Murine Cytomegalovirus m02 Gene Family Protects against Natural Killer Cell-Mediated Immune Surveillance (original) (raw)
Abstract
The murine cytomegalovirus m02 gene family encodes putative type I membrane glycoproteins named m02 through m16. A subset of these genes were fused to an epitope tag and cloned into an expression vector. In transfected and murine cytomegalovirus-infected cells, m02, m04, m05, m06, m07, m09, m10, and m12 localized to cytoplasmic structures near the nucleus, whereas m08 and m13 localized to a filamentous structure surrounding the nucleus. Substitution mutants lacking the m02 gene (SM_sub_m02) or the entire m02 gene family (SM_sub_m02-16) grew like their wild-type parent in cultured cells. However, whereas SM_sub_m02 was as pathogenic as the wild-type virus, SM_sub_m02-16 was markedly less virulent. SM_sub_m02-16 produced less infectious virus in most organs compared to wild-type virus in BALB/c and C57BL/6J mice, but it replicated to wild-type levels in the organs of immunodeficient γc/Rag2 mice, lacking multiple cell types including natural killer cells, and in C57BL/6J mice depleted of natural killer cells. These results argue that one or more members of the m02 gene family antagonize natural killer cell-mediated immune surveillance.
More than a quarter of the coding potential of the murine cytomegalovirus (MCMV) and human cytomegalovirus genomes is devoted to putative glycoproteins. As yet, the function of most of these proteins remains unexplored. The MCMV genome encodes two families of related glycoproteins, the m02 family at the left end and the m145 family at the right end of the conventional MCMV map (26). Human cytomegalovirus also codes for two glycoprotein families, RL11 and US6 (7). Although the gene families in the mouse and human viruses do not have significant sequence homology, their positions in their respective genomes are relatively well conserved.
The m02 gene family includes 15 putative membrane glycoproteins that exhibit modest sequence homology. The members of this family have common motifs, including hydrophobic regions at their amino and carboxy termini, which may correspond to a signal and an anchor sequence, respectively, as well as several N-linked glycosylation motifs. These sequence features suggest that m02 family members encode type I membrane glycoproteins that have a large extracellular region, a transmembrane domain, and a small cytoplasmic tail. The sequences of the m02 gene family members are more similar to each other than to other MCMV genes, but their homology is limited. A Blast search for homologs in the GenBank database did not identify significant matches for any of the m02 gene family members. Sequence alignments of the 15 members show that the genes can be clustered into three subgroups, m02 to m06, m07 to m10, and m11 to m16, based on the conservation of cysteine residues, and some motifs and repeats are found in each of these subgroups (26).
Two members of the m02 family have been shown to target major histocompatibility complex (MHC) class I molecules and interfere with their function. Cells stably expressing m06 were shown to almost completely lose cell surface expression of Lq class I molecules, which are targeted to the lysosome (27). The m04 protein forms a complex with MHC class I molecules, but instead of causing its retention or degradation, the complex migrates to the cell surface, perhaps to protect cells from natural killer (NK) cell killing (19).
Here we report our initial analysis of the m02 gene family. All m02 gene family members that were studied localized to cytoplasmic structures. The gene family is not required for MCMV growth in vitro. However, a mutant virus lacking the gene family, SM_sub_m02-16, exhibits reduced pathogenicity in infected mice. The m02 gene family was found to facilitate MCMV replication in vivo by controlling NK cell-mediated responses.
MATERIALS AND METHODS
Viruses and cells.
The Smith strain (33) of MCMV was obtained from the American Type Culture Collection (ATCC) (VR-1399), and plaque-purified derivatives were used in these studies. Virus stocks were produced and titered on the 10.1 mouse embryo fibroblast cell line (12), which was propagated in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum. Viral growth also was also examined in the IC21 mouse macrophage cell line (ATCC TIB-186), which was propagated in RPMI-1640 medium (ATCC 30-2001) supplemented with 10% fetal bovine serum.
To produce mutant viruses lacking the m02 gene and the whole m02 gene family, two plasmids were designed that would create substitution mutations upon recombination with the viral genome in mouse cells. The m02 coding region with about 1.0 kb of flanking region on each side (bp 450 to 3420 genomic region; sequence numbers from reference 26) was amplified by PCR and cloned into pUC19. Then the _Mlu_I-_Sgf_I fragment of the m02 coding region (bp 1109 to 1819) was replaced with the enhanced green fluorescent protein (EGFP) cassette (_Xho_I-_Sgf_I fragment) from pGET007 (34), so that the EGFP coding region controlled by the simian virus 40 (SV40) promoter and polyadenylation site was inserted in the same direction as the m02 coding region (Fig. 1A). This SM_sub_m02 mutant construct was mixed with purified wild-type Smith viral DNA (1:2 ratio) and used to transfect 10.1 cells with Lipofectamine (Boehringer Mannheim).
FIG. 1.
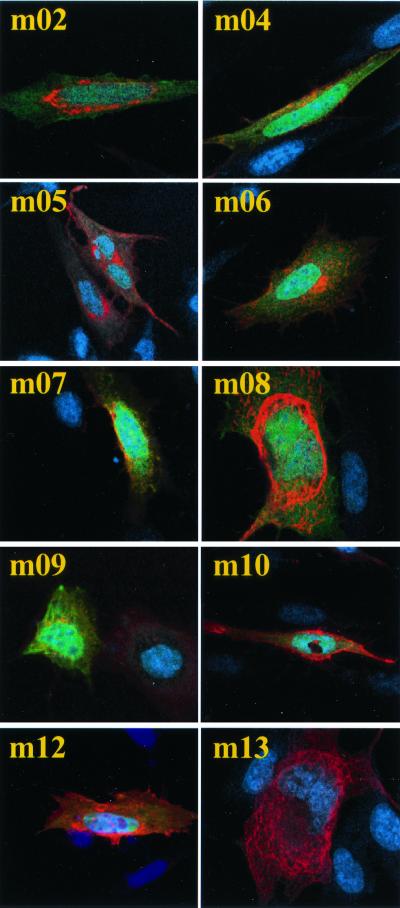
Intracellular localization of m02 gene family members. Constructs expressing viral proteins fused to an HA epitope were transfected into 10.1 cells, and cells were processed for immunofluorescence 24 h later. Nuclei were stained with Hoechst dye (blue), and m02 gene family members were detected using an antibody to HA plus an Alexa-568-labeled secondary antibody (red). The green color results from expression of EGFP in transfected cells, and the yellow color corresponds to coincident green and red staining.
Three rounds of plaque purification generated two pure populations of independently isolated viruses carrying the substitution mutation, termed SM_sub_m02 and SM_sub_m02′. Similarly, the SM_sub_m02-16 and SM_sub_m02-16′ substitution mutants lacking the whole m02 gene family were generated by homologous recombination between the wild-type viral DNA and the SM_sub_m02-16 knockout construct. This construct was derived from the previous one by replacing the m02 right-hand flanking region (bp 1819 to 3420) with the m16 right-hand flanking region (bp 15500 to 17000).
Analysis of viral nucleic acids and protein.
Viral DNA was prepared by lysing infected cells in extraction buffer (0.14 M NaCl, 1.5 mM MgCl2, 10 mM Tris-HCl [pH 8.6], 0.5% NP-40, 1 mM dithiothreitol) when 100% cytopathic effect was visible, pelleting the cellular debris, and digesting the supernatant with proteinase K (50 μg/ml). After extraction with phenol-chloroform and precipitation with ethanol plus sodium acetate, ≈1-μg aliquots of DNA were subjected to Southern blot analyses using probes that were 32P labeled by random priming.
For immunofluorescence, infected cells were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 and 0.05% Tween 20 in phosphate-buffered saline, treated with blocking solution (0.5% bovine serum albumin and 10% goat serum in phosphate-buffered saline), sequentially incubated with a primary antibody against an influenza virus hemagglutinin (HA) epitope (16B12; Berkeley Antibody Company) and Alexa-568-labeled goat anti-rabbit immunoglobulin (Ig) secondary antibody in blocking solution, and subjected to confocal microscopy. Nuclei were stained with Hoechst dye (Molecular Probes).
Pathogenesis in mice.
Virus was concentrated by centrifugation through a 15% sucrose cushion and titered by plaque assay on 10.1 cells. BALB/c and C57BL/6J (Jackson Laboratories), Rag2 knockout and γc/Rag2 double knockout (Taconic), and CD1 knockout mice (24) mice were injected intraperitoneally with virus. To compare the pathogenicity of different viruses, mice were infected and monitored for survival. To assay viral spread, mice were sacrificed by CO2 inhalation, and organs were homogenized by trituration as a 10% solution in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum. The total amount of infectious virus per organ was determined by plaque assay of the homogenates on 10.1 cells. The rabbit polyclonal antibody anti-asialo GM1 (Wako Chemicals) was injected intravenously 1 day prior to viral infection. Experimental protocols were approved by the Princeton University Animal Welfare Committee and were consistent with regulations stipulated by the American Association for Accreditation of Laboratory Animal Care and those in the Animal Welfare Act (Public Law 99-198).
Fluorescence-activated cell sorting.
The livers of mock-, wild type-, and SM_sub_m02-16-infected mice were harvested at 3 days postinfection and transformed into single-cell suspensions, and lymphocytes were purified with Lympholyte (Cedarlane Laboratories Ltd., Ontario, Canada). The lymphocytes were incubated for 4 h at 37°C with brefeldin A (5 μg/ml; Sigma), cell surface stained with CD3-indocarbocyanine and DX5-fluorescein isothiocyanate antibodies (Pharmingen), and then permeabilized and incubated with gamma interferon-phycoerythrin antibody (Pharmingen). The samples were washed and analyzed with a Becton Dickinson FACSort machine.
NK lysis assay.
YAC-1 cells (2 × 106) (20) in the log phase of growth were resuspended in 2 ml of medium (RPMI supplemented with 10% fetal bovine serum) and labeled with 10 μCi of [3H]thymidine for 4 h at 37°C and 5% CO2. Six-week-old female C57BL/6J mice were either mock injected, injected with a rabbit polyclonal IgG preparation, or injected with rabbit polyclonal antibody anti-asialo GM1. Their spleens were harvested at 1 day postinfection and homogenized into single-cell suspensions, and lymphocytes were purified with Lympholyte solution. Either 106, 3 × 105, 105, 3 × 104, 104 or no lymphocytes were plated in triplicate in a 96-well plate (100 μl per well), and 104 labeled YAC-1 cells (100 μl) were added to each well. The effector and target cells were incubated for 5 h at 37°C and harvested, and the cpm were counted in a scintillation counter. The percent lysis was calculated as 100 × [(maximum cpm − experimental cpm)/maximum cpm], the maximum cpm being the counts from YAC-1 cells alone.
RESULTS
Intracellular localization of m02 gene family members.
Plasmid vectors expressing m02 gene family members were produced by cloning open reading frames (ORFs) with a carboxy-terminal influenza virus HA epitope (HA tag) into pAdTrack-CMV (13) (gift from T. C. He, John Hopkins Oncology Center). Genes cloned into the multiple cloning site of this vector are expressed under the control of the immediate-early human cytomegalovirus promoter. The vector also contains the EGFP gene controlled by a separate promoter. An HA-tagged MCMV M78 ORF, which encodes a G protein-coupled receptor homologue, was cloned into pAdTrack-CMV as a control.
The subcellular localization of m02 gene family members was examined by confocal microscopy of 10.1 cells that were transfected with the expression constructs and then either mock infected (Fig. 1) or infected with wild-type MCMV (data not shown). Proteins expressed by m02 family members were detected at 24 h after transfection by using a primary antibody to the HA epitope followed by an Alexa-568-labeled secondary antibody (red), and nuclei were stained (blue) with Hoechst dye. Transfected cells are expected to be green because the expression construct carries the EGFP gene, but in some cells the expression level of the m02 family member was very high, and as a result, the EGFP fluorescence is difficult to observe at the exposure used to visualize the m02 family member.
The localization was identical in infected and mock-infected cells. All m02 gene family members accumulated in the cytoplasmic compartment, and with the exception of m08 and m13, their position near the nucleus suggests that they might localize to the vicinity of the endoplasmic reticulum and Golgi apparatus. m08 and m13 seem to localize to a cytoskeletal structure within the cell cytoplasm and perinuclear area. Surprisingly, none of the m02 gene family proteins appeared to localize to the plasma membrane, although we cannot rule out the accumulation of small amounts of the proteins in this compartment.
Construction and in vitro growth of mutant viruses lacking m02 or the entire m02 gene family.
To analyze the role of the m02 gene family in the replication cycle of MCMV, recombinant viruses lacking the m02 gene (SM_sub_m02 and SM_sub_m02′) or the whole m02 gene family (SM_sub_m02-16 and SM_sub_m02-16′) were constructed. In the m02 substitution mutants, the MCMV bp 1109 to 1819 genomic region (sequence numbers from reference 26) was replaced by an EGFP gene controlled by the SV40 promoter and polyadenylation site, while in substitution mutants lacking the entire gene family this cassette replaced the bp 1109 to 15500 genomic region (Fig. 2A). The two independent isolates of the SM_sub_m02 and SM_sub_m02-16 substitution mutants were analyzed by PCR assay to confirm their purity and recombination fidelity (Fig. 2B).
FIG. 2.
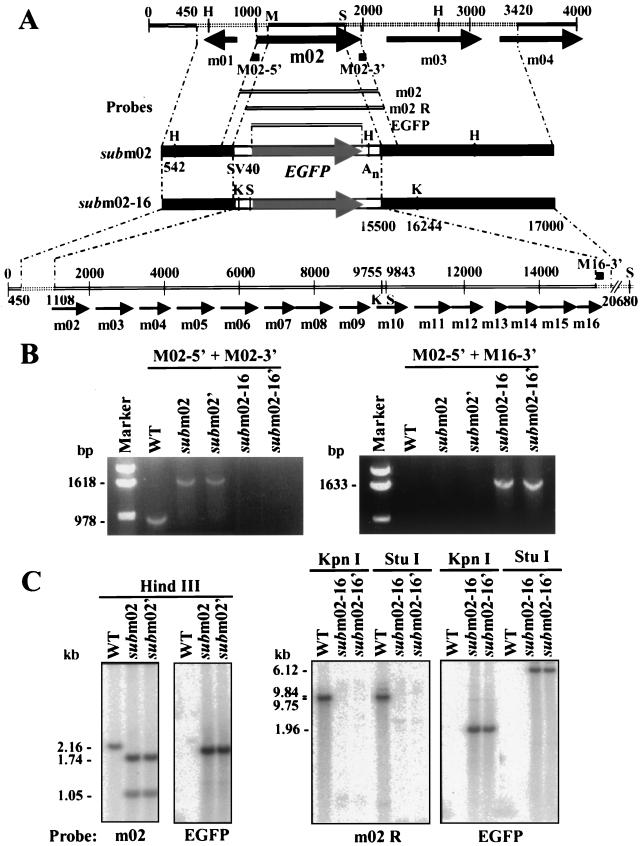
SM_sub_m02 and SM_sub_m02-16 construction and physical characterization. (A) Schematic representation of the MCMV m02 genomic region with open reading frames designated by arrows. Plasmids used to generate the SM_sub_m02 and SM_sub_m02-16 substitution mutants, the PCR primers (M02-5′, M02-3′, and M16-3′) used for PCR amplification, and the probes (m02, m02 R, and EGFP) used for Southern blot analysis are shown. Key nucleotide sequence numbers and restriction endonuclease cleavage sites (H, _Hin_dIII; X, _Xho_I; K, _Kpn_I; S, _Stu_I) are indicated. The EGFP substitution in SM_sub_m02 and SM_sub_m02-16 is controlled by the SV40 early promoter (SV40) and polyadenylation site (An). (B) PCR analysis of mutant virus DNAs. Diagnostic DNA segments from wild-type (WT), SM_sub_m02, and SM_sub_m02-16 viruses were amplified by PCR using primers hybridizing to the 5′ and 3′ ends of m02 region open reading frames. The expected sizes (in base pairs) of the PCR products and the primers used are indicated beside the gels and are consistent with the sizes of the marker DNAs. (C) Southern blot analysis of SM_sub_m02 and SM_sub_m02-16. DNA was isolated from 10.1 cells infected at a multiplicity of 0.01 PFU/cell with wild-type virus, SM_sub_m02, or SM_sub_m02-16, digested with the indicated restriction enzymes, and probed with the PCR-amplified m02 (bp 999 to 1976), m02 R (bp 1109 to 1976), or EGFP 32P-labeled probes. The expected sizes (in kilobase pairs) of DNA fragments are indicated.
Viral DNA was amplified with primers specific for the m02 gene (M02-5′ and M02-3′) or the m02 gene family (M02-5′ and M16-3′). The m02 gene-specific primers directed the production of a PCR product from the wild type and the two SM_sub_m02 DNAs, but not from the two SM_sub_m02-16 DNAs because the M02-3′ primer had no binding site. The m02 gene family primers did not direct the synthesis of a PCR product from the wild type or SM_sub_m02 due to the distance between their binding sites on the template DNAs, but they successfully amplified the EGFP gene and m02 gene family extremities from the two SM_sub_m02-16 DNAs. Viral DNA from the wild type, SM_su_bm02, and SM_sub_m02-16 was also digested with several restriction enzymes and subjected to Southern blot analysis (Fig. 2C). The probes hybridized to fragments of the expected sizes, further confirming that the two recombinant viruses are free of contaminating wild-type virus and carry the desired mutations.
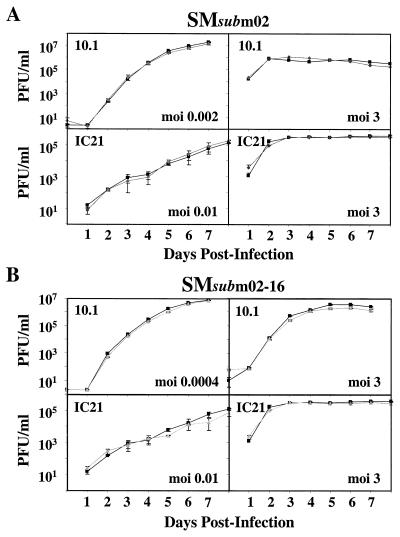
The mutant viruses were purified without the need for complementation, demonstrating that the m02 gene family is not essential for MCMV growth in cultured cells. Growth curves of the mutant viruses were performed in 10.1 fibroblast and IC21 macrophage cell lines to determine whether the gene family influences the efficiency of MCMV replication in cultured cells. Macrophages are involved in the spread, immune surveillance, and latency of MCMV infection (11). Cells were infected at relatively low (<0.01 PFU/cell) and high (3 PFU/cell) multiplicities, and the production of virus was monitored by plaque assay. The recombinant viruses grew with the same kinetics and to the same final yield as the wild-type virus (Fig. 3A and B), indicating that the m02 gene family is not required for MCMV growth in these cell types.
FIG. 3.
m02 gene family is not required for MCMV growth in cultured fibroblasts or macrophages. 10.1 or IC21 cells were infected at the indicated multiplicities of infection (moi) with the wild-type (▪), SM_sub_m02 (⧫) (A), or SM_sub_m02-16 (▴) (B) virus. Supernatants were harvested at the indicated times, and virus titers were quantified by plaque assay on 10.1 cells. Three independently infected cultures were assayed for each time point.
m02 gene family is required for normal replication and pathogenesis in mice.
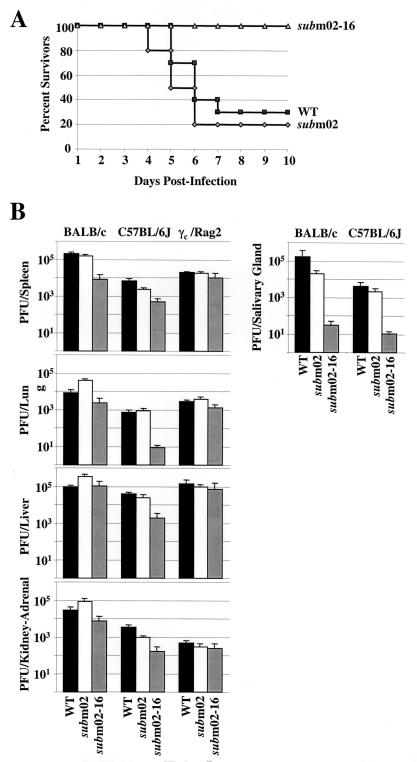
To compare the virulence of the wild-type and mutant viruses, a 50% lethal dose (LD50) experiment was performed (Fig. 4A). Four-week-old BALB/c mice were challenged with a lethal intraperitoneal dose (5 × 106 PFU) of the wild type, SM_sub_m02, or SM_sub_m02-16, and their survival was monitored. In the days following the virus injection, mice infected with the wild-type and SM_sub_m02 viruses looked sick, but SM_sub_m02-16-infected mice appeared normal. More than 70% of the wild-type- and SM_sub_m02-infected mice died by the seventh day postinfection, whereas all SM_sub_m02-16-infected mice were alive at this time. Therefore, SM_sub_m02 is as pathogenic as the wild-type virus, while SM_sub_m02-16 is significantly less virulent.
FIG. 4.
Pathogenesis and replication of SM_sub_m02 and SM_sub_m02-16 in mice. (A) Survival curves. Female BALB/c mice were intraperitoneally injected with 5 × 106 PFU of wild-type (▪), SM_sub_m02 (⋄), or SM_sub_m02-16 (▵) virus and monitored for survival. (B) Accumulation of infectious virus in acutely and persistently infected organs. Female mice were intraperitoneally infected with 5 × 106 PFU (BALB/c and C57BL/6J) or 3 × 105 PFU (γc/Rag2 mice) of wild-type virus (WT), SM_sub_m02, or SM_sub_m02-16. Spleen, liver, kidney, adrenal gland, and lung were harvested 3 days later. Female BALB/c and C57BL/6J mice were intraperitoneally infected with 106 PFU of virus, and their salivary glands were harvested 14 days later. Organs were stored frozen (−80°C) until virus titers in organ homogenates were determined by plaque assay. Each data point represents the average plus standard deviation for at least three animals.
The in vivo role of the m02 gene family was examined further by monitoring viral replication in individual organs. BALB/c and C57BL/6J mice (6 weeks old) were injected intraperitoneally with 5 × 106 PFU of wild-type or mutant virus. BALB/c mice are more susceptible to MCMV infection than C57BL/6J mice. Major histocompatibility complex-linked genes (2, 5, 10) as well as non-major histocompatibility complex-linked genes (10, 25, 29) have been identified as determinants of strain susceptibility. Acutely infected organs were harvested at 3 days postinfection, and the salivary glands were harvested at 14 days postinfection.
In both mouse strains, SM_sub_m02 grew like wild-type virus, while SM_sub_m02-16 generated reduced yields in every organ compared to wild-type virus (Fig. 4B), with the possible exception of the liver in BALB/c mice. Tropism for the salivary gland is central to the biology of cytomegaloviruses, since persistent and recurrent shedding from the salivary gland is believed to be the principal means by which these viruses spread within host populations. The SM_sub_m02-16 growth defect is more pronounced in the salivary gland at 14 days postinfection (Fig. 4B). The other isolates of the mutant viruses, SM_sub_m02′ and SM_sub_m02-16′, grew similarly to SM_sub_m02 and SM_sub_m02-16, suggesting that the observed defects do not result from random mutations (data not shown).
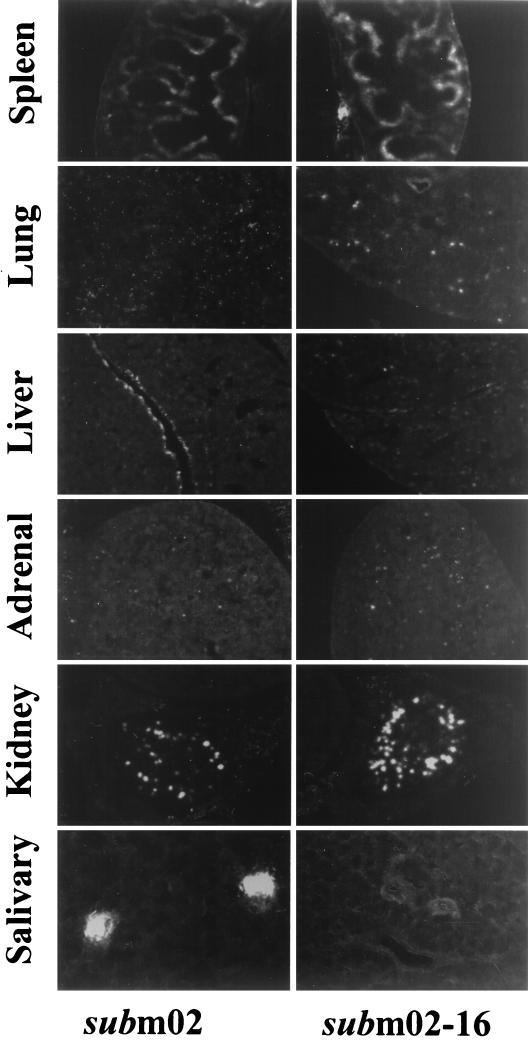
We confirmed the growth of the virus in various organs by monitoring EGFP fluorescence in tissue sections (Fig. 5). SM_sub_m02 and SM_sub_m02-16 contain EGFP expression cassettes (Fig. 2A), and since SM_sub_m02 exhibited pathogenicity and yields in various organs similar to those of its wild-type parent, it was used as a pseudo-wild-type control for comparison with SM_sub_m02-16 in this experiment. Four-week-old female BALB/c mice were infected intraperitoneally with 2.3 × 107 PFU of SM_sub_m02 or SM_sub_m02-16 virus. The spleen, liver, lungs, kidney, and adrenal gland were harvested at 3 days postinfection, and salivary glands were harvested at 14 days postinfection.
FIG. 5.
Expression of the EGFP marker gene carried by SM_sub_m02 and SM_sub_m02-16 in acutely and persistently infected organs of mice. BALB/c mice were infected with 2.3 × 107 PFU of SM_sub_m02 or SM_sub_m02-16, and their organs were harvested and quick-frozen at −80°C. Spleen, liver, lung, adrenal gland, and kidney were harvested at 3 days postinfection, while the salivary gland was harvested at 14 days postinfection. Thin sections (20 μm) were made using a cryostat and were observed and photographed using a confocal fluorescent microscope.
The two mutants produced similar fluorescence patterns in acutely infected organs, even though SM_sub_m02 produced more infectious virus than SM_sub_m02-16. Uninfected organs were examined (data not shown), ruling out the possibility that autofluorescence confounded the results. MCMV replicates primarily in sinusoid lining cells within the red pulp of the spleen (21), and sinusoid lining cells exhibited high levels of EGFP expression in our experiment. A lethal dose of MCMV has been reported to cause necrosis and spreading foci of infection in the liver (17), and we observed fluorescent cells throughout the liver. Earlier work has indicated that immunosuppressed mice develop histological evidence of interstitial pneumonitis, while the lungs of immunocompetent mice appear normal after infection with MCMV (30). However, we detected extensive fluorescence in the lungs of immunocompetent mice. This discrepancy might due to the route of inoculation of the virus, which was different in our experiment than the earlier work, or it might result from the higher sensitivity of our assay. Mims and Gould (22) reported that interstitial tissues, glomeruli, and tubular epithelium of the kidneys are infected, and we observed fluorescent cells throughout the kidney. The adrenal cortex has been reported to be more extensively infected than the medulla (22), consistent with our fluorescence data. Persistent infection of the submaxillary salivary glands produce “acinar foci” of infection (16, 22, 28). We observed foci of infection in SM_sub_m02-infected mice, but none were detected in SM_sub_m02-16-infected mice, consistent with the low yield of SM_sub_m02-16 in this organ.
Taken together with the in vitro results (Fig. 3), the in vivo experiments (Fig. 4 and 5) suggest that the m02 gene family plays an important role in the virus-host interaction rather than at the cellular level.
SM_sub_m02-16 grows normally in γc/Rag2 knockout mice.
SM_sub_m02-16 grew normally in cultured cells (Fig. 3) and exhibited a replication defect in two wild-type mouse strains (Fig. 4), suggesting that the m02 gene family might be involved in the control of immune surveillance. Consequently, we investigated the replication of the mutant viruses in immune-deficient γc/Rag2 double knockout mice. These mice lack the common cytokine receptor gamma chain (γc) and the recombinase-activating gene 2 (Rag2) in a C57BL/6J background (4, 32). They fail to produce mature B (B220+) and T (Thy1+) cells, NK, and NK T cells (NK1.1+).
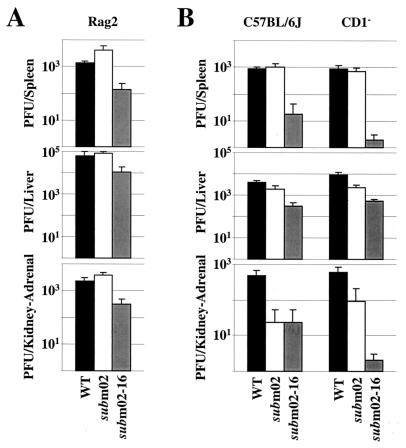
Six-week-old γc/Rag2 knockout mice were intraperitoneally injected with 3 × 105 PFU of wild-type or mutant viruses, their organs were harvested 3 days later, and virus was titered (Fig. 4B). SM_sub_m02 and SM_sub_m02-16 grew identically to wild-type virus in all organs during the acute phase of the infection in the γc/Rag2 knockout mice. This indicates that one or several of the cell types missing in this mouse strain normally controls the growth of SM_sub_m02-16. In other words, one or more members of the m02 gene family apparently antagonize host immune functions to facilitate the replication of MCMV.
B and T cells would not be expected to inhibit viral replication at 3 days postinfection. Nevertheless, to eliminate the possibility that these cells interfere with the growth of SM_sub_m02-16, we compared the titers of the wild-type and mutant viruses in Rag2 knockout mice, which lack mature B and T lymphocytes as well as NK T cells, but contain NK cells (Fig. 6A). The SM_sub_m02-16 virus grew more poorly than wild-type virus in each organ tested, indicating that B, T, and NK T cells are not the limiting factor in the growth of SM_sub_m02-16.
FIG. 6.
B, T, and NK T cells are not required to inhibit SM_sub_m02-16 replication during the acute phase of infection in the mouse. (A) SM_sub_m02-16 growth defect in Rag2 knockout mice. Six-week-old female Rag2 knockout mice (C57BL/6J background) were intraperitoneally infected with 5 × 106 PFU of the wild type (WT), SM_sub_m02, or SM_sub_m02-16, their organs were harvested at 3 days postinfection, and virus was titered. (B) SM_sub_m02-16 growth defect in CD1 knockout (CD1−) mice. Six-week-old female wild-type C57BL/6J and CD1 knockout (C57BL/6J background) mice were intraperitoneally infected with 106 PFU of the wild type, SM_sub_m02, or SM_sub_m02-16, their organs were harvested at 3 days postinfection, and virus was titered. Each bar represents the average titer per organ plus the standard deviation for at least three animals.
To confirm that the NK T cells are not causing the reduced titers in SM_sub_m02-16-infected mice, the replication of the mutant virus was also analyzed in CD1 knockout mice, which specifically lack this cell type (Fig. 6B). SM_sub_m02-16 exhibited its growth defect in the mutant mouse strain, arguing that NK T cells are not the cell population inhibiting its growth.
SM_sub_m02-16 grows normally in NK cell-depleted mice.
NK cells are known to be critical for the control of MCMV infection during the first few days following intraperitoneal inoculation, prior to the development of cytotoxic-T-cell-mediated clearance mechanisms (3, 31, 35). To assess the role of NK cells in the clearance of mutant viruses compared to wild-type virus, the replication of these viruses was compared in mice whose NK cells had been neutralized by antibody injection.
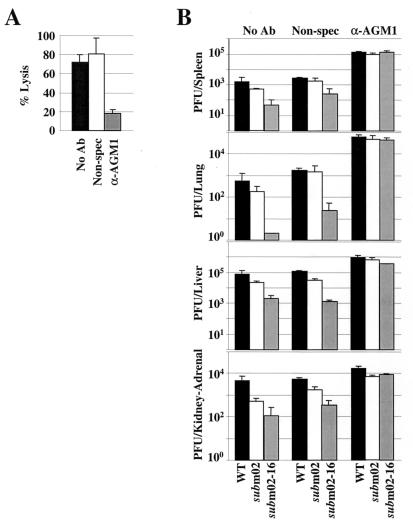
A group of 6-week-old C57BL/6J mice were depleted for NK cell function by a single intravenous injection of purified IgG from a rabbit polyclonal antibody specific for asialo ganglio-_N_-tetraosylceramide (anti-asialo GM1). Another group of mice were intravenously injected with a nonspecific rabbit IgG preparation, and a third group were injected with buffer. The neutralization of NK cells by anti-asialo GM1 treatment was confirmed by flow cytometry analysis (data not shown) and by NK cell lysis assay (Fig. 7A). One day after the antibody injection, the mice were intraperitoneally infected with 5 × 106 PFU of either wild-type virus, SM_sub_m02, or SM_sub_m02-16. Organs were harvested 3 days later and virus was titered (Fig. 7B). In mice depleted of NK cells, SM_sub_m02-16 grew in all organs tested as well as the SM_sub_m02 and wild-type viruses did, whereas the injection of a nonspecific antibody had little effect. This experiment argues that NK cells play a critical role in the clearance of the SM_sub_m02-16 virus.
FIG. 7.
NK cells inhibit the growth of SM_sub_m02-16 during the acute phase of infection in the mouse. (A) Lack of NK cell cytolytic activity in NK cell-depleted mice. Six-week-old female C57BL/6J mice were intravenously injected with no antibody (No Ab), a nonspecific rabbit polyclonal IgG preparation (Non-spec), or a rabbit anti-asialo GM1 IgG preparation (α-AGM1). One day later the spleens were harvested and homogenized as a single-cell suspension, and lymphocytes were purified and subjected to an NK cell lysis assay. The percent lysis was calculated at an effector-to-target cell ratio of 10:1. (B) Replication of wild-type virus (WT), SM_sub_m02, and SM_sub_m02-16 in NK cell-depleted mice. Six-week-old female C57BL/6J mice were intravenously injected with no antibody, a nonspecific rabbit polyclonal IgG preparation, or an anti-asialo GM1 IgG preparation 1 day prior to intraperitoneal infection with 5 × 106 PFU of virus. Organs were harvested 3 days later, homogenates were prepared, and infectious virus was quantified by plaque assay. Each bar corresponds to the average titer per organ of at least three mice plus the standard deviation.
It has been suggested that the anti-asialo GM1 antibody also affects the activity of B, T, and NK T cells, but, as shown above (Fig. 6A), the growth of SM_sub_m02-16 is inhibited in Rag2 knockout mice, which lack these three cell types.
DISCUSSION
The MCMV m02 gene family members appear to code for membrane glycoproteins because they contain potential signal and anchor sequences in addition to N-linked glycosylation sites (26). A subset of genes from the m02 family were successfully expressed in transfected cells as fusion proteins with an HA epitope, and they all localized to cytoplasmic structures (Fig. 1). Their localization was not altered by the infection of cells with wild-type virus (data not shown). None of the proteins appeared to accumulate in the plasma membrane, although we cannot rule out the presence of small amounts of the proteins in this compartment. Presumably, m02 family members are associated with the membranes of organelles in the cytoplasm, although the filamentous appearance of m08 and m13 fluorescence suggests that a portion of these proteins might associate with a component of the cytoskeleton when they are overexpressed. Consistent with our results, m06-associated MHC I molecules were previously shown to be targeted to lysosomes and to be degraded rapidly in a compartment marked by the lysosome-associated membrane protein (LAMP1+) (27).
The m02 family of proteins adds to a growing list of cytomegalovirus glycoproteins found in the cytoplasm but not detected on the cell surface. For example, m152, a member of the MCMV m145 glycoprotein gene family, localizes to the endoplasmic reticulum-Golgi intermediate compartment/_cis_-Golgi (38); M78, a G protein-coupled receptor homolog, accumulates in the Golgi compartment (23); and the human cytomegalovirus type I transmembrane proteins encoded by US3, US6, and US11 localize to the endoplasmic reticulum (1, 14, 36). Some viral proteins, such as m06 (27), localize to the region of the endoplasmic reticulum and Golgi apparatus to antagonize MHC class I function; whereas others, such as M78 (23), appear to localize in this region to be incorporated into virions.
To study the function(s) of the m02 gene family, we constructed MCMV substitution mutants lacking the m02 ORF or the entire m02 gene family, termed SM_sub_m02 and SM_sub_m02-16, respectively (Fig. 2). A mutant similar to SM_sub_m02-16, named Δm01-m17 and containing a deletion in the _Hin_dIII A region, had been constructed previously, but it is derived from another MCMV strain (Vancouver), and the exact extent of the deletion and its growth properties were not described (19, 27). The two mutant viruses replicated with the same kinetics and to the same final yield as their wild-type parent in cultured fibroblasts (10.1 cells) and macrophages (IC21 cells) (Fig. 3). This finding is similar to what has been reported for the US6 gene family, which encode a set of glycoproteins in human cytomegalovirus. A mutant lacking the IRS1 through US11 ORFs grew normally in cultured human fibroblasts (18).
The role of the m02 gene family in pathogenesis was evaluated in mice. Whereas SM_sub_m02 is as virulent as its parent, SM_sub_m02-16 is markedly attenuated (Fig. 4A). SM_sub_m02 replicates like the parent virus in all organs of both mouse strains in the acute phase of the infection (3 days) and shows only a slight reduction of titers in the salivary glands at 14 days postinfection (Fig. 4B). These results suggest that m02 is dispensable for virus growth in these organs and that the presence of the EGFP gene in the viral genome does not significantly affect viral replication in vivo. In contrast, SM_sub_m02-16 produced less infectious progeny in acutely infected organs, and >500-fold less virus accumulated in salivary glands at 14 days after infection (Fig. 4B).
Although SM_sub_m02-16 produced less progeny than SM_sub_m02 in acutely infected organs, there was little difference in the amount of fluorescence produced by the two viruses’ EGFP marker genes in acutely infected organs (Fig. 5). This could be interpreted to mean that SM_sub_m02-16 infected cells in the target organs as efficiently as the other viruses, but an NK response limited the yield of progeny from the infected cells. SM_sub_m02-16 generated significantly less fluorescence in persistently infected salivary glands than did SM_sub_m02, consistent with its dramatically reduced titer in this organ.
We have shown here that m02 is dispensable for viral replication and spread in the mouse, and earlier work has shown that the same is true for m09 (37). Presumably, then, the attenuation of SM_sub_m02-16 does not result from the lack of m02 and m09 and other members of the gene family must be involved in the virus-host interaction. Therefore, we analyzed the growth of the SM_sub_m02-16 mutant virus in mice lacking a complete immune system.
SM_sub_m02-16 grew like the wild-type virus during the acute phase of infection in γc/Rag2 immunodeficient mice lacking NK, NK T, T, and B cells (Fig. 4B). This demonstrates that the mutant is competent to replicate and spread in acutely infected organs, but it is limited by the action of one or several of the cell types missing in the γc/Rag2 mice. Experiments analyzing the growth of SM_sub_m02-16 in mice lacking NK T cells (CD1 knockout mice) or B, T, and NK T cells (Rag2 knockout mice) (Fig. 6) and in NK cell-depleted mice (Fig. 7) showed that NK cells antagonize the growth of SM_sub_m02-16. One or several members of the m02 gene family are therefore protecting against NK cell-mediated clearance of infected cells during the acute infection. It is conceivable that the m04 gene, which has been shown to favor cell surface accumulation of MHC class I molecules (19), might protect infected cells from attack by NK cells, since NK cells attack cells deficient in MHC class I antigen. However, it is not yet known if m04 can antagonize NK cell function. The identification of the m02 family member(s) responsible for this function will require the construction and analysis of additional mutant viruses.
Other MCMV genes have previously been shown to be involved in the avoidance of NK cell function. MCMV mutants lacking the m131/129 chemokine homolog (Δm131/129) replicated identically to the wild type in vivo at 2 and 3 days postinfection (9). Nevertheless, the mutants were then cleared more rapidly from the spleen and liver during acute infection compared with wild-type MCMV. The accelerated clearance of the mutants was mediated by NK cells and CD4+ CD8+ T lymphocytes (9). The attenuation of an MCMV m152 deletion mutant during the acute phase of infection is also NK and CD8+ T cell dependent (15). The MCMV MHC class I homologue m144 was shown to associate with β2-microglobulin, but unlike human cytomegalovirus UL18, was unable to bind peptides (6). A mutant from which the m144 gene had been deleted was shown to be attenuated as a result of its inability to control antiviral NK cell activity (8).
Although MCMV has multiple genes that function to protect the virus-infected cell from NK cells, deletion of any one of them can impair replication of the virus in the host. Consequently, we can conclude that the different genes required for resistance to NK cells do not simply encode redundant functions. Either they antagonize different aspects of the NK cell response, they work through different or complementary mechanisms, or some of the viral gene products mediate additional, as yet undiscovered functions in addition to their NK cell-related role.
The m02 gene family might also interact with NK cells to regulate their proliferation or the production of cytokines, rather than to block their cytolytic action. Indeed, we found that the level of gamma interferon in NK cells from the livers of SM_sub_m02-16-infected mice was half of that in cells from in wild-type virus-infected mice (data not shown). As yet, we are not able to interpret this observation in terms of mechanism, but it reinforces the view that one or more m02 family members alter NK cell activity.
Acknowledgments
This work was supported by grants from the National Cancer Institute (CA82396 and CA85786).
We thank L. Enquist and B. Wing for critical reading of the manuscript. We also thank P. Robinson for assistance with in vivo studies, J. Goodhouse for help with confocal microscopy, and A. Levine (Princeton University) for 10.1 cells. S.A.O. was supported by a Ph.D. fellowship (BD/13342/97), granted by PRAXIS XXI through Programa Gulbenkian de Doutoramento em Biologia e Medicina (Portugal). P.L. was supported by the Juvenile Diabetes Foundation International.
REFERENCES
- 1.Ahn, K., A. Angulo, P. Ghazal, P. A. Peterson, Y. Yang, and K. Fruh. 1996. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc. Natl. Acad. Sci. USA 93**:**10990–10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan, J. E., and G. R. Shellam. 1984. Genetic control of murine cytomegalovirus infection: virus titers in resistant and susceptible strains of mice. Arch. Virol. 81**:**139–150. [DOI] [PubMed] [Google Scholar]
- 3.Bukowski, J. F., B. A. Woda, and R. M. Welsh. 1984. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J. Virol. 52**:**119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao, X., E. W. Shores, J. Hu-Li, M. R. Anver, B. L. Kelsall, S. M. Russell, J. Drago, M. Noguchi, A. Grinberg, E. T. Bloom, et al. 1995. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity 2**:**223–238. [DOI] [PubMed] [Google Scholar]
- 5.Chalmer, J. E., J. S. Mackenzie, and N. F. Stanley. 1977. Resistance to MCMV linked to the major histocompatibility complex of the mouse. J. Gen. Virol. 37**:**107–114. [DOI] [PubMed] [Google Scholar]
- 6.Chapman, T. L., and P. J. Bjorkman. 1998. Characterization of a murine cytomegalovirus class I major histocompatibility complex (MHC) homolog: comparison to MHC molecules and to the human cytomegalovirus MHC homolog. J. Virol. 72**:**460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chee, M. S., A. T. Bankier, S. Beck, R. Bohni, C. M. Brown, R. Cerny, T. Horsnell, C. A. Hutchinson 3d, T. Kouzarides, J. A. Martignetti, S. C. Satchwell, P. Tomlinson, K. M. Weston, and B. G. Barrell. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154**:**125–169. [DOI] [PubMed] [Google Scholar]
- 8.Farrell, H. E., H. Vally, D. M. Lynch, P. Fleming, G. R. Shellam, A. A. Scalzo, and N. J. Davis-Poynter. 1997. Inhibition of natural killer cells by a cytomegalovirus MHC class I homologue in vivo. Nature 386**:**510–514. [DOI] [PubMed] [Google Scholar]
- 9.Fleming, P., N. Davis-Poynter, M. Degli-Esposti, E. Densley, J. Papadimitriou, G. Shellam, and H. Farrell. 1999. The murine cytomegalovirus chemokine homolog, m131/129, is a determinant of viral pathogenicity. J. Virol. 73**:**6800–6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grundy, J. E., J. S. Mackenzie, and N. F. Stanley. 1981. Influence of H-2 and non-H-2 genes on resistance to MCMV infection. Infect. Immun. 32**:**277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson, L. K., J. S. Slater, Z. Karabekian, H. W. Virgin, I. V., C. A. Biron, M. C. Ruzek, N. Van Rooijen, R. P. Ciavarra, R. M. Stenberg, and A. E. Campbell. 1999. Replication of murine cytomegalovirus in differentiated macrophages as a determinant of viral pathogenesis. J. Virol. 73**:**5970–5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvey, D. M., and A. J. Levine. 1991. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev. 5**:**2375–2385. [DOI] [PubMed] [Google Scholar]
- 13.He, T.-C., S. Zhou, L. T. Da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95**:**2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hengel, H., J. O. Koopmann, T. Flohr, W. Muranyi, E. Goulmy, G. J. Hammerling, U. H. Koszinowski, and F. Momburg. 1997. A viral ER-resident glycoprotein inactivates the MHC-encoded peptide transporter. Immunity 6**:**623–632. [DOI] [PubMed] [Google Scholar]
- 15.Hengel, H., U. Reusch, A. Gutermann, H. Ziegler, S. Jonjic, P. Lucin, and U. H. Koszinowski. 1999. Cytomegaloviral control of MHC class I function in the mouse. Immunol. Rev. 168**:**167–176. [DOI] [PubMed] [Google Scholar]
- 16.Henson, D., and A. J. Strano. 1972. Mouse cytomegalovirus. Necrosis of infected and morphologically normal submaxillary gland acinar cells during termination of chronic infection. Am. J. Pathol. 68**:**183–202. [PMC free article] [PubMed] [Google Scholar]
- 17.Henson, D., R. D. Smith, and J. Gehrke. 1966. Non-fatal mouse cytomegalovirus hepatitis: combined morphologic, virologic and immunologic observations. Am. J. Pathol. 49**:**871–888. [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, T. R., and V. P. Muzithras. 1992. A cluster of dispensable genes within the human cytomegalovirus genome short component: IRS1, US1 through US5, and the US6 family. J. Virol. 66**:**2541–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleijnen, M. F., J. B. Huppa, P. Lucin, S. Mukherjee, H. Farrell, A. E. Campbell, U. H. Koszinowski, A. B. Hill, and H. L. Ploegh. 1997. A mouse cytomegalovirus glycoprotein, gp34, forms a complex with folded class I MHC molecules in the ER which is not retained but is transported to the cell surface. EMBO J. 16**:**685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matzinger, P. 1991. The JAM test: a simple assay for DNA fragmentation and cell death. J. Immunol. Methods 145**:**185–192. [DOI] [PubMed] [Google Scholar]
- 21.Mercer, J. A., C. A. Wiley, and D. H. Spector. 1988. Pathogenesis of murine cytomegalovirus infection: identification of infected cells in the spleen during acute and latent infections. J. Virol. 62**:**987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mims, C. A., and J. Gould. 1978. Splenic necrosis in mice infected with cytomegalovirus. J. Infect. Dis. 137**:**587–591. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira, S. A., and T. E. Shenk. 2001. Murine cytomegalovirus M78 protein, a G protein-coupled receptor homologue, is a constituent of the virion and facilitates accumulation of immediate-early viral mRNA. Proc. Natl. Acad. Sci. USA 98**:**3237–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park, S.-H., D. Guy-Grand, F. A. Lemonnier, C. R. Wang, A. Bendelac, and B. Jabri. 1999. Selection and expansion of CD8aa+TCRab+ intestinal intraepithelial lymphocytes in the absence of both classical major histocompatibility complex class I and nonclassical CD1 molecules. J. Exp. Med. 190**:**885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinnan, G. V., and J. F. Manischewitz. 1987. Genetically determined resistance to lethal MCMV infection is mediated by interferon-dependent and -independent restriction of virus replication. J. Virol. 61**:**1875–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rawlinson, W. D., H. E. Farrell, and B. G. Barrell. 1996. Analysis of the complete DNA sequence of the murine cytomegalovirus. J. Virol. 70**:**8833–8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reusch, U., W. Muranyi, P. Lucin, H. G. Burgert, H. Hengel, and U. H. Koszinowski. 1999. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J. 18**:**1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruebner, B. H., T. Hirano, R. Slusser, J. Osborn, and D. N. Medearis. 1966. Cytomegalovirus infection. Viral ultrastructure with particular reference to the relationship of lysosomes to cytoplasmic inclusions. Am. J. Pathol. 48**:**971–989. [PMC free article] [PubMed] [Google Scholar]
- 29.Scalzo, A. A., N. A. Fitzgerald, A. Simmons, A. B. La Vista, and G. R. Shellam. 1990. Cmv-1, a genetic locus that controls MCMV replication in the spleen. J. Exp. Med. 171**:**1469–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shanley, J. D., and E. L. Pesanti. 1985. The relation of viral replication to interstitial pneumonitis in murine cytomegalovirus lung infection. J. Infect. Dis. 151**:**454–458. [DOI] [PubMed] [Google Scholar]
- 31.Shellam, G. R., J. E. Allan, J. M. Papadimitriou, and G. J. Bancroft. 1981. Increased susceptibility to cytomegalovirus infection in beige mutant mice. Proc. Natl. Acad. Sci. USA 78**:**5104–5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shinkai, Y., G. Rathbun, K. P. Lam, E. M. Oltz, V. Stewart, M. Mendelsohn, J. Charron, M. Datta, F. Young, A. M. Stall, and F. W. Alt. 1992. RAG-2 deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68**:**855–867. [DOI] [PubMed] [Google Scholar]
- 33.Smith, M. G. 1954. Propagation of salivary gland virus of the mouse in tissue cultures. Proc. Soc. Exp. Biol. Med. 86**:**435–440. [DOI] [PubMed] [Google Scholar]
- 34.Tullis, G. E., and T. Shenk. 2000. Efficient replication of adeno-associated virus type 2 vectors: a _cis_-acting element outside of the terminal repeats and a minimal size. J. Virol. 74**:**11511–11521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welsh, R. M., J. O. Brubaker, M. Vargas-Cortes, and C. L. O’Donnell. 1991. Natural killer (NK) cell response to virus infections in mice with severe combined immunodeficiency: the stimulation of NK cells and the NK cell-dependent control of virus infections occur independently of T and B cell function. J. Exp. Med. 173**:**1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiertz, E. J., T. R. Jones, L. Sun, M. Bogyo, H. J. Geuze, and H. L. Ploegh. 1996. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell 84**:**769–779. [DOI] [PubMed] [Google Scholar]
- 37.Zhan, X., M. Lee, J. Xiao, and F. Liu. 2000. Construction and characterization of murine cytomegaloviruses that contain transposon insertions at open reading frames m09 and M83. J. Virol. 74**:**7411–7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziegler, H., R. Thale, P. Lucin, W. Muranyi, T. Flohr, H. Hengel, H. Farrell, W. Rawlinson, and U. H. Koszinowski. 1997. A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis-Golgi compartments. Immunity 6**:**57–66. [DOI] [PubMed] [Google Scholar]