Functional Evaluation of DC-SIGN Monoclonal Antibodies Reveals DC-SIGN Interactions with ICAM-3 Do Not Promote Human Immunodeficiency Virus Type 1 Transmission (original) (raw)
Abstract
DC-SIGN, a type II membrane-spanning C-type lectin that is expressed on the surface of dendritic cells (DC), captures and promotes human and simian immunodeficiency virus (HIV and SIV) infection of CD4+ T cells in trans. To better understand the mechanism of DC-SIGN-mediated virus transmission, we generated and functionally evaluated a panel of seven monoclonal antibodies (MAbs) against DC-SIGN family molecules. Six of the MAbs reacted with myeloid-lineage DC, whereas one MAb preferentially bound DC-SIGNR/L-SIGN, a homolog of DC-SIGN. Characterization of hematopoietic cells also revealed that stimulation of monocytes with interleukin-4 (IL-4) or IL-13 was sufficient to induce expression of DC-SIGN. All DC-SIGN-reactive MAbs competed with intercellular adhesion molecule 3 (ICAM-3) for adhesion to DC-SIGN and blocked HIV-1 transmission to T cells that was mediated by THP-1 cells expressing DC-SIGN. Similar but less efficient MAb blocking of DC-mediated HIV-1 transmission was observed, indicating that HIV-1 transmission to target cells via DC may not be dependent solely on DC-SIGN. Attempts to neutralize DC-SIGN capture and transmission of HIV-1 with soluble ICAM-3 prophylaxis were limited in success, with a maximal inhibition of 60%. In addition, disrupting DC-SIGN/ICAM-3 interactions between cells with MAbs did not impair DC-SIGN-mediated HIV-1 transmission. Finally, forced expression of ICAM-3 on target cells did not increase their susceptibility to HIV-1 transmission mediated by DC-SIGN. While these findings do not discount the role of intercellular contact in facilitating HIV-1 transmission, our in vitro data indicate that DC-SIGN interactions with ICAM-3 do not promote DC-SIGN-mediated virus transmission.
Dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN; also called CD209) is a C-type lectin abundantly expressed in myeloid cell-derived DC and binds to and fosters interactions with T cells via ICAM-3 binding. The ability of myeloid cell-derived DC to efficiently transmit human immunodeficiency virus type 1 (HIV-1) to CD4+ T cells (4, 5, 11, 12, 17-19, 31) and the earlier identification of the C-type lectin as a ligand of HIV envelope (Env) glycoprotein gp120 (9) prompted an investigation of whether DC-SIGN accorded DC their virus transmission property (14). These studies demonstrated that DC-SIGN expression on transformed cells is sufficient to enable efficient capture and _trans_-presentation of HIV-1 to target CD4+ T cells. Moreover, this transmission function is dependent on DC-SIGN interaction with HIV-1 Env.
Subsequent work has demonstrated that HIV-1, HIV-2, and simian immunodeficiency virus (SIV) can also be efficiently captured and transmitted via DC-SIGN, suggesting a conserved role for DC-SIGN in the transmission and pathogenesis of primate lentiviruses (1, 14, 26). DC-SIGN expression in cis can also enhance HIV and SIV infection, suggesting that DC or other primary cells induced to express DC-SIGN may increase their susceptibility to infection even with suboptimal expression of canonical viral receptors (22).
The extracellular domain of DC-SIGN comprises seven 23-residue tandem repeats and a C-terminal C-type carbohydrate recognition domain (CRD). Binding and transmission of HIV-1 mediated by DC-SIGN are dependent on the presence of the CRD (26). Furthermore, HIV-1 capture and transmission can be inhibited by soluble mannan, indicating that Env glycosylation may be important in the DC-SIGN and HIV-1 interaction (14). Structural analysis of the DC-SIGN CRD has indicated a selectivity for N-linked glycoproteins displaying high-mannose oligosaccharides (10).
DC-SIGN-related molecules have been identified in other species, including mouse (2, 6, 25), macaque (2, 13), and chimpanzee (13). Mice express as many as five related genes, including one DC-SIGN homolog. Macaque DC-SIGN has been shown to function in primate lentivirus capture and transmission (2, 13). Murine DC-SIGN has not yet been functionally evaluated (25); however, murine SIGNR1, a SIGN-related molecule, appears to lack lentivirus transmission activity (2).
A human homolog of DC-SIGN, named DC-SIGNR (for DC-SIGN related) or L-SIGN (for liver/lymph node SIGN; also called CD209L) has also been identified; the gene encoding this homolog lies within 15 kb of DC-SIGN on chromosome 19 in a head-to-head orientation (3, 30). Similar to DC-SIGN, DC-SIGNR/L-SIGN binds ICAM-3 and captures and enhances HIV-1, HIV-2, and SIV infection of T cells in trans (3, 26, 27).
DC-SIGN is a major ICAM-3 receptor on DC and is important in establishing the initial, transient interaction between DC and T cells (15). Like HIV/SIV Env, ICAM-3 binding to DC-SIGN requires an interaction with the CRD, is calcium dependent, and can be inhibited by soluble mannan (14). The mechanism of DC-SIGN-mediated transmission of HIV-1 to CD4+ T cells has not been elucidated. It is conceivable that contacts between DC-SIGN on virus-presenting cells and ICAM-3 displayed on virus acceptor/target cells aid in virus transfer by increasing the general synaptic area between the cells, orienting DC-SIGN presentation of virus toward CD4 and a viral coreceptor, increasing the duration that the cells are in contact for or fostering a change in DC-SIGN structure that enhances release of virus. Although DC-SIGN-mediated HIV-1 transmission does not require ICAM-3 expression on target cells, T cells that express ICAM-3 are more suitable targets for DC-SIGN infection enhancement than other cell lines (14). However, whether interactions between DC-SIGN and ICAM-3 aid in HIV-1 transmission via DC-SIGN was not clearly investigated. Indeed, previous studies have demonstrated that leukocyte function-associated molecule 1 (LFA-1) interactions with intercellular adhesion molecules can contribute to cell-to-cell transmission of HIV-1 (20, 21).
Here we report the characterization of a panel of seven mouse monoclonal antibodies (MAbs) raised against human DC-SIGN and L-SIGN. Reactivity of the antibodies was confirmed on myeloid lineage hematopoietic cells. The MAbs were also examined for their ability to block DC-SIGN interactions with either ICAM-3 or HIV-1. Finally, using blocking MAbs, we investigated the mechanism of DC-SIGN-mediated HIV-1 transmission. Specifically, we assessed whether interactions between donor cells expressing DC-SIGN and target cells expressing ICAM-3 enabled a cell-to-cell microenvironment facilitating virus transmission from the donor cell membrane to the receptor complex of the target cell membrane.
MATERIALS AND METHODS
Isolation of ICAM-3 cDNA.
Human ICAM-3 cDNA was isolated from PCR amplification of human T-cell cDNA, subcloned into a murine leukemia virus pMX vector (24), and verified by DNA sequencing. The PCR primers used for ICAM-3 cDNA amplification were I3-5Bgl2 (5′-GCG ATA GAC TGT CAG ATC TCT GTC AGA ATG GCC-3′) and I3-3R1 (5′-CTT TGA TCC CGA ATT CCA GCG TCA CTC AGC-3′).
Antibodies.
A panel of seven MAbs against DC-SIGN or L-SIGN were generated by R&D Systems (Minneapolis, Minn.). The MAbs were obtained by screening hybridoma supernatants of BALB/c mice immunized with NIH 3T3/BABE-DC-SIGN or NIH 3T3/BABE-L-SIGN cells for the ability to stain DC-SIGN or L-SIGN. All other antibodies were purchased from B-D/PharMingen unless noted otherwise.
Cell culture.
NIH 3T3/DC-SIGN and NIH 3T3/L-SIGN cell lines were stably transduced with pMX vectors encoding DC-SIGN or L-SIGN, respectively, and subjected to fluorescence-activated cell sorting (FACS) with the DC-SIGN- and L-SIGN-cross-reactive MAb 526(X) for gene expression.
THP-1 and THP-1/DC-SIGN cell lines were provided by Douglas Kwon and Dan Littman (New York University Medical Center). THP-1/DC-SIGN cells were subsequently subjected to FACS four times to obtain high expression of DC-SIGN.
Immature DC were generated as described previously (29). Peripheral blood mononuclear cells (PBMC) were separated from buffy coats of healthy donors (Vanderbilt Medical Center) by using Ficoll-Hypaque (Pharmacia). Briefly, CD14+ monocytes were purified using the MACS system (Milteni Biotech) and cultured in the presence of 100 ng of interleukin-4 (IL-4; R&D Systems) and 50 ng of granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D Systems) per ml for 4 to 6 days. At day 7, the cells expressed high levels of HLA-DR, major histocompatibility complex class I, CD11b, CD11c, DC-SIGN, and ICAM-1, moderate levels of LFA-1 and CD86, and low levels of CD14.
Hut/CC chemokine receptor (CCR) 5 (Hut/CCR5) cells are the transformed human T-cell line Hut78 stably transduced with CCR5. HEK293T cells are human embryonic kidney cells containing a single temperature-sensitive allele of simian virus 40 (SV40) large T antigen.
GHOST/R5 and GHOST/X4/R5 cells, which do not express endogenous ICAM-3, are HIV indicator cells derived from human osteosarcoma cells (7).
The GHOST/R5/ICAM-3 cell line was generated by stable transduction of GHOST/R5 cells with the murine leukemia virus vector MX-ICAM-3. The transduced GHOST/R5/ICAM-3 cells were positively sorted for ICAM-3 expression by using phycoerythrin (PE)-conjugated mouse antibody against ICAM-3 (Caltag Laboratories). Staining of GHOST/R5/ICAM-3 cells with anti-human ICAM-3 confirmed a high level of ICAM-3 expression on the stable transductants (data not shown).
The THP-1, THP-1/DC-SIGN, and Hut/CCR5 cells described above were maintained in RPMI 1640 (Life Technologies/Invitrogen) supplemented with 10% fetal bovine serum (FBS; HyClone Laboratories). HEK293T, NIH 3T3, NIH 3T3/DC-SIGN, NIH 3T3/L-SIGN, GHOST/R5, and GHOST/R5/ICAM-3 cells were grown in Dulbecco's modified Eagle's medium (Life Technologies/Invitrogen) supplemented with 10% FBS. DC were maintained in OptiMEM medium (Life Technologies/Invitrogen) supplemented with 20% FBS (HyClone Laboratories) and specific cytokines as indicated.
Flow cytometry.
To assess the reactivity of DC-SIGN and L-SIGN with the MAbs, NIH 3T3/DC-SIGN, NIH 3T3/L-SIGN, and DC were FACS stained against the MAb panel. For staining, 2 × 105 cells were incubated in ice-cold phosphate-buffered saline (PBS) containing 2% FBS, 0.02% sodium azide (FACS buffer), and 2 μg of MAbs per ml in a total volume of 100 μl. After 30 min at 4°C, the cells were washed with the FACS buffer and recovered in 100 μl of FACS buffer containing 2 μg of fluorescein isothiocyanate (FITC)-conjugated antibody against mouse immunoglobulin G (IgG) (Caltag Laboratories) per ml. Cells were incubated for 30 min at 4°C, washed with FACS buffer, and analyzed with a FACSCalibur apparatus (Becton Dickinson).
ICAM-3 adhesion assay.
Soluble, recombinant ICAM-3 was obtained from R&D Systems. Carboxylate-modified TransFluorSpheres (1.0 μm; 488 nm excitation/645 nm emission; Molecular Probes) were coated with ICAM-3 as described previously (16). The fluorescent bead adhesion assay was performed as described previously (15) with modifications. Briefly, THP-1 and THP-1/DC-SIGN cells (2 × 105) were resuspended in 20 mM Tris-HCl (pH 7.4)-150 mM NaCl-1 mM CaCl2-2 mM MgCl2-0.5% bovine serum albumin (BSA)-20 nM sodium azide (adhesion buffer) and incubated with each MAb or mouse IgG isotype control (10 μg/ml) for 10 min at room temperature. For THP-1/DC-SIGN cells, ICAM-3-coated fluorescent beads were added and incubated for 30 min at 37°C, while for DC, incubation was performed at 4°C to minimize nonspecific adsorption. Adhesion of ICAM-3 to DC-SIGN was determined by measuring the detectable percentage of cells that bound fluorescent beads, using flow cytometry on a FACSCalibur (Becton Dickinson).
Virus stocks.
Single-round infectious, pseudotyped HIV-1 stocks were generated by calcium phosphate cotransfections of HEK293T cells with the proviral vector plasmid NL-Luc-E−R− (HIV-Luc) containing a firefly luciferase reporter gene (8) and expression plasmids for either R5-tropic HIV-1JRFL (HIV-Luc/JRFL) or X4-tropic HIV-1HXB2 (HIV-Luc/HXB2) envelope glycoproteins. Virus stocks were evaluated by limiting dilution on GHOST/X4/R5 cells.
HIV-1 infection assays.
HIV-1 capture and transmission assays were performed as described previously (3). In brief, THP-1, THP-1/DC-SIGN, or DC donor cells (2.5 × 105) were incubated with pseudotyped HIV-1 (multiplicity of infection, ≈0.1) in a total volume of 400 μl for 3 h to allow cellular adsorption of the virus. After 3 h, cells were washed with 1 ml of PBS and cocultured with Hut/CCR5 target cells (105) in the presence of 10 μg of Polybrene per ml in 1 ml of cell culture medium. For the ICAM-3, mannan, or DC-SIGN antibody-blocking assay, cells were incubated with either soluble recombinant human ICAM-3/Fc chimera (10 μg/ml; R&D Systems) or mannan (20 μg/ml; Sigma) and MAbs against DC-SIGN or L-SIGN (10 μg/ml), respectively, for 30 min at 37°C before virus addition. Pretreatment with MAbs under these conditions did not lead to surface downmodulation of DC-SIGN. Cell lysates were obtained 2 days after infection and analyzed for luciferase activity with a commercially available kit (Promega).
For the capture and transmission assays using GHOST/R5 or GHOST/R5/ICAM-3 as target cells, cells (5 × 104/well) were seeded in 24-well plates 1 day before coculturing with donor cells. In these assays, the suspended THP-1 and THP-1/DC-SIGN donor cells were removed from the cocultivation after a 5-h incubation. Adherent target cells were then washed with 1 ml of medium and cultured in 1 ml of fresh medium for 2 days before the luciferase activity was measured. To inhibit DC-SIGN and ICAM-3 interactions, fresh DC-SIGN MAb 531(D) and anti-human ICAM-3 MAb (R&D Systems, antibody clone 76205.11) were added daily.
RESULTS
Reactivity of antibodies against DC-SIGN and L-SIGN.
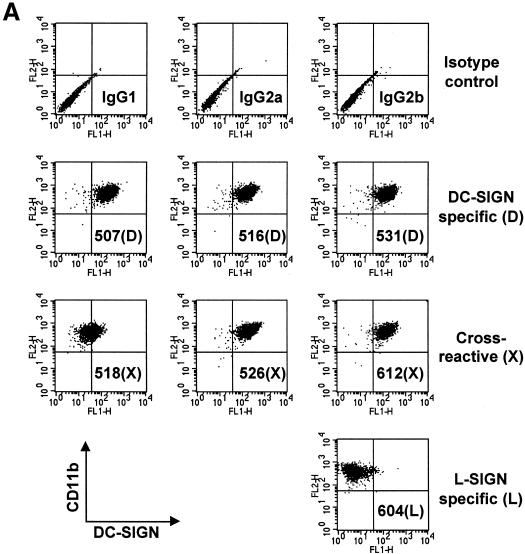
Staining of NIH 3T3/DC-SIGN cells and NIH 3T3/L-SIGN cells demonstrated that three of the seven MAbs, 507(D), 516(D), and 531(D), were DC-SIGN specific; three MAbs, 518(X), 526(X), and 612(X), were cross-reactive; and MAb 604(L) was L-SIGN specific (Table 1). Staining of parental NIH 3T3 cells was uniformly negative (data not shown). Furthermore, MAbs 507(D), 516(D), and 526(X) were found to recognize Rhesus macaque DC-SIGN (33). The reactivity of the MAbs was also confirmed by staining of human immature DC with each of the seven MAbs and anti-human CD11b (Fig. 1A ). DC were highly positive for CD11b, one of the surface markers of immature DC. Six of the DC-SIGN-reactive MAbs positively stained DC. However, the L-SIGN-specific MAb 604(L) showed minimal binding to DC (Fig. 1A).
TABLE 1.
Reactivity of mouse MAbs against DC-SIGN and L-SIGN
| Antibody clonea | % Positive cells (mean fluorescence)b | Reactivityc | Isotype | |
|---|---|---|---|---|
| NIH 3T3/DC-SIGN | NIH 3T3/L-SIGN | |||
| 507(D) | 99.2 (512) | 0.2 (30) | DC-SIGN, mac-DC-SIGN | IgG2b |
| 516(D) | 98.8 (169) | 0.05 (21) | DC-SIGN, mac-DC-SIGN | IgG2a |
| 531(D) | 97.8 (254) | 0.0 (NA) | DC-SIGN | IgG1 |
| 518(X) | 98.5 (66) | 95.4 (92) | DC-SIGN, L-SIGN | IgG2a |
| 526(X) | 98.8 (170) | 98.6 (175) | DC-SIGN, L-SIGN, mac-DC-SIGN | IgG2a |
| 612(X) | 98.8 (182) | 99.1 (309) | DC-SIGN, L-SIGN | IgG2a |
| 604(L) | 1.3 (40) | 99.5 (650) | L-SIGN | IgG2b |
| IgG1 | 0.4 (27) | 0.0 (NA) | NA | |
| IgG2a | 0.2 (27) | 0.0 (NA) | NA | |
| IgG2b | 0.4 (29) | 0.03 (22) | NA |
FIG. 1.
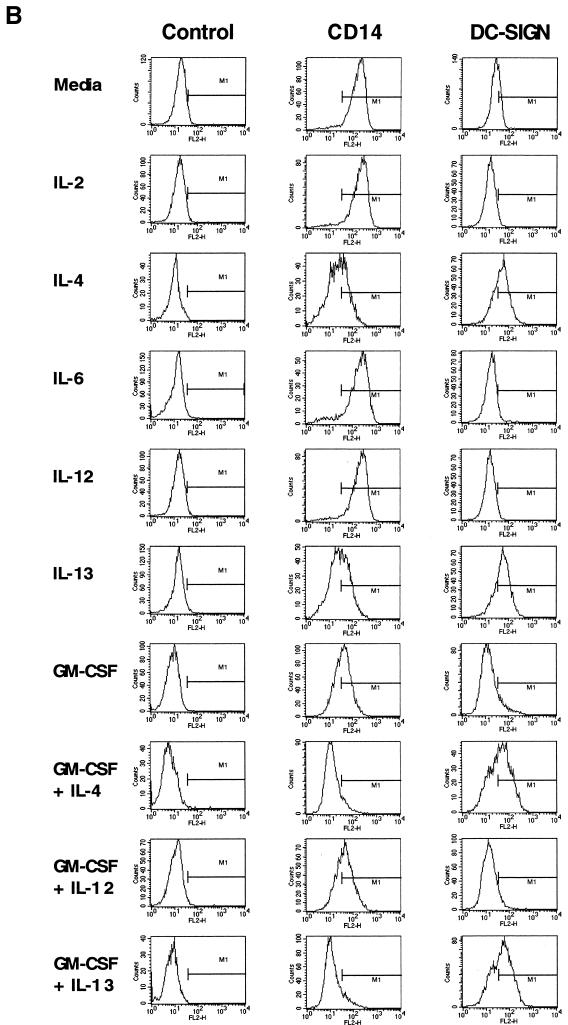
DC-SIGN expression on myeloid lineage cells. (A) DC stained with MAbs against DC-SIGN or L-SIGN. DC were first incubated with each of the MAbs, after which FITC-conjugated anti-mouse IgG and PE-conjugated anti-human CD11b were used for double staining. PE-conjugated mouse IgG2a and FITC-conjugated mouse IgG1, IgG2a, and IgG2b were used as isotype control antibodies (top row). (B) DC-SIGN expression of cytokine-treated monocytes. As depicted to the left of the FACS panel sets, purified CD14+ monocytes were cultured for 4 days in the presence of no cytokines, IL-2 (200 U/ml), IL-4 (100 ng/ml), IL-6 (1,000 U/ml), IL-12 (20 ng/ml), IL-13 (100 ng/ml), or GM-CSF (50 ng/ml), singly or in combination. Cells were stained with DC-SIGN MAb 507(D) or isotype control antibody (IgG2b), followed by PE-conjugated goat anti-mouse Ig antibody, or stained with PE-conjugated CD14 antibody. Antibody staining (FL2) is depicted by the histogram plots along the x axis.
Cytokines induce DC-SIGN expression on monocytes.
Because in vitro generation of monocyte-derived DC requires stimulation with GM-CSF and IL-4, we asked whether signaling from both cytokines was required for DC-SIGN expression. Culture of monocytes with IL-4 or the related cytokine IL-13 was sufficient to induce DC-SIGN expression (Fig. 1B). In contrast, GM-CSF, IL-2, IL-12, or IL-6 alone or in combination was not effective in stimulating DC-SIGN expression (Fig. 1B and data not shown). The expression of DC-SIGN correlated with the downregulation of CD14 (Fig. 1B). These results suggest a critical role for signals relayed from the IL-4/IL-13 receptors for induction of DC-SIGN expression.
Antibodies block DC-SIGN/ICAM-3 interactions.
To determine whether the MAbs could compete for ICAM-3 binding to DC-SIGN, we used a previously described flow cytometric assay (15) to measure the adhesion of ICAM-3-coated fluorescent beads to THP-1/DC-SIGN cells or immature DC. Bead adhesion experiments with DC were performed at 4°C to minimize the multiple, nonspecific auxiliary processes that DC use to sample their extracellular environments.
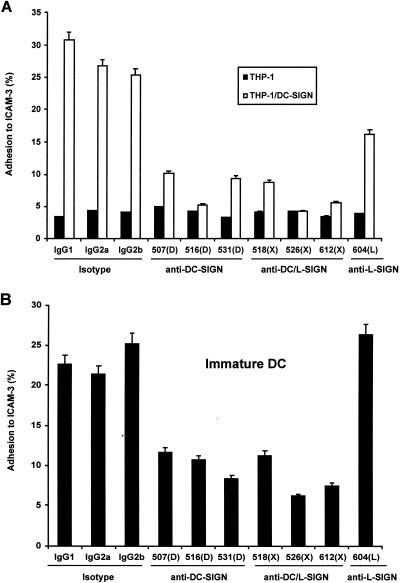
Both DC-SIGN-specific and cross-reactive MAbs were able to impair ICAM-3 adhesion to DC-SIGN at different efficiencies (Fig. 2). Background binding of ICAM-3 to THP-1 cells was uniformly less than 5% in repeat experiments. The detectable adhesion of ICAM-3-coated beads to THP-1/DC-SIGN cells was 25 to 31% in the presence of 10 μg of mouse IgG1, IgG2a, and IgG2b isotype control antibodies per ml (Fig. 2A). Addition of 10 μg of DC-SIGN-specific MAbs 507(D), 516(D), and 531(D) per ml reduced ICAM-3 bead binding to 10, 5, and 9%, respectively, whereas the cross-reactive MAbs 518(X), 526(X), and 612(X) reduced the adhesion to 8, 4, and 6%, respectively. At the same antibody concentration, the L-SIGN-specific MAb 604(L) also diminished ICAM-3 binding, albeit more modestly, to 16%.
FIG. 2.
ICAM-3 adhesion to DC-SIGN is inhibited by DC-SIGN MAbs. (A) Adhesion of ICAM-3 to THP-1/DC-SIGN cells was measured by FACS with a fluorescent bead adhesion assay (15) as described in Materials and Methods. Adhesion of ICAM-3 to THP-1 parental cells was less than 5%. (B) Adhesion of ICAM-3 to immature DC. Mouse IgG1, IgG2a, or IgG2b was used as the isotype control antibody. One representative experiment of two is shown.
Similar ICAM-3 adhesion results were obtained with monocyte-derived DC (Fig. 2B). The binding of ICAM-3-coated beads to DC was 21 to 25% in the presence of mouse IgG1, IgG2a, and IgG2b isotype control antibodies. The DC-SIGN-specific MAbs 507(D), 516(D), and 531(D) reduced the adhesion to 12, 11, and 8%, respectively, and the cross-reactive MAbs 518(X), 526(X), and 612(X) reduced the adhesion to 11, 6, and 7%, respectively (Fig. 2B). The L-SIGN-specific MAb 604(L) did not impair any ICAM-3 adhesion (26%) to DC.
Antibody neutralization of HIV-1 transmission from THP-1/DC-SIGN cells.
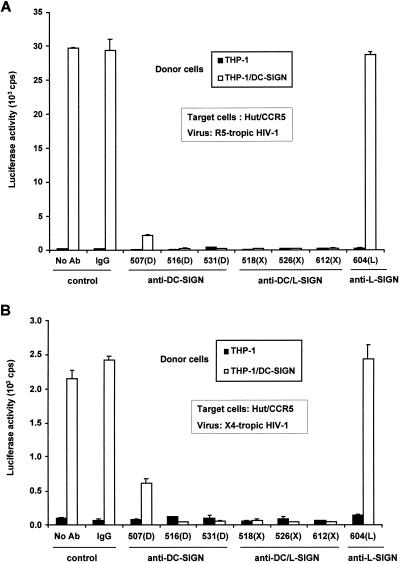
DC-SIGN promotes efficient infection in trans of cells that express HIV-1 receptor molecules (14), and DC-SIGN interactions with HIV-1 Env are required for this transmission. Thus, we reasoned that MAbs raised against native DC-SIGN could disrupt these interactions and be useful in exploring the mechanism of DC-SIGN-mediated HIV-1 transmission. To test this hypothesis, a single-round infectious HIV-luciferase vector pseudotyped with R5-tropic HIV-1JRFL or X4-tropic HIV-1HXB2 Env was incubated with THP-1/DC-SIGN donor cells and then cocultured with the human T-cell target line Hut/CCR5. Compared with THP-1 donor cell controls, HIV-1 transmission was enhanced 200- to 300-fold by THP-1/DC-SIGN cells (Fig. 3).
FIG. 3.
HIV-1 transmission mediated by THP-1/DC-SIGN cells is blocked with the DC-SIGN-specific MAbs. HIV-1 capture and transmission of R5-tropic HIV-Luc/JRFL (A) and X4-tropic HIV-Luc/HXB2 (B) was tested. THP-1 or THP-1/DC-SIGN donor cells were incubated with each of the MAbs (10 μg/ml) for 30 min at 37°C, and then HIV-luciferase pseudotyped virus was incubated with the cells for 3 h at 37°C. The cells were washed and cocultured with Hut/CCR5 target cells in the presence of Polybrene. HIV-1 infection was determined after 2 days by measuring the luciferase activity. No Ab, treatment without antibody. Mouse IgG was used as a nonspecific antibody control. Each data set represents the mean of three or four separate wells of infected cells. cps, counts per second.
To ascertain the neutralization potential of anti-DC-SIGN MAbs, THP-1 or THP-1/DC-SIGN donor cells were incubated with MAbs against DC-SIGN or L-SIGN before exposure to HIV-luciferase-pseudotyped virus, after which washed donor cells were cocultured with Hut/CCR5 target cells. With the exception of 604(L), the six DC-SIGN-reactive MAbs efficiently neutralized the transmission of X4- or R5-tropic HIV-1 (Fig. 3). The DC-SIGN-specific MAb 507(D) appeared to be the least efficient in blocking either type of HIV-1, suggesting that it interacted with a region of DC-SIGN that is less critical for HIV-1 capture and transmission or that it had a reduced affinity for DC-SIGN. As expected, the L-SIGN-specific MAb 604(L) was unable to inhibit virus transmission by DC-SIGN.
To exclude any direct effects of the MAbs on HIV-1 infection of the target cell, the Hut/CCR5 cells were challenged with HIV-1 in the presence of each of the MAbs against DC-SIGN and L-SIGN. No significant difference in direct infection was observed for either R5- or X4-tropic HIV-1 with or without the MAbs (data not shown).
DC-SIGN plays a significant role in HIV-1 transmission by DC.
To investigate the blocking efficiency of MAbs against DC-SIGN for HIV-1 transmission by DC, immature DC were used as donor cells in the HIV-1 capture and transmission assay. The monocyte-differentiated DC were more that 99% pure by DC-SIGN, HLA-DR, and CD11b staining but completely negative for CD3 and CD14 (data not shown).
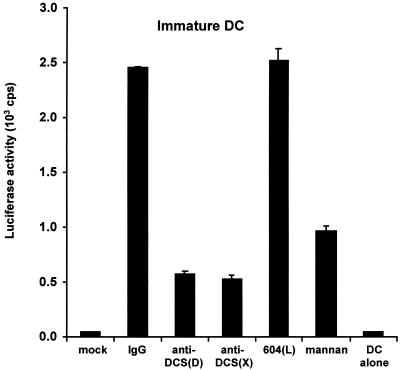
To observe the maximum blocking efficiency of HIV-1 transmitted by DC with DC-SIGN-reactive MAbs, we used cocktails of DC-SIGN-specific or cross-reactive MAbs based on the results of individual MAb inhibition tests (data not shown). In contrast to the results with THP-1/DC-SIGN cells, we found that DC-mediated transmission of HIV-1 to the Hut/CCR5 target cells could be blocked only partially with a cocktail of MAbs against DC-SIGN (Fig. 4.). To evaluate the DC background infection, DC were first pulsed with HIV-1 for 3 h and washed to remove unbound virus as in all the samples; cells were then cultured alone without Hut/CCR5 target cells. Under these conditions, minimal infection of DC was observed relative to mock controls of DC in the absence of virus (Fig. 4). Relative to HIV-1 transmission results with mouse IgG control antibody, a cocktail of DC-SIGN-specific MAbs 507(D), 516(D), and 531(D) inhibited the transmission to 23%, whereas a cocktail of the cross-reactive MAbs 518(X), 526(X), and 612(X) reduced the transmission to 22%. No inhibition of the transmission was observed with the L-SIGN-specific MAb 604(L). In addition, preincubation with mannan reduced the HIV-1 transmission by DC to only 39% (Fig. 4).
FIG. 4.
HIV-1 transmission mediated by DC is blocked by DC-SIGN MAbs. Transmission of R5-tropic HIV-Luc/JRFL using DC as donor cells and Hut/CCR5 as target cells was performed as described for Fig. 3. DC cocultured with Hut/CCR5 cells not exposed to HIV-1 were used as a mock-infected control. Mouse IgG was used as a nonspecific antibody control. Anti-DCS(D), cocktail containing the DC-SIGN-specific MAbs 507(D), 516(D), and 531(D) (10 μg/ml combined). Anti-DCS(X): cocktail containing the cross-reactive MAbs 518(X), 526(X), and 612(X) (10 μg/ml combined). The L-SIGN-specific MAb 604(L) was used at 10 μg/ml; mannan was used at 20 μg/ml. DC alone were incubated with the virus, washed to remove unbound virus, and then cultured without Hut/CCR5 target cells. One representative experiment out of two is shown. cps, counts per second.
Weak cross-competition of ICAM-3 with HIV-1 for DC-SIGN.
ICAM-3 binds DC-SIGN efficiently, and ICAM-3 expressed by resting T cells is important in their initial contact with DC (15). The precise amino acid residues within the CRD of DC-SIGN that are necessary for HIV-1 Env interaction versus ICAM-3 interaction have not been determined.
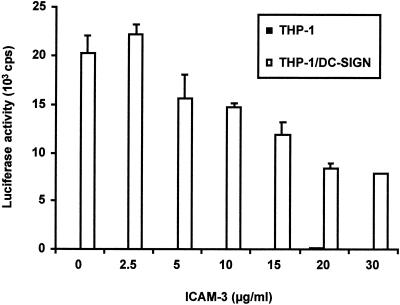
Because DC-SIGN-reactive MAbs could impair ICAM-3 binding and neutralize HIV-1 transmission, we explored whether ICAM-3 binding to DC-SIGN would preclude HIV-1 interaction. To test this, THP-1 or THP-1/DC-SIGN donor cells were incubated with increasing amounts of soluble ICAM-3 before being pulsed with HIV-luciferase pseudotypes. Donor cells were then washed to remove free virus, and target cells were added. Relative to control samples with no soluble ICAM-3, the transmission of R5-tropic HIV-1 by THP-1/DC-SIGN was impaired to 77, 73, 59, 41, and 39% of the control level as the ICAM-3 concentration for incubation was increased to 5, 10, 15, 20, and 30 μg/ml, respectively (Fig. 5). These results suggest that soluble ICAM-3 is not able to compete efficiently for HIV-1 Env binding to DC-SIGN. Similar results were obtained with DC as donor cells and with different R5- and X4-tropic HIV isolates (data not shown).
FIG. 5.
Soluble ICAM-3 partially inhibits HIV-1 transmission mediated by DC-SIGN. THP-1 or THP-1/DC-SIGN donor cells were incubated with increasing amounts of soluble ICAM-3 for 30 min at 37°C. Hut/CCR5 target cells and R5-tropic HIV-Luc/JRFL were used for the capture and transmission assay as described for Fig. 3. Each data set represents the mean of four separate wells of infected cells. One representative experiment out of two is shown. cps, counts per second.
Forced expression of ICAM-3 in target cells does not enhance DC-SIGN-mediated HIV-1 transmission.
Given that DC-SIGN and ICAM-3 interactions are thought to promote contact between DC and T cells, we were curious whether these numerous donor cell-to-target cell contacts facilitate HIV-1 transmission. To test this hypothesis, we sought to either manipulate the expression of ICAM-3 in our capture and transmission experiments or disrupt these contacts between cells.
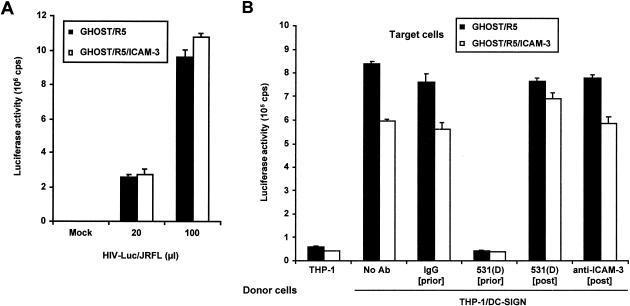
Human ICAM-3 subcloned from T-cell cDNA was used to produce GHOST/R5/ICAM-3 cells. Challenge of GHOST/R5 cells that were positive or negative for ICAM-3 with R5-tropic HIV-1 indicated that the sensitivities of the two lines of cells to HIV-1 infection were comparable (Fig. 6A). However, despite the presence of ICAM-3 on GHOST/R5/ICAM-3 cells, DC-SIGN-mediated transmission of HIV-1 to GHOST/R5/ICAM-3 cells was not better and, in fact, was somewhat lower than that to ICAM-3-negative GHOST/R5 cells (Fig. 6B). Similarly, blocking DC-SIGN and ICAM-3 interactions with MAb 531(D) or anti-ICAM-3 added after virus adsorption to THP-1/DC-SIGN cells and before target cell addition did not impair virus transmission (Fig. 6B). In contrast, incubation of THP-1/DC-SIGN cells with DC-SIGN MAb 531(D) before pulsing with HIV-1 completely inhibited the transmission to target cells (Fig. 6B).
FIG. 6.
Expression of ICAM-3 in GHOST/R5 target cells does not enhance HIV-1 transmission mediated by DC-SIGN. (A) Direct infection of GHOST/R5 and GHOST/R5/ICAM-3 cells. Cells were infected with different amounts of HIV-Luc/JRFL pseudotyped virus as indicated. Luciferase activity was measured 2 days after infection. Cells not exposed to virus were used as a mock-infected control. (B) HIV-1 transmission to ICAM-3-positive or -negative GHOST/R5 cells mediated by DC-SIGN. THP-1 or THP-1/DC-SIGN donor cells were incubated with DC-SIGN-specific MAb 531(D) or mouse IgG control (10 μg/ml) for 30 min at 37°C ([prior]) and then pulsed with HIV-Luc/JRFL for 3 h at 37°C. The washed cells were then added to GHOST/R5 or GHOST/R5/ICAM-3 target cells, respectively. The DC-SIGN-specific MAb 531(D) and anti-ICAM-3 (10 μg/ml) were kept in the cocultivation for 5 h ([post]), and then donor cells were removed and target cells were washed with medium and cultured in 1 ml of fresh medium in the presence of MAb 531(D) or anti-ICAM-3 (refreshed daily) for 2 days before luciferase activity was measured ([post]). Each data set represents the mean of three separate wells of infected cells. One representative experiment out of two is shown. cps, counts per second.
DISCUSSION
We have described a panel of MAbs that recognize and discriminate among DC-SIGN family molecules, providing an additional tool in characterizing DC-SIGN function and understanding its role in primate lentivirus pathogenesis. Staining analysis of DC-SIGN- and L-SIGN-expressing NIH 3T3, HEK293T, and THP-1 cells (data not shown for all cell types) demonstrated that among the seven MAbs, three were DC-SIGN specific, three were cross-reactive with DC-SIGN and L-SIGN, and one was L-SIGN specific.
In addition, myeloid-lineage DC also reacted with those MAbs that recognized DC-SIGN. In the course of creating DC through differentiation of CD14+ monocytes, we observed that treatment with either IL-4 or IL-13 was sufficient to induce DC-SIGN expression on these cells. IL-4 and IL-13 are the cytokines typically expressed by Th2 CD4+ T cells. Thus, it is conceivable that the interaction of Th2 CD4+ T cells with monocytes or macrophages might create a microenvironment in which HIV-1 is more readily transmitted from monocytes/macrophages to either Th2 CD4+ T cells or other monocytes/macrophages. Of interest, IL-4 and IL-13 signals activate the STAT-6 transcription factor, whereas cytokines that do not induce DC-SIGN do not activate STAT-6 (23) Thus, it will be interesting to examine whether STAT-6 or transcription factors induced by STAT-6, such as GATA3 (28), participate in the induction of DC-SIGN transcription.
Functional characterization of the panel of DC-SIGN family MAbs revealed that they may be useful in studying DC-SIGN-ligand interactions. The DC-SIGN-specific or -cross-reactive MAbs inhibited the adhesion between DC-SIGN and ICAM-3. As expected, the L-SIGN-specific MAb 604(L) was not able to block adhesion of DC to ICAM-3. However, 604(L) slightly decreased ICAM-3 adhesion to THP-1/DC-SIGN cells, suggesting cross-reactivity that was not detected by FACS staining. Notably, five times more antibody was used in the ICAM-3 blocking assays than in the FACS staining analysis. Background binding of ICAM-3 to THP-1 cells was uniformly less than 5% in repeat experiments.
Except for MAb 507(D), HIV-1 transmission via THP-1/DC-SIGN cells to the Hut/CCR5 target cells was completely inhibited to background levels with the MAbs recognizing DC-SIGN. Although MAb 507(D) reacted with DC-SIGN strongly in the FACS staining assays, its relatively weaker neutralization of virus transmission suggests different sites of DC-SIGN for MAb 507(D) binding and virus interactions. As expected, L-SIGN-specific MAb 604(L) could not block HIV-1 capture and transmission by THP-1/DC-SIGN cells.
The mechanism by which DC-SIGN MAbs block HIV Env or ICAM-3 interactions remains to be determined. Given that both ligands are blocked, the DC-SIGN MAbs might affect the conformation or exposure of the CRD. Further investigation of DC-SIGN mutant molecules will allow mapping of epitopes that also influence HIV-1 Env or ICAM-3 binding.
Neutralizing DC-SIGN or L-SIGN MAbs may also be used to elucidate the role of DC-SIGN and L-SIGN in vivo in animal models or in vitro in organ culture systems. Unexpectedly, we observed that virus transmission by DC could be only partially impaired with the cocktails of DC-SIGN-specific or -cross-reactive MAbs. In agreement with this result, virus transmission by DC was reduced only to 59 to 78% with each of six individual DC-SIGN-reactive MAbs (10 μg/ml) relative to mouse IgG control antibody (data not shown). Consistent with earlier data, the L-SIGN-specific MAb 604(L) was not able to block the virus transmission mediated by DC. We also found that incubation with 20 μg of mannan per ml could only block HIV-1 transmission by DC to 39%, despite the fact that virus transmission via THP-1/DC-SIGN cells could be completely inhibited by mannan incubation (data not shown). These data suggest that although DC-SIGN is important for DC-mediated HIV-1 transmission to target cells, there may be additional factors that the virus uses in this process. In fact, Rhesus macaque DC efficiently transmit primate lentiviruses in the absence of DC-SIGN expression (33).
Besides the role of DC-SIGN in HIV-1 transmission, direct contact of HIV-1-exposed DC with T cells through adhesion molecules or other unknown factors and mechanisms may also contribute to the general property of virus transmission by DC. In the initial description of DC-SIGN function in HIV-1 transmission, we noted that CD4+ T cells were consistently better targets than 293T-CD4-CCR5 cells (14). Some of the known interactions that occur between DC and T cells are contacts between DC-SIGN and ICAM-3, LFA-1 and ICAM-1, LFA-3 and CD2, and the antigen-presenting complexes with their cognate T-cell receptors. Because the role of adhesion interactions mediated by DC-SIGN had not been formally examined within the context of HIV-1 transmission, we investigated whether DC-SIGN/ICAM-3 interactions create a microenvironment that favors transmission to T cells. In addition, others have demonstrated that direct contact of HIV-1-infected DC with T cells is required for efficient virus transmission and subsequent virus production (32).
In examining the possible role of ICAM-3, we found that exposure of DC-SIGN to soluble ICAM-3 was not sufficient to fully neutralize HIV-1 transmission mediated by THP-1/DC-SIGN cells, even if the concentration of ICAM-3 was increased to 30 μg/ml. Similar results were obtained when DC were used as donor cells in the HIV-1 capture and transmission assay (data not shown). Nonetheless, a more careful examination of DC-SIGN interactions with HIV-1 Env should provide the basis for the development of potential antiviral drugs that can be used prophylactically or therapeutically in disrupting DC-SIGN capture and transmission of HIV-1. Indeed, recent crystallographic studies of the DC-SIGN and DC-SIGNR CRD complexed with high-mannose oligosaccharide highlight contact points within the CRD which might serve as targets in developing novel antiviral agents against HIV-1 (10).
Initial experiments had indicated that HIV-1 capture and transmission could not be inhibited by using soluble ICAM-3 or ICAM-3 MAbs in the coculture stage of DC or THP-1/DC-SIGN donor cells and target T cells (data not shown). However, it was possible that the soluble ICAM-3 or the antibodies did not fully block DC-SIGN/ICAM-3 interactions between the donor and target cells. We thus chose to employ a system in which DC-SIGN/ICAM-3 interactions could be strictly controlled. Because ICAM-3-negative GHOST/R5 cells are readily infected by HIV-1, it was tested whether ICAM-3 expression might increase their susceptibility to DC-SIGN-mediated HIV-1 transmission. Our data indicate that the presence or absence of ICAM-3 on target GHOST/R5 cells did not affect their susceptibility to direct infection by HIV-1 or transmission mediated by DC-SIGN.
Similarly, attempts to block DC-SIGN/ICAM-3 interactions during the coculture of THP-1/DC-SIGN donor cells and GHOST/R5/ICAM-3 target cells did not impair HIV-1 transmission. In fact, we found that the transmission of HIV-1 mediated by DC-SIGN to the ICAM-3-positive GHOST/R5/ICAM-3 cells was slightly lower than that to ICAM-3-negative GHOST/R5 cells (Fig. 6B). In summary, no direct role for ICAM-3 in the DC-SIGN-mediated transmission of HIV-1 is apparent. The fact that different nonhematopoietic target cells can be used in HIV-1 transmission from DC-SIGN-expressing donor cells suggests that the effect of DC-SIGN is virus specific or restricted to the HIV-1 receptor molecules.
A recent study (2) mapped the determinants recognized by a panel of 16 MAbs raised against recombinant DC-SIGN to the repeat region, the lectin-binding domain, and the extreme C terminus of DC-SIGN. Although all of the MAbs bound to DC-SIGN on the surface of cells, none of the MAbs potently inhibited binding of soluble ICAM-3 to DC-SIGN, nor did any of the MAbs effectively block virus transmission. The DC-SIGN MAbs used in this study were developed against cell surface-expressed DC-SIGN and were competent in blocking ICAM-3 binding and neutralizing HIV-1 capture and transmission, suggesting that the HIV-1 Env and ICAM-3 binding sites are conformational. Three of these MAbs that recognized Rhesus macaque DC-SIGN also blocked HIV-1 and SIV transmission mediated by Rhesus macaque DC-SIGN (33). Thus, these MAbs may be useful to test the role of DC-SIGN in primate lentivirus transmission in in vivo models.
In summary, we have characterized a panel of seven MAbs reactive to DC-SIGN family molecules, some of which recognize primary human DC. Examination of cytokine-treated monocytes indicates that these MAbs will be useful in characterizing DC development. These studies also suggest that DC-SIGN may contribute to viral pathogenesis in other cell types, given the ability to induce DC-SIGN expression in human monocytes. The DC-SIGN-reactive MAbs compete with ICAM-3 for specific adhesion to DC-SIGN and inhibit R5- and X4-tropic HIV-1 transmission to T cells, suggesting that the DC-SIGN-MAb interaction sites may be useful as targets in antiviral therapy. Consistent with our studies using Rhesus macaque DC, less-efficient MAb neutralization of human DC-mediated HIV-1 transmission indicated that these cells are also capable of transmitting HIV-1 to target cells in a DC-SIGN-independent manner. Finally, although interactions between DC-SIGN and ICAM-3 may be important in initiating DC and CD4+ T-cell contact, they appear to be neither essential nor contributory in the cell-to-cell transmission of HIV-1 via DC-SIGN.
Acknowledgments
We thank Chad Borchert, Monica Tsang, and R&D Systems for help in developing DC-SIGN monoclonal antibodies, Douglas Kwon and Dan Littman for providing THP-1/DC-SIGN cells, and Arman Bashirova and Mary Carrington for sharing reagents and ideas. We thank Zandrea Ambrose, Anne Arthur, Jörg Baumann, and Vinay Pathak for helpful comments on the manuscript. We also thank members of the KewalRamani lab for scientific discussions.
Funding for this research was provided by the National Cancer Institute's intramural Center for Cancer Research, which supports the HIV Drug Resistance Program. D.U. was supported by NIH grant RO1-AI42284.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
REFERENCES
- 1.Baribaud, F., S. Pöhlmann, and R. W. Doms. 2001. The role of DC-SIGN and DC-SIGNR in HIV and SIV attachment, infection, and transmission. Virology 286**:**1-6. [DOI] [PubMed] [Google Scholar]
- 2.Baribaud, F., S. Pöhlmann, T. Sparwasser, M. T. Kimata, Y. K. Choi, B. S. Haggarty, N. Ahmad, T. Macfarlan, T. G. Edwards, G. J. Leslie, J. Arnason, T. A. Reinhart, J. T. Kimata, D. R. Littman, J. A. Hoxie, and R. W. Doms. 2001. Functional and antigenic characterization of human, rhesus macaque, pigtailed macaque, and murine DC-SIGN. J. Virol. 75**:**10281-10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashirova, A. A., T. B. Geijtenbeek, G. C. van Duijnhoven, S. J. van Vliet, J. B. Eilering, M. P. Martin, L. Wu, T. D. Martin, N. Viebig, P. A. Knolle, V. N. KewalRamani, Y. van Kooyk, and M. Carrington. 2001. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 193**:**671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257**:**383-387. [DOI] [PubMed] [Google Scholar]
- 5.Cameron, P. U., M. G. Lowe, S. M. Crowe, U. O'Doherty, M. Pope, S. Gezelter, and R. M. Steinman. 1994. Susceptibility of dendritic cells to HIV-1 infection in vitro. J. Leukoc. Biol. 56**:**257-265. [DOI] [PubMed] [Google Scholar]
- 6.Caminschi, I., K. M. Lucas, M. A. O'Keeffe, H. Hochrein, Y. Laabi, T. C. Brodnicki, A. M. Lew, K. Shortman, and M. D. Wright. 2001. Molecular cloning of a C-type lectin superfamily protein differentially expressed by CD8α− splenic dendritic cells. Mol. Immunol. 38**:**365-373. [DOI] [PubMed] [Google Scholar]
- 7.Cecilia, D., V. N. KewalRamani, J. O'Leary, B. Volsky, P. Nyambi, S. Burda, S. Xu, D. R. Littman, and S. Zolla-Pazner. 1998. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J. Virol. 72**:**6988-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206**:**935-944. [DOI] [PubMed] [Google Scholar]
- 9.Curtis, B. M., S. Scharnowske, and A. J. Watson. 1992. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 89**:**8356-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feinberg, H., D. A. Mitchell, K. Drickamer, and W. I. Weis. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294**:**2163-2166. [DOI] [PubMed] [Google Scholar]
- 11.Frankel, S. S., R. M. Steinman, N. L. Michael, S. R. Kim, N. Bhardwaj, M. Pope, M. K. Louder, P. K. Ehrenberg, P. W. Parren, D. R. Burton, H. Katinger, T. C. VanCott, M. L. Robb, D. L. Birx, and J. R. Mascola. 1998. Neutralizing monoclonal antibodies block human immunodeficiency virus type 1 infection of dendritic cells and transmission to T cells. J. Virol. 72**:**9788-9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frankel, S. S., B. M. Wenig, A. P. Burke, P. Mannan, L. D. Thompson, S. L. Abbondanzo, A. M. Nelson, M. Pope, and R. M. Steinman. 1996. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science 272**:**115-117. [DOI] [PubMed] [Google Scholar]
- 13.Geijtenbeek, T. B., G. Koopman, G. C. van Duijnhoven, S. J. van Vliet, A. C. van Schijndel, A. Engering, J. L. Heeney, and Y. van Kooyk. 2001. Rhesus macaque and chimpanzee DC-SIGN act as HIV/SIV gp120 trans-receptors, similar to human DC-SIGN. Immunol. Lett. 79**:**101-107. [DOI] [PubMed] [Google Scholar]
- 14.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances _trans_-infection of T cells. Cell 100**:**587-597. [DOI] [PubMed] [Google Scholar]
- 15.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100**:**575-585. [DOI] [PubMed] [Google Scholar]
- 16.Geijtenbeek, T. B., Y. van Kooyk, S. J. van Vliet, M. H. Renes, R. A. Raymakers, and C. G. Figdor. 1999. High frequency of adhesion defects in B-lineage acute lymphoblastic leukemia. Blood 94**:**754-764. [PubMed] [Google Scholar]
- 17.Granelli-Piperno, A., E. Delgado, V. Finkel, W. Paxton, and R. M. Steinman. 1998. Immature dendritic cells selectively replicate macrophage-tropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72**:**2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granelli-Piperno, A., V. Finkel, E. Delgado, and R. M. Steinman. 1999. Virus replication begins in dendritic cells during the transmission of HIV-1 from mature dendritic cells to T cells. Curr. Biol. 9**:**21-29. [DOI] [PubMed] [Google Scholar]
- 19.Granelli-Piperno, A., B. Moser, M. Pope, D. Chen, Y. Wei, F. Isdell, U. O'Doherty, W. Paxton, R. Koup, S. Mojsov, N. Bhardwaj, I. Clark-Lewis, M. Baggiolini, and R. M. Steinman. 1996. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J. Exp. Med. 184**:**2433-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hioe, C. E., L. Bastiani, J. E. Hildreth, and S. Zolla-Pazner. 1998. Role of cellular adhesion molecules in HIV type 1 infection and their impact on virus neutralization. AIDS Res. Hum. Retroviruses 14(Suppl. 3)**:**S247-S254. [PubMed] [Google Scholar]
- 21.Hioe, C. E., P. C. Chien, Jr., C. Lu, T. A. Springer, X. H. Wang, J. Bandres, and M. Tuen. 2001. LFA-1 expression on target cells promotes human immunodeficiency virus type 1 infection and transmission. J. Virol. 75**:**1077-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, B., G. Leslie, E. Soilleux, U. O'Doherty, S. Baik, E. Levroney, K. Flummerfelt, W. Swiggard, N. Coleman, M. Malim, and R. W. Doms. 2001. cis expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J. Virol. 75**:**12028-12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelms, K., A. D. Keegan, J. Zamorano, J. J. Ryan, and W. E. Paul. 1999. The IL-4 receptor: signaling mechanisms and biologic functions. Annu. Rev. Immunol. 17**:**701-738. [DOI] [PubMed] [Google Scholar]
- 24.Onishi, M., S. Kinoshita, Y. Morikawa, A. Shibuya, J. Phillips, L. L. Lanier, D. M. Gorman, G. P. Nolan, A. Miyajima, and T. Kitamura. 1996. Applications of retrovirus-mediated expression cloning. Exp. Hematol. 24**:**324-329. [PubMed] [Google Scholar]
- 25.Park, C. G., K. Takahara, E. Umemoto, Y. Yashima, K. Matsubara, Y. Matsuda, B. E. Clausen, K. Inaba, and R. M. Steinman. 2001. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int. Immunol. 13**:**1283-1290. [DOI] [PubMed] [Google Scholar]
- 26.Pöhlmann, S., F. Baribaud, B. Lee, G. J. Leslie, M. D. Sanchez, K. Hiebenthal-Millow, J. Munch, F. Kirchhoff, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J. Virol. 75**:**4664-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pöhlmann, S., E. J. Soilleux, F. Baribaud, G. J. Leslie, L. S. Morris, J. Trowsdale, B. Lee, N. Coleman, and R. W. Doms. 2001. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc. Natl. Acad. Sci. USA 98**:**2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rengarajan, J., S. J. Szabo, and L. H. Glimcher. 2000. Transcriptional regulation of Th1/Th2 polarization. Immunol. Today 21**:**479-483. [DOI] [PubMed] [Google Scholar]
- 29.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179**:**1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soilleux, E. J., R. Barten, and J. Trowsdale. 2000. DC-SIGN; a related gene, DC-SIGNR; and CD23 form a cluster on 19p13. J. Immunol. 165**:**2937-2942. [DOI] [PubMed] [Google Scholar]
- 31.Steinman, R. M., K. Inaba, S. Turley, P. Pierre, and I. Mellman. 1999. Antigen capture, processing, and presentation by dendritic cells: recent cell biological studies. Hum. Immunol. 60**:**562-567. [DOI] [PubMed] [Google Scholar]
- 32.Tsunetsugu-Yokota, Y., S. Yasuda, A. Sugimoto, T. Yagi, M. Azuma, H. Yagita, K. Akagawa, and T. Takemori. 1997. Efficient virus transmission from dendritic cells to CD4+ T cells in response to antigen depends on close contact through adhesion molecules. Virology 239**:**259-268. [DOI] [PubMed] [Google Scholar]
- 33.Wu, L., A. A. Bashirova, T. D. Martin, L. Villamide, E. Mehlhop, A. O. Chertov, D. Unutmaz, M. Pope, M. Carrington, and V. N. KewalRamani. 2002. Rhesus macaque dendritic cells efficiently transmit primate lentiviruses independently of DC-SIGN. Proc. Natl. Acad. Sci. USA 99**:**1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]