Disruption of T helper 2-immune responses in Epstein–Barr virus-induced gene 3-deficient mice (original) (raw)
Abstract
Epstein–Barr virus-induced gene 3 (EBI3) is a widely expressed IL-12p40-related protein that associates as a heterodimer with either IL-12p35 or an IL-12p35 homologue, p28, to create a new cytokine (IL-27). To define the function of EBI3 in vivo, we generated knockout mice in which the ebi3 gene was targeted by homologous recombination. EBI3−/− mice exhibited normal numbers of both naive and mature CD4+ and CD8+ T cells and B cells, but markedly decreased numbers of invariant natural killer T cells (iNKT) as defined by staining with an α-galactosylceramide (αGalCer)-loaded CD1d-tetramer. iNKT cells from EBI3−/− mice exhibited decreased IL-4 and, to a lesser extent, IFN-γ production after αGalCer stimulation in vitro. A sustained decrease in IL-4 production was also observed in EBI3−/− mice after αGalCer stimulation in vivo in contrast to IFN-γ production, which was only transiently decreased under such stimulation. Notably, EBI3−/− mice were resistant to the induction of immunopathology associated with oxazolone-induced colitis, a colitis model mediated primarily by T helper (Th) 2-type cytokine production by iNKT cells. In contrast, trinitrobenzene sulfonic acid-induced colitis, a predominantly Th1-mediated colitis model, was unaffected. Thus, EBI3 plays a critical regulatory role in the induction of Th2-type immune responses and the development of Th2-mediated tissue inflammation in vivo, which may be mediated through the control of iNKT cell function.
Keywords: cytokines, colitis, T helper cells, dendritic cells, natural killer T cells
The adaptive immune system has developed several highly effective strategies against pathogens that include differentiation of naive CD4+ T cells into effector T cells producing T helper (Th) 1 (e.g., IFN-γ) or Th2 (e.g., IL-4 and IL-5) type cytokines (1, 2). It has been proposed that the cytokine environment present at the earliest phases of T cell priming is one of the major variables influencing Th cell development. Although a fundamental role for cytokines such as IL-12 and IL-4 in determining such Th1 and Th2 cell development, respectively, is well accepted, the sources and regulation of these cytokines in vivo have been a matter of debate (3, 4). IL-12 and the highly related cytokine IL-23 are produced by monocytes, macrophages, and/or subsets of dendritic cells (DC), such as CD8α-positive DC in mice, and bind IL-12 receptors whereupon they activate signal transducer and activator of transcription (Stat) 4, thus stimulating T cells to produce IFN-γ (5, 6). IL-4, on the other hand, which binds IL-4 receptors stimulating Stat 6, is produced by many different cell types, including eosinophils, basophils, mast cells, and invariant natural killer T cells (iNKT cells).
iNKT cells of rodents and humans are a lymphocyte lineage that shares cell surface markers with both conventional T cells and natural killer cells, that contributes to the immune response related to tumors, infections, and autoantigens, and that plays a key role in certain types of peripheral tolerance and hypersensitivity responses (7–9). iNKT cells are characterized by the use of an invariant T cell antigen receptor (TCR)-α chain (Vα14Jα281 in mouse; Vα24JαQ in human) that preferentially associates with certain TCR-β chains and recognizes glycolipid antigens such as α-galactosylceramide (αGalCer) when presented by CD1d, a MHC class I-like protein expressed on antigen-presenting cells. On engagement with CD1d bearing a glycolipid antigen such as αGalCer, iNKT cells rapidly secrete a broad range of cytokines and chemokines, including both IL-4 and IFN-γ consistent with a Th0 phenotype. As such, iNKT cells have been suggested to significantly contribute to conventional T cell differentiation, either directly through this property of secreting IL-4 and IFN-γ at the initiation of an immune response, or indirectly through effects on DC subsets (10, 11). iNKT cells are, in particular, the earliest source of T cell-derived IL-4 in vivo such that, in the absence of CD1d and, consequently, the iNKT cell lineage, anti-CD3-induced IL-4 production is absent. This early production of IL-4 by iNKT cells has been directly linked to the regulation of certain, but not all, Th2-mediated immune responses (9, 12–14). However, the factors that regulate IL-4 production by iNKT cells are unknown.
Epstein–Barr virus-induced gene 3 (EBI3) was initially discovered as a transcriptionally activated gene in Epstein–Barr virus-infected human B lymphocytes (15). It encodes a 34-kDa hematopoietin receptor that lacks a membrane-anchoring motif, and that bears structural similarities to the p40 chain of IL-12. In humans, EBI3 protein is expressed in vivo by DCs of lymphoid tissues and at very high levels by placental syncytiotrophoblasts (15–17). EBI3 has been shown to form heterodimers with either IL-12p35 or p28, a novel IL-12p35-related polypeptide (18). Although IL-12p35/EBI3 has no described biologic function, p28/EBI3 (IL-27) has been recently shown to function as a proliferation factor for naive, but not memory, CD4+ T cells and to synergize with IL-12 to stimulate IFN-γ production (18). EBI3, however, is expressed much more widely than p28, including contexts in which Th1-associated cytokine tone is diminished, raising the possibility that EBI3 has functions other than promoting Th1 cytokine production, acting either as a homodimer or in association with other heterodimeric binding partners (15, 19). Thus, to gain a more broad understanding of EBI3 function, we generated EBI3−/− mice. An examination of these mice has revealed that EBI3 is both an essential growth and Th2-differentiating factor for iNKT cells that is associated with regulation of Th2-mediated immunopathology, as defined in a hapten-mediated model of experimental colitis.
Methods
Mice.
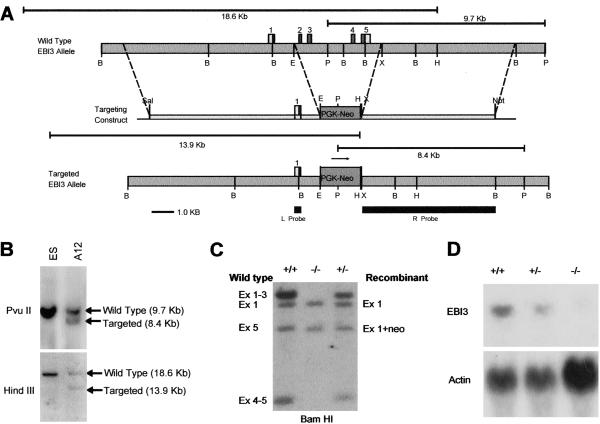
All animals were bred and maintained under specific pathogen-free conditions in our animal facilities. The ebi3 gene was disrupted by homologous recombination in 129SvJ embryonic stem (ES) cells (Fig. 1A) and confirmed by Southern blot hybridization (Fig. 1B). Chimeric mice were generated by injection of ES cells into C57BL/6 blastocysts. Heterozygous offspring of one chimeric founder were identified by PCR amplification of genomic DNA and interbred to generate homozygous animals of mixed C57BL/6 and 129SvJ background. The generation of EBI3−/− homozygotes was confirmed by Southern blotting of genomic DNA (Fig. 1C) and Northern blotting of RNA from splenocytes (Fig. 1D). Additional proof of deletion was obtained by using PCR to amplify introns 2 and 4 from genomic DNA templates. These reactions yielded the expected products of 336 bp (intron 2) and 341 bp (intron 4) using DNA templates from WT or heterozygous animals but failed to generate detectable products from knockout DNA (data not shown). All studies were approved by the Harvard Medical School Standing Committee on Animals.
Fig 1.
Generation of the EBI3−/− mouse strain. (A) Exons 2–5, encoding amino acids 24–228 of the EBI3 protein, were replaced by the Neo gene cassette under control of the phosphoglycerate kinase promoter (PGK-Neo) inserted in the same orientation as the ebi3 gene. (B) Southern analysis of _Pvu_II- and _Hin_dIII-digested genomic DNA from ganciclovir/G-418-resistant embryonic stem cell clone A12 using 32P-labeled right (R) and left (L) probes. Clones showing the expected recombinant band at 8.4 kb with the R probe (Upper) were further screened by hybridization with the L probe (Lower), which revealed the expected recombinant band of 13.9 kb. (C) Southern analysis of genomic DNA from homozygous WT (+/+), homozygous mutant (−/−), and heterozygous (+/−) mice. _Bam_HI-digested DNA was hybridized with 32P-labeled EBI3 cDNA. _Bam_HI fragments predicted to contain the left side of exon 1 (2.8 kb) are present in all three samples at equal levels, whereas fragments containing the right side of exon 1 and exons 2 and 3 (3.2 kb), and containing exons 4 and 5 (0.95 kb) are undetectable (−/− mice) or are present at half molar levels (heterozygotes). A band at ≈2.4 kb detected in all lanes corresponds to the right end of exon 5 in WT and heterozygous DNA, and to a rearranged _Bam_HI fragment of identical size containing the right side of exon 1 linked to the Neo gene in heterozygous and knockout mouse DNA. (D) Northern blot of RNA from spleens of EBI3 WT (+/+), heterozygous (+/−), and knockout (−/−) mice hybridized to 32P-labeled EBI3 cDNA (EBI3) or beta-actin (Actin) probes. EBI3 transcripts of 1.8 kb are detected in WT (+/+) and heterozygous (+/−) mouse RNA but are undetectable in knockout RNA (−/−).
Preparation of Lymphocytes.
Single-cell suspensions from spleen and liver cells were obtained by forcing the organs through a 70-μm nylon strainer in Hanks' balanced salt solution containing 2% FCS (Life Technologies, Gaithersburg, MD). Total liver cells were further fractionated on a 40/60% Percoll gradient (Pharmacia Biotech) by centrifugation at 800 × g for 20 min at 4°C, and erythrocytes were lysed.
Antibodies and Flow Cytometry.
Flow cytometry was performed by standard methods with FITC- or phycoerythrin (PE)-labeled anti-mouse CD3ɛ, CD4, CD8, CD45RB, CD62L, B220, and CD11c mAbs (PharMingen). αGalCer-specific lymphocytes were identified with PE-labeled CD1d-αGalCer tetramer complexes (20).
Stimulation of CD4+ T cells.
Splenocytes were incubated with anti-CD4-coated magnetic beads followed by positive selection (Dynal, Lake Success, NY). IL-4 and IFN-γ in supernatants from 105 CD4+ T cells were determined after stimulation with plate-bound anti-CD3 (5 μg/ml; PharMingen) and anti-CD28 (1 μg/ml; PharMingen) by ELISA (PharMingen). Purified CD4+ T cells were also stimulated with plate-bound, anti-CD3 (5 μg/ml) and anti-CD28 (1 μg/ml) antibodies in the presence of anti-IL-12 (10 μg/ml) and anti-IFN-γ (10 μg/ml) antibodies and IL-4 1,000 units/ml for 72 h. Cells were washed and restimulated with anti-CD3 (5 μg/ml) and anti-CD28 (1 μg/ml) before cytokine analysis.
Stimulation of DC.
Splenocytes were incubated with CD11c-coupled microbeads (Miltenyi Biotec, Auburn, CA) and positively selected over a magnetic cell sorting column. DC (5 × 105) were stimulated with 1 μg/ml LPS (Escherichia coli LPS; Sigma) and Sac (Calbiochem, 1/1,000) for 48 h, and IL-12 levels were determined in the supernatants by ELISA (PeproTech, Rocky Hill, NJ).
Stimulation of iNKT Cells.
CD1d-transfected C1R cells (105; ref. 21) were incubated with either 0.1 μg/ml αGalCer or βGalCer and iNKT cells derived from enriched T cells from the liver of either EBI3−/− or WT mice, by using mouse T cell enrichment columns (R & D Systems). The supernatants were screened for IFN-γ and IL-4 by ELISA (PharMingen). For in vivo stimulation, one dose of 100 μg/kg αGalCer (provided by Kirin Brewery, Tokyo) or vehicle control (DMSO) was administered i.p., and IFN-γ and IL-4 were assessed in the serum after 2, 4, and 24 h.
Experimental Colitis Models.
Colitis induction was performed according to previously described methods (22). In brief, colitis was induced 7 days after sensitization with either 150 μl of trinitrobenzene sulfonic acid (TNBS; 2.5% in 50% ethanol) or oxazolone (3% in 100% ethanol). For induction of colitis, mice received by intrarectal administration 150 μl of a solution of TNBS 1.5% or oxazolone 1% dissolved in 0.9% NaCl and mixed with an equal volume of ethanol (50% ethanol). Lamina propria T cells were isolated from freshly obtained colonic specimens by standard methods (22). Tissues obtained at the indicated time points were examined for evidence of colitis by using five previously established criteria for colitis activity: hypervascularization, presence of mononuclear cells, epithelial hyperplasia, epithelial injury, and presence of granulocytes (22).
Results
Decreased IL-4 Production by Spleen T Cells from EBI3−/− Mice.
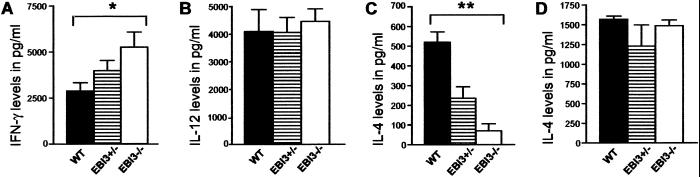
EBI3−/− mice were generated as described in Methods. These mice bred normally and exhibited no gross or histologic abnormalities in various organs including liver, kidney, spleen, colon, lung, and heart (data not shown). Because the EBI3/p28 heterodimer has been shown to stimulate the proliferation of naive CD4+ T cells and synergize with IL-12 to promote IFN-γ production, we first examined the phenotypes of, and cytokine production by, spleen cells from EBI3−/− and WT control mice. EBI3−/− and WT animals exhibited similar numbers of spleen mononuclear cells expressing markers for B cells, CD8+ T cells, and CD4+ T cells and similar proportions of naive vs. memory CD4+ T cells as defined by CD45RB and CD62L staining (data not shown). CD4+ T cells isolated from spleens of EBI3−/− mice, however, produced moderately more IFN-γ than cells from WT control mice after in vitro stimulation with anti-CD3 and anti-CD28 antibodies (Fig. 2A). Heterozygous mice exhibited an intermediate pattern of IFN-γ production. However, WT and EBI3−/− mice exhibited the same proportions of spleen cells expressing CD11c and similar quantities of IL-12 production by CD11c+ spleen cells (Fig. 2B) and by total adherent cells (data not shown) after in vitro stimulation with lipopolysaccharide and Staphylococcus aureus Cowan strain, indicating that the increase in IFN-γ production observed was not simply due to a role for EBI3 as an antagonist of IL-12 production. In contrast, IL-4 secretion by EBI3−/− CD4+ T cells in response to similar direct ex vivo stimulation was strikingly reduced in comparison with that observed with WT cells (Fig. 2C). As noted for IFN-γ, T cells from heterozygous mice produced IL-4 at intermediate levels. These results suggested that EBI3−/− animals maintained a relatively smaller proportion of Th2-differentiated CD4+ T cells in vivo. However, after in vitro differentiation under a Th2-inducing environment consisting of spleen CD4+ T cell stimulation with anti-CD3 plus anti-CD28 in the presence of IL-4 plus anti-IL-12 and anti-IFN-γ, T cells from EBI3−/− mice were prompted to secrete IL-4 at levels similar to that of cells from WT mice (Fig. 2D). Notably, when WT cells were not restimulated with anti-CD3 plus anti-CD28 after in vitro differentiation, little IL-4 could be detected in the supernatant, supporting the stimulated T cells as the source of the IL-4 (data not shown). These data suggest that the primary defect for reduced IL-4 production in EBI3−/− mice was not due to intrinsic defects in T cell function in that, when provided an exogenous source of IL-4, T cells could be differentiated to produce Th2-associated cytokines.
Fig 2.
IL-4 defect in EBI3−/− mice. (A) EBI3−/− CD4+ T cells from spleen stimulated with anti-CD3 plus anti-CD28 monoclonal antibodies produce more IFN-γ in comparison with CD4+ T cells from WT control mice. Heterozygous mice exhibited an intermediate pattern of cytokine production. Data are representative of three experiments. (B) Similar levels of IL-12 production by DC from WT and EBI3−/− spleens. Data are representative of two experiments. (C) CD4+ T cells from EBI3−/− mice produce significantly less IL-4 than WT animals when stimulated as in A. (D) IL-4 production in EBI3−/− mice is similar to WT mice in a Th2-inducing environment. Data are representative of three experiments. Bars represent means and standard errors of the means (n = 5). *, P < 0.05, compared with the respective WT control group. **, P < 0.002, compared with the respective WT control group.
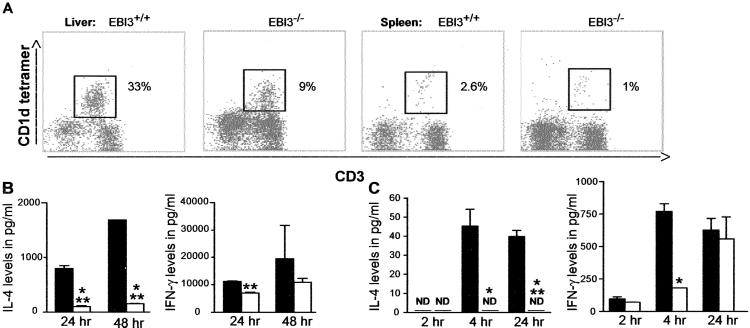
Reduced Number and IL-4 Secretion by iNKT Cells in EBI3−/− Animals.
The significant diminution in IL-4 production by spleen CD4+ T cells after anti-CD3 stimulation in vitro and the ability to reverse this effect through differentiation of CD4+ T cells in the presence of IL-4 suggested that EBI3−/− animals might have a defect in a cell type important to Th2 differentiation. One such cell type is the iNKT cell, which is responsible for the rapid production of IL-4 and IFN-γ in vitro and in vivo after anti-CD3 stimulation (12, 13). We therefore determined the numbers of iNKT in the liver and spleen as defined by staining with anti-CD3 and a αGalCer-loaded CD1d tetramer (20). EBI3−/− animals exhibited a consistent and statistically significant (P < 0.05) reduction in the number of iNKT cells in the liver (WT, 47.3 ± 9.0%; EBI3+/−, 22.6 ± 6.1%; EBI3−/−, 19.2 ± 9.6%) and spleen (WT, 3.7 ± 0.7%; EBI3+/−, 2.4 ± 0.4%; EBI3−/−, 1.8 ± 1.0%) relative to the numbers of iNKT cells detected in WT mice (Fig. 3A). We next determined the ability of iNKT cells from EBI3−/− mice to produce IL-4 and IFN-γ after stimulation with a CD1d-restricted glycolipid antigen. To do so, intrahepatic lymphocytes were isolated from EBI3−/− and WT animals and stimulated in vitro with αGalCer or βGalCer as control as described in Methods. To compensate for the reduced number of iNKT cells in the EBI3−/− mice in comparison with the WT animals, the quantities of cytokine produced were normalized to the number of CD3+CD1d-tetramer+ cells present in each sample analyzed. As can be seen in Fig. 3B (Left), EBI3−/− iNKT cells exhibited a strong impairment on a per cell basis in their capacity to produce IL-4 in response to stimulation with the CD1d-restricted antigen, αGalCer. Similarly, a lesser but still significant decrease in IFN-γ production by iNKT cells was observed at 24 h after αGalCer stimulation in EBI3−/− mice in comparison with the WT controls (Fig. 3B Right). To determine whether this functional impairment in IL-4 and IFN-γ production also applied in vivo, we next assessed the serum levels of IL-4 and IFN-γ after in vivo activation of iNKT cells. Groups of EBI3−/− and WT mice were injected with 2 μg of αGalCer, and serum cytokines were assessed at 2, 4, and 24 h after injection. EBI3−/− mice exhibited nearly undetectable levels of serum IL-4 at 2, 4, and 24 h in comparison with WT animals, which exhibited significant quantities of IL-4 at 4 and 24 h after in vivo αGalCer stimulation (Fig. 3C). In comparison, IFN-γ levels in the serum were decreased only at 2 and 4 h in the EBI3−/− mice relative to the WT controls. By 24 h after in vivo αGalCer stimulation, EBI3−/− and WT mice exhibited similar levels of IFN-γ in the serum (Fig. 3C). Thus, in the absence of EBI3, iNKT cells exhibit a major reduction in numbers and ability to produce IL-4, suggesting an important growth and differentiating effect of EBI3 on iNKT cells in vivo. In contrast, although a defect in iNKT cell-mediated IFN-γ production can also be detected in EBI3−/− mice, it is of lesser severity and is likely superceded by contributions from other cell types after iNKT cell activation.
Fig 3.
iNKT cell defect in EBI3−/− mice. (A) Liver and spleen mononuclear cells were stained with anti-CD3 and an αGalCer-loaded CD1d tetramer. iNKT cell numbers are reduced in the liver and spleen of EBI3−/− mice compared with WT animals. A representative flow cytometry analysis is shown. (B) Intrahepatic lymphocytes from WT (filled bars) or EBI3−/− (open bars) were stimulated with αGalCer. IFN-γ (Right) and IL-4 (Left) levels were determined by ELISA at 24 and 48 h. EBI3−/− iNKT cells exhibited decreased αGalCer-stimulated IL-4 production. No cytokines were detected with the control lipid βGalCer (data not shown). (C) WT (filled bars) and EBI3−/− mice (open bars) received 2 μg of αGalCer i.p., and serum IL-4 and IFN-γ levels were assessed at 2, 4, and 24 h by ELISA. EBI3−/− mice exhibit impaired IL-4 production after in vivo stimulation with αGalCer at all time points, whereas IFN-γ production is decreased only at 4 h after stimulation. ND, not detectable; *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
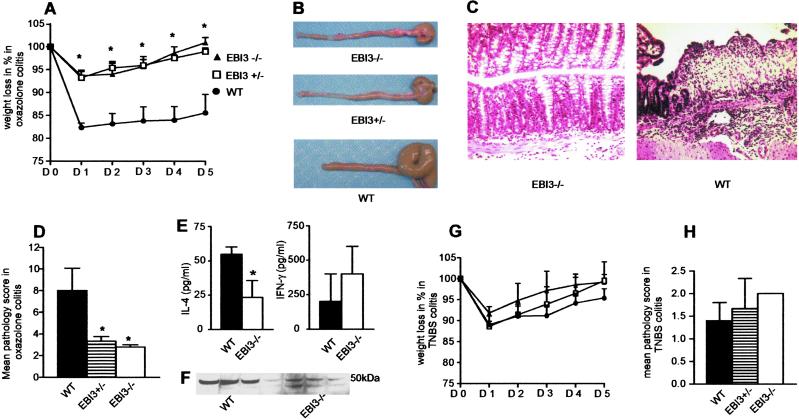
EBI3 Deficiency Is Associated with Decreased Th2-Mediated Immunopathology.
iNKT cells have recently been linked to the regulation of several immune-mediated diseases (23, 24), including inflammatory bowel disease (IBD) (25). Specifically, recent evidence suggests that oxazolone colitis, previously shown to depend predominantly on Th2-associated cytokines (26), is mediated by iNKT cells (27). Moreover, EBI3 expression is increased in humans with active ulcerative colitis (19), a form of IBD thought to represent a modified Th2 response (28). Accordingly, EBI3−/− and WT control mice were treated with oxazolone to induce a hapten-associated hypersensitivity colitis model that is primarily mediated by iNKT cell-dependent production of Th2 cytokines, including IL-4 and IL-13 (26, 27). Interestingly, EBI3−/− mice were almost completely protected from the development of oxazolone-induced colitis. In the absence of EBI3, mice exposed to oxazolone exhibited only transiently, low levels of weight loss (Fig. 4A), markedly diminished macroscopic evidence of colitis as defined by colon shortening and hemorrhage (Fig. 4B), and a dramatic decrease in histological evidence of colonic inflammatory changes (Fig. 4 C and D). Interestingly, mice heterozygous for the EBI3 allele also exhibited nearly complete protection from oxazolone colitis (Fig. 4 A_–_D). This lack of a direct correlation between EBI3 gene dosage and the clinicopathologic response to hapten-mediated colitis induction is reminiscent of previous observations with other cytokines and cytokine-related transcription factors, such as tumor necrosis factor-α and t-bet, respectively (29, 30). These results in heterozygous mice presumably reflect a threshold of susceptibility to tissue injury from EBI3 that has important therapeutic implications such that complete neutralization of EBI3 may be unnecessary to achieve a clinical response. Consistent with the previously reported role of the Th2 cytokine IL-4 in oxazolone-mediated colitis (26), this reduction in colonic inflammation was accompanied by significantly reduced IL-4 production and a trend toward increased IFN-γ production of lamina propria mononuclear cells from EBI3−/− in comparison with WT control mice (Fig. 4E). Splenic T cells from oxazolone-treated EBI3−/− mice also exhibited little evidence of nuclear GATA-3 expression as defined by Western blotting in comparison with WT animals that contained significant levels of this transcription factor after such treatment (Fig. 4F). Given that high levels of GATA-3 are normally associated with IL-4 production (31), this result is consistent with the decrease in IL-4 levels observed in the EBI3−/− animals. In contrast to oxazolone colitis, EBI3−/− mice exhibited an inflammatory response in the colon after administration of TNBS, a colitis model shown to be mediated primarily by Th1 cells (32) that was indistinguishable from the response of WT mice as defined by clinical (Fig. 4G) and histopathologic (Fig. 4H) criteria. These observations are consistent with a significant disruption of Th2-type responses in EBI3−/− animals.
Fig 4.
Selective protection from Th2-mediated immunopathology in EBI3−/− mice. (A) Weight loss after induction of oxazolone colitis was limited to 5% in both EBI3−/− and EBI3+/− mice in comparison with 15% in WT littermates. Both EBI3−/− and EBI3+/− exhibited complete clinical recovery on day 5 after disease induction. *, P < 0.02. (B) Almost complete absence of macroscopic signs of disease in EBI3−/− and EBI3+/− mice 5 days after induction of oxazolone colitis. (C) WT mice exhibit severe histopathologic evidence of colitis, whereas EBI3−/− mice show only mild inflammatory signs. (D) Significant reduction in inflammatory score in EBI3−/− and EBI3+/− mice compared with WT mice. (E) Significantly reduced IL-4 production and a trend toward increased IFN-γ production of lamina propria mononuclear cells from EBI3−/− mice in comparison with WT control mice. (F) Nuclear extracts from spleen T cells from oxazolone-treated WT (n = 3) and EBI3−/− (n = 4) mice analyzed by Western blotting for GATA-3 (50-kDa band) expression. Control Western blotting for β-actin showed equal loading in all lanes (data not shown). (G) Similar clinical response (weight loss) after TNBS administration in EBI3−/− and EBI3+/− mice in comparison with WT mice. No significant weight loss was observed in an ethanol control group of WT and EBI3−/− mice (data not shown). (H) No significant difference in pathological score among EBI3−/−, EBI3+/−, and WT mice in TNBS-induced colitis. Bars represent means and standard errors of the means. Data are representative of three experiments with five mice per group. *, P < 0.05 (in D and E), compared with the respective WT control groups.
Discussion
Recent studies have shown that the EBI3 molecule forms homodimers or heterodimers with either IL-12p35 or p28 (18, 33). Although the biologic function(s) of EBI3/EBI3 or IL-12p35/EBI3 dimers are unknown, dimeric p28/EBI3 (IL-27) has been shown to have two major functions based on studies with recombinant proteins in vitro: as a growth factor for naive CD4+ T cells and as a facilitator of IL-12-induced IFN-γ production (18).
In the current report, we have examined the in vivo role of EBI3 in immune regulation through deletion of the EBI3 gene. These studies have revealed some interesting findings that revise our understanding of EBI3. Although IL-27 has been shown to be a growth factor for naive CD4+ T cells (18), EBI3−/− animals exhibited normal numbers of B cells and T cells, both CD4+ and CD8+, including a normal ratio of naive vs. memory cells, based on CD45RB and CD62L expression. Interestingly and consistent with a role of EBI3 as a T cell growth factor, when expressed as a heterodimer with p28, EBI3−/− mice exhibited a significant diminution in the number of iNKT cells, suggesting that these cells are more strictly dependent on the growth-promoting effects of either IL-27 or other potential EBI3 heterodimers. As a corollary, our studies suggest that the lack of the growth-promoting effects of EBI3 for accumulating normal numbers of conventional T cells can be compensated by other cytokine growth factors in vivo.
However, in marked contrast to what would be predicted by an absence of IL-27, EBI3 deficiency was not associated with a diminution in anti-CD3-induced IFN-γ production but rather a relative increase in IFN-γ production in association with a significant decrease in IL-4 secretion. Importantly, these cytokine responses were not associated with an increase in IL-12 production by DC, suggesting that the increased IFN-γ production was likely to be secondary to diminished IL-4 production and its inhibitory effects on Th1 pathways (34). Thus, a major in vivo phenotype of EBI3 deficiency is diminished Th2 tone, with a presumed corollary release of Th1 pathways. Given that this diminution in IL-4 production by anti-CD3-stimulated CD4+ T cells could be reversed by differentiation of CD4+ T cells under Th2-inducing conditions and occurred in association with a diminution in iNKT cell number and a significant reduction in their ability to secrete IL-4, our studies would tend to support a major role for EBI3 in regulating the growth and Th2-secreting behaviors of iNKT cells and consequently Th2 differentiation of conventional CD4+ T cells. Consistent with this finding, EBI3-deficiency was also associated with resistance to development of the hapten-mediated colitis associated with oxazolone administration, a model which is critically Th2 dependent (26) and mediated by iNKT cells (27). This defect in iNKT cell secretory function in the absence of EBI3 was not limited to IL-4 production and also included decreased IFN-γ production, consistent with previous studies in CD1d-deficient (12, 13, 14), but not β2-microglobulin-deficient (35), animals that also lack iNKT cells. Moreover, the deficiency in IFN-γ production by iNKT cells, perhaps due to loss of IL-27 production (18), seemed to be less pronounced and was possibly compensated for by IFN-γ production by other cell types. One such cell type may be the NK cell (36). NK cells are responsible for significant IFN-γ production by 6 h after CD1d-mediated stimulation of iNKT cells, kinetics that are consistent with our observations that IFN-γ levels in EBI3−/− and WT mice were similar at 24 h after αGalCer stimulation. The fact that IFN-γ production by iNKT cells was less disabled and might be compensated for by other cell types presumably explains the lack of differences observed in the immunopathology associated with the model of TNBS administration as applied here. Whether EBI3 deficiency affects other immune mechanisms that may impact on the severity of oxazolone colitis cannot be excluded. Finally, given the apparent dichotomy between the severity of cytokine production observed and relative modest reduction in iNKT cell number, our results suggest a major role for EBI3 in iNKT cell development as well as cellular proliferation.
Our results thus suggest that EBI3 is an essential component of a secreted factor or group of factors necessary for Th cells to differentiate into Th2 cells. It is possible that this pathway is mediated through an IL-27 growth and/or differentiation factor effect on iNKT cells. Alternatively, our studies might predict that EBI3 interacts directly with either iNKT and/or conventional T cells through a partnership with either EBI3 as a homodimer, IL-12p35, or another yet to be defined heterodimeric binding partner. The fact that EBI3 expression can be identified in cells types wherein either IL-12p35 or p28 are not present supports this latter possibility (16, 18, 19).
Acknowledgments
We thank Drs. E. Cahir-McFarland (Brigham and Women's Hospital, Boston) and M. Exley (Beth Israel-Deaconess Medical Center, Boston) for helpful discussions and Dr. L. Degenstein for blastocyst injections (University of Chicago). This work was supported by grants from the Ter Meulen Fund, Royal Netherlands Academy of Arts and Sciences (to E.E.S.N.); by grants from the Innovationsstiftung Rheinland-Pfalz, the Sonderforschungsbereich 458, and the Gerhard Hess program of the Deutsche Forschungsgemeinschaft, and by a J. William Fulbright scholarship for senior scientists (to M.F.N.); by National Science Foundation of Switzerland Grant 81BE-64629 (to N.C.); by the Naito Foundation (H.I.); by National Institutes of Health Grant T32CA0954 (to J.T.); by National Institutes of Health Grant CA62375 and an Eastern Virginia Medical School Institutional Grant (to M.B.); and by National Institutes of Health Grants DK44319, DK53056, and DK51362 and the Harvard Digestive Diseases Center, Harvard Medical School (R.S.B.).
Abbreviations
- EBI3, Epstein–Barr virus-induced gene 3
- iNKT cells, invariant natural killer T cell
- αGalCer, α-galactosylceramide
- Th, T helper
- DC, dendritic cell
- TNBS, trinitrobenzene sulfonic acid
References
- 1.Thierfelder W. E., van Deursen, J. M., Yamamoto, K., Tripp, R. A., Sarawar, S. R., Carson, R. T., Sangster, M. Y., Vignali, D. A., Doherty, P. C., Grosveld, G. C. & Ihle, J. N. (1996) Nature 382**,** 171-174. [DOI] [PubMed] [Google Scholar]
- 2.Asnagli H. & Murphy, K. M. (2001) Curr. Opin. Immunol. 13**,** 242-247. [DOI] [PubMed] [Google Scholar]
- 3.Murphy T. L., Cleveland, M. G., Kulesza, P., Magram, J. & Murphy, K. M. (1995) Mol. Cell. Biol. 15**,** 5258-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tew J. G., Wu, J., Fakher, M., Szakal, A. K. & Qin, D. (2001) Trends Immunol. 22**,** 361-367. [DOI] [PubMed] [Google Scholar]
- 5.Maldonado-Lopez R., De Smedt, T., Michel, P., Godfroid, J., Pajak, B., Heirman, C., Thielemans, K., Leo, O., Urbain, J. & Moser, M. (1999) J. Exp. Med. 189**,** 587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oppmann B., Lesley, R., Blom, B., Timans, J. C., Xu, Y., Hunte, B., Vega, F., Yu, N., Wang, J., Singh, K., et al. (2000) Immunity 13**,** 715-725. [DOI] [PubMed] [Google Scholar]
- 7.Cui J., Shin, T., Kawano, T., Sato, H., Kondo, E., Toura, I., Kaneko, Y., Koseki, H., Kanno, M. & Taniguchi, M. (1997) Science 278**,** 1623-1626. [DOI] [PubMed] [Google Scholar]
- 8.Nieuwenhuis E. E., Matsumoto, T., Exley, M., Schleipman, R. A., Glickman, J., Bailey, D. T., Corazza, N., Colgan, S. P., Onderdonk, A. B. & Blumberg, R. S. (2002) Nat. Med. 8**,** 588-593. [DOI] [PubMed] [Google Scholar]
- 9.Askenase P. W. (2001) Clin. Exp. Immunol. 125**,** 345-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fearon D. T. & Locksley, R. M. (1996) Science 272**,** 50-53. [DOI] [PubMed] [Google Scholar]
- 11.Abbas A. K., Murphy, K. M. & Sher, A. (1996) Nature 383**,** 787-793. [DOI] [PubMed] [Google Scholar]
- 12.Mendiratta S. K., Martin, W. D., Hong, S., Boesteanu, A., Joyce, S. & Van Kaer, L. (1997) Immunity 6**,** 469-477. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y. H., Chiu, N. M., Mandal, M., Wang, N. & Wang, C. R. (1997) Immunity 6**,** 459-467. [DOI] [PubMed] [Google Scholar]
- 14.Singh N., Hong, S., Scherer, D. C., Serizawa, I., Burdin, N., Kronenberg, M., Koezuka, Y. & Van Kaer, L. (1999) J. Immunol. 163**,** 2373-2377. [PubMed] [Google Scholar]
- 15.Devergne O., Hummel, M., Koeppen, H., Le Beau, M. M., Nathanson, E. C., Kieff, E. & Birkenbach, M. (1996) J. Virol. 70**,** 1143-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devergne O., Coulomb-L'Hermine, A., Capel, F., Moussa, M. & Capron, F. (2001) Am. J. Pathol. 159**,** 1763-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto S. I., Suzuki, T., Nagai, S., Yamashita, T., Toyoda, N. & Matsushima, K. (2000) Blood 96**,** 2206-2214. [PubMed] [Google Scholar]
- 18.Pflanz S., Timans, J. C., Cheung, J., Rosales, R., Kanzler, H., Gilbert, J., Hibbert, L., Churakova, T., Travis, M., Vaisberg, E., et al. (2002) Immunity 16**,** 779-790. [DOI] [PubMed] [Google Scholar]
- 19.Christ A. D., Stevens, A. C., Koeppen, H., Walsh, S., Omata, F., Devergne, O., Birkenbach, M. & Blumberg, R. S. (1998) Gastroenterology 115**,** 307-313. [DOI] [PubMed] [Google Scholar]
- 20.Naidenko O. V., Maher, J. K., Ernst, W. A., Sakai, T., Modlin, R. L. & Kronenberg, M. (1999) J. Exp. Med. 190**,** 1069-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicol A., Nieda, M., Koezuka, Y., Porcelli, S., Suzuki, K., Tadokoro, K., Durrant, S. & Juji, T. (2000) Immunology 99**,** 229-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neurath M. F., Weigmann, B., Finotto, S., Glickman, J., Nieuwenhuis, E., Iijima, H., Mizoguchi, A., Mizoguchi, E., Mudter, J., Galle, P. R., et al. (2002) J. Exp. Med. 195**,** 1129-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh A. K., Wilson, M. T., Hong, S., Olivares-Villagomez, D., Du, C., Stanic, A. K., Joyce, S., Sriram, S., Koezuka, Y. & Van Kaer, L. (2001) J. Exp. Med. 194**,** 1801-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong S., Wilson, M. T., Serizawa, I., Wu, L., Singh, N., Naidenko, O. V., Miura, T., Haba, T., Scherer, D. C., Wei, J., et al. (2001) Nat. Med. 7**,** 1052-1056. [DOI] [PubMed] [Google Scholar]
- 25.Saubermann L. J., Beck, P., De Jong, Y. P., Pitman, R. S., Ryan, M. S., Kim, H. S., Exley, M., Snapper, S., Balk, S. P., Hagen, S. J., et al. (2000) Gastroenterology 119**,** 119-128. [DOI] [PubMed] [Google Scholar]
- 26.Boirivant M., Fuss, I. J., Chu, A. & Strober, W. (1998) J. Exp. Med. 188**,** 1929-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heller F., Fuss, I. J., Nieuwenhuis, E. E., Blumberg, R. S. & Strober, W. (2002) Immunity 17**,** 629-638. [DOI] [PubMed] [Google Scholar]
- 28.Fuss I. J., Neurath, M., Boirivant, M., Klein, J. S., de la Motte, C., Strong, S. A., Fiocchi, C. & Strober, W. (1996) J. Immunol. 157**,** 1261-1270. [PubMed] [Google Scholar]
- 29.Amiot F., Boussadia, O., Cases, S., Fitting, C., Lebastard, M., Cavaillon, J. M., Milon, G. & Dautry, F. (1997) Eur. J. Immunol. 27**,** 1035-1042. [DOI] [PubMed] [Google Scholar]
- 30.Szabo S. J., Sullivan, B. M., Stemmann, C., Satoskar, A. R., Sleckman, B. P. & Glimcher, L. H. (2002) Science 295**,** 338-342. [DOI] [PubMed] [Google Scholar]
- 31.Zheng W. & Flavell, R. A. (1997) Cell 89**,** 587-596. [DOI] [PubMed] [Google Scholar]
- 32.Neurath M. F., Fuss, I., Kelsall, B. L., Stuber, E. & Strober, W. (1995) J. Exp. Med. 182**,** 1281-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devergne O., Birkenbach, M. & Kieff, E. (1997) Proc. Natl. Acad. Sci. USA 94**,** 12041-12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seder R. A., Paul, W. E., Davis, M. M. & Fazekas de St Groth, B. (1992) J. Exp. Med. 176**,** 1091-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris S. C., Coffman, R. L. & Finkelman, F. D. (1998) J. Immunol. 160**,** 3299-3304. [PubMed] [Google Scholar]
- 36.Carnaud C., Lee, D., Donnars, O., Park, S. H., Beavis, A., Koezuka, Y. & Bendelac, A. (1999) J. Immunol. 163**,** 4647-4650. [PubMed] [Google Scholar]