Resistance of α-synuclein null mice to the parkinsonian neurotoxin MPTP (original) (raw)
Abstract
Parkinson's disease (PD) is most commonly a sporadic illness, and is characterized by degeneration of substantia nigra dopamine (DA) neurons and abnormal cytoplasmic aggregates of α-synuclein. Rarely, PD may be caused by missense mutations in α-synuclein. MPTP, a neurotoxin that inhibits mitochondrial complex I, is a prototype for an environmental cause of PD because it produces a pattern of DA neurodegeneration that closely resembles the neuropathology of PD. Here we show that α-synuclein null mice display striking resistance to MPTP-induced degeneration of DA neurons and DA release, and this resistance appears to result from an inability of the toxin to inhibit complex I. Contrary to predictions from in vitro data, this resistance is not due to abnormalities of the DA transporter, which appears to function normally in α-synuclein null mice. Our results suggest that some genetic and environmental factors that increase susceptibility to PD may interact with a common molecular pathway, and represent the first demonstration that normal α-synuclein function may be important to DA neuron viability.
The concept of genetic predisposition to disease suggests that one's genes influence susceptibility to environmental insult. However, the relationship between genetic and environmental factors is poorly understood; most models of disease focus on single genes or toxins. A major challenge of postgenomic biology will be to link the molecular pathways modified by disease-associated alleles to the environmental factors implicated in disease susceptibility.
There is increasing evidence for genetic susceptibility to Parkinson's disease (PD) (1–3). Additionally, dysfunction of a common molecular pathway has been implicated in the familial and sporadic forms of PD. Mutations in the gene that encodes α-synuclein cause a rare form of dominantly inherited PD, and α-synuclein is an abundant protein in Lewy bodies, the proteinaceous neuronal inclusions that are the pathological hallmark of sporadic PD (4–6). The α-synuclein pathway is also implicated in an autosomal recessive form of PD caused by mutations in the gene encoding parkin (7, 8). Epidemiological and twin studies suggest that environmental factors alter susceptibility to PD (9). The fact that exposure of humans to the environmental toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) causes a syndrome that mimics the core neurological symptoms and relatively selective dopamine (DA) neuron degeneration of PD lends support to this concept (10, 11).
We asked whether a model neurotoxin for an environmental cause of PD might act on a molecular pathway implicated in genetic and sporadic forms of the disease by generating α-synuclein null mice, and testing whether they display altered sensitivity to MPTP-induced degeneration of substantia nigra (SN) DA neurons.
Methods
Animal Generation.
A 5.7-kb _Eco_RV mouse α-synuclein fragment (Fig. 1A) was used to generate the targeting construct. A DNA fragment containing, in order, LoxP–phosphoglycerate kinase–Neomycin–transcription blocking “STOP” sequence (12)–LoxP–mutant human α-synuclein cDNA (“STOP Cassette,” Fig. 1A) was cloned 7 bp upstream of the start ATG. The mutant human α-synuclein cDNA contains a point mutation changing amino acid 53 from alanine to threonine, but is not transcribed because it is downstream of the “STOP” sequence (Fig. 1 D and E). Chimeric mice generated from this construct were bred with 129/Sv females and heterozygous mice were then bred to generate α-synuclein null mice. The null mutation was maintained on an inbred 129/Sv background for all experiments. Mice were used in accordance with the National Institutes of Health guidelines for the use of live animals and the animal protocol was approved by the Institutional Animal Care and Use Committee of Columbia University.
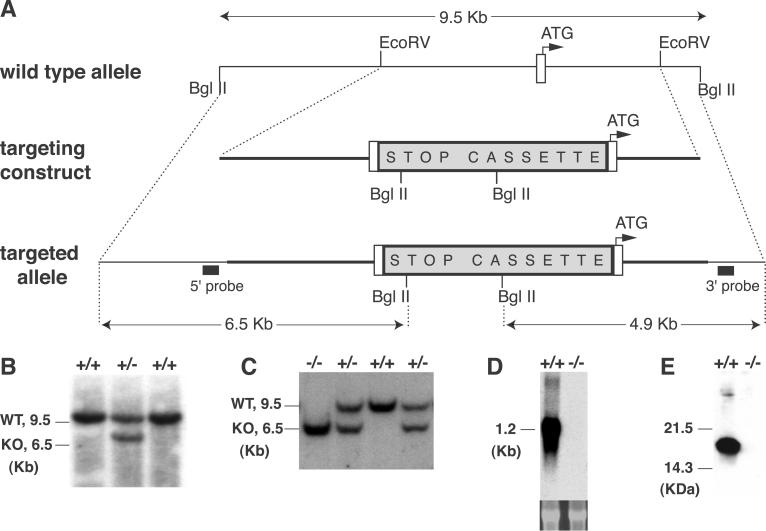
Fig 1.
Targeted disruption of the α-synuclein locus by homologous recombination. (A) Schematic drawing of the wild-type (WT) locus, the targeting construct, and the recombined allele. A null allele was generated by blocking transcription from the α-synuclein allele with a “STOP” cassette (12) inserted upstream of the start ATG. The location of hybridization probes for Southern blot analysis (5′ probe, 3′ probe) are shown. (B) Southern blot analysis of embryonic stem cell clones. DNA was digested with _Bgl_II and was hybridized with the 5′ probe. The WT allele produced a 9.5-kb fragment, and the targeted allele produced a 6.5-kb fragment. _Bgl_II-digested DNA was also hybridized with the 3′ probe (data not shown). (C) Southern blot analysis of genomic DNA from littermates of a α-synuclein heterozygote mating. Tail DNA was isolated and analyzed as in B. (D) Northern blot analysis of whole brain mRNA. Total RNA was hybridized with α-synuclein cDNA probe containing the entire coding region. The lower panel shows ethidium bromide staining of the 28s ribosomal bands. (E) Western blot analysis of whole brain protein blotted with an α-synuclein antibody (Transduction Laboratories).
MPTP Studies.
MPTP handling and safety measures were in accordance with our published guidelines (13). Eight-week-old α-synuclein null mice and littermate controls derived from heterozygous matings were treated with the indicated MPTP regimen, and after pentobarbital (35 mg/kg, i.p) anesthesia, animals were perfused with cold 4% (wt/vol) paraformaldehyde in 0.1M PBS (pH 7.1), and brains were postfixed in the same buffer. Serial coronal sections (30 μm) spanning the entire midbrain and the mid striatum were cut and collected free floating and processed for tyrosine hydroxylase (TH) immunoreactivity (polyclonal antibody, 1:1,000; Calbiochem) by using standard methods. The total number of TH-positive SNpc neurons was stereologically counted by using an unbiased optical fractionator method (14) that is not affected by either the volume of reference (SNpc) or the size of the counted elements (neurons). Densitometric analysis of striatal TH-immunostained sections was performed by using a computerized image analysis system as described (15). Differences were analyzed using one-way ANOVA; where appropriate, Newman–Keuls post hoc analysis was used to test pair-wise comparisons. The null hypothesis was rejected at the 0.05 level.
Primary Neuronal Culture.
Postnatal (P1–P3) ventral midbrain neurons were cultured on rat astrocyte monolayers as described (16). Briefly, ventral midbrain sections from wild-type or α-synuclein pups were pooled, and incubated in papain (20 units/ml) with kynurenate (500 μM) at 32°C under continuous oxygenation with gentle agitation for 2 h. The tissue segments were dissociated by gentle trituration, resuspended in serum-free medium, and plated onto 1-cm2 astrocyte covered glass coverslips at a density of 80,000 cells per dish. Cultures were maintained in serum-free media, and all experiments were conducted in serum-free media on 14-day-old cultures. All TH+ neurons were counted on each plate. Within each genotype, the TH+ count from each experimental plate was compared with the average value of the control plates to derive a “% control” value. For all cell culture experiments, 2–5 plates per genotype were used for each condition, and were repeated with independent litters of mice. Experiments were pooled for statistical analysis.
Microdialysis.
Mice were anesthetized with chloral hydrate (400 mg/kg i.p.) and placed in a stereotaxic frame. Dialysis probes (membrane length, 2 mm; made as described in ref. 17) were implanted into the right striatum. The stereotaxic coordinates for implantation of microdialysis probes were: anterior–posterior, +0.6 mm; dorsal–ventral, −4.2 mm; and lateral, 2.0 mm relative to bregma. Placement of the probe was verified by histological examination subsequent to the experiments. After surgery, animals were returned to their home cages with free access to food and water. Twenty-four hours after surgery, the dialysis probe was connected to a syringe pump and perfused at 1.5 μl/min with ACSF composed of 147 mM NaCl, 3.5 mM KCl, 1.0 mM CaCl2, 1.2 mM MgCl2, 1.0 mM NaH2PO4, and 25.0 mM NaHCO3 (pH 7.2 ± 0.2). After a 1-h equilibration period, the perfusates were collected every 15 min. Four control samples were taken in the hour before the MPTP injection to determine baseline. DA levels were measured using standard HPLC methods.
Results
Generation of α-Synuclein Null Mice.
We generated α-synuclein null mice by using homologous recombination to insert a transcriptional blocking cassette (12) upstream of the start ATG of the gene (Fig. 1). We confirmed that this “stop” cassette abolished transcription and translation of α-synuclein (Fig. 1). The α-synuclein null mice are viable, fertile, and indistinguishable from their wild-type littermates. Breeding of heterozygote α-synuclein null mice produced all genotypes at the expected Mendelian frequency. No gross or microscopic abnormalities were observed in the brains of the α-synuclein null mice (data not shown).
α-Synuclein Null Mice Are Resistant to MPTP.
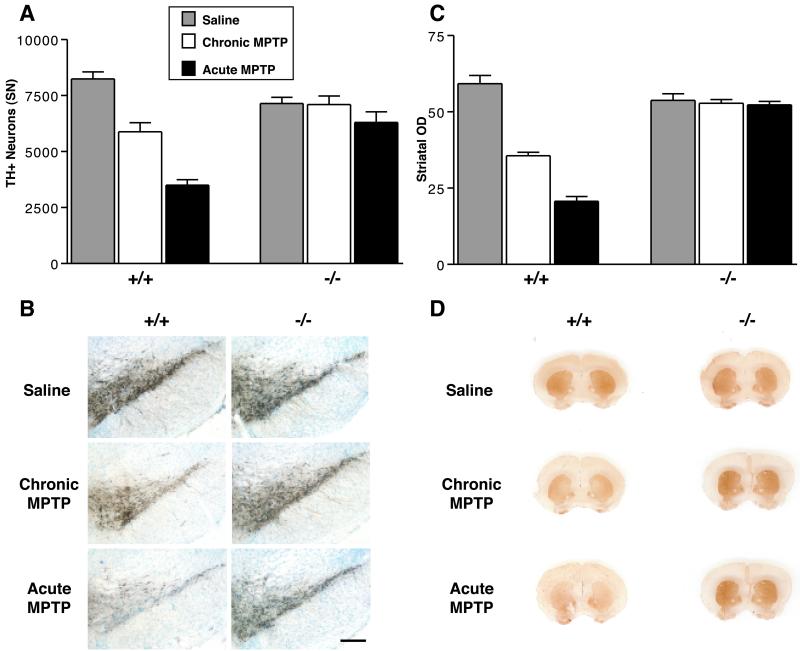
In mice, the regimen of MPTP administration has been shown to determine the mode of SN DA neuron cell death. An acute regimen of MPTP causes necrotic death of DA neurons, whereas a chronic regimen causes these cells to die via an apoptotic pathway (18, 19). We found that α-synuclein mutant mice display striking resistance to both forms of MPTP-induced neurodegeneration (Fig. 2). In the chronic regimen, MPTP-treated wild type mice display significant losses of both TH-positive (TH+) SN cell bodies (29% decrease) and striatal nerve terminals (40% decrease), whereas MPTP-treated α-synuclein mutant mice do not differ significantly from saline-treated controls on either measure. More strikingly, wild-type mice treated with the acute MPTP regimen exhibit an even greater loss of TH+ SN cell bodies (58% decrease) and striatal nerve terminals (65% decrease), but the α-synuclein mutant mice again show no significant neurodegeneration in this paradigm. Because MPTP can down-regulate phenotypic markers such as TH (20), we also counted Nissl stained neurons to confirm that loss of TH staining represents actual neuronal loss. Similar to the findings with TH staining, wild-type mice display a significant loss of Nissl-stained neurons in both MPTP regimens, whereas α-synuclein mutant mice are resistant to both paradigms: wild type: saline = 12,805 ± 871, chronic MPTP = 10,589 ± 1,342, acute MPTP = 7,716 ± 1,097; α-synuclein null: saline = 11,423 ± 534, chronic MPTP = 11,132 ± 2,451, acute MPTP = 9,751 ± 918 (for chronic and acute MPTP, P < 0.05 compared with saline injected mice, Newman–Keuls post hoc. Data: means ± SEM for 4–9 mice per group).
Fig 2.
α-Synuclein−/− dopaminergic neurons are strikingly resistant to MPTP-induced neurodegeneration. α-Synuclein−/− mice and wild-type littermate controls were treated with a chronic (30 mg/kg/d for 5 d) or acute (80 mg/kg for 1 d) MPTP regimen, and were killed 21 d after the final chronic dose or 7 d after the acute dose. n = 4–9 mice per group. (A) Stereological counts of DA TH+ cell bodies after saline (gray bar), chronic MPTP (white bar), or acute MPTP (black bar). (B) TH antibody staining of the SN pars compacta (DA) after the two different MPTP regimens. (C) Quantitation (OD) of the intensity of striatal TH staining; legend as in A. (D) TH antibody staining of the striatum after two different MPTP regimens. (Error bars = SEM.) Both MPTP regimens produced significant reductions of TH+ DA neurons and striatal immunostaining in wild-type mice, but no significant changes in α-synuclein−/− mice (P < 0.05, Newman–Keuls post hoc).
MPTP Metabolism and Monoamine Transporter Function Are Normal in α-Synuclein Null Mice.
Although the normal function of α-synuclein is just beginning to be elucidated, much is known about the molecular pathway through which MPTP exerts neurotoxicity (11). Therefore, to determine the point of intersection between the pathways for α-synuclein and MPTP, we examined which aspect of the MPTP pathway was altered in the α-synuclein mutant mice. MPTP is a pro-toxin that is converted to the active compound 1-methyl-4-phenylpyridinium (MPP+) by monoamine oxidase B (predominantly glial) after crossing the blood–brain barrier. To exert its toxicity MPP+ must be transported into neurons by neurotransmitter transporters, and once inside the neuron it is thought to act by inhibiting mitochondrial complex I, resulting in cellular energy failure (21). The selective toxicity of MPP+ for DA neurons derives, at least in part, from its high affinity for the DA transporter (DAT) (22). MPP+ is also sequestered into synaptic vesicles by the vesicular monoamine transporter (VMAT), and sequestration into vesicles decreases MPP+ toxicity by preventing its interaction with mitochondria (23, 24). According to this framework, cellular resistance occurs if synaptic alterations prevent MPP+ from reaching the mitochondria (“pre-complex I”) or if the cell is better able to withstand the consequences of complex I inhibition (“post-complex I”).
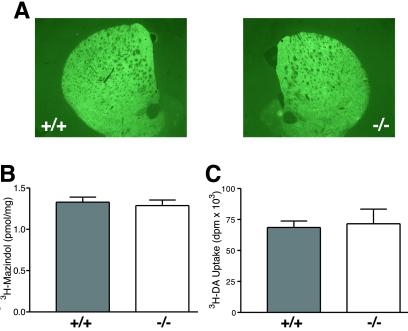
We first determined that normal levels of MPP+ were reaching the DA neurons of the mutant mice by assessing striatal MPP+ levels in both MPTP regimens; these studies show that there is no significant genotype difference in the amount of MPP+ reaching the striatum in either MPTP regimen. For the acute regimen, MPP+ levels 90 min after the final 20 mg/kg injection of MPTP were: wild type = 18.5 ± 0.4, KO = 19.1 ± 0.4 μg/g striatal tissue (n = 6 wild type, 7 knockout). For the chronic regimen, MPP+ levels 90 min after single i.p. injection of 30 mg/kg MPTP were: wild type = 10.6 ± 0.9, knockout = 9.2 ± 0.9 μg/g striatal tissue (n = 10 wild type, 11 knockout). We then asked whether abnormalities of the pre-complex I pathway might be responsible for the MPTP resistance of α-synuclein mutant mice, by analyzing the level and activity of DAT and VMAT in the mutant mice. Although it has been suggested that α-synuclein is involved in trafficking DAT to the membrane (25), quantification of DAT by using immunofluorescence or receptor autoradiography demonstrated that DAT levels are normal in the striatum of α-synuclein mutant mice (Fig. 3 A and B). DAT function, as measured by [3H]DA uptake, was normal in primary cultures of mutant DA neurons (Fig. 3C). Additionally, no abnormality in the kinetics of DAT or VMAT were found in experiments in striatal synaptosomes or isolated synaptic vesicles (Table 1).
Fig 3.
The amount and activity of the DAT is normal in α-synuclein mutant mice. (A and B) Analysis of DAT in the striatum of wild type and α-synuclein mutant mice using DAT antibody staining (A) and [3H]mazindol (30 nM) autoradiography (B) revealed no genotype-related differences. Immunofluorescence was measured by obtaining all images at the same empirically defined exposure that contained no saturated pixels, and all images were taken at the same rostrocaudal striatal plane. (C) [3H]DA uptake in ventral midbrain cultures.
Table 1.
Kinetics of [3H]DA and [3H]MPP+ handling in striatal synaptosomes and synaptic vesicles
| Ligand/Substrate | +/+ | −/− |
|---|---|---|
| Synaptosomes | ||
| [3H]DA | ||
| _K_m | 80 ± 6 | 72 ± 10 |
| _V_max | 2.4 ± 1.2 | 2.1 ± 1.1 |
| [3H]MPP+ | ||
| _K_m | 302 ± 60 | 298 ± 73 |
| _V_max | 1.7 ± 0.9 | 1.9 ± 1.3 |
| [3H]DTBZ | ||
| _K_d | 3.4 ± 0.6 | 3.6 ± 0.6 |
| _B_max | 8.9 ± 0.5 | 8.9 ± 0.8 |
| Vesicles | ||
| [3H]MPP+ | ||
| Transport rate | 0.64 ± 0.2 | 0.85 ± 0.3 |
α-Synuclein Null DA Neurons Have a Pre-Complex I Alteration That Prevents MPP+ from Reaching Mitochondrial Complex I.
To further probe the mechanism for the MPTP resistance of α-synuclein null mice, we used primary cultures of postnatal midbrain neurons. Because these cultures are prepared by plating wild-type or α-synuclein null neurons onto wild-type rat astrocytes, only the neuronal genotype differs between the cultures. Therefore, in addition to its tractability, a particular advantage of this system is that it can be used to identify cell autonomous effects in the plated neurons.
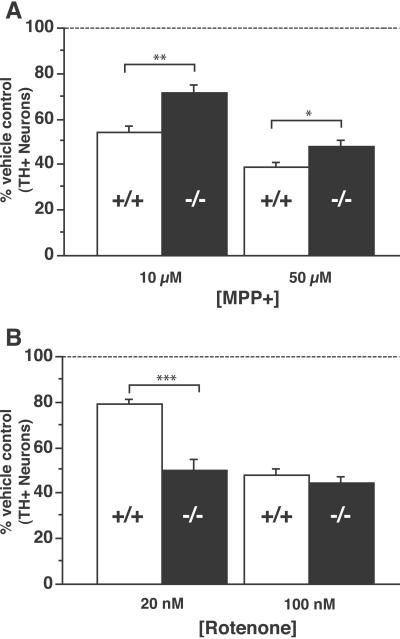
We first confirmed that cultured α-synuclein null DA neurons retain resistance to MPP+ (Fig. 4A), suggesting that a cell autonomous effect of α-synuclein null DA neurons makes them resistant to MPP+. The fact that the degree of resistance observed in vitro is less striking than observed in vivo suggests that additional factors contributing to resistance in the animals may not be captured in the in vitro setting. The presence of exogenous neuroprotectants (superoxide dismutase 1, glial-derived neurotrophic factor) in the cultures (added as part of the standard primary culture protocol) also likely contribute to this difference.
Fig 4.
Dissociation between MPTP- and rotenone-induced death of α-synuclein null DA neurons. Ventral midbrain cultures were treated for two days with 10 or 50 μM MPP+ (A) and 20 or 100 nM rotenone (B). α-Synuclein mutant DA neurons displayed significant resistance to death at both concentrations of MPP+, but did not show resistance to rotenone; there were significantly fewer α-synuclein−/− TH+ neurons at the 20 nM rotenone dose (P < 0.05, Newman–Keuls post hoc). For each condition, n = 5–10 plates per genotype. Values are represented as a percentage of untreated controls. (Error bars = SEM.)
We next tested the sensitivity of cultured DA neurons to rotenone, a mitochondrial toxin that selectively inhibits complex I at the same site as MPP+. In contrast to MPP+, rotenone is highly lipophilic, does not depend on DAT for cellular entry, and is not sequestered into synaptic vesicles. Therefore, rotenone would bypass a pre-complex I site of resistance, but if a postcomplex I mechanism accounts for the MPTP resistance, the mutant neurons should also be resistant to rotenone. Rotenone was found to bypass the resistance of α-synuclein mutant DA neurons (Fig. 4B), and in fact, α-synuclein null DA neurons were significantly more sensitive to 20 nM rotenone. Interestingly, the enhanced sensitivity to rotenone may reflect a synergistic effect of complex I inhibition and DA toxicity (26–28), as α-synuclein null mice display abnormalities in the synaptic handling of DA (29). The clear dissociation between the effects of MPP+ and rotenone suggests that the MPTP resistance of α-synuclein null mice is caused by a pre-complex I alteration that prevents MPP+ from inhibiting complex I.
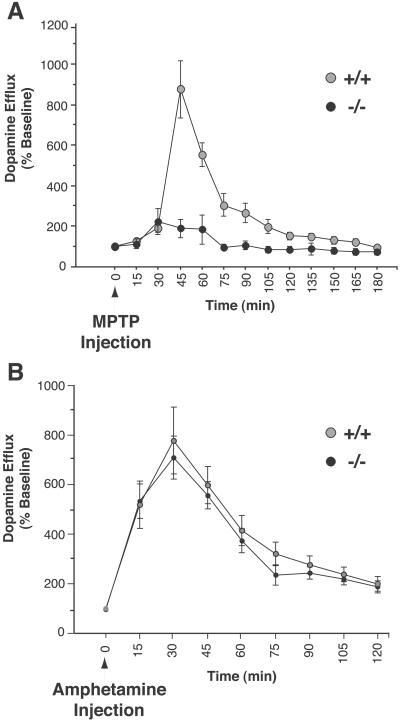
To further probe the pre-complex I pathway, we studied an acute in vivo effect of MPTP, MPP+-induced efflux of striatal dopamine. This response is believed to result from complex I-related energy failure, because it occurs with the same time course as the lactate elevation that occurs secondary to MPP+-induced complex I inhibition, and other interventions that inhibit mitochondrial respiration also produce this effect (30–32). We reasoned that if a pre-complex I abnormality was preventing MPP+ from accessing and inhibiting mitochondrial complex I, as suggested by the rotenone experiment, there should be deficient MPTP-induced DA efflux in the α-synuclein null mice. Using in vivo microdialysis in awake freely moving animals, we observed a greater than 60% decrease in the efflux of striatal dopamine in α-synuclein mutant mice (Fig. 5A). Like amphetamine, MPTP-induced efflux of DA is thought to occur via reversal of the DAT. Therefore, to control for the possibility that deficient MPTP-induced DA efflux might reflect an inability of DAT to mediate reverse transport in α-synuclein mutants, we measured amphetamine-induced DA release, and found no difference between mutant and wild-type mice (Fig. 5B). This result further suggests that DAT function is normal in α-synuclein mutant mice, and is consistent with the concept that the differences observed in MPTP-induced DA efflux relate to complex I inhibition.
Fig 5.
MPTP-induced DA efflux is deficient in α-synuclein null mice. (A) In vivo microdialysis of striatal dopamine efflux after a single i.p injection of 30 mg/kg MPTP. Gray circle, wild type; black circle, α-synuclein−/−. (B) α-Synuclein mutant mice display normal amphetamine-induced DA release. Legend as in A. Microdialysis values are represented as a percentage of the average of four baseline measurements taken in the hour preceding MPTP injection; each animal was used as its own baseline control. There were no significant differences in baseline values. n = 3–8 mice per genotype.
Discussion
Our experiments suggest that in the absence of α-synuclein function, MPP+ is unable to inhibit mitochondrial complex I. This conclusion is supported by three observations: (i) rotenone can bypass the resistance of α-synuclein mutant DA neurons, (ii) MPTP-induced efflux of dopamine is significantly reduced in the α-synuclein mutant mice, and (iii) α-synuclein mutant mice are resistant to regimens of MPTP that lead to different modes of cell death, a finding inconsistent with a role for α-synuclein in a discrete post-complex I cell death-related pathway (Fig. 2).
Pre-complex I-mediated MPTP resistance could result from either decreased DAT-mediated cellular entry of MPP+ or enhanced vesicular storage of MPP+. Although a report using transient transfection suggests that α-synuclein interacts with DAT and is involved in trafficking it to the plasma membrane (25), our studies in whole animals, striatal tissue, cultured DA neurons, and synaptosomes suggest that DAT function is normal in α-synuclein null mice (Figs. 3 and 5B, and Table 1).
Vesicular sequestration of MPP+, the second component of the pre-complex I pathway, promotes resistance to MPTP (23, 24). Although our data on isolated vesicles suggest that VMAT kinetics are normal in α-synuclein mutant mice, increased vesicular sequestration of MPP+ may be related to a change in a dynamic property of vesicles not measured in an in vitro preparation, such as movement of vesicles between different pools. A number of observations are consistent with a role for α-synuclein in vesicular dynamics (29, 33, 34). Alternatively, there may be a direct requirement for α-synuclein in MPTP-mediated neurotoxicity, consistent with the suggestion that α-synuclein-dopamine adducts may be an important mediator of toxicity in DA neurons (35).
It is not known whether the mutations in α-synuclein that cause dominantly inherited PD alter the normal function of the protein, or endow it with a novel property, such as the ability to aggregate. Findings that overexpression of wild-type α-synuclein in mice and flies is toxic, and that α-synuclein accumulates but does not aggregate in parkin-related PD, are consistent with the possibility that an increase in normal α-synuclein function leads to cellular toxicity (7, 36, 37). The current study lends further support to the hypothesis that the normal function of α-synuclein is important to DA neuron viability, and raises the possibility that alterations in normal α-synuclein function may modify the vulnerability of DA neurons to an environmental toxin.
The finding that α-synuclein null mice are resistant to MPTP-induced neurodegeneration provides an example of how a disease-related gene can modulate susceptibility to a relevant environmental insult. Together with the fact that the α-synuclein pathway has also been implicated in sporadic and parkin-related PD, this finding suggests that α-synuclein participates in a pathway of central importance to the survival of dopaminergic neurons.
Acknowledgments
We thank Ms. N. Romero and Ms. L. Zhang for expert animal care, Leonidas Stefanis for providing a synuclein cDNA, and Luca Santarelli, Brett Lauring, and Eric Schon for helpful comments on the manuscript. Thanks to Stanley Fahn for his invaluable mentorship and support. Thanks also to Michio Hirano and Ramon Marti for HPLC analysis and Monica Mendelsohn for ES cell injection. This work was supported by the Parkinson's Disease Foundation, Lowenstein Foundation, Goldman Foundation, National Institute of Neurological Disorders and Stroke, and National Institute on Drug Abuse. W.D. is the recipient of a Howard Hughes Medical Institute Postdoctoral Fellowship for Physicians, and M.V. is the recipient of the Human Frontier Science Program Fellowship.
Abbreviations
- PD, Parkinson's disease
- DA, dopamine
- MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- SN, substantia nigra
- DAT, DA transporter
- MPP+, 1-methyl-4-phenylpyridinium
- VMAT, vesicular monoamine transporter
- TH, tyrosine hydroxylase
See commentary on page 13972.
References
- 1.Scott W. K., Nance, M. A., Watts, R. L., Hubble, J. P., Koller, W. C., Lyons, K., Pahwa, R., Stern, M. B., Colcher, A., Hiner, B. C., et al. (2001) J. Am. Med. Assoc. 286**,** 2239-2244. [DOI] [PubMed] [Google Scholar]
- 2.Kruger R., Vieira-Saecker, A. M., Kuhn, W., Berg, D., Muller, T., Kuhnl, N., Fuchs, G. A., Storch, A., Hungs, M., Woitalla, D., et al. (1999) Ann. Neurol. 45**,** 611-617. [DOI] [PubMed] [Google Scholar]
- 3.Martin E. R., Scott, W. K., Nance, M. A., Watts, R. L., Hubble, J. P., Koller, W. C., Lyons, K., Pahwa, R., Stern, M. B., Colcher, A., et al. (2001) J. Am. Med. Assoc. 286**,** 2245-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polymeropoulos M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., et al. (1997) Science 276**,** 2045-2047. [DOI] [PubMed] [Google Scholar]
- 5.Kruger R., Kuhn, W., Muller, T., Woitalla, D., Graeber, M., Kosel, S., Przuntek, H., Epplen, J. T., Schols, L. & Riess, O. (1998) Nat. Genet. 18**,** 106-108. [DOI] [PubMed] [Google Scholar]
- 6.Spillantini M. G., Schmidt, M. L., Lee, V. M., Trojanowski, J. Q., Jakes, R. & Goedert, M. (1997) Nature 388**,** 839-840. [DOI] [PubMed] [Google Scholar]
- 7.Shimura H., Schlossmacher, M. G., Hattori, N., Frosch, M. P., Trockenbacher, A., Schneider, R., Mizuno, Y., Kosik, K. S. & Selkoe, D. J. (2001) Science 293**,** 263-269. [DOI] [PubMed] [Google Scholar]
- 8.Chung K. K., Zhang, Y., Lim, K. L., Tanaka, Y., Huang, H., Gao, J., Ross, C. A., Dawson, V. L. & Dawson, T. M. (2001) Nat. Med. 7**,** 1144-1150. [DOI] [PubMed] [Google Scholar]
- 9.Marion S. A. (2001) Adv. Neurol. 86**,** 163-172. [PubMed] [Google Scholar]
- 10.Langston J. W., Ballard, P., Tetrud, J. W. & Irwin, I. (1983) Science 219**,** 979-980. [DOI] [PubMed] [Google Scholar]
- 11.Przedborski S. & Vila, M. (2001) Clin. Neurosci. Res. 1**,** 407-418. [Google Scholar]
- 12.Lakso M., Sauer, B., Mosinger, B., Jr., Lee, E. J., Manning, R. W., Yu, S. H., Mulder, K. L. & Westphal, H. (1992) Proc. Natl. Acad. Sci. USA 89**,** 6232-6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Przedborski S., Jackson-Lewis, V., Naini, A. B., Jakowec, M., Petzinger, G., Miller, R. & Akram, M. (2001) J. Neurochem. 76**,** 1265-1274. [DOI] [PubMed] [Google Scholar]
- 14.Liberatore G. T., Jackson-Lewis, V., Vukosavic, S., Mandir, A. S., Vila, M., McAuliffe, W. G., Dawson, V. L., Dawson, T. M. & Przedborski, S. (1999) Nat. Med. 5**,** 1403-1409. [DOI] [PubMed] [Google Scholar]
- 15.Burke R. E., Cadet, J. L., Kent, J. D., Karanas, A. L. & Jackson-Lewis, V. (1990) J. Neurosci. Methods 35**,** 63-73. [DOI] [PubMed] [Google Scholar]
- 16.Przedborski S., Khan, U., Kostic, V., Carlson, E., Epstein, C. J. & Sulzer, D. (1996) J. Neurochem. 67**,** 1383-1392. [DOI] [PubMed] [Google Scholar]
- 17.Trillat A. C., Malagie, I., Scearce, K., Pons, D., Anmella, M. C., Jacquot, C., Hen, R. & Gardier, A. M. (1997) J. Neurochem. 69**,** 2019-2025. [DOI] [PubMed] [Google Scholar]
- 18.Tatton N. A. & Kish, S. J. (1997) Neuroscience 77**,** 1037-1048. [DOI] [PubMed] [Google Scholar]
- 19.Jackson-Lewis V., Jakowec, M., Burke, R. E. & Przedborski, S. (1995) Neurodegeneration 4**,** 257-269. [DOI] [PubMed] [Google Scholar]
- 20.Tatton W. G., Kwan, M. M., Verrier, M. C., Seniuk, N. A. & Theriault, E. (1990) Brain Res. 527**,** 21-31. [DOI] [PubMed] [Google Scholar]
- 21.Nicklas W. J., Youngster, S. K., Kindt, M. V. & Heikkila, R. E. (1987) Life Sci. 40**,** 721-729. [DOI] [PubMed] [Google Scholar]
- 22.Javitch J. A. & Snyder, S. H. (1984) Eur. J. Pharmacol. 106**,** 455-456. [DOI] [PubMed] [Google Scholar]
- 23.Reinhard J. F., Diliberto, E. J., Jr., Viveros, O. H. & Daniels, A. J. (1987) Proc. Natl. Acad. Sci. USA 84**,** 8160-8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y., Peter, D., Roghani, A., Schuldiner, S., Prive, G. G., Eisenberg, D., Brecha, N. & Edwards, R. H. (1992) Cell 70**,** 539-551. [DOI] [PubMed] [Google Scholar]
- 25.Lee F. J., Liu, F., Pristupa, Z. B. & Niznik, H. B. (2001) FASEB J. 15**,** 916-926. [DOI] [PubMed] [Google Scholar]
- 26.Nakao N., Nakai, K. & Itakura, T. (1997) Brain Res. 777**,** 202-209. [DOI] [PubMed] [Google Scholar]
- 27.McLaughlin B. A., Nelson, D., Erecinska, M. & Chesselet, M. F. (1998) J. Neurochem. 70**,** 2406-2415. [DOI] [PubMed] [Google Scholar]
- 28.Burrows K. B., Nixdorf, W. L. & Yamamoto, B. K. (2000) J. Pharmacol. Exp. Ther. 292**,** 853-860. [PubMed] [Google Scholar]
- 29.Abeliovich A., Schmitz, Y., Farinas, I., Choi-Lundberg, D., Ho, W. H., Castillo, P. E., Shinsky, N., Verdugo, J. M., Armanini, M., Ryan, A., et al. (2000) Neuron 25**,** 239-252. [DOI] [PubMed] [Google Scholar]
- 30.Ferger B., Eberhardt, O., Teismann, P., de Groote, C. & Schulz, J. B. (1999) J. Neurochem. 73**,** 1329-1332. [DOI] [PubMed] [Google Scholar]
- 31.Rollema H., Kuhr, W. G., Kranenborg, G., De Vries, J. & Van den Berg, C. (1988) J. Pharmacol. Exp. Ther. 245**,** 858-866. [PubMed] [Google Scholar]
- 32.Buyukuysal R. L. & Mete, B. (1999) J. Neurochem. 72**,** 1507-1515. [DOI] [PubMed] [Google Scholar]
- 33.Murphy D. D., Rueter, S. M., Trojanowski, J. Q. & Lee, V. M. (2000) J. Neurosci. 20**,** 3214-3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lotharius J., Barg, S., Wiekop, P., Lundberg, C., Raymon, H. K. & Brundin, P., (2002) J. Biol. Chem., ePub, M205518200v1. [DOI] [PubMed]
- 35.Conway K. A., Rochet, J. C., Bieganski, R. M. & Lansbury, P. T., Jr. (2001) Science 294**,** 1346-1349. [DOI] [PubMed] [Google Scholar]
- 36.Masliah E., Rockenstein, E., Veinbergs, I., Mallory, M., Hashimoto, M., Takeda, A., Sagara, Y., Sisk, A. & Mucke, L. (2000) Science 287**,** 1265-1269. [DOI] [PubMed] [Google Scholar]
- 37.Feany M. B. & Bender, W. W. (2000) Nature 404**,** 394-398. [DOI] [PubMed] [Google Scholar]