Global traffic and disease vector dispersal (original) (raw)
Abstract
The expansion of global air travel and seaborne trade overcomes geographic barriers to insect disease vectors, enabling them to move great distances in short periods of time. Here we apply a coupled human–environment framework to describe the historical spread of Aedes albopictus, a competent mosquito vector of 22 arboviruses in the laboratory. We contrast this dispersal with the relatively unchanged distribution of Anopheles gambiae and examine possible future movements of this malaria vector. We use a comprehensive database of international ship and aircraft traffic movements, combined with climatic information, to remap the global transportation network in terms of disease vector suitability and accessibility. The expansion of the range of Ae. albopictus proved to be surprisingly predictable using this combination of climate and traffic data. Traffic volumes were more than twice as high on shipping routes running from the historical distribution of Ae. albopictus to ports where it has established in comparison with routes to climatically similar ports where it has yet to invade. In contrast, An. gambiae has rarely spread from Africa, which we suggest is partly due to the low volume of sea traffic from the continent and, until very recently, a European destination for most flights.
Keywords: Aedes albopictus, air travel, Anopheles gambiae, biological invasion, shipping
Throughout history the opening of travel and trade routes between countries has been accompanied by the spread of microbes and their vectors (1). Significant events include the 14th- to 17th-century plague pandemics, the 19th-century cholera epidemics, and the escape of Aedes aegypti from West Africa, facilitating yellow fever epidemics in North American port cities in the 19th and early 20th centuries (2). The most devastating disease vector introduction in recent history was that of Anopheles gambiae (3, 4), the most important malaria vector in Africa, to northeastern Brazil in 1930. Although malaria was already endemic in the area, the greater vectorial capacity of this mosquito species, relative to local species, sparked Plasmodium falciparum malaria epidemics costing 16,000 lives (5) before eradication of An. gambiae by an effective, yet costly, control program (6).
During the last 50 years air travel passenger numbers have grown by nearly 9% per annum (7); shipping traffic has increased by >27% since 1993 (8). This growth is associated with rising numbers of cases of biological invasion. Gradual mixing of the world’s biota represents a global problem, with consequences ranging from native species’ decline to threats to human health (9). Biological invasion is a multistep process involving initial dispersal, establishment, and spread (10, 11). Invasion research has increased considerably over the past 15 years, yet the initial dispersal phase, on which the other stages rely, remains the least studied (9). Recent biological invasions have involved both disease vectors (12) and the expansion or reemergence of vector-borne diseases (13).
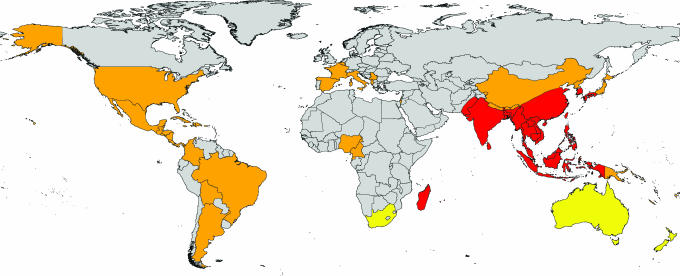
Modern transport facilitates the movement of disease vectors farther and faster than ever before. Aircraft have been proven capable of transporting disease vectors through experiments (14), the phenomenon of airport malaria (15), and incoming flight surveys (16). Air travel is also suspected to be directly responsible for the dispersal and consequent establishment of exotic mosquitoes on many Pacific islands, including Hawaii (12, 17). Modern container ships are known to have introduced numerous alien mosquito species, including the Asian tiger mosquito, Aedes albopictus (18), to new areas (12). Ae. albopictus is of medical and public health concern because it has been shown in the laboratory to be a highly efficient vector of 22 arboviruses, including dengue, yellow, and West Nile fever viruses (19, 20). In the wild, however, its efficiency as a vector appears to be generally low (19, 20), although it has been implicated in recent dengue fever outbreaks in the absence of the principal vector, Ae. aegypti (20, 21). From its Old World distribution reported in 1930 (Fig. 1), Ae. albopictus expanded its range first to the Pacific Islands (20) and then, within the last 20 years, to other countries in both the Old World and the New World (Fig. 1) (20, 22), principally through ship-borne transportation of eggs and larvae in tires (23).
Fig. 1.
Old World distribution of Ae. albopictus (red), countries reporting established breeding populations of Ae. albopictus in the last 30 years (orange), and countries reporting Ae. albopictus interception at ports (yellow).
Although distance may no longer represent a significant barrier to vector movement, climate at the point of entry still presents a fundamental constraint to establishment, because small invertebrates are very sensitive to the weather. Here we combine data on global air and sea traffic volumes with climatic data to examine the effects of these constraints on global disease vector dispersal and establishment. By remapping the global transport network in terms of climatic similarity, we examine both how predictable was the spread of Ae. albopictus and what might be the potential for future dispersal of both Ae. albopictus and An. gambiae.
Results
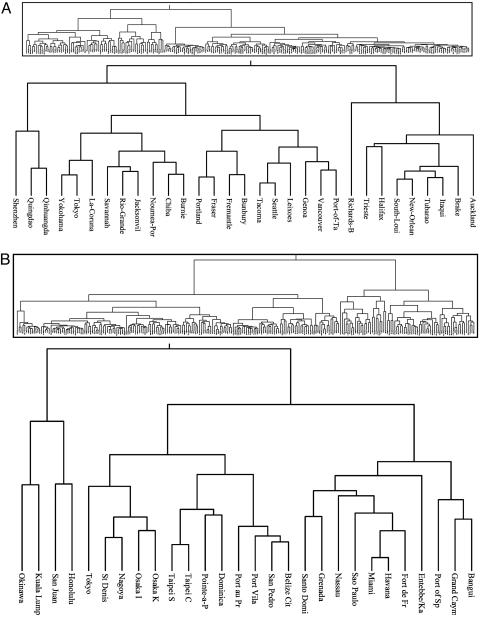
Fig. 2 displays climatic dendrograms for the global shipping network (Fig. 2A) and the global air travel network (Fig. 2B). The dendrograms are climate-based phenetic trees that represent the global transport network remapped in terms of suitability for each disease vector. The cutoff level on the seaports dendrogram that included 90% of ports within the Old World distribution of Ae. albopictus encompassed a single branch. This branch consisted of two major subbranches reflecting the tropical and temperate Ae. albopictus races (24), with the remaining 10% of ports displaying quite distinct environments (Mormugao, New-Mangalore, and Kuching). Ninety percent of airports within the preexpansion distribution of Ae. albopictus were encompassed in a single branch of the airport dendrogram, but temperate and tropical races were again distinguishable within this branch.
Fig. 2.
Climatic similarity dendrogram for the major seaports of the world (A Inset) and the major international airports of the world (B Inset), with a sample of the branches relating to the spread of Ae. allopictus in close-up on each.
From >25,000 possible sea routes and >6,000 air routes, Table 1 shows the top 10 shipping and air travel routes identified as having the highest relative invasion risk (defined in Materials and Methods) for Ae. albopictus and whether or not this species has been recorded at the arrival port. We attempted to quantify the relative importance of sea traffic volume and local climate in the establishment of Ae. albopictus by examining all 68 ports within the invasion risk dendrogram branches. Some (n = 21) ports were within the original range of Ae. albopictus, but, of the other 47, to date just over half are in regions colonized or where breeding populations have been found. Within this group of 47 ports, the average climatic distances of the invaded (n = 24) and noninvaded (n = 23) ports were identical, but average sea traffic volumes were significantly (2.43 times) greater in the former than the latter (41.84 ship visits per annum per route for invaded ports and 17.24 for noninvaded ones; t = 2.343, P = 0.0024).
Table 1.
Ae. albopictus shipping and air travel risk routes
| Rank | From | To | Ae. albopictus found? | Ae. albopictus established? |
|---|---|---|---|---|
| Shipping | ||||
| 1 | Chiba, Japan | New Orleans | Y | Y |
| 2 | Chiba, Japan | Genoa, Italy | Y | Y |
| 3 | Chiba, Japan | Fraser, Canada | N | ? |
| 4 | Chiba, Japan | Brisbane, Australia | Y | ? |
| 5 | Chiba, Japan | Auckland | Y | ? |
| 6 | Chiba, Japan | South Louisiana | Y | Y |
| 7 | Yokohama, Japan | Fraser, Canada | N | ? |
| 8 | Kobe, Japan | Fraser, Canada | N | ? |
| 9 | Chiba, Japan | Miami | Y | Y |
| 10 | Yokohama, Japan | Genoa, Italy | Y | Y |
| Air | ||||
| 1 | Tokyo Narita, Japan | Honolulu | Y | Y |
| 2 | Osaka Kansai, Japan | Honolulu | Y | Y |
| 3 | Nagoya, Japan | Honolulu | Y | Y |
| 4 | Tokyo Narita, Japan | Seattle | Y | ? |
| 5 | Tokyo Narita, Japan | Brisbane, Australia | Y | ? |
| 6 | Fukuoka, Japan | Honolulu | Y | Y |
| 7 | Seoul, South Korea | Honolulu | Y | Y |
| 8 | Tokyo Hareda, Japan | Honolulu | Y | Y |
| 9 | Taipei Chang, Taiwan | Seattle | Y | ? |
| 10 | Tokyo Narita, Japan | Portland, OR | Y | ? |
Fig. 3 shows the 10 sea traffic routes identified with the strongest risk factors for importation and establishment of each of four principal members of the An. gambiae complex. The results of the air traffic analysis for the African mosquitoes are not displayed here because the current European bias in flight routes from Africa and the climatic differences between the two continents mean that few risk routes were identified.
Fig. 3.
The 10 sea traffic routes identified with the strongest risk factors for importation and establishment of each of four principal members of the An. gambiae complex. (A) An. gambiae sensu stricto (s.s.). (B) An. arabiensis. (C) An. quadriannulatus. (D) An. melas.
Discussion
There is excellent correspondence between the predicted top 10 risk routes in Table 1 and the global spread or interception of Ae. albopictus. Three of the top 10 shipping routes run from Japan to the southeast United States, where some of the earliest breeding populations of Ae. albopictus were found and identified as originating from Japan (12). Genoa, the destination of two more routes from Japan, was one of the earliest European cities to report Ae. albopictus, which was established there by 1990 (25); the vector has since become the most important local nuisance mosquito (20). Of the remaining routes in Table 1, interceptions of Ae. albopictus in tire shipments from Japan to Australasia have been made in both Brisbane (26) and Auckland (27), but the species appears not to have established, probably an endorsement of the strict local inspection and fumigation policies (12). There is no documented evidence of invasion of Fraser, Canada, by Ae. albopictus. Given the climatic similarity of ports in Japan and Fraser and the level of shipping traffic between the two, Fraser should be considered at high risk, especially because Ae. albopictus has already been intercepted at nearby Seattle (28).
Traffic volumes within the global transport network have been proposed as important in determining biological invasion success (29–31). The role of shipping depends on a range of factors, such as ship type and destination port (32). Although it has been suggested that invasion pressure (the number of arriving propagules) is not a simple function of total ship arrivals, the present analysis suggests that the combination of traffic volumes and climatic suitability can explain a great deal of the global spread of Ae. albopictus and that, when the climatic suitabilities of sites are similar, shipping volume alone appears to determine invasion probability.
Although air travel is not implicated in the spread of Ae. albopictus, 6 of the 10 highest-risk air transport routes (Table 1) link the original range of Ae. albopictus with Honolulu in Hawaii, where this species has become established (33), whereas the remaining destinations have all reported interceptions of this mosquito. Recent work suggests that air travel was indeed the most likely route of invasion for Ae. albopictus to Hawaii (17). Details on Hawaiian ports were not available in the sea traffic database.
Although the efficiency of Ae. albopictus as a disease vector remains unclear, the An. gambiae complex of African mosquitoes are proven efficient vectors of P. falciparum malaria. Their establishment outside Africa, which has been the subject of recent speculation (34), could have devastating consequences (6). The results in Fig. 2 show that An. gambiae sensu stricto and Anopheles melas, from the hotter, wetter parts of tropical West and Central Africa, are more likely to thrive in climatically equivalent zones in Southeast Asia and Central/South America. In contrast, Anopheles arabiensis prefers drier climates, which match those of the Middle East and Canary Islands, whereas Anopheles quadriannulatus prefers relatively cooler habitats in southern Africa that are matched most closely by those elsewhere in South Africa, Mediterranean Europe, and Japan. In each case, the combination of local climate and volume of sea traffic determines the areas at greatest risk of invasion. Unlike the Ae. albopictus risk routes, however, there is no evidence of An. gambiae invasion at any of these destinations to date. This absence of invasion may be partly explained by the relatively low sea traffic volumes originating from most African ports, which are only 1/10th (An. melas and An. quadriannulatus inhabited areas), 1/20th (An. arabiensis), and 1/30th (An. gambiae sensu stricto) of the average volumes of traffic on the Ae. albopictus routes in Table 1. These figures appear to reinforce the conclusion about the importance of traffic volume, although An. gambiae sensu lato may also be less prone to move than Ae. albopictus because it is less well adapted to anthropogenic breeding sites, such as tires (3, 4), and is less frequently found in urban areas (35).
In contrast to the sea routes, which originate in Africa and have distant destinations in ecologically equivalent environments, the air travel network from the African continent is primarily to European destinations, outside of the climatic suitability limits of An. gambiae s.l. The air transport destinations most at risk are, therefore, generally to be found within the continent itself or are very close to it. Thus, we identify existing potential risks of air transfer of An. arabiensis to the Middle East and of An. quadriannulatus from Mozambique to Southern Europe (Lisbon). The air transport climate dendrogram also identifies climatic similarities to Africa in both South America and Southeast Asia, but these were not linked by any direct flights in the database used for this analysis. Recently, however, long-haul flights from Africa direct to Washington, DC; Beijing; Hong Kong; and Bangkok, Thailand, have been opened, considerably increasing the risk of introducing malaria vectors from Africa.
There are currently no confirmed examples of vector establishment arising from air travel (12), although there is circumstantial evidence that this has occurred (12, 16, 36). This mode of transport, however, may be more important in delivering the disease, rather than the vector, via infected human passengers (37, 38). Furthermore, the continuing rise in imported and airport malaria cases (39, 40), the spread of insecticide resistance in mosquitoes, and antimalarial drug-resistant malaria parasites (41, 42) are all reflections of an expanding global air transport network, increased travel to malarious countries (43), and declining aircraft disinsection (16).
Successful biological invaders can be difficult to predict given the lack of evidence of a universal trait related to invasion (44). The identification here, however, of the principal routes of movement of Ae. albopictus from thousands of possible alternatives is evidence that seaports and airports within the global transport network at greatest risk of future spread of disease vectors can be highlighted through a combination of climate and traffic data. Such an approach can, therefore, be used to prioritize monitoring, inspection, and quarantine efforts. Future challenges involve incorporating more sophisticated climatic data and extra variables, as well as information on shipping and aircraft specifics, intraspecies competition, vector infection rates, human populations at risk, seasonality, breeding site availability, disinsection, and land transport routes.
It is being increasingly recognized that future progress in the fields of emerging diseases and ecology requires a holistic perspective incorporating multiple-scale social, physical, and biological dimensions (45). The study of disease vector dispersal must encompass scales from the study of microbial pathogens and arthropod vectors to the global scale of ecological processes affected by humans. Continuing globalization will increase the relative risks of establishment of a range of vectors, as will many aspects of global climate change (46). There is no room for complacency because, whereas quarantine services must succeed every time, disease vectors need succeed only once to establish new bridgeheads from which to invade new regions or continents.
Materials and Methods
Transport Matrices and Climatic Signatures.
Estimates of the amount of traffic among the 243 most visited international seaports in 2000 (30) yielded measures of traffic volumes on a total of 29,403 routes. Data on total air passenger numbers moving between the world’s 278 principal international airports in 2000 were also obtained (OAG Worldwide). Air and sea transport matrices were produced, with each cell containing the number of air passenger or ship movements between each port. The locations of the seaports and airports were superimposed onto nine gridded global climate surfaces (47) representing means, maximums, and minimums of temperature, rainfall, and humidity, each rescaled to the same range of values. Each 10 × 10 (≈18 × 18 km at the equator) grid square covering the seaport/airport location was identified. To ensure a representative climate measure, up to eight land pixels surrounding each seaport/airport grid square were identified where possible. Any seaports/airports located on islands too small to be represented by the climate surfaces were eliminated from the analysis, reducing the sample size to 241 seaports and 259 airports. The selected grid square data from the nine climate surfaces thus formed the climatic “signature” of each port.
Hierarchical Clustering and Climatic Envelopes.
The distance in climatic space between each seaport/airport signature and every other seaport/airport signature was calculated to produce separate seaport and airport “climatic dissimilarity” matrices. Euclidean distance measures were used because there were generally too few pixels for any more sophisticated measures of environmental distance. In the few cases where they could be applied, more complex statistical measures of distance did not affect the results. To remap the global transportation network in terms of disease vector accessibility, each climatic dissimilarity matrix was subjected to hierarchical clustering by using an agglomerative algorithm (phylip v3.63, University of Washington). The clustering results were then translated into dendrograms based on centroid linkage to map the world’s seaport and airport connections by climatic similarity.
To examine how predictable was the spread of Ae. albopictus, each seaport and airport location was overlaid on a map of the mosquito’s Old World range (Fig. 1) (20, 24, 48) and classified as either inside or outside the historical distribution. Those seaports/airports within the distribution were located on the relevant dendrogram. The dendrogram branch that encompassed at least 90% of the ports was designated as defining the limits of Ae. albopictus ’ “climatic suitability envelope.” In doing this, we allowed for the fact that Ae. albopictus has both temperate (diapausing) and tropical (nondiapausing) races with distinct environmental requirements and different original geographical distributions (24).
Assuming the availability of suitable habitats and breeding sites and no interspecific competition, the chance of a vector becoming established on introduction is considered highest if the climate at any new (previously uninvaded) site is similar to that at sites within its historical distribution (49). Those seaports/airports not within the historical distribution of Ae. albopictus, but linked via a dendrogram branch within the environmental envelope, were therefore considered to be similar enough climatically for there to be a risk of vector establishment.
Where sufficient seaport and airport data existed within the predicted range of each mosquito (50), the above procedure was repeated for the malaria vectors, An. gambiae sensu stricto, An. arabiensis, An. quadriannulatus, and An. melas.
Risk Matrices.
The risks of invasion were quantified as a function of climatic similarity and traffic volume between the origin and destination seaport/airport by rescaling independently the climatic dissimilarity and sea/air traffic matrices to a range between 0 (climates of the seaports/airports in question very different; no traffic between them) and 1.0 (climates identical or traffic high). The product of these rescaled measures was taken to represent relative invasion risk for the sites on the dendrograms.
Acknowledgments
We are indebted to Briony Boon, John Drake, Carlos Guerra, Beth Purse, Sarah Randolph, Paul Reiter, Dave Smith, and Robert Snow for comments on earlier drafts of the manuscript, as well as two anonymous reviewers for valuable input. We are especially grateful to John Drake for the generous supply of the sea traffic estimates. A.J.T. and S.I.H. are funded by Research Career Development Fellowship 069045 (to S.I.H.) from the Wellcome Trust.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.McNeill W. H. Plagues and People. New York: Anchor; 1976. [Google Scholar]
- 2.Tatem A. J., Rogers D. J., Hay S. I. Adv. Parasitol. 2006;62:297–347. doi: 10.1016/S0065-308X(05)62009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillies M. T., De Meillon B. The Anopheline of Africa South of the Sahara. Johannesburg: South African Inst. for Medical Research; 1968. [Google Scholar]
- 4.Gillies M. T., Coetzee M. A Supplement to the Anopheline of Africa South of the Sahara. Johannesburg: South African Inst. for Medical Research; 1987. [Google Scholar]
- 5.Soper F. L., Wilson D. B. Anopheles gambiae in Brazil: 1930 to 1940. New York: Rockefeller Foundation; 1943. [Google Scholar]
- 6.Killeen G. F., Fillinger U., Kiche I., Gouagna L. C., Knols B. G. J. Lancet Infect. Dis. 2002;2:618–627. doi: 10.1016/s1473-3099(02)00397-3. [DOI] [PubMed] [Google Scholar]
- 7.Upham P., Thomas C., Gillingwater D., Raper D. J. Air Transport Manage. 2003;9:145–151. [Google Scholar]
- 8.Zachcial M., Heideloff C. ISL Shipping Statistics Yearbook 2003. Bremen, Germany: Inst. of Shipping Economics and Logistics; 2003. [Google Scholar]
- 9.Puth L. M., Post D. M. Ecol. Lett. 2005;8:715–721. [Google Scholar]
- 10.Elton C. S. The Ecology of Invasions by Animals and Plants. London: Methuen; 1958. [Google Scholar]
- 11.Shigesada N., Kawasaki K. Biological Invasions: Theory and Practice. Oxford: Oxford Univ. Press; 1997. [Google Scholar]
- 12.Lounibos L. P. Annu. Rev. Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- 13.Gratz N. G. Annu. Rev. Entomol. 1999;44:51–75. doi: 10.1146/annurev.ento.44.1.51. [DOI] [PubMed] [Google Scholar]
- 14.Russell R. C. Travel Med. Int. 1989;7:26–31. [Google Scholar]
- 15.Isaäcson M. Bull. W. H. O. 1989;67:737–743. [PMC free article] [PubMed] [Google Scholar]
- 16.Gratz N. G., Steffen R., Cocksedge W. Bull. W. H. O. 2000;78:995–1004. [PMC free article] [PubMed] [Google Scholar]
- 17.Kilpatrick A. M., Gluzberg Y., Burgett J., Daszak P. Ecohealth. 2004;1:205–209. [Google Scholar]
- 18.Hawley W. A. J. Am. Mosquito Control Assoc. 1988;4:1–40. [PubMed] [Google Scholar]
- 19.Gubler D. J. Lancet. 2003;3:751–752. doi: 10.1016/s1473-3099(03)00826-0. [DOI] [PubMed] [Google Scholar]
- 20.Gratz N. G. Med. Vet. Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 21.Effler P. V., Pang L., Kitsutani P., Vorndam V., Nakata M., Ayers T., Elm J., Tom T., Reiter P., Rigau-Perez J. G., et al. Emerging Infect. Dis. 2005;11:742–749. doi: 10.3201/eid1105.041063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore C. G., Mitchell C. J. Emerging Inf. Dis. 1997;3:329–334. doi: 10.3201/eid0303.970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiter P. J. Am. Mosquito Control Assoc. 1998;14:83–94. [PubMed] [Google Scholar]
- 24.Hawley W. A., Reiter P., Copeland S., Pumpuni C. B., Craig G. B. Science. 1987;236:1114–1115. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- 25.Romi R., Di Luca M., Majori G. J. Am. Mosquito Control Assoc. 1999;15:425–427. [PubMed] [Google Scholar]
- 26.Kay B. H., Ives W. A., Whelan P. I., Barker-Hudson P., Fanning I. D., Marks E. N. Med. J. Aust. 1990;153:31–34. doi: 10.5694/j.1326-5377.1990.tb125460.x. [DOI] [PubMed] [Google Scholar]
- 27.Laird M., Calder L., Thornton R. C., Syme R., Holder P. W., Mogi M. J. Am. Mosquito Control Assoc. 1994;10:14–23. [PubMed] [Google Scholar]
- 28.Centers for Disease Control. Morbid. Mortal. Wkly. Rep. 1986;35:649–651. [Google Scholar]
- 29.Levine J. M., D’Antonio C. M. Conserv. Biol. 2003;17:322–326. [Google Scholar]
- 30.Drake J. M., Lodge D. M. Proc. R. Soc. London Ser. B; 2004. pp. 575–580. [Google Scholar]
- 31.Lockwood J. L., Cassey P., Blackburn T. Trends Ecol. Evol. 2005;20:223–228. doi: 10.1016/j.tree.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Verling E., Ruiz G. M., Smith L. D., Galil B., Miller A. W., Murphy K. R. Proc. R. Soc. London Ser. B; 2005. pp. 1249–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell C. J. J. Vector Ecol. 1995;20:44–58. [Google Scholar]
- 34.Powell J. R., Coluzzi M. International Herald Tribune, Opinion. 2004. Jul 21,
- 35.Hay S. I., Guerra C. A., Tatem A. J., Atkinson P. M., Snow R. W. Nat. Rev. Microbiol. 2005;3:81–90. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mangili A., Gendreau M. A. Lancet. 2005;365:989–996. doi: 10.1016/S0140-6736(05)71089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wenzel R. P. N. Engl. J. Med. 1996;334:981–982. doi: 10.1056/NEJM199604113341509. [DOI] [PubMed] [Google Scholar]
- 38.Hufnagel L., Brockmann D., Geisel T. Proc. Natl. Acad. Sci. USA. 2004;101:15124–15129. doi: 10.1073/pnas.0308344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muentener P., Schlagenhauf P., Steffen R. Bull. W. H. O. 1999;77:560–566. [PMC free article] [PubMed] [Google Scholar]
- 40.Toovey S., Jamieson A. Travel Med. Infect. Dis. 2003;1:167–175. doi: 10.1016/j.tmaid.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Roper C., Pearce R., Nair S., Sharp B., Nosten F., Anderson T. Science. 2004;305:1124. doi: 10.1126/science.1098876. [DOI] [PubMed] [Google Scholar]
- 42.Okeke I. Science. 2004;306:2039–2040. doi: 10.1126/science.306.5704.2039c. [DOI] [PubMed] [Google Scholar]
- 43.Loutan L. Int. J. Antimicrob. Agents. 2003;21:158–163. doi: 10.1016/s0924-8579(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 44.Gewin V. PLoS Biol. 2005;3:2066–2071. [Google Scholar]
- 45.Wilcox B. A., Colwell R. R. Ecohealth. 2005;2:244–257. [Google Scholar]
- 46.Rogers D. J., Randolph S. E., Lindsay S., Thomas C. Health Effects of Climate Change in the U.K. London: U.K. Dept. of Health; 2001. [Google Scholar]
- 47.New M., Lister D., Hulme M., Makin I. Climate Res. 2002;21:1–25. [Google Scholar]
- 48.Reiter P., Darsie R. F. Mosquito News. 1984;44:396–399. [Google Scholar]
- 49.Rogers D. J., Randolph S. E. Nat. Rev. Microbiol. 2003;1:231–237. doi: 10.1038/nrmicro776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rogers D. J., Randolph S. E., Snow R. W., Hay S. I. Nature. 2002;415:710–715. doi: 10.1038/415710a. [DOI] [PMC free article] [PubMed] [Google Scholar]