
Adenovirus vectors.
All viruses used in this study were based on adenovirus type 5 and were E1-deleted first-generation adenovirus vectors with a further partial deletion in E3. Expression cassettes were inserted into the E1 region and contained the human cytomegalovirus intermediate-early promoter (hCMV), the transgene, and a simian virus 40 (SV40) poly(A). Vectors expressed either β-galactosidase (Z) or firefly luciferase (L), and their capsids were of four classes: (i) AdZ and AdL, with wild-type capsids (retaining both CAR and integrin binding functions); (ii) AdZF* and AdLF*, with capsids defective for CAR binding due to site-directed mutagenesis of the CAR binding domain in the fiber knob (12, 20); (iii) AdLF*PB* with capsids defective for CAR binding and integrin binding, due to ablation of the RGD sequence in the penton base protein (28); and (iv) AdZFpK7 with capsids retaining CAR and integrin binding functions, but with an insertion of seven polylysine residues incorporated into the fiber H1 loop to confer additional binding to heparan sulfate (27). The construction, propagation, and purification of all vectors used in this study have been described previously (8, 12, 20, 27, 28). All vector preparations were determined to be free from contamination with lipopolysaccharide by the Limulus amebocyte gel clot assay (BioWhittaker, Wockingham, Berkshire, United Kingdom). Vectors were used on the basis of physical particle concentration (determined by optical absorbance as described in reference 15) and were diluted in sterile phosphate-buffered saline (PBS) immediately before injection. During production, the titers of all vectors were determined by focus-forming unit (FFU) assays. FFU were determined in immunofluorescent-focus assays, and all vectors were propagated as described recently (9). Particle/FFU ratios varied between 9 and 73 for the vectors used in these studies, and ratio variability was unrelated to particular mutations in vector capsid structure. Identical results are described in reference 9, demonstrating that particle activity is not significantly compromised in mutant vectors.
Animals and surgical procedures.
Adult Sprague-Dawley rats with a body weight of 250g (Charles River Breeding Laboratories) were anesthetized with halothane and were placed in a stereotaxic apparatus that was modified for use with inhalational anesthetic. Animals were injected unilaterally in the left striatum (0.6 mm forward and 3.4 mm lateral from bregma, 5.0 mm vertical from dura) with 1 × 109 or 5 × 107 particles of AdZ, AdZF*, AdZFpK7, AdL, AdLF*, or AdLF*PB* (n = 4 per group). The high doses of vector were administered in a volume of 4 μl, and the low doses were administered in a volume of 2 μl. Control animals were injected with 4 μl of virus storage buffer (3% sucrose, 10 mM Tris [pH 7.8], 150 mM NaCl, 10 mM MgCl2) or 2 μl of sterile PBS. Each injection was performed over a period of 10 min. Three days after the intrastriatal injection, rats were injected intraperitoneally with pentobarbitone and were transcardially perfused and fixed with heparinized saline and 4% paraformaldehyde in PBS. Brains were postfixed in 4% paraformaldehyde for 12 h and were cut into 40-μm-thick sections with a vibratome, prior to analysis by horseradish peroxidase-based immunohistochemistry. To determine cell types transduced by AdZ and AdZF*, animals were injected with 109 particles of AdZ or AdZF* in 4 μl of saline (n = 3 per group) as described above. Brains were perfused and fixed 3 days after vector injection as described above and were cut into 20-μm-thick sections with a vibratome, before analysis by fluorescence-based immunohistochemistry.
Immunohistochemistry.
To determine levels of vector-mediated transgene expression and inflammation, free-floating peroxidase immunohistochemistry was performed on 40-μm serial brain sections as described by Thomas et al. (22), with the following primary antibodies: anti-β-galactosidase (1:1,000; Promega), antiluciferase (1:2,000; Promega), anti-ED1 (macrophages and activated microglial cells, 1:1,000; Serotec), anti-CD43 (brain infiltrating lymphocytes, 1:500; Serotec), anti-major histocompatibility complex (MHC) class I (activated glial cells and infiltrating immune cells, 1:200; Serotec), and anti-adenovirus hexon (1:1,000; Serotec). All primary antibodies were mouse monoclonal antibodies, except for antiluciferase antibody, which was raised in goat. Secondary antibodies were biotinylated rabbit anti-mouse immunoglobulin G (IgG) or biotinylated swine anti-goat IgG (1:200; DAKO) and were detected by the Vectastain Elite ABC horseradish peroxidase method (Vector Labs, Peterborough, United Kingdom). Fluorescent double labeling of cell types transduced by AdZ versus AdZF* was performed on 20-μm-thick brain sections, as described in reference 27, by using rabbit anti-β-galactosidase antibody (1:750; a kind gift of R. Goya, School of Medicine, University of La Plata, Argentina), mouse anti-CNP (2′,3′-cyclic nucleotide 3′-phosphodiesterase, a cytoplasmic protein expressed exclusively by oligodendrocytes; 1:1,000; Promega), and mouse anti-ED1 (activated macrophages, 1:1,000; Serotec) as primary antibodies. Secondary antibodies were donkey anti-mouse IgG (conjugated with fluorescein, 1:100; Jackson ImmunoResearch Laboratories, Inc.) and swine anti-rabbit IgG (conjugated with rhodamine, 1:200; DAKO). Fluorescent sections were analyzed with an Olympus Vanox-T microscope with BP490:O515 and BP545:O590 exciter-barrier filter combinations for fluorescein and rhodamine, respectively.
Quantification of immunohistochemical staining.
Quantitative image analysis to determine the area occupied by cells immunohistochemically stained with anti-β-galactosidase, antiluciferase, anti-ED1, anti-CD43, or anti-MHC class I antibodies within 40-μm brain sections was performed with a Leica Quantimet 600 image analysis system controlled by QWIN software (Leica Microsystems, Cambridge, United Kingdom) as described previously (24, 25). To quantitate transgene expression (luciferase or β-galactosidase) in each of the brains, we measured the area of immmunoreactivity in five 40-μm sections, spaced at regular 360-μm intervals through the injected striatum. The anatomical area occupied by transgene-positive cells in each of the five sections was summed for each brain. This sampling method covered >90% of the transduced area from each brain. Single brain sections containing the needle track (and thus displaying the highest levels of immunoreactivity) were used to quantitate levels of ED1, MHC class I, or CD43 immunoreactivity. Student's t test was used to determine the degree of statistical significance between values from different experimental groups.
RESULTS
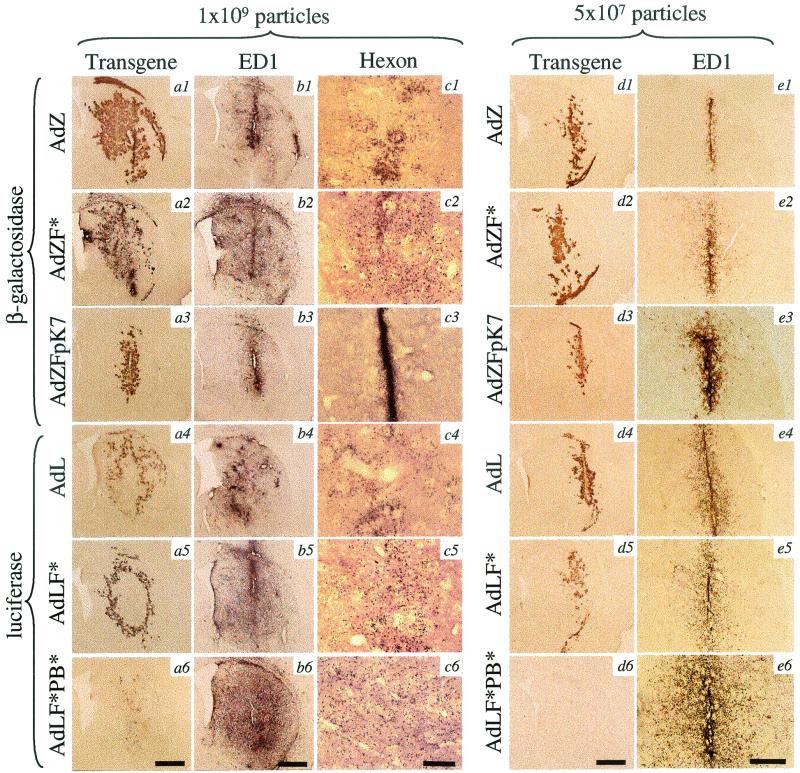
Equal numbers of particles of capsid-modified adenovirus vectors (AdZF*, AdLF*, AdLF*PB*, or AdZFpK7) or vectors with wild-type capsids (AdZ and AdL) were injected into the brains of adult rats (n = 4 per group). (AdZF*and AdLF* contained mutant fiber proteins defective for CAR binding, AdLF*PB* contained CAR binding-defective fibers and mutant penton base proteins defective for integrin binding, and AdZFpK7 was competent for CAR and integrin binding, but incorporated an additional polylysine insertion into the fiber knob.) Three days after the intrastriatal injection, animals were killed, and the brains were analyzed by immunohistochemistry to detect transduction, inflammation, and vector diffusion (Fig. 1). At a dose of 109 vector particles, both AdZ and AdZF* mediated widespread β-galactosidase expression throughout the ipsilateral striatum, indicating that interaction of the adenovirus fiber protein with the cell surface receptor CAR is not a prerequisite for brain transduction (Fig. 1a1 and 1a2). Administration of the same number of particles of the CAR and integrin-binding-competent AdZFpK7 resulted in a pattern of transduction that was more localized to the needle track (Fig. 1a3). Injection of 109 particles of AdL and AdLF* transduced equivalent, widespread anatomical areas of striatum (similar to the corresponding vectors AdZ and AdZF*); however, luciferase-immunoreactive cells were confined to the periphery of a large area devoid of immune staining (Fig. 1a4 and 1a5). This area of loss of immunoreactivity was likely indicative of luciferase toxicity, occurring when cells were infected with high multiplicities of vector (23). A total of 109 particles of the doubly-ablated vector AdLF*PB* (defective for both CAR and integrin binding) transduced only very few cells, which were diffusely scattered over the ipsilateral striatum (Fig. 1a6).
FIG. 1.
Immunohistochemical detection of vector-mediated transgene expression (β-galactosidase or luciferase; a1 to a6 and d1 to d6), macrophage activation (ED1; b1 to b6 and e1 to e6), and vector diffusion (hexon; c1 to c6) in 40-μm brain sections 3 days after injection into the brain of 1 × 109 (a to c) or 5 × 107 (d to e) particles of vector. Scale bars: panels a6, b6, and d6, 1 mm; c6, 250 μm; e6, 500 μm.
To exclude the possibility that transduction by AdLF*PB* was not seen due to the toxicity of the high vector dose, we injected a lower dose of 5 × 107 particles of each vector (again, n = 4 for each vector group) and again analyzed the brains 3 days postinjection (Fig. 1d1 to 1d6). AdZ, AdZF*, AdL, and AdLF* transduced equivalent anatomical areas of the brain, but at this lower dose, the pattern of transduction was less widespread, and the large lesion indicative of luciferase toxicity was not observed in brains transduced by AdL and AdLF*. The lower dose of AdZFpK7 again resulted in a pattern of transduction that was more restricted to the needle track than AdZ and AdZF*. No transgene expression was seen in brain injected with AdLF*PB*.
The anatomical area of brain occupied by activated microglial cells and macrophages roughly overlapped with the area of brain transduced by the five vectors AdZ, AdZF*, AdZFpK7, AdL, and AdLF*: i.e., at the high dose of 109 particles, ED1 immunoreactive cells were found throughout the injected striatum after injection of AdZ, AdL, AdZF*, and AdLF*, but were localized to the needle track after injection of AdZFpK7 (Fig. 1b1 to 1b5). In contrast, although the doubly-ablated vector AdLF*PB* failed to mediate significant brain transduction, it elicited more widespread ED1 immunoreactivity than the other vectors; at the high dose of 109 particles, ED1 immunoreactive cells were found to densely populate the entire injected striatum (Fig. 1b6). At the low dose of 5 × 107 particles, ED1 immunoreactivity elicited by all vectors was more localized to the needle track compared with the higher dose (Fig. 1e1 to 1e6). At this lower dose, the pattern of ED1 staining in brains injected with AdLF*PB* was more widespread than those in brains injected with AdZ, AdZF*, AdL, AdLF*, or AdZFpK7 (Fig. 1e6).
To determine how far the different vectors diffused from the needle track, brain sections were stained with an antibody that detected the hexon protein of the adenovirus capsid (Fig. 1c1 to 1c6). Hexon immunoreactivity in brains injected with AdZ, AdZF*, AdL, AdLF*, and AdLF*PB* was detected over a wide area of the striatum, roughly overlapping with the area of transduction and ED1 immunoreactivity (Fig. 1c1, 1c2, 1c4, 1c5, and 1c6). Hexon staining in brains injected with AdZFpK7 was restricted to the needle track (Fig. 1c3). Unlike the patterns of β-galactosidase immunoreactivity, which revealed the morphology of the transduced cells and allowed identification of the transduced neural cell types, the pattern of hexon immunoreactivity did not reveal which particular cell types contained the doubly-ablated vector AdLF*PB*. The pattern of hexon staining in brains injected with AdZ, AdZF*, AdL, AdLF*, and AdLF*PB* was particulate; whether this represents intracellular sequestration of virion particles in lysosomes or endosomes remains to be determined.
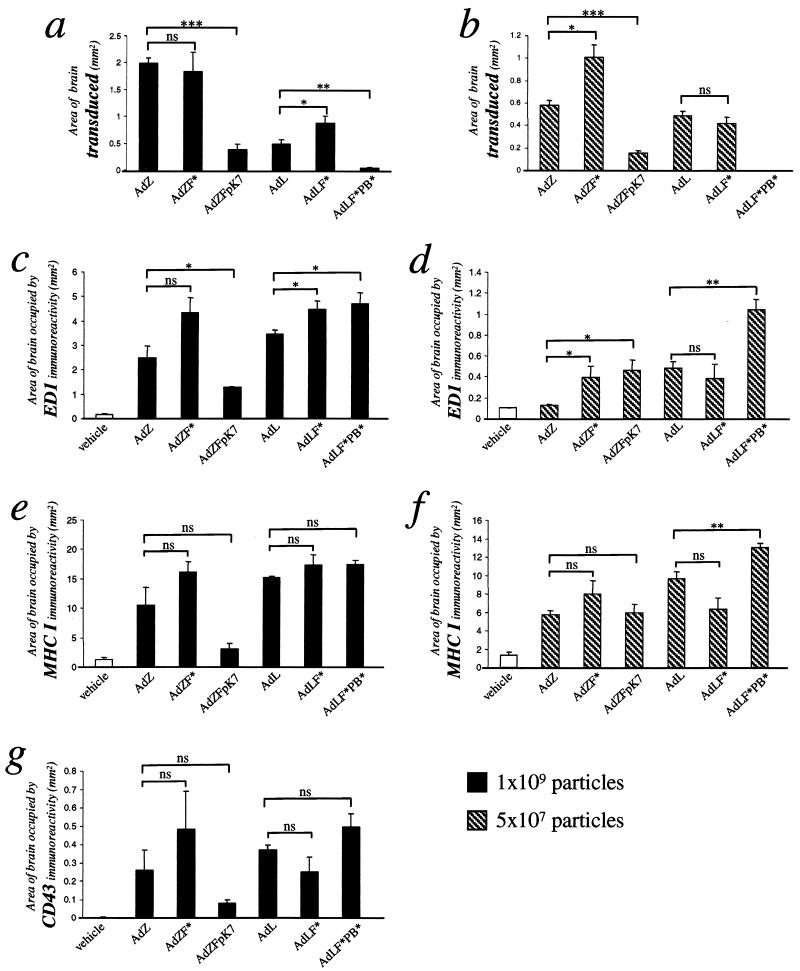
The anatomical area occupied by transduced cells or by ED1, MHC class I, or CD43 immunoreactivity was quantified by image analysis (Fig. 2). Quantification of luciferase or β-galactosidase immunoreactivity confirmed that ablation of CAR binding did not decrease overall brain transduction: i.e., there was no significant decrease in the area of transduction mediated by AdZF* compared with AdZ or AdLF* compared with AdL (Fig. 2a and b). In contrast, insertion of polylysine into the fiber knob resulted in a three- to fourfold decline in the area of transgene expression compared with vectors with wild-type capsids (AdZFpK7 versus AdZ, Fig. 2a and b). At the high dose, areas of ED1 (activated macrophages), MHC class I (upregulated on activated microglial cells and brain-infiltrating immune cells), and CD43 (brain-infiltrating lymphocytes) immunoreactivity were also lower in brains injected with AdZFpK7 than in brains injected with AdZ or AdZF*, reflecting the fact that the extent of inflammation was restricted to the brain area surrounding the needle track after injection of 109 particles of AdZFpK7 (Fig. 2c to g). Strikingly, although the doubly-ablated vector AdLF*PB* did not efficiently transduce the brain, the areas of inflammation as evidenced by macrophage and microglial cell activation (ED1 and MHC class I, respectively) and lymphocyte infiltration (CD43) elicited by injection of this vector were higher than the levels of inflammation elicited by AdL and AdLF*, again reflecting the more widespread anatomical scattering of these inflammatory cells (Fig. 2c to g). The particulate nature of hexon immunoreactivity made quantitation of this area difficult to determine accurately (hence not shown), since immunostaining had to be evaluated at high magnification.
FIG. 2.
Quantitative analysis of the area of 40-μm brain sections occupied by transduced cells (a and b), ED1 immunoreactivity (c and d), MHC class I immunoreactivity (e and f), and CD43 immunoreactivity (g) 3 days after injection of 1 × 109 (a, c, e, and g) or 5 × 107 (b, d, and f) particles of vector. No CD43 immunoreactive cells were seen in brains injected with the low doses of vector (hence, graph not shown). Error bars show the standard error of the mean from each experimental group (∗, P ≤ 0.05; ∗∗, P ≤ 0.005; ∗∗∗, P ≤ 0.0005).
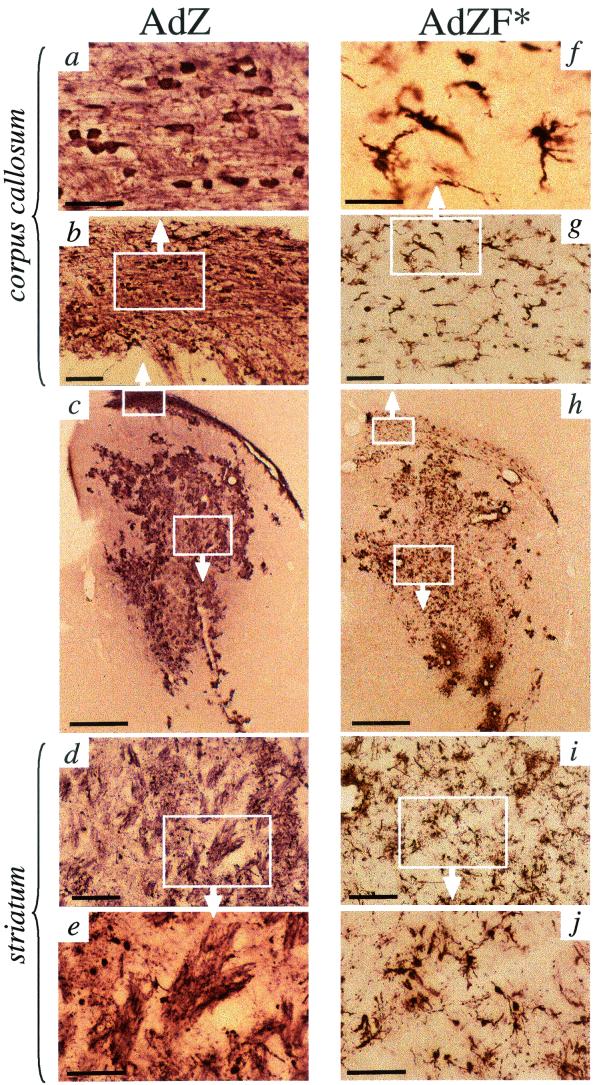
Although ablation of CAR binding had little effect upon the total levels of vector-mediated brain transduction, dramatic differences in the neural cell populations transduced by AdZ versus AdZF* were observed (Fig. 3 and 4). Microglial cells, neurons, and other glial cells were transduced by AdZ, but β-galactosidase immunoreactivity was predominantly localized to the cross-sections of white matter bundles coursing through the striatum (Fig. 3c to e). The corpus callosum was also heavily stained in brains injected with AdZ; analysis of this region at high magnification showed fiber-like patterns of β-galactosidase immunoreactivity and numerous small spherical β-galactosidase-positive cell bodies approximately 10 μm in diameter (Fig. 3a and b). The main constituents of white matter in the CNS are neuronal axons and oligodendrocytes, which provide the myelin sheaths surrounding the axons. The β-galactosidase immunoreactivity in the white matter-like structures in brains injected with AdZ was unlikely to represent projecting axons of transduced neurons, since too few transduced neuronal cell bodies were seen in other areas of the brain. Furthermore, few neuronal cell bodies are found in white matter tracts, and in AdZ-injected brains, many transduced cell bodies were seen in the corpus callosum.
FIG.3.
Immunohistochemical detection of the transgene product β-galactosidase in 40-μm brain sections from animals intrastriatally injected with AdZ (a to e) or AdZF* (f to j). Differences in the morphology of cell types transduced by AdZ versus AdZF* were clearly visible, particularly at high magnification in the corpus callosum (a versus f) and striatum (e and j). Scale bars: panels a and f, 50 μm; b and g, 100 μm; c and h, 1 mm; d and i, 200 μm; e and j, 100 μm.
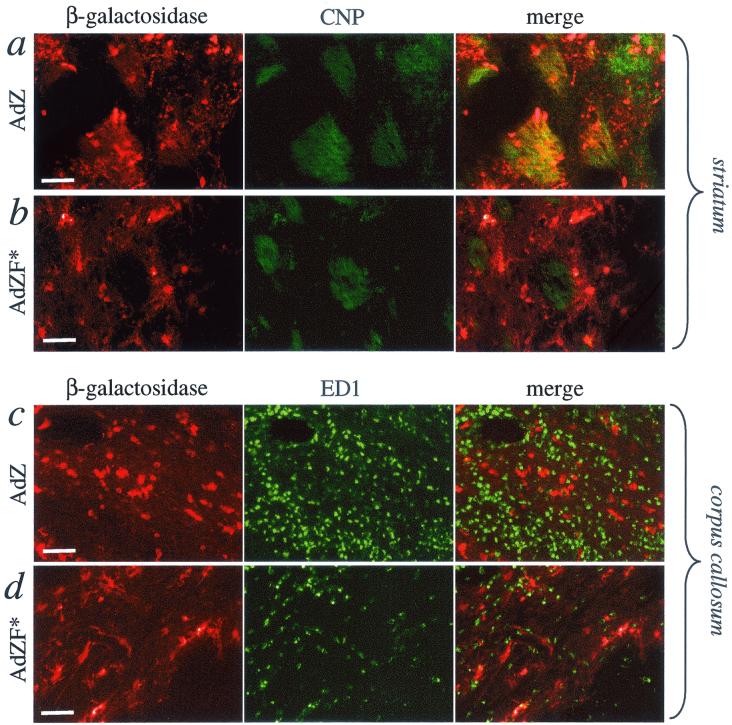
FIG. 4.
Immunohistochemical colocalization of β-galactosidase expression with the oligodendrocyte marker CNP (a and b) or the marker of activated macrophages ED1 (c and d) in 20-μm sections from brains injected with AdZ (a and c) or AdZF* (b and d). Scale bars (shown in β-galactosidase panels), 50 μm.
To exclude the possibility that β-galactosidase immunoreactive cell bodies in the corpus callosum were infiltrating macrophages, brain sections were immunohistochemically double stained with anti-β-galactosidase antibody and the marker for activated macrophages, ED1 (Fig. 4c). Although the white matter in AdZ-injected brains was heavily infiltrated with macrophages, ED1 immunoreactive cells did not colocalize with the β-galactosidase-positive cells. To confirm that the majority of neural cells transduced by AdZ were indeed oligodendrocytes, brain sections were double labeled with anti-β-galactosidase and anti-CNP antibodies. β-Galactosidase-positive cells strongly colocalized with areas of CNP immunoreactivity (Fig. 4a).
Brains injected with AdZF* exhibited a different pattern of transduction (Fig. 3f to j). The majority of the cells transduced by AdZF* were of microglial, neuronal, and, possibly, astroglial-like morphology. Oligodendrocytes were not transduced; β-galactosidase staining of white-matter bundles in the striatum was not seen (Fig. 3h to j) and β-galactosidase immunoreactive cells in AdZF*-injected brains did not colocalize with CNP immunoreactivity (Fig. 4b). Although β-galactosidase-positive cells were seen in the corpus callosum (Fig. 3f to g), these were exclusively of microglial-like morphology, and again, few β-galactosidase-positive cells colocalized with macrophage immunoreactivity (Fig. 4d).
DISCUSSION
It is well established that injection of adenovirus vectors into the CNS triggers an acute dose-dependent inflammatory response (distinct from the later, adenovirus-specific T-cell responses) characterized by microglial and astrocyte activation and infiltration of immune cells from the circulation (23). The rapidity with which cytokines are released after intracranial injection of adenovirus vectors strongly suggests that the adenovirus capsid is itself the trigger for inflammation, independently of any viral gene expression or transgene expression (6). The mechanisms through which adenovirus capsids elicit acute inflammation in the brain remain unknown. Several studies have indicated that cells infected by adenovirus vectors are stimulated to release proinflammatory cytokines (3, 4, 7, 13, 14, 17). Other studies have suggested that release of cytokines by transduced cells is stimulated by intracellular signaling pathways activated by the adenovirus infection process (4). Thus, a hypothetical trigger for brain inflammation after intracranial injection of adenovirus vectors could be the release of cytokines by infected neural cells, which in turn could trigger the activation of large numbers of noninfected microglial cells and astrocytes, resulting in the recruitment of macrophages and other immune cells across the blood-brain barrier to the site of the vector injection.
In this study, we investigated whether the capacity of adenovirus vectors to induce acute brain inflammation was linked to their capacity to infect and transduce neural cells, and, furthermore, whether inflammation was connected with the route of adenovirus infection (i.e., through interaction with CAR and integrin receptors or other cellular receptors [e.g., those containing heparan sulfate]). To this end, we injected into the brain parenchyma a panel of different adenovirus vectors with either wild-type capsids, capsids with extended tropism for cell surface heparan sulfate, mutant capsids defective for CAR binding, or mutant capsids defective for binding of both CAR and integrin.
We observed that the inflammatory potential of each adenovirus vector was independent of its capacity to infect and transduce neural cells via CAR and integrin receptors. Adenovirus mutants that were defective for both CAR binding and integrin binding failed to mediate brain transduction; however, these vectors elicited more widespread and thus higher levels of inflammation than those vectors that successfully transduced cells. Ablation of only CAR binding function dramatically altered vector tropism for neural cells but did not significantly alter the capacity of the vectors to induce brain inflammation. Interestingly, and contrary to our expectations, the vector AdZFpK7, which retained functional CAR and integrin binding domains, but incorporated a polylysine insertion into the fiber knob to promote binding to heparan sulfate, mediated transduction that was restricted to an area close to the needle track. The observation that both the spread of inflammation mediated by this vector and the area occupied by hexon immunoreactivity were also restricted to the needle track suggests that addition of the polylysine domain limited the diffusion of the vector through the brain parenchyma, perhaps by facilitating vector binding to heparan sulfate moieties within the extracellular matrix.
Although CAR binding and integrin binding are clearly not required to elicit brain inflammation after intracranial injection of adenovirus vectors, exactly what constitutes the primary trigger for the inflammatory response in the absence of infection and transduction remains unclear. Zsengellér et al. (29) have demonstrated that it is the internalization of adenovirus into alveolar macrophages, but not airway epithelial or vascular endothelial cells, that initiates acute proinflammatory cytokine signaling during lung infection with adenovirus. Internalization of vector particles by macrophages is unlikely to be the primary trigger for initiating proinflammatory signaling within the brain, since macrophages are recruited into the brain as a response to intraparenchymal cytokine signals. Resident brain microglial cells are derived from macrophage precursors and perform macrophage-like functions within the brain (19). Whether CAR- and integrin-independent internalization of adenovirus particles into microglial cells early after infection is the primary trigger for a cascade of inflammation or whether other cellular elements (e.g., perivascular macrophages) provide the initial stimulus for macrophage recruitment and infiltration remains to be determined.
This study has several implications for neurological gene therapy using retargeted adenovirus vectors. We show that redirection of vector tropism can be achieved simply by ablating binding to the primary receptor CAR, without incorporation of additional ligands into the capsid architecture. To achieve complete retargeting, our data suggest that CAR- and integrin-deficient vectors that do not transduce any neural cell types could be used as a capsid “backbone” into which to introduce alternative ligands for specific binding to specific neural cell populations. The observation that the insertion of polylysine into the fiber prevented diffusion of vector particles through the brain, rather than increasing the extent of transduction, highlights the importance of selecting ligands that do not interact with components of the extracellular matrix. Our data indicate that there is an inverse correlation between the efficiency with which vector particles bind to neural cells or extracellular components and the spread of vector-mediated brain inflammation. That is, the vector AdZFpK7, which binds CAR, integrins, and heparan sulfate with high efficiency, did not diffuse far from the needle track, and the area of inflammation was similarly restricted. In contrast, the vector AdLF*PB*, which did not bind CAR or integrins, diffused over a large area and elicited more widespread inflammation than the wild-type vector. Thus, the efficiency with which different ligands promote vector binding and infection is likely to determine both the spread of brain transduction and brain inflammation mediated by retargeted adenovirus vectors.
Acknowledgments
This work was supported by grant 3087 from the Parkinson's Disease Society (United Kingdom), the Wellcome Trust (United Kingdom), and European Union/Biomed Program grants BMH4-CT98-3277, BMH4-CT98-0297, and QLK3-CT-1999-00356 (to M.G.C. and P.R.L.). P.R.L. was a research fellow of the Lister Institute of Preventive Medicine. P.E. was supported by a SPRING studentship from the Parkinson’s Disease Society (United Kingdom).
REFERENCES
- 1.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275**:**1320-1323. [DOI] [PubMed] [Google Scholar]
- 2.Bergelson, J. M., A. Krithivas, L. Celi, G. Droguett, M. S. Horwitz, T. Wickham, R. L. Crowell, and R. W. Finberg. 1998. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J. Virol. 72**:**415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borgland, S. L., G. P. Bowen, N. C. W. Wong, T. A. Liebermann, and D. A. Muruve. 2000. Adenovirus vector-induced expression of the C-X-C chemokine IP-10 is mediated through capsid-dependent activation of NF-κB. J. Virol. 74**:**3941-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruder, J. T., and I. Kovesdi. 1997. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J. Virol. 71**:**398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrnes, A. P., J. E. Rusby, M. J. A. Wood, and H. M. Charlton. 1995. Adenovirus gene transfer causes inflammation in the brain. Neuroscience 66**:**1015-1024. [DOI] [PubMed] [Google Scholar]
- 6.Cartmell, T., T. D. Southgate, G. S. Rees, M. G. Castro, P. R. Lowenstein, and G. N. Luheshi. 1999. Interleukin-1 mediates a rapid inflammatory response after injection of adenoviral vectors into the brain. J. Neurosci. 19**:**1517-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clesham, G. J., P. J. Adam, D. Proudfoot, P. D. Flynn, S. Efstathiou, and P. L. Weissberg. 1998. High adenoviral loads stimulate NFκB-dependent gene expression in human vascular smooth muscle cells. Gene Ther. 5**:**174-180. [DOI] [PubMed] [Google Scholar]
- 8.Einfeld, D. A., D. E. Brough, P. W. Roelvink, I. Kovesdi, and T. J. Wickham. 1999. Construction of a pseudoreceptor that mediates transduction by adenoviruses expressing a ligand in fiber or penton base. J. Virol. 73**:**9130-9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Einfeld, D. A., R. Schroeder, P. W. Roelvink, A. Lizonova, C. Richter King, I. Kovesdi, and T. J. Wickham. 2001. Reducing the native tropism of adenovirus vectors requires removal of both CAR and integrin interactions. J. Virol. 75**:**11284-11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hidaka, C., E. Milano, P. L. Leopald, J. M. Bergelsone, N. R. Hackett, R. W. Finberg, T. J. Wickham, I. Kovesdi, P. Roelvink, and R. G. Crystak. 1999. CAR-dependent and CAR-independent pathways of adenovirus vector-mediated gene transfer and expression in human fibroblasts. J. Clin. Investig. 193**:**579-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jooss, K., H. C. J. Ertl, and J. M. Wilson. 1998. Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver. J. Virol. 72**:**2945-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirby, I., E. Davison, A. J. Beavil, C. P. C. Soh, T. J. Wickham, P. W. Roelvink, I. Kovesdi, B. J. Sutton, and G. Santis. 1999. Mutations in the DG loop of adenovirus type 5 fiber knob protein abolish high-affinity binding to its cellular receptor CAR. J. Virol. 73**:**9508-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leland Booth, J., and J. P. Metcalf. 1999. Type-specific induction of interleukin-8 by adenovirus. Am. J. Respir. Cell Mol. Biol. 21**:**521-527. [DOI] [PubMed] [Google Scholar]
- 14.Lieber, A., C.-Y. He, L. Meuse, D. Schowalter, I. Kirillova, B. Winther, and M. A. Kay. 1997. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 71**:**8798-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittereder, N., K. L. March, and B. C. Trapnell. 1996. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 70**:**7498-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molinier-Frenkel, V., H. Gahery-Segard, M. Mehtali, C. Le Boulaire, S. Ribault, P. Boulanger, T. Tursz, J.-G. Gulet, and F. Farace. 2000. Immune response to recombinant adenovirus in humans: capsid components from viral input are targets for vector-specific cytotoxic T lymphocytes. J. Virol. 74**:**7678-7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noah, T. L., I. A. Wortman, P. C. Hu, M. W. Leigh, and R. C. Boucher. 1996. Cytokine production by cultured human bronchial epithelial cells infected with a replication-deficient adenoviral gene transfer vector or wild-type adenovirus type 5. Am. J. Respir. Cell Mol. Biol. 14**:**417-424. [DOI] [PubMed] [Google Scholar]
- 18.O'Neal, W. K., H. Zhou, N. Morral, C. Langston, R. J. Parks, F. L. Graham, S. Kochanek, and A. L. Beaudet. 2000. Toxicity associated with repeated administration of first-generation adenovirus vectors does not occur with a helper-dependent vector. Mol. Med. 6**:**179-195. [PMC free article] [PubMed] [Google Scholar]
- 19.Perry, V. H., and S. Gordon. 1997. Microglia and macrophages, p. 155-172. In R. W. Keane and W. F. Hickey (ed.), Immunology of the nervous system. Oxford University Press, Oxford, United Kingdom.
- 20.Roelvink, P. W., G. Mi Lee, D. A. Einfeld, I. Kovesdi, and T. J. Wickham. 1999. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science 286**:**1568-1571. [DOI] [PubMed] [Google Scholar]
- 21.Schiedner, G., N. Morral, R. J. Parks, Y. Wu, S. C. Koopmans, C. Langston, F. L. Graham, A. L. Beaudet, and S. Kochanek. 1998. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat. Genet. 18**:**180-183. [DOI] [PubMed] [Google Scholar]
- 22.Thomas, C. E., E. Abordo-Adesida, T. C. Maleniak, D. Stone, C. A. Gerdes, and P. R. Lowenstein. 2000. Gene transfer to the brain using adenovirus vectors. Curr. Protocols Neurosci. 4.24.1-4.24.37. [DOI] [PubMed]
- 23.Thomas, C. E., D. Birkett, I. Anozie, M. G. Castro, and P. R. Lowenstein. 2001. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol. Ther. 3**:**36-46 [DOI] [PubMed] [Google Scholar]
- 24.Thomas, C. E., G. Schiedner, S. Kochanek, M. G. Castro, and P. R. Lowenstein. 2000. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc. Natl. Acad. Sci. USA 97**:**7482-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas, C. E., G. Schiedner, S. Kochanek, M. G. Castro, and P. R. Lowenstein. 2001. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum. Gene Ther. 12**:**839-846. [DOI] [PubMed] [Google Scholar]
- 26.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73**:**309-319. [DOI] [PubMed] [Google Scholar]
- 27.Wickham, T. J., P. W. Roelvink, D. E. Brough, and I. Kovesdi. 1996. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat. Biotechnol. 14**:**1570-1573. [DOI] [PubMed] [Google Scholar]
- 28.Wickham, T. J., M. E. Carrion, and I. Kovesdi. 1995. Targeting of adenovirus penton base to new receptors through replacement of its RGD motif with other receptor-specific peptide motifs. Gene Ther. 2**:**750-756. [PubMed] [Google Scholar]
- 29.Zsengellér, Z., K. Otake, S. A. Hossain, P. Y. Berclaz, and B. C. Trapnell. 2000. Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection. J. Virol. 74**:**9655-9667. [DOI] [PMC free article] [PubMed] [Google Scholar]