Intranasal Vaccination with a Recombinant Vesicular Stomatitis Virus Expressing Cottontail Rabbit Papillomavirus L1 Protein Provides Complete Protection against Papillomavirus-Induced Disease (original) (raw)
Abstract
Immunizations with live recombinant vesicular stomatitis viruses (rVSV) expressing foreign viral proteins have successfully protected animals from challenges with several heterologous viruses. We developed an rVSV expressing the major capsid protein (L1) of cottontail rabbit papillomavirus (CRPV) and tested the efficacy of protection following CRPV challenge. An rVSV expressing L1 of CRPV (VSV-L1) was characterized for the protective ability afforded by intranasal, intradermal, or intramuscular vaccination in rabbits subsequently challenged with CRPV. Protein expression of L1 in VSV-L1 was confirmed by radioimmunoprecipitation assays. Nuclear localization of L1 was demonstrated by indirect immunofluorescence assays. Immunized rabbits elicited significant VSV neutralization and VLP-L1 enzyme-linked immunosorbent assay titers. VSV-L1 vaccination was not associated with weight loss or any other adverse clinical signs in the rabbit model. VSV shedding in nasal secretions occurred in some rabbits, peaking at 4 to 6 days after intranasal vaccination, with no further shedding after day 6. Specific humoral immunity to the L1 protein was consistently seen after a single VSV-L1 vaccination when administered through an intradermal or intramuscular route or after a boost via the intranasal route. Rabbits were completely protected from CRPV-induced papillomas after VSV-L1 vaccination and boost given intranasally or intramuscularly. Vaccination with VSV-L1 is a novel approach to prevent papillomavirus-induced disease and demonstrates a potential strategy for developing a human papillomavirus vaccine that can be given without injection.
High-risk human papillomavirus (HPV) is the etiologic agent associated with over 90% of cervical cancer cases (41). Human papillomaviruses comprise more than 80 distinct types, some of which are sexually transmitted and predominantly infect genital skin and mucosa. Most genital warts are caused by sexually transmitted HPV types 6 and 11, whereas types 16 and 18 are most frequently associated with cervical cancer (2). HPV-associated malignancies are leading causes of cancer-related deaths in the world, and among women, cervical cancer is second only to breast cancer in incidence and mortality (23). In developing nations, cervical cancer is, in fact, the leading cause of death among women between the ages of 35 and 45 (40). More than 500,000 new cases of cervical cancer are reported worldwide each year. HPVs are also associated with penile, vulvar, anal, respiratory, and cutaneous neoplasms (24, 40). Unfortunately, current therapies for premalignant neoplasms associated with HPV infection are inadequate (2, 7). The cost burden for screening for HPV-induced cervical neoplasia exceeds 5 billion dollars per year in the United States alone (D. R. Lowy, unpublished data).
Prophylactic vaccination has been the most effective public health measure to reduce morbidity and mortality associated with viral infections. Since HPV infections cause cervical neoplasia, vaccines targeting HPV antigens should be effective at preventing or treating HPV-associated neoplasia. zur Hausen postulated that HPV stimulation of the immune system was critical to control of cervical neoplasia (41), and in fact, immune responses to HPV are likely to control susceptibility to infection, the severity of disease, remission, and the potential for oncogenicity (12). Further evidence is given in cases where immunosuppression associated with organ transplantation, pregnancy, old age, or human immunodeficiency virus infection increases the prevalence of HPV infection and cervical neoplasia (32). Unfortunately, current approaches to HPV vaccines fail to induce long-lasting immune system recognition of the virus. Since HPV infects and replicates in mucosal membranes, a vaccine with specific mucosal tissue tropism that can generate mucosal and systemic immunity would be advantageous.
The cottontail rabbit papillomavirus (CRPV) rabbit model has several advantages for HPV research. CRPV and the HPV genomes share significant sequence homology, and their respective genes encode proteins with similar functions (11). CRPV infection follows a highly predictable course of disease (4, 5, 17, 34). Infection of the epidermis by CRPV via scarification of rabbits results in the formation of multiple solitary cutaneous papillomas within 3 to 4 weeks of inoculation. These papillomas form at the sites of inoculation and develop into confluent growths a few weeks later. Malignant progression proceeds through a series of clinical and histological stages, as in HPV-associated disease (23, 37). Spontaneous regression of CRPV papillomas occurs in less than 10% of infected rabbits. Over time, papillomas progress to squamous cell carcinomas in 60 to 75% of rabbits. The prevalence of papilloma induction is virus dose dependent (5). Papillomas can be induced at multiple sites on a given animal, where they remain localized to the site of inoculation. This allows repeated inspection and biopsy of many papillomas on a single rabbit and provides built-in controls for host genotype.
L1, a 55-kDa protein, is the major viral capsid protein, constituting 90 to 95% of the papillomavirus virion. L1 is both the primary attachment protein for viral entry and the major target for neutralizing antibodies. L1 elicits strong type-specific neutralizing antibody responses that protect against subsequent infection in vaccinated animals, which identifies this protein as the primary candidate vaccine target for prophylaxis (31). Systemic vaccination of dogs by using the canine oral papillomavirus L1 protein protected against challenge with canine oral papillomavirus (1, 36). In the rabbit, immunization with bacterial fusion proteins of L1 as well as virus-like particles (VLPs) of CRPV protected against papilloma formation (6, 8, 9, 15, 21, 22). Immunization with L1 fusion proteins requires very large quantities of nondenatured protein (250 μg) with adjuvant, followed by several booster injections (21, 22). Nondenatured L1 VLPs, given with or without adjuvant and followed by two booster injections (50 μg of antigen each), also provided significant protection against papilloma formation (6, 8). Although HPV-VLP vaccines have entered early clinical trials, it is not clear that they will induce adequate and persistent L1-specific immunity in people, since VLPs do not replicate in vivo.
Live viral vaccines have traditionally offered the most effective protection against viral infections. Such vaccines induce strong cellular and humoral immune responses due to the intracellular synthesis of specific antigens at high levels over a prolonged period. However, achieving sufficient protein expression without vector pathogenicity is a major challenge for live viral vaccines. In the last few years, Rose and colleagues have pioneered the use of vesicular stomatitis virus (VSV) as an expression and vaccine vector (3, 18, 19, 26-28, 30).
VSV is a nonsegmented, negative-strand RNA virus with an 11-kb genome that encodes five major proteins: nucleocapsid (N), phosphoprotein (P), matrix (M), glycoprotein (G), and an RNA-dependent RNA polymerase (L). Expression of each protein occurs from a separate mRNA, and these mRNAs are transcribed sequentially in the cytoplasm (28, 29). No upward limit to the amount of foreign sequence inserted in the VSV genome has been identified. At least two additional transcription units, totaling 4.5 kb, can be added to and successfully expressed from the VSV genome (18).
Recombinant VSVs (rVSVs) expressing foreign viral proteins are considered good vaccine candidates, because VSV elicits strong humoral and cellular immune responses. Additionally, VSV can infect through mucosal surfaces and elicit strong systemic immunity and possibly local mucosal immunity (26). The potential for generating mucosal immunity is a feature that is particularly attractive for an HPV vaccine, since the site of infection and replication is usually genital mucosal membranes. VSV recombinants are attenuated relative to wild-type VSV (27), and even the wild type has only negligible pathogenicity in humans who have been infected (33). Furthermore, very few people have been infected with VSV, which limits the prevalence of preexposure immunity in the general population. The practical advantages of using VSV vectors are many. The virus grows to very high titers (5 × 109 PFU/ml) and can be propagated in almost all mammalian cells, as well as in many other cell types. It can be assayed in an overnight plaque assay and is easily prepared in milligram quantities. Immunization of rabbits with rVSVs expressing the major capsid protein of CRPV (VSV-L1) may provide a model for noninjectable prophylactic vaccination and prevention of papillomavirus-induced disease.
MATERIALS AND METHODS
Viruses and inocula.
rVSVs were grown and titers were determined as previously described for rVSV-hemagglutinin (26). Plaque-purified recombinant VSV-L1 was grown and titers were determined on baby hamster kidney (BHK) cells (BHK-21; American Type Culture Collection). Recombinants were thawed and diluted with Dulbecco's modified Eagle medium (DMEM) (with l-glutamine, sodium pyruvate, and high levels of glucose and bicarbonate [3.7 g/liter]) (product no. 56499; JRH Biosciences, Lenexa, Kans.) to appropriate titers immediately prior to inoculation. For virus booster injections in experiment 2, VSV-L1 was semipurified through 10% sucrose by centrifugation at 38,000 rpm for 1 h at 4°C in a Beckman L8-70 M Ultracentrifuge, using an SW41 rotor. The pellet was resuspended in 400 μl of Tris-EDTA buffer, pH 7.6.
CRPV stock K216 was used for challenge (35). The CRPV was thawed and diluted immediately prior to challenge.
Generation of pVSV-CRPV-L1.
The L1 gene (1,510 bp) was amplified from the CRPV-pLAII clone (38) with the upstream primer 5′-GCGCTCGAGATCATGGCAGTGTGGCTGTCTACG-3′ and the downstream primer 5′-CCCAGCGCGGCCGCGCTAGCTTAAGTACGTCTCTTGCG-3′ to introduce restriction endonuclease sites _Xho_I and _Nhe_I, facilitating directional cloning into the full-length VSV plasmid (pVSVMXA2XN2). The underlined sequences correspond to L1 open reading frame start and stop codons, respectively. The full-length VSV plasmid and L1 PCR products were cleaved at the _Xho_I and _Nhe_I cloning sites by restriction endonuclease digestions. The L1 fragment was ligated into the _Xho_I and _Nhe_I sites of VSV (between the genes encoding the G and L proteins with T4 DNA ligase).
VSV-L1 was recovered on BHK-21 cells (American Type Culture Collection) as described previously (19). Briefly, BHK cells were infected with recombinant vaccinia virus vTF7-3 expressing T7 RNA polymerase (10) at a multiplicity of infection of 10 and incubated for 1 h in DMEM containing 5% fetal bovine serum and 100 U of penicillin-streptomycin (maintenance medium; Life Technologies, Gibco-BRL, Grand Island, N.Y.)/ml. Full-length pVSV-CRPV-L1 and support plasmids (pBS-NT0, pBS-PT0, and pBS-LT0), all under the control of T7 promoters, were transfected into vaccinia virus-infected cells and incubated for 4 to 5 h in serum-free DMEM. The medium was then replaced with maintenance medium, and the cultures were incubated for 2 days. The supernatant was collected, filtered through a 0.2-μm-pore-diameter sterile filter to remove the remaining vaccinia virus, and passaged onto fresh BHK-21 cells. The medium was collected when a cytoplasmic effect (CPE) was noticed (∼2 days later) and filtered again through a 0.1-μm-pore-diameter sterile filter.
Indirect immunofluorescent assays.
BHK cells were seeded onto glass coverslips at 5 × 104 cells/3.5-cm2 plate. Cells were infected with supernatants from VSV recovery assays (1 ml/plate) the following day. Cells were incubated at 37°C for 4 to 16 h, washed with phosphate-buffered saline (PBS), and fixed in 3% paraformaldehyde for 20 min at room temperature and washed twice with PBS-glycine (10 mM). Coverslips were removed and incubated at 37°C for 30 to 45 min with primary antibody (mouse monoclonal antibodies I1 and I14 directed to VSV-G protein [gift from Douglas Lyles, Department of Microbiology and Immunology, Wake Forest University School of Medicine, Winston-Salem, North Carolina] or polyclonal rabbit sera specific for CRPV-L1 protein [35]) at 1:100 dilutions in PBS containing 10 mM glycine and 5 mg of bovine serum albumin (BSA)/ml (PBS-glycine-BSA). After incubation with primary antibody, coverslips were rinsed twice in PBS-glycine, incubated at 37°C for 30 to 45 min with secondary antibody (fluorescein isothiocyanate-conjugated goat anti-mouse [Jackson ImmunoResearch, West Grove, Pa.] or Texas red-conjugated goat anti-rabbit [Jackson ImmunoResearch]) at 1:50 in PBS-glycine-BSA. After incubation with secondary antibody, coverslips were rinsed twice in PBS-glycine and mounted in a glycerol-0.1 M _n_-propylgallate solution on glass slides, cell side down. Cells were examined using a Nikon Microphot-FX fluorescent microscope with a 40× Planapochromat objective.
Radiolabeled immunoprecipitation assays.
BHK cells were infected with rVSVs (multiplicity of infection, 5 to 10) and incubated at 37°C in maintenance medium for 1 h. The medium was aspirated and replaced with 1 ml of maintenance medium, and the plates were incubated for an additional 3 h. The cells were washed twice with PBS, and 1 ml of labeling medium (15% DMEM, 80% DMEM minus methionine, 100 μCi of 35S-Translabel [Easy Tag EXPRESS protein labeling mix, product no. NEG772; NEN Life Sciences, Boston, Mass.], 5% fetal bovine serum) was added to each plate. For radiolabeled cell extracts, cells were incubated for 1 h, washed twice in PBS, and lysed in 500 μl of ice-cold sodium dodecyl sulfate (SDS) lysis buffer (50 mM Tris [pH 8.0], 0.5% SDS, 1 mM dithiothreitol) for 20 to 30 min on ice. Lysates were heated at 100°C for 2 min before adding 4 volumes of radioimmunoprecipitation assay (RIPA) buffer (1% NP-40, 1% deoxycholate, 0.1% SDS, 150 mM NaCl, 10 mM Tris-HCl [pH 7.4]). Lysates were vortexed vigorously and transferred to cold 1.5-ml Eppendorf tubes, and debris was pelleted by centrifugation at 4°C. Supernatants were transferred to fresh, prechilled 1.5-ml Eppendorf tubes and stored at −20°C.
Primary antibody to L1 was added to 200 to 300 μl of radiolabeled cell extracts at a dilution of 1:100, and SDS was added to achieve a 0.2% final concentration. Antibody-extract solutions were incubated at 37°C for 45 min. Samples were precipitated by adding 30 μl of fixed Staphylococcus aureus (Pansorbin; Calbiochem) and incubating at 37°C for 30 min. Precipitates were pelleted by centrifugation and washed in 1.5 ml of RIPA buffer three times and pelleted again. Pellets were resuspended in 30 to 40 μl of sample buffer containing 2-mercaptoethanol, boiled for 3 to 5 min, and pelleted again. Supernatants were carefully removed and loaded onto SDS-10% polyacrylamide gel electrophoresis (SDS-10% PAGE) gels.
ELISA.
The enzyme-linked immunosorbent assay (ELISA) was used to determine specific serum antibody titers to CRPV VLPs (prepared in a baculovirus expression system and composed of CRPV L1 and L2 proteins; VLP-L1). The association between specific serum L1 antibodies reacting to VLP-L1 antigen and CRPV neutralization was previously demonstrated (35). Ninety-six-well flat-bottom polystyrene microtiter plates (Nunc MaxiSorp, Roskilde, Denmark) were coated by adding 50 ng of either VLP-L1 or green fluorescent protein (GFP; Clontech Laboratories Inc., Palo Alto, Calif.) in 100 μl of PBS/well. After incubation overnight at 4°C, plates were washed with PBS-0.5% Tween 20 (wash buffer) and blocked with 3% gelatin-PBS for 1 h at 37 °C. Twofold titrations of dilutions of rabbit sera in a final volume of 100 μl of 0.5% BSA-PBS were added per well, and the plates were incubated at 37°C for 1 h and then rinsed with wash buffer. Subsequently, 100 μl of 1:10,000 polyvalent horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin (Biosource, Camarillo, Calif.) was added to each well, and each plate was then incubated for 1 h at 37°C. The plates were washed, and the assay was developed by adding 100 μl of 3,3′,5,5′tetramethylbenzidine (TMB) peroxidase substrate (Kirkegaard & Perry Labs Inc., Gaithersburg, Md.) to each well. Reactions were terminated by the addition of 100 μl of 1 N HCl, and absorbencies were measured in a Benchmark Microplate Reader (Bio-Rad Laboratories, Hercules, Calif.), using a wavelength of 415 nm. Control values were subtracted from those for experimental rabbits, and an absorbance value of 0.2 was set as the cutoff for positive VLP-L1 titers.
Immunization of rabbits.
All animal procedures were approved by the Yale University Animal Care and Use Committee. Two-kilogram, specific-pathogen-free, random-bred New Zealand White rabbits (Millbrook Farm Inc., Concord, Mass.) were singly housed in an isolated room upon arrival. Rabbits were acclimated for 1 week before the study began. Rabbits were sedated with 1 mg of acepromazine (Boehringer Ingelheim Vetmedica, Inc., St. Joseph, Mo.)/kg of body weight subcutaneously and identified by permanent tattoo or indelible ink on the ear. Sera were collected on the day of immunization and at various time points postimmunization.
For experiment 1, VSV-L1, VSV-GFP (5 × 105 PFU/rabbit in 200 μl of DMEM), or medium was administered intranasally. Delayed-type hypersensitivity was measured in VSV-L1-vaccinated rabbits by introducing 10 μg of denatured L1 protein in 3 M urea [purified with the pET-20b+ expression system (Novagen, Madison, Wis.) using BL21(DE3)] intradermally 7 weeks after primary VSV-L1 immunization.
Rabbits in experiment 2 were given VSV-L1 inocula (5 × 105 PFU/rabbit in 200 μl of DMEM) through an intranasal, intradermal, intramuscular, or intravaginal route. Eight weeks after primary immunization, rabbits received a high-dose booster injection of homologous VSV-L1 (1.1 × 107 PFU/rabbit in 150 μl of DMEM) given by the same route. Rabbits receiving intravaginal VSV-L1 did not receive booster immunizations. All rabbits were observed daily for adverse clinical signs, inappetence, or diarrhea.
Recovery of viruses from nasal swabs.
For the rabbits in experiment 1, nasal swabs were collected postimmunization on days 1 to 14, using sterile cotton applicators wetted with serum-free DMEM, and transferred to tubes containing 1.5 ml of DMEM-penicillin-streptomycin. Samples were stored at 4°C. To detect recoverable virus from initial infections with recombinant viruses, BHK cells (∼80% confluent in 6-well plates) were infected with 200 μl (per well) of 10-fold serial dilutions of medium from nasal swabs within 24 h of collection. Cells were incubated for 1 h at room temperature and washed in PBS. Two milliliters of maintenance medium was added to each well, and the plates were incubated at 37°C and observed daily for 2 to 3 days for virus-associated CPE. To confirm the identity of recovered viruses, supernatants were collected from cells positive for viral CPE and screened for GFP expression by direct immunofluorescence or for L1 expression by indirect immunofluorescence assay as described above.
VSV neutralization assay.
Sera were heat inactivated at 56°C for 30 to 60 min and diluted with PBS in serial twofold dilutions in a 96-well plate. Generally, 25 μl of serum was mixed with 75 μl of PBS, and 50 μl of the resulting mixture was then transferred for serial dilutions. Dilutions were incubated in the presence of 200 PFU of wild-type rVSV (rwtVSV) at 37°C for 30 to 45 min. Approximately 500 BHK cells were added to each well, and plates were incubated at 37°C with 5% CO2 for 2 days. In each assay, sera were diluted and analyzed in duplicate. Neutralization titers were determined by total inhibition of virus CPE on cells.
CRPV challenge.
Rabbits were challenged with CRPV 10 to 13 weeks after the initial VSV-L1 immunization as previously described (25). Three unvaccinated rabbits were also challenged for each experiment. Rabbits were sedated with 2 to 3 mg of acepromazine/kg, given subcutaneously. An area of fur 4 in. wide by 6 in. long was clipped on the right flank. CRPV stock was diluted in PBS to 1:50, 1:200, and 1:800 concentrations (for high-, moderate-, and low-challenge doses, respectively). Three sites per rabbit were infected with each dilution by applying 30 μl to the skin, followed by scarification. Papilloma formation was monitored weekly after challenge for 8 weeks. For individual rabbits, the mean number of days to papilloma formation was determined by averaging the sum of the numbers of days that each site (out of nine) was free of papillomas following CRPV challenge. Sites remaining papilloma negative for the entire study were counted as having been papilloma free for 56 days (8 weeks). Each papilloma was measured in three dimensions (length, width, and height). Levels of protection were determined by the following equation: [1 − (total papilloma volume of vaccinated rabbit/average papilloma volume of control rabbits)] × 100.
RESULTS
Expression of CRPV-L1 in rVSV.
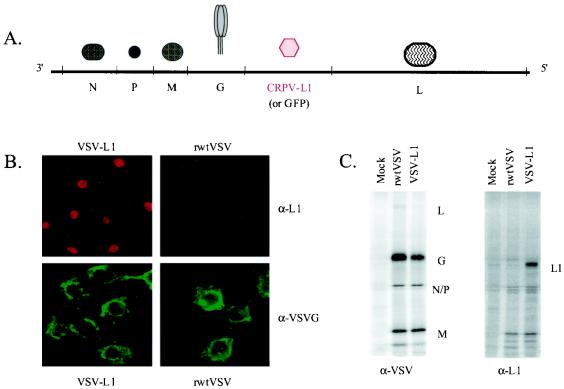
In order to determine the potential of a VSV-based vaccine for preventing papillomavirus-induced disease, we constructed and recovered an rVSV encoding the CRPV major capsid L1 gene between the G and L genes (Fig. 1A). Expression of the 55-kDa CRPV-L1 protein by VSV-L1 was characterized in vitro by indirect immunofluorescence assays and radioimmunoprecipitation assays. Using indirect immunofluorescence, surface and cytoplasmic expression of VSV-G protein was detected in both rwtVSV- and VSV-L1-infected cells (Fig. 1B). In contrast, L1 expression was clearly localized to the nucleus of VSV-L1-infected BHK cells. CRPV-L1 was not distinguishable in infected whole-cell extracts (data not shown). Because expression of L1 was lower than expected, radioimmunoprecipitation, followed by SDS-PAGE analysis, was used to concentrate the protein. A 55-kDa protein was present in VSV-CRPV-L1-infected whole-cell extracts but not in infected cell supernatant or rwtVSV- or mock-infected BHK cells (Fig. 1C).
FIG. 1.
(A) Schematic representation of the VSV-L1 genome. The genome of VSV-L1 is shown in 3′ → 5′ orientation, and the open reading frames of the major VSV proteins are indicated. The CRPV-L1 gene was inserted between the VSV G and L genes. The GFP gene in the control VSV-GFP is also inserted between the VSV G and L genes. (B) Immunofluorescence of L1 expression from VSV-L1. VSV-G protein expression is seen in both VSV-L1- and rwtVSV-infected cells and is primarily localized at the plasma membrane (lower two panels). In contrast, L1 protein expression is highly localized to the nuclear compartment and is seen only in VSV-L1-infected cells (upper left panel). (C) Immunoprecipitation of L1 expression from VSV-L1. VSV proteins (L, G, N, P, and M) are present in both rwtVSV and VSV-L1 extracts (left panel). In contrast, L1 is present only in VSV-L1 extracts (right panel).
Low-dose intranasal rVSV immunization.
In an initial experiment to assess both the safety and efficacy of immunization with rVSVs, rabbits were either given single, low-dose, intranasal vaccinations with VSV-L1 or VSV-GFP or were inoculated with medium (unvaccinated). VSV shedding in nasal secretions, ranging from 0 to 3 days in duration, was detected in some rabbits throughout the first week following immunization. However, the duration of VSV shedding was an insensitive predictor of postvaccination serum VSV-neutralizing antibody titers and subsequent protection against CRPV challenge. Rabbits showed no adverse effects from vaccination. Positive serum VSV-neutralizing antibody titers (defined as >1:8) were seen in all VSV-GFP- and 4 of 5 VSV-L1-immunized rabbits (Table 1). However, the titers were significantly higher for rabbits receiving VSV-GFP than for those receiving VSV-L1 (P = 0.0316; Student's t test, with equal variances assumed). In tissue culture, VSV-GFP also showed increased replication kinetics (data not shown) compared to VSV-L1, suggesting that L1 protein production may slow viral replication.
TABLE 1.
VSV-neutralizing antibody responses
| Vaccination strategy | Vaccinea | Rabbit no. | Neutralizing antibody titer to VSV (relative to time of immuniation)d | ||||
|---|---|---|---|---|---|---|---|
| Primary (PFU, 5 × 105) | Booster (PFU, 1 × 107) | wk 3 | wk 5 | wk 7-8 | wk 10-13 | ||
| Expt 1 | VSV-GFP (i.n.) | 1 | 1:4,096 | 1:4,096 | 1:8,192 | 1:8,192 | |
| 2 | 1:1,024 | 1:2,048 | 1:2,048 | 1:2,048 | |||
| 3 | 1:2,048 | 1:4,096 | 1:4,096 | 1:16,384 | |||
| 4 | 1:4,096 | 1:1,024 | 1:4,096 | 1:2,048 | |||
| 5 | 1:1,024 | 1:1,024 | 1:2,048 | 1:2,048 | |||
| VSV-L1 (i.n.)b | 6 | 1:2,048 | 1:2,048 | 1:2,048 | 1:8,192 | ||
| 7c | — | — | — | — | |||
| 8 | 1:2,048 | 1:1,024 | 1:2,048 | 1:16,384 | |||
| 9 | 1:128 | 1:256 | 1:512 | 1:512 | |||
| 10 | 1:1,024 | 1:512 | 1:2,048 | 1:8,192 | |||
| Expt 2 | VSV-L1 (i.d.) | VSV-L1 (i.d.) | 1 | ND | 1:256 | 1:256 | 1:1,024 |
| 2 | ND | 1:512 | 1:1,024 | 1:4,096 | |||
| 3 | ND | 1:512 | 1:512 | 1:8,192 | |||
| VSV-L1 (i.n.) | VSV-L1 (i.n.) | 4 | ND | — | — | 1:1,024 | |
| 5 | ND | 1:128 | 1:128 | 1:2,048 | |||
| 6 | ND | 1:1,024 | 1:512 | 1:4,096 | |||
| VSV-L1 (i.m.) | VSV-L1 (i.m.) | 7 | ND | 1:256 | 1:128 | 1:8,192 | |
| 8 | ND | 1:256 | 1:256 | 1:8,192 | |||
| 9 | ND | 1:1,024 | 1:512 | 1:8,192 | |||
| VSV-L1 (i.vag.) | 10 | ND | — | — | — | ||
| 11 | ND | — | — | — | |||
| 12 | ND | — | — | — |
All rabbits vaccinated with VSV-GFP developed serum antibodies to GFP (data not shown) and 3 of 5 rabbits vaccinated with VSV-L1 developed high levels of serum antibodies to VLP-L1 (Table 2). To assess delayed hypersensitivity to L1 protein, rabbits vaccinated with VSV-L1 were given intradermal denatured L1 protein. Because of the presence of urea in the antigen, the results were inconclusive. However, serum VLP-L1 antibody titers following this procedure were not affected, remaining within a twofold dilution of previous titers.
TABLE 2.
Antibody responses to CRPV VLP-L1 detected by ELISA
| Vaccination strategy | Vaccinea | Rabbit no. | ELISA VLP-L1 antibody titer (relative to time of immunization)d | Level of protection (%)e | ||
|---|---|---|---|---|---|---|
| Primary (PFU, 5 × 105) | Booster (PFU, 1 × 107) | wk 7-8 | wk 10-13 | |||
| Expt 1 | VSV-L1 (i.n.)b | 6 | >1:12,800 | 1:6,400 | 100.0 | |
| 7c | — | — | 79.3 | |||
| 8 | >1:12,800 | 1:6,400 | 100.0 | |||
| 9 | — | — | 0.0 | |||
| 10 | 1:6,400 | 1:3,200 | 98.3 | |||
| Expt 2 | VSV-L1 (i.d.) | VSV-L1 (i.d.) | 1 | 1:6,400 | >1:12,800 | 100.0 |
| 2 | >1:12,800 | >1:12,800 | 99.4 | |||
| 3 | 1:1,600 | 1:6,400 | 100.0 | |||
| VSV-L1 (i.n.) | VSV-L1 (i.n.) | 4 | — | 1:6,400 | 100.0 | |
| 5 | — | 1:3,200 | 100.0 | |||
| 6 | >1:12,800 | >1:12,800 | 100.0 | |||
| VSV-L1 (i.m.) | VSV-L1 (i.m.) | 7 | 1:3,200 | 1:3,200 | 100.0 | |
| 8 | 1:3,200 | >1:12,800 | 100.0 | |||
| 9 | >1:12,800 | >1:12,800 | 100.0 | |||
| VSV-L1 (i.vag.) | 10 | — | — | NA | ||
| 11 | — | — | NA | |||
| 12 | — | — | NA |
Single, low-dose intranasal VSV-L1 vaccination conferred significant, although incomplete, protection against CRPV-induced papilloma formation (Fig. 2). Papillomas in one VSV-GFP-immunized rabbit regressed, consistent with the rate of spontaneous regression observed in CRPV infections of outbred rabbits (14, 17). Immunologic responses from vaccination with VSV-GFP did not alter responses to CRPV challenge. Significant variability in VSV-neutralizing and VLP-L1 antibody titers among rabbits was observed with single-dose intranasal VSV-L1 vaccination. In a separate experiment, treating rabbits with a boost of low-dose (5 × 105 PFU) intranasal VSV-L1 was ineffective at correcting the variability in titers (data not shown). There was no improvement in either VSV or VLP-L1 titers if primary VSV-neutralizing antibodies were detected. Therefore, we sought to determine the influence of vaccine administration route on subsequent titer variability, using low-dose VSV-L1 vaccinations followed by high-dose (107 PFU) boost.
FIG. 2.
Protection from CRPV challenge in experiment 1. Average percentages of papilloma-free sites and average papilloma volumes (natural log) after CRPV challenge were determined in rabbits immunized once with intranasal VSV-L1 vaccine (gray bar and ▴), rabbits immunized once with intranasal VSV-GFP vaccine (diagonal line bar and ▪), and unimmunized control rabbits (black bar and ×). The results for high-challenge (A), moderate-challenge (B), and low-challenge (C) CRPV doses are shown. Error bars represent standard errors of the mean.
Response to VSV following vaccination by different routes (experiment 2).
For comparison of effectiveness of VSV-L1 vaccination by different routes, rabbits were given VSV-L1 immunizations and boosts through an intradermal, intranasal, or intramuscular route. A group of rabbits also received one dose of intravaginal VSV-L1 without a boost. A 20-fold increased concentration of virus was used for boosting, since in earlier experiments we had shown that boosting with a low dose of VSV-L1 was ineffective, presumably because of neutralization of the virus. No adverse clinical signs were observed in any rabbits following any inoculations. At 5 weeks following primary immunization, significant VSV-neutralizing antibody titers developed for all rabbits (6 of 6) immunized with VSV-L1 through an intradermal or intramuscular route, 2 of 3 rabbits immunized through the intranasal route, and 0 of 3 rabbits immunized through the intravaginal route (Table 1). Vaginal administration of the vaccine was technically challenging and resulted in inconsistent dosing. Because of these challenges and the failure of any rabbits in this group to seroconvert to VSV or L1, these rabbits did not receive a boost.
At 5 and 8 weeks following primary immunization, VSV-neutralizing antibody titers were not significantly different between rabbits receiving VSV-L1 through any of the routes (P > 0.38, analysis of variance [single factor]; α < 0.1). <0.1).>Homologous VSV vaccine boosts through the same route increased VSV-neutralizing antibody titers for all rabbits (intradermal route, P < 0.0919; intranasal route, _P_ < 0.0387; intramuscular route, _P_ < 0.0006 [Student's _t_ test; two samples paired for means]). Following booster immunizations, VSV-neutralizing antibody titers were significantly higher and most consistent in rabbits immunized through the intramuscular route (intramuscular route versus intradermal, _P_ < 0.0725; intramuscular versus intranasal, _P_ < 0.0015 [Student's _t_ test; two samples, equal variances assumed). No differences were seen between postbooster VSV-neutralizing antibody titers in rabbits immunized through the intranasal or intradermal route (_P_ > 0.2).
Immune responses to L1 induced by vaccination (experiment 2).
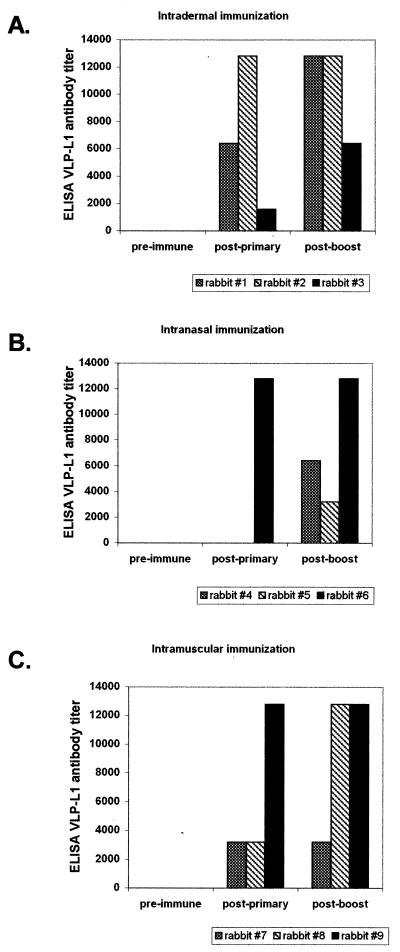
Following primary vaccination, L1-specific antibodies developed in all rabbits immunized with VSV-L1 through an intradermal (3 of 3) or intramuscular (3 of 3) route but in only 1 of 3 rabbits immunized through the intranasal route (Fig. 3). The two nonresponders (rabbits 4 and 5) also had the poorest VSV-neutralizing antibody response. Following a homologous VSV-L1 boost, all serum VLP-L1 titers either were equal or increased and all initially nonresponding rabbits seroconverted to high VLP-L1 titers (Table 2). Thus, there was considerable variability with the intranasal route of administration which was overcome with a boost of a high dose of homologous vaccine.
FIG. 3.
Antibody to VLP-L1 in rabbits in experiment 2. Rabbits were immunized through the intradermal (A), intranasal (B), or intramuscular (C) route, and antibody titers were determined by ELISA using VLP-L1 protein. Results are shown for rabbits tested prior to immunization (pre-immune), 8 weeks after the primary injection (post-primary), and 13 weeks after the primary injection (i.e., 5 weeks after the homologous VSV-L1 booster injection).
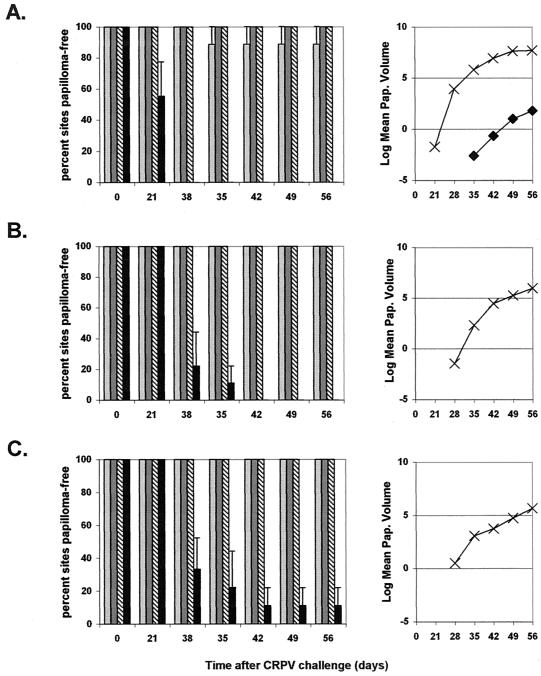
Protection from CRPV challenge (experiment 2).
All rabbits immunized with VSV-L1 through the intramuscular (3 of 3) or intranasal (3 of 3) route and 2 of 3 rabbits immunized through the intradermal route were completely protected against CRPV-induced papilloma formation (Fig. 4). However, 1 of 3 rabbits in the intradermal group developed papillomas at one challenge site (high-challenge dose). The time to papilloma onset was delayed, appearing 35 days after CRPV challenge in the immunized rabbit versus 29.7 ± 4.6 days (mean ± standard deviation) in the control rabbits (P < 0.0003 [Student's t test; equal variances assumed]). The number of papilloma-free sites was significantly greater in all VSV-L1-immunized groups, with 27 of 27 sites papilloma free for rabbits vaccinated with VSV-L1 through either the intranasal or intramuscular route and 26 of 27 sites papilloma free for rabbits vaccinated through the intradermal route versus 1 of 27 in the control group (Fig. 4). Total papilloma volume for the only rabbit immunized through intradermal VSV-L1 with papillomas was 18.4 mm3 postchallenge, compared with an average of 2,918 mm3 for control rabbits (P < 0.0680 [Student's t test; equal variances assumed]). From these data, we conclude that the immunization route and dosage level of the vaccine significantly influence responses to VSV-L1 vaccination. Complete protection from CRPV-induced papilloma formation was achievable with intramuscular or intranasal VSV-L1 vaccination. Our intranasal VSV-L1 vaccine is unique among current papillomavirus vaccine candidates, being the first noninjected means by which complete prevention of papilloma formation is possible.
FIG. 4.
Protection from CRPV challenge in experiment 2. Average percentages of papilloma-free sites and average papilloma volumes (natural log) are shown for rabbits immunized with VSV-L1 vaccine through intradermal (light gray bar and ♦), intranasal (dark gray bar and ▪), and intramuscular (diagonal line bar and ▴) routes and for unvaccinated control rabbits (black bar and ×). The results for high-challenge (A), moderate-challenge (B), and low-challenge (C) CRPV doses are shown. Error bars represent standard errors of the mean.
DISCUSSION
Vaccination of rabbits with a VSV recombinant expressing CRPV-L1 protein induced specific humoral immunity against CRPV-L1 protein and protected against subsequent papillomavirus challenge. Incomplete protection of rabbits was seen in an initial experiment using a single low intranasal dose, but complete protection was seen in a subsequent experiment in which rabbits were given boosts with a high dose. This dose-response observation can be inferred only with the intranasal route of administration, since single-dose vaccination was not assessed using the other administration routes.
Significantly higher VSV-neutralizing antibody titers were seen among rabbits responding to rVSVs expressing GFP versus L1. It is possible that L1 protein expression is inhibitory to VSV replication or is cytotoxic. Supporting such a hypothesis, VSV-L1 replicated to lower titers in tissue culture than did VSV-GFP (titers were reduced by 10- to 100-fold). The rVSV in vitro cellular L1 expression level was also low compared to the expression of other foreign proteins in the rVSV system (26, 27). This was demonstrated by the need to immunoprecipitate L1 protein from infected cellular extracts for protein detection, which is normally not necessary for VSV recombinants. The explanation for the low level of expression is not known but could be related to codon usage in CRPV-L1, since with bovine papillomavirus and HPV16-L1, codon substitutions for more common tRNAs dramatically increased expression (20, 39).
Studies of rVSV immunization in mice suggest that specific antibody titers to VSV and foreign antigens continue to rise over time without subsequent booster injections; however, the reason for this remains unclear (27). We also observed a gradual rise over 13 weeks in VSV neutralization titers in experiment 1, but not within shorter intervals. One possible explanation for the increase in titer is an increase in antibody affinity for VSV rather than an increase in antibody amount. Antibodies to VLP-L1 displayed peak titers 7 to 8 weeks following immunization and remained high at the time of challenge. In experiment 2, significant and acute rises in titers to VSV and VLP-L1 were seen after booster injections. The booster dose was increased 20-fold to help overcome immediate neutralization by preexisting antibodies of VSV generated in the primary vaccination. The administration of boosts of an equal dose of homologous virus was advantageous only for those rabbits failing to respond to primary vaccination. The duration of immunity afforded by immunization with VSV-L1 remains to be determined.
The route of immunization was an important variable in the responses that were generated to VSV-L1 in the rabbit model. Administration of VSV-L1 through intradermal and intramuscular routes provided significant antibody responses and protection against challenge. Intranasal administration provides an attractive method to optimize mucosal immunity, which is important for preventing cases of HPV, for which the primary site of infection is genital mucosa. In addition, a noninjectable method of delivery might facilitate vaccine administration and reduce overall costs, especially in developing nations, in which cervical cancer is the leading cause of death among women between the ages of 35 and 45 (40). Although a low vaccine dose given through the intranasal route is highly successful for mice, it is possible that a higher dose is necessary for rabbits, since the lower dose produced highly variable VSV- and VLP-L1 titers in those experiments. This variability may limit the use of this route as a one-dose immunization, although use of a higher titer or higher-level expression vector might overcome this problem. The success of boosting with high titer virus to overcome problems with initial intranasal vaccinations supports this idea. We attempted intravaginal immunization with VSV-L1, but technical problems with delivery and retention of inocula in experiment 2 made it impossible to evaluate the efficacy of immunization by that route.
The optimization of several factors in future studies may allow complete protection using a single dose. Such factors include increasing the level of L1 protein expression from rVSV, changing the location of L1 expression from intranuclear to intracytoplasmic, and/or changing the dose, schedule, and number of inoculations. It is possible that the nuclear localization of L1 limits its expression or presentation to immune surveillance for production of antibody. We could not detect L1 protein in infectious supernatants of VSV-L1-infected BHK cells (tested through immunoprecipitation assays and SDS-PAGE analysis). It is possible that L1 expressed in mammalian cells is not released from cells as VLPs, as it is in insect cells (16). Altering the sequence of L1 to allow release of L1 from cells might enhance L1 presentation to the immune system. Additionally, the expression levels of VSV proteins and foreign proteins expressed in VSV are increasingly attenuated according to the 3′ → 5′ orientation of the expression site, with the expression levels of the 3′-oriented sites least attenuated and those of the 5′-oriented sites most attenuated (27). Therefore, insertion of L1 into a site nearer the upstream N gene may improve protein expression and enhance the immune response to the L1 protein.
Live attenuated rVSV vectors induce very strong humoral and cellular immunity in vaccinated animals and provide lasting, protective immunity directed toward foreign viral antigens (13, 27, 30). The results of this report demonstrate the feasibility of expressing viral proteins from DNA viruses within the rVSV system and the potential of preventing papillomavirus-induced disease using a noninjected live intranasal VSV-L1 vaccine.
Acknowledgments
J. Reuter and A. Roberts contributed equally to the experimental data presented in this report.
A. Roberts was supported by a Cancer Research Institute fellowship. Work was funded in part by American Cancer Society IRG 58-012-42 and NIH grant R01AI24345.
We thank Mark Shlyankevich for technical assistance, Karl Haglund for editorial expertise, and John Schiller for thoughtful comments and review of the manuscript.
REFERENCES
- 1.Bell, J. A., J. P. Sundberg, S. J. Ghim, J. Newsome, and A. B. Jenson. 1994. Formalin-inactivated vaccine protects against mucosal papillomavirus infection: a canine model. Pathobiology 62**:**194-198. [DOI] [PubMed] [Google Scholar]
- 2.Beutner, K. R., and S. Tyring. 1997. Human papillomavirus and human disease. Am. J. Med. 102**:**9-15. [DOI] [PubMed] [Google Scholar]
- 3.Boritz, E., J. Gerlach, J. E. Johnson, and J. K. Rose. 1999. Replication-competent rhabdoviruses with human immunodeficiency virus type 1 coats and green fluorescent protein: entry by a pH-independent pathway. J. Virol. 73**:**6937-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandsma, J. L. 1994. Animal models for HPV vaccine development. Papillomavirus Rep. 5**:**105-111. [Online.] http://www.leeds.ac.uk/lmi/pvr/pvrmain.html. [Google Scholar]
- 5.Brandsma, J. L. 1994. Animal models of human papillomavirus-associated oncogenesis. Intervirology 37**:**189-200. [DOI] [PubMed] [Google Scholar]
- 6.Breitburd, F., R. Kirnbauer, N. L. Hubbert, B. Nonnenmacher, C. Trin-Dinh-Desmarquet, G. Orth, J. T. Schiller, and D. R. Lowy. 1995. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 69**:**3959-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Car, J., S. A. Khan, and J. M. Best. 1993. Towards vaccines against human papillomavirus type-16 genital infections. Vaccine 11**:**603-611. [DOI] [PubMed] [Google Scholar]
- 8.Christensen, N. D., C. A. Reed, N. M. Cladel, R. Han, and J. W. Kreider. 1996. Immunization with virus-like particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J. Virol. 70**:**960-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnelly, J. J., D. Martinez, K. U. Jansen, R. W. Ellis, D. L. Montgomery, and M. A. Liu. 1996. Protection against papillomavirus with a polynucleotide vaccine. J. Infect. Dis. 173**:**314-320. [DOI] [PubMed] [Google Scholar]
- 10.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83**:**8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gissmann, L., V. Diehl, H.-J. Schultz-Coulon, and H. zur Hausen. 1982. Molecular cloning and characterization of human papilloma virus DNA derived from a laryngeal papilloma. J. Virol. 44**:**393-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gissmann, L., I. Jochmus, I. Nindl, and M. Muller. 1993. Immune response to genital papillomavirus infections in women. Prospects for the development of a vaccine against cervical cancer. Ann. N. Y. Acad. Sci. 690**:**80-85. [DOI] [PubMed] [Google Scholar]
- 13.Haglund, K., I. Leiner, K. Kerksiek, L. Buonocore, E. Pamer, and J. K. Rose. 2002. Robust recall and long-term memory T-cell responses induced by prime-boost regimens with heterologous live viral vectors expressing human immunodeficiency virus type 1 Gag and Env proteins. J. Virol. 76**:**7506-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopfl, R. M., N. D. Christensen, M. G. Angell, and J. W. Kreider. 1993. Skin test to assess immunity against cottontail rabbit papillomavirus antigens in rabbits with progressing papillomas or after papilloma regression. J. Investig. Dermatol. 101**:**227-231. [DOI] [PubMed] [Google Scholar]
- 15.Jansen, K. U., M. Rosolowsky, L. D. Schultz, H. Z. Markus, J. C. Cook, J. J. Donnelly, D. Martinez, R. W. Ellis, and A. R. Shaw. 1995. Vaccination with yeast-expressed cottontail rabbit papillomavirus (CRPV) virus-like particles protects rabbits from CRPV-induced papilloma formation. Vaccine 13**:**1509-1514. [DOI] [PubMed] [Google Scholar]
- 16.Kirnbauer, R., F. Booy, N. Cheng, D. R. Lowy, and J. T. Schiller. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 89**:**12180-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreider, J. W., and G. L. Bartlett. 1985. Shope rabbit papilloma-carcinoma complex. A model system of HPV infections. Clin. Dermatol. 3**:**20-26. [DOI] [PubMed] [Google Scholar]
- 18.Kretzschmar, E., L. Buonocore, M. J. Schnell, and J. K. Rose. 1997. High-efficiency incorporation of functional influenza virus glycoproteins into recombinant vesicular stomatitis viruses. J. Virol. 71**:**5982-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawson, N. D., E. A. Stillman, M. A. Whitt, and J. K. Rose. 1995. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. USA 92**:**4477-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leder, C., J. A. Kleinschmidt, C. Wiethe, and M. Müller. 2001. Enhancement of capsid gene expression: preparing the human papillomavirus type 16 major structural gene L1 for DNA vaccination purposes J. Virol. 75**:**9201-9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, Y. L., L. A. Borenstein, R. Selvakumar, R. Ahmed, and F. O. Wettstein. 1992. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology 187**:**612-619. [DOI] [PubMed] [Google Scholar]
- 22.Lin, Y. L., L. A. Borenstein, R. Ahmed, and F. O. Wettstein. 1993. Cottontail rabbit papillomavirus L1-protein based vaccines: protection is achieved only with a full-length, nondenatured product. J. Virol. 67**:**4154-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz, N., and F. X. Bosch. 1992. HPV and cervical cancer: review of case-control and cohort studies, p. 251-261. In N. Munoz and F. X. Bosch (ed.), The epidemiology of human papillomavirus and cervical cancer. Scientific Publication No. 119. International Agency for Research on Cancer, Lyon, France.
- 24.Orth, G. 1987. Epidermodysplasia verruciformis, p. 199-243. In N. P. Salzman and P. M. Howley (ed.), The Papovaviridae, vol. 2. The papillomaviruses. Plenum, New York, N.Y.
- 25.Reuter, J. D., D. L. Gomez, J. H. Brandsma, J. K. Rose, and A. J. Roberts. 2001. Optimization of cottontail rabbit papilloma virus challenge technique. J. Virol. Methods 98**:**127-134. [DOI] [PubMed] [Google Scholar]
- 26.Roberts, A., E. Kretzschmar, A. S. Perkins, J. Forman, R. Price, L. Buonocore, Y. Kawaoka, and J. K. Rose. 1998. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J. Virol. 72**:**4704-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts, A., L. Buonocore, R. Price, J. Forman, and J. K. Rose. 1999. Attenuated vesicular stomatitis viruses as vaccine vectors. J. Virol. 73**:**3723-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose, J. K., and M. Schubert. 1987. Rhabdovirus genome and their products, p. 129-166. In R. R. Wagner (ed.), The rhabdoviruses. Plenum, New York, N.Y.
- 29.Rose, J. K., and M. A. Whitt. 2001. Rhabdoviridae: the viruses and their replication, p. 1221-1244. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott, Williams and Wilkins, New York, N.Y.
- 30.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106**:**539-549. [DOI] [PubMed] [Google Scholar]
- 31.Schiller, J. T. 1999. Papillomavirus-like particle vaccines for cervical cancer. Mol. Med. Today 5**:**209-215. [DOI] [PubMed] [Google Scholar]
- 32.Schneider, A., and L. A. Koutsky. 1992. Natural history and epidemiologic features of genital HPV infection, p. 25-52. In N. Munoz and F. X. Bosch (ed.), The epidemiology of human papillomavirus and cervical cancer. Scientific Publication No. 119. International Agency for Research on Cancer, Lyon, France.
- 33.Shah, K. V., and P. M. Howley. 1996. Papillomaviruses, p. 2077-2109. In B. N. Fields and D. M. Knipe (ed.), Virology. Raven Press, Ltd., New York, N.Y.
- 34.Shope, R. E. 1933. Infectious papillomatosis of rabbits. J. Exp. Med. 58**:**607-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundaram, P., R. E. Tigelaar, and J. L. Brandsma. 1997. Intracutaneous vaccination of rabbits with the cottontail rabbit papillomavirus (CRPV) L1 gene protects against virus challenge. Vaccine 15**:**664-671. (Erratum, **16:**655, 1998.) [DOI] [PubMed] [Google Scholar]
- 36.Suzich, J. A., S. J. Ghim, F. J. Palmer-Hill, W. I. White, J. K. Tamura, J. A. Bell, J. A. Newsome, A. B. Jenson, and R. Schlegel. 1995. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosa papillomas. Proc. Natl. Acad. Sci. USA 92**:**11553-11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Syverton, J. T. 1952. The pathogenesis of rabbit papilloma to carcinoma sequence. Ann. N. Y. Acad. Sci. 54**:**1126-1140. [DOI] [PubMed] [Google Scholar]
- 38.Wettstein, F. O., and J. G. Stevens. 1980. Distribution and state of viral nucleic acid in tumors induced by Shope papilloma virus. Cold Spring Harbor Conference. Cell Prolif. 7**:**301-307. [Google Scholar]
- 39.Zhou, J., W. J. Liu, S. W. Peng, X. Y. Sun, and I. Frazer. 1999. Papillomavirus capsid protein expression level depends on the match between codon usage and tRNA availability. J. Virol. 73**:**4972-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.zur Hausen, H. 1994. Molecular pathogenesis of cancer of the cervix and its causation by specific papillomavirus types. Curr. Top. Microbiol. Immunol. 186**:**131-156. [DOI] [PubMed] [Google Scholar]
- 41.zur Hausen, H., and E. M. de Villiers. 1994. Human papillomavirus. Annu. Rev. Microbiol. 48**:**427-447. [DOI] [PubMed] [Google Scholar]