The N- and C-terminal RNA recognition motifs of splicing factor Prp24 have distinct functions in U6 RNA binding (original) (raw)
Abstract
Prp24 is an essential yeast U6 snRNP protein with four RNA recognition motifs (RRMs) that facilitates the association of U4 and U6 snRNPs during spliceosome assembly. Genetic interactions led to the proposal that RRMs 2 and 3 of Prp24 bind U6 RNA, while RRMs 1 and 4 bind U4 RNA. However, the function of each RRM has yet to be established through biochemical means. We compared the binding of recombinant full-length Prp24 and truncated forms lacking RRM 1 or RRM 4 with U6 RNA. Contrary to expectations, we found that the N-terminal segment containing RRM 1 is important for high-affinity binding to U6 RNA and for discrimination between wild-type U6 RNA and U6 with point mutations in the 3′ intramolecular stem–loop. In contrast, deletion of RRM 4 and the C terminus did not significantly alter the affinity for U6 RNA, but resulted in the formation of higher order Prp24·U6 complexes. Truncation and internal deletion of U6 RNA mapped three Prp24-binding sites, with the central site providing most of the affinity for Prp24. A newly identified temperature-sensitive lethal point mutation in RRM 1 is exacerbated by mutations in the U6 RNA telestem, as is a mutation in RRM 2, but not one in RRM 3. We propose that RRMs 1 and 2 of yeast Prp24 bind the same central site in U6 RNA that is bound by the two RRMs of human Prp24, and that RRMs 3 and 4 bind lower affinity flanking sites, thereby restricting the stoichiometry of Prp24 binding.
Keywords: pre-mRNA splicing, U6 RNA, Prp24, RRM (RNA recognition motif)
INTRODUCTION
Intron removal from nuclear precursor messenger RNA (pre-mRNA) in eukaryotes is performed by a multimega-dalton ribonucleoprotein complex called the spliceosome. The spliceosome is composed primarily of five small nuclear ribonucleoprotein particles (snRNPs), each of which contains one small nuclear RNA (U1, U2, U4, U5, and U6 snRNA) and several proteins (Brow 2002). It was thought that the snRNPs assemble onto the pre-mRNA in a stepwise fashion, but recent evidence suggests the existence of a preassembled “holospliceosome” complex containing all five snRNPs that binds as a complete unit to the intron (Stevens et al. 2002). Regardless of the mechanism of spliceosome assembly, specific RNA rearrangements mediated by protein factors with annealing or helicase activities must occur for the assembled spliceosome to become catalytically active (Staley and Guthrie 1998; Brow 2002).
U6 may be the most structurally dynamic spliceosomal RNA. Much of a cell’s U6 RNA exists as the solitary U6 snRNP, in which U6 RNA forms two stable intramolecular stem–loops (Fortner et al. 1994). The 5′ stem–loop of U6 appears not to change structure during the splicing cycle and is not well conserved, but the 3′ intramolecular stem–loop (ISL) (Huppler et al. 2002) is highly dynamic and well conserved. To be incorporated into the spliceosome, U6 RNA must base pair with U4 RNA to form the U4/U6 bi-snRNP (Hashimoto and Steitz 1984; Rinke et al. 1985; Brow and Guthrie 1988), which requires unwinding of the U6 ISL. After assembly of the complete spliceosome on an intron, U4 RNA unwinds from U6, allowing the ISL to reform and adjacent sequences to base pair with U2 RNA (Cheng and Abelson 1987; Yean and Lin 1991; Madhani and Guthrie 1992; Kuhn et al. 1999; Staley and Guthrie 1999). The U2/U6 complex is thought to participate in catalysis of the two transesterification reactions of pre-mRNA splicing (Yean et al. 2000; Valadkhan and Manley 2001; Hilliker and Staley 2004; Sashital et al. 2004). After the exons are joined, the spliceosome is disassembled and U6 snRNP is released to begin the splicing cycle anew.
Given that both the U6 ISL and the U4/U6 intermolecular stems are very stable, with melting temperatures above 50°C at physiological salt concentration (Brow and Guthrie 1988; Sashital et al. 2003), it is likely that their interconversion is assisted by proteins and may involve metastable intermediate structures. Indeed, dissociation of the human U4/U6 RNA complex is facilitated by formation of a long-range intramolecular helix in U6 RNA that extends the ISL (Brow and Vidaver 1995). Yeast U6 RNA apparently does not spontaneously form such a structure, as the yeast U4/ U6 RNA complex is much more stable than the human complex in vitro. However, genetic studies suggest the formation of an analogous structure, called the telestem, in yeast U6 RNA in vivo (Brow and Vidaver 1995). Both biochemical and genetic data implicate the U6 snRNP protein Prp24 in binding and stabilization of the U6 telestem (Shannon and Guthrie 1991; Jandrositz and Guthrie 1995; Vidaver et al. 1999; Ryan et al. 2002).
It is well established that Prp24 mediates the recycling of U4 and U6 snRNPs in vitro to produce U4/U6 bi-snRNP for subsequent rounds of splicing (Raghunathan and Guthrie 1998). Recombinant Prp24 stimulates the formation of U4/U6 RNA complex from in vitro-transcribed U4 and U6 RNAs, although this reaction is not as efficient as the annealing of U4 and U6 snRNPs in cell extracts by recombinant Prp24 (Ghetti et al. 1995; Raghunathan and Guthrie 1998). One reason that U4/U6 annealing may be more efficient in cell extracts is that another component of the U6 snRNP, the Lsm protein complex, also promotes U4/U6 association (Achsel et al. 1999; Vidal et al. 1999; Verdone et al. 2004). Upon base pairing of U4 and U6 RNAs, Prp24 apparently dissociates from the complex (Shannon and Guthrie 1991; Jandrositz and Guthrie 1995), while the Lsm complex remains bound, at least until activation of the spliceosome (Stevens et al. 2002; Chan et al. 2003). We and others have proposed that Prp24 may return to the U4/U6 complex during spliceosome activation to assist in U4/U6 unwinding (Shannon and Guthrie 1991; Ghetti et al. 1995; Vidaver et al. 1999). Thus, Prp24 likely acts as an RNA chaperone or matchmaker (Pontius and Berg 1992; Portman and Dreyfuss 1994; Herschlag 1995), promoting U4/ U6 RNA association and, perhaps, dissociation.
Saccharomyces cerevisiae Prp24 contains four RNA recognition motifs (RRMs) (Fig. 1A ▶), although the C-terminal RRM is quite degenerate and was not recognized until orthologs of the yeast protein were identified. Prp24 also has a highly conserved decapeptide at its C terminus (Bell et al. 2002; Rader and Guthrie 2002), which we call the “SNFFL box” after its most conserved residues (SNDDFRKMFL). Two-hybrid studies have shown that Prp24 interacts with subunits of the heteroheptameric Lsm complex (Fromont-Racine et al. 2000), and that the SNFFL box is necessary for the Prp24–Lsm5 interaction, suggesting that Prp24 may interact with the Lsm complex via the SNFFL box (Rader and Guthrie 2002). Although all Prp24 orthologs contain the SNFFL box at the C-terminal end, they contain anywhere from one to four RRMs (Rader and Guthrie 2002). The most well-characterized ortholog, human Prp24, or p110 (Bell et al. 2002; Liu et al. 2002), contains two RRMs, which are proposed to correspond to RRMs 2 and 3 of yeast Prp24, based on a sequence alignment (Bell et al. 2002). Human Prp24 binds primarily to residues 38–57 of human U6 RNA and residues 10–30 of the variant human U6 RNA found in the “ATAC” spliceosome, U6atac (Bell et al. 2002; Damianov et al. 2004), which correspond to residues 44–63 of yeast U6.
FIGURE 1.
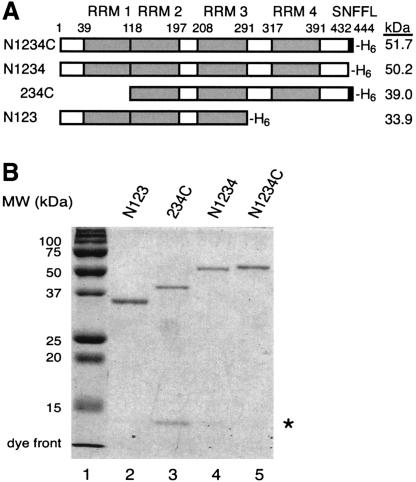
Recombinant Prp24 proteins. (A) Primary structures of Prp24 N- and C-terminal truncation constructs. The domain structure of the full-length protein, N1234C, is represented schematically at the top with four RNA recognition motifs (RRMs 1–4), the SNFFL box, and the 6xHis tag (H6) at the C terminus. N- and C-terminal residues of the constructs are indicated by position number. The name of each protein truncation construct is indicated at left and the calculated molecular weight (kDa; including the 6xHis tag) is shown at right. (B) Purified Prp24 N- and C-terminal truncation constructs were analyzed by 12% SDS-PAGE and Coomassie Blue staining. The asterisk indicates an unidentified contaminant.
It is currently unknown whether all four RRMs in S. cerevisiae Prp24 are necessary for its function. _Trans_-acting suppressors of cold-sensitive mutations in U4 and U6 RNA that interfere with U4/U6 pairing map to RRMs 2 and 3 of Prp24, suggesting that these two RRMs normally stabilize free U6 RNA (Shannon and Guthrie 1991; Vidaver et al. 1999). A triple alanine substitution in RRM 2 is lethal, while the analogous mutation in RRM 3 confers temperature-sensitive growth (Vidaver et al. 1999). Interestingly, while a triple alanine substitution in RRM 4 is also temperature sensitive, an analogous substitution in RRM 1 has no effect on the viability of yeast cells (Vidaver et al. 1999; Rader and Guthrie 2002). These results suggest that RRMs 2, 3, and 4 are important for Prp24 function, while RRM 1 is not, although one cannot exclude the possibility that the residues mutated in RRM 1 are not critical for its function. It has been proposed that RRMs 1 and 4 of yeast Prp24 bind to U4 RNA, and may be at least partially redundant with one another (Rader and Guthrie 2002).
To further assess the functions of the N- and C-terminal RRMs of yeast Prp24, we generated recombinant full-length protein as well as truncated forms lacking RRM 1 or RRM 4, and assayed binding to U6 RNA in vitro. Surprisingly, we found that RRM 1 is important for high-affinity binding to U6, and is required for the discrimination of wild-type and mutant ISL sequences. In contrast, deletion of RRM 4 and the C terminus has no significant effect on the affinity of Prp24 for U6 RNA, but results in the formation of higher order Prp24·U6 complexes. Our sequence analyses indicate that human Prp24 RRMs 1 and 2 are most similar to yeast Prp24 RRMs 1 and 2, respectively, and we find that the high-affinity yeast Prp24-binding site on U6 RNA corresponds well to the previously determined human Prp24-binding site. Alignment of recently discovered Prp24 orthologs reveals that the previous triple alanine substitution in RRM 1 did not alter conserved residues, and we show that substitution of a conserved residue in RRM 1 confers temperature-sensitive lethality to yeast cells, underscoring the importance of RRM 1 in Prp24 function. Temperature-sensitive mutations in either RRM 1 or 2, but not in RRM 3, are lethal at normal growth temperature when combined with mutations in the U6 RNA telestem, which is adjacent to the high-affinity Prp24-binding site. Our results indicate that RRMs 1 and 2 of Prp24 are primarily responsible for the high-affinity binding of U6 RNA, and that this interaction is conserved from yeast to humans. RRMs 3 and 4 apparently bind lower affinity sites on U6 RNA, and thereby restrict the stoichiometry of Prp24 binding.
RESULTS
Expression and purification of Prp24 N- and C-terminally truncated proteins
To determine whether RRMs 1 and 4 are important for binding to U6 RNA, we expressed and purified from Escherichia coli recombinant full-length, N-terminally truncated, and C-terminally truncated versions of Prp24 carrying a C-terminal His6 tag (Fig. 1A ▶). Full-length protein is denoted by N1234C. N represents the N-terminal region of the protein from amino acids 1–39, the numbers 1–4 represent each individual RRM and associated spacer regions, and C represents the SNFFL box. The truncation constructs are named after the domains that are retained as follows: N1234, 234C, and N123. Figure 1B ▶ shows the purity and relative sizes of the recombinant proteins, as detected by SDS-PAGE and Coomassie Blue staining. The identity of the low molecular-weight contaminant in the 234C preparation is unknown. This contaminant could alter the apparent Kd, but not the adjusted Kd (see below).
Measurement of the affinity of Prp24 for U6 RNA
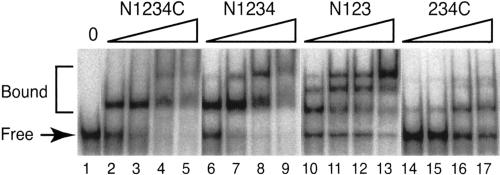
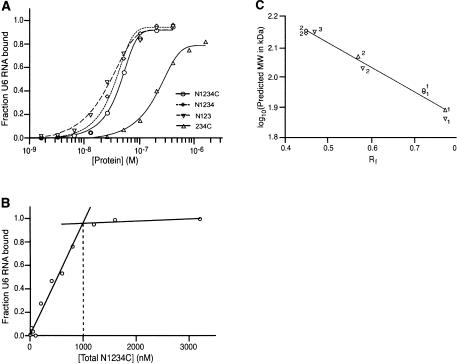
The recombinant proteins were incubated with radiolabeled S. cerevisiae U6 RNA synthesized in vitro by T7 RNA polymerase (see Fig. 4A ▶, below), and assayed for binding by gel-mobility shift (Ghetti et al. 1995). Incubation of full-length Prp24 (N1234C) with U6 RNA results in the formation of a single slow-migrating species that most likely corresponds to one molecule of N1234C bound to one molecule of U6 RNA (Fig. 2 ▶, lanes 2,3). At higher concentrations of protein (Fig. 2 ▶, lanes 4,5), a more slowly migrating higher order complex is detected. The apparent Kd [Kd(app.)] for N1234C binding to U6 RNA under these conditions is ~40 nM (Fig. 3A ▶; Table 1 ▶), which is similar to the previously reported value of 95 nM (Ghetti et al. 1995). The twofold difference in apparent Kd may be due to the fact that Ghetti et al. (1995) used a large excess of E. coli tRNA competitor in the binding reactions, while we did not. Furthermore, Ghetti et al. (1995) used filter-binding assays rather than gel-shift experiments to calculate apparent Kd values. The Hill coefficient of the N1234C interaction with U6 RNA is 1.5 (Table 1 ▶). A Hill coefficient of greater than one suggests some cooperativity between two molecules of N1234C, although we think it is likely that the major shifted species contains only one molecule of N1234C.
FIGURE 4.
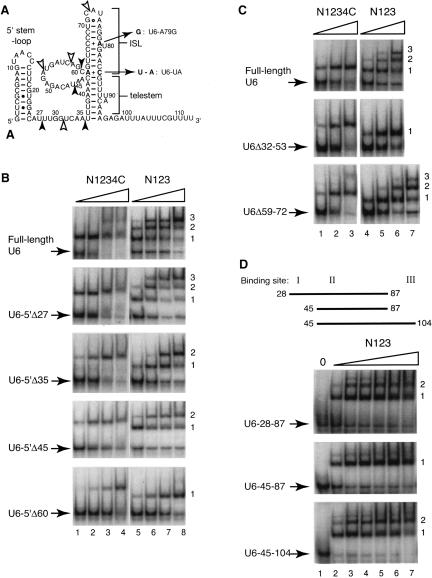
Binding of full-length and C-terminally truncated Prp24 to truncated and internally deleted U6 RNAs. (A) Proposed secondary structure of S. cerevisiae U6 RNA. Here, the ISL includes residues 62–85, and the telestem includes residues 36–43 and 86–95. Not shown is the potential “central stem” pairing between residues 30–34 and 54–58 (Fortner et al. 1994). The filled arrowheads denote the 5′ ends of the 5′-truncated U6 constructs shown in Figure 4B. Open arrowheads denote the endpoints of the internal deletions of U6 constructs shown in Figure 4C. Nucleotides altered by U6 mutations U6-UA and U6-A79G (see below) are indicated in bold. (B) Gel mobility-shift analysis of 5′-truncated U6 RNAs. The RNA-binding activities of N1234C (lanes 1–4) and N123 (lanes 5–8) for full-length and 5′ end-truncated U6 RNAs as indicated were monitored by gel mobility-shift analysis. Complexes and free RNA (indicated by arrows) were resolved on a 6% native polyacrylamide gel. The wedge represents increasing protein concentrations, i.e., 50, 100, 200, and 400 nM. Complexes are numbered 1 through 3 at the right. (C) Gel mobility-shift analysis of internally deleted U6 RNAs. RNA-binding activities of N1234C (lanes 1–3) and N123 (lanes 4–7) for the internally deleted U6 RNAs U6-Δ32–53 and U6-Δ59–72 were assayed as in B. The wedges represent increasing protein concentrations, i.e., 25, 50, and 100 nM (lanes 1–3,4–6) and 200 nM (lane 7). (D) Gel mobility-shift analysis of U6 RNA oligonucleotides U6-28–87, 45–87, and 45–104. U6 RNA oligonucleotides are depicted by straight lines containing combinations of N123-binding sites I, II, and/or III. RNA-binding activities of N123 for the RNAs were assayed as in B. (Lane 1) A control without added protein; the wedges represent increasing protein concentrations, i.e., 25, 50, 100, 200, 400, and 800 nM (lanes 2–7).
FIGURE 2.
U6 RNA-binding activities of the Prp24 truncation constructs monitored by gel mobility-shift analysis. Complexes and free RNA were resolved on a 6% native polyacrylamide gel. (Lane 1) A control without added protein; the wedges represent increasing protein concentrations, i.e., 50, 100, 200, and 400 nM.
FIGURE 3.
Affinity and stoichiometry of full-length and truncated Prp24 binding to U6 RNA. (A) Binding curves for N1234C (○), N1234 (⋄), N123 (▿), and 234C (▵). The data are from the experiments shown in Figure 2 ▶. Binding of each Prp24 construct was assayed three times, and the deviation from the mean Kd was no more than 10%. (B) Determination of concentration of binding-competent Prp24. A total of 42 nM 32P-labeled U6 RNA was mixed with varying concentrations of N1234C. The fraction of 32P-labeled U6 bound was plotted against the concentration of total N1234C. The linear increase in fraction RNA bound saturates at 1000 nM N1234C. (C) The predicted log molecular weights (in kDa) of protein–RNA complexes in the gel-shift analysis (Fig. 2 ▶) were plotted against the measured Rf values of the complexes. The points were fitted with a straight line (correlation coefficient = 0.98). Symbols are as in A. Numbers located next to the points correspond to the predicted stoichiometry of protein molecules bound to one molecule of U6 RNA.
TABLE 1.
Kd (in nM), Bmax, and Hill coefficient (n) of U6 RNA·protein complexes
| Construct | Kd (app.) | Kd (adj.) | Bmax | n |
|---|---|---|---|---|
| N1234C | 43 ± 11 | 1.8 ± 0.5 | 1.0 | 1.5 |
| N1234 | 27 ± 5 | 1.0 ± 0.2 | 1.0 | 1.6 |
| N123 | 24 ± 2 | 1.4 ± 0.1 | 1.0 | 1.3 |
| 234C | 224 ± 43 | 8.3 ± 1.6 | 0.85 ± 0.06 | 1.3 |
To relate apparent Kd values to actual Kd values, we determined the concentration of binding-competent protein by adding increasing amounts of protein to a high concentration of U6 RNA until saturation of U6 RNA binding was achieved (Fig. 3B ▶; Polach and Uhlenbeck 2002). The concentration of total U6 RNA divided by the concentration of total protein needed for all of the RNA to be bound gave the relative concentration of binding-competent protein in each preparation. These values ranged from 3.6% to 5.9%, and the Kd values were adjusted accordingly [Kd(adj.); Table 1 ▶]. The low concentration of binding-competent protein in each preparation could be due to a large fraction of nonfunctional protein, a high stoichiometry of binding, or some combination of these two properties. Thus, the affinity of Prp24 for U6 RNA appears to be significantly higher than previously thought, yielding an adjusted Kd of ~2 nM.
The N terminus and RRM 1 contribute to U6 RNA-binding affinity, while RRM 4 and the C terminus restrict the stoichiometry of Prp24 binding to U6
Deletion of the N terminus and RRM 1 of Prp24 (234C), results in an approximately fivefold decrease in affinity for U6 RNA (Fig. 2 ▶, lanes 14–17; Fig. 3A ▶). Furthermore, even at the highest protein concentration tested (1.6 μM), free U6 RNA is still present. Binding of U6 RNA by 234C saturates at 85% of the total RNA (Table 1 ▶; Fig. 3A ▶). These results indicate that RRM 1 and/or the N terminus contribute significantly to U6 RNA binding.
In contrast, deletion of RRM 4 and the C-terminal sequences (N123) had no significant effect on the affinity for U6 RNA (Table 1 ▶; Fig. 3A ▶). However, removal of RRM 4 resulted in the appearance of multiple higher order complexes, even at low protein concentration (Fig. 2 ▶, lanes 10–13), while deletion of the SNFFL box alone (N1234) had only a minor effect on higher order complex formation (Fig. 2 ▶, lanes 6–9). These results indicate that RRM 4 and/or the C terminus restrict the stoichiometry of Prp24 binding to U6 RNA.
To test whether the three complexes formed between N123 and U6 RNA have a mobility consistent with a stoichiometry of one, two, or three protein molecules per RNA molecule, the Rf value of each complex was determined by dividing the distance traveled by the distance free U6 migrated. The log of the expected molecular weight of each complex was plotted against the measured Rf value of that complex. The plot produced a set of points that fit quite well to a straight line (Fig. 3C ▶), suggesting that the proposed stoichiometry of the complexes is correct. An alternative explanation is that one molecule of protein binds multiple copies of U6. To address this possibility, we conducted in vitro binding in a mixture of radiolabeled full-length U6 and a truncated version of U6 (U6-Δ59-72) (Fig. 4A ▶; Eschenlauer et al. 1993). N123·U6-Δ59-72 complexes have a Kd similar to that of N123·U6 complexes, but migrate faster through the gel. If two different-sized RNAs were binding to one N123 molecule, we would expect to observe higher order protein–RNA complexes with mobilities intermediate to those of the N123·U6 and N123·U6-Δ59-72 complexes. We did not see intermediates (data not shown), suggesting that the higher order complexes in the gel shifts contain a single RNA molecule and two or three protein molecules.
Binding sites of Prp24 on U6 RNA
To map the location of the binding site(s) on U6 RNA for full-length Prp24 and N123, we created a series of 5′-end truncated U6 RNA mutants (Fig. 4A ▶) as follows: U6-5′Δ27, U6-5′Δ35, U6-5′Δ45, and U6-5′Δ60. In each case, two G residues were added at the 5′ end to promote efficient transcription initiation by T7 RNA polymerase. N1234C binding of all the U6-5′-deletion constructs resulted in formation of only one slower migrating complex (Fig. 4B ▶, lanes 1–4). However, the fraction of U6 bound at a given concentration of N1234C decreased steadily with progressive 5′ truncation, as can be seen most clearly by examining the 100-nM protein concentration (Fig. 4B ▶, lane 2). Therefore, binding of U6 RNA by N1234C is partially, but not completely dependent on sequences between positions 1 and 60.
As with full-length U6, binding of U6-5′Δ27 to N123 resulted in the formation of three complexes (Fig. 4B ▶, lanes 5–8). However, binding of U6-5′Δ35 and U6-5′Δ45 to N123 resulted in the formation of only two complexes (Complexes 1 and 2 in Fig. 4B ▶, lanes 5–8). Further increases in N123 concentration did not result in Complex 3 formation for either U6-5′Δ35 or U6-5′Δ45 (data not shown). Thus, it is likely that the upstream border of an N123-binding site (here called site I) exists between nucleotides 28 and 35 in U6. U6-5′Δ60 and N123 form only one slower migrating species (Complex 1, Fig. 4B ▶, lanes 5–8), indicating that at least a portion of a second N123-binding site is located between nucleotides 46 and 60 (site II), and that a third N123-binding site exists somewhere between nucleotides 61 and the 3′ end of U6 RNA. The 3′ truncation of U6 maps the 3′ border of the third site somewhere between nucleotides 96 and 103 (site III; data not shown). As seen with full-length U6 RNA, the affinity of N123 for all the truncated RNAs is comparable to or greater than that of N1234C, reinforcing our conclusion that neither RRM 4 nor the C terminus contribute to the net affinity of Prp24 for U6 RNA.
U6 RNA with an internal deletion of nucleotides 32–53 (Eschenlauer et al. 1993) forms only one complex with N123, and thus is missing two binding sites (Fig. 4C ▶, lanes 4–6). In contrast, U6 RNA lacking nucleotides 59–72 forms all three complexes, although formation of complex 3 is less efficient (Fig. 4C ▶, lanes 4–7). Both internal deletions affect binding affinity to full-length Prp24, with the deletion of nucleotides 32–53 being more disruptive (Fig. 4C ▶, lanes 1–3). These results help narrow down the 5′ and 3′ borders of the second site to nucleotides 46 and 58, respectively. Assuming that the sites do not overlap, the first N123-binding site lies within nucleotides 27–45.
To further confirm the presence of three N123-binding sites in U6, we tested N123 binding to three RNA oligonucleotides predicted to contain sites I and II (U6-28–87), site II only (U6-45–87), and sites II and III (U6-45–104). As expected, we saw two higher order complexes for both U6-28–87 and U6-45–104 RNAs, but only one complex for U6–45–87 (Fig. 4D ▶). The apparent Kd of N123 for U6-45–87 is ~25 nM, similar to that observed for full-length U6. These results indicate that the high-affinity N123-binding site on U6 is site II (nt 47–58). The location of site II corresponds very well to the high-affinity binding site of human Prp24 on human U6 RNA, which is equivalent to yeast residues 44–63 (Bell et al. 2002; Damianov et al. 2004).
RRM 1 contributes to the recognition of the U6 ISL sequence by Prp24
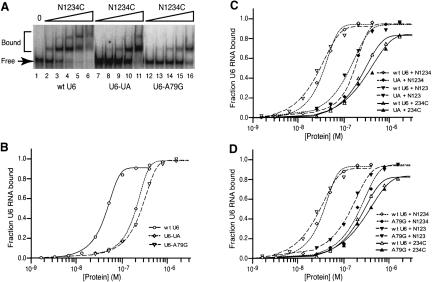
To determine whether binding of Prp24 to U6 RNA in vitro is sensitive to point mutations that affect U6 RNA function in vivo, we tested binding to U6-UA and U6-A79G (Fig. 4A ▶). Each mutation replaces an A+C base pair in the ISL with a Watson–Crick base pair, and each confers a cold-sensitive growth defect (Fortner et al. 1994). N1234C affinity for U6-UA and U6-A79G is five- to sixfold less than for wild-type U6 (Fig. 5A,B ▶; Table 2 ▶), which demonstrates that Prp24 binding to U6 RNA in vitro is sequence specific. Prp24 may directly contact the A+C base pairs in the ISL, or mutation of these residues may alter the secondary or tertiary structure of U6 in a way that perturbs the structure of other regions important for recognition by the protein.
FIGURE 5.
U6-UA and U6-A79G mutations decrease affinity for full-length Prp24, N1234, and N123, but not the RRM 1 deletion construct 234C. (A) Prp24-binding activities of wild-type U6 (lanes 2–6), U6-UA (lanes 7–11), or U6-A79G (lanes 12–16) were monitored by gel mobility-shift analysis. (Lane 1) A control without added protein; the wedges represent increasing N1234C concentrations, i.e., 25, 50, 100, 200, and 400 nM. (B) N1234C-binding curves for wild-type U6 (○), U6-UA (⋄), and U6-A79G (▿). (C) Binding curves for N1234 with wild-type U6 (⋄) and U6-UA (♦), N123 with wild-type U6 (▿) and U6-UA (▾), and 234C with wild-type U6 (▵) and U6-UA (▴). (D) Binding curves for Prp24 constructs as in C, except U6-UA is replaced by U6-A79G. The data are from single representative experiments. Binding of each Prp24 construct was assayed three times, and the deviation from the mean Kd was no more than 10%.
TABLE 2.
Kd (in nM), Bmax, and Hill coefficient (n) of mutant U6 RNA·N1234C complexes
| Construct | Kd (app.) | Kd (adj.) | Bmax | n |
|---|---|---|---|---|
| wt-U6 | 43 ± 11 | 1.8 ± 0.4 | 1.0 | 1.5 |
| U6-UA | 190 ± 20 | 7.6 ± 0.8 | 1.0 | 1.8 |
| U6-A79G | 250 ± 20 | 10 ± 0.8 | 1.0 | 1.5 |
To pinpoint which domain of Prp24 confers sensitivity to the U6-UA and U6-A79G mutations, we tested binding of N1234, N123, and 234C to the mutant RNAs. Like N1234C, N1234 and N123 both exhibited an approximately fivefold decrease in binding affinity to U6-UA and U6-A79G in comparison to wild-type U6 (Fig. 5C,D ▶), suggesting that the SNFFL box and RRM 4 do not recognize a site affected by the mutations. Interestingly, 234C binding was relatively unaffected by both the U6-UA and U6-A79G mutations, indicating that RRM 1 may normally contact regions of U6 RNA whose structure is altered by these substitutions. The simplest interpretation of these data is that RRM 1 of Prp24 binds the U6 ISL region in a sequence-specific fashion.
Substitution of a highly conserved residue in RRM 1 of Prp24 confers temperature-sensitive growth
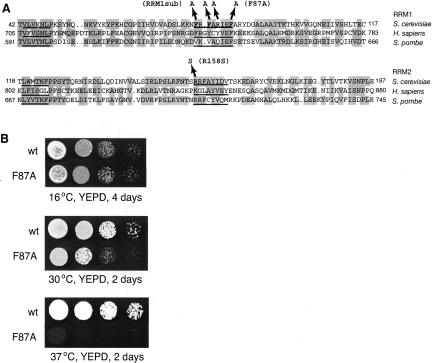
The close correspondence between the high-affinity Prp24-binding sites of yeast and human U6 RNA, and our discovery that RRM 1 of yeast Prp24 is important for high-affinity binding to U6 RNA, suggest that human RRM 1 may be homologous to yeast RRM 1, and not to yeast RRM 2 as previously proposed (Bell et al. 2002). We therefore used the WU BLAST 2.0 program on the Saccharomyces Genome Database (www.yeastgenome.org) to compare human Prp24 RRM 1 with all translated open-reading frames from S. cerevisiae. The only yeast Prp24 RRM that scored as a match was RRM 1. Likewise, human Prp24 RRM 2 recovered RRM 2 of yeast Prp24, but not RRMs 1, 3, or 4. Figure 6A ▶ shows an alignment of RRMs 1 and 2 from S. cerevisiae, humans, and the fission yeast Schizosaccaromyces pombe. Interestingly, this alignment reveals that the triple alanine substitution that we made previously in RRM 1 (RRM1sub) does not alter the most conserved residues in the RNP-1 motif, which may explain why it does not confer a growth defect (Vidaver et al. 1999). We therefore revised our alanine substitution strategy to target the conserved phenylalanine residue at position 87. Strikingly, the prp24-F87A allele confers a recessive slow growth phenotype at 30°C and is lethal at 37°C (Fig. 6B ▶). Thus, in agreement with our biochemical results, RRM 1 is important for Prp24 function in vivo.
FIGURE 6.
A temperature-sensitive lethal mutation in RRM 1 of Prp24. (A) Sequence alignment of the S. cerevisiae RRMs 1 and 2 with homologous RRMs in H. sapiens and S. pombe. The shaded regions indicate conservation of the physicochemical properties of the residues. The RNP-2 and RNP-1 motifs (Kenan et al. 1991; Burd and Dreyfuss 1994) are underlined. The three residues replaced by alanine substitutions in the RRM1sub allele and a temperature-sensitive mutation in RRM 2 (prp24-R158S) (Vidaver et al. 1999) are indicated. The conserved phenylalanine residue at position 87 in RRM 1 was selected for alanine substitution. (B) Yeast strains containing either the wild-type (WT) or prp24-F87A allele on a low-copy plasmid and a disruption of the genomic PRP24 locus were plated to YEPD medium as serial 10-fold dilutions and incubated at the indicated temperatures and times.
Genetic interactions of Prp24 mutations with U6 telestem mutations
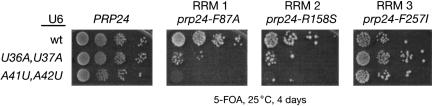
prp24-R158S and prp24-F257I are temperature-sensitive mutations in the RNP-1 motif of RRMs 2 and 3, respectively, that were originally selected as suppressors of the A62G cold-sensitive mutation in U6 RNA (Vidaver et al. 1999). Destabilizing telestem mutations in U6 were also shown to suppress A62G cold sensitivity, and the temperature sensitivity of prp24-R158S, but not prp24-F257I, is enhanced (exacerbated) by these mutations (Vidaver et al. 1999). To determine whether RRM 1 behaves similarly to RRM 2 or RRM 3 in this regard, we tested PRP24 alleles containing mutations in each of these domains in combination with the U6 telestem destabilizing mutations snr6-U36A,U37A and snr6-A41U,A42U (Vidaver et al. 1999). The F87A mutation in RRM 1 and the R158S mutation in RRM 2 both enhance U6-U36A,U37A and U6-A41U,A42U, while the RRM 3 mutation F257I does not (Fig. 7 ▶). This result indicates that alteration of the telestem exacerbates the defect caused by the mutations in RRMs 1 or 2, but not by the mutation in RRM3.
FIGURE 7.
Interaction of mutations in the U6 telestem with mutations in RRMs 1 and 2 of Prp24. Yeast strains with chromosomal disruptions of the Prp24 and U6 RNA genes, and bearing either the wild-type Prp24 allele (PRP24) or temperature-sensitive allele prp24-F87A (RRM 1), prp24-R158S (RRM 2), or prp24-F257I (RRM 3) on a _HIS3_-marked plasmid, as well as wild-type U6 on a counter-selectable _URA3_-marked plasmid, were transformed with plasmids bearing the wild-type U6 allele (U6-wt), U6-U36A,U37A, or U6-A41U,A42U on a _TRP1_-marked plasmid. Starting with cultures of equal cell density, serial 10-fold dilutions were plated to medium containing 5-FOA to select against the wild-type U6 plasmid, and incubated at 25°C for 4 d.
DISCUSSION
U6 RNA undergoes remarkably large changes in conformation during the splicing cycle. While some of this conformational flexibility is undoubtedly programmed into the U6 RNA sequence, U6 RNA’s structural transitions must be assisted by protein cofactors. Prp24 is a prime candidate for a U6 RNA chaperone, given its presence in the U6 snRNP, its known activity in promoting U4/U6 RNA association, and its genetic interactions with mutations in U6 and U4 RNAs that alter their pairing. To begin to assess the RNA chaperone activity of Prp24, we determined the U6 RNA-binding activity of recombinant full-length, N-terminally truncated and C-terminally truncated forms of the protein. We found that deletion of RRM 1 and the N terminus (234C) results in a fivefold decrease in the affinity for U6 RNA, revealing a previously unknown function for this region in U6 binding. Deletion of RRM 4 and the SNFFL box (N123), in contrast, results in no significant change in affinity for U6 RNA, but the appearance of multiple higher order complexes. Our results indicate that the C-terminal portion of Prp24, including RRM 4, restricts the stoichiometry of Prp24 binding to U6 RNA, most likely by occupying RRM-binding sites in the RNA. Furthermore, we show that deletion of RRM 1 and the N terminus greatly reduces the ability of Prp24 to discriminate between wild-type U6 and U6 containing ISL mutations U6-UA and U6-A79G. In agreement with our in vitro results that implicate RRM 1 in Prp24 function, we identified a temperature-sensitive lethal point mutation in the RNP-1 motif of RRM 1, F87A, which allowed us to compare the genetic interactions of RRM 1 with previously determined genetic interactions of RRMs 2 and 3. We found that RRM 1 acts like RRM 2, but not like RRM 3, with respect to mutations in the telestem.
RRMs 1 and 2 of yeast Prp24 are homologous to the two RRMs of human Prp24
Several prior observations suggested that RRMs 2 and 3 of yeast Prp24 are primarily responsible for binding to U6 RNA. First, spontaneous suppressors of a cold-sensitive mutation in the U6 ISL mapped to RRMs 2 and 3, but not to other domains of Prp24 (Vidaver et al. 1999). Second, triple alanine substitutions in RRMs 2 and 3 of Prp24 are lethal and temperature-sensitive lethal, respectively, while a similarly positioned mutation in RRM 1 confers no growth defect (Vidaver et al. 1999). Third, initial sequence alignments of human and yeast Prp24 indicated that RRMs 2 and 3 of the yeast protein are most similar to the two RRMs of the human protein (Bell et al. 2002).
The results presented in this study alter our interpretation of these prior observations. BLAST analysis indicates that RRMs 1 and 2, not 2 and 3, of yeast Prp24 are most similar to the two RRMs of human Prp24. The resulting alignment shows that the original triple alanine substitution does not alter conserved residues (Fig. 6A ▶), and alanine substitution of a single conserved phenylalanine residue at the C terminus of the RNP-1 motif of RRM 1 severely diminishes Prp24 function in vivo, consistent with an essential role of RRM 1 in Prp24 function. The in vitro-binding studies suggest that one essential role of RRM 1 is sequence-specific binding of U6 RNA. The conclusion that RRMs 1 and 2 of yeast Prp24 are primarily responsible for U6 RNA binding is further supported by the enhancement (exacerbation) of mutations in RRMs 1 and 2, but not RRM 3, when combined with mutations in either the upper or lower telestem. While the molecular mechanism of this enhancement in unclear, the fact that it affects RRMs 1 and 2 similarly is consistent with the hypothesis that these two RRMs share a common function that involves the telestem.
Although RRM 1 appears to be important for high-affinity binding to U6 RNA, the lack of conservation in the N-terminal portion of RNP-1, coupled with our mutational data, suggest that it does not contact RNA in a canonical fashion. A possible explanation for these observations is that the lack of a linker region between RRMs 1 and 2 constrains their structure in a manner that precludes the conventional interaction of RNA with the RNP-1 motif of RRM 1. Instead, other regions of RRM 1 may be involved in contacting the RNA. Interestingly, the NMR structure of hamster nucleolin RRMs 1 and 2 bound to the nucleolin recognition element (NRE) shows that the residues corresponding to Prp24 F87 do not directly contact the RNA (Allain et al. 2000), yet point mutations in these residues were isolated in an in vivo screen for loss of NRE binding (Bouvet et al. 1997). The F87A mutation in Prp24 may similarly affect the conformation of RRM 1 in such a way that binding affinity to U6 RNA is decreased.
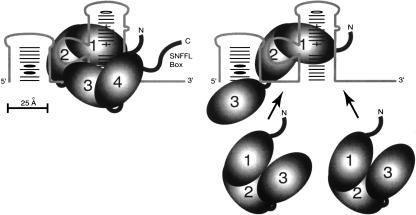
We cannot exclude the possibility that RRM 1 participates in intramolecular protein–protein interactions rather than (or in addition to) intermolecular protein–RNA interactions, and in doing so, modifies the RNA-binding activities of one or more of the other RRMs. In this regard, it is interesting that RRM 1 of Dutch-elm fungus Prp24, which also has four RRMs, was recently classified as a possible U2AF homology motif, or UHM (Kielkopf et al. 2004). UHMs are RRM-like domains that mediate protein–protein interactions. The prototypical UHMs are in U2AF35 and U2AF65, and in each case, the UHM binds to a tryptophan residue of its polypeptide ligand. Conceivably, RRM 1 of yeast Prp24 is also a UHM. The only tryptophan residue in yeast Prp24 is in the RNP-2 motif of RRM 2, suggesting the possibility that RRM 1 binds and stabilizes RRM 2. This tryptophan residue is conserved or substituted with another aromatic residue (tyrosine or phenylalanine) in more than a dozen fungal orthologs, as well as in human Prp24 (Fig. 6A ▶). RRM 1 of Dutch-elm fungus has a low isoelectric point (pI = 6.8), which is a hallmark of a UHM. However, RRM 1 of S. cerevisiae Prp24 has a higher isoelectric point (pI = 9.5), suggesting that it may not be a UHM. We therefore favor a model in which the primary function of RRM 1 is direct interaction with U6 RNA (Fig. 8 ▶). Interestingly, RRM 3 is highly acidic (pI = 4.9), suggesting that it might mediate protein–protein interactions.
FIGURE 8.
Model for Prp24 interaction with U6 RNA. (Left) The full-length Prp24 molecule is shown binding U6. RRMs 1 and 2 are depicted interacting with site II (nts 47–58), RRM 3 with site I (nts 28–35), RRM 4 with site III (nts 96–103), while the SNFFL box is positioned adjacent to the Lsm-binding site near the 3′ terminus of U6. On the right, three N123 molecules are shown interacting with regions I–III in U6. RRMs 1 and 2 of one molecule bind site II in a similar fashion as for full-length Prp24. It is not clear which RRMs of N123 bind sites I and III.
The primary binding site of Prp24 on U6 RNA is conserved from yeast to humans
The elevated binding stoichiometry of the N123 construct allowed us to map three Prp24-binding sites on U6 RNA. These sites are located in putative single-stranded regions immediately upstream of the telestem, between the telestem and ISL, and immediately downstream of the telestem. The simplest interpretation of our results is that a single molecule of wild-type Prp24 binds all three sites, while a single molecule of N123 can bind only one site (Fig. 8 ▶). Notably, binding of full-length Prp24 to U6 RNA in vitro results in the strong protection of two of these three regions from hydroxyl radical cleavage, residues 30–39 and 50–58 (Ghetti et al. 1995). Given the fact that N123 binds a truncated U6 RNA containing only the central site (U6-45–87) with approximately the same affinity as full-length U6, we infer that the central site is responsible for the high-affinity binding of Prp24. The flanking sites remain exposed and are bound with lower affinity by additional molecules of N123 as the protein concentration is increased.
Why does deletion of one RRM (RRM 4) expose two sites? While it is possible that RRM 4 and its flanking sequences are responsible for binding both sites, we prefer the explanation that the deletion of conserved sequences immediately C-terminal to RRM 3 in the N123 construct disrupts the RNA-binding activity of RRM 3. Thus, our favored model shows RRM 3 interacting with sequences upstream of the telestem, and RRM 4 binding sequences downstream of the telestem adjacent to the Lsm-binding site (Fig. 8 ▶). Thus, in this model, the C-terminal SNFFL box is properly positioned to interact with the Lsm complex (Achsel et al. 1999; Vidal et al. 1999; Rader and Guthrie 2002).
Binding of the second and third N123 molecules to the low-affinity sites could be mediated by RRMs 1 and/or 2. Interestingly, sequences flanking the telestem are similar to sequences in the central, high-affinity Prp24-binding site. In particular, the sequence AGAGAU appears both at positions 49–54 and 95–100. Also, the sequence GGUCAA at positions 30–35 is similar to GAUCAG at positions 55–60. RRMs 1 and 2 of a single N123 molecule may bind these sequences cooperatively in the central site, while additional N123 molecules bind separately to the flanking sites. When full-length Prp24 binds U6 RNA, the flanking sites are presumably occupied by RRMs 3 and 4. However, the higher order Prp24·U6 complex observed at high concentrations of full-length protein could reflect displacement of RRM 3 and/or 4 by another molecule of Prp24. Alternatively, there may be a low-affinity binding site for an additional molecule of full-length Prp24 in the 5′ stem–loop, since 5′-truncated U6 appears not to form the higher order complex (Fig. 4B ▶). It is not clear whether the secondary binding sites of Prp24 on U6 RNA are of functional relevance, or are simply “parking spaces” for RRMs 3 and 4 when they are not engaged in other interactions.
We show here that the high-affinity binding site for yeast Prp24 is mostly or entirely contained within nucleotides 45–87 of yeast U6 RNA (Fig. 4D ▶). This region corresponds almost exactly to the loop between the telestem and ISL, and the ISL itself. It is unlikely that the ISL contributes much to Prp24 binding, since deletion of the 5′ half of the ISL (Δ59–72) does not substantially disrupt any of the binding sites (Fig. 4C ▶), and Prp24 exhibits little or no detectable binding to the ISL alone (data not shown). Thus, the high-affinity binding site of yeast Prp24 most likely lies within residues 45–58 of yeast U6 RNA, a region contained entirely within the sequences corresponding to the human Prp24-binding site in human U6 RNA (yeast residues 44–63). Since the yeast and human U6 RNAs are identical at 12 of 14 positions in this interval (yeast residues 45–58), we conclude that yeast and human Prp24 bind nearly identical sequences with their respective RRMs 1 and 2.
Genetic interactions between Prp24 RRMs and the U6 telestem: Implications for the spliceosome activation cascade
We showed previously that mutations that disrupt the lower telestem of yeast U6 RNA enhance (exacerbate) the effect of a mutation in RRM 2, but not RRM 3 (Vidaver et al. 1999). Here, we show that mutations expected to disrupt the upper telestem have an even stronger synthetic effect with the RRM 2 mutation, while still failing to interact with the RRM 3 mutation (Fig. 7 ▶). Furthermore, we show that a mutation in RRM 1 acts just like a mutation in RRM 2 in this regard. In light of our proposal that the telestem promotes U4/U6 dissociation (Vidaver et al. 1999), these genetic interactions suggest that binding of RRMs 1 and 2 to U6 RNA promotes its dissociation from U4 RNA. To do so would require that RRMs 1 and 2 bind within nucleotides 45–58 of U6 when U6 is paired with U4 RNA, which should be possible, since these residues lie mostly outside of the U4/U6 base-paired region. Indeed, binding of recombinant yeast Prp24 to the U4/U6 RNA complex in vitro strongly protects residues 50–57 from hydroxyl radical cleavage (Ghetti et al. 1995).
In considering how Prp24 might bind U6 RNA during spliceosome activation, it is intriguing that the primary Prp24-binding site colocalizes with the 5′ splice-site pairing sequences. Current evidence (for review, see Brow 2002; Chan et al. 2003) indicates that the ACA sequence at positions 43–45 of U6 base pairs with positions 4–6 of the intron early in the spliceosome activation process. Later, possibly after U4/U6 unwinding, 5′ splice-site pairing shifts to the ACA sequence at positions 47–49. This latter site abuts or overlaps the primary Prp24-binding site, suggesting that both interactions could not occur simultaneously. A plausible model is that Prp24 binds U6 RNA when it is base paired to the 5′ splice site via the upstream ACA sequence. Prp24-assisted isomerization of U6 RNA results in displacement of U4 RNA, and subsequent release of Prp24 (perhaps catalyzed by a DEXD/H-box protein) allows the 5′ splice site to shift to the downstream ACA sequence.
The absence of a genetic interaction between the telestem mutations and the RRM 3 mutation suggests that RRM 3 does not have an important role in U4/U6 unwinding. RRMs 3 and 4 may primarily promote U4/U6 association, perhaps by binding to U4 RNA and/or U4 snRNP proteins. The absence of RRMs 3 and 4 in human Prp24 could be explained by the fact that human Prp24 binds the 90K U4 snRNP protein through an extended N-terminal domain not present in yeast Prp24 (Medenbach et al. 2004). Thus, yeast and human Prp24s may use different interactions to promote U4/U6 association.
Conclusions
Our results suggest that the current model for Prp24 function requires significant revision. In particular, we propose a more central role for RRM 1 in Prp24 function than has been proposed previously. Although we cannot exclude the involvement of RRM 3 in binding to U6 RNA, our results are most compatible with a model in which RRMs 1 and 2 are primarily responsible for anchoring Prp24 to U6. This model is appealing, because it implies that the most fundamental interaction in Prp24 function, binding of U6 RNA, is conserved from yeast to humans. Ultimately, structural analysis of the Prp24·U6 complex will be required to validate our model. Our in vitro-binding data suggest that a complex consisting of RRMs 1 and 2 of yeast Prp24 and residues 45–58 of yeast U6 RNA is a promising starting point for structural studies.
MATERIALS AND METHODS
Plasmids and strains
E. coli pET expression vectors used to produce purified protein were prepared as follows. NdeI–XhoI DNA fragments were generated by PCR using pRS314-PRP24 (Vidaver et al. 1999) as template and inserted into NdeI–XhoI-digested pET21b (Novagen). Primer pair 24START-5′ and 24END-3′ was used to generate N1234C, 24START-5′ and 24ΔSNF-3′ for N1234, 24RRM2-5′ and 24END-3′ for 234C, and 24START-5′ and 24RRM3-3′ for N123 (Table 3 ▶).
TABLE 3.
Oligonucleotides used in this study
| Primer name | Sequence |
|---|---|
| 24START-5′ | 5′-CGCCATATGGAGTATGGACATCACGCT-3′ |
| 24RRM2-5′ | 5′-CGCCATATGACAGAATGCACATTATGG-3′ |
| 24RRM3-3′ | 5′-CCGCTCGAGTGGTTTTTTATCAGCCAAG-3′ |
| 24RRM3-5′ | 5′-CGCCATATGGAGGGGCGAGAGATTATGATAC-3′ |
| 24RRM4-5′ | 5′-CGCCATATGTTTCCACTTTCAGATAAG-3′ |
| 24ΔSNF-3′ | 5′-CCGCTCGAGCATCTGCTCTTGTTTGTC-3′ |
| 24END-3′ | 5′-CCGCTCGAGCTCACCTAGAAACATCTT-3′ |
| T7U6-5′ | 5′-CGGAATTCTAATACGACTCACTATAGGTTCGCGAAGTAACCC-3′ |
| T7U6-5′Δ27 | 5′-CGGAATTCTAATACGACTCACTATAGGTTGGTCAATTTGAAACAATACA-3′ |
| T7U6-5′Δ35 | 5′-CGGAATTCTAATACGACTCACTATAGGTTTGAAACAATACAGAGATGAT-3 |
| T7U6-5′Δ45 | 5′-CGGAATTCTAATACGACTCACTATAGGTACAGAGATGATCAGCAGT-3′ |
| T7U6-5′Δ60 | 5′-CGGAATTCTAATACGACTCACTATAGGCAGTTCCCCTGCATAAG-3′ |
| 3′-6DBbsBam | 5′-CAAAGAGATTTATTTCGTTTTCGGTCTTCGGATCCGCG-3′ |
| U6 RNaseHa | 5′-mCmGmAmAmGmAmCmCmGdAdAdAdAmCmGmAmAmAmUmAmA-3′ |
| Prp24Seq1 | 5′-GGAGTATGGACATCACGC-3′ |
| 24RRM1-F87Ab | 5′-CGCTGAAGAAGAACTTTCGTTTTGCACGTATTGAAGCTGCC-3′ |
| PRP24 5′PCR | 5′-CCAGGTTCTTCAGATGTCC-3′ |
pRS313-prp24-F87A was generated by mutagenesis of pRS314-PRP24 (Vidaver et al. 1999) using oligonucleotide 24RRM1-F87A (Table 3 ▶) as described (Kunkel et al. 1987; Vieira and Messing 1987). Resulting clones were sequenced with oligonucleotides PRP24-5′PCR, Prp24Seq1, 24RRM2-5′, 24RRM3-5′, and 24RRM4-5′ (Table 3 ▶). The mutation was then subcloned by isolating the SacI–RsrII fragment from pRS314-prp24-F87A and ligating into SacI–RsrII cut pRS313-PRP24 (Vidaver et al. 1999).
Mutant alleles of PRP24 cloned into pRS313 were tested for their ability to function as the sole PRP24 gene in the cell by transformation (Gietz et al. 1995) into a yeast strain containing a disruption of the chromosomal copy of PRP24 (LL101; MATa his3 leu2 trp1 ura3 met2 can1 ade2 lys2 prp24_-Δ_1::ADE2 [_pUN50-PRP24_]) (Vidaver et al. 1999), selection on -His medium, and subsequent plating to synthetic complete medium containing 0.75 mg/mL 5-fluoroorotic acid (5-FOA). Serial 10-fold dilutions of the 5-FOA-resistant strains were plated on YEPD medium.
To test for genetic interactions between alleles of PRP24 and SNR6 (the U6 RNA gene), variants of a yeast strain containing a disruption of the chromosomal copies of SNR6 and PRP24 (LL200; MATa _his3 leu2 trp1 ura3 met2 can1 ade2 lys2 prp24_Δ_1::ADE2 snr6_Δ::LEU2 [pUN50-_PRP24_], [YCp50-39D6]) (Vidaver et al. 1999) were constructed with pRS313-PRP24, -prp24-R158S, -prp24-F257I (Vidaver et al. 1999), or -prp24-F87A (described above) in place of pUN50-PRP24. SNR6 alleles pSE358-SNR6 (wt-U6) (Fortner et al. 1994), -_snr6_-U36A,U37A, or -_snr6_-A41U,A42U (Vidaver et al. 1999) were transformed into LL200 strains containing the PRP24 allele of interest, selected on -Trp medium, and tenfold dilutions were plated to synthetic complete medium containing 5-FOA to select against the wild-type SNR6 plasmid.
Preparation of recombinant Prp24 proteins
Recombinant proteins with a carboxyl-terminal 6×His tag were prepared as follows. pET21b expression vectors were transformed into Escherichia coli strain BL21-DE3, which was then grown to a density of 0.6 O.D. and induced with 1 mM IPTG for 3 h at 37°C. Cell pellet from a 50-mL induced culture was resuspended in 2 mL Buffer A (500 mM NaCl, 20 mM Tris-Cl at pH 8.0, and 5 mM β-mercaptoethanol) containing 30 mM imidazole and stored overnight at −20°C. After an additional freeze-thaw cycle (30 min at 4°C, −20°C overnight), 250 μL of 10 mg/mL lysozyme (Sigma) in Buffer A was added to the lysate and incubated for 30 min at 4°C. The lysate was then sonicated at power level 2 (Fisher Scientific Sonic Dismembrator Model 100) and cleared by microcen-trifugation for 15 min at 4°C. A total of 2 mL of lysate was incubated with 2 mL of a 50% slurry of Ni-NTA resin (Qiagen) in Buffer A + 30 mM imidazole and rotated overnight at 4°C. The slurry was loaded into a disposable 2 mL column and washed with 10 mL of buffer A + 30 mM imidazole and 4 mL of buffer A + 10% glycerol + 30 mM imidazole. The his-tagged recombinant proteins were eluted with 4 mL of buffer A + 10% glycerol + 80 mM imidazole and 6 mL of buffer A + 10% glycerol + 100 mM imidazole. Peak fractions were dialyzed in three changes of buffer B (100 mM NaCl, 20 mM Tris-Cl at pH 8.0, 5 mM β-mercapto-ethanol, 0.1 mM EDTA) with increasing concentrations of glycerol as follows: 20%, 30%, and 50%. The concentration of protein was determined by Bradford assay (Bio-Rad), and the proteins were stored at −70°C.
Synthesis of full-length and 5′-truncated or internally deleted U6 RNA
DNA template for transcription of full-length U6 was made by PCR amplification of the wild-type U6 RNA gene (SNR6). The 5′ oligonucleotide (T7U6-5′) contains a T7 promoter and one additional G nucleotide prior to the 5′ end of U6 for more efficient initiation of T7 transcription (Table 3 ▶). Because of efficient non-templated addition of nucleotides at the 3′ end of U6 RNA made by run-off transcription, we used a 3′ oligonucleotide that contains additional sequence past the 3′ end of U6 that includes BbsI and BamHI restriction sites (3′-6DBbsBam; Table 3 ▶). To produce a discrete U6 3′ end, we used an RNase H cleavage method to process the in vitro-transcribed RNA (Lapham and Crothers 1996). The PCR product was gel purified (Qiagen QIAquick Gel Extraction Kit), and 0.1 μg of DNA was transcribed in 50 μL with T7 RNA polymerase (USB) in the presence of 1 mM ATP, CTP, and GTP, 0.1 mM UTP, and 1 μCi/μL [α-32P]UTP (1 μCi = 37 kBq) at 37°C for 4 h. For trace-labeled U6 RNA (used for the experiment shown in Fig. 3B ▶), 1 mM ATP, CTP, GTP, UTP, and 0.125 μCi/μL [α-32P]UTP were used. The transcription reaction was digested with 5 U of RQ1 DNase (Promega), phenol/chloroform extracted, and ethanol precipitated. RNA was purified on a 8.3-M urea, 6% polyacrylamide gel [20:1 acrylamide:bis-acryl-amide] and resuspended in TE (10 mM Tris, 1 mM EDTA at pH 8.0). Typically, 20 pmol of U6 RNase H oligo (Table 3 ▶) were added to RNA produced from a 50-μL transcription in a final volume of 10 μL, and the mixture was heated for 3 min at 95°C. The heat block was then removed from the heating unit and cooled at room temperature for 1.5 h to 37°C. RNase H buffer (at final concentrations of 20 mM HEPES-KOH (pH 8.0), 50 mM KCl, 10 mM MgCl2, 1 mM DTT) and 10 U of RNase H (USB) were added for a total reaction volume of 15 μL, and incubation at 37°C was continued for 3 h. RNase H-processed RNA was gel purified as before. The 5′ end-truncated U6 RNAs were made by PCR amplification with the appropriate 5′-end primers (T7U6-5′Δ27, T7U6-5′Δ35, T7U6-5′Δ45, or T7U6-5′Δ60; Table 3 ▶) and 3′-6DBbsBam. Internally deleted U6 RNAs Δ32–53 and Δ59–72 were made by PCR amplification of pΔAB6 and pΔBE6 derivatives (Eschenlauer et al. 1993) with primers T7U6-5′ and 3′-6DBbsBam. Discrete 3′ ends for these RNAs were produced by the same method as above. Gel-purified RNAs were quantitated by scintillation counting. U6-28-87, U6-45-87, and U6-45-104 were ordered from Dharmacon and 5′-end labeled with polynucleotide kinase and [α-32P]ATP. Free radiolabel was removed by application of the sample onto a Sephadex G-25 spin column (Amersham).
Protein–RNA binding assays
Prp24·U6 binding was performed in 10 μL of RNA-binding buffer (100 mM NaCl, 30 mM Tris at pH 7.5, 4% glycerol, 0.2 mM EDTA, and 2 mM DTT), 8 mM MgCl2, 40 μg/mL BSA, 32P-labeled RNA at 2 nM, and indicated concentrations of protein. E. coli tRNA at a concentration of 0.01 ng/μL (0.4 nM) was also present in the experiments shown in Figure 2 ▶. The binding reactions were incubated at room temperature for 30 min prior to loading directly onto a 6% polyacrylamide gel (30:1 acrylamide:bis-acryl-amide; 50 mM Tris, 50 mM boric acid, 1 mM EDTA at pH 8.3; 20 cm × 10 cm × 1.0 mm) that was prerun at 75 V for 1 h at 4°C. Samples were electrophoresed at 4°C for 2 h at 75 V. Gels were dried on Whatman 3MM paper, exposed to a PhosphorImager screen, and results were analyzed on a Molecular Dynamics PhosphorImager using ImageQuant software.
The results of RNA-binding titration assays from a single representative experiment were expressed as the fraction of RNA bound and plotted as a function of Prp24 polypeptide concentration using the Prism software package (Graphpad). The data were fitted to a one-site hyperbolic binding function (Y = Bmax·X ÷{Kd+X}), where Y is the fraction of U6 RNA bound (the sum of all complexes) and X is the concentration of Prp24 polypeptide. Bmax was defined to be 1.0 for all instances in which all of the RNA was bound at high concentrations of protein, and the Kd was determined by the one-site binding function as the protein concentration at which 50% of the RNA formed an RNA–protein complex. Because 234C appeared to be unable to bind all of the U6 RNA even at the highest protein concentrations tested, Bmax for 234C was determined by extrapolation of the one-site binding function hyperbola. The Kd of 234C was then determined with the calculated Bmax. The Hill coefficient (n) was calculated by finding the slope of a straight line fitted to points from a plot of log [θ/(1-θ)] vs. log nM protein, where θ is fraction of U6 RNA that is bound.
Acknowledgments
We thank Kris McConnell for initiating construction of the ΔSN-FFL allele; Allison Morin for help with construction of the SNR6 telestem alleles and strains; David Page, Jr., Tom Record, and members of the Brow and Dahlberg labs for helpful discussions; and C. Joel McManus, Sam Butcher, and Jim Keck for critical reading of the manuscript. This work was supported by grant GM54018 from the National Institutes of Health (NIH) to D.A.B. S.K. was supported in part by NIH Training Grant GM08349.
REFERENCES
- Achsel, T., Brahms, H., Kastner, B., Bachi, A., Wilm, M., and Luhrmann, R. 1999. A doughnut-shaped heteromer of human Sm-like proteins binds to the 3′-end of U6 snRNA, thereby facilitating U4/U6 duplex formation in vitro. EMBO J. 18**:** 5789–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain, F.H., Bouvet, P., Dieckmann, T., and Feigon, J. 2000. Molecular basis of sequence-specific recognition of pre-ribosomal RNA by nucleolin. EMBO J. 19**:** 6870–6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, M., Schreiner, S., Damianov, A., Reddy, R., and Bindereif, A. 2002. p110, a novel human U6 snRNP protein and U4/U6 recycling factor. EMBO J. 21**:** 2724–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet, P., Jain, C., Belasco, J.G., Amalric, F., and Erard, M. 1997. RNA recognition by the joint action of two nucleolin RNA-binding domains: Genetic analysis and structural modeling. EMBO J. 16**:** 5235–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brow, D.A. 2002. Allosteric cascade of spliceosome activation. Annu. Rev. Genet. 36**:** 333–360. [DOI] [PubMed] [Google Scholar]
- Brow, D.A. and Guthrie, C. 1988. Spliceosomal RNA U6 is remarkably conserved from yeast to mammals. Nature 334**:** 213–218. [DOI] [PubMed] [Google Scholar]
- Brow, D.A. and Vidaver, R.M. 1995. An element in human U6 RNA destabilizes the U4/U6 spliceosomal RNA complex. RNA 1**:** 122–131. [PMC free article] [PubMed] [Google Scholar]
- Burd, C.G. and Dreyfuss, G. 1994. Conserved structures and diversity of functions of RNA-binding proteins. Science 265**:** 615–621. [DOI] [PubMed] [Google Scholar]
- Chan, S.P., Kao, D.I., Tsai, W.Y., and Cheng, S.C. 2003. The Prp19p-associated complex in spliceosome activation. Science 302**:** 279–282. [DOI] [PubMed] [Google Scholar]
- Cheng, S.C. and Abelson, J. 1987. Spliceosome assembly in yeast. Genes & Dev. 1**:** 1014–1027. [DOI] [PubMed] [Google Scholar]
- Damianov, A., Schreiner, S., and Bindereif, A. 2004. Recycling of the U12-type spliceosome requires p110, a component of the U6atac snRNP. Mol. Cell. Biol. 24**:** 1700–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenlauer, J.B., Kaiser, M.W., Gerlach, V.L., and Brow, D.A. 1993. Architecture of a yeast U6 RNA gene promoter. Mol. Cell. Biol. 13**:** 3015–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortner, D.M., Troy, R.G., and Brow, D.A. 1994. A stem/loop in U6 RNA defines a conformational switch required for pre-mRNA splicing. Genes & Dev. 8**:** 221–233. [DOI] [PubMed] [Google Scholar]
- Fromont-Racine, M., Mayes, A., Brunet-Simon, A., Rain, J.C., Colley, A., Dix, I., Decourty, L., Joly, N., Ricard, F., Beggs, J., et al. 2000. Genome-wide protein interaction screens reveal functional networks involving Sm-like proteins. Yeast 17**:** 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti, A., Company, M., and Abelson, J. 1995. Specificity of Prp24 binding to RNA: A role for Prp24 in the dynamic interaction of U4 and U6 snRNAs. RNA 1**:** 132–145. [PMC free article] [PubMed] [Google Scholar]
- Gietz, R.D., Schiestl, R.H., Willems, A.R., and Woods, R.A. 1995. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11**:** 355–360. [DOI] [PubMed] [Google Scholar]
- Hashimoto, C. and Steitz, J.A. 1984. U4 and U6 RNAs coexist in a single small nuclear ribonucleoprotein particle. Nucleic Acids Res. 12**:** 3283–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschlag, D. 1995. RNA chaperones and the RNA folding problem. J. Biol. Chem. 270**:** 20871–20874. [DOI] [PubMed] [Google Scholar]
- Hilliker, A.K. and Staley, J.P. 2004. Multiple functions for the invariant AGC triad of U6 snRNA. RNA 10**:** 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppler, A., Nikstad, L.J., Allmann, A.M., Brow, D.A., and Butcher, S.E. 2002. Metal binding and base ionization in the U6 RNA in-tramolecular stem-loop structure. Nat. Struct. Biol. 9**:** 431–435. [DOI] [PubMed] [Google Scholar]
- Jandrositz, A. and Guthrie, C. 1995. Evidence for a Prp24 binding site in U6 snRNA and in a putative intermediate in the annealing of U6 and U4 snRNAs. EMBO J. 14**:** 820–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenan, D.J., Query, C.C., and Keene, J.D. 1991. RNA recognition: Towards identifying determinants of specificity. Trends Biochem. Sci. 16**:** 214–220. [DOI] [PubMed] [Google Scholar]
- Kielkopf, C.L., Lucke, S., and Green, M.R. 2004. U2AF homology motifs: Protein recognition in the RRM world. Genes & Dev. 18**:** 1513–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn, A.N., Li, Z., and Brow, D.A. 1999. Splicing factor Prp8 governs U4/U6 RNA unwinding during activation of the spliceosome. Mol. Cell 3**:** 65–75. [DOI] [PubMed] [Google Scholar]
- Kunkel, T.A., Roberts, J.D., and Zakour, R.A. 1987. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 154**:** 367–382. [DOI] [PubMed] [Google Scholar]
- Lapham, J. and Crothers, D.M. 1996. RNase H cleavage for processing of in vitro transcribed RNA for NMR studies and RNA ligation. RNA 2**:** 289–296. [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., Li, J., Kim, B.O., Pace, B.S., and He, J.J. 2002. HIV-1 Tat protein-mediated transactivation of the HIV-1 long terminal repeat promoter is potentiated by a novel nuclear Tat-interacting protein of 110 kDa, Tip110. J. Biol. Chem. 277**:** 23854–23863. [DOI] [PubMed] [Google Scholar]
- Madhani, H.D. and Guthrie, C. 1992. A novel base-pairing interaction between U2 and U6 snRNAs suggests a mechanism for the catalytic activation of the spliceosome. Cell 71**:** 803–817. [DOI] [PubMed] [Google Scholar]
- Medenbach, J., Schreiner, S., Liu, S., Luhrmann, R., and Bindereif, A. 2004. Human U4/U6 snRNP recycling factor p110: Mutational analysis reveals the function of the tetratricopeptide repeat domain in recycling. Mol. Cell. Biol. 24**:** 7392–7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polach, K.J. and Uhlenbeck, O.C. 2002. Cooperative binding of ATP and RNA substrates to the DEAD/H protein DbpA. Biochemistry 41**:** 3693–3702. [DOI] [PubMed] [Google Scholar]
- Pontius, B.W. and Berg, P. 1992. Rapid assembly and disassembly of complementary DNA strands through an equilibrium intermediate state mediated by A1 hnRNP protein. J. Biol. Chem. 267**:** 13815–13818. [PubMed] [Google Scholar]
- Portman, D.S. and Dreyfuss, G. 1994. RNA annealing activities in HeLa nuclei. EMBO J. 13**:** 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader, S.D. and Guthrie, C. 2002. A conserved Lsm-interaction motif in Prp24 required for efficient U4/U6 di-snRNP formation. RNA 8**:** 1378–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan, P.L. and Guthrie, C. 1998. A spliceosomal recycling factor that reanneals U4 and U6 small nuclear ribonucleoprotein particles. Science 279**:** 857–860. [DOI] [PubMed] [Google Scholar]
- Rinke, J., Appel, B., Digweed, M., and Lührmann, R. 1985. Localization of a base-paired interaction between small nuclear RNAs U4 and U6 in intact U4/U6 ribonucleoprotein particles by psoralen cross-linking. J. Mol. Biol. 185**:** 721–731. [DOI] [PubMed] [Google Scholar]
- Ryan, D.E., Stevens, S.W., and Abelson, J. 2002. The 5′ and 3′ domains of yeast U6 snRNA: Lsm proteins facilitate binding of Prp24 protein to the U6 telestem region. RNA 8**:** 1011–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sashital, D.G., Allmann, A.M., Van Doren, S.R., and Butcher, S.E. 2003. Structural basis for a lethal mutation in U6 RNA. Biochemistry 42**:** 1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sashital, D.G., Cornilescu, G., McManus, C.J., Brow, D.A., and Butcher, S.E. 2004. U2-U6 RNA folding reveals a group II intron-like domain and a four-helix junction. Nat. Struct. Mol. Biol. 11**:** 1237–1242. [DOI] [PubMed] [Google Scholar]
- Shannon, K.W. and Guthrie, C. 1991. Suppressors of a U4 snRNA mutation define a novel U6 snRNP protein with RNA-binding motifs. Genes & Dev. 5**:** 773–785. [DOI] [PubMed] [Google Scholar]
- Staley, J.P. and Guthrie, C. 1998. Mechanical devices of the spliceosome: Motors, clocks, springs, and things. Cell 92**:** 315–326. [DOI] [PubMed] [Google Scholar]
- ———. 1999. An RNA switch at the 5′ splice site requires ATP and the DEAD box protein Prp28p. Mol. Cell 3**:** 55–64. [DOI] [PubMed] [Google Scholar]
- Stevens, S.W., Ryan, D.E., Ge, H.Y., Moore, R.E., Young, M.K., Lee, T.D., and Abelson, J. 2002. Composition and functional characterization of the yeast spliceosomal penta-snRNP. Mol. Cell 9**:** 31–44. [DOI] [PubMed] [Google Scholar]
- Valadkhan, S. and Manley, J. 2001. Splicing-related catalysis by protein-free snRNAs. Nature 413**:** 701–707. [DOI] [PubMed] [Google Scholar]
- Verdone, L., Galardi, S., Page, D., and Beggs, J.D. 2004. Lsm proteins promote regeneration of pre-mRNA splicing activity. Curr. Biol. 14**:** 1487–1491. [DOI] [PubMed] [Google Scholar]
- Vidal, V.P.I., Verdone, L., Mayes, A.E., and Beggs, J.D. 1999. Characterization of U6 snRNA-protein interactions. RNA 5**:** 1470–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver, R.M., Fortner, D.M., Loos-Austin, L.S., and Brow, D.A. 1999. Multiple functions of Saccharomyces cerevisiae splicing protein Prp24 in U6 RNA structural rearrangements. Genetics 153**:** 1205–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira, J. and Messing, J. 1987. Production of single-stranded plasmid DNA. Methods Enzymol. 153**:** 3–11. [DOI] [PubMed] [Google Scholar]
- Yean, S.L. and Lin, R.J. 1991. U4 small nuclear RNA dissociates from a yeast spliceosome and does not participate in the subsequent splicing reaction. Mol. Cell. Biol. 11**:** 5571–5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yean, S.L., Wuenschell, G., Termini, J., and Lin, R.J. 2000. Metal-ion coordination by U6 small nuclear RNA contributes to catalysis in the spliceosome. Nature 408**:** 881–884. [DOI] [PMC free article] [PubMed] [Google Scholar]