Regulation of Endosome Sorting by a Specific PP2A Isoform (original) (raw)
Abstract
The regulated sorting of proteins within the _trans_-Golgi network (TGN)/endosomal system is a key determinant of their biological activity in vivo. For example, the endoprotease furin activates of a wide range of proproteins in multiple compartments within the TGN/endosomal system. Phosphorylation of its cytosolic domain by casein kinase II (CKII) promotes the localization of furin to the TGN and early endosomes whereas dephosphorylation is required for efficient transport between these compartments (Jones, B.G., L. Thomas, S.S. Molloy, C.D. Thulin, M.D. Fry, K.A. Walsh, and G. Thomas. 1995. EMBO [Eur. Mol. Biol. Organ.] J. 14:5869–5883). Here we show that phosphorylated furin molecules internalized from the cell surface are retained in a local cycling loop between early endosomes and the plasma membrane. This cycling loop requires the phosphorylation state-dependent furin-sorting protein PACS-1, and mirrors the trafficking pathway described recently for the TGN localization of furin (Wan, L., S.S. Molloy, L. Thomas, G. Liu, Y. Xiang, S.L. Ryback, and G. Thomas. 1998. Cell. 94:205–216). We also demonstrate a novel role for protein phosphatase 2A (PP2A) in regulating protein localization in the TGN/endosomal system. Using baculovirus recombinants expressing individual PP2A subunits, we show that the dephosphorylation of furin in vitro requires heterotrimeric phosphatase containing B family regulatory subunits. The importance of this PP2A isoform in directing the routing of furin from early endosomes to the TGN was established using SV-40 small t antigen as a diagnostic tool in vivo. The role of both CKII and PP2A in controlling multiple sorting steps in the TGN/endosomal system indicates that the distribution of itinerant membrane proteins may be acutely regulated via signal transduction pathways.
Keywords: furin, endosome, PP2A, sorting, PACS-1
Two compelling and fundamental questions in cell biology are to identify the mechanisms by which proteins are routed to their correct intracellular compartments and how these sorting steps are regulated. The broad importance and complexity of the _trans_-Golgi network (TGN)/endosomal sorting system (for review see Robinson et al., 1996) has been highlighted by recent studies of such diverse processes as delivery of vacuolar proteins in yeast (Cowles et al., 1997_a_ ,b; Piper et al., 1997; Voos and Stevens, 1998), transcytosis in polarized cells (Apodaca et al., 1996; Odorizzi et al., 1996; Zacchi et al., 1998), the mobilization of major histocompatability complex class 2 and Glut-4–containing endosomes (for review see Keller and Simons, 1997), and synaptic vesicle biogenesis (Cameron et al., 1991; West et al., 1997; Partoens et al., 1998). Despite their central role to the physiology of cells, the regulation of these complex trafficking systems remain largely undetermined.
One factor that contributes to the dynamic capacity of the TGN/endosomal sorting system is regulation by phosphorylation. The link between protein trafficking and phosphorylation provides a means by which cells can rapidly and reversibly alter the distribution and function of a variety of transmembrane proteins. Kinase and phosphatase activities have been shown to control both general and cargo-specific trafficking. Phospholipid kinases and phosphatases (for reviews see Stack et al., 1995; De Camilli et al., 1996; Shepherd et al., 1996; Woscholski and Parker, 1997) modulate membrane dynamics throughout the TGN/endosomal system, including budding from the TGN (Simon et al., 1996; Chen et al., 1997; Jones et al., 1998) and recycling from endosomal compartments (Cardone and Mostov, 1995; Spiro et al., 1996; Chung et al., 1997; Luo and Chang, 1997; Malide and Cushman, 1997). Although protein kinase activities have long been recognized as important modulators of receptor transduction complexes at the cell surface and in signaling endosomes (Bevan et al., 1995; Wang et al., 1996; Grimes et al., 1997), there is growing evidence that protein kinases and phosphatases also control the sorting of itinerant membrane proteins (for review see Seaman et al., 1996). Regulation of protein traffic by phosphorylation can occur via both general e.g., modification of adaptin binding to clathrin (Wilde and Brodsky, 1996), and TGN export (Ohashi and Huttner, 1994; Austin and Shields, 1996) and specific, cargo-directed mechanisms. Examples of the latter include the transcytosis of the polymeric immunoglobulin receptor (Apodaca and Mostov, 1993; Okamoto et al., 1994), internalization of T cell receptors (CTLA-4 [Bradshaw et al., 1997] and CD4 [Pelchen-Matthews et al., 1993]), as well as the TGN localization of the endoprotease furin (Jones et al., 1995; Takahashi et al., 1995; Dittié et al., 1997; Wan et al., 1998).
Whereas a few of the itinerant membrane protein–directed kinases involved in regulation of protein trafficking have been identified (e.g., CKII, Jones et al., 1995), both the phosphatases and the machinery responsible for the differential sorting of phosphoproteins remain largely uncharacterized. Furin is an excellent model for defining the cellular machinery involved in phosphorylation state-dependent protein sorting within the TGN/endosomal system. The endoprotease is routed through multiple proprotein processing compartments by virtue of defined trafficking signals within its cytosolic domain (cd)1 (Jones et al., 1995; Schäfer et al., 1995). Although the steady-state localization of furin is predominantly in the TGN (Bosshart et al., 1994; Molloy et al., 1994; Schäfer et al., 1995; Shapiro et al., 1997), the protease cycles between this compartment and the cell surface via an endosomal pathway (Molloy et al., 1994; Jones et al., 1995; Liu et al., 1997).
Internalization of furin from the cell surface and export from the TGN are directed by canonical tyrosine and/or dileucine based clathrin-coated pit recruitment motifs interacting with the clathrin sorting machinery (Ohno et al., 1996; Schäfer et al., 1995; Wan et al., 1998). Localization of furin to the TGN, however, requires a cluster of acidic residues (AC)1 that constitute a CKII phosphorylation site (Bosshart et al., 1994; Jones et al., 1995; Schäfer et al., 1995). Phosphorylation of this AC motif by CKII regulates the TGN localization of the protease by promoting its retrieval from immediate post-TGN compartments (Wan et al., 1998). The phosphofurin acidic cluster-sorting protein (PACS-1) directs this TGN retrieval step by linking the phosphorylated furin-cd to the clathrin sorting machinery (Wan et al., 1998).
Despite their importance for establishing processing compartments within endosomes and at the cell surface (Liu et al., 1997), the factors which control sorting of furin in peripheral compartments have not been well characterized. Phosphorylation of the furin-cd has, however, been implicated in the trafficking of the protease in the endosomal system (Jones et al., 1995). This observation indicates dual roles for both CKII and AC motifs in protein sorting. Furthermore, the ability of a phosphatase inhibitor (tautomycin) to alter the routing of internalized furin suggested that dephosphorylation is a critical determinant of furin sorting in early endosomes (Jones et al., 1995). The identity of the furin phosphatase, and the mechanism(s) by which these various factors act to regulate endosomal sorting, however, are not known.
The emerging complexity of the protein phosphatase (PP) 1 and 2A families suggests a myriad of roles for these enzymes. Indeed, isoforms of PP1 containing catalytic and regulatory or targeting subunits have been shown to control glycogen metabolism and myosin dephosphorylation (for review see Hubbard and Cohen, 1993). PP2A has been linked to the regulation of mitogen-signaling pathways, microtubule dynamics, and control of gene expression in the cell cycle (for reviews see Hubbard and Cohen, 1993; Mayer-Jaekel and Hemmings, 1994; Barford, 1996). The active form of PP2A in vivo is predominantly a heterotrimer which consists of a catalytic moiety (C subunit), as well as an A subunit which mediates the binding of variable regulatory subunits. Although neither phosphatase has been shown to direct protein trafficking, the demonstrated importance of subunit composition in determining their function in vivo suggests such a role is feasible.
A variety of PP2A regulatory subunit families genes, and splice variants have been reported (McCright et al., 1996; Zolnierowicz et al., 1996), yet specialized functions for these phosphatase isoforms remain to be established. Three unique PP2A regulatory subunit gene families have been identified to date in mammals based upon the characterization of tissue-specific isoforms and homology cloning. The B/PR55 family includes α, β, and γ gene products, whereas the B′/B56 family contains 5 genes (α, β, γ, δ, and ε) some of which express multiple splice variants (γ1–5, δ1–3). The PR72/130 regulatory subunits are the product of differential splicing of a single gene. Although some of these regulatory subunits are associated with isoforms of PP2A highly expressed in particular tissues or cell types (Strack et al., 1998), their roles in vivo are largely undetermined.
Here we report the identity of a furin phosphatase and the importance of furin-cd phosphorylation state in regulating multiple steps in the trafficking of the endoprotease in vivo. We demonstrate that furin undergoes phosphorylation-dependent local cycling between early endosomes and the cell surface. This peripheral cycling loop mirrors that reported for the TGN localization of the protease (Wan et al., 1998), and requires the phosphorylation state-dependent sorting protein PACS-1. Analyses in vitro and in vivo show that the movement of furin between early endosomes and the TGN is regulated by specific PP2A isoforms containing B family regulatory subunits. These findings demonstrate a novel role for PP2A and reveal the importance of phosphorylation/dephosphorylation in the acute regulation of protein sorting within the TGN/endosomal system.
Materials and Methods
Antibodies and Reagents
Antibodies against PP2A regulatory subunits have been described previously (Kamibayashi et al., 1994; Tehrani et al., 1996). PP2A catalytic subunit antibody was obtained from B. Wadzinsky (Vanderbilt University, Nashville, TN). mAb M1 was from Eastman Kodak Co. (Rochester, NY). PP1 and PP2A catalytic subunits were provided by A.A. DePaoli-Roach (Indiana University, Indianapolis, IN). All chemical reagents were obtained from Sigma Chemical Co. (St. Louis, MO) unless otherwise noted.
Cell Culture and Immunofluorescence Analyses
BSC-40 and PACS-1 control (C1) and antisense (AS19) cells were cultured as previously described (Thorne et al., 1989; Wan et al., 1998). HeLa cells expressing TS-Dyn I under control of Tet-suppressing elements were obtained from S. Schmid (The Scripps Research Institute, La Jolla, CA), and maintained as described (Damke et al., 1995). For immunofluorescence analyses, cells were cultured directly on glass coverslips to a density of 50–80% confluence before experimental manipulation as described in figure legends. Cells were fixed in 4% paraformaldehyde and processed for immunofluorescence as previously described (Molloy et al., 1994). mAb M1 was detected using either FITC- or TXR-conjugated goat anti– mouse IgG2b-specific secondary antibodies (Fisher Scientific Co., Pittsburgh, PA).
Cloning and Expression Vectors
Epitope-tagged furin (fur/f), the CKII phosphorylation site point mutants (fur/f-DDD and fur/f-ADA), and the glutathione-S-transferase (GST)– Furcd fusion protein were generated previously (Molloy et al., 1994; Jones et al., 1995). Dynamin I WT and K44E cDNAs were each excised from pSVL (Herskovits et al., 1993) using EcoRI. The inserts were then blunted with Klenow and cloned into the vaccinia recombination vector pZVneo cut with StuI. SV-40 small t WT and the truncated small t mut 3 (Sontag et al., 1993) were excised from pCMV5 using EcoRI and BamHI, and then blunted with Klenow and inserted into pZVneo cut with StuI. Recombinant vaccinia expressing the various constructs were generated by standard methods (VanSlyke et al., 1995).
Surface Labeling and Uptake Analysis
HeLa cells expressing the temperature-sensitive dominant-negative dynamin I were cultured in 35-mm plates for 3 d at permissive temperature in the absence of tetracycline. The cells were then infected with vaccinia virus expressing fur/f with either the serine to alanine (fur/f-ADA) or serine to aspartic acid (fur/f-DDD) substitutions within the cd CKII site (multiplicity of infection [m.o.i.] = 10) and allowed to express at nonpermissive temperature, 37°C. At 6 h postinfection the cells were placed on ice, rinsed with cold PBS and surface proteins were labeled for 1 h using 0.5 mg/ml EZ-link NHS-SS-biotin (Pierce Chemical Co., Rockford, IL) in PBS. After labeling, the cells were rinsed three times with PBS containing 50 mM glycine to quench unreacted biotin, and then refed with prewarmed medium (except for 0 time points) and placed at permissive temperature (31°C) for 10, 20, 30, or 40 min. At each time point, cells were transferred to ice, rinsed with cold PBS, and then either harvested directly in modified RIPA buffer (mRIPA, 50 mM Tris-HCl, pH 8, 150 mM NaCl, 1% NP-40 [Calbiochem-Novabiochem Corp., La Jolla, CA] and 1% sodium deoxycholate) for immunoprecipitation of total labeled furin using mAb M1 as previously described (Molloy et al., 1994), or were stripped of remaining surface biotin by washing three times for 10 min in Tris-buffered saline (TBS; 10 mM Tris-HCl, pH 8, 150 mM NaCl) with MesNa (50 mM). The surface-stripped samples were rinsed with PBS supplemented with Hepes (10 mM, pH 7.5) and harvested in mRIPA for immunoprecipitation of internalized labeled furin with mAb M1. The immunoprecipitates were resolved by SDS-PAGE on 8% gels, transferred to nitrocellulose membranes and probed with avidin HRP (1:2,000 dilution of 1 mg/ml in TBS + 0.05% Triton X-100) and developed by chemiluminescence (Renaissance; NEN Life Science Products, Boston, MA) to detect biotinylated furin. In double-strip analyses, cells processed as described above after the initial 20-min uptake period were then incubated for a second 20-min period at permissive temperature to allow for recycling of the internal pool to the cell surface. After the second chase period, cells were placed on ice and either harvested directly (control) or subjected to a second MesNa strip as described above before harvesting and analysis by Western blot.
PP2A Expression and Assays
Baculoviruses expressing PP2A catalytic, A, Bα, and Bβ subunits have been described previously (Kamibayashi et al., 1994). The recombinant baculovirus encoding the B′α subunit was generated as described (Tehrani et al., 1996). For expression experiments, Sf9 cells growing in log phase were seeded in 25-cm flasks (3 × 106 cells/flask) and infected with baculovirus recombinants (m.o.i. = 2). The cells were harvested at 64–72 h postinfection by trituration and then pelleted and washed with PBS. The washed cell pellet was then resuspended in 0.5 ml of ice-cold harvest buffer (50 mM Tris, pH 7.0, 1 mM EDTA, and 2 mM DTT with pepstatin, leupeptin, PMSF, aprotinin, and E64) and the cells broken open by passage (10×) through a 25-gauge needle. The lysates were clarified by low speed centrifugation at 500 g and brought to 10% glycerol before performing phosphatase assays (see below).
Phosphatase Assays
Substrates for phosphatase assays were prepared by phosphorylating GST–Furcd fusion protein and phosphorylase b in vitro with CKII or phosphorylase kinase, respectively. The phosphorylation reactions (100 μl) contained 50 μg of substrate, and 100 μM of 32P-labeled ATP (3,000 cpm/ pmol). After phosphorylation for 1 h at 30°C, the reactions were run over two G25 spin columns to remove free ATP. Dephosphorylation assays were conducted in a 50-μl reaction containing lysates, buffer (25 mM Tris, pH 7.0, 0.2 mM MnCl2, 1 mM DTT, and 0.2 mg/ml BSA) and ∼10 μM phosphorylated substrate (either phosphorylase a or GST–Furcd). Aliquots (5 μl in triplicate) of each reaction were removed at 15 and 30 min of incubation and applied to P81 filter paper (Whatman Inc., Clifton, NJ) squares that were washed three times with 75 mM phosphoric acid to remove free phosphate and then dried and subjected to scintillation counting. In some cases, the aliquots were mixed with SDS sample buffer, resolved by SDS-PAGE, and then analyzed using a PhosphoImager (model 445SI; Molecular Dynamics Inc., Sunnyvale, CA). Both measurement techniques gave identical results.
Microcystin Affinity Chromatography
Affinity chromatography for purification of endogenous furin-directed phosphatase from BSC-40 cell extract was performed essentially as described (Moorhead et al., 1994). In brief, microcystin (MC)-LR (LC Laboratories, Woburn, MA) was derivatized with aminoethanethiol to generate a primary amine group for coupling to NHS-activated sepharose (Pharmacia Biotech Inc., Piscataway, NJ). Approximately 0.25 mg of derivatized MC was linked to 1 ml of Sepharose resin. For the preparation of extracts, three 15-cm plates of confluent BSC-40 cells were rinsed three times with cold PBS and one time with harvest buffer (see above). The cells were then scraped from the plates and lysed by passage (10×) through a 25-gauge needle. The extract was then clarified by low-speed centrifugation (10 min at 500 g) and brought to 10% glycerol. The clarified extract was then cycled over the MC column (0.5 ml/min) for 1 h at 4°C to allow binding of the phosphatase. The column was then washed with 10 vol of harvest buffer and eluted with 3M NaSCN. Each eluted fraction (10 ml) was dialyzed over night against harvest buffer before assaying for phosphorylase a and GST–Furcd-directed phosphatase activities as described above.
Results
Importance of Furin-Cd Dephosphorylation for Early Endosome to TGN Sorting
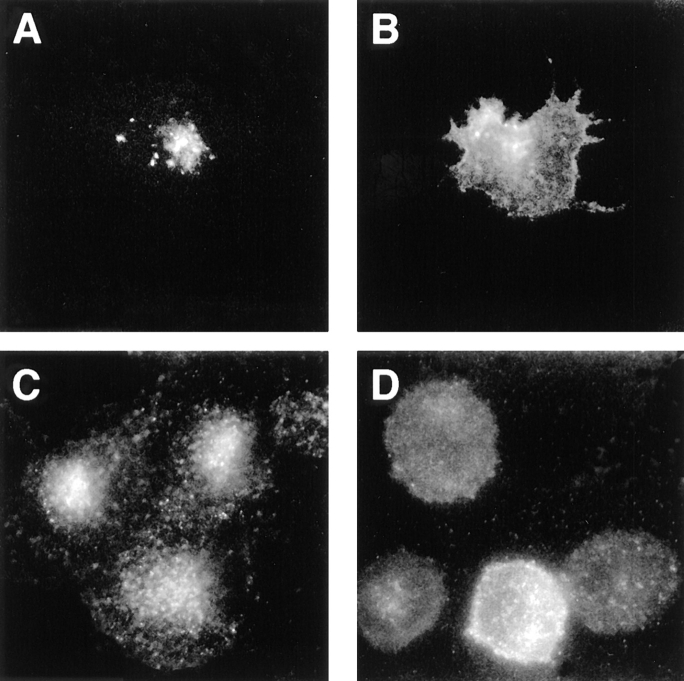
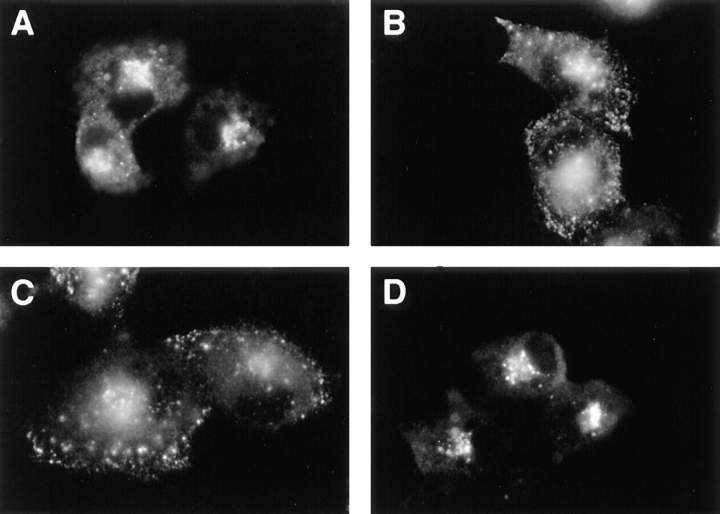
Phosphorylation of the furin-cd by CKII is necessary for maintaining the steady-state distribution of the endoprotease in the TGN and also for the localization of cell surface-internalized furin to early endosomes. By contrast, sorting of furin from early endosomes to the TGN requires dephosphorylation of the furin-cd (Jones et al., 1995). These findings were confirmed by antibody uptake studies (Fig. 1). Incubation of cells expressing epitope (FLAG)- tagged furin (fur/f) with mAb M1 demonstrated the retrieval of cell surface fur/f to the TGN (Fig. 1 A). However, treatment of cells with tautomycin, a potent inhibitor of PP1 and PP2A (Takai et al., 1995), resulted in the accumulation of internalized fur/f in peripheral punctate structures characteristic of early endosomes (Fig. 1 B). The effect of tautomycin is specific for a furin-directed phosphatase since (a) this drug has no effect on the internalization of a nonphosphorylatable furin construct, fur/ fS773,775A (ADA) (Jones et al., 1995), and (b) internalization of a phosphorylation mimic furin construct, fur/ fS773,775D (DDD), also resulted in accumulation in the peripheral punctate compartments (Fig. 1 C). Double-labeling experiments showed that internalized fur/f-DDD and transferrin colocalized within these peripheral vesicles (Fig. 1, D and E). Together these results demonstrate that after internalization, phosphorylated furin localizes to early endosomes and sorting of the endoprotease from this compartment to the TGN requires the activity of a tautomycin-sensitive furin phosphatase.
Figure 1.
Phosphorylation-dependent routing of furin in early endosomes (top). Epitope-tagged furin showing the FLAG insertion (cross-hatched), the catalytic (shaded) and transmembrane domains (stippled), as well as the sequence of the cd with CKII phosphorylation site substitutions. (Bottom) BSC-40 cells were infected with vaccinia recombinants (m.o.i. = 10) expressing either fur/f with the native cytosolic domain (A and B) or with serine to aspartic acid substitutions within the CKII site (fur/f-DDD, C–E). At 6 h postinfection, the cells were incubated with mAb M1 (15 μg/ml) and TRITC-transferrin (40 ng/ml) (E) for 1 h before fixation and processing for immunofluorescence. The cells in B were treated with 100 nM tautomycin during the uptake period. Internalized mAb M1 was visualized using FITC goat anti–mouse IgG (A–D).
Surface Recycling of Furin
The dynamic nature of the early endosomal sorting system suggests that the phosphorylation state-dependent accumulation of furin in early endosomes is not likely due to static retention in these structures. Therefore, we tested the possibility that this accumulation reflected a directed cycling of phosphorylated furin between the cell surface and early endosomes. To facilitate quantitative analyses of internalization and recycling, we first investigated the mechanism of furin endocytosis as a means by which to increase the surface pool of the protease. The presence of functional tyrosine- and dileucine-based internalization signals within the furin-cd suggested that endocytosis occurs via a clathrin-dependent pathway (Schäfer et al., 1995; Ohno et al., 1996). Therefore, we expressed a dominant-negative form of the GTPase dynamin I, K44E, to effectively block clathrin-dependent endocytosis (Herskovits et al., 1993; van der Bliek et al., 1993) and examined its effect on furin trafficking. Immunofluorescence analysis of furin internalization from the cell surface (Fig. 2) showed that although overexpression of native dynamin I had no effect on uptake (compare Fig. 2 A with Fig. 1 A), the K44E dominant-negative mutant blocked uptake and resulted in accumulation of the enzyme at the cell surface (Fig. 2 B). This effect on furin internalization mimicked the block in TfR endocytosis as observed with TRITC-transferrin uptake (Fig. 2, C [Dyn I] and D [K44E]).
Figure 2.
Internalization of furin is dependent upon dynamin function. BSC-40 cells were infected with vaccinia recombinants (m.o.i. = 5 each) expressing fur/f and either native dynamin I (A and C) or the dominant-negative dynamin construct K44E (B and D) in the presence of 10 μM hydroxyurea. At 16 h postinfection, cells were incubated with either mAb M1 (A and B) or TRITC-transferrin (C and D) for 1 h in culture before fixation and analysis by immunofluorescence. The extended culture time postinfection was required to allow sufficient expression levels for optimum K44E block.
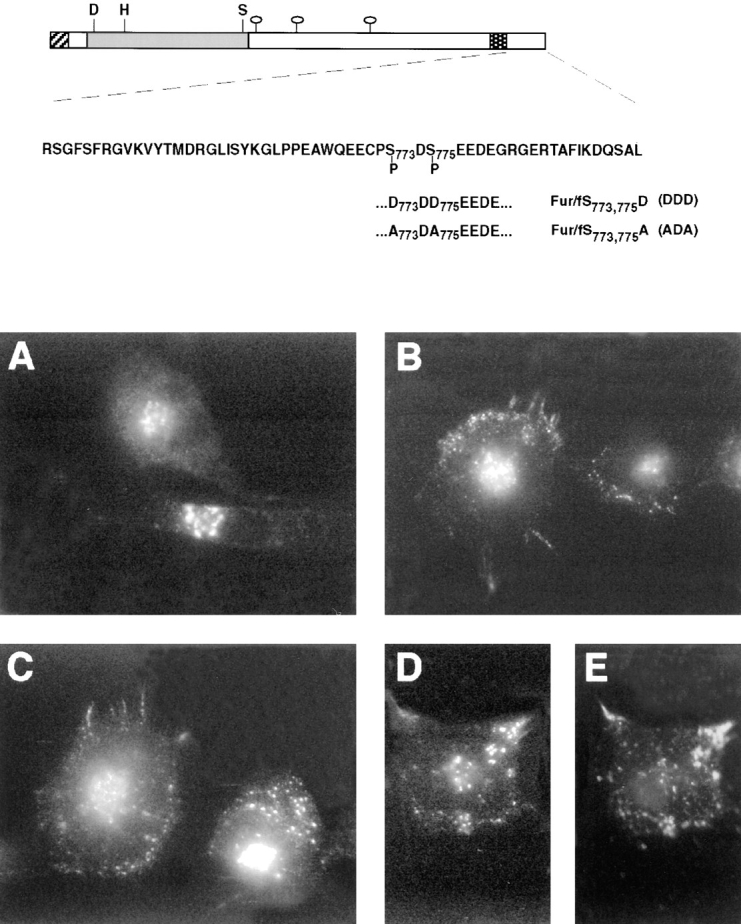
Based on this result, quantitative analyses of furin trafficking were performed in stably transfected HeLa cells expressing a temperature-sensitive and reversible dominant-negative form of dynamin I (TS-Dyn I). Incubation of cells expressing fur/f with TS-Dyn I at nonpermissive temperature resulted in a dramatic increase in the amount of furin detected by surface biotinylation when compared with control cells at permissive temperature (data not shown). Therefore, we used this system to compare the trafficking of fur/f-DDD, to fur/f-ADA (Fig. 3). After surface labeling, cells expressing either fur/f-DDD or fur/f-ADA were chased for the increasing times to allow endocytosis/recycling. At each time point duplicate samples were either processed to detect internalized (I) or total (T) surface labeled furin (Fig. 3 A). The ratio of protected/total furin was plotted quantitatively as a percentage of surface furin internalized versus time at the permissive temperature (Fig. 3 B). At time points up to 20 min the internalization of fur/f-DDD and fur/f-ADA were similar. However, at longer incubation times the percentage of fur/ f-DDD in internal compartments decreased while the percentage of internalized fur/f-ADA continued to increase. Additional double-strip experiments showed that a significant portion (∼50%) of the fur/f-DDD found in internal compartments at the 20-min time point was reexpressed at the cell surface during a subsequent 20-min chase (Fig. 3 B, inset). Reexpression of substantial amounts of fur/f-DDD at the cell surface within 20–30 min is consistent with the recycling time of transferrin receptor in HeLa cells (Bleil and Bretscher, 1982), and suggests that the phosphorylated form of furin undergoes a similar local cycling between early endosomes and the cell surface.
Figure 3.
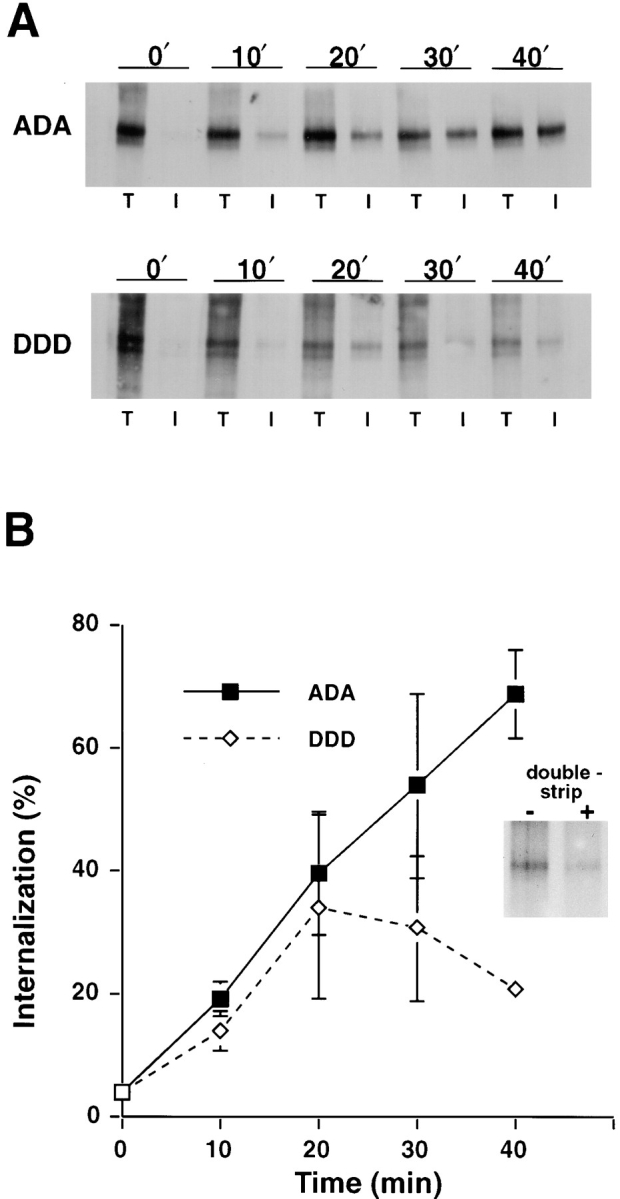
Recycling of phosphorylated furin from endosomes to the cell surface. (A) HeLa TS-Dyn I cells were infected with vaccinia recombinants (m.o.i. = 10) expressing fur/f mutants mimicking either nonphosphorylated (fur/f ADA) or constitutively phosphorylated furin (fur/f DDD). After accumulation at the cell surface during incubation at nonpermissive temperature (37°C) for 6 h, surface proteins were biotinylated (refer to Materials and Methods) and then allowed to internalize for the indicated times at permissive temperature (31°C). The total (T) and internalized (I) pools of labeled furin were then determined by immunoprecipitation and Western analysis. (B) The percent of furin internalized at each time point was quantified by densitometric analysis (data averaged from four independent experiments, error bars = SEM). (Inset) Double-strip analyses were conducted in which the pool of internalized furin following an initial 20-min chase period was assessed for reexpression at the cell surface. After a second 20-min chase, total biotinylated furin was compared with surface localized furin using a second MesNa strip (− and +, respectively).
PACS-1 Is Required for Early Endosome Localization of Phosphorylated Furin
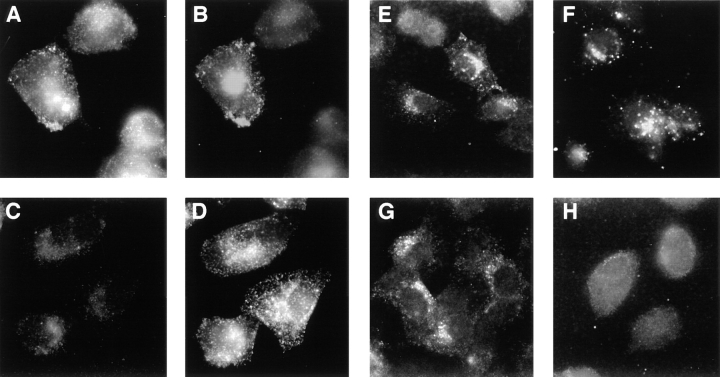
The cell surface/early endosome recycling of furin requires multiple sorting motifs within the enzyme's cd. Internalization of furin from the cell surface is directed by the canonical tyrosine and di-leucine-based endocytosis signals (Schäfer et al., 1995). Recycling from the early endosome to the cell surface, however, requires the CKII-catalyzed phosphorylation of the furin-cd AC (Fig. 3). Whereas the hydrophobic endocytosis signals bind directly to the clathrin adaptor AP-2 (Ohno et al., 1996), association of the phosphorylated furin-cd AC with the clathrin sorting machinery requires a connector protein, PACS-1 (Wan et al., 1998). A requirement for PACS-1 for the early endosomal localization of phosphorylated furin was demonstrated by monitoring furin internalization in PACS-1–deficient cells (Fig. 4). Parallel plates of control or PACS-1–deficient cells expressing fur/f were incubated with mAb M1 for 1 h in the presence of tautomycin. In agreement with Fig. 1, tautomycin treatment caused the accumulation of internalized fur/f in transferrin-containing early endosomes (Fig. 4 A). By contrast, in the absence of PACS-1, internalized fur/f failed to localize to early endosomes and instead showed a faint and dispersed staining pattern throughout the cytosol (Fig. 4 C). Early endosomal localization of internalized transferrin, however, was unaffected by PACS-1 depletion (Fig. 4, compare B with D). The inability of internalized fur/f to localize to early endosomes in the PACS-1–deficient cells was not the result of defective furin endocytosis since after short times of antibody uptake both the control and PACS-1–deficient cells showed similar punctate staining patterns (Fig. 4, E and G). Rather, the faint staining pattern in panel B reflects a shared requirement for PACS-1 in the localization of phosphorylated furin both to early endosomes and to the TGN (Wan et al., 1998). Indeed, mAb M1 uptake in the absence of tautomycin showed that in control cells fur/f is retrieved to the TGN and to a peripheral endosome/lysosome population (Fig. 4 F). In PACS-1 antisense cells, however, internalized fur/f fails to localize to either the TGN or to the peripheral endosome/lysosome population (Fig. 4 H). Together, these observations show that the sorting, but not the internalization, of furin requires PACS-1.
Figure 4.
Endocytic sorting of furin requires the phosphorylated cd binding protein PACS-1. Fur/f was expressed in control cells (A, B, E, and F) and PACS-1–deficient antisense cells (C, D, G, and H) using vaccinia recombinants (m.o.i. = 10). At 4 h postinfection, the cells were exposed to mAb M1 as a marker for endocytosed furin and TRITC-labeled transferrin (B and D) for either 1 h (A–D, F, and H) or 10 min. (E and G) before fixation and processing for immunofluorescence microscopy. The cells in A–D were exposed to 100 nM tautomycin during the 1-h incubation period with mAb M1 and transferrin. Internalized mAb M1 was visualized using FITC-conjugated goat anti–mouse IgG2b (A, C, and E–H).
Identification of Furin Phosphatase
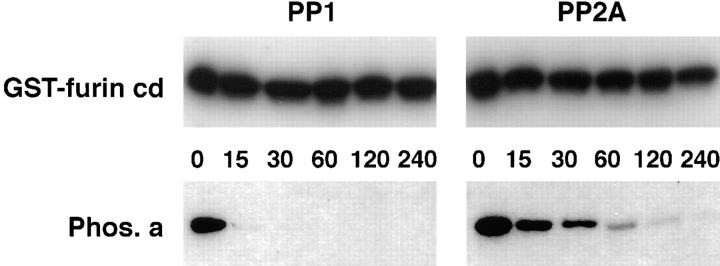
After internalization into early endosomes, phosphorylated furin may either (a) undergo a PACS-1–dependent recycling to the cell surface (Figs. 3 and 4) or (b) be directed to the TGN by a tautomycin-sensitive furin phosphatase (refer to Fig. 1) (Jones et al., 1995). The sensitivity to tautomycin suggested the furin phosphatase is either PP1 or PP2A. Therefore, the ability of both PP1 and PP2A catalytic (C) subunits to dephosphorylate the phosphorylated furin-cd in vitro was determined (Fig. 5). Surprisingly, neither phosphatase was able to dephosphorylate the furin-cd, whereas both enzymes efficiently dephosphorylated a control substrate, phosphorylase a. Similar results were obtained for PP2B (data not shown).
Figure 5.
Dephosphorylation of furin by purified phosphatase. PP1 and PP2A catalytic subunits were incubated with either 32P-labeled GST-Furcd fusion protein (GST–furincd) phosphorylated with CKII, or a 32P-labeled control substrate (phosphorylase a, Phos. a) for the indicated times (in min). Aliquots from the dephosphorylation reactions were then separated by SDS-PAGE and analyzed by autoradiography. Parallel experiments analyzed by quantitative filter paper assays also indicated no measurable dephosphorylation of the furin-cd by either enzyme (data not shown).
Whereas neither the PP1 nor the PP2A catalytic subunits were active against the phosphorylated furin-cd, both cell extracts and bovine brain cytosol contained a tautomycin- and okadaic acid-sensitive furin phosphatase activity (Fig. 6 A). Because of the high total protein concentrations required to detect furin phosphatase activity, we were unable to identify the phosphatase by differential sensitivity to these inhibitors. Therefore, an affinity chromatography procedure was used to isolate the furin phosphatase. Cell extract containing furin phosphatase activity was applied to a microcystin (a potent PP1/PP2A inhibitor) affinity column (Moorhead et al., 1994, 1995). Although microcystin normally covalently binds to both PP1 and PP2A (MacKintosh et al., 1995), derivatization for coupling to the column matrix blocks the reactive group and changes the reagent to a tight-binding reversible inhibitor. Bound material was then eluted with NaSCN, dialyzed and assayed for phosphatase activity using either the phosphorylated furin-cd or phosphorylase a as substrate (Fig. 6 B). The microcystin affinity column efficiently bound both the phosphorylase a and furin-cd phosphatase activities from the load sample. However, only the phosphorylase a activity could be recovered from the eluate. The elution conditions were not responsible for the loss of furin-directed activity since treatment of extract with NaSCN followed by dialysis did not inactivate the furin phosphatase. Furthermore, neither the pooling of all eluted fractions from the column before dialysis nor the addition of the column flow-through to the assay was able to restore furin-directed phosphatase activity (data not shown). These results suggested that the depletion of furin phosphatase activity was not simply the result of separating the catalytic moiety from a required cofactor during purification.
Figure 6.
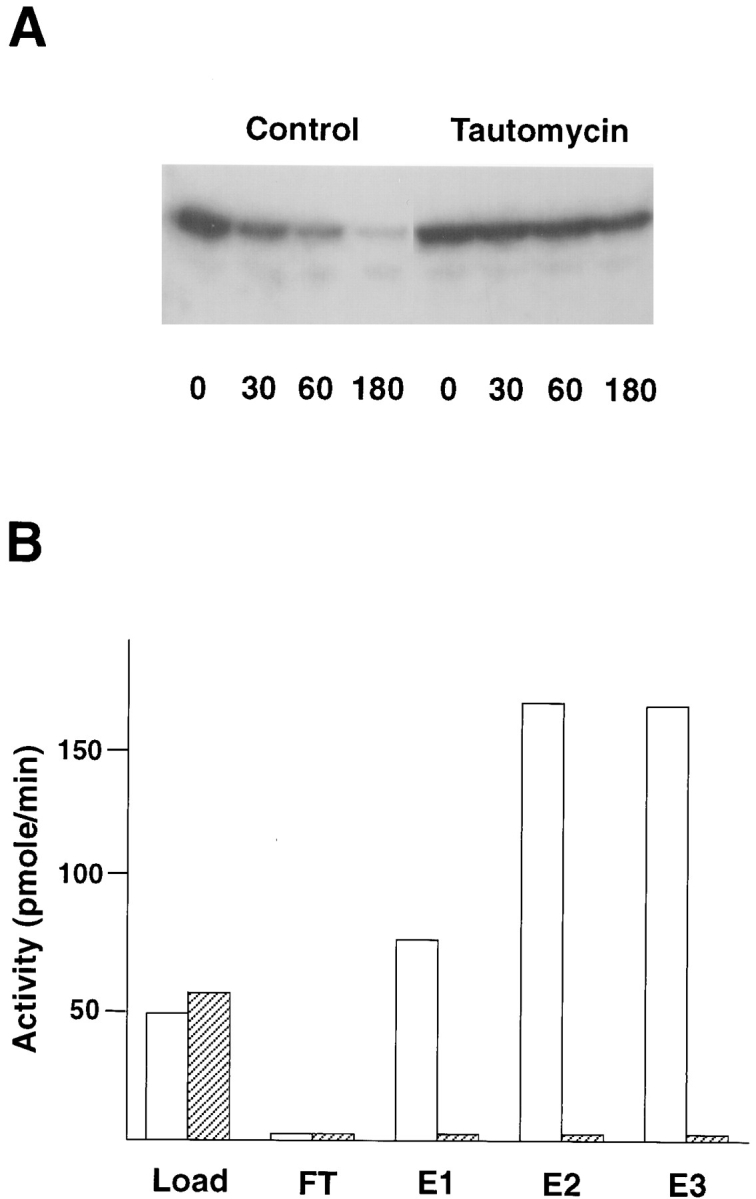
Endogenous furin-directed phosphatase activity. (A) Extracts from bovine brain (shown) and cultured cells were incubated with 32P-labeled GST–Furcd in vitro in the absence (Control) or presence of 100 nM tautomycin for the indicated times (in min). Aliquots of the reactions were separated by SDS-PAGE and analyzed by autoradiography. (B) Affinity chromatography of endogenous furin-directed phosphatase activity in BSC-40 cells. Cell extract was applied to a microcystin affinity column and the bound phosphatase eluted with 3M NaSCN. Aliquots of the original extract (Load), the unbound protein (FT), and eluted material (E1–3) were assayed for total PP1/PP2A activity using 32P-labeled phosphorylase a (open bars) and furin-directed phosphatase activity using 32P-labeled GST–Furcd (hatched bars). The total activities were calculated based on fraction volume and expressed as pmol/min. Assays were performed in triplicate with relative error (one standard deviation) of less than 5%. Similar results were obtained in several independent experiments.
The enhanced total phosphorylase a–directed activity recovered in the eluate fractions is consistent with previous reports (Moorhead et al., 1994, 1995), and reflects the purification of PP1 away from endogenous inhibitors. Interestingly, despite the ability of microcystin to bind and inhibit both PP1 and PP2A, only PP1 isoforms from muscle myofibrils and glycogen particles have been successfully isolated using this affinity technique (Moorhead et al., 1994, 1995). It is possible, therefore, that exposure of bound PP2A to the high concentrations of chaotropic agent required to dissociate it from the resin leads to an irreversible inhibition of the enzyme. These results, together with those in Fig. 5, indicate that the furin phosphatase is indeed a PP1/PP2A-like enzyme (tautomycin-sensitive, binds to the microcystin column). Furthermore, the inability to recover the furin phosphatase activity from the affinity column implicates PP2A.
Role of PP2A Regulatory Subunits in Determining Specificity for Furin
Although the PP2A C subunit is active towards numerous substrates in vitro, the predominant form of the enzyme in vivo is a trimeric complex with additional A and B subunits (for reviews see Barford, 1996; Hubbard and Cohen, 1993; Mayer-Jaekel and Hemmings, 1994). The A subunit promotes association of the C subunit with one of a variety of regulatory B subunits. Recent cloning studies reveal several unique families of regulatory subunits with multiple members derived from both separate genes and alternative splicing. Although the role of complex formation was originally envisioned as restricting the otherwise broad substrate specificity of the isolated C subunit, recent studies show that the B subunits can act as positive regulators to enhance catalytic activity toward particular substrates both in vitro and in vivo (Sontag et al., 1996). Moreover, the distinct subcellular localization of the different B subunits could act to target isoforms of PP2A to particular locations within the cell (Sontag et al., 1995; McCright et al., 1996; Okamoto et al., 1996). Together, these findings suggest a potentially high degree of isoform-specific PP2A characteristics.
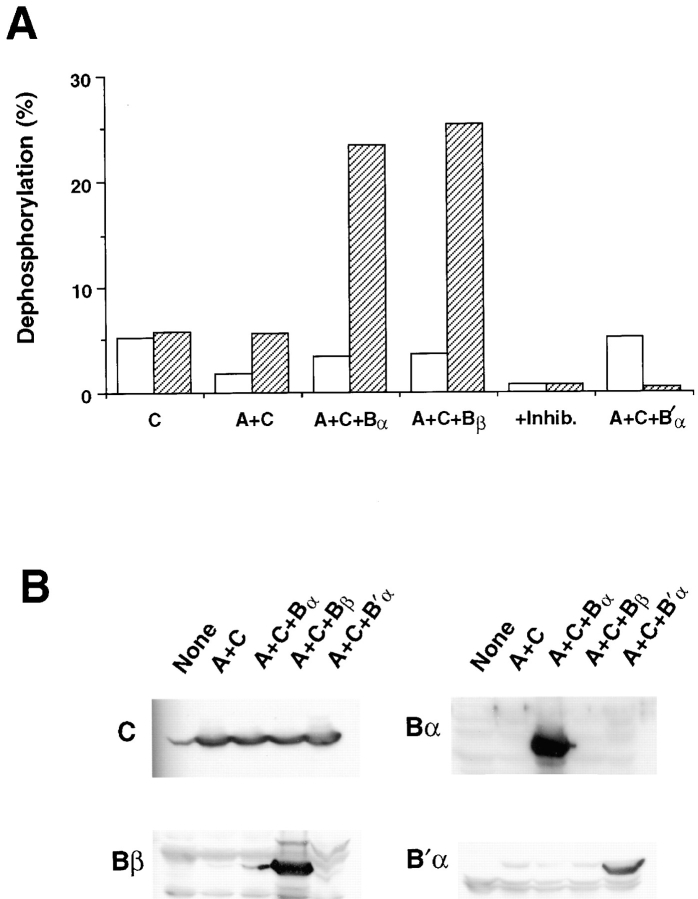
To test the possibility that such isoform-specific composition could modulate the activity of PP2A toward furin, we adopted a strategy of phosphatase isoform reconstitution by baculovirus expression (Kamibayashi et al., 1994). Sf9 cells were coinfected with baculovirus recombinants expressing combinations of the C, A, and one of the various B subunits (Fig. 7 A). Furin phosphatase activity was strictly dependent on B subunit composition. Whereas expression of C subunit alone or coexpression of C and A subunits failed to generate increased furin phosphatase activity, coexpression of the C and A subunits with B family regulatory subunits (Bα and Bβ) resulted in a selective increase in furin phosphatase activity (two- to fourfold). Importantly, the furin phosphatase activity was B family– specific since coexpression of C and A subunits with a member of the B′ family, B′α, failed to stimulate furin phosphatase activity. Indeed, B′α selectively inhibited the basal furin-directed activity. This effect is most likely due to displacement of endogenous Sf9 cell B family regulatory subunits from holoenzyme complexes by the overexpressed B′α, which has a higher affinity for the AC complex in vitro (Tehrani et al., 1996). As expected, both the phosphorylase a and furin phosphatase activities were inhibited completely by submicromolar concentrations of okadaic acid and tautomycin. Furthermore, Western blot analyses of the Sf9 extracts using subunit-specific antisera show that (a) the appropriate regulatory subunits were indeed expressed by the recombinant baculoviruses and (b) similar levels of catalytic subunit were expressed in each combinatorial infection (Fig. 7 B). This latter observation indicates that the dramatic changes in furin dephosphorylation are not the result of differential stabilization of the catalytic subunit, but rather reflect the inherent ability of the regulatory subunits to facilitate the recognition of furin by PP2A holoenzyme.
Figure 7.
Isoform-specific dephosphorylation of furin by PP2A. (A) Baculovirus recombinants (m.o.i. = 2) were used to express PP2A subunits, either alone or in combination, in Sf9 cells. At 64–72 h postinfection, cells were harvested and lysates were assayed for phosphatase activity with both 32P-labeled phosphorylase a (open bars) and phosphorylated 32P-labeled GST–Furcd (hatched bars). Noninfected Sf9 cells (data not shown) expressed a detectable level of endogenous furin-directed phosphatase activity which was not increased by expression of the catalytic subunit either alone or in combination with the A subunit (C, and A+C, respectively). The C and A subunits were also expressed with either the α or β isoforms of the B family of regulatory subunits (A+C+Bα and A+C+Bβ, respectively), as well as the α subunit from the unrelated B′ family (A+C+B′α). As a control, lysates from A+C+_Bβ–_expressing cells were exposed to 100 nM tautomycin and okadaic acid (+Inhib.) during the phosphatase assays. Assays were performed in triplicate with relative error <5%. The data shown are representative of several independent experiments. (B) Western analysis of PP2A subunit expression. Lysates of Sf9 cells infected with baculovirus recombinants encoding the indicated PP2A subunits were resolved by SDS-PAGE and screened by Western blot using antisera specific for the catalytic subunit or the Bα, Bβ, or B′α regulatory subunits.
PP2A Regulates Furin Trafficking In Vivo
The in vitro data described above clearly implicate PP2A isoforms containing B family subunits in the dephosphorylation of furin. The characterization of SV-40 virus-transforming factors has also provided a mechanism through which to probe the importance of these particular PP2A isoforms in vivo. The small t antigen of SV-40 contributes to the transforming capacity of the large T antigen, at least in part, by disrupting the mitogen-activated protein (MAP) kinase signaling pathway. Recent studies have shown that this effect of small t derives from the ability of the SV-40 protein to displace B family regulatory subunits from PP2A, resulting in a change in PP2A substrate specificity and hyperphosphorylation of several members of the MAP kinase cascade (Sontag et al., 1993). We used this characteristic of small t antigen to examine directly the importance of PP2A for the correct routing of furin in vivo.
To test this possibility, replicate plates of BSC-40 cells were coinfected with vaccinia virus recombinants expressing fur/f and either small t or an inactive small t mutant (mut3) lacking the PP2A binding site (Sontag et al., 1993). The effect of small t expression on the recycling of furin from the cell surface was then assessed in immunofluorescence studies as described above (Fig. 8). As seen previously, antibody uptake showed that recycling furin is primarily localized to the TGN/late endosome in control cells (Fig. 8 A). Coexpression of small t, however, resulted in an accumulation of the internalized furin in peripheral endosome-like structures (Fig. 8 C) as seen in cells treated with tautomycin (Fig 8 B). This effect required the PP2A-binding function of small t since the inactive mut3 construct had no discernible effect on furin trafficking (Fig. 8 D). These results complement the in vitro data described in Fig. 7 and show that PP2A isoforms containing B family regulatory subunits control the dephosphorylation dependent transfer of furin from early endosomes to the TGN.
Figure 8.
Disruption of endogenous PP2A affects furin trafficking in vivo. BSC-40 cells were infected with vaccinia recombinants (m.o.i. = 5 each) expressing fur/f alone (A and B) or in combination with viruses expressing either SV-40 small t (C) or truncated and inactive small t mut3. At 6 h (D) postinfection, the cells were incubated with mAb M1 for 1 h in culture before fixation. The cells in B were incubated in the presence of 100 nM tautomycin during mAb M1 uptake The cells were then processed for immunofluorescence to localize internalized furin.
Discussion
The mechanisms by which the endosomal system achieves and controls its complex sorting functions are largely unknown. Studies of furin recycling, however, demonstrate several factors that affect sorting within the early endosomal system. Here we report the identification of cellular machinery that directs the phosphorylation state-dependent trafficking of furin through endosomal compartments. Cell surface furin is endocytosed in a clathrin-/dynamin I–dependent step by direct interaction of the tyrosine- and dileucine-like internalization motifs with the AP-2 adaptor (refer to Fig. 2) (Schäfer et al., 1995; Ohno et al., 1996). After localization to transferrin containing early endosomes (refer to Fig. 1), furin undergoes a phosphorylation state-dependent sorting step that is controlled by the activities of CKII, and PP2A isoforms containing B family regulatory subunits (Jones et al., 1995) (refer to Fig. 7). Phosphorylated furin molecules bind the connector protein PACS-1 and are placed in a local cycling loop between the early endosome and the cell surface (refer to Figs. 3 and 4). By contrast, furin molecules dephosphorylated by PP2A are sorted to the TGN (refer to Figs. 5–8).
Several findings indicate that phosphorylation/dephosphorylation of the furin-cd by CKII and PP2A directly affect furin trafficking in the TGN/endosomal system (Jones et al., 1995; Dittié et al., 1997; Wan et al., 1998). For example, the accumulation of furin in early endosomes observed upon treatment of cells with tautomycin (refer to Fig. 1) (Jones et al., 1995) is replicated by the fur/f-DDD point mutant designed to mimic phosphorylation at the CKII site. Furthermore, this effect of tautomycim requires an intact, phosphorylatable CKII site in the furin-cd (Jones et al., 1995). Similarly, the effect of depletion of the phosphorylation state-dependent furin-binding protein PACS-1 upon trafficking is selective for furin and proteins containing related AC motifs (Wan et al., 1998). Although these results suggest direct effects on furin sorting, it seems likely that CKII and PP2A also have additional global roles in controlling membrane traffic within the TGN/endosomal system.
Regulation of Isoform-specific Phosphatase Function
The regulation of furin trafficking by the combined activities of CKII and PP2A indicates a link between signaling pathways and control of protein localization within the TGN/endosomal system. Together with their known function in cell cycle progression, our studies support a broad role for CKII and PP2A in regulating diverse cellular processes. Although modulation of CKII activity in vivo has not been demonstrated (Allende and Allende, 1995), PP2A can be regulated in several ways. The importance of holoenzyme composition in generating a furin-directed phosphatase (refer to Fig. 7) illustrates the high level of substrate specificity determined by subunit composition. Similarly, the B family is required for the PP2A-catalyzed dephosphorylation of tau both in vitro and in vivo. Although a selective role for B′ family subunits has not been demonstrated in mammalian cells, deletion of the B′ homologue in yeast, Rts1, results in a temperature-sensitive growth defect that can be rescued by rabbit B′ family subunits (Zhao et al., 1997). Together, these studies show that the PP2A regulatory subunits can act as positive effectors for select substrates.
Regulatory subunit composition may also modulate PP2A by targeting the phosphatase to select compartments. For example, B′ subunits differentially partition between the nucleus and cytoplasm (McCright et al., 1996), whereas Bα subunits target a population of PP2A to microtubules where they are positioned for efficient dephosphorylation of tau (Sontag et al., 1995). The microtubule-associated PP2A also undergoes cell cycle-dependent modulation of its activity, indicating an additional level of regulation. The observation that both the catalytic and regulatory subunits of PP2A are phosphoproteins (Mayer-Jaekel and Hemmings, 1994; McCright et al., 1996) introduces the possibility that this second tier of regulation represents rapid, reversible changes in PP2A function linked to second messenger signaling pathways.
Role of PP2A in Protein Sorting
The effect of SV-40 small t expression on furin sorting (Fig. 8) provides an unequivocal demonstration of PP2A's role in directing trafficking in the endocytic pathway. The selective displacement of B family regulatory subunits by small t offers a sensitive and direct diagnostic tool for delineating the role of PP2A isoforms in vivo. Our data do not, however, exclude a potential contribution by PP1 to the regulation of furin sorting. At limiting concentrations in vitro, PP1 is at least an order of magnitude more sensitive to tautomycin whereas PP2A is more sensitive to okadaic acid (Takai et al., 1995). Initial studies of the phosphorylation state-dependent sorting of furin, however, showed that, at low concentrations (100 nM), tautomycin affected sorting while similar concentrations of okadaic acid had no effect. Okadaic acid at high concentrations (e.g., 1 μM) not only affected furin trafficking, but also caused a dramatic dispersal of the paranuclear staining, consistent with disruption of the microtubule network and fragmentation of the TGN and Golgi (Lucocq, 1992; Reaven et al., 1993; Horn and Banting, 1994), precluding the use of this inhibitor in evaluating protein sorting. This preferential inhibition of furin trafficking by tautomycin could reflect cell type differences in the permeability of the inhibitors and/or their relative ability to penetrate the cellular compartments associated with PP2A-dependent sorting. Although the empirical determination of effective concentrations by examination of residual PP1 and PP2A activities in treated cells can facilitate the application of inhibitors as diagnostic tools (Favre et al., 1997), the possibility of resistant or sensitive pools of the enzymes remains a complicating factor.
TGN and Endosomal Protein Sorting Share Common Machinery
The requirement of PACS-1 for the sorting of phosphorylated furin in early endosomes points to several commonalties between the early endosome/cell surface furin cycling loop and the phosphorylation- and PACS-1–dependent localization of the endoprotease to the TGN (Wan et al., 1998). In the model shown in Fig. 9, the cell surface/early endosome local cycling loop represents a mirror image of a TGN/endosome cycling pathway. As for endocytosis of cell surface furin to early endosomes (Schäfer et al., 1995), efficient export of TGN-localized furin to a post-TGN endosomal compartment requires the canonical tyrosine- and/or dileucine-based internalization signals (Wan et al., 1998). These hydrophobic sorting signals bind directly to the clathrin adaptor AP-2 at the cell surface, whereas budding from the TGN uses AP-1 (Alconada et al., 1996; Honing et al., 1996; Ohno et al., 1996; Wan et al., 1998). Although furin in the peripheral recycling pathway colocalizes with TfR, demonstrating its presence in early endosomes, the identity of the post-TGN recycling compartment is not established. The recovery of phosphorylated furin from immature secretory granules in neuroendocrine cells (Dittié et al., 1997), however, provides an analogy for the TGN recycling loop. PACS-1 directs the retrieval step of both cycling loops by linking phosphorylated furin to the clathrin sorting machinery. Binding assays in vitro show PACS-1 connects the phosphorylated furin-cd to AP-1, which is consistent with a role for this adaptor in the TGN localization of membrane proteins. This finding, however, does not exclude the possibility that other adaptors (e.g., AP-3) may also mediate retrieval. The composition of the adaptor species used in the PACS-1–directed recycling between early endosomes and the plasma membrane has yet to be established. Interestingly, the prevalence on endosomes of clathrin-coated buds which contain neither α or γ adaptin (Stoorvogel et al., 1996) indicates that unique adaptor species may mediate the retrieval step within the peripheral cycling loop.
Figure 9.
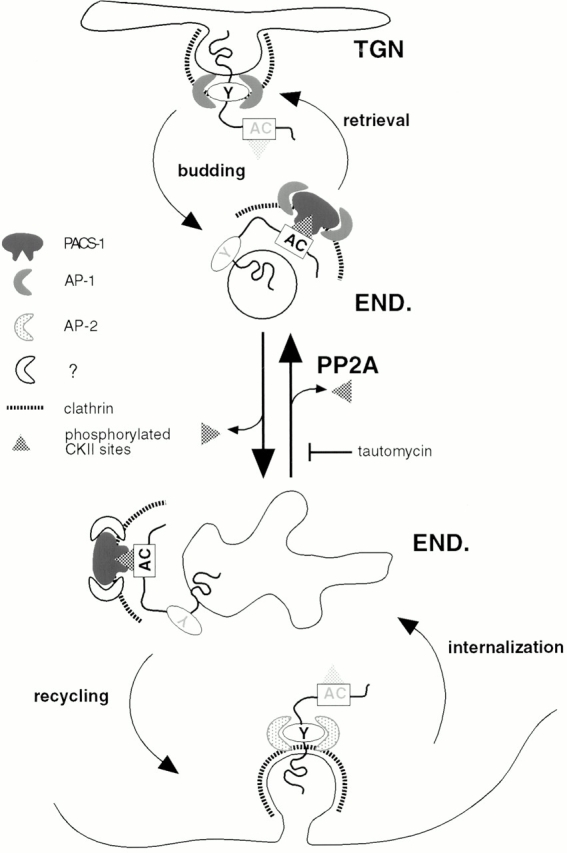
Model of furin trafficking in the endosomal system. Furin molecules are internalized via dynamin-sensitive, clathrin-dependent endocytosis. Within early endosomes shared by TfR, furin phosphorylated by CKII is directed toward a cell surface recycling pathway by virtue of its selective interaction with PACS-1, which links phosphorylated furin to components of the clathrin sorting machinery (Wan et al., 1998). This endosome to plasma membrane recycling pathway mirrors the local cycling loop which localizes furin to the TGN (Wan et al., 1998). Furin molecules dephosphorylated by PP2A isoforms containing B regulatory subunits are sorted from the plasma membrane/early endosome cycling loop to a TGN retrieval pathway. Black shading of either the tyrosine or AC motifs denotes their “active” state whereas gray shading reflects a “silencing” of these signals.
As reflected in Fig. 9, depletion of PACS-1 leads to sorting defects at both the level of endosomal trafficking and TGN localization. This model, however, does not address why nonphosphorylatable furin, fur/f-ADA (which does not bind PACS-1), displays some trafficking characteristics unique from fur/f in PACS-1 antisense cells (compare Fig. 4 and Wan et al., 1998 with Takahashi et al., 1995 and Jones et al., 1995). Most notable is the finding that fur/f-ADA mislocalizes from either the TGN or early endosomes to larger punctate membrane structures whereas fur/f in PACS-1 antisense cells shows very fine, dispersed vesicular staining. There are several possible explanations for these results, including (a) the presence of additional phosphorylated-furin binding proteins, (b) multiple roles for PACS-1 in organizing endosomal sorting compartments, and (c) the presence of binding proteins which interact selectively with the nonphosphorylated furin cd but not with fur/f-ADA. This latter possibility implies that nonphosphorylated AC motifs constitute positive sorting signals for trafficking within the TGN/endosomal system.
Phosphorylation-dependent Sorting Regulates Furin Processing Compartments
While PACS-1 directs the retrieval of phosphorylated furin in both the TGN and peripheral cycling loops, the transport of furin between these loops requires dephosphorylation by PP2A. This dephosphorylation-dependent trafficking step provides a mechanism by which cells may control the distribution of furin between the biosynthetic and plasma membrane/endosomal processing compartments. Furin at the cell surface is tethered by interaction with the actin binding protein ABP-280 (Liu et al., 1997). This interaction modulates the rate of furin internalization and may also act to generate processing sites at the cell surface (e.g., bacterial toxin activation) (Gordon et al., 1995). The cycling of furin between the cell surface and endosomes may reflect a requirement for the formation of peripheral processing compartments in which the enzyme can be concentrated with its substrates. In addition, the internal milieu of the early endosomes (e.g., acidic pH) facilitates the processing of some substrates such as pseudomonas and diphtheria toxins (Gordon et al., 1995). Thus, due to the highly dynamic and transitory nature of the endosomal system, continuous cycling of phosphorylated furin between the plasma membrane and early endosomes could be the best mechanism for maintaining functional concentrations of the protease within the peripheral processing compartments. Similarly, the TGN cycling loop might function to optimize processing of substrates in the biosynthetic pathway. This concept is consistent with the observation that, in some cells, endogenous furin can be predominantly localized to a post-TGN processing compartment (Sariola et al., 1995).
A Broad Role for PACS-1/PP2A in Protein Sorting
The PACS-1–directed trafficking of furin suggests that the localization of additional proteins containing acidic cluster motifs may be regulated via the phosphorylation state-dependent sorting machinery. Indeed, a number of itinerant secretory pathway membrane proteins including the cation-independent (Meresse et al., 1990) and cation-dependent (Mauxion et al., 1996) mannose 6 phosphate receptors, carboxypeptidase D (Xin et al., 1997), and sortilin (Petersen et al., 1997) have CKII phosphorylatable ACs within their cytosolic domains. In the case of the MPRs this phosphorylation has been linked to the efficient function of the receptor, however, it is not clear how this modification effects trafficking. Interestingly, many herpes virus envelope glycoproteins (e.g., VZV-gE, HSV-1-gE, and HCMV-gB) have phosphorylatable AC sorting motifs on their cds (Edson et al., 1987; Norais et al., 1996; Yao et al., 1993). Recent data indicate that the phosphorylation of these sites can influence trafficking of the envelope proteins (Alconada et al., 1996; Fish et al., 1998) and that PACS-1 binds VZV-gE cytosolic domain (Wan et al., 1998), raising the possibility that the CKII-mediated sorting system is used in viral biogenesis and/or spread.
Our studies of the regulation of furin sorting between the early endosome and plasma membrane also reveal several parallels with the cellular machinery that controls the phosphorylation-dependent resensitization of G protein– coupled receptors. The trafficking of both furin and βAR depend upon (a) the phosphorylation state of the cytosolic domain (Ferguson et al., 1995, 1996), (b) the presence of a phosphorylation state-dependent connector protein (either PACS-1 or β arrestin) (Goodman et al., 1996) that provides a link to the clathrin-sorting machinery, and (c) dephosphorylation by specific endosome-associated PP2A isoforms (Pitcher et al., 1995).
In summary, our results reveal the integrated roles of identified components of the endosomal trafficking machinery that direct the phosphorylation state-dependent sorting of furin. The importance of kinase (CKII) and isoform-specific phosphatase (PP2A) activities in regulating protein routing also indicates that these sorting events are likely under the control of second messenger systems. Future studies, therefore, will focus on how this dynamic endosomal sorting system responds to intracellular signaling pathways in order to control the distribution and activity of membrane proteins in vivo.
Acknowledgments
We thank B.G. Jones (University of Sheffield, Sheffield, UK) for contributions to the early part of this study. We also wish thank S.L. Schmid for the TS-Dyn I cell line, A.A. DePaoli-Roach for purified PP1 and PP2A, Wadzinsky for PP2A catalytic subunit antibody, R. Valle (Worcester Foundation, Shrewsbury, MA) for dynamin I and K44E plasmids, and P. Cohen (University of Dundee, Dundee, UK) for helpful discussions.
This work was supported by National Institutes of Health grants to G. Thomas (DK-44629 and DK-37274).
Abbreviations used in this paper
AC
acidic cluster
cd
cytosolic domain
GST
glutathione-S-transferase
MC
microcystin
m.o.i.
multiplicity of infection
PACS-1
phosphofurin acidic cluster-sorting protein
PP2A
protein phosphatase 2A
Footnotes
Address all correspondence to G. Thomas, Vollum Institute, Oregon Health Sciences University, Portland, OR 97201. Tel.: (503) 494-6955. Fax: (503) 494-4534. E-mail: thomasg@ohsu.edu
C. Kamibayashi's present address is Simmons Cancer Center, University of Texas Southwestern Medical Center, Dallas, TX 75235.
References
- Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO (Eur Mol Biol Organ) J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- Allende JE, Allende CC. Protein kinases. 4. Protein kinase CK2: an enzyme with multiple substrates and a puzzling regulation. FASEB (Fed Am Soc Exp Biol) J. 1995;9:313–323. doi: 10.1096/fasebj.9.5.7896000. [DOI] [PubMed] [Google Scholar]
- Apodaca G, Cardone MH, Whiteheart SW, DasGupta BR, Mostov KE. Reconstitution of transcytosis in SLO-permeabilized MDCK cells: existence of an NSF-dependent fusion mechanism with the apical surface of MDCK cells. EMBO (Eur Mol Biol Organ) J. 1996;15:1471–1481. [PMC free article] [PubMed] [Google Scholar]
- Apodaca G, Mostov KE. Transcytosis of placental alkaline phosphatase-polymeric immunoglobulin receptor fusion proteins is regulated by mutations of Ser664. J Biol Chem. 1993;268:23712–23719. [PubMed] [Google Scholar]
- Austin CD, Shields D. Formation of nascent secretory vesicles from the trans-Golgi network of endocrine cells is inhibited by tyrosine kinase and phosphatase inhibitors. J Cell Biol. 1996;135:1471–1483. doi: 10.1083/jcb.135.6.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barford D. Molecular mechanisms of the protein serine/threonine phosphatases. Trends Biochem Sci. 1996;21:407–412. doi: 10.1016/s0968-0004(96)10060-8. [DOI] [PubMed] [Google Scholar]
- Bevan AP, Burgess JW, Drake PG, Shaver A, Bergeron JJ, Posner BI. Selective activation of the rat hepatic endosomal insulin receptor kinase. Role for the endosome in insulin signaling. J Biol Chem. 1995;270:10784–10791. doi: 10.1074/jbc.270.18.10784. [DOI] [PubMed] [Google Scholar]
- Bleil JD, Bretscher MS. Transferrin receptor and its recycling in HeLa cells. EMBO (Eur Mol Biol Organ) J. 1982;1:351–355. doi: 10.1002/j.1460-2075.1982.tb01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshart H, Humphrey J, Deignan E, Davidson J, Drazba J, Yuan L, Oorschot V, Peters PJ, Bonifacino JS. The cytoplasmic domain mediates localization of furin to the trans-Golgi network en route to the endosomal/lysosomal system. J Cell Biol. 1994;126:1157–1172. doi: 10.1083/jcb.126.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw JD, Lu P, Leytze G, Rodgers J, Schieven GL, Bennett KL, Linsley PS, Kurtz SE. Interaction of the cytoplasmic tail of CTLA-4 (CD152) with a clathrin-associated protein is negatively regulated by tyrosine phosphorylation. Biochemistry. 1997;36:15975–15982. doi: 10.1021/bi971762i. [DOI] [PubMed] [Google Scholar]
- Cameron PL, Sudhof TC, Jahn R, De Camilli P. Colocalization of synaptophysin with transferrin receptors: implications for synaptic vesicle biogenesis. J Cell Biol. 1991;115:151–164. doi: 10.1083/jcb.115.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone M, Mostov K. Wortmannin inhibits transcytosis of dimeric IgA by the polymeric immunoglobulin receptor. FEBS (Fed Eur Biochem Soc) Lett. 1995;376:74–76. doi: 10.1016/0014-5793(95)01251-8. [DOI] [PubMed] [Google Scholar]
- Chen YG, Siddhanta A, Austin CD, Hammond SM, Sung TC, Frohman MA, Morris AJ, Shields D. Phospholipase D stimulates release of nascent secretory vesicles from the trans-Golgi network. J Cell Biol. 1997;138:495–504. doi: 10.1083/jcb.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JK, Sekiya F, Kang HS, Lee C, Han JS, Kim SR, Bae YS, Morris AJ, Rhee SG. Synaptojanin inhibition of phospholipase D activity by hydrolysis of phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 1997;272:15980–15985. doi: 10.1074/jbc.272.25.15980. [DOI] [PubMed] [Google Scholar]
- Cowles CR, Odorizzi G, Payne GS, Emr SD. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997a;91:109–118. doi: 10.1016/s0092-8674(01)80013-1. [DOI] [PubMed] [Google Scholar]
- Cowles CR, Snyder WB, Burd CG, Emr SD. Novel Golgi to vacuole delivery pathway in yeast: identification of a sorting determinant and required transport component. EMBO (Eur Mol Biol Organ) J. 1997b;16:2769–2782. doi: 10.1093/emboj/16.10.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Baba T, van der Bliek AM, Schmid SL. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol. 1995;131:69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P, Emr SD, McPherson PS, Novick P. Phosphoinositides as regulators in membrane traffic. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- Dittié AS, Thomas L, Thomas G, Tooze SA. Interaction of furin in immature secretory granules from neuroendocrine cells with the AP-1 adaptor complex is modulated by casein kinase II phosphorylation. EMBO (Eur Mol Biol Organ) J. 1997;16:4859–4870. doi: 10.1093/emboj/16.16.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson CM, Hosler BA, Waters DJ. Varicella-zoster virus gpI and herpes simplex virus gE: phosphorylation and Fc binding. Virology. 1987;161:599–602. doi: 10.1016/0042-6822(87)90157-7. [DOI] [PubMed] [Google Scholar]
- Favre B, Turowski P, Hemmings BA. Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J Biol Chem. 1997;272:13856–13863. doi: 10.1074/jbc.272.21.13856. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Downey WER, Colapietro AM, Barak LS, Menard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Menard L, Barak LS, Koch WJ, Colapietro AM, Caron MG. Role of phosphorylation in agonist-promoted beta 2-adrenergic receptor sequestration. Rescue of a sequestration-defective mutant receptor by beta ARK1. J Biol Chem. 1995;270:24782–24789. doi: 10.1074/jbc.270.42.24782. [DOI] [PubMed] [Google Scholar]
- Fish KN, Soderberg-Naucler C, Nelson JA. A tautomycin sensitive phosphatase regulates the cycling of HCMV gB at the plasma membrane in a cell specific manner. J Virol. 1998;72:6657–6664. doi: 10.1128/jvi.72.8.6657-6664.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2- adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Gordon VM, Klimpel KR, Arora N, Henderson MA, Leppla SH. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect Immun. 1995;63:82–87. doi: 10.1128/iai.63.1.82-87.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes ML, Beattie E, Mobley WC. A signaling organelle containing the nerve growth factor-activated receptor tyrosine kinase, TrkA. Proc Natl Acad Sci USA. 1997;94:9909–9914. doi: 10.1073/pnas.94.18.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits JS, Burgess CC, Obar RA, Vallee RB. Effects of mutant rat dynamin on endocytosis. J Cell Biol. 1993;122:565–578. doi: 10.1083/jcb.122.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honing S, Griffith J, Geuze HJ, Hunziker W. The tyrosine-based lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles. EMBO (Eur Mol Biol Organ) J. 1996;15:5230–5239. [PMC free article] [PubMed] [Google Scholar]
- Horn M, Banting G. Okadaic acid treatment leads to a fragmentation of the trans-Golgi network and an increase in expression of TGN38 at the cell surface. Biochem J. 1994;301:69–73. doi: 10.1042/bj3010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard MJ, Cohen P. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem Sci. 1993;18:172–177. doi: 10.1016/0968-0004(93)90109-z. [DOI] [PubMed] [Google Scholar]
- Jones BG, Thomas L, Molloy SS, Thulin CD, Fry MD, Walsh KA, Thomas G. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO (Eur Mol Biol Organ) J. 1995;14:5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Alb JG, Jr, Phillips SE, Bankaitis VA, Howell KE. A phosphatidylinositol 3-kinase and phosphatidylinositol transfer protein act synergistically in formation of constitutive transport vesicles from the trans-Golgi network. J Biol Chem. 1998;273:10349–10354. doi: 10.1074/jbc.273.17.10349. [DOI] [PubMed] [Google Scholar]
- Kamibayashi C, Estes R, Lickteig RL, Yang SI, Craft C, Mumby MC. Comparison of heterotrimeric protein phosphatase 2A containing different B subunits. J Biol Chem. 1994;269:20139–20148. [PubMed] [Google Scholar]
- Keller P, Simons K. Post-Golgi biosynthetic trafficking. J Cell Sci. 1997;110:3001–3009. doi: 10.1242/jcs.110.24.3001. [DOI] [PubMed] [Google Scholar]
- Liu G, Thomas L, Warren RA, Enns CA, Cunningham CC, Hartwig JH, Thomas G. Cytoskeletal protein ABP-280 directs the intracellular trafficking of furin and modulates proprotein processing in the endocytic pathway. J Cell Biol. 1997;139:1719–1733. doi: 10.1083/jcb.139.7.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucocq J. Mimicking mitotic Golgi disassembly using okadaic acid. J Cell Sci. 1992;103:875–880. doi: 10.1242/jcs.103.4.875. [DOI] [PubMed] [Google Scholar]
- Luo W, Chang A. Novel genes involved in endosomal traffic in yeast revealed by suppression of a targeting-defective plasma membrane ATPase mutant. J Cell Biol. 1997;138:731–746. doi: 10.1083/jcb.138.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh RW, Dalby KN, Campbell DG, Cohen PT, Cohen P, MacKintosh C. The cyanobacterial toxin microcystin binds covalently to cysteine-273 on protein phosphatase 1. FEBS (Fed Eur Biochem Soc) Lett. 1995;371:236–240. doi: 10.1016/0014-5793(95)00888-g. [DOI] [PubMed] [Google Scholar]
- Malide D, Cushman SW. Morphological effects of wortmannin on the endosomal system and GLUT4- containing compartments in rat adipose cells. J Cell Sci. 1997;110:2795–2806. doi: 10.1242/jcs.110.22.2795. [DOI] [PubMed] [Google Scholar]
- Mauxion F, Le Borgne R, Munier-Lehmann H, Hoflack B. A casein kinase II phosphorylation site in the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor determines the high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J Biol Chem. 1996;271:2171–2178. doi: 10.1074/jbc.271.4.2171. [DOI] [PubMed] [Google Scholar]
- Mayer-Jaekel RE, Hemmings BA. Protein phosphatase 2A—a ‘menage a trois'. Trends Cell Biol. 1994;4:287–291. doi: 10.1016/0962-8924(94)90219-4. [DOI] [PubMed] [Google Scholar]
- McCright B, Rivers AM, Audlin S, Virshup DM. The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J Biol Chem. 1996;271:22081–22089. doi: 10.1074/jbc.271.36.22081. [DOI] [PubMed] [Google Scholar]
- Meresse S, Ludwig T, Frank R, Hoflack B. Phosphorylation of the cytoplasmic domain of the bovine cation-independent mannose 6-phosphate receptor. Serines 2421 and 2492 are the targets of a casein kinase II associated to the Golgi-derived HAI adaptor complex. J Biol Chem. 1990;265:18833–18842. [PubMed] [Google Scholar]
- Molloy SS, Thomas L, VanSlyke JK, Stenberg PE, Thomas G. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO (Eur Mol Biol Organ) J. 1994;13:18–33. doi: 10.1002/j.1460-2075.1994.tb06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhead G, MacKintosh C, Morrice N, Cohen P. Purification of the hepatic glycogen-associated form of protein phosphatase-1 by microcystin-Sepharose affinity chromatography. FEBS (Fed Eur Biochem Soc) Lett. 1995;362:101–105. doi: 10.1016/0014-5793(95)00197-h. [DOI] [PubMed] [Google Scholar]
- Moorhead G, MacKintosh RW, Morrice N, Gallagher T, MacKintosh C. Purification of type 1 protein (serine/threonine) phosphatases by microcystin-Sepharose affinity chromatography. FEBS (Fed Eur Biochem Soc) Lett. 1994;356:46–50. doi: 10.1016/0014-5793(94)01232-6. [DOI] [PubMed] [Google Scholar]
- Norais N, Hall JA, Gross L, Tang D, Kaur S, Chamberlain SH, Burke RL, Marcus F. Evidence for a phosphorylation site in cytomegalovirus glycoprotein gB. J Virol. 1996;70:5716–5719. doi: 10.1128/jvi.70.8.5716-5719.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Pearse A, Domingo D, Trowbridge IS, Hopkins CR. Apical and basolateral endosomes of MDCK cells are interconnected and contain a polarized sorting mechanism. J Cell Biol. 1996;135:139–152. doi: 10.1083/jcb.135.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi M, Huttner WB. An elevation of cytosolic protein phosphorylation modulates trimeric G-protein regulation of secretory vesicle formation from the trans-Golgi network. J Biol Chem. 1994;269:24897–24905. [PubMed] [Google Scholar]
- Ohno H, Fournier MC, Poy G, Bonifacino JS. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J Biol Chem. 1996;271:29009–39015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- Okamoto CT, Song W, Bomsel M, Mostov KE. Rapid internalization of the polymeric immunoglobulin receptor requires phosphorylated serine 726. J Biol Chem. 1994;269:15676–15682. [PubMed] [Google Scholar]
- Okamoto K, Kamibayashi C, Serrano M, Prives C, Mumby MC, Beach D. p53-dependent association between cyclin G and the B′ subunit of protein phosphatase 2A. Mol Cell Biol. 1996;16:6593–6602. doi: 10.1128/mcb.16.11.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partoens P, Slembrouck D, Quatacker J, Baudhuin P, Courtoy P, Potter W. Retrieved constituents of large dense-cored vesicles and synaptic vesicles intermix in stimulation-induced early endosomes of noradrenergic neurons. J Cell Sci. 1998;111:681–689. doi: 10.1242/jcs.111.6.681. [DOI] [PubMed] [Google Scholar]
- Pelchen-Matthews A, Parsons IJ, Marsh M. Phorbol ester-induced downregulation of CD4 is a multistep process involving dissociation from p56lck, increased association with clathrin-coated pits, and altered endosomal sorting. J Exp Med. 1993;178:1209–1222. doi: 10.1084/jem.178.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CM, Nielsen MS, Nykjaer A, Jacobsen L, Tommerup N, Rasmussen HH, Roigaard H, Gliemann J, Madsen P, Moestrup SK. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J Biol Chem. 1997;272:3599–3605. doi: 10.1074/jbc.272.6.3599. [DOI] [PubMed] [Google Scholar]
- Piper RC, Bryant NJ, Stevens TH. The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J Cell Biol. 1997;138:531–545. doi: 10.1083/jcb.138.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher JA, Payne ES, Csortos C, DePaoli-Roach AA, Lefkowitz RJ. The G-protein-coupled receptor phosphatase: a protein phosphatase type 2A with a distinct subcellular distribution and substrate specificity. Proc Natl Acad Sci USA. 1995;92:8343–8347. doi: 10.1073/pnas.92.18.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven E, Tsai L, Maffe B, Azhar S. Effect of okadaic acid on hepatocyte structure and function. Cell Mol Biol Res. 1993;39:275–488. [PubMed] [Google Scholar]
- Robinson MS, Watts C, Zerial M. Membrane dynamics in endocytosis. Cell. 1996;84:13–21. doi: 10.1016/s0092-8674(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Sariola M, Saraste J, Kuismanen E. Communication of post-Golgi elements with early endocytic pathway: regulation of endoproteolytic cleavage of Semliki Forest virus p62 precursor. J Cell Sci. 1995;108:2465–2475. doi: 10.1242/jcs.108.6.2465. [DOI] [PubMed] [Google Scholar]
- Schäfer W, Stroh A, Berghofer S, Seiler J, Vey M, Kruse ML, Kern HF, Klenk HD, Garten W. Two independent targeting signals in the cytoplasmic domain determine trans-Golgi network localization and endosomal trafficking of the proprotein convertase furin. EMBO (Eur Mol Biol Organ) J. 1995;14:2424–2435. doi: 10.1002/j.1460-2075.1995.tb07240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MN, Burd CG, Emr SD. Receptor signalling and the regulation of endocytic membrane transport. Curr Opin Cell Biol. 1996;8:549–556. doi: 10.1016/s0955-0674(96)80034-2. [DOI] [PubMed] [Google Scholar]
- Shapiro J, Sciaky N, Lee J, Bosshart H, Angeletti RH, Bonifacino JS. Localization of endogenous furin in cultured cell lines. J Histochem Cytochem. 1997;45:3–12. doi: 10.1177/002215549704500102. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Reaves BJ, Davidson HW. Phosphoinositide 3-kinases and membrane traffic. Trends Cell Biol. 1996;6:92–97. doi: 10.1016/0962-8924(96)80998-6. [DOI] [PubMed] [Google Scholar]
- Simon JP, Ivanov IE, Adesnik M, Sabatini DD. The production of post-Golgi vesicles requires a protein kinase C-like molecule, but not its phosphorylating activity. J Cell Biol. 1996;135:355–370. doi: 10.1083/jcb.135.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- Sontag E, Nunbhakdi-Craig V, Bloom GS, Mumby MC. A novel pool of protein phosphatase 2A is associated with microtubules and is regulated during the cell cycle. J Cell Biol. 1995;128:1131–1144. doi: 10.1083/jcb.128.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron. 1996;17:1201–1207. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- Spiro DJ, Boll W, Kirchhausen T, Wessling-Resnick M. Wortmannin alters the transferrin receptor endocytic pathway in vivo and in vitro. Mol Biol Cell. 1996;7:355–367. doi: 10.1091/mbc.7.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack JH, Horazdovsky B, Emr SD. Receptor-mediated protein sorting to the vacuole in yeast: roles for a protein kinase, a lipid kinase and GTP-binding proteins. Annu Rev Cell Dev Biol. 1995;11:1–33. doi: 10.1146/annurev.cb.11.110195.000245. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W, Oorschot V, Geuze HJ. A novel class of clathrin-coated vesicles budding from endosomes. J Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S, Zaucha JA, Ebner FF, Colbran RJ, Wadzinski BE. Brain protein phosphatase 2A: developmental regulation and distinct cellular and subcellular localization by B subunits. J Comp Neurol. 1998;392:515–527. [PubMed] [Google Scholar]
- Takahashi S, Nakagawa T, Banno T, Watanabe T, Murakami K, Nakayama K. Localization of furin to the trans-Golgi network and recycling from the cell surface involves Ser and Tyr residues within the cytoplasmic domain. J Biol Chem. 1995;270:28397–28401. doi: 10.1074/jbc.270.47.28397. [DOI] [PubMed] [Google Scholar]
- Takai A, Sasaki K, Nagai H, Mieskes G, Isobe M, Isono K, Yasumoto T. Inhibition of specific binding of okadaic acid to protein phosphatase 2A by microcystin-LR, calyculin-A and tautomycin: method of analysis of interactions of tight-binding ligands with target protein. Biochem J. 1995;306:657–665. doi: 10.1042/bj3060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani MA, Mumby MC, Kamibayashi C. Identification of a novel protein phosphatase 2A regulatory subunit highly expressed in muscle. J Biol Chem. 1996;271:5164–5170. doi: 10.1074/jbc.271.9.5164. [DOI] [PubMed] [Google Scholar]
- Thorne BA, Caton LW, Thomas G. Expression of mouse proopiomelanocortin in an insulinoma cell line. Requirements for beta-endorphin processing. J Biol Chem. 1989;264:3545–3552. [PubMed] [Google Scholar]
- van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanSlyke, J.K., L. Thomas, and G. Thomas. 1995. Use of Vaccinia Virus Vectors to Study Neuropeptide Processing. In Peptidases and Neuropeptide Processing. Vol. 23. A.I. Smith, editor. Academic Press, San Diego. CA. 45– 64.
- Voos W, Stevens TH. Retrieval of resident late-Golgi membrane proteins from the prevacuolar compartment of Saccharomyces cerevisiae is dependent on the function of Grd19p. J Cell Biol. 1998;140:577–590. doi: 10.1083/jcb.140.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Molloy SS, Thomas L, Liu G, Xiang Y, Rybak SL, Thomas G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94:205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- Wang Z, Tung PS, Moran MF. Association of p120 ras GAP with endocytic components and colocalization with epidermal growth factor (EGF) receptor in response to EGF stimulation. Cell Growth Differ. 1996;7:123–133. [PubMed] [Google Scholar]
- West MA, Bright NA, Robinson MS. The role of ADP-ribosylation factor and phospholipase D in adaptor recruitment. J Cell Biol. 1997;138:1239–1254. doi: 10.1083/jcb.138.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde A, Brodsky FM. In vivo phosphorylation of adaptors regulates their interaction with clathrin. J Cell Biol. 1996;135:635–645. doi: 10.1083/jcb.135.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woscholski R, Parker PJ. Inositol lipid 5-phosphatases—traffic signals and signal traffic. Trends Biochem Sci. 1997;22:427–431. doi: 10.1016/s0968-0004(97)01120-1. [DOI] [PubMed] [Google Scholar]
- Xin X, Varlamov O, Day R, Dong W, Bridgett MM, Leiter EH, Fricker LD. Cloning and sequence analysis of cDNA encoding rat carboxypeptidase D. DNA Cell Biol. 1997;16:897–909. doi: 10.1089/dna.1997.16.897. [DOI] [PubMed] [Google Scholar]
- Yao Z, Jackson W, Grose C. Identification of the phosphorylation sequence in the cytoplasmic tail of the varicella-zoster virus Fc receptor glycoprotein gpI. J Virol. 1993;67:4464–4473. doi: 10.1128/jvi.67.8.4464-4473.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchi P, Stenmark H, Parton RG, Orioli D, Lim F, Giner A, Mellman I, Zerial M, Murphy C. Rab17 regulates membrane trafficking through apical recycling endosomes in polarized epithelial cells. J Cell Biol. 1998;140:1039–1053. doi: 10.1083/jcb.140.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Boguslawski G, Zitomer RS, DePaoli-Roach AA. Saccharomyces cerevisiaehomologs of mammalian B and B′ subunits of protein phosphatase 2A direct the enzyme to distinct cellular functions. J Biol Chem. 1997;272:8256–8262. doi: 10.1074/jbc.272.13.8256. [DOI] [PubMed] [Google Scholar]
- Zolnierowicz S, Van Hoof C, Andjelkovic N, Cron P, Stevens I, Merlevede W, Goris J, Hemmings BA. The variable subunit associated with protein phosphatase 2A0 defines a novel multimember family of regulatory subunits. Biochem J. 1996;317:187–194. doi: 10.1042/bj3170187. [DOI] [PMC free article] [PubMed] [Google Scholar]