Trimethyltin-Induced Neurogenesis in the Murine Hippocampus (original) (raw)
. Author manuscript; available in PMC: 2006 Feb 6.
Published in final edited form as: Neurotox Res. 2004;5(8):623–627. doi: 10.1007/BF03033182
Abstract
Neurogenesis continues to occur in the mature rodent brain with one of the most prominent sources for new neurons being the subgranular layer (SGL) of the dentate gyrus (DG) in the hippocampus. A number of factors can stimulate this process including synaptic activity and injury. To determine if this process would occur upon a direct injury to the dentate region, we exposed young, 21 day old male CD-1 mice to the hippocampal toxicant, trimethyltin (TMT). An acute i.p. injection of TMT (2 mg/kg) produced extensive damage and loss of dentate granule neurons within 72 h. This active period of degeneration was accompanied by an increase in the generation of progenitor cells within the SGL as identified by BrdU uptake and Ki-67 immunostaining. As additional markers for neurogenesis, both nestin and doublecortin showed increased staining patterns within the blades of the dentate. In these young weanling mice, the level of proliferation was sufficient to significantly repopulate the dentate region by 4 weeks post-TMT, suggesting a high level of regenerative potential. Our data indicate a significant level of neurogenesis occurring during the active process of degeneration and in an environment of microglia activation. The TMT-induced injury offers a model system for further examination of the process of neurogenesis, neural adaptation, and the influence of inflammatory factors and glia interactions.
Keywords: Trimethyltin, Neurogenesis, Hippocampus, Neurodegeneration, Apoptosis, Microglia
INTRODUCTION
It is now generally accepted that neurogenesis in the mature rodent routinely occurs in two specific areas of the brain, the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus, and the subventricular zone (SVZ) of the anterior lateral ventricles (Gage, 2002; Kennea and Mehmet, 2002; Parent and Lowenstein, 2002). New neurons, differentiating from progenitor cells in the subgranular layer of the dentate gyrus, can be induced by a number of factors and migrate into the granular cell layer where they are often indistinguishable from the surrounding granule cell neurons (Markakis and Gage, 1999; Takagi et al., 1999; Covolan et al., 2000; Cameron and McKay, 2001; Jin et al., 2001; Parent and Lowenstein, 2002; van Praag et al., 2002).
To determine if neurogenesis can be stimulated by a direct injury to the dentate granule cells, we employed a chemical-induced model of hippocampal damage using the prototypic neurotoxicant, trimethyltin (TMT). As has been previously described, acute exposure of mice to TMT results in extensive damage to dentate granule cells (Reuhl et al., 1983), accompanied by early activation of glial cells (Bruccoleri et al., 1998; Eskes et al., 2003). The mechanism of neuronal death induced by TMT has been linked to apoptosis and necrosis (Bruccoleri and Harry, 2000; Fiedorowicz et al., 2001), inflammation (Bruccoleri et al., 1998; Harry and Lefebvre d'Hellencourt, 2003), and signaling via the cyclooxygenase pathway (Geloso et al., 2002). In addition to the characterization of hippocampal damage, recent work has demonstrated an early elevation of cell proliferation and expression of cell cycle genes within the hippocampus (McPherson et al., 2003) suggestive of injury-induced neurogenesis. This interpretation is substantiated in the current study by the increased proliferation within the dentate regions, the localization of cells within the dentate immunopositive for neurogenesis markers, and the significant repopulation of the dentate region.
METHODS
Animals
Twenty-one day old CD-1 male mice (Charles River Breeding Laboratories, Raleigh, NC, USA) were randomly assigned to experimental groups and administered a single intraperitoneal (i.p.) injection of either 2 mg/kg trimethyltin hydroxide (TMT, originally obtained from Alfa Products, Danvers, MA, USA) or saline (injection vol., 2 ml/kg). Each animal was injected with bromodeoxyuridine (BrdU; 50 mg/kg i.p.) at the time of TMT dosing, and twice daily for 3 days (Takagi et al., 1999). Animals were randomly assigned to time points for examination and individually housed in a dual corridor, semi-barrier animal facility at ambient temperature (21 ± 2oC), humidity (50% ± 10%) under a 12-h light/dark cycle. Autoclaved NIH 31 rodent chow and deionized, reverse osmotic-treated water were available ad libitum. All procedures were performed in compliance with an animal protocol approved by the NIEHS/NIH Animal Care and Use Committee.
Morphological Examination
At 72 h and 1, 2 or 4 weeks following saline or TMT injection, animals (15 per group) were lightly anesthesized by CO2, decapitated, the brains quickly excised, bisected on midsaggital plane and immersion fixed in 4% paraformaldehyde/0.1M PBS, pH 7.2, overnight at room temperature (RT). Brains were rinsed with PBS, dehydrated in ethanol, embedded in paraffin, and 8-μm sections cut through the hippocampus. Sections were cleared and rehydrated, subjected to heat-induced epitope retrieval (HIER) in 0.01 M citrate buffer (pH 6.0) using a decloaking chamber (Biocare Medical, Walnut Creek, CA) for 3 min, rinsed, and maintained at RT for 20 min. Routine hematoxylin and eosin (H&E) staining visualized tissue cellularity. Cell counts of neurons were recorded on 10 randomly selected hippocampal sections per animal. Using an ocular counting grid the number of viable neurons within 3 identified grid locations of the dentate (total area per grid section 0.105 mm2) was measured at 72 h and 4 weeks post-TMT.
For BrdU histochemistry, 3–5 sections per animal were incubated in 2N HCl (37oC, 30 min), trypsinized (0.01% trypsin in 0.5M Tris-HCl; 37oC, 3 min), endogenous peroxidase activity quenched with 3% H2O2 (10 min), and endogenous biotin activity quenched using a commercial avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA, USA). BrdU incorporation was localized by a 1 h incubation with 1:400 rat monoclonal anti-BrdU (Accurate Chemical and Scientific Corp, Westbury, NY, USA), Vectastain _Elite_® ABC Rat IgG, and DAB chromagen (Vector Labs). Immunofluoresence for BrdU was detected with either AlexaFluor®488 or 594. Proliferation was also identified with a mouse monoclonal antiserum for Ki-67 (1:100; NCL-L-Ki67-MM1, Nova Castra, Newcastle upon Tyne, UK) (Kee et al., 2002), a mouse-on-mouse peroxidase kit (MOM™, Vector Labs) as visualized by DAB. Apoptotic cells were identified by terminal deoxynucleotidyl transferase-mediated dUTP-biotin in situ end-labeling (TUNEL) using a commercially available kit (Intergen, Purchase, NY, USA).
Astrocytes were identified using an Alexa Fluor®594 conjugated mouse monoclonal anti-glial fibrillary acidic protein (GFAP; 1:200; 1.5 h, RT; Molecular Probes, Eugene, OR, USA). Microglia were identified by lectin binding (Griffonia (Bandeiraea) simplicifolia, IB4). Briefly, sections were pre-incubated in PBS containing cations (0.1 mM CaCl2, MgCl2, MnCl2) and 0.1% Triton X-100, for 30 min RT, incubated with IB4 (1:200; 1.5 h, RT; Molecular Probes) conjugated to AlexaFluor®594. Mature neurons were identified with immunostaining for NeuN (1:100; Chemicon International, Temecula, CA, USA) detected with anti-mouse IgG or streptavidin AlexaFluor®488. As additional markers of neurogenesis (Kempermann et al., 2003; Jin et al., 2001), mouse monoclonal anti-nestin (1:100; Chemicon) was detected using a MOM™ kit and goat anti-mouse IgG-AlexaFluor®594 (Molecular Probes) and goat polyclonal anti-doublecortin (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA) using a Vectastain Elite® ABC goat IgG kit and Novared® (Vector) chromagen, and counterstained with hematoxylin QS.
Light and fluorescence microscopy was performed with a Leica DMRBE microscope (Wetzlar, Germany) equipped with differential interference contrast (DIC/Nomarski) optics, epifluorescence, and motorized Z-control. Digital images were acquired using a SpotRT™ cooled, charged-couple device camera (Diagnostic Instruments, Sterling Heights, MI) under the control of Metamorph™ software (Universal Imaging Co., Downingtown, PA, USA).
RESULTS
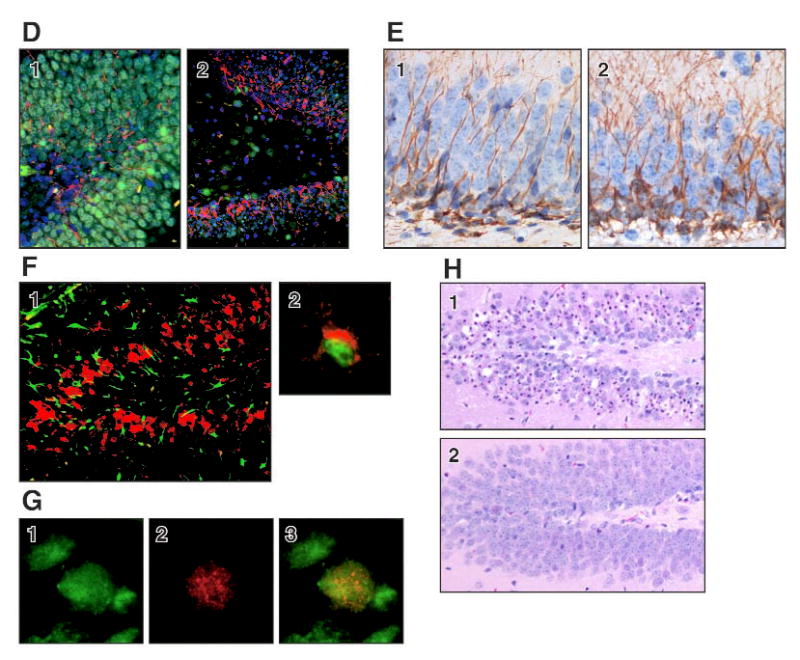
A significant level of neuronal cell death occurred between 24 and 72 h within the dentate granule cell layer characterized by nuclear pyknosis, karyolysis, and cell loss (FIG. 1 H.1), and positive TUNEL staining (FIG. 1 C.1). Astrocyte reactivity and microglia activation occurred concurrent with the progression of neuronal death (FIG. 1 F.1). A proliferative response was detected in the dentate region of the hippocampus both with BrdU incorporation (FIG. 1 A; C.3) and with Ki-67 (FIG. 1B). In control animals, this response was limited to a few cells in the subgranular layer (FIG. 1A.1; B.1). At 72 h post-TMT, positive cells were seen along the subgranular layer. However, a significant number of positive cells were found within the blades of the dentate, identified as a 2 cell layer distance from the subgranular layer (FIG. 1A.2; B.2). By 7 days, TUNEL staining subsided (FIG. 1C.2), however, BrdU staining remained prominent (FIG. 1C.3). The merged image of sections double labeled with lectin and BrdU suggested that the response was not due to microglia proliferation (FIG. 1F.2). Immunostaining for nestin as a marker of neurogenesis supported the active process of newly generated cells within the dentate. In the absence of neurodegeneration, a limited staining pattern for nestin was seen along the inner blade of the dentate with thin processes through the dentate cell layer (FIG. 1D.1). During the active time of cell death and neuronal loss nestin positive cells showed hypertrophy and distribution throughout the blades of the dentate (FIG. 1D.2). In the weanling mouse, cells immunopositive for doublecortin were seen within the dentate located along the subgranular layer and extended processes through the blades of the dentate into the molecular layer (FIG. 1E.1). By 72 h, exposure to TMT produced an increased staining density of doublecortin+ cells and processes as compared to the control hippocampus (FIG. 1E.2). A significant replacement of neurons lost at 3 days (FIG. 1C.1; H.1) was evident in the dentate region and was maintained at 4 weeks post-TMT (FIG. 1H.2).
FIGURE 1. Representative immunohistochemical staining in the dentate gyrus.
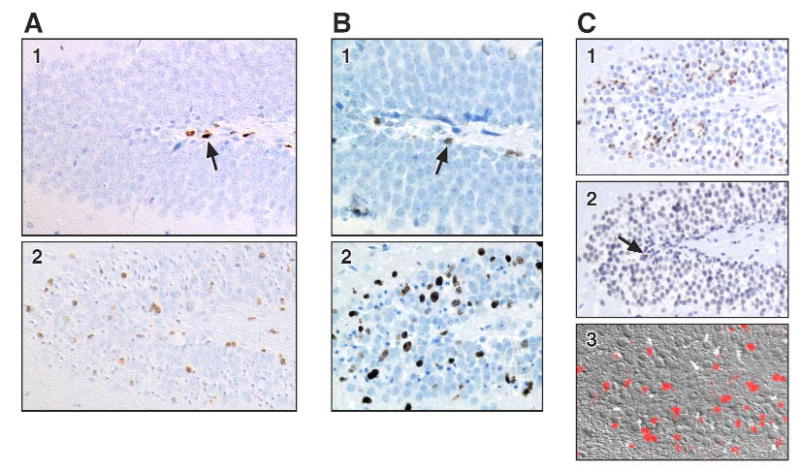
A. BrdU incorporation in the dentate gyrus (DG). 1) Brown BrdU positive cells (arrow) are present in the subgranular layer in saline control. 2) At 72 h post-TMT, BrdU positive cells are distributed throughout the DG. Hematoxylin counterstain. Differential Interference Constrast microscopy (DIC).
B. Ki-67 immunoreactivity for proliferation in the DG. 1) Saline controls (arrow) and 2) 72 h post-TMT. Hematoxylin counterstain.
C. Representative TUNEL staining (brown) in the DG at 1) 72 h and 2) 7 days post-TMT (arrow). 3) DIC image of fluorescent BrdU+ cells (red) in the DG at 7 days post-TMT.
D. NeuN+ mature neuron (green), nestin+ progenitor cells (red) in DG. 1) control. 2) At 72 h post-TMT, an increase in the number and density of nestin+ cells was seen throughout the DG. DAPI counterstain (blue).
E. Doublecortin+ progenitor cells (DAB - brown) within the DG of 1) control and 2) 72 h post-TMT weanling mice. Hematoxylin counterstain.
F. 1) Lectin+ microglia (red) and GFAP+ astrocytes (green) in the DG at 72 h post-TMT. 2) Merged image of BrdU+ (green) cell with lectin+ microglia (red) in contact.,
G. Two weeks post-TMT, representative individual images of 1) BrdU+ (red) and 2) NeuN+ mature neuron (green) and merged image.
H. H&E staining of DG. 1) 72 h post-TMT, neuronal nuclear pyknosis, karyolysis, and cell loss. 2) Replacement of neurons by 4 weeks.
Consistent with the morphology, cell counting of the dentate cell layer (10 sections per animal/15 animals per group) using an ocular counting grid showed a significant decrease of 35% ± 7% in the number of viable granule neurons at 72 h followed by a return to within control levels by 4 weeks (Area 1 - Control: 63 ± 3, TMT: 63.1 ± 4.2; Area 2 - Control 40.83 ± 4.1, TMT: 43.75 ± 6.3; Area 3 - Control: 50.33 ± 1.4, TMT: 54.13 ± 5.6). Merged images from the double staining of BrdU+ and NeuN+ cells at 2 weeks post-TMT (FIG. 1G) suggested that while degeneration occurred within the first 3 days (FIG. 1H.1), a proportion of the cell proliferation required to re-populate the dentate region by 4 weeks (FIG. 1H.2) also occurred.
DISCUSSION
Injury-induced neurogenesis within the hippocampus has been demonstrated in various models, however, the level of replacement in the adult has been somewhat limited and the temporal association not clearly delineated. Focal damage within the hippocampus induced by the administration of TMT resulted not only in degeneration of the dentate granule cell layer but also in the proliferation of neural progenitor cells. The level of regenerative potential maintained in these young weanling mice is such that the newly generated neurons were able to significantly repopulate the neuronally depleted dentate region. While the interpretation of cell fate from markers of DNA synthesis can be complicated by synthesis occurring in degenerating cells, the BrdU staining pattern occurring in the absence of active cell death at 7 days post-TMT and the significant level of repopulation by 4 weeks post-TMT support neurogenesis as an active mechanism of repair. This proliferative response occurred during the active process of neuronal degeneration, microglia phagocytosis of neuronal debri, and under highly inflammatory conditions. These findings are in agreement with the previously reported observation of an activation of cell cycle pathways in both glia and newly generated neurons following TMT (McPherson et al., 2003). It is known that during development, post-mitotic neurons generated from precursor cells continue to express cell cycle components for a short time period after terminal differentiation (Freeman et al., 1994; Padmanabhan et al., 1999). Thus, the proliferation of neuronal progenitor cells proposed in the current study is consistent with the co-localization of cyclin D with newly generated neurons in the TMT-induced injury response (McPherson et al., 2003).
The results of this study demonstrate an early activation of neurogenesis in concert with neuronal degeneration and suggest a possible signaling role for inflammatory factors in the activation of neural progenitor cell generation. The microenvironment for newly generated neurons was characterized by the presence of microglia actively phagocytizing neuronal debris in the dentate. The specific individual roles for microglia initiated cell-cell interactions determining cell death and cell survival have not been elucidated; however, it has been suggested that activated glial cells may exert neuroprotective and pro-regenerative functions that may be essential for the occurrence of neuronal regeneration (Streit et al., 2000). It is possible that glia and the associated inflammatory response both contribute to the induction of neuronal progenitor cells as a mechanism of hippocampal repair. The extent of the neurogenesis generated in young mice following TMT offers a model system for further examination of the process of neurogenesis and the individual cell-cell interactions that determine neuronal fate.
References
- Bruccoleri A, Harry GJ. Chemical-induced hippocampal neurodegeneration and elevations in TNFalpha, TNFbeta, IL-1alpha, IP-10, and MCP-1 mRNA in osteopetrotic (op/op) mice. J Neurosci Res. 2000;62:146–155. doi: 10.1002/1097-4547(20001001)62:1<146::AID-JNR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Bruccoleri A, Brown H, Harry GJ. Cellular localization and temporal elevation of tumor necrosis factor-alpha, interleukin-1alpha, and transforming growth factor-beta mRNA in hippocampal injury response induced by trimethyltin. J Neurochem. 1998;71:1577–1587. doi: 10.1046/j.1471-4159.1998.71041577.x. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Covolan L, Riberiro LT, Longo BM, Mello LE. Cell damage and neurogenesis in the dentate granule cell layer of adult rats after pilocarpine- or kainate-induced status epilepticus. Hippocampus. 2000;10:169–180. doi: 10.1002/(SICI)1098-1063(2000)10:2<169::AID-HIPO6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Eskes C, Juillerat-Jeanneret L, Leuba G, Honegger P, Monnet-Tschudi F. Involvement of microglia-neuron interactions in the tumor necrosis factor-alpha release, microglial activation, and neurodegeneration induced by trimethyltin. J Neurosci Res. 2003;71:583–590. doi: 10.1002/jnr.10508. [DOI] [PubMed] [Google Scholar]
- Fiedorowicz A, Figiel I, Kaminska B, Zaremba M, Wilk S, Oderfeld-Nowak B. Dentate granule neuron apoptosis and glia activation in murine hippocampus induced by trimethyltin exposure. Brain Res. 2001;912:116–127. doi: 10.1016/s0006-8993(01)02675-0. [DOI] [PubMed] [Google Scholar]
- Freeman RS, Estus S, Johnson EM., Jr Analysis of cell cycle-related gene expression in postmitotic neurons: selective induction of cyclin D1 during programmed cell death. Neuron. 1994;12:343–355. doi: 10.1016/0896-6273(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geloso M, Vercelli A, Corvino V, Repici M, Boca M, Haglid Z, Zelano G, Michetti F. Cyclooxygenase-2 and caspase 3 expression in trimethyltin-induced apoptosis in the mouse hippocampus. Exp Neurol. 2002;175:152–160. doi: 10.1006/exnr.2002.7866. [DOI] [PubMed] [Google Scholar]
- Harry GJ and C Lefebvre d'Hellencourt (2003) The neuroinflammatory components of the trimethyltin (TMT) model of hippocampal neurodegeneration, In: Neuroinflammation, 2nd Edition, Mechanisms and Management, Wood PL, Ed. (Humana Press, Totowa, NJ, USA), pp 301–329.
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Meth. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kennea NL, Mehmet H. Neural stem cells. J Pathol. 2002;197:536–550. doi: 10.1002/path.1189. [DOI] [PubMed] [Google Scholar]
- Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- McPherson CA, Kubik J, Wine RN, Lefebvre d'Hellencourt C, Harry GJ. Alterations in cyclin A, B, and D1 in mouse dentate gyrus following TMT-induced hippocampal damage. Neurotoxicity Res. 2003;5:339–354. doi: 10.1007/BF03033154. [DOI] [PubMed] [Google Scholar]
- Padmanabhan J, Park DS, Greene LA, Shelanski ML. Role of cell cycle regulatory proteins in cerebellar granule neuron apoptosis. J Neurosci. 1999;19:8747–8756. doi: 10.1523/JNEUROSCI.19-20-08747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Lowenstein DH. Seizure-induced neurogenesis: are more new neurons good for an adult brain? Prog Brain Res. 2002;135:121–131. doi: 10.1016/S0079-6123(02)35012-X. [DOI] [PubMed] [Google Scholar]
- Reuhl KR, Smallridge EA, Chang LW, Mackenzie BA. Developmental effects of trimethyltin intoxication in the neonatal mouse. I. Light microscopic studies. Neurotoxicology. 1983;4:19–28. [PubMed] [Google Scholar]
- Streit WJ, Hurley SD, McGraw TS, Semple-Rowland SL. Comparative evaluation of cytokine profiles and reactive gliosis supports a critical role for interleukin-6 in neuron-glia signaling during regeneration. J Neurosci Res. 2000;61:10–20. doi: 10.1002/1097-4547(20000701)61:1<10::AID-JNR2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Nozaki K, Takahashi J, Yodoi J, Ishikawa M, Hashimoto N. Proliferation of neuronal precursor cells in the dentate gyrus is accelerated after transient forebrain ischemia in mice. Brain Res. 1999;831:283–287. doi: 10.1016/s0006-8993(99)01411-0. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]