The TAK1-NLK Mitogen-Activated Protein Kinase Cascade Functions in the Wnt-5a/Ca2+ Pathway To Antagonize Wnt/β-Catenin Signaling (original) (raw)
Abstract
Wnt signaling controls a variety of developmental processes. The canonical Wnt/β-catenin pathway functions to stabilize β-catenin, and the noncanonical Wnt/Ca2+ pathway activates Ca2+/calmodulin-dependent protein kinase II (CaMKII). In addition, the Wnt/Ca2+ pathway activated by Wnt-5a antagonizes the Wnt/β-catenin pathway via an unknown mechanism. The mitogen-activated protein kinase (MAPK) pathway composed of TAK1 MAPK kinase kinase and NLK MAPK also negatively regulates the canonical Wnt/β-catenin signaling pathway. Here we show that activation of CaMKII induces stimulation of the TAK1-NLK pathway. Overexpression of Wnt-5a in HEK293 cells activates NLK through TAK1. Furthermore, by using a chimeric receptor (β2AR-Rfz-2) containing the ligand-binding and transmembrane segments from the β2-adrenergic receptor (β2AR) and the cytoplasmic domains from rat Frizzled-2 (Rfz-2), stimulation with the β-adrenergic agonist isoproterenol activates activities of endogenous CaMKII, TAK1, and NLK and inhibits β-catenin-induced transcriptional activation. These results suggest that the TAK1-NLK MAPK cascade is activated by the noncanonical Wnt-5a/Ca2+ pathway and antagonizes canonical Wnt/β-catenin signaling.
The Wnt proteins constitute a large family of extracellular signaling molecules that control a variety of developmental processes, including cell proliferation, cell fate specification, and embryonic patterning (3, 21, 34). These Wnt proteins activate different cytoplasmic signaling pathways by binding to members of the Frizzled family of prospective receptors. Signaling in response to members of the Wnt-1 class leads to activation of Dishevelled, which then represses the function of glycogen synthase kinase 3β (GSK-3) activity (17, 22). In this canonical Wnt signaling pathway, in the absence of Wnt signal, GSK-3 promotes the phosphorylation and degradation of β-catenin. Stimulation of the Wnt pathway represses GSK-3 activity, which in turn reduces the degradation of β-catenin, leading to its accumulation. As β-catenin levels increase, it forms complexes with members of the T-cell factor/lymphoid enhancer factor (TCF/LEF) classes of architectural high-mobility group box transcription factors to regulate expression of target genes. In contrast, the so-called “noncanonical Wnt pathway” mediated by the Wnt-5a subclass triggers intracellular Ca2+ release to activate Ca2+-sensitive enzymes, such as protein kinase C (PKC) and Ca2+/calmodulin-dependent kinase II (CaMKII) (11, 12, 27, 28). This can be mimicked by expression of rat Frizzled-2 (Rfz-2), but not Rfz-1 (27). Reciprocally, Rfz-1, but not Rfz-2, couples to the canonical Wnt-1 pathway (36). Thus, Wnt-1 and Rfz-1 activate the β-catenin pathway, but do not elevate intracellular Ca2+, whereas Wnt-5a and Rfz-2, which do not activate β-catenin signaling, nevertheless elevate levels of intracellular Ca2+.
Members of the Wnt-1 class are able to induce a secondary axis in Xenopus embryos when misexpressed on the ventral side. They transform C57mg mammary epithelial cells. In both assays, the cellular response is attributed to activation of the β-catenin pathway. On the other hand, members of the Wnt-5a class do not induce formation of a secondary axis in Xenopus, nor do they transform C57mg cells (4, 18, 35). Furthermore, Wnt-5a is able to antagonize the Wnt/β-catenin pathway. Expression of Wnt-5a can partially block altered cell morphology of C57mg cells induced by stable expression of Wnt-1 (20). In Xenopus, coexpression of Wnt-5a with Wnt-8 blocks the ability of Wnt-8 to induce a secondary axis (30). These results suggest that Wnt-5a modulates the activity of the canonical Wnt/β-catenin pathway via activation of the Wnt/Ca2+ pathway. Consistent with this, activation of Ca2+ signaling in Xenopus by ectopic expression of the serotonin receptor can block activation of the Wnt/β-catenin pathway by Wnt-8 (28). Thus, the antagonism between these distinct Wnt pathways regulates cell proliferation and cell fate specification during development. It is therefore important to understand how Wnt-5a/Ca2+ antagonizes Wnt/β-catenin signaling.
Recent evidence indicates that the canonical Wnt/β-catenin signaling pathway is regulated by a mitogen-activated protein kinase (MAPK) pathway composed of TAK1 MAPK kinase kinase (MAPKKK), and NLK MAPK. Evidence for the involvement of the MAPK pathway comes from genetic analyses of a Wnt/β-catenin signaling pathway in Caenorhabditis elegans (15, 23, 26) and the ability of TAK1 and NLK to regulate β-catenin-TCF-function in mammalian cells (8). These studies provide evidence that TAK1 stimulates NLK activity. Active NLK then phosphorylates TCF and prevents the β-catenin-TCF complex from binding DNA, thereby inhibiting the ability of β-catenin-TCF to activate transcription. Thus, TAK1 and NLK appear to act in a pathway parallel to the Wnt/β-catenin pathway.
Components of the signaling pathway that lie upstream of the TAK1-NLK cascade have been undefined. Recent studies indicate that elevated intracellular Ca2+ can activate the MAPK pathways: CaMKII activates the ERK MAPK in several different cell types (33), and the CaMKII-MAPK pathway regulates neuronal cell fate determination in C. elegans (24, 29). This raises the possibility that CaMKII acts upstream to activate the TAK1-NLK MAPK pathway. In this study, we investigated the relationship between the noncanonical Wnt-5a/Ca2+ and TAK1-NLK MAPK pathways. We show that the Wnt-5a-mediated Ca2+ signaling activates the TAK1-NLK pathway via CaMKII.
MATERIALS AND METHODS
Cell culture and transfection.
Cells of the human embryonic kidney line HEK293 were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. 293 cells in 100-mm-diameter plates were transfected with the expression plasmids (10 μg) by calcium phosphate precipitation.
Reporter gene assays.
293 cells (1.6 × 105 cells per well) were seeded into six-well, 35-mm-diameter plates. Cells were transfected by the calcium phosphate precipitate method at 24 h after seeding with the TOPFLASH reporter gene plasmid along with each expression vector as indicated. The total DNA concentration (1.7 μg) was kept constant by supplementation with empty vector DNAs. Luciferase activity was determined with the Promega luciferase assay system. β-Galactosidase (β-Gal) vector (0.1 μg) under the control of the β-actin promoter was used for normalizing transfection efficiencies. The values shown are the average of one representative experiment in which each transfection was performed in duplicate.
In vitro kinase assays.
Polyclonal rabbit antibody to NLK (anti-NLK) was produced against peptides corresponding to amino acids 496 to 515 of mouse NLK. The rabbit anti-TAK1 polyclonal antibody, bacterially expressed MKK6, and LEF-1 were described previously (8, 19). Aliquots of immunoprecipitates were incubated with MKK6 or LEF-1 (1 μg) in 10 μl of kinase buffer containing 10 mM HEPES (pH 7.4), 1 mM dithiothreitol (DTT), 5 mM MgCl2, and 5 μCi of [γ-32P]ATP at 25°C for 2 min. Samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and phosphorylated proteins were visualized by autoradiography.
CaMK activity assays.
The activity of CaMKII was determined with the Upstate Biotechnology CaM Kinase II Assay kit. Aliquots of crude cell lysates were incubated with the specific substrate peptide KKALRRQETVDAL in 50 μl of reaction buffer containing 16 mM MOPS (morpholineethanesulfonic acid [pH 7.2]), 20 mM β-glycerol phosphate, 0.8 mM sodium orthovanadate, 0.6 mM CaCl2 (pH 7.4), 0.8 mM DTT, 8 μg of calmodulin per ml, 1 mM EGTA, 15 mM MgCl2, 100 μM ATP, and 10 μCi of [γ-32P]ATP at 30°C for 10 min. The phosphorylated substrate was then separated from the residual [γ-32P]ATP by using phosphocellulose paper and quantitated with a scintillation counter.
Generation of cell lines stably expressing the β2AR-Rfz-2 chimeric receptor.
To establish stable cell line that expresses β2-adrenergic receptor-rat Frizzled-2 chimera (β2AR-Rfz-2), 293 cells in 100-mm plates were transfected with pcDneo (1 μg) and pcDNA3 harboring the cDNA construct encoding β2AR-Rfz-2 (10 μg) by calcium phosphate precipitation. Clones were selected in medium containing G418 (500 μg/ml). Thirteen independent colonies were cloned, and expression of β2AR-Rfz-2 was determined by reverse transcription-PCR (RT-PCR).
RESULTS AND DISCUSSION
CaMKII activates the TAK1-NLK pathway.
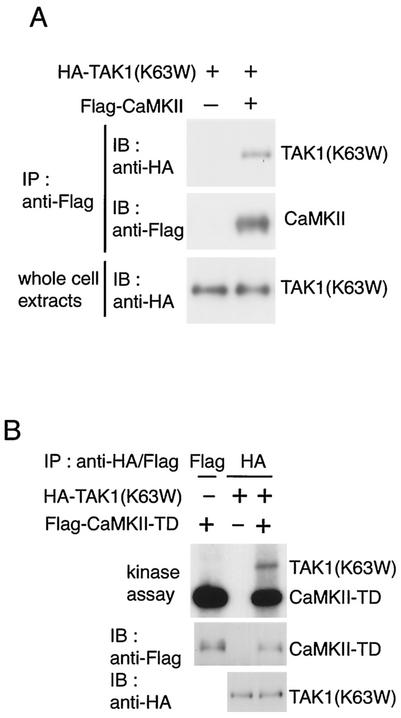
To explore the possibility that CaMKII acts upstream to activate the TAK1-NLK MAPK pathway, we investigated whether CaMKII physically interacts with TAK1 in intact cells. Hemagglutinin (HA)-tagged kinase-inactive TAK1 [HA-TAK1(K63W)] was cotransfected with Flag epitope-tagged CaMKII into HEK293 cells. Cell extracts were immunoprecipitated with anti-Flag antibody, followed by immunoblotting with anti-HA antibody. In cells cotransfected with CaMKII, an association between TAK1 and CaMKII was detected (Fig. 1A).
FIG. 1.
CaMKII interacts with and phosphorylates TAK1. (A) Interaction between CaMKII and TAK1. 293 cells were transfected with Flag-CaMKII and HA-TAK1(K63W) as indicated. Cell extracts were immunoprecipitated (IP) with anti-Flag antibody. The immunoprecipitates were immunoblotted with anti-HA (top panel) and anti-Flag (middle panel) antibodies. Whole-cell extracts were immunoblotted (IB) with anti-HA antibody (bottom panel). (B) Phosphorylation of TAK1 by CaMKII. 293 cells were transfected with Flag-CaMKII(T286D) (TD) and HA-TAK1(K63W) as indicated. Immunoprecipitated complexes with anti-Flag or anti-HA antibody were incubated with [γ-32P]ATP and analyzed by autoradiography (top panel). The immunoprecipitates were immunoblotted with anti-Flag (middle panel) and anti-HA (bottom panel) antibodies.
We next tested whether CaMKII phosphorylates TAK1. 293 cells were cotransfected with HA-TAK1(K63W) and Flag-tagged constitutively active CaMKII(T286D), generated by replacement of Thr-286 with Asp to mimic autophosphorylation of CaMKII. Cell extracts were subjected to immunoprecipitation with anti-HA antibody, followed by both immunoblotting and in vitro kinase assay (Fig. 1B). CaMKII(T286D) was detected in TAK1(K63W) immunoprecipitates. When the immunoprecipitates were incubated with [γ-32P]ATP, CaMKII(T286D) was autophosphorylated and TAK1(K63W) became phosphorylated. These results suggest that activated CaMKII induces phosphorylation of TAK1. However, we failed to detect phosphorylation of purified TAK1 proteins by CaMKII in vitro (data not shown). Thus, whether TAK1 is a direct substrate of CaMKII remains to be determined.
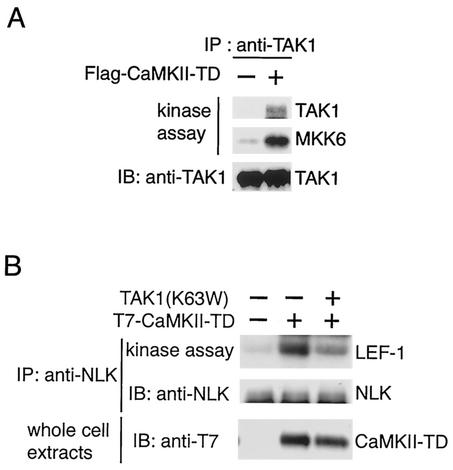
We wished to determine whether TAK1 itself can be activated by CaMKII. To this end, 293 cells were transfected with CaMKII(T286D). Because TAK1 contains autophosphorylation sites, autophosphorylation is an easy method to detect its activity (10). Endogenous TAK1 protein was immunoprecipitated from the cells lysates with anti-TAK1 antibody, and its autophosphorylation activity was measured. TAK1 autophosphorylation was stimulated by CaMKII(T286) transfection (Fig. 2A, top panel). Endogenous TAK1 immunoprecipitated from the cells was then assayed with MKK6 as a substrate to confirm that enhanced autophosphorylation is a reflection of enhanced TAK1 activity. In cells transfected with CaMKII(T286D), phosphorylation of MKK6 by TAK1 was enhanced (Fig. 2A, middle panel). Thus, activated CaMKII increases TAK1 activity.
FIG. 2.
Activation of TAK1 and NLK by CaMKII. (A) Activation of endogenous TAK1. 293 cells were transfected with Flag-CaMKII(T286D) (TD) as indicated. Endogenous TAK1 was immunoprecipitated (IP) with anti-TAK1 antibody. The immunoprecipitates were subjected to an in vitro phosphorylation assay by autophosphorylation of TAK1 (top panel) and bacterially expressed MKK6 as an exogenous substrate (middle panel). The immunoprecipitates were analyzed by immunoblotting (IB) with anti-TAK1 antibody (bottom panel). (B) Activation of endogenous NLK by CaMKII. 293 cells were transfected with expression plasmids encoding T7-CaMKII(T286D) (TD) and TAK1(K63W) as indicated. Endogenous NLK was immunoprecipitated with anti-NLK antibody. The immunoprecipitates were subjected to an in vitro phosphorylation assay with bacterially expressed LEF-1 as an exogenous substrate (top panel). The immunoprecipitates were analyzed by immunoblotting with anti-NLK antibody (middle panel). Whole-cell extracts were immunoblotted with anti-T7 antibody (bottom panel).
To test whether CaMKII activates the TAK1-NLK pathway, we investigated the possibility that CaMKII activates NLK activity. We transfected CaMKII(T286D) in 293 cells and assayed endogenous NLK kinase activity following immunoprecipitation with bacterially expressed LEF-1 protein as a substrate (Fig. 2B). Transfection of CaMKII(T286D) stimulated NLK kinase activity. To determine whether TAK1 is actually required for CaMKII-mediated NLK activation, we studied the effect of a dominant-negative form of TAK1, TAK1(K63W), on the activation of NLK by CaMKII(T286D). TAK1(K63W) significantly blocked activation of NLK in response to CaMKII, consistent with the possibility that CaMKII activates NLK via TAK1. These results support the hypothesis that CaMKII leads to activation of the TAK1-NLK cascade.
Calcium signaling activates NLK via CaMKII.
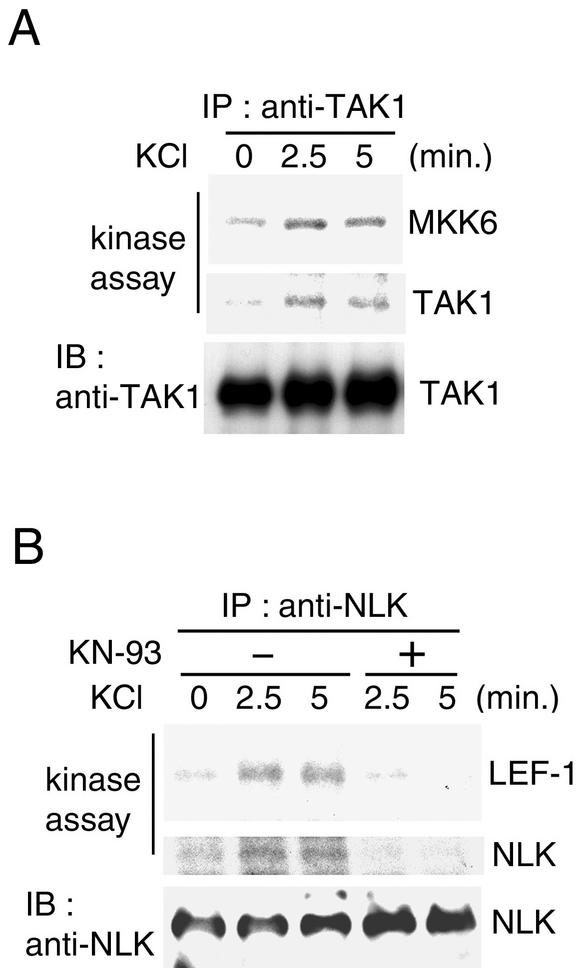
We examined whether Ca2+ signaling activates the TAK1-NLK pathway under physiological conditions. Membrane depolarization caused by extracellular high K+ concentration induces Ca2+ influx through voltage-dependent Ca2+ channels. Extracts were prepared from PC12 cells, either untreated or stimulated with KCl for various times, and subjected to immunoprecipitation with anti-TAK1 antibody. These TAK1 immunoprecipitates were assayed for TAK1 kinase activity toward itself and with MKK6 as a substrate (Fig. 3A). Endogenous TAK1 was activated within 2.5 to 5 min of KCl addition.
FIG. 3.
Calcium-induced activation of TAK1 and NLK. (A) Calcium-induced activation of TAK1. PC12 cells were treated with 75 mM KCl for the indicated periods. Cell extracts were immunoprecipitated (IP) with anti-TAK1 antibody. The immunoprecipitates were subjected to an in vitro phosphorylation assay with bacterially expressed MKK6 as an exogenous substrate (top panel) and autophosphorylation of TAK1 (middle panel). The immunoprecipitates were analyzed by immunoblotting (IB) with anti-TAK1 antibody (bottom panel). (B) Calcium-induced activation of NLK. PC12 cells pretreated with or without 20 μM KN-93 for 20 min were treated with 75 mM KCl for the indicated periods. Cell extracts were immunoprecipitated with anti-NLK antibody. The immunoprecipitates were subjected to an in vitro phosphorylation assay with bacterially expressed LEF-1 as an exogenous substrate (top panel) and autophosphorylation of NLK (middle panel). The immunoprecipitates were analyzed by immunoblotting with anti-NLK antibody (bottom panel).
When endogenous NLK kinase activity following immunoprecipitation was assayed with LEF-1 protein as a substrate, NLK was also activated in response to KCl stimulation (Fig. 3B, top panel). Previous studies have shown that activation of NLK correlates with NLK autophosphorylation (8). Endogenous NLK was immunoprecipitated from the cells, and we measured its ability to autophosphorylate. The kinase assays revealed that NLK prepared from KCl-treated cells phosphorylated NLK itself (meddle panel). To analyze whether KCl-induced NLK activation involves CaMKII, PC12 cells were treated with KCl in the absence or presence of CaMKII inhibitor KN-93. CaMKII inhibitor effectively inhibited the NLK activation induced by high K+ levels. These results suggest that CaMKII activates NLK in response to Ca2+ influx.
CaMKII antagonizes Wnt/β-catenin signaling.
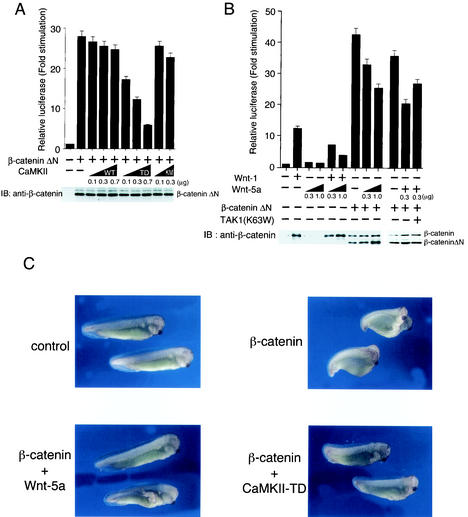
The fact that CaMKII activates the TAK1-NLK pathway suggests that CaMKII functions as an antagonist of Wnt/β-catenin signaling. To explore this possibility, we tested the effect of CaMKII on the canonical Wnt signal transduction pathway that leads to the accumulation of the β-catenin—TCF complex (2). Turnover of β-catenin in the absence of Wnt-1 signaling requires its N-terminal region, and deletion of this region (β-cateninΔN) results in the accumulation of β-catenin, thus mimicking constitutive Wnt-1 signaling (1). β-CateninΔN was transiently coexpressed in 293 cells together with a luciferase reporter plasmid driven by a TCF-responsive promoter, TOPFLASH (31, 32). Transient expression of β-cateninΔN resulted in activation of this reporter (Fig. 4A), while no activity was observed when a FOPFLASH reporter, which lacks TCF binding sites, was cotransfected (data not shown). Coexpression of constitutively active CaMKII(T286D) repressed the activation of β-cateninΔN-induced reporter transcription in a dose-dependent manner. In contrast, wild-type CaMKII or a kinase-inactive mutant of CaMKII, CaMKII(K42M), had no inhibitory effect (Fig. 4A). These results indicate that CaMKII antagonizes the canonical Wnt pathway at a point downstream of β-catenin.
FIG. 4.
Effects of CaMKII and Wnt-5A on Wnt/β-catenin signaling. (A) Effects of CaMKII on TCF-dependent reporter gene activity. 293 cells were transfected with luciferase reporter plasmid (0.1 μg), β-cateninΔN expression plasmid (0.5 μg), and the indicated amounts of plasmids encoding CaMKII (wild type [WT]), CaMKII(T286D) (TD), and CaMKII(K24 M) (KM). After 24 h of incubation, cells were harvested, and luciferase activity was measured. The values shown are the average of one representative experiment in which each transfection was performed in duplicate. Equal amounts of cell lysates were immunoblotted (IB) with anti-β-catenin antibody. (B) Effects of Wnt-1 and Wnt-5a on β-catenin stabilization. 293 cells were transfected with luciferase reporter (0.1 μg), β-cateninΔN (0.5 μg), Wnt-1 expression plasmid (0.5 μg), TAK1(K63W) (0.2 μg), and the indicated amounts of Wnt-5a expression plasmid. Cell lysates were used for measuring luciferase activity. Equal amounts of cell lysates were immunoblotted with anti-β-catenin antibody. (C) Effect of Wnt-5a and CaMKII on β-catenin-induced axis formation in Xenopus embryos. β-Catenin mRNA (100 pg) was injected into two ventral blastomeres at the four-cell stage with Wnt-5a or CaMKII(T286D) (TD) mRNA (5 pg) as indicated. Embryos were examined for axial duplications at the tadpole stage. Injection of β-catenin led to secondary axis formation with head in 52% of the injected embryos (n = 50), injection of β-catenin plus Wnt-5a had the same result in 0% of the embryos (n = 50), and injection with β-catenin plus CaMKII-TD had the same result in 14% of the embryos (n = 50).
Wnt-5a interferes with the canonical Wnt/β-catenin pathway.
Recent evidence has shown that CaMKII is activated by Wnt-5a via the Fz receptors (12), which include Rfz-2 (27). Furthermore, Wnt-5a interferes with Wnt/β-catenin signaling (30). To study this interaction in 293 cells, we examined the effects of Wnt-5a on Wnt-1-induced accumulation of β-catenin and transcriptional activation (Fig. 4B). As observed previously (4), expression of Wnt-1, but not of Wnt-5a, resulted in increased steady-state levels of β-catenin. However, expression of Wnt-5a had no effect on Wnt-1-mediated induction of β-catenin, whereas expression of Wnt-5a repressed Wnt-1-induced transcriptional activation of the TCF-responsive TOPFLASH reporter construct. These results demonstrate that Wnt-5a represses Wnt-1 signaling without affecting the up-regulation of cytosolic β-catenin and suggests that the antagonism between Wnt-1 and Wnt-5a occurs downstream of β-catenin. Consistent with this possibility, cotransfection of Wnt-5a with β-cateninΔN inhibited transactivation by β-cateninΔN. To determine whether TAK1 indeed is the mediator of Wnt-5a to antagonize β-catenin signaling, we examined the effect of a dominant-negative TAK1(K63W) on the Wnt-5a-mediated inhibition of β-cateninΔN-induced transcriptional activation. As shown in Fig. 4B, TAK1(K63W) partially reversed the blocking effect of Wnt-5a on transactivation by β-cateninΔN. These results suggest that Wnt-5a can inhibit β-catenin-mediated transcriptional stimulation via TAK1.
It is well established that the Wnt/β-catenin pathway plays a crucial role in the development of the Xenopus embryonic axis (16, 21, 34). We also used the induction of secondary axes by ectopic expression of β-catenin in Xenopus embryos as an assay for the role of Wnt-5a and CaMKII in the canonical Wnt pathway in vivo (Fig. 4C). Injection of β-catenin mRNA into the vegetal ventral region of early-cleavage-stage embryos leads to the induction of a secondary embryonic axis (5). Coinjection of Wnt-5a or CaMKII(T286D) mRNA effectively blocked the induction of this secondary axis caused by β-catenin mRNA. These results support the possibility that Wnt-5a and CaMKII interfere with the canonical Wnt/β-catenin pathway at a point downstream of β-catenin.
Wnt-5a activates the TAK1-NLK pathway via CaMKII.
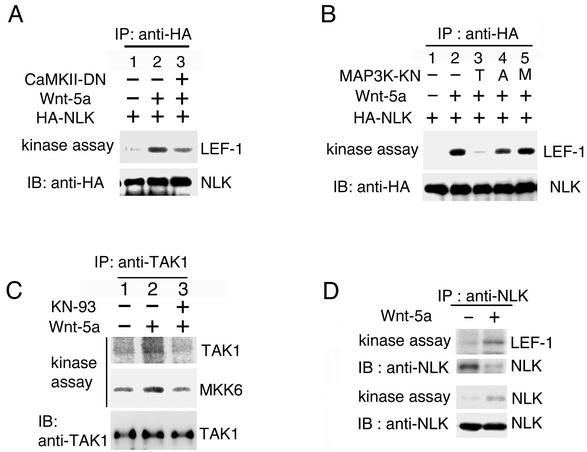
The results presented above raise the possibility that Wnt-5a antagonizes the canonical Wnt/β-catenin pathway by activating the TAK1-NLK pathway. To test this possibility, we determined whether NLK activity is modulated by Wnt-5a signaling. HA epitope-tagged NLK (HA-NLK) was cotransfected with Wnt-5a in 293 cells. The NLK protein was immunoprecipitated from the cell lysates, and its kinase activity was measured in vitro. Cotransfection of Wnt-5a resulted in an increase in NLK kinase activity (Fig. 5A and B, lane 2). We next examined whether Wnt-5a-induced activation of NLK is mediated via CaMKII and TAK1. A COOH-terminally-truncated version of kinase-dead CaMKII, CaMKII(K42 M) (positions 1 to 271), is known to act to interfere with the activity of endogenous CaMKII (12). We showed that CaMKII(K42 M)(1-271) was able to reduce activation of NLK in response to Wnt-5a (Fig. 5A, lane 3). A dominant-negative TAK1(K63W) also inhibited NLK activation induced by Wnt-5a (Fig. 5B, lane 3). Dominant-negative mutants of other members of the MAPKKK family, ASK1(K709 M) and MTK1(K1371R), had no effect on NLK activation by Wnt-5a (Fig. 5B, lanes 4 and 5). These results indicate that the dominant-negative effect on NLK activation is specific for TAK1(K63W).
FIG. 5.
Activation of TAK1 and NLK by Wnt-5a. (A and B) Activation of NLK by Wnt-5a is dependent on CaMKII and TAK1. 293 cells were transfected with the indicated expression plasmids. HA-NLK was immunoprecipitated (IP) with anti-HA antibody. The immunocomplexes were used for in vitro kinase assays with bacterially expressed LEF-1 as an exogenous substrate (upper panels) and immunoblotted (IB) with anti-HA antibody (lower panels). CaMKII-DN, CaMKII(K42 M) (1-271); T, TAK1(K63W); A, ASK1(K709 M); M, MTK1(K1371R). (C) Activation of endogenous TAK1 by Wnt-5a. 293 cells were transfected with Wnt-5a as indicated. Cells were treated with or without KN-93 (20 μM). Endogenous TAK1 was immunoprecipitated with anti-TAK1 antibody. The immunoprecipitates were subjected to an in vitro phosphorylation assay with autophosphorylation of TAK1 (top panel) and bacterially expressed MKK6 as an exogenous substrate (middle panel). The immunoprecipitates were analyzed by immunoblotting with anti-TAK1 antibody (bottom panel). (D) Activation of endogenous NLK by Wnt-5a. 293 cells were transfected with Wnt-5a as indicated. Endogenous NLK was immunoprecipitated with anti-NLK antibody. The immunoprecipitates were subjected to an in vitro phosphorylation assay with LEF-1 as a substrate (top panel) and autophosphorylation of NLK (third panel). The immunoprecipitates were analyzed by immunoblotting with anti-NLK antibody (second and bottom panels).
To confirm that Wnt-5a activates the TAK1-NLK pathway, we analyzed the kinase activities of endogenous TAK1 and NLK from 293 cells transfected with Wnt-5a. TAK1 was immunoprecipitated from the cell lysates and incubated in a kinase reaction with MKK6 as a substrate. Transfection of Wnt-5a resulted in an increase in TAK1 activity toward itself and MKK6 (Fig. 5C, lane 2). This activation was efficiently blocked by CaMKII inhibitor KN-93 (lane 3), suggesting that CaMKII is involved in mediating the ability of Wnt-5a to activate TAK1. When we assayed endogenous NLK kinase activity following immunoprecipitation with LEF-1 protein as a substrate, transfection of Wnt-5a stimulated NLK kinase activity and its autophosphorylation activity (Fig. 5D). Taken together, these results support the hypothesis that Wnt-5a activates the TAK1-NLK signaling pathway via CaMKII.
Activation of the TAK1-NLK pathway is a direct response to receptor stimulation.
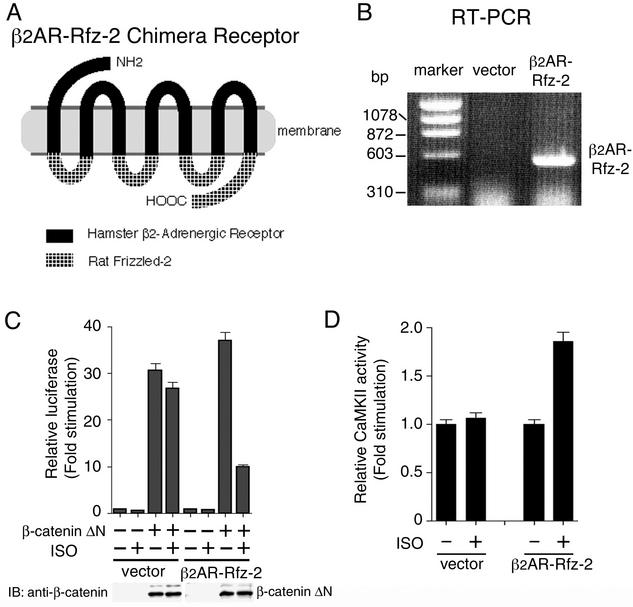
To test whether the activation of the TAK1-NLK pathway by Wnt-5a is a rapid response to receptor activation or a belated physiological reflection of this activation, we used a chimeric receptor (β2AR-Rfz-2) containing the extracellular and transmembrane regions of the hamster β2AR and the intracellular domains of Rfz-2 (Fig. 6A). This circumvented the need for purified, active Wnt-5a ligand. This chimeric receptor has the potential to be activated by soluble drugs of well-known pharmacology (14). 293 cells were stably transfected with an expression vector harboring the β2AR-Rfz-2 chimera. Clones (293-β2AR-Rfz-2) expressing mRNA encoding the Rfz-2 chimeric receptor in large amounts were identified by RT-PCR and propagated (Fig. 6B).
FIG. 6.
Activation of CaMKII by inducible Rfz-2. (A) Schematic representation of the β2AR-Rfz-2 construct. (B) RT-PCR of β2AR-Rfz-2 stably expressed in 293 cells. The RNA of 293 cells harboring the empty expression vector or the vector expressing β2AR-Rfz-2 chimera was reverse transcribed and amplified. The molecular markers indicate the relative size in base pairs of the amplified products. (C) Effect of β2AR-Rfz-2 on TCF-dependent reporter gene activity. Cells of 293-β2AR-Rfz-2 and 293 stably transfected with control vector were transfected with luciferase reporter plasmid (0.1 μg) and β-cateninΔN expression plasmid (0.5 μg) as indicated. Cells were treated with or without the β-adrenergic agonist ISO (100 μM), and luciferase activity was measured. The values shown are the average of one representative experiment in which each transfection was performed in duplicate. Equal amounts of cell lysates were immunoblotted (IB) with anti-β-catenin antibody. (D) Activation of endogenous CaMKII by β2AR-Rfz-2. Cells of 293-β2AR-Rfz-2 cells and 293 cells stably transfected with control vector were treated with or without ISO (50 μM) for 5 min. Cell extracts were assayed for CaMKII activity. The values shown are the average of one representative experiment in which each transfection was performed in duplicate.
We measured the effect of the β-adrenergic agonist isoproterenol (ISO) on the activation of β-catenin pathway in 293-β2AR-Rfz-2 cells. Treatment with ISO blocked β-cateninΔN-induced transcriptional activation in 293-β2AR-Rfz-2 cells, but not in 293 cells stably transfected with control vector (Fig. 6C). Because CaMKII is activated by Wnt-5a via Rfz-2 in Xenopus embryos (12), we used this assay to ensure that the β2AR-Rfz-2 chimera functioned in a manner resembling that of wild-type Rfz-2. We observed that treatment of the 293-β2AR-Rfz-2 cells with ISO activated endogenous CaMKII within 5 min (Fig. 6D). These results establish that the β2AR-Rfz-2 chimera elicits a downstream-signaling response in a β-adrenergic agonist-stimulatable manner and reflect the normal signaling activity of Rfz-2.
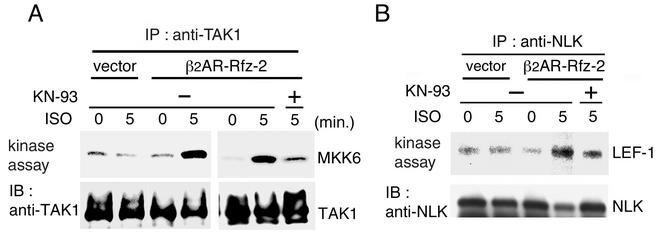
We next investigated whether the TAK1-NLK pathway was activated in response to activating Rfz-2 signaling with β2AR-Rfz-2. When stimulated with the β-adrenergic agonist ISO, TAK1 activation was observed in 293-β2AR-Rfz-2 cells within 5 min of stimulation with ISO (Fig. 7A). NLK was also activated in response to ISO stimulation in 293-β2AR-Rfz-2 cells, but not 293 cells stably transfected with control vector (Fig. 7B). To test further the involvement of CaMKII in mediating the ability of Rfz-2 activation to activate TAK1 and NLK, we examined the effect of the CaMKII inhibitor KN-93 on activation. KN-93 effectively blocked the activation of endogenous TAK1 and NLK induced by ISO treatment (Fig. 7A and B). These results suggest that Rfz-2 requires CaMKII to activate the TAK1-NLK pathway.
FIG. 7.
Activation of TAK1 and NLK by inducible Rfz-2. Cells of 293-β2AR-Rfz-2 and 293 stably transfected with control vector were pretreated with or without KN-93 (50 μM) for 50 min. Then they were treated with ISO (50 μM) for the indicated time periods. Endogenous TAK1 (A) and NLK (B) were immunoprecipitated (IP) with anti-TAK1 and anti-NLK antibodies, respectively. The immunocomplexes were used for in vitro kinase assays (upper panels). The amounts of immunoprecipitated TAK1 and NLK were determined by immunoblotting (IB) with anti-TAK1 and anti-NLK antibodies, respectively (lower panels).
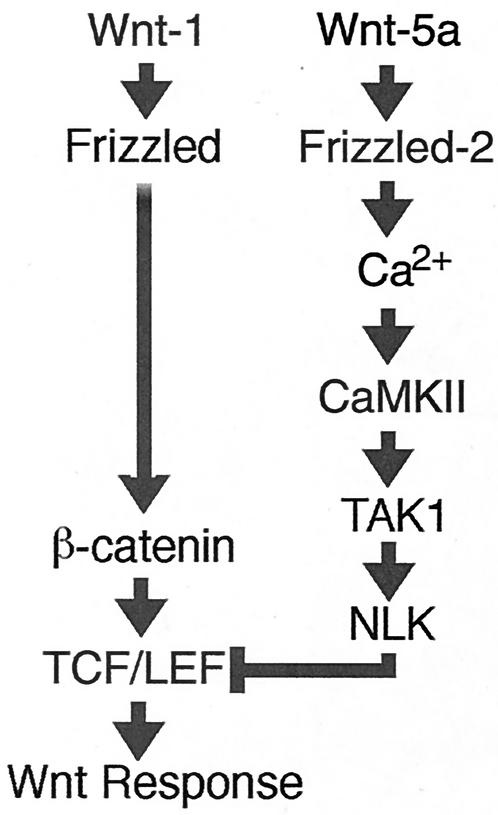
Wnt-1 signaling stabilizes cytosolic β-catenin, which, in turn, forms a complex with one of the TCF/LEF transcription factors and thereby activates expression of specific target genes (3, 21, 34). In contrast, Wnt-5a and Rfz-2 both stimulate Ca2+ release and activate CaMKII (11, 12, 27, 28). Wnt-5a also is able to antagonize the Wnt/β-catenin pathway (30). Recent evidence also implicates the TAK1-NLK MAPK pathway in the antagonism of Wnt/β-catenin signaling (8). Active NLK phosphorylates TCF and prevents the β-catenin-TCF complex from binding DNA, thereby inhibiting the ability of β-catenin-TCF to activate transcription. Based on these data and the results of the present study, we propose a model in which the Wnt/Ca2+ pathway activated by Wnt-5a antagonizes the Wnt/β-catenin pathway by activating CaMKII, which in turn activates the TAK1-NLK MAPK pathway (Fig. 8). Thus, these distinct Wnt pathways converge in an antagonistic manner.
FIG. 8.
Model for interaction between the Wnt/β-catenin and Wnt/Ca2+ pathways. (See the text for details.)
Recent studies suggest that the antagonism between these distinct Wnt pathways regulates opposing cell fates during development of vertebrates. For example, the Wnt/β-catenin pathway specifies dorsal cell fates, whereas the Wnt/Ca2+ pathway promotes ventral cell fates by inducing the activity of CaMKII (12). In support of these findings, a mutant form of a Frizzled receptor that acts to block Wnt signals promotes dorsal cell fates when expressed ventrally in Xenopus embryos and acts independently of β-catenin (9). Furthermore, activation of the Wnt/Ca2+ pathway blocks convergent extension movements during Xenopus gastrulation by interfering with the Wnt/β-catenin pathway at two different levels (13). PKC, activated by the Wnt/Ca2+ pathway, blocks the Wnt/β-catenin pathway upstream of β-catenin, whereas CaMKII inhibits the Wnt/β-catenin signaling cascade downstream of β-catenin. Thus, a finely balanced activity of distinct Wnt signaling cascades is required for proper development. Besides antagonism of Wnt pathways in Xenopus, an opposing cross talk of distinct antagonism between Wnt proteins has also been observed in Drosophila. Specifically, Dwnt-4 antagonizes the function of wingless in the embryonic ectoderm and Dfz-3 attenuates wingless signaling (6, 25). Moreover, similar to Wnt-5a, Dwnt-4 also blocks the ability of Wnt-8 to induce an ectopic axis in Xenopus (6). Therefore, functionally distinct Wnt activities and their interactions are conserved from flies to vertebrates. However, Dwnt-4 and wingless can elicit similar cellular responses during imaginal development (7). The molecular bases underlying the ability of wingless and Dwnt-4 to perform antagonistic or similar signaling activities remain to be explored.
Our elucidation of the mechanism of antagonism between the Wnt/Ca2+ and Wnt/β-catenin pathways also has implications for understanding Wnt antagonism in cell transformation. Specifically, in C57mg mammary epithelial cells, gain of function of Wnt-1, which activates the β-catenin pathway, promotes cell transformation. In contrast, loss of function of Wnt-5a in the same cells is also transforming (20). Given the considerable interest in understanding the roles of the Wnt/β-catenin pathway in diverse human cancers (20, 21, 22), our model (Fig. 8) should foster insights into how this transforming activity might be controlled by other Wnt pathways.
Acknowledgments
We thank T. Akiyama, B. Brott, H. Clevers, A. Kikuchi, and J. Munguia for materials; E. Nishida for helpful discussions; and M. Lamphier for critical reading of the manuscript. This work was supported by special grants for CREST; Advanced Research on Cancer from the Ministry of Education, Culture and Science of Japan; the Asahi Glass Foundation; the Daiko Foundation; the Uehara Foundation; and the Yamanouchi Foundation for Research on Metabolic Disorders (K.M.).
REFERENCES
- 1.Aberle, H., A. Bauer, J. Stappert, A. Kispert, and R. Kemler. 1997. β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16**:**3797-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrens, J., J. P. von Kries, M. Kuhl, L. Bruhn, D. Wedlich, R. Grosschedl, and W. Birchmeier. 1996. Functional interaction of β-catenin with the transcription factor LEF-1. Nature 382**:**638-642. [DOI] [PubMed] [Google Scholar]
- 3.Cardigan, K. M., and R. Nusse. 1997. Wnt signaling: a common theme in animal development. Genes Dev. 11**:**3286-3305. [DOI] [PubMed] [Google Scholar]
- 4.Du, S. J., S. M. Purcell, J. L. Christian, L. L. McGrew, and R. T. Moon. 1995. Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol. Cell. Biol. 15**:**2625-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funayama, N., F. Fagotto, P. McCrea, and B. M. Gumbiner. 1995. Embryonic axis induction by the armadillo repeat domain of β-catenin: evidence for intracellular signaling. J. Cell Biol. 128**:**959-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gieseler, K., Y. Graba, M. C. Mariol, E. L. Wilder, A. Martinez-Arias, P. Lemaire, and J. Pradel. 1999. Antagonist activity of DWnt-4 and wingless in the Drosophila embryonic ventral ectoderm and in heterologous Xenopus assays. Mech. Dev. 85**:**123-131. [DOI] [PubMed] [Google Scholar]
- 7.Gieseler, K., E. Wilder, M. C. Mariol, M. Buratovitch, H. Berenger, Y. Graba, and J. Pradel. 2001. DWnt4 and wingless elicit similar cellular responses during imaginal development. Dev. Biol. 232**:**339-350. [DOI] [PubMed] [Google Scholar]
- 8.Ishitani, T., J. Ninomiya-Tsuji, S. Nagai, M. Nishita, M. Meneghini, N. Barker, M. Waterman, B. Bowerman, H. Clevers, H. Shibuya, and K. Matsumoto. 1999. The TAK1-NLK-MAPK-related pathway antagonizes signaling between β-catenin and transcription factor TCF. Nature 399**:**798-802. [DOI] [PubMed] [Google Scholar]
- 9.Itoh, K., and S. Y. Sokol. 1999. Axis determination by inhibition of Wnt signaling in Xenopus. Genes Dev. 13**:**2328-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kishimoto, K., K. Matsumoto, and J. Ninomiya-Tsuji. 2000. TAK1 mitogen-activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. J. Biol. Chem. 275**:**7359-7364. [DOI] [PubMed] [Google Scholar]
- 11.Kuhl, M., L. C. Sheldahl, M. Park, J. R. Miller, and R. T. Moon. 2000. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 16**:**279-283. [DOI] [PubMed] [Google Scholar]
- 12.Kuhl, M., L. C. Sheldahl, C. C. Malbon, and R. T. Moon. 2000. Ca2+/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J. Biol. Chem. 275**:**12701-12711. [DOI] [PubMed] [Google Scholar]
- 13.Kuhl, M., K. Geis, L. C. Scheldahl, T. Pukrop, R. T. Moon, and D. Wedlich. 2001. Antagonistic regulation of convergent extension movements in Xenopus by Wnt/β-catenin and Wnt/Ca2+ signaling. Mech. Dev. 106**:**61-76. [DOI] [PubMed] [Google Scholar]
- 14.Liu, X., T. Liu, D. C. Slusarski, J. Yang-Snyder, C. C. Malbon, R. T. Moon, and H. Wang. 1999. Activation of a frizzled-2/β-adrenergic receptor chimera promotes Wnt signaling and differentiation of mouse F9 teratocarcinoma cells via Gαo and Gαt. Proc. Natl. Acad. Sci. USA 96**:**14383-14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meneghini, M. D., T. Ishitani, J. C. Carter, N. Hisamoto, J. Ninomiya-Tsuji, C. J. Thorpe, D. R. Hamill, K. Matsumoto, and B. Bowerman. 1999. MAP kinase and Wnt pathways converge to down-regulate an HMG domain repressor in Caenorhabditis elegans. Nature 399**:**793-797. [DOI] [PubMed] [Google Scholar]
- 16.Miller, J. R., and R. T. Moon. 1996. Signal transduction through β-catenin and specification of cell fate during embryogenesis. Genes Dev. 10**:**2527-2539. [DOI] [PubMed] [Google Scholar]
- 17.Miller, J. R., A. M. Hocking, J. D. Brown, and R. T. Moon. 1999. Mechanism and function of signal transduction by the Wnt/β-catenin and Wnt/Ca2+ pathways. Oncogene 18**:**7860-7872. [DOI] [PubMed] [Google Scholar]
- 18.Moon, R. T., J. D. Brown, and M. Torres. 1997. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 4**:**157-162. [DOI] [PubMed] [Google Scholar]
- 19.Ninomiya-Tsuji, J., K. Kishimoto, A. Hiyama, J. Inoue, Z. Cao, and K. Matsumoto. 1999. The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398**:**252-256. [DOI] [PubMed] [Google Scholar]
- 20.Olson, D. J., and D. M. Gibo. 1998. Antisense Wnt-5a mimics wnt-1-mediated C57MG mammary epithelial cell transformation. Exp. Cell Res. 241**:**134-141. [DOI] [PubMed] [Google Scholar]
- 21.Peifer, M., and P. Polakis. 2000. Wnt signaling in oncogenesis and embryogenesis: a look outside the nucleus. Science 287**:**1606-1609. [DOI] [PubMed] [Google Scholar]
- 22.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14**:**1837-1851. [PubMed] [Google Scholar]
- 23.Rocheleau, C. E., Y. Yasuda, T. H. Shin, R. Lin, H. Sawa, H. Okano, J. R. Priess, R. J. Davis, and C. C. Mello. 1999. WRM-1 activates the LIT-1 protein kinase to transduce anterior/posterior polarity signals in C. elegans. Cell 97**:**717-726. [DOI] [PubMed] [Google Scholar]
- 24.Sagasti, A., N. Hisamoto, J. Hyodo, M. Tanaka-Hino, K. Matsumoto, and C. I. Bargmann. 2001. The CaMKII UNC-43 activates the MAPKKK NSY-1 to execute a lateral signaling decision required for asymmetric olfactory neuron fates. Cell 105**:**221-232. [DOI] [PubMed] [Google Scholar]
- 25.Sato, A., T. Kojima, K. Ui-Tei, Y. Miyata, and K. Saigo. 1999. Dfrizzled-3, a new Drosophila Wnt receptor, acting as an attenuator of Wingless signaling in wingless hypomorphic mutants. Development 126**:**4421-4430. [DOI] [PubMed] [Google Scholar]
- 26.Shin, T. H., J. Yasuda, C. E. Rocheleau, R. Lin, M. Soto, Y. Bei, R. J. Davis, and C. C. Mello. 1999. MOM-4, a MAP kinase kinase kinase related protein, activates WRM-1/LIT-1 kinase to transduce anterior/posterior polarity signals in C. elegans. Mol. Cell 4**:**275-280. [DOI] [PubMed] [Google Scholar]
- 27.Slusarski, D. C., V. G. Corces, and R. T. Moon. 1997. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signaling. Nature 390**:**410-413. [DOI] [PubMed] [Google Scholar]
- 28.Slusarski, D. C., J. Yang-Snyder, W. B. Busa, and R. T. Moon. 1997. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5a. Dev. Biol. 182**:**114-120. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka-Hino, M., A. Sagasti, N. Hisamoto, M. Kawasaki, S. Nakano, J. Ninomiya-Tsuji, C. I. Bargmann, and K. Matsumoto. 2002. SEK-1 MAPKK mediates Ca2+ signaling to determine neuronal asymmetric development in C. elegans. EMBO Rep. 3**:**56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torres, M. A., J. A. Yang-Snyder, S. M. Purcell, A. A. DeMarais, L. L. McGrew, and R. T. Moon. 1996. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5a class and by a dominant negative cadherin in early Xenopus development. J. Cell Biol. 133**:**1123-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Wetering, M., M. Oosterwegel, D. Dooijes, and H. Clevers. 1991. Identification and cloning of TCF-1, a T lymphocyte-specific transcription factor containing a sequence-specific HMG box. EMBO J. 10**:**123-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van de Wetering, M., J. Castrop, V. Korinek, and H. Clevers. 1996. Extensive alternative splicing and dual promoter usage generate Tcf-1 protein isoforms with differential transcription control properties. Mol. Cell. Biol. 16**:**745-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watt, W. C., and D. R. Storm. 2001. Odorants stimulate the ERK/mitogen-activated protein kinase pathway and activate cAMP-response element-mediated transcription in olfactory sensory neurons. J. Biol. Chem. 276**:**2047-2052. [DOI] [PubMed] [Google Scholar]
- 34.Wodarz, A., and R. Nusse. 1998. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14**:**59-88. [DOI] [PubMed] [Google Scholar]
- 35.Wong, G. T., B. J. Gavin, and A. P. McMahon. 1994. Differential transformation of mammary epithelial cells by Wnt genes. Mol. Cell. Biol. 14**:**6278-6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang-Snyder, J., J. R. Miller, J. D. Brown, C. J. Lai, and R. T. Moon. 1996. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr. Biol. 6**:**1302-1306. [DOI] [PubMed] [Google Scholar]