Positive and Negative Regulation of Mast Cell Activation by Lyn via the Fc∊RI (original) (raw)
. Author manuscript; available in PMC: 2006 Nov 15.
Abstract
Aggregation of the high affinity receptor for IgE (Fc∊RI) induces activation of mast cells. In this study we show that upon low intensity stimulation of Fc∊RI with monomeric IgE, IgE plus anti-IgE, or IgE plus low Ag, Lyn (a Src family kinase) positively regulates degranulation, cytokine production, and survival, whereas Lyn works as a negative regulator of high intensity stimulation with IgE plus high Ag. Low intensity stimulation suppressed Lyn kinase activity and its association with Fc∊RI β subunit, whereas high intensity stimulation enhanced Lyn activity and its association with Fc∊RI β. The latter induced much higher levels of Fc∊RI β phosphorylation and Syk activity than the former. Downstream positive signaling molecules, such as Akt and p38, were positively and negatively regulated by Lyn upon low and high intensity stimulations, respectively. In contrast, the negative regulators, SHIP and Src homology 2 domain-containing protein tyrosine phosphatase-1, interacted with Fc∊RI β, and their phosphorylation was controlled by Lyn. Therefore, we conclude that Lyn-mediated positive vs negative regulation depends on the intensity of the stimuli. Studies of mutant Fc∊RI β showed that Fc∊RI β subunit-ITAM (ITAM motif) regulates degranulation and cytokine production positively and negatively depending on the intensity of Fc∊RI stimulation. Furthermore, Lyn-mediated negative regulation was shown to be exerted via the Fc∊RI β-ITAM.
Mast cells are the major effector cells in acute and chronic allergic reactions and host defense against certain parasites and bacteria (1). IgE-dependent activation of mast cells induces the release of preformed proinflammatory chemical mediators (such as histamine and serotonin), proteases, proteoglycans, and nucleotides and the secretion of de novo synthesized lipids (such as leukotrienes and PGs) and polypeptides (such as cytokines and chemokines). These substances contribute to the development of allergy and other forms of inflammation.
The high affinity receptor (Fc∊RI) on murine mast cells consists of four subunits: an IgE-binding α subunit; a signal-amplifying, receptor-stabilizing β subunit; and two disulfide-bonded γ subunits that are the major signal transducer (2). The aggregation of Fc∊RI can be induced by stimulation of IgE-sensitized mast cells with multivalent Ag (stimulation modes hereafter referred to as IgE + low Ag and IgE + high Ag depending on the Ag concentration used) or anti-IgE Ab (referred to as IgE + anti-IgE). A recent study suggested that receptor aggregation can be achieved by binding of monomeric IgE as well (3). In a widely accepted model (4), it is believed that receptor aggregation leads to the activation of β subunit-associated Lyn, an Src family protein tyrosine kinase (PTK),4 and Lyn phosphorylates tyrosine residues in the ITAM motifs (ITAMs) in the cytoplasmic regions of β and γ subunits. Phosphorylated β and γ ITAMs recruit Lyn and Syk (another PTK with two tandem Src homology 2 (SH2) domains N-terminal to the catalytic domain), respectively. Another Src family PTK, Fyn, was also shown to associate with Fc∊RI and to play a complementary role, particularly by activating PI3K (5). These PTKs phosphorylate numerous targets and activate several signaling pathways, including the PI3K, phospholipase C/Ca2+, and several MAPK pathways (4, 6). These signaling events eventually lead to degranulation and cytokine production.
In addition to the above positive regulatory role, a negative regulatory role for Lyn was shown by numerous studies, particularly those of B cell signaling (reviewed in Ref. 7). However, the role of Lyn in mast cells has been controversial: lyn_−/_− mice failed to exhibit passive cutaneous anaphylaxis reactions (an in vivo model for IgE/Ag-dependent mast cell functions) (8), but young lyn_−/_− mice instead exhibited enhanced anaphylactic reactions (9); and degranulation was defective (10), normal (11), or enhanced (5) in IgE + Ag-stimulated lyn_−/_− cells depending on the study. Despite these confusions, negative regulatory functions of Lyn have been well documented; the promoter activity of the IL-2 gene and the production of TNF-α and IL-2 were increased in lyn_−/_− mast cells (10), potentially accounting for the increased degranulation and cytokine gene expression, the compensatory enhanced activation of Fyn (5, 12), the reduced SHIP activities (12), and the prolonged activation of MAPKs such as ERK1, ERK2, JNK1, and p38 observed in lyn_−/_− mast cells (10).
Despite the rapid progress in our understanding of Fc∊RI signaling, we still do not know conditions where Lyn works as a positive vs a negative regulator and mechanisms by which Lyn perform such opposite functions. Our recent data provide a novel insight into this issue. Stimulation with monomeric, highly cytokinergic (HC) IgE, IgE + anti-IgE, and IgE + low Ag induced slower internalization of Fc∊RI than that with IgE + high Ag; receptor internalization induced by the former modes of stimulation was abrogated by Lyn deficiency, but that by the latter was almost intact in lyn_−/_− cells (13). To avoid unnecessary complexities, we stimulated only Fc∊RI, not known inhibitory receptors such as FcγRIIB, paired Ig-like receptor B, gp49B1, or leukocyte mono-Ig-like receptor 1 (another inhibitory receptor mast cell function-associated Ag is not expressed in bone marrow-derived mast cells (BMMC); data not shown). Our data indicate that Lyn plays a positive regulatory role in survival, degranulation, and cytokine production when mast cells are stimulated with low intensity stimuli such as monomeric IgE, IgE + anti-IgE, and IgE + low Ag, whereas these activation events were negatively regulated by Lyn upon high intensity stimulation with IgE + high Ag. Lyn appears to use its associated protein, Fc∊RI β, as a pivotal molecule to negatively regulate downstream events upon the latter stimulation.
Materials and Methods
Abs and other reagents
Anti-DNP IgE mAbs, H1 DNP-∊-206 and SPE-7, were described previously (3). Anti-IgE mAb B1E3 and polyclonal anti-SHIP Ab were provided by D. H. Conrad (Virginia Commonwealth University, Richmond, VA) and J. C. Cambier (National Jewish Medical and Research Center, Denver, CO), respectively. Commercial sources of other Abs are as follows: anti-phosphotyrosine mAb 4G10 from Upstate Cell Signaling Solutions; anti-Lyn, anti-Syk, anti-p38, and anti-Akt from Santa Cruz Biotechnology; anti-ERK from Zymed Laboratories; anti-phospho-p44/42 MAPK (Thr202/Tyr204), anti-phospho-p38 MAPK (Thr180/Tyr182), anti-phospho-Akt (Ser473), anti-phospho Src (Tyr416), and anti-phospho-SHIP1 (Tyr1020) from Cell Signaling Technology. DNP3-BSA and DNP21-BSA were purchased from Biosearch Technologies.
Cell culture and Fc∊RI stimulation
Bone marrow cells from wild-type (wt) and mutant mice were cultured in IL-3-containing medium for 4–6 wk to generate mast cells (BMMC) with >95% purity (c-Kit+ Fc∊RI+ by flow cytometry). The following mutant mice were used: lyn_−/− (14) and Fc_∊_RI β−/− (15). Fc_∊_RI β−/−_lyn_−/_− double-knockout mice were generated by crossing the single-knockout mice. For IgE + Ag or IgE + anti-IgE stimulation, BMMC were first sensitized by a 24-h incubation with 1 μg/ml H1 DNP-∊-206 IgE. BMMC washed twice with buffer were stimulated with the indicated concentration of Ag (DNP3-BSA or DNP21-BSA) or anti-IgE mAb B1E3. For monomeric IgE stimulation, naive BMMC were incubated with a typical HC IgE, SPE-7, for the indicated periods.
Retroviral transduction
Retroviral transduction of Fc_∊_RI β−_/− or Fc_∊_RI β−/_− lyn_−/_− mast cells was performed as described previously (16). Briefly, pMX-puro plasmids harboring wt (YYY) or mutant (FFF) Fc∊RI β cDNAs (17) were transfected into packaging cells to generate recombinant retroviruses. BMMC in culture medium containing IL-3 and stem cell factor (SCF) were infected with the viruses. Mass populations of puromycin-resistant cells were used for Fc∊RI stimulation.
Measurements of histamine and cytokines
Amounts of histamine in BMMC or in culture supernatants from BMMC that had been stimulated through Fc∊RI were measured as previously described (3). Supernatants of Fc∊RI-stimulated BMMC were measured by ELISA for IL-6, IL-13, and TNF-α (BD Pharmingen).
Immunoblotting analysis
Mast cells were lysed in 1% Nonidet P-40-containing lysis buffer (20 mM Tris-HCl (pH 8.0), 0.15 M NaCl, 1 mM EDTA, 1 mM sodium orthovana-date, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 25 μM _p_-nitrophenyl _p_′-guanidinobenzoate, 1 μM pepstatin, and 0.1% sodium azide). Cell lysates were either directly analyzed by SDS-PAGE, followed by immunoblotting, or immunoprecipitated with an Ab before the immune complexes were analyzed by SDS-PAGE and immunoblotting. Proteins reactive with primary Ab were visualized with an HRP-conjugated secondary Ab and ECL reagents (PerkinElmer).
In vitro kinase assays
Lyn and Syk molecules were immunoprecipitated from unstimulated or Fc∊RI-stimulated mast cells with the appropriate Abs. Immune complexes were washed and incubated in the kinase buffer (50 mM HEPES (pH 7.4), 10 mM MgCl2, 10 mM MnCl2, and 0.1 μM ATP) in the presence of 10 μCi of [γ-32P]ATP. Reaction products were analyzed by SDS-PAGE and blotted onto polyvinylidene difluoride membranes before exposure to x-ray films.
Results
Lyn positively and negatively regulates biological outcomes of Fc∊RI aggregation in low and high intensity stimulations, respectively
To seek conditions for Lyn's positive vs negative regulatory functions, we studied the effects of the different modes of Fc∊RI stimulation on other consequences of mast cell activation in wt and lyn_−/_− BMMCs. Withdrawal of IL-3 from culture medium induced dramatic apoptosis in BMMC (Fig. 1A), as shown previously (18, 19). However, Fc∊RI stimulation with monomeric HC IgE (SPE-7), IgE + anti-IgE (B1E3), or IgE + low Ag (1–100 ng/ml DNP3-BSA or 1 ng/ml DNP21-BSA) increased survival in wt BMMC, but these survival effects were strongly reduced or abolished by Lyn deficiency. By contrast, IgE + high Ag stimulation (1000 ng/ml DNP3-BSA or 10–1000 ng/ml DNP21-BSA) had no or reduced survival effects in wt BMMC, but this mode of stimulation significantly enhanced survival in lyn_−/_− BMMC.
FIGURE 1.
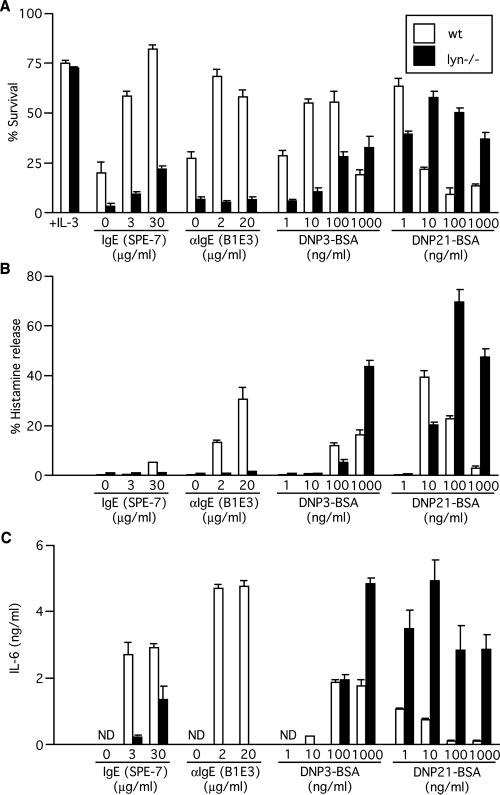
Survival, histamine release, and IL-6 production in response to various modes of Fc∊RI stimulation. BMMCs from wt and lyn_−/_− mice were incubated with or without the indicated concentrations of a highly cytokinergic IgE (3), SPE-7, for 60 h (survival), 45 min (histamine release), or 20 h (cytokine secretion). Alternatively, BMMCs were sensitized by incubation with 1 μg/ml anti-DNP IgE 206 for 24 h. After washing, IgE-sensitized BMMCs were incubated with the indicated concentrations of DNP3-BSA or DNP21-BSA or anti-IgE mAb B1E3 in the absence of growth factors for 60 h (survival), 45 min (histamine release), or 20 h (cytokine secretion). In A (leftmost columns), survival of the cells cultured in the presence of IL-3 is also shown. Experiments were performed in triplicate. Results shown are representative of three independent cultures from three or four mice for each genotype. ND, not detectable.
Degranulation and cytokine production are cardinal features of Fc∊RI-induced mast cell activation. Thus, we compared these responses to different modes of stimulation between wt and lyn_−/_− cells. Similar to survival effects, Fc∊RI stimulation with HC IgE, IgE + anti-IgE, or IgE + low Ag (100 ng/ml DNP3-BSA or 10 ng/ml DNP21-BSA) induced histamine release in wt BMMC, but histamine release was reduced or abolished in lyn_−/_− BMMC (Fig. 1B). In contrast, IgE + high Ag stimulation (1000 ng/ml DNP3-BSA or 100-1000 ng/ml DNP21-BSA) induced higher levels of histamine release in lyn_−/_− cells than in wt cells. Similar patterns of IL-6, IL-13, and TNF-α production were observed. Fc∊RI stimulation with HC IgE, IgE + anti-IgE, or IgE + low Ag (10 ng/ml DNP3-BSA) induced the production of higher levels of IL-6, IL-13, and TNF-α in wt cells than in lyn_−/_− cells (Fig. 1C and data not shown); IgE + high Ag stimulation (1000 ng/ml DNP3-BSA or 1–1000 ng/ml DNP21-BSA) induced higher levels of IL-6, IL-13, and TNF-α in lyn_−/_− cells than in wt cells. As described previously (13), HC IgE, IgE + anti-IgE, and IgE + low Ag are considered to represent low intensity stimulation and IgE + high Ag high intensity stimulation. These results collectively indicate that low intensity stimulation requires Lyn to exert biological effects such as survival, degranulation, and cytokine production in addition to receptor internalization; therefore, Lyn plays a positive regulatory function under this mode of stimulation. In contrast, under high intensity stimulation, Lyn's role is predominantly negative regulation.
High intensity stimulation induces higher levels of Lyn activation and Fc∊RI β phosphorylation than low intensity stimulation
To investigate the mechanism of Lyn's dual functions in Fc∊RI signaling, we first compared Lyn activity among different modes of Fc∊RI stimulation in wt cells. The catalytic activity of Lyn is suppressed by phosphorylation at Tyr507 at the C terminus, and phosphorylation at Tyr396 in the activation loop is required for full activation (20). As shown in Fig. 2, A and B, high intensity stimulation with 100 ng/ml DNP21-BSA induced a significant increase in both kinase activity and Tyr396 phosphorylation within 1.5 min of stimulation, with their peaks at ∼5 min. By contrast, stimulation with 1 ng/ml DNP21-BSA induced a reduction in kinase activity and Tyr396 phosphorylation. These results indicate that kinase activity and Tyr396 phosphorylation of Lyn can be down- or up-regulated by IgE + low Ag or IgE + high Ag, respectively. They also suggest that a low range of Lyn activity exhibits predominantly a positive regulatory function, but a high range of Lyn activity plays a negative role.
FIGURE 2.
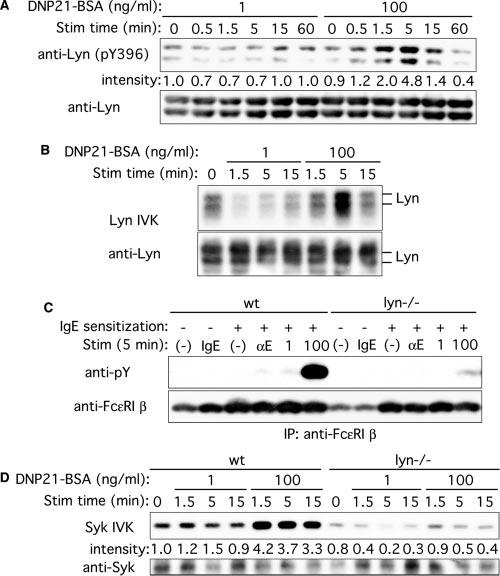
Lyn kinase activity, tyrosine phosphorylation of Fc∊RI β, and Syk kinase activity upon stimulation with low and high concentrations of Ag. A and B, Wt BMMC were sensitized with IgE and stimulated with 1 or 100 ng/ml DNP21-BSA for the indicated periods. Cell lysates were directly analyzed by immunoblotting with anti-phospho-Src (Tyr416) that interacts with Tyr396-phosphorylated Lyn, followed by reprobing the same blot with anti-Lyn Ab (A). Lyn was immunoprecipitated from lysates and submitted to in vitro kinase (IVK) assays (B). The same blot was probed with anti-Lyn Ab. C, wt and lyn_−/_− BMMCs were incubated without or with 5 μg/ml SPE-7 IgE (IgE). Alternatively, these cells were IgE sensitized and stimulated with 2 μg/ml anti-IgE (αE) or 1 or 100 ng/ml DNP21-BSA for 5 min. Fc∊RI β subunit was immunoprecipitated and submitted to immunoblotting with anti-phosphotyrosine mAb, followed by reprobing with anti-Fc∊RI β mAb. D, Wt and lyn_−/− BMMCs were sensitized with IgE and stimulated with 1 or 100 ng/ml DNP21-BSA for the indicated periods. Syk was immunoprecipitated from lysates and submitted to in vitro kinase assays. The same blot was probed with anti-Syk Ab. Relative band intensities of p56_lyn phosphorylation (A) and Syk (D) normalized against their amounts, as determined by densitometry, are shown. The results shown were reproduced in an additional experiment using an independent set of BMMCs.
We next examined the activation status of two major Lyn targets, Fc∊RI β subunit and Syk. Tyrosine phosphorylation of Fc∊RI β activity correlated well with catalytic activity of Lyn in wt cells under high and low intensity stimulations (Fig. 2, C and D); Fc∊RI β phosphorylation and Syk activity were drastically reduced in lyn_−/_− cells upon high and low intensity stimulations. Therefore, the catalytic activity of Lyn and the phosphorylation of Lyn's direct targets are strictly controlled by the intensity of Fc∊RI stimulation.
Activities of p38 and Akt are positively and negatively regulated by Lyn in low intensity and high intensity stimulations, respectively
MAPKs and Akt are important positive regulators of Fc∊RI-induced mast cell activation and cell survival. Therefore, we assessed the activities of these signaling molecules under different modes of Fc∊RI stimulation. Stimulation with both low (1 ng/ml) and high (100 ng/ml) Ag concentrations induced robust phosphorylation and thus activation of MAPKs (ERK1, ERK2, and p38) and Akt in wt cells (Fig. 3). In contrast, there was little or no activation of these kinases in lyn_−/_− cells when stimulated by 1 ng/ml DNP21-BSA (Fig. 3) or other low intensity stimuli (HC IgE or IgE+anti-IgE; data not shown). ERK1/2 phosphorylation was of a higher intensity and more sustained after high intensity stimulation than after low intensity stimulation. p38 phosphorylation was of similar intensity and duration after low or high intensity stimulation. Neither ERK1/2 nor p38 phosphorylation was enhanced in lyn_−/_− cells, and p38, but not ERK1/2, phosphorylation, lasted longer in these cells. In contrast, Akt phosphorylation was both increased and more sustained in lyn_−/_− cells. These results indicate that Lyn regulates downstream events, such as p38 and Akt, positively and negatively depending on the Ag concentration and the intensity of stimulation. They also indicate that low intensity stimulation requires Lyn to induce activation of these downstream events, whereas high intensity stimulation activates a Lyn-dependent, negative regulatory pathway that appears to limit the duration of activation of p38 and Akt, eventually resulting in inhibition of biological outcomes.
FIGURE 3.
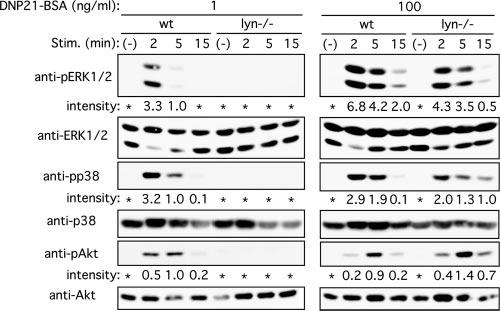
Phosphorylation of ERK, p38, and Akt in wt and lyn_−/_− mast cells upon stimulation with low and high concentrations of Ag. Wt and lyn_−/_− BMMC were sensitized with IgE and stimulated with 1 or 100 ng/ml DNP21-BSA for the indicated periods. Cell lysates were directly analyzed by immunoblotting with anti-phospho-ERK (Thr202/Tyr204), anti-phospho-p38 (Thr180/Tyr182), or anti-phospho-Akt (Ser473), followed by reprobing the same blot with anti-ERK, anti-p38, or pAkt, respectively. Relative band intensities of ERK1, p38, and Akt phosphorylation normalized against their amounts are shown. Phosphorylation induced at 1 ng/ml DNP21-BSA for 5 min is set at 1.0. *, p < 0.1. Results shown are representative of three experiments using independent sets of BMMCs.
Phosphorylation of negative regulatory molecules, SHIP and SH2 domain-containing protein tyrosine phosphatase-1 (SHP-1), requires Lyn and is dependent on Ag concentrations
We next investigated the roles of Lyn in the regulation of negative signaling molecules under low intensity and high intensity stimulations. SHIP plays an important negative regulatory role in mast cells (21), and targeting to the plasma membrane is crucial for its activity and phosphorylation (22). Stimulation with 100 ng/ml DNP21-BSA induced stronger phosphorylation of SHIP in wt cells than did 1 ng/ml DNP21-BSA (Fig. 4A). Consistent with a previous report (12), SHIP phosphorylation was almost abolished in lyn_−/_− cells stimulated with 1 or 100 ng/ml DNP21-BSA (Fig. 4B).
FIGURE 4.
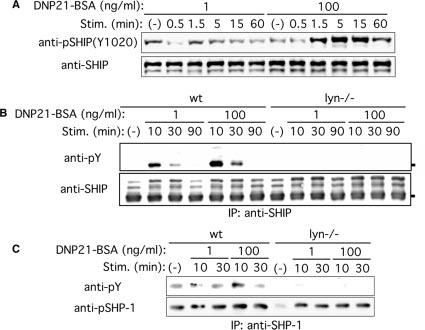
Phosphorylation of SHIP and SHP-1 in wt and lyn_−/_− mast cells upon stimulation with low and high concentrations of Ag. wt and lyn_−/_− BMMC were sensitized with IgE and stimulated with 1 or 100 ng/ml DNP21-BSA for the indicated periods. Cell lysates were directly analyzed by immunoblotting with anti-phospho-SHIP1 (Tyr1020; A) or were first immunoprecipitated with anti-SHIP Ab, then immune complexes were analyzed by immunoblotting with anti-phosphotyrosine mAb (B). C, SHP-1 was immunoprecipitated, and immune complexes were subjected immunoblotting with anti-phosphotyrosine mAb. The blots were reprobed with Abs that recognize SHIP or SHP-1 regardless of the phosphorylation status. Results shown are representative of two similar experiments.
SHP-1, an SHIP, is another potential negative regulator for mast cell activation (6, 23). Phosphorylation of SHP-1 was stronger upon stimulation with 100 ng/ml DNP21-BSA than with 1 ng/ml DNP21-BSA, and it was abolished in lyn_−/_− cells stimulated with 1 or 100 ng/ml DNP21-BSA (Fig. 4C). Thus, these results suggest that negative signaling pathways involving SHIP and SHP-1 are activated more vigorously by high intensity than by low intensity stimulation in a Lyn-dependent manner.
Fc∊RI β subunit is associated with negative signaling molecules, SHIP and SHP-1
Previous studies showed that phosphorylated peptides corresponding to Fc∊RI β ITAM can bind several negative signaling molecules, including SHIP (17, 24) and SHP-1 (25). Coimmunoprecipitation experiments showed that a small fraction of the Fc∊RI β pool was associated with SHIP in unstimulated mast cells expressing wt (YYY) Fc∊RI β subunit, and the extent of this association was increased upon stimulation with 100 ng/ml DNP21-BSA (Fig. 5A). The association of Fc∊RI β with SHIP and Tyr1020 phosphorylation of SHIP were dramatically reduced in mast cells expressing Fc∊RI β subunit-ITAM mutant FFF (Fig. 5, A and B). Although SHIP phosphorylation is dependent on Lyn (Fig. 4B), there was no physical association between Lyn and SHIP, as assessed by coimmunoprecipitation and immunoblotting (data not shown). In contrast, SHP-1 was constitutively associated with the Fc∊RI β subunit. This association was not dependent on Fc∊RI β ITAM, although tyrosine phosphorylation of SHP-1 was still dependent on Fc∊RI β ITAM (Fig. 5C). These results indicate that Fc∊RI β interacts with both SHIP and SHP-1 and regulates tyrosine phosphorylation of these negative regulators in a manner that is dependent on the tyrosine residues in the Fc∊RI β-ITAM.
FIGURE 5.
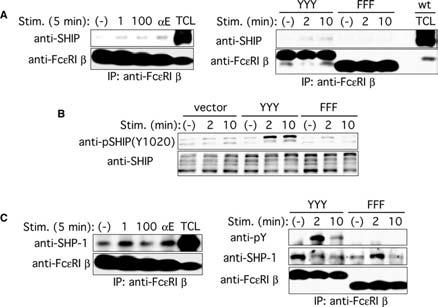
Fc∊RI β interacts with SHIP and SHP-1, and tyrosine phosphorylation of SHIP and SHP-1 requires the intact ITAM of the Fc∊RI β subunit. A, wt BMMC were sensitized with IgE and stimulated with 1 or 100 ng/ml DNP21-BSA or 2 μg/ml anti-IgE mAb B1E3 for 5 min (left panel). Fc_∊_RI β−_/− BMMCs transduced with wt (YYY) or mutant (FFF) Fc∊RI β were sensitized and stimulated with 100 ng/ml DNP21-BSA for the indicated periods (right panel). Fc∊RI β immunoprecipitates were analyzed by immunoblotting with anti-SHIP Ab. The same blots were reprobed with anti-Fc∊RI β mAb. B, Fc_∊_RI β−/− BMMCs transduced with wt (YYY) or mutant (FFF) Fc∊RI β were sensitized and stimulated with 100 ng/ml DNP21-BSA for the indicated periods. Cell lysates were analyzed by immunoblotting with anti-phospho-SHIP1 (Tyr1020) Ab. The same blot was reprobed with anti-SHIP Ab. C, wt BMMC were sensitized with IgE and stimulated with 1 or 100 ng/ml DNP21-BSA or 2 μg/ml anti-IgE mAb B1E3 for 5 min (left panel). Fc_∊_RI β−/_− BMMCs transduced with wt (YYY) or mutant (FFF) Fc∊RI β were sensitized and stimulated with 100 ng/ml DNP21-BSA for the indicated periods (right panel). Fc∊RI β immunoprecipitates were analyzed by immunoblotting with anti-SHP-1 Ab (left) or anti-phosphotyrosine mAb (right). The same blots were reprobed with anti-Fc∊RI β mAb (left) or with anti-SHP-1 and anti-Fc∊RI β mAb after cutting them into two pieces of higher and lower _M_r portions of ∼40 kDa (right). The results shown were reproduced in an additional experiment using an independent set of BMMCs and retrovirally transduced cells.
Lyn association with Fc∊RI β subunit is increased upon high intensity stimulation
Previous studies showed that Fc∊RI β subunit is constitutively associated with Lyn, and this association is increased upon Fc∊RI stimulation (26, 27). We confirmed this association by immunoprecipitation and immunoblotting (Fig. 6A). Stimulation with 100 ng/ml DNP21-BSA dramatically increased the Lyn/Fc∊RI β association within 1.5 min, and the association level reached a peak at 5 min. By contrast, this association was not increased, but, rather, was decreased, upon stimulation with 1 ng/ml DNP21-BSA (Fig. 6A). Another low intensity stimulus, IgE + anti-IgE, also did not increase this association. As shown previously (10), tyrosine phosphorylation of Fc∊RI β was severely impaired by Lyn deficiency (Fig. 6B). We next examined whether the Lyn/Fc∊RI β association takes place in lipid rafts or soluble fractions. As shown in Fig. 6C, both Lyn and Fc∊RI β subunit were localized in raft as well as soluble fractions. The amount of Fc∊RI β subunit in raft fractions was increased upon stimulation with a high concentration of Ag, whereas stimulation with a low concentration of Ag concentration decreased it (Fig. 6C). In contrast, Lyn levels in rafts did not change with high or low concentration Ag stimulation. Concordant with the increased levels of Fc∊RI β in raft fractions under high intensity stimulation, more Fc∊RI β subunit was coimmunoprecipitated with Lyn in raft fractions when stimulated with a high concentration of Ag (Fig. 6D). These results are also consistent with higher levels of tyrosine phosphorylation of Fc∊RI β subunit upon high intensity stimulation (Figs. 2C and 6B), because tyrosine-phosphorylated Fc∊RI β binds the SH2 domain of Lyn (28). The concordance between Lyn activity and Fc∊RI β phosphorylation under high and low intensity stimulations combined with the ability of Fc∊RI β ability to bind to negative signaling molecules suggest that Fc∊RI β subunit serves as an intermediary molecule for Lyn-mediated negative signaling under high intensity stimulation.
FIGURE 6.
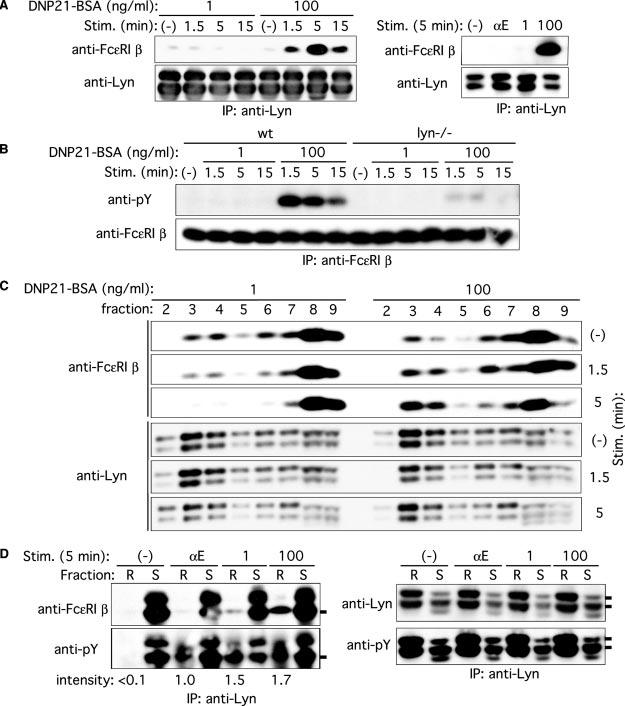
Decreased and increased Fc∊RI β/Lyn association and Fc∊RI β localization to lipid rafts upon stimulation with low and high concentrations of Ag, respectively. A, wt BMMC were sensitized with IgE and stimulated with 1 or 100 ng/ml DNP21-BSA or 2 μg/ml anti-IgE mAb B1E3 (αE) for the indicated periods. Cell lysates were immunoprecipitated with anti-Lyn, and Lyn immune complexes were subjected to immunoblotting with anti-Fc∊RI β mAb. The same blots were reprobed with anti-Lyn Ab. Results representative of two experiments are shown. B, wt and lyn_−/_− BMMC were sensitized with IgE and stimulated with 1 or 100 ng/ml DNP21-BSA for the indicated periods. Fc∊RI β was immunoprecipitated and analyzed by immunoblotting with anti-phosphotyrosine mAb, followed by reprobing with anti-Fc∊RI β. C, wt BMMC were sensitized with IgE and stimulated with 1 or 100 ng/ml DNP21-BSA for the indicated periods. Cell lysates were fractionated by ultracentrifugation through a sucrose density gradient. Fractions were analyzed by immunoblotting with anti-Fc∊RI β mAb or anti-Lyn. Fractions 2–4 contain lipid rafts, and fractions 6–9 contain soluble proteins. The reduced and increased raft localizations of Fc∊RI β depending on Ag concentration were reproduced in two additional experiments. D, wt BMMC were sensitized with IgE and stimulated with 1 or 100 ng/ml DNP21-BSA or 2 μg/ml anti-IgE mAb B1E3 (αE) for 5 min. Cell lysates were fractioned by ultracentrifugation, and raft (R) and soluble fractions (S) were pooled and immunoprecipitated with anti-Lyn. Lyn immune complexes were analyzed by immunoblotting with anti-Fc∊RI β, anti-phosphotyrosine, or anti-Lyn mAb. Relative band intensities of Fc∊RI β phosphorylation in lipid rafts are shown. Phosphorylation induced with anti-IgE is set at 1.0.
Fc∊RI β subunit-ITAM regulates biological functions positively and negatively depending on the intensity of stimulation
Recent studies demonstrated that the N-terminal Tyr residue (Y219 in the human sequence) within the Fc∊RI β ITAM plays a predominant role in signal amplification through the interaction with Lyn, and the middle Tyr residue (Y225) in between the N- and C-terminal canonical Tyr residues plays a negative regulatory role (17, 29). Therefore, we analyzed biological functions induced by different modes of stimulation in Fc_∊_RI β−_/_− mast cells expressing wt (YYY) or mutant (FFF) Fc∊RI β subunit-ITAM. Fc∊RI expression, as measured by flow cytometry, was restored to levels comparable to those in wt cells (data not shown). When stimulated with low intensity stimulation, e.g., IgE + anti-IgE, FFF cells exhibited lower levels of degranulation and cytokine production compared with YYY cells; however, degranulation and cytokine production were increased in FFF than in YYY cells when given high intensity stimulation, 100 ng/ml DNP21-BSA (Fig. 7, A and B). However, stimulation with l ng/ml DNP21-BSA resulted in similarly low levels of degranulation and cytokine production in both YYY and FFF cells. Therefore, Fc∊RI β subunit-ITAM regulates biological functions positively and negatively depending on the intensity of Fc∊RI stimulation.
FIGURE 7.
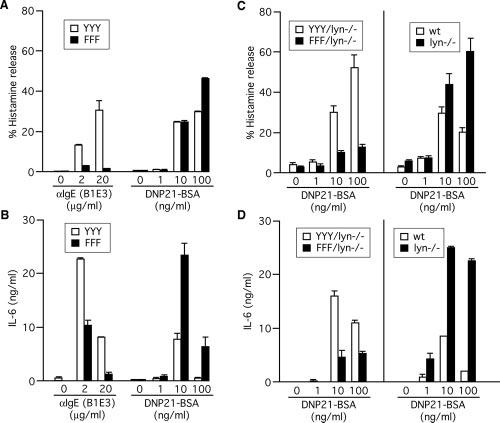
Lyn-dependent phenocopy of degranulation and cytokine production in lyn_−/− mast cells by FFF cells. A and B, Fc_∊_RI β−/− BMMCs transduced with wt (YYY) or mutant (FFF) Fc∊RI β were sensitized and stimulated with the indicated concentrations of DNP21-BSA or anti-IgE mAb B1E3 for 45 min (histamine release) or 20 h (cytokine production). Histamine released and IL-6 secreted into culture supernatants were measured. C and D, Fc_∊_RI β−/−_lyn_−/_− (double-knockout) BMMCs transduced with wt (YYY) or mutant (FFF) Fc∊RI β were sensitized and stimulated with the indicated concentrations of DNP21-BSA as described above. Results with wt and lyn_−/_− BMMCs cultured in SCF-containing medium (same as the retrovirally transduced cells) are also shown for comparison. Results representative of two experiments using independently transduced cells are shown.
Lyn negatively regulates mast cell activation through the Fc∊RI β subunit-ITAM
The pattern of biological functions regulated by the Fc∊RI β-ITAM (Fig. 7, A and B) was very similar to that by Lyn, particularly in response to high intensity stimulation (compare Fig. 1, B and C, and Fig. 7, A and B). These observations may suggest that negative regulatory roles of Lyn are exerted specifically through the Fc∊RI β-ITAM. To test this possibility, BMMC from Fc_∊_RI β−_/−_lyn_−/_− (double-knockout) mice were reconstituted with wt or mutant Fc∊RI β. Fc∊RI expression on the surface of reconstituted cells was comparable to that on wt and lyn_−/_− cells (data not shown). YYY/lyn_−/_− cells restored IgE + Ag-induced histamine release to levels comparable to those in lyn_−/_− cells (Fig. 7C). By contrast, FFF/lyn_−/_− cells exhibited drastically lower levels of histamine release upon stimulation with 1–100 ng/ml DNP21-BSA, unlike Lyn-sufficient FFF cells. IL-6 production was also largely restored in IgE + Ag-stimulated YYY/lyn_−/_− cells (Fig. 7D). However, stimulation with 1–100 ng/ml DNP21-BSA resulted in reduced IL-6 production in FFF/lyn_−/_− cells compared with YYY/lyn_−/_− cells. These results strongly suggest that the negative regulation of mast cell activation by Lyn is exerted through the Fc∊RI β-ITAM. They also indicate that the positive regulation of degranulation and cytokine production induced by the higher Ag concentration range (10 –100 ng/ml DNP21-BSA) was independent of Lyn.
Discussion
The present study indicates that Lyn plays a positive regulatory role in survival, degranulation, and cytokine production when mast cells were stimulated with low intensity stimuli, whereas these activation events were negatively regulated by Lyn upon high intensity stimulation. Reconstitution and signaling analyses strongly suggest that Lyn uses the Fc∊RI β-ITAM as a pivotal module to negatively regulate downstream events upon the latter mode of stimulation.
Throughout this study, we have used an operational classification of low intensity and high intensity stimuli, which are based on the rate of receptor internalization induced by different modes of Fc∊RI stimulation (13). The former include monomeric IgE, IgE + anti-IgE, and IgE + low Ag, whereas the latter include IgE + high Ag. Conveniently, almost all biological outcomes induced by the former and latter stimuli were positively and negatively regulated, respectively, by Lyn. Although the biological readouts induced by monomeric IgE and IgE + anti-IgE followed this pattern of Lyn dependency very faithfully, the division between IgE + low Ag and IgE + high Ag varied depending on biological readouts; for example, 10 ng/ml DNP21-BSA behaved as a low intensity stimulus in its capacity to induce degranulation (Fig. 1B), but as a high intensity stimulus in survival and cytokine production (Fig. 1, A and C). Assuming that all the tested stimuli signal only through the Fc∊RI, but not other receptors, this variability in Ag concentrations in defining high intensity vs low intensity stimuli may reflect the thresholds required for different activation events: survival seems to require a weaker strength of stimulation than degranulation or cytokine production (Fig. 1). These thresholds seem to be dependent on the maturity of mast cells, because, for example, the decreased histamine release with 10 ng/ml DNP21-BSA in Lyn-deficient regular BMMCs (that had been cultured in IL-3 alone) was not seen in more mature, Lyn-deficient mast cells (that had been cultured in IL-3 plus SCF for ≥2 wk). Another point worthy of comment is that the signaling and biological consequences of IgE + anti-IgE stimulation are different from those of IgE + Ag, although IgE + anti-IgE seems to have been traditionally considered to be equivalent to IgE + Ag.
Although biological measurements indicate that Lyn plays a positive regulatory role in the low Ag concentration range, we do not completely understand how Lyn works as a positive regulator. In addition, there seems to be another signaling molecule that plays a positive regulatory molecule(s) in the high Ag concentration range in an Fc∊RI β-ITAM-dependent manner. Thus, degranulation and cytokine production induced by stimulation with 10–100 ng/ml DNP21-BSA were much higher in YYY/lyn_−/_− than in FFF/lyn_−/_− cells (Fig. 7, C and D). Because multiple Src PTKs are expressed in mast cells (5, 26), a reasonable candidate for this positive regulator should be Fyn, another Src PTK. Parravicini et al. (5) showed that Fyn deficiency impairs degranulation and that the Fyn kinase-dependent pathway does not require Lyn or linker for activation of T cells for its initiation. Furthermore, Fyn activity is enhanced in lyn_−/_− mast cells, in part through the loss of negative regulation by C-terminal Src kinase (Csk), because phosphorylation of the adaptor, Csk-binding protein, that can recruit Csk is largely dependent on Lyn (9). Consistent with the positive regulatory role of Fyn, Lyn/Fyn doubly-deficient mast cells did not significantly de-granulate (9) or produce cytokines in response to a wide range of Ag stimulation (K. Asai, C. A. Lowell, and T. Kawakami, data not shown). It remains to be explored whether Fyn plays an exclusively positive regulatory role.
Where do these regulatory events take place? Current literature suggests the involvement of lipid rafts, membrane microdomains rich in cholesterol, sphingolipids, GPI-anchored proteins, and several signaling molecules, including Src PTKs, in mast cell activation (reviewed in Ref. 30). It is remarkable that a larger amount of Fc∊RI β becomes associated with Lyn and is recruited to lipid rafts upon stimulation by 100 ng/ml than by 1 ng/ml DNP21-BSA, whereas both the Fc∊RI β/Lyn association and raft localization of Fc∊RI β were decreased upon stimulation by 1 ng/ml DNP21-BSA (Fig. 6). These observations might suggest that Lyn-mediated negative regulation by high intensity stimulation is initiated in lipid rafts, whereas Lyn-mediated positive regulation by low intensity stimulation might be initiated outside rafts. Indeed, negative regulatory molecules downstream of Lyn, such as SHIP and SHP-1, can be recruited to lipid rafts (31-33). Recent studies demonstrated that defects in cholesterol synthesis result in exaggerated activation of mast cells (M. Kovarova and J. Rivera, personal communication), suggesting that negative regulatory events are associated with lipid rafts. In contrast, the possibility was shown for the initiation of mast cell activation by Lyn outside lipid rafts; palmitoylation site-mutant Lyn is anchored to the plasma membrane, but exhibits reduced localization into lipid rafts and can initiate the tyrosine phosphorylation of Fc∊RI subunits, Syk and linker of activation of T cells, along with an increase in the concentration of intracellular Ca2+ (34). These arguments are made based on the current method of fractionation of lipid rafts. However, as suggested previously (35), it is possible that the initial activation by Lyn might take place in a microlipid raft environment that cannot be isolated by this method.
This and other (5, 9, 10, 12) studies have underlined the importance of Lyn in negative regulation of mast cell activation through the Fc∊RI. Although local concentrations of IgE or allergen in allergic inflammation sites are difficult to determine, it might be possible that mast cells are exposed to very high levels of allergen in such locales as nasal mucosae in polinosis patients in high seasons of allergy. In such situations, Lyn's role might be important in suppressing mast cell activation, as shown for high intensity stimulation in this study. Teleological considerations suggest that there might be additional molecules that initiate similar negative regulatory functions if the negative regulatory role of Lyn is critical for the regulation of mast cell activation. However, no such molecules have been reported yet, although other Src PTKs are obvious candidates. As we have shown the stimulation conditions for the positive vs negative regulation by Lyn, we will be able to embark on future expeditions to characterize the initial activation processes of mast cell activation.
Acknowledgments
We are grateful to Drs. John C. Cambier, Daniel H. Conrad, and Yasuko Furumoto for providing Abs, and to members of the Kawakami laboratory for providing the BMMCs used in this study.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
1
This work was supported by National Institutes of Health Grants AI50209 and AI/GM38348 (to T.K.). This is Publication 720 from the La Jolla Institute for Allergy and Immunology.
4
Abbreviations used in this paper: PTK, protein tyrosine kinase; BMMC, bone marrow-derived mast cell; Csk, C-terminal Src kinase; HC, highly cytokinergic; SCF, stem cell factor; SH, Src homology; SHP-1, SH2-containing protein tyrosine phosphatase-1; wt, wild type.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Galli SJ, Maurer M, Lantz CS. Mast cells as sentinels of innate immunity. Curr. Opin. Immunol. 1999;11:53–59. doi: 10.1016/s0952-7915(99)80010-7. [DOI] [PubMed] [Google Scholar]
- 2.Kinet JP. The high-affinity IgE receptor (Fc∊RI): from physiology to pathology. Annu. Rev. Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 3.Kitaura J, Song J, Tsai M, Asai K, Maeda-Yamamoto M, Mocsai A, Kawakami Y, Liu FT, Lowell CA, Barisas BG, et al. Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the Fc∊RI. Proc. Natl. Acad. Sci. USA. 2003;100:12911–12916. doi: 10.1073/pnas.1735525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc∊RI. Nature. 402:B24–B30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- 5.Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, Samelson LE, O'Shea JJ, Rivera J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat. Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- 6.Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat. Rev. Immunol. 2002;2:773–786. doi: 10.1038/nri914. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity. 2005;22:9–18. doi: 10.1016/j.immuni.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 9.Odom S, Gomez G, Kovarova M, Furumoto Y, Ryan JJ, Wright HV, Gonzalez-Espinosa C, Hibbs ML, Harder KW, Rivera J. Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J. Exp. Med. 2004;199:1491–1502. doi: 10.1084/jem.20040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawakami Y, Kitaura J, Satterthwaite AB, Kato RM, Asai K, Hartman SE, Maeda-Yamamoto M, Lowell CA, Rawlings DJ, Witte ON, et al. Redundant and opposing functions of two tyrosine kinases, Btk and Lyn, in mast cell activation. J. Immunol. 2000;165:1210–1219. doi: 10.4049/jimmunol.165.3.1210. [DOI] [PubMed] [Google Scholar]
- 11.Nishizumi H, Yamamoto T. Impaired tyrosine phosphorylation and Ca2+ mobilization, but not degranulation, in lyn-deficient bone marrow-derived mast cells. J. Immunol. 1997;158:2350–2355. [PubMed] [Google Scholar]
- 12.Hernandez-Hansen V, Smith AJ, Surviladze Z, Chigaev A, Mazel T, Kalesnikoff J, Lowell CA, Krystal G, Sklar LA, Wilson BS, et al. Dysregulated Fc∊RI signaling and altered Fyn and SHIP activities in Lyn-deficient mast cells. J. Immunol. 2004;173:100–112. doi: 10.4049/jimmunol.173.1.100. [DOI] [PubMed] [Google Scholar]
- 13.Kitaura J, Xiao W, Maeda-Yamamoto M, Kawakami Y, Lowell CA, Kawakami T. Early divergence of Fc∊ receptor I signals for receptor up-regulation and internalization from degranulation, cytokine production, and survival. J. Immunol. 2004;173:4317–4323. doi: 10.4049/jimmunol.173.7.4317. [DOI] [PubMed] [Google Scholar]
- 14.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 15.Hiraoka S, Furumoto Y, Koseki H, Takagaki Y, Taniguchi M, Okumura K, Ra C. Fc receptor β subunit is required for full activation of mast cells through Fc receptor engagement. Int. Immunol. 1999;11:199–207. doi: 10.1093/intimm/11.2.199. [DOI] [PubMed] [Google Scholar]
- 16.Kawakami Y, Kitaura J, Yao L, McHenry RW, Newton AC, Kang S, Kato RM, Leitges M, Rawlings DJ, Kawakami T. A Ras activation pathway dependent on Syk phosphorylation of protein kinase C. Proc. Natl. Acad. Sci. USA. 2003;100:9470–9475. doi: 10.1073/pnas.1633695100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furumoto Y, Nunomura S, Terada T, Rivera J, Ra C. The Fc∊RIβ immunoreceptor tyrosine-based activation motif exerts inhibitory control on MAPK and IκB kinase phosphorylation and mast cell cytokine production. J. Biol. Chem. 2004;279:49177–49187. doi: 10.1074/jbc.M404730200. [DOI] [PubMed] [Google Scholar]
- 18.Mekori YA, Oh CK, Metcalfe DD. IL-3-dependent murine mast cells undergo apoptosis on removal of IL-3: prevention of apoptosis by c-kit ligand. J. Immunol. 1993;151:3775–3784. [PubMed] [Google Scholar]
- 19.Yee NS, Paek I, Besmer P. Role of kit-ligand in proliferation and suppression of apoptosis in mast cells: basis for radiosensitivity of white spotting and steel mutant mice. J. Exp. Med. 1994;179:1777–1787. doi: 10.1084/jem.179.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu. Rev. Cell. Dev. Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 21.Huber M, Helgason CD, Damen JE, Liu L, Humphries RK, Krystal G. The src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc. Natl. Acad. Sci. USA. 1998;95:11330–11335. doi: 10.1073/pnas.95.19.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phee H, Jacob A, Coggeshall KM. Enzymatic activity of the Src homology 2 domain-containing inositol phosphatase is regulated by a plasma membrane location. J. Biol. Chem. 2000;275:19090–19097. doi: 10.1074/jbc.M001093200. [DOI] [PubMed] [Google Scholar]
- 23.Xie ZH, Zhang J, Siraganian RP. Positive regulation of c-Jun N-terminal kinase and TNF-α production but not histamine release by SHP-1 in RBL-2H3 mast cells. J. Immunol. 2000;164:1521–1528. doi: 10.4049/jimmunol.164.3.1521. [DOI] [PubMed] [Google Scholar]
- 24.Kimura T, Sakamoto H, Appella E, Siraganian RP. The negative signaling molecule SH2 domain-containing inositol-polyphosphate 5-phosphatase (SHIP) binds to the tyrosine-phosphorylated β subunit of the high affinity IgE receptor. J. Biol. Chem. 1997;272:13991–13996. doi: 10.1074/jbc.272.21.13991. [DOI] [PubMed] [Google Scholar]
- 25.Kimura T, Zhang J, Sagawa K, Sakaguchi K, Appella E, Siraganian RP. Syk-independent tyrosine phosphorylation and association of the protein tyrosine phosphatases SHP-1 and SHP-2 with the high affinity IgE receptor. J. Immunol. 1997;159:4426–4434. [PubMed] [Google Scholar]
- 26.Eiseman E, Bolen JB. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita T, Mao SY, Metzger H. Aggregation of the high-affinity IgE receptor and enhanced activity of p53/56lyn protein-tyrosine kinase. Proc. Natl. Acad. Sci. USA. 1994;91:11251–11255. doi: 10.1073/pnas.91.23.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kihara H, Siraganian RP. Src homology 2 domains of Syk and Lyn bind to tyrosine-phosphorylated subunits of the high affinity IgE receptor. J. Biol. Chem. 1994;269:22427–22432. [PubMed] [Google Scholar]
- 29.On M, Billingsley JM, Jouvin MH, Kinet JP. Molecular dissection of the FcRβ signaling amplifier. J. Biol. Chem. 2004;279:45782–45790. doi: 10.1074/jbc.M404890200. [DOI] [PubMed] [Google Scholar]
- 30.Holowka D, Baird B. Fc∊RI as a paradigm for a lipid raft-dependent receptor in hematopoietic cells. Semin. Immunol. 2001;13:99–105. doi: 10.1006/smim.2000.0301. [DOI] [PubMed] [Google Scholar]
- 31.Petrie RJ, Schnetkamp PP, Patel KD, Awasthi-Kalia M, Deans JP. Transient translocation of the B cell receptor and Src homology 2 domain-containing inositol phosphatase to lipid rafts: evidence toward a role in calcium regulation. J. Immunol. 2000;165:1220–1227. doi: 10.4049/jimmunol.165.3.1220. [DOI] [PubMed] [Google Scholar]
- 32.Su MW, Yu CL, Burakoff SJ, Jin YJ. Targeting Src homology 2 domain-containing tyrosine phosphatase (SHP-1) into lipid rafts inhibits CD3-induced T cell activation. J. Immunol. 2001;166:3975–3982. doi: 10.4049/jimmunol.166.6.3975. [DOI] [PubMed] [Google Scholar]
- 33.Kosugi A, Sakakura J, Yasuda K, Ogata M, Hamaoka T. Involvement of SHP-1 tyrosine phosphatase in TCR-mediated signaling pathways in lipid rafts. Immunity. 2001;14:669–680. doi: 10.1016/s1074-7613(01)00146-7. [DOI] [PubMed] [Google Scholar]
- 34.Kovarova M, Tolar P, Arudchandran R, Draberova L, Rivera J, Draber P. Structure-function analysis of Lyn kinase association with lipid rafts and initiation of early signaling events after Fc∊ receptor I aggregation. Mol. Cell. Biol. 2001;21:8318–8328. doi: 10.1128/MCB.21.24.8318-8328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furumoto Y, Gonzalez-Espinosa C, Gomez G, Kovarova M, Odom S, Parravicini V, Ryana JJ, Rivera J. Rethinking the role of Src family protein tyrosine kinases in the allergic response: new insights on the functional coupling of the high affinity IgE receptor. Immunol. Res. 2004;30:241–253. doi: 10.1385/ir:30:2:241. [DOI] [PubMed] [Google Scholar]