Cushing's syndrome without excess cortisol (original) (raw)
In patients taking inhaled corticosteroids the biochemical detection of a suppressed hypothalamicpituitary-adrenal axis is well documented.1,2 Fluticasone propionate is the most potent inhaled glucocorticoid,3,4 and adrenocortical insufficiency has been reported in 12% of patients on a high dose of inhaled fluticasone.1,5 The incidence of addisonian crises is lower, but crises may occur during intercurrent illness or after dose reduction or withdrawal.5
Itraconazole is an orally active antifungal triazole that inhibits cytochrome P450 dependent CYP3A4 and consequently decreases the clearance of synthetic glucocorticoids.6 The combination of long term inhaled steroid with oral itraconazole may exacerbate suppression of the hypothalamic-pituitary-adrenal axis. In a cohort of 25 patients with cystic fibrosis, six were reported to have adrenal insufficiency.7 However, overt Cushing's syndrome as a result of this drug combination is less well understood. We report the rapid development and the resolution of iatrogenic Cushing's syndrome in a patient taking itraconazole for six weeks in addition to inhaled fluticasone.
Case report
A 55 year old man with bronchiectasis and asthma developed an exacerbation of allergic bronchopulmonary aspergillosis. His regular medication included long acting and short acting inhaled β2 agonists (formoterol and terbutaline respectively), the leucotriene receptor antagonist montelukast (10 mg once daily), alendronate (75 mg weekly), amitriptyline (50 mg daily at night), and codeine phosphate (60 mg as required). In addition, he had been taking inhaled fluticasone (1-1.5 mg twice daily) for over two years with no evidence of Cushing's syndrome. He had required oral steroids on only two occasions in the preceding 12 months. His last course of oral prednisolone was one month previously and was for one week at 30 mg once daily.
He was prescribed itraconazole 100 mg once daily, which was then increased to 200 mg once daily after four weeks because of a deterioration in symptom control. The patient was on holiday six weeks after starting to take itraconazole, when he noted that his face had become swollen. Of his own volition, he stopped taking itraconazole. The patient presented three weeks later, complaining of being tired, bruising easily, and being unable to fasten either his trousers or shirt. He was noted to have a buffalo hump, acne, a proximal myopathy, and obvious moon face (figure). There was also evidence of impaired glucose tolerance (not noted previously) with a random glucose of 9.2 mmol/l. Blood pressure was 150/80 mm Hg, with no postural drop.
Figure 1.
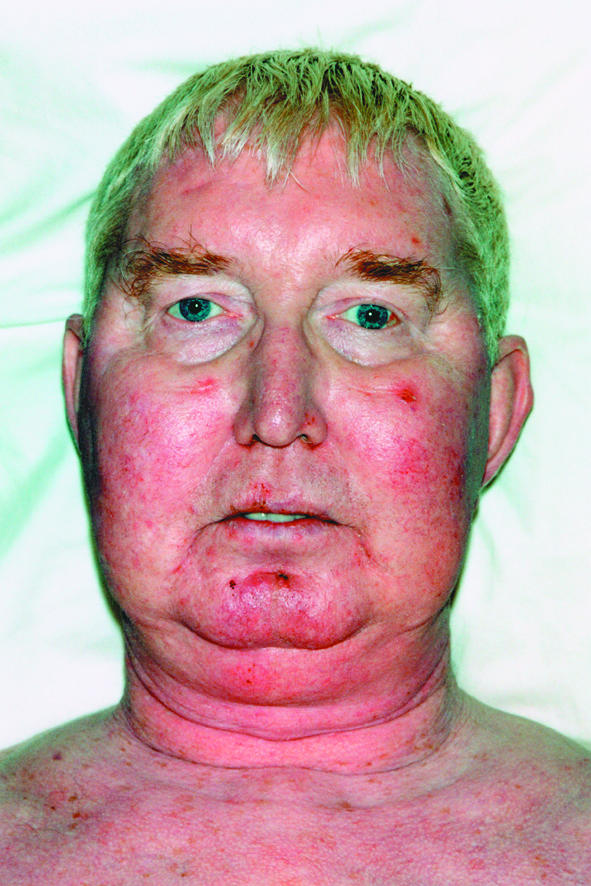
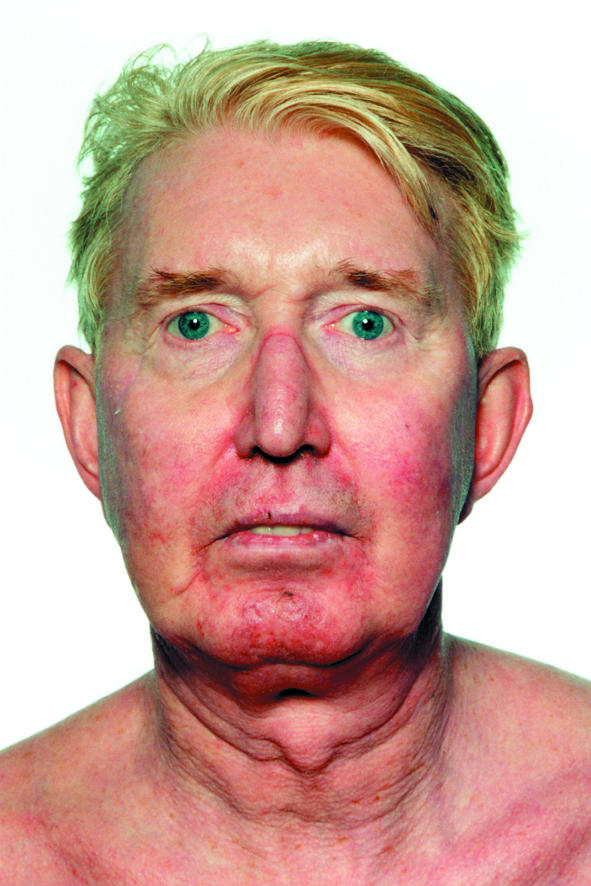
Left: Patient's facial appearance six weeks after start of itraconazole treatment. Right: Facial appearance six months after patient stopped taking itraconazole
Initial investigation detected no serum cortisol or urine cortisol (24 hour), and no adrenocorticotrophic hormone. A short synacthen test (250 μg tetracosactide) was clearly abnormal, with a baseline serum cortisol concentration of < 20 nmol/l (normal range 190-650 nmol/l), rising to 82 nmol/l and 118 nmol/l at 30 and 60 minutes respectively. Adrenal antibody assay was negative and computed tomography of the adrenal glands was normal. The patient was advised about steroid supplementation in the event of intercurrent illness.
The patient showed little response clinically to the addition of itraconazole and no further deterioration after stopping it. Hence, no other steroid sparing agent was used. Six months after stopping itraconazole, the patient felt that his clinical appearance had returned to normal (figure). There was still evidence of a suppressed hypothalamic-pituitary-adrenal axis, consistent with long term inhaled steroid use: a repeat short synacthen test showed a baseline cortisol concentration of 21 nmol/l, rising to 164 nmol/l and 233 nmol/l at 30 and 60 minutes respectively. One month later a 9 am cortisol test showed a concentration of 507 nmol/l.
Discussion
This patient probably developed clinical Cushing's syndrome secondary to increased systemic concentrations of fluticasone. This was associated with subsequent adrenal insufficiency due to the suppression of pituitary adrenocorticotrophic hormone. A reduction in fluticasone clearance occurs as a result of the inhibition of the cytochrome P450 CYP3A4 enzyme system by itraconazole.6 Although itraconazole also has the potential to directly inhibit adrenal steroidogenesis, this usually happens at a much higher dose. In addition, there was no concomitant rise in adrenocorticotrophic hormone to suggest that the dominant effect of itraconazole was a reduction in adrenal steroidogenesis.
With prolonged use, fluticasone alone has previously been associated with adrenal suppression and a cushingoid appearance.2 The combination of itraconazole and inhaled steroids has occasionally been reported to cause Cushing's syndrome. This is usually over a prolonged period of administration at higher doses of itraconazole than used here: long term use at 800 mg a day in one patient;8 four months at 200 mg twice daily in another;9 and a total accumulated dose of 635 g in another patient.7 One report has documented the development of Cushing's syndrome after two weeks of itraconazole in combination with inhaled budesonide, and another report documented development after two months.10,11
Itraconazole may also have an important acute effect on the clearance of inhaled steroid that may not be clinically apparent. A randomised, double blind, two phase crossover study in 10 participants has shown that 200 mg itraconazole once daily for five days increases the half life of inhaled budesonide from 1.6 hours to 6.2 hours and, compared with placebo, leads to a 60% increase in the peak plasma concentration.12 This effect was associated with a considerable suppression of endogenous cortisol production. Similarly four days of itraconazole markedly increases concentrations of methylprednisolone and increases suppression of endogenous cortisol secretion after a single dose of oral methylprednisolone.13
In patients with marked adrenal suppression who require hydrocortisone replacement therapy, consideration should be given to the long half life of itraconazole.14 Patients who are given standard doses of hydrocortisone for adrenal replacement therapy may become cushingoid. Even without steroid replacement therapy the recovery of adrenal function may take up to 10 months.7 Any steroid replacement therapy should probably be conservative and closely monitored.
Importantly, adrenal suppression does not occur in every patient treated with a combination of inhaled steroid and itraconazole. Variation in CYP3A4 activity, in glucocorticoid receptor sensitivity, or in glucocorticoid receptor polymorphisms may influence the response of the individual.15 However, this clinical scenario can occur with other cytochrome P450 inhibitors, as recently reported with ritonavir in combination with inhaled fluticasone.16
Allergic bronchopulmonary aspergillosis is the most common allergic bronchopulmonary mycosis in humans, occurring in 7-14% of asthmatic patients dependent on steroids and in 6% of patients with cystic fibrosis.17 Glucocorticoids are the mainstay of treatment with itraconazole having an established steroid sparing role. Some of this apparent steroid sparing effect may be secondary to the interaction between itraconazole and fluticasone, producing raised systemic fluticasone levels. Adrenal suppression and iatrogenic Cushing's syndrome secondary to concomitant inhaled steroid and itraconazole is a potentially common and serious drug interaction of which both endocrinologists and general physicians should be aware.
Inhaled steroid plus itraconazole can lead to adrenal suppression and iatrogenic Cushing's syndrome
Contributors: The case was identified and managed by PAC, PP, and DRW. The idea for the article was conceived by DRW and PAC. DRW and CSA did the literature search, and DRW and CSA wrote the article. DRW is the guarantor.
Funding: No special funding.
Competing interests: None declared.
References
- 1.Sim D, Griffiths A, Armstrong D, Clarke C, Rodda C, Freezer N. Adrenal suppression from high-dose inhaled fluticasone propionate in children with asthma. Eur Respir J (2003);21: 633-6. [DOI] [PubMed] [Google Scholar]
- 2.Wilson AM, Blumsohn A, Jung RT, Lipworth BJ. Asthma and Cushing's syndrome. Chest (2000);117: 593-4. [DOI] [PubMed] [Google Scholar]
- 3.Lipworth BJWA. Dose response to inhaled corticosteroids: benefits and risks. Semin Respir Crit Care Med (1998);19: 625-46. [Google Scholar]
- 4.Wilson AM, McFarlane LC, Lipworth BJ. Effects of repeated once daily dosing of three intranasal corticosteroids on basal and dynamic measures of hypothalamic-pituitary-adrenal-axis activity. J Allergy Clin Immunol 1998;101(4 part1): 470-4. [DOI] [PubMed] [Google Scholar]
- 5.Wong J, Black P. Acute adrenal insufficiency associated with high dose inhaled steroids. BMJ 1992;304: 1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatakrishnan K, von Moltke LL, Greenblatt DJ. Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin Pharmacokinet (2000);38: 111-80. [DOI] [PubMed] [Google Scholar]
- 7.Skov M, Main KM, Sillesen IB, Muller J, Koch C, Lanng S. Iatrogenic adrenal insufficiency as a side-effect of combined treatment of itraconazole and budesonide. Eur Respir J (2002);20: 127-33. [DOI] [PubMed] [Google Scholar]
- 8.Main KM, Skov M, Sillesen IB, Dige-Petersen H, Muller J, Koch C, et al. Cushing's syndrome due to pharmacological interaction in a cystic fibrosis patient. Acta Paediatr (2002);91: 1008-11. [DOI] [PubMed] [Google Scholar]
- 9.Parmar JS, Howell T, Kelly J, Bilton D. Profound adrenal suppression secondary to treatment with low dose inhaled steroids and itraconazole in allergic bronchopulmonary aspergillosis in cystic fibrosis. Thorax (2002);57: 749-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolland MJ, Bagg W, Thomas MG, Lucas JA, Ticehurst R, Black PN. Cushing's syndrome due to interaction between inhaled corticosteroids and itraconazole. Ann Pharmacother 2004;38(1): 46-9. [DOI] [PubMed] [Google Scholar]
- 11.De Wachter E, Malfroot A, De Schutter I, Vanbesien J, De Schepper J. Inhaled budesonide induced Cushing's syndrome in cystic fibrosis patients, due to drug inhibition of cytochrome P450. J Cyst Fibros 2003;2(2): 72-5. [DOI] [PubMed] [Google Scholar]
- 12.Raaska K, Niemi M, Neuvonen M, Neuvonen PJ, Kivisto KT. Plasma concentrations of inhaled budesonide and its effects on plasma cortisol are increased by the cytochrome P4503A4 inhibitor itraconazole. Clin Pharmacol Ther (2002);72: 362-9. [DOI] [PubMed] [Google Scholar]
- 13.Lebrun-Vignes B, Archer VC, Diquet B, Levron JC, Chosidow O, Puech AJ, et al. Effect of itraconazole on the pharmacokinetics of prednisolone and methylprednisolone and cortisol secretion in healthy subjects. Br J Clin Pharmacol (2001);51: 443-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant SM, Clissold SP. Itraconazole. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in superficial and systemic mycoses. Drugs (1989);37: 310-44. [DOI] [PubMed] [Google Scholar]
- 15.Van Rossum EF, Lamberts SW. Polymorphisms in the glucocorticoid receptor gene and their associations with metabolic parameters and body composition. Recent Prog Horm Res (2004);59: 333-57. [DOI] [PubMed] [Google Scholar]
- 16.Clevenbergh P, Corcostegui M, Gerard D, Hieronimus S, Mondain V, Chichmanian RM, et al. Iatrogenic Cushing's syndrome in an HIV-infected patient treated with inhaled corticosteroids (fluticasone propionate) and low dose ritonavir enhanced PI containing regimen. J Infect (2002);44: 194-5. [DOI] [PubMed] [Google Scholar]
- 17.Soubani AO, Chandrasekar PH. The clinical spectrum of pulmonary aspergillosis. Chest (2002);121: 1988-99. [DOI] [PubMed] [Google Scholar]