Impaired Wound Contraction in Stromelysin-1–Deficient Mice (original) (raw)
Abstract
Objective
To determine whether the deletion of stromelysin-1, a single metalloproteinase gene product, will alter the time course and quality of dermal wound repair in mice.
Summary Background Data
After dermal injury, a highly coordinated program of events is initiated by formation of a fibrin clot, followed by migration of keratinocytes, contraction of the dermis, recruitment of inflammatory macrophages, formation of granulation tissue with angiogenesis, and finally tissue remodeling. Matrix metalloproteinases are rapidly induced in the dermis and granulation tissue and at the leading edge of the epidermis in the healing wounds.
Methods
Incisional and circular full-thickness wounds 2 to 10 mm were made in the dermis of stromelysin-1–deficient and wild-type mice. The wounds were analyzed for rate of cellular migration and epithelialization. The wound contraction was examined by immunohistochemical staining for α-smooth muscle actin and fluorescent staining for fibrillar actin.
Results
Independent of the age of the animal, excisional wounds in stromelysin-1–deficient mice failed to contract and healed more slowly than those in wild-type mice. Cellular migration and epithelialization were unaffected in the stromelysin-1–deficient animals. The functional defect in these mice is failure of contraction during the first phase of healing because of inadequate organization of actin-rich stromal fibroblasts.
Conclusions
Excisional dermal wound healing is impaired in mice with a targeted deletion in the stromelysin-1 gene. Incisional wound healing is not affected. These data implicate stromelysin-1 proteolysis during early wound contraction and indicate that stromelysin-1 is crucial for the organization of a multicellular actin network.
Perturbation of the balance between extracellular matrix (ECM) degradation and deposition contributes to a number of pathologic conditions characterized by abnormal healing and chronic inflammation. 1–3 Matrix metalloproteinases are expressed in migrating keratinocytes, the adjacent dermis, and granulation tissue of healing wounds, and their altered functions have been implicated in disease processes characterized by abnormal healing. 4–7
Stromelysin-1 (matrix metalloproteinase-3) degrades proteoglycans, laminin, fibronectin, the nonhelical domains of collagen types IV and IX, propeptides of type I collagen, and denatured collagens and activates collagenase-1. 8–11 It is synthesized primarily by fibroblasts and to a lesser extent by activated macrophages and keratinocytes adjacent to sites of injury. 12–14 Stromelysin-1 is found in settings where active ECM remodeling occurs, including the stroma of normally healing rabbit corneal wounds, 7 stromal cells and keratinocytes of chronic ulcers, 14 and burn wound fluid from humans. 15 With the possible exception of interstitial collagenase, metalloproteinase activities are overlapping. Compensatory mechanisms have been observed in stromelysin-1–deficient (_Str-1_-/-) mice in studies of uterine involution 16 and collagen-induced arthritis. 17
We designed a study to investigate the impact of disruption of the stromelysin-1 gene on incisional and excisional dermal wound healing in mice. In contrast to previous studies on mice with a disrupted stromelysin-1 gene, 16,17 this study describes a phenotype in _Str-1_-/- mice that is not compensated by other metalloproteinases. Our results demonstrate that under the stress of excisional wound repair, deletion of stromelysin-1 results in a failure of wound contraction and significantly delayed healing.
METHODS
Generation of _Str-1_-/- Mice
Str-1-/- mice were generated through gene targeting by homologous recombination in murine embryonic stem cells, as described previously, 16,17 and maintained on 129Sv background. Young (younger than 4 months of age) and old (9 to 16 months of age) wild-type and _Str-1_-/- mice were used in the studies as indicated.
Wounding
All procedures were performed in a laminar animal operating suite under aseptic conditions according to protocols approved by the University of California, San Francisco, Committee on Animal Research. The mice were anesthetized with Metofane general anesthesia (Pitman-Moore, Mundelein, IL). Full-thickness incisional wounds were created on the backs of _Str-1_-/- mice (n = 10 total mice, 28 wounds) and wild-type mice (n = 10 total mice, 29 wounds) and sutured with 5-0 Surgilene (Davis and Geck, Wayne, NJ). Wounds were left exposed to air and harvested after 1, 2, 3, 7, and 10 days. Full-thickness circular excisional wounds 2, 5, 7, and 10 mm in diameter were made on the backs of _Str-1_-/- mice (n = 10 mice, 40 wounds) and wild-type mice (n = 13 mice, 48 wounds) and harvested after 20 days. Wounds were measured (greatest horizontal and vertical diameters) and photographed daily. Full-thickness circular excisional wounds 7 mm in diameter were created and harvested after 12 hours and 1, 2, 3, 5, 7, 10, or 14 days (n = 6 _Str-1_-/- mice, n = 5 wild-type mice; 50 wounds). All wounds were fixed in 10% phosphate-buffered formalin, processed, and sectioned for histologic analysis.
For the actin detection studies, an additional set of 7-mm wounds were created on _Str-1_-/- and wild-type mice (n = 44 wounds), harvested after 4, 12, 24, 48, and 72 hours, fixed, processed, and sectioned transversely and cross-sectionally. At the time of harvest, the mice were killed by Metofane anesthesia and cervical dislocation.
Immunohistochemistry and Staining of Filamentous Actin
Alpha-smooth muscle actin (αSMA) was detected with a mouse antihuman αSMA monclonal antibody (Sigma, St. Louis, MO). The antibody was purified by affinity chromatography using a protein A column (Bio-Rad, Oakland, CA) and biotinylated using the EZ-Link Sulfo-NHS-LC-Biotinylation kit (Pierce, Rockford, IL). Sections were incubated with biotinylated anti-αSMA antibody (40 μg/ml concentration) for 1 hour at room temperature. The biotinylated anti-αSMA antibodies were detected using the avidin–biotin conjugate method (ABC kit; Vector Laboratories, Burlingame, CA). Immunostaining was performed on at least five sections per sample for the following samples: unwounded normal wild-type and _Str-1_-/- skin (n = 4), normal and _Str-1_-/- wounds harvested after 12 hours (n = 4), 1 day (n = 10), 2 days (n = 6), 3 days (n = 4), 5 days (n = 10), 7 days (n = 8), 14 days (n = 12), and 20 days (n = 8). Blood vessels that served as positive controls stained strongly in both _Str-1_-/- mice and control mice. Negative controls (nonimmune goat serum) had no staining.
To detect filamentous actin, cross-sectional tissue samples from _Str-1_-/- and normal wounds harvested at 4 (n = 4), 12 (n = 4), 24 (n = 10), 48 (n = 10), and 72 (n = 8) hours were analyzed. Transverse sections from another set of wounds harvested after 4 (n = 4) and 24 (n = 4) hours were also stained. Sections were incubated overnight at room temperature with FITC-phalloidin (Sigma) in 2.5 μg/ml Tris-buffered saline to detect filamentous actin. 18,19
Wound Breaking Strength
Incisional wounds (3 cm) were created on the backs of _Str-1_-/- mice (n = 7) and normal wild-type mice (n = 7) and sutured as described above. The mice were killed 10 days after wounding, and three strips of skin (8-mm wide × 30-mm long) were harvested from the wounded area. Breaking strength was tested on a tensometer. 20
Data Analysis
Mean values and standard errors of the mean were calculated and compared where appropriate with a Student’s t test. A value of p < 0.05 was considered statistically significant.
RESULTS
Full-thickness sutured incisional wounds (10 mm) and full-thickness circular excisional wounds (2, 5, 7, and 10 mm in diameter) were created on the backs of _Str-1_-/- mice and wild-type mice and allowed to heal. The incisional wounds all closed by 72 hours after wounding in both _Str-1_-/- (2.1 ± 0.2 days, 10 mice, 28 wounds) and wild-type mice (1.8 ± 0.2 days, n = 10 mice, 29 wounds). In contrast, excisional wounds healed more slowly in the _Str-1_-/- mice than in the wild-type mice (Fig. 1). Time for excisional wound closure did not differ between young _Str-1_-/- mice (younger than 4 months old, n = 4 mice, 16 wounds) and old _Str-1_-/- mice (9 to 16 months old, n = 6 mice, 24 wounds). Macroscopically the excisional wounds in wild-type mice contracted primarily in the cranial-caudal direction during the first 48 hours of healing and then closed at a rate of 50% per day (n = 13 mice, 48 wounds). In contrast, in the _Str-1_-/- mice (n = 10 mice, 40 wounds), excisional wounds did not contract during the first days of healing, showing an initial increase in wound area, followed by a slow diminution as the wounds healed concentrically, with a 5% to 10% decrease in area per day (p < 0.001 at each time point, Student’s t test).
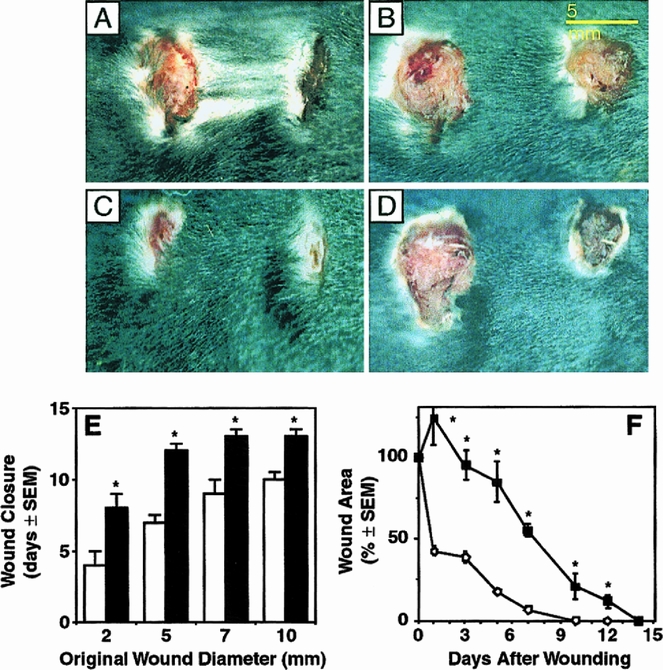
Figure 1. Healing excisional wounds in normal wild-type (A, C) and _Str-1_-/- mice (B, D). In all panels, 7-mm (original diameter) wounds are on the left and 5-mm (original diameter) wounds are on the right. The appearance of wounds 1 day after wounding (A, B) and 7 days after wounding (C, D) is shown. (E) Time for excisional wound closure in wild-type (open bars) and _Str-1_-/- mice (closed bars). (F) Wound areas, expressed as a percentage of the original wound area, in healing excisional wounds (circle, wild-type mice; square, _Str-1_-/- mice). Elliptical wound areas were calculated as πr1 × r2 = area, where r1 is the horizontal wound radius and r2 is the vertical wound radius.
We next determined the cellular events during the abnormal healing in the mutant mice. Sutured incisional wounds (10 mm) were created on the backs of _Str-1_-/- and wild-type mice and then harvested between 1 and 20 days. Circular excisional wounds 7 mm in diameter were created on a second set of wild-type and _Str-1_-/- mice and then harvested after 4 hours to 14 days. We first determined whether stromelysin-1 was required for keratinocyte migration, as has been found for plasminogen. 21 In the incisional wounds, wedges of migrating keratinocytes appeared in both the _Str-1_-/- wounds and normal wounds within 24 hours. By 72 hours after wounding, all the incisional wounds in both sets of mice displayed microscopic wound closure (defined as complete reepithelialization). Dermal and epidermal architecture of both wounded and unwounded skin appeared histologically normal in the _Str-1_-/- mice. There were no detectable differences in inflammatory infiltrates, granulation tissue, orneovascularization. When incisional wounds harvested from _Str-1_-/- mice and wild-type mice 10 days after wounding were analyzed for wound breaking strength, no difference was detected in mean wound strength between the _Str-1_-/- mice (mean break strength 262 g, n = 7 wounds) and the normal mice (mean break strength 255 g, n = 7 wounds).
In the excisional wounds, wedges of migrating keratinocytes appeared in both the _Str-1_-/- wounds and normal wounds within 24 hours after wounding (Fig. 2 ). The microscopic characteristics of the _Str-1_-/- migrating keratinocytes were indistinguishable from those of normal wounds. Although keratinocytes migrated at normal rates in the Str-1-/- wounds, the defect in early wound contraction increased the distance over which keratinocytes were required to migrate. Therefore, microscopic wound closure occurred later in the _Str-1_-/- mice (11 ± 1 days, n = 6 mice) than in wild-type mice (8 ± 1 days, n = 5 mice; p < 0.01, Student’s t test). Because incisional wounds healed normally in the _Str-1_-/- mice and epithelial cell migration and other aspects of the healing process were normal in the _Str-1_-/- mice, these data suggest that the primary defect involves the inability of the wound to contract.
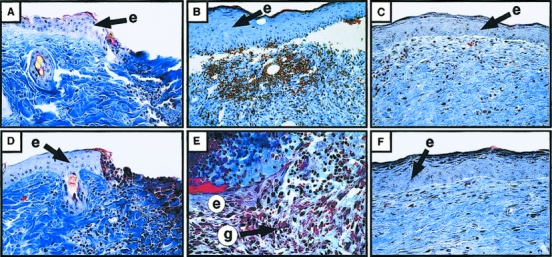
Figure 2. Histologic analysis (×100) of 7-mm excisional wounds from wild-type and _Str-1_-/- mice stained with Mallory’s trichrome. After 1 day, both the wild-type (A) and _Str-1_-/- (D) wounds showed a wedge of migrating keratinocytes in the epithelium. After 7 days, the wild-type (B) wounds were microscopically closed, whereas the _Str-1_-/- wounds (E) remained open, although keratinocyte migration appeared histologically normal. After 14 days, both sets of wounds had closed and the dermal and epidermal architecture in the wild-type (C) and _Str-1_-/- (F) wounds appeared normal. (e, epithelium; g, granulation tissue)
Two cell types have been implicated in wound contraction, myofibroblasts 22 and dermal fibroblasts. 18,19 Few cells containing αSMA, a marker of myofibroblasts, were detected by immunohistochemistry in either the _Str-1_-/- wounds or the wild-type wounds during the first 4 days of healing (Fig. 3). Cells staining positively for αSMA were detected 7 days after wounding in the dermis of both _Str-1_-/- and wild-type mice but were no longer present in 8/8 wild-type wounds and 7/8 _Str-1_-/- wounds after 14 days. No αSMA containing cells were detectable 20 days after wounding. Thus, there was no defect in formation of αSMA-positive myofibroblasts in the mutant mice.
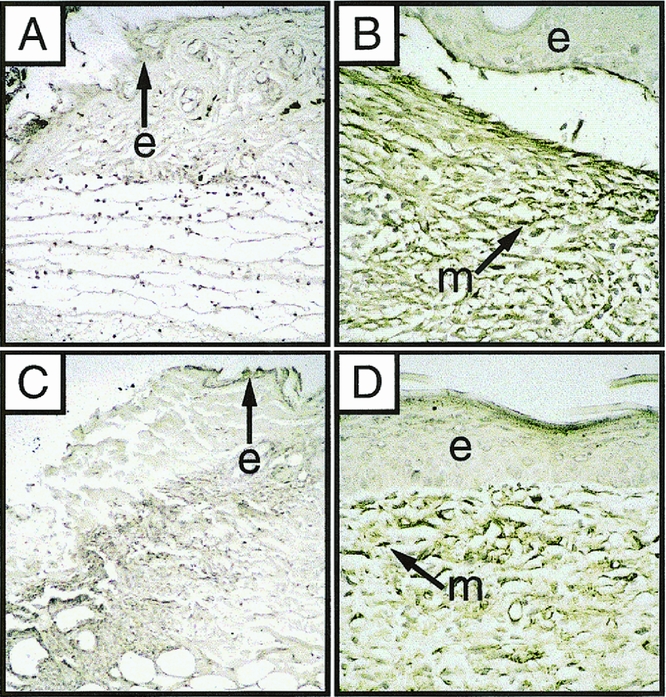
Figure 3. Photomicrograph (×100) of 7-mm excisional wounds from wild-type and _Str-1_-/- mice immunostained for αSMA. Brown pigment marks the myofibroblasts containing αSMA (m, myofibroblast; e, epithelium). One day after wounding, both the wild-type (A) and _Str-1_-/- wounds (C) show few αSMA-containing cells. After 7 days, both wild-type (B) and _Str-1_-/- wounds (D) have abundant myofibroblasts.
Early wound contraction may be mediated by formation of organized actin bundles in dermal fibroblasts. 18,19 Because the healing defect in the _Str-1_-/- mice was greatest during the earliest phase of contraction, we analyzed filamentous actin in transverse and cross-sections from _Str-1_-/- and wild-type mice harvested between 4 and 72 hours after wounding by staining with FITC-labeled phalloidin. We observed diffuse actin staining throughout the epidermis in both wild-type and mutant animals at all time points examined. However, an organized network of fibroblasts staining strongly for bundles of actin filaments was observed as a rim at the dermal–granulation tissue interface as early as 4 hours after wounding and persisted through 72 hours in wild-type wounds (Fig. 4). In contrast, loose, disorganized filamentous actin staining without any discernible network structure developed in the fibroblasts in the _Str-1_-/- wounds. These results point to a defect in actin assembly and intercellular organization of fibroblasts to form the multicellular network needed to facilitate wound contraction in the _Str-1_-/- mice.
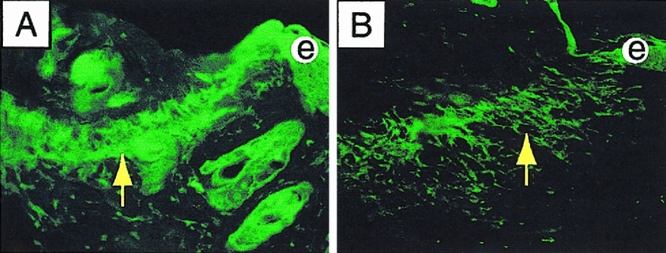
Figure 4. Photomicrograph (×100) of 7-mm excisional wounds stained with FITC-phalloidin. Transverse section from wild-type wounds harvested after 24 hours displays a well-organized actin network at the dermal–granulation tissue interface (A, arrow). Transverse section from _Str-1_-/- wounds harvested after 24 hours displays a loose and disorganized actin network (B, arrow). (e, epithelium)
DISCUSSION
Our data show that stromelysin-1 plays a crucial role in wound healing by modulating early wound contraction. Wound contraction involves interactions between wound fibroblasts and the surrounding extracellular matrix. Two theories have been advanced to explain wound contraction. Myofibroblasts, which are highly contractile and present in granulation tissue, have been implicated as the important cell type. 22–25 Other investigators have argued that the organization of intracellular actin bundles and a rim of organized, densely packed network of fibroblasts may be responsible for initiating the first phase of wound contraction. 18,19,26–28
We observed that normal wounds contract during the first 48 hours of healing, when few myofibroblasts are present. This indicates that faulty myofibroblast migration or differentiation could not contribute to the mechanism underlying delayed wound contraction in the _Str-1_-/- mice before the fifth day of healing. Our observation that contraction occurred before detection of myofibroblasts in the granulation tissue suggests that although myofibroblasts may be important for long-term wound contraction, scar formation, and matrix remodeling, they are not involved in the first phase of wound contraction. Our data suggest that the organized network of cells containing actin filaments at the edge of the normal wounds may initiate wound contraction. We observed that a tightly woven network of cells containing filamentous actin formed within 4 hours at the junction between the dermis and the granulation tissue in normal healing wounds. In contrast, _Str-1_-/- wounds had loose, disorganized cells staining for actin, without a clearly organized network structure. Stromelysin-1 is necessary for the assembly of this actin network and for the events that allow early wound contraction to proceed.
Fibroblast traction on ECM has been proposed as the mechanism underlying the first phase of wound contraction. 29,30 This requires normal adherence of cells to ECM and normal ECM architecture. The ability of fibroblasts to contract collagen gels _in vitro_depends on cell adhesion receptors interacting with type I collagen and laminin and on cytokines. 31 Contraction of collagen gels also induces collagenase and stromelysin-1. 32 Because the substrates for stromelysin-1 are located throughout the ECM and on cell surfaces, we hypothesize that stromelysin-1 mediates the remodeling of cell–ECM interactions and/or liberation of cytokines or other factors required for organization of a network of actin-containing wound fibroblasts. Stromelysin-1 may also be crucial for modifying the provisional matrix so that it is capable of transmitting the mechanical force necessary for contraction. In the absence of an appropriate scaffold, wound fibroblast migration, attachment and detachment, organization, or traction may be impaired.
Despite the profound defect in wound contraction, all other aspects of healing were normal in the _Str-1_-/- mice. Stromelysin-1 is expressed in basal keratinocytes adjacent to the wound edge but not in the leading edge of migrating keratinocytes in chronic ulcerative wounds. 14 Keratinocyte migration, and thereby wound healing, is compromised in mice lacking plasminogen. 21 Keratinocyte migration and reepithelialization proceeded normally in the _Str-1_-/- mice. Our data support the assertion that stromelysin-1 is not required for active keratinocyte migration or proliferation. Wound breaking strength, which reflects collagen synthesis and long-term ECM remodeling, 20,33,34 was unaffected in the mutant mice, suggesting that stromelysin-1 is not crucial for these processes. Finally, although aging may alter the ECM, resulting in delayed cutaneous wound healing, 33–35 the delayed healing seen in the _Str-1_-/- mice was independent of age. This indicates that the fundamental process requiring stromelysin-1 is unrelated to the age-dependent changes in the ECM. Taken together, these data indicate that stromelysin-1 exerts a unique function early in healing that is critical for initiating wound contraction. It will be interesting to determine whether or when other matrix metalloproteinases expressed during wound healing exert specific functions.
Acknowledgment
The authors thank Morgan T. Rosenbach for assistance in preparing the figures.
Footnotes
Correspondence: Kelli M. Bullard, MD, Box 0570, Fetal Treatment Center, Room HSW-1601, University of California, San Francisco, San Francisco, CA 94143-0570.
Supported by the Society for University Surgeons Surgical Research Fellowship and grants from NIH (AR 41118 and DE 10306), and a contract from OHER, U.S. Department of Energy DE-AC03-76-SF01012.
Accepted for publication January 5, 1999.
References
- 1.Bullen EC, Longaker MT, Updike DL, et al. Tissue inhibitor of metalloproteinases-1 is decreased and activated gelatinases are increased in chronic wounds. J Invest Dermatol 1995; 104: 236–240. [DOI] [PubMed] [Google Scholar]
- 2.Airola K, Vaalamo M, Reunala T, Saarialho-Kere UK. Enhanced expression of interstitial collagenase, stromelysin-1, and urokinase plasminogen activator in lesions of dermatitis herpetiformis. J Invest Dermatol 1995; 105: 184–189. [DOI] [PubMed] [Google Scholar]
- 3.Saarialho-Kere UK, Vaalamo M, Airola K, et al. Interstitial collagenase is expressed by keratinocytes that are actively involved in reepithelialization in blistering skin disease. J Invest Dermatol 1995; 104: 982–988. [DOI] [PubMed] [Google Scholar]
- 4.Salo T, Makela M, Kylmaniemi M, Autio-Harmainen H, Larjava H. Expression of matrix metalloproteinase-2 and -9 during early human wound healing. Lab Invest 1994; 70: 176–182. [PubMed] [Google Scholar]
- 5.Saarialho-Kere UK, Kovacs SO, Pentland AP, et al. Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest 1993; 92: 2858–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saarialho-Kere UK, Chang ES, Welgus HG, Parks WC. Distinct localization of collagenase and tissue inhibitor of metalloproteinases expression in wound healing associated with ulcerative pyogenic granuloma. J Clin Invest 1992; 90: 1952–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girard MT, Matsubara M, Kublin C, et al. Stromal fibroblasts synthesize collagenase and stromelysin during long-term tissue remodeling. J Cell Science 1993; 104: 1001–1011. [DOI] [PubMed] [Google Scholar]
- 8.Murphy G, Cockett MI, Stephens PE, et al. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J 1987; 248: 265–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito A, Nagase H. Evidence that human rheumatoid synovial matrix metalloproteinase 3 is an endogenous activator of procollagenase. Arch Biochem Biophys 1988; 267: 211–216. [DOI] [PubMed] [Google Scholar]
- 10.He CS, Wilhelm SM, Pentland AP, et al. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci USA 1989; 86: 2632–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welgus HG. Stromelysin: Structure and function. Agents Actions 1991; 35: 61–67. [PubMed] [Google Scholar]
- 12.Wilhelm SM, Collier IE, Kronberger A, et al. Human skin fibroblast stromelysin: Structure, glycosylation, substrate specificity, and differential expression in normal and tumorigenic cells. Proc Natl Acad Sci USA 1987; 84: 6725–6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welgus HG, Campbell EJ, Cury JD, et al. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest 1990; 86: 1496–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saarialho-Kere UK, Pentland AP, Birkedal-Hansen H, et al. Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J Clin Invest 1994; 94: 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young PK, Grinnell F. Metalloproteinase activation cascade after burn injury: A longitudinal analysis of the human wound environment. J Invest Dermatol 1994; 103: 660–664. [DOI] [PubMed] [Google Scholar]
- 16.Rudolph-Owen LA, Hulboy DL, Wilson CL, Mudgett J, Matrisian LM. Coordinate expression of matrix metalloproteinase family members in the uterus of normal, matrilysin-deficient, and stromelysin-1–deficient mice. Endocrinology 1997; 138: 4902–4911. [DOI] [PubMed] [Google Scholar]
- 17.Mudgett JS, Hutchinson NI, Chartrain NA, et al. Susceptibility of stromelysin-1–deficient mice to collagen-induced arthritis and cartilage destruction. Arth Rheum 1998; 41: 110–121. [DOI] [PubMed] [Google Scholar]
- 18.Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature 1992; 360: 179–183. [DOI] [PubMed] [Google Scholar]
- 19.Brock J, Midwinter K, Lewis J, Martin P. Healing of incisional wounds in the embryonic chick wing bud: Characterization of the actin purse-string and demonstration of a requirement for Rho activation. J Cell Biol 1996; 135: 1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mellin TN, Mennie RJ, Cashen DE, et al. Acidic fibroblast growth factor accelerates dermal wound healing. Growth Factors 1992; 7: 1–14. [DOI] [PubMed] [Google Scholar]
- 21.Romer J, Bugge TH, Pyke C, et al. Impaired wound healing in mice with a disrupted plasminogen gene. Nature Medicine 1996; 2: 287–292. [DOI] [PubMed] [Google Scholar]
- 22.Darby I, Skalli O, Gabbiani G. α-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 1990; 63: 21–29. [PubMed] [Google Scholar]
- 23.Germain L, Jean A, Auger FA, Garrel DR. Human wound healing fibroblasts have greater contractile properties than dermal fibroblasts. J Surg Res 1994; 57: 268–273. [DOI] [PubMed] [Google Scholar]
- 24.Cass DL, Sylvester KG, Yang EY, et al. Myofibroblast persistence in fetal sheep wounds is associated with scar formation. J Pediatr Surg 1997; 32: 1017–1102. [DOI] [PubMed] [Google Scholar]
- 25.Hembry RM, Bernanke DH, Hayashi K, et al. Morphologic examination of mesenchymal cells in healing wounds of normal and tight skin mice. Am J Pathol 1986; 125: 81–89. [PMC free article] [PubMed] [Google Scholar]
- 26.Kubler MD, Watt FM. Changes in the distribution of actin-associated proteins during epidermal wound healing. J Invest Dermatol 1993; 100: 785–789. [DOI] [PubMed] [Google Scholar]
- 27.Bement WM, Forscher P, Mooseker MS. A novel cytoskeletal structure involved in purse-string wound closure and cell polarity maintenance. J Cell Biol 1993; 121: 565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross J, Farinelli W, Sadow P, et al. On the mechanism of skin wound “contraction”: A granulation tissue “knockout” with a normal phenotype. Proc Natl Acad Sci USA 1995; 92: 5982–5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris AK, Stopak D, Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature 1981; 290: 249–251. [DOI] [PubMed] [Google Scholar]
- 30.Stopak D, Harris AK. Connective tissue morphogenesis by fibroblast traction. I. Tissue culture observations. Devel Biol 1982; 90: 383–398. [DOI] [PubMed] [Google Scholar]
- 31.Carver W, Molano I, Reaves TA, et al. Role of the α1β1 integrin complex in collagen gel contraction in vitro by fibroblasts. J Cell Physiol 1995; 165: 425–437. [DOI] [PubMed] [Google Scholar]
- 32.Unemori EN, Werb Z. Reorganization of polymerized actin: A possible trigger for induction of procollagenase in fibroblasts cultured in and on collagen gels. J Cell Biol 1986; 103: 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen TI, Kissmeyer-Nielsen P, Laurberg S, Christensen H. Impaired wound healing but unaltered colonic healing with increasing age: An experimental study in rats. Eur Surg Res 1995; 27: 250–257. [DOI] [PubMed] [Google Scholar]
- 34.Van de Kerkhof PC, Van Bergen B, Spruijt K, Kuiper JP. Age-related changes in wound healing. Clin Exper Dermatol 1994; 19: 369–374. [DOI] [PubMed] [Google Scholar]
- 35.Quaglino D, Fornieri C, Nanney LB, Davidson JM. Extracellular matrix modifications in rat tissues of different ages. Correlations between elastin and collagen type I mRNA expression and lysyl-oxidase activity. Matrix 1993; 13: 481–490. [PubMed] [Google Scholar]