Interruption of tumor dormancy by a transient angiogenic burst within the tumor microenvironment (original) (raw)
Abstract
Tumor growth is currently viewed as a phenomenon associated with neovascularization and sustained production of angiogenic factors, but whether a transient angiogenic switch may trigger tumor growth remains unclear. Here, we report that leukemia cells (MOLT-3) were poorly angiogenic and remained dormant when injected s.c. into immunodeficient mice. However, progressive growth of lymphoid tumors was invariably recorded when irradiated angiogenic cells from Kaposi’s sarcoma (KS-IMM) were locally coinjected with MOLT-3 cells or administered later. The persistence of KS-IMM cells in vivo was tracked by flow cytometry and real-time PCR analysis, and it was limited to a few days, during which angiogenesis was induced and preceded tumor growth. The engraftment of other types of poorly tumorigenic cancer cells was also greatly improved by irradiated KS-IMM cells. Moreover, short-term treatment with angiogenic factors, including basic FGF or VEGF, either given as recombinant factors or delivered by retroviral vectors, also accelerated tumor growth. These findings may emphasize that tumor angiogenesis is a process requiring a higher amount of angiogenic factors for its induction than maintenance.
Keywords: angiogenesis, basic FGF, VEGF, leukemia
Tumor dormancy is a clinically relevant phenomenon that has been observed in patients who have been treated for primary cancer and relapsed after a long disease-free period (1, 2). Hypothetically, malignant cells, shed from the primary mass, are able to remain viable to express their tumorigenic potential at a later date as a consequence of a shift in the balance between host and tumor. The nature of the shift responsible for releasing these dormant cells from growth restraints is complex and, currently, poorly understood (3, 4).
Historically, failure to induce an angiogenic response has been proposed as one of the mechanisms that may be responsible for the dormant state (5); experimental evidence in favor of this hypothesis includes the finding of a reduced blood vessel density in dormant micrometastases of some experimental tumors (6) and the observation that up-regulation of angiogenic factors such as VEGF and basic (b)FGF in poorly tumorigenic cancer cell lines can reverse their engraftment potential and lead to progressively growing tumors (7–10).
The escape of tumors from dormancy is considered to depend, at least in some experimental systems, on the so-called angiogenic switch, a discrete event that can be triggered by various signals including genetic mutations, hypoxia, and other metabolic stress, mechanical stress, and the immune/inflammatory response (11). In some cases, escape could be the result of a selection process made possible by the heterogeneity of tumors in terms of the production of angiogenic factors, which results in the emergence of angiogenic and, hence, more tumorigenic cancer variants. In this respect, angiogenic profiling of early and late breast cancer samples has shown that the latter often acquire expression of a broader angiogenic pattern (12). Alternatively, micrometastases may escape dormancy because of down-regulation of circulating angiogenesis inhibitors, as can occur after removal of a primary tumor in some experimental models (13, 14).
Generally, it is assumed that a sustained production of angiogenic factors is required for tumor growth, and it has not been elucidated whether a short-term angiogenic burst may suffice to break dormancy. This is an important issue, because, should the latter be true, it would imply that conversion to a tumorigenic phenotype does not necessarily require highly angiogenic cancer cells but may be initiated by a transient angiogenic spark of the host tissues. We attempted to elucidate this issue in an experimental model of tumor dormancy and investigated the events occurring after administration of irradiated Kaposi’s sarcoma cells as a prototype of angiogenic feeder cells.
Results
Establishment of a Model of Tumor Dormancy.
Several studies have shown that some human T cell leukemia-derived cells have reduced tumorigenicity in immunodeficient mice, associated with reduced expression of angiogenic factors (15, 16). In a preliminary experiment with a T cell leukemia-derived cell line, we implanted s.c. 5 × 106 MOLT-3 cells bilaterally in the flanks of six nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice and found that these cells were not tumorigenic: Tumors did not form in any of the mice injected over a 6-month observation period. To investigate whether injected MOLT-3 cells persisted in a dormant state, we dissected the s.c. tissue of the mice at the end of an 8-week observation period. In most cases, we could not find any evidence of occult tumors at macroscopic examination of the injection sites; however, in 22% of the injection sites, we found a small white mass in the s.c. tissue (Fig. 1A Left), which was confirmed to be formed by viable MOLT-3 cells by cytofluorimetric analysis for CD5, a surface marker expressed at high levels by these cells (Fig. 1B). Intriguingly, RT-PCR analysis of the RNA extracted from MOLT-3 cells with a panel of primers specific for several angiogenic factors and cytokines (listed in Table 1, which is published as supporting information on the PNAS web site) disclosed that they weakly expressed VEGF and were negative for all the other factors analyzed (Fig. 1C), suggesting that the lack of engraftment could depend on insufficient angiogenesis.
Fig. 1.
Engraftment of MOLT-3 cells is fostered by coadministration of Kaposi’s sarcoma cells. (A) Macroscopic appearance of the tumors formed by MOLT-3 cells in NOD/SCID mice. (Left) Picture obtained 8 weeks after the bilateral s.c. injection of 5 × 106 MOLT-3 cells. Although these cells failed to produce a visible mass in living mice, small tumors were found at autopsy in 11 of 50 sites injected with MOLT-3 cells (22%); tumor volumes ranged between 180 and 310 mm3. (Right) Picture obtained 8 weeks after bilateral s.c. injection of 5 × 106 MOLT-3 cells plus 5 × 106 irradiated KS-IMM cells; tumors were found in 56 of 56 injection sites (100%), and their volumes ranged between 1,240 and 1,780 mm3. (B) Flow-cytometric analysis of _in vitro_-cultured MOLT-3 and KS-IMM cells (in vitro) or single-cell suspensions obtained through mechanical dissociation of the tumors (ex vivo). Cells were incubated with a phycoerythrin-labeled anti-human CD5 mAb and analyzed on an EPICS-Elite cytofluorimeter. The percentage of positive cells is indicated. (C) Expression of angiogenic factors and cytokines in MOLT-3 and KS-IMM cells. PCR products were evaluated by electrophoresis on 1.5% agarose gels with ethidium bromide staining. (D) Tumor growth curves of s.c. xenotransplants in NOD/SCID mice. MOLT-3 cells (5 × 106 cells per injection, n = 6 mice per group) were injected either alone (▾) or with other cell types. The tumor volume was plotted as a function of time (weeks after transplantation). Groups (•) and (■) received MOLT-3 cells inoculated with equal numbers of irradiated KS-IMM cells or irradiated MOLT-3 cells, respectively; group (○) received MOLT-3 cells into one flank and irradiated KS-IMM cells into the contralateral flank. In a group of mice (▿), irradiated KS-IMM cells were injected 21 days after MOLT-3 cell injection. The tumor volumes of the experimental group (•) evaluated after 9 weeks differed significantly (P = 0.021, according to Wilcoxon’s paired test) compared with the (▿) experimental group and the (▾), (○), and (■) groups.
Engraftment of Lymphoid Malignant Cells Is Fostered by Coadministration of Irradiated Kaposi’s Sarcoma Cells.
To determine whether it could be possible to reverse the nontumorigenic phenotype, we coinjected equal numbers of MOLT-3 cells along with tumor cells from an _in vitro_-established cell line, termed KS-IMM, derived from a highly angiogenic Kaposi’s sarcoma (17). KS-IMM cells were found to broadly express angiogenic factors and chemokines (Fig. 1C). To avoid outgrowth of KS-IMM cells when coinjected with MOLT-3 cells, the former were irradiated at 45 Gy before injection, a dose that completely arrested their proliferation in vitro and rendered them nontumorigenic without modulating the expression of angiogenic factors (data not shown).
Coinjection of MOLT-3 and KS-IMM cells resulted in progressive tumor growth at all injection sites. To characterize these tumors, mice were killed 8 weeks after tumor-cell injection. Tumors formed by MOLT-3 cells coinjected with KS-IMM cells were, on average, 5-fold larger than dormant tumors formed by MOLT-3 cells alone [tumor volumes were 1,600 ± 110 mm3 and 260 ± 50 mm3, respectively, calculated on six samples, (P < 0.001)] and had a reddish appearance (Fig. 1A Right). Staining with anti-CD5, a marker expressed by MOLT-3 but not by KS-IMM cells, followed by FACS analysis, showed that tumors were predominantly formed by MOLT-3 cells (Fig. 1B).
Coadministration of irradiated KS-IMM cells resulted not only in the engraftment of MOLT-3 cells in 100% of the injection sites (12 of 12) but also in the progressive growth of these tumors (Fig. 1D). Furthermore, coinjection of irradiated MOLT-3 cells in a 1:1 ratio with viable MOLT-3 cells did not result in tumors (Fig. 1D), thus indicating that the type of cell coinjected did play a substantial role. This feeder effect is local; in fact, when we injected MOLT-3 cells into one flank and irradiated KS-IMM cells into the other flank, tumor growth never occurred (Fig. 1D). In four independent experiments, the s.c. injection of MOLT-3 cells into NOD/SCID mice yielded 0 of 50 progressively growing tumors, with evidence of dormant tumor foci in 11 of 50 sites in 11 different mice (22%); coinjection of MOLT-3 and irradiated KS-IMM cells yielded 56 of 56 progressive tumors in 28 different mice (χ2 square for trends = 96.144, with one degree of freedom, P < 0.0001). Interestingly, injection of MOLT-3 cells in KS-IMM-conditioned medium in both flanks of NOD/SCID mice (n = 8) was followed by engraftment of the lymphoid cells and the formation of tumors in 50% of the injection sites, thus showing a role of soluble factors produced by KS-IMM cells in the phenomenon observed.
To test whether coinjection of KS-IMM cells was strictly required to promote the engraftment of the lymphoid tumors, we first injected 5 × 106 MOLT-3 cells bilaterally into the flanks of six NOD/SCID mice, followed 3 weeks later by the administration of 5 × 106 irradiated KS-IMM cells at the same site. This process resulted in the progressive growth of tumors, which were formed by MOLT-3 cells (Fig. 1D). This finding confirmed that MOLT-3 cells injected alone remain viable and potentially tumorigenic in NOD/SCID mice for at least 3 weeks.
Finally, we observed that the s.c. growth of other poorly tumorigenic cell lines, including an Epstein–Barr virus (EBV)+ lymphoblastoid cell line and the colorectal carcinoma cell line MICOL-29, was efficiently promoted by KS-IMM cell coadministration (see Fig. 5, which is published as supporting information on the PNAS web site), thus indicating that the phenomenon observed is not restricted to MOLT-3 cells.
The Persistence of Irradiated KS-IMM Cells in Vivo Is Short-Term.
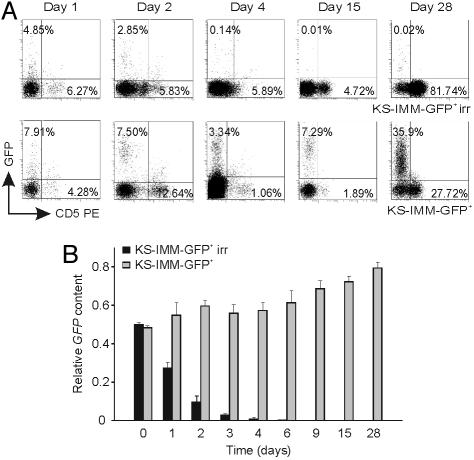
Having established that KS-IMM cells favor the engraftment of nontumorigenic cancer cells, we wondered whether these cells should be present long-term or whether they were required only for a short time period. To investigate the duration of KS-IMM cell survival in vivo, we tagged KS-IMM cells with GFP by retroviral-vector-mediated gene transfer, allowing us to measure by FACS analysis the percentage of KS-IMM cells within the tumor mass at different time points after the injection of a 1:1 mix of MOLT-3 and irradiated KS-IMM cells. This experiment indicated that irradiated GFP+ KS-IMM cells disappear rapidly from the injection site and that, 4 days later, they were virtually undetectable (Fig. 2A); on the other hand, control nonirradiated KS-IMM cells were detected at different time points after their coinjection with MOLT-3 cells, and their percentage increased with time (Fig. 2A). To rule out that transgene silencing may have masked detection of GFP-tagged KS-IMM cells, we also measured by real-time PCR analysis the amount of GFP transgene in cells recovered from the injection site at different time points. This experiment confirmed that irradiated KS-IMM cells rapidly disappear in vivo, because they were almost undetectable by PCR 4 days after injection (Fig. 2B). We thus conclude that KS-IMM cells are not required throughout the entire course of the experiment and that they likely contribute to create a tumorigenic microenvironment at the beginning of tumor growth.
Fig. 2.
The persistence of irradiated KS-IMM cells in vivo is short-term. (A) Nonirradiated and irradiated GFP+ KS-IMM cells (5 × 106) were coinjected s.c. with MOLT-3 cells in a 1:1 ratio into NOD/SCID mice. The percentage of GFP+ KS-IMM cells in the tumor mass at different time points after the injection was measured by FACS analysis and is reported on the y axis; the percentage of CD5+ MOLT-3 cells was also determined and is indicated on the x axis. (Upper) Irradiated GFP+ KS-IMM cells disappeared rapidly from the injection site, and, 4 days later, they were virtually undetectable. (Lower) Control nonirradiated KS-IMM cells were detected at different time points after their coinjection with MOLT-3 cells, and their percentage increased with time. (B) Measurement by real-time PCR analysis of the amount of the GFP gene in cells recovered from the injection site at different time points. Results are expressed as the relative GFP content in each sample, assuming the undiluted DNA from the KS-IMM GFP+ cell line as 1. Detection of the GFP gene was rapidly lost after injection of irradiated KS-IMM cells, whereas it persisted in controls receiving nonirradiated KS-IMM cells. One representative experiment of two is shown.
Evidence That Angiogenesis Is Implicated in This Phenomenon.
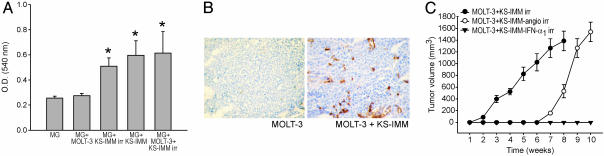
To investigate whether the broad expression of angiogenic factors in KS-IMM cells detected by RT-PCR experiments correlated with their capability of inducing angiogenesis, we performed an in vivo assay by entrapping KS-IMM cells in Matrigel pellets and analyzing the angiogenic response 6 days after implantation of the pellets in the s.c. tissue of NOD/SCID mice. This experiment showed that irradiated KS-IMM cells can induce a strong angiogenic response, in terms of both hemoglobin content of the pellets (Fig. 3A) and the number of microvessels detected in the Matrigel (Fig. 3B), in agreement with published data (18, 19). On the other hand, MOLT-3 cells induced only a minimal angiogenic response in this in vivo assay, which was increased after coinjection with KS-IMM cells (Fig. 3B).
Fig. 3.
Induction of angiogenesis by KS-IMM precedes tumor growth, and angiogenesis inhibitors counteract the engraftment of MOLT-3 cells. (A) Induction of angiogenesis in the s.c. Matrigel-pellet model by inclusion of cells in the pellet. The hemoglobin content per 100 mg of pellet is expressed as OD540. The bars indicate mean ± SD; data were pooled from two independent experiments, each with six pellets per experimental group. The asterisks mark OD significantly different compared with control group (P = 0.021, according to Wilcoxon’s paired test). (B) Immunohistochemical analysis of vascularization of Matrigel pellets isolated from MOLT-3-injected and MOLT-3 plus KS-IMM-injected NOD/SCID mice 6 days after injection. Anti-CD31 staining showed microvessels penetrating the Matrigel sponges only in MOLT-3 plus KS-IMM samples. (C) Angiogenesis inhibitors counteract KS-mediated engraftment of MOLT-3 cells. KS-IMM cells were transduced by retroviral vectors producing either murine angiostatin or murine IFNα. These cells were then irradiated and coinjected s.c. with MOLT-3 cells in a 1:1 ratio into NOD/SCID mice. Tumor growth curves of xenotransplants (5 × 106 MOLT-3 cells, n = 6 mice per group) of MOLT-3 cells in combination with nontransduced (•), angiostatin-producing (○) or IFNα-producing (▾) KS-IMM cells are shown. Angiostatin significantly delayed (P = 0.021), and IFNα completely prevented (P = 0.021), the generation of progressively growing MOLT-3 tumors.
Because angiogenesis seemed to be involved in facilitating the engraftment of MOLT-3 cells by KS-IMM cells, we sought to establish whether endogenous angiogenesis inhibitors could interfere with this process. To investigate this question, we transduced KS-IMM cells with retroviral vectors encoding either murine angiostatin or IFN-α1, two molecules with marked antiangiogenic effects (20–22). Next, we injected six NOD/SCID mice with MOLT-3 cells mixed with equal numbers of irradiated KS-IMM-angiostatin or KS-IMM-IFN-α1 cells and measured tumor growth. We found that IFN-α1-producing KS-IMM cells were not able to promote the growth of MOLT-3 cells (Fig. 3C); on the other hand, angiostatin delayed, but did not completely block, the tumor-promoting effect of KS-IMM cells (Fig. 3C). These findings reinforce the notion that KS-induced angiogenesis is a crucial event in this model.
Short-Term VEGF and bFGF Expression Suffices to Cause Escape from Tumor Dormancy.
To gain further evidence that MOLT-3 dormancy was due to lack of angiogenesis and that a transient angiogenic spike may suffice to interrupt it, we performed the following experiments. First, we injected MOLT-3 cells in Matrigel pellets embedded with prototypic angiogenic factors, including bFGF or VEGF. Progressive tumor growth was observed at all injection sites, although the angiogenic factors were administered only at the time of MOLT-3 injection (Fig. 4A). The kinetics of tumor growth after injection of MOLT-3 cells with bFGF was similar to that observed with MOLT-3 plus KS-IMM cells, whereas VEGF-induced tumors developed more slowly. Staining with anti-CD31 and microvessel density (MVD) calculation indicated 4.4 ± 1.3 and 7.6 ± 0.5 vessels per high-magnification (×400) microscopic field in established tumors formed by MOLT-3 and MOLT-3 plus KS-IMM cells, respectively (n = 6 samples per group). Tumors induced by a single injection of recombinant bFGF or VEGF behaved similarly to KS-induced tumors, and their MVD values were 8.0 ± 0.5 and 7.4 ± 0.5, respectively (Fig. 4B). Thus, MVD was significantly lower in dormant versus progressively growing tumors (P < 0.05).
Fig. 4.
Effects of angiogenic factors on MOLT-3 cell engraftment. (A) Effect of angiogenic factors on MOLT-3 tumorigenicity in NOD/SCID mice. MOLT-3 cells (5 × 106 cells per injection, n = 6 mice per group) were injected in Matrigel either without (▿) or with (▾) bFGF (100 ng/ml), VEGF (•) (100 ng/ml), or irradiated KS-IMM cells (○). The tumor volume (mm3) was plotted as a function of time (weeks after transplantation). (B) Immunohistochemical analysis of 7-week-old dormant and progressively growing tumors. Shown is a representative CD31 staining of microvessels of dormant and progressively growing MOLT-3 tumors, induced by KS-IMM cell coadministration, recombinant VEGF, or bFGF. (Scale bars, 100 μm.) (C) Tumor growth curves of retroviral-vector-transduced MOLT-3 cells in NOD/SCID mice. Parental MOLT-3 cells or its derivatives, releasing either bFGF (▾) or VEGF (•) (5 × 106 cells per injection, n = 6 mice per group), were injected either alone or in combination with irradiated MOLT-3-GFP+ (■), MOLT-3-bFGF (▿), or MOLT-3-VEGF (○) cells in a 1:1 ratio. The tumor volume was plotted as a function of time (weeks after transplantation). The tumor volumes of the experimental groups (▾), (•), (▿), and (○) evaluated 6 weeks after the beginning of the experiment significantly differed (P = 0.021) compared with the control groups. (D) Hematoxylin and eosin staining of tumors generated in the presence of sustained (MOLT-3bFGF) or short-term (MOLT-3 plus MOLT-3bFGF irr) bFGF release; one representative sample of three analyzed is shown. (Scale bars, 100 μm.)
Finally, we performed tumor-growth studies using MOLT-3 cells transduced by retroviral vectors encoding human bFGF or murine VEGF, which were shown to express these angiogenic factors (data not shown), and observed that these cells become consistently tumorigenic (Fig. 4C). Interestingly, we found that irradiated MOLT-3 cells releasing VEGF or bFGF became capable of sustaining the progressive growth of parental MOLT-3 cells (Fig. 4C). In these experiments, we observed that the tumors developed more rapidly when MOLT-3 cells constitutively producing bFGF or VEGF were injected compared with the kinetics measured in mice receiving parental MOLT-3 cells with feeder-irradiated MOLT-3-bFGF or MOLT-3-VEGF (Fig. 4C); on the other hand, the incidence of tumors did not differ among the different experimental groups. Notably, tumors whose growth was induced by short-term bFGF production contained appreciable areas of necrosis, mainly in the central part of the tumor mass, which were absent in those generated by MOLT-3 cells constitutively producing bFGF (Fig. 4D).
Interruption of Tumor Dormancy Leads to Selection of a More Aggressive MOLT-3 Subpopulation.
In previous studies, breaking dormancy was followed by selection of overtly tumorigenic and angiogenic tumor subpopulations (9, 23). To evaluate whether this process occurred in our model as well, MOLT-3 cells obtained from tumors induced by short-course bFGF were purified and injected s.c. into new recipient mice in the absence of KS-IMM feeder or angiogenic factors. We found that cells obtained from progressively growing tumors became tumorigenic, albeit with reduced growth rates compared with MOLT-3 cells constitutively producing bFGF, which were used as a positive control; on the other hand, MOLT-3 cells from dormant tumors remained nontumorigenic in new hosts (see Fig. 6_A_, which is published as supporting information on the PNAS web site). Moreover, MOLT-3 cells from progressively growing tumors induced angiogenesis in the Matrigel assay compared with parental MOLT-3 cells or MOLT-3 cells from dormant tumors (Fig. 6_B_).
Interestingly, however, selection of a more aggressive tumor subpopulation was not associated with detectable increase in the expression of angiogenic factors at the mRNA level, as indicated by microarray analysis (see Table 2, which is published as supporting information on the PNAS web site). Moreover, VEGF protein levels produced by MOLT-3 cells obtained from progressively growing tumors were not higher that those measured in parental or dormant MOLT-3 cells (Fig. 6_C_). Because progressively growing tumors contained MOLT-3 cells relatively resistant to apoptosis in vivo (Fig. 6_D_), we hypothesize that their increased survival could be one of the mechanisms involved in sustaining progressive tumor growth.
Discussion
In this study, we established a model of tumor dormancy with poorly angiogenic human leukemic cells in NOD/SCID mice. Although traditionally regarded as an angiogenesis-independent disease, there is increasing evidence that leukemia also requires angiogenesis for its growth (24–27). Furthermore, recent studies have suggested that the xenotransplantability of human leukemia and lymphoma cell lines may depend, at least in part, on their production of VEGF and their angiogenic potential (16).
This experimental model was used to test the hypothesis that a short-term perturbation in the tumor microenvironment could suffice to break tumor dormancy. Our key finding is that irradiated KS-IMM cells locally administered not only enabled nontumorigenic MOLT-3 cells to engraft very efficiently in immunodeficient hosts, but they also interrupted a state of dormancy, as shown by the formation of progressively growing tumors after administration of KS-IMM cells 3 weeks after MOLT-3 injection. Importantly, the phenomenon observed is not unique to the MOLT-3 leukemia cell line but may, rather, apply to tumor types of different histological origin.
Historically, the ability of feeder cells to improve tumor engraftment was known as the “Revesz effect” (28), and it has been subsequently confirmed by several groups in many tumor models (29–31), including leukemia (32). Moreover, recent studies have addressed the role of fibroblasts in promoting tumorigenesis by using experimental models of breast (33) and prostate cancer (31); altogether, these data show that the stromal microenvironment plays a critical role in determining the rate and incidence of tumorigenesis, yet the mechanisms behind this phenomenon remain largely unexplored.
We hypothesize that factors produced by KS-IMM cells may turn on angiogenesis in the microenvironment. The involvement of angiogenesis in this phenomenon was suggested by the dramatic induction of angiogenesis in vivo by KS-IMM cells mixed with MOLT-3 cells in a short-term assay. Moreover, our findings that irradiated MOLT-3 cells encoding VEGF or bFGF also induce tumor growth strongly support the hypothesis that short-term angiogenesis is causing escape from tumor dormancy. Finally, the observation that transient local production of angiogenesis inhibitors, including angiostatin and IFN-α1, delayed tumor growth further reinforces the importance of early events in this experimental model in determining the final outcome, i.e., dormancy versus progressive tumor growth.
One alternative explanation for the phenomenon described here was that KS-IMM cells or the angiogenic factors used to induce tumor growth may have direct effects on MOLT-3 cells. However, we could not find evidence of any in vitro effect of KS-IMM-conditioned medium or recombinant VEGF or bFGF on the proliferation of MOLT-3 cells, nor did this medium protect them from apoptosis after growth-factor deprivation (see Fig. 7, which is published as supporting information on the PNAS web site). More importantly, MOLT-3 cells pretreated with conditioned medium of KS cells or cocultured for 48 h in the presence of KS-IMM cells did not become tumorigenic, thus implying that the phenomenon observed depends mainly on the influence of KS cells on the host microenvironment. On the other hand, because MOLT-3 cells express low levels of FGFR3 by RT-PCR analysis (data not shown), it remains possible that bFGF could also exert some protective effect on tumor cells, i.e., by sustaining their initial survival in vivo before angiogenesis is induced. This possibility, among others, could explain the increased potency of bFGF compared with VEGF in this model.
Once angiogenesis is switched on, and endothelial cells have escaped from their quiescent state, low-level production of VEGF, as provided by the MOLT-3 cells in our study, may suffice to sustain progressive tumor growth. Notably, although sustained production of bFGF or VEGF led to accelerated tumor growth rates compared with a short-term pulse with the same factors, and the former tumors were more florid and lacked necrotic areas, the overall tumor incidence was comparable. These findings, although highlighting the differences between sustained and short-term angiogenesis on the promotion of tumor growth, indirectly suggest that rapid selection of highly angiogenic variants of MOLT-3 cells did not occur. The findings that growing tumors did not substantially differ from dormant tumors in terms of their angiogenic profile or the amounts of VEGF produced support this suggestion. On the other hand, in transplantation experiments, MOLT-3 cells from growing tumors were angiogenic in a Matrigel assay and maintained their tumorigenic potential, as observed in other models of tumor dormancy (9, 23). How can this apparent paradox be explained? Although full characterization of the molecular events occurring in vivo will require additional studies, it seems that MOLT-3 cells from growing tumors are relatively resistant to apoptosis, compared with dormant MOLT-3 cells; conceivably, increased in vivo survival contributes to initiation of tumor growth.
Overall, our findings imply that tumor angiogenesis could be regarded as a process requiring more angiogenic factors for its induction than maintenance, and some recent studies in murine models lend some support for this provocative hypothesis. In fact, Yoshiji et al. (8), by using a tetracycline-regulated retroviral-vector system, have found that VEGF is essential for initial, but not continued, in vivo growth of human breast cancer cells; in addition, Giavazzi et al. (10) have found that modulation of tumor angiogenesis by conditional expression of bFGF affects early, but not established, endometrial carcinoma growth. On the other hand, the observation that treatment with angiogenesis inhibitors (34) or VEGF withdrawal (35) have dramatic consequences on the viability of established tumors indicates that tumors are heterogeneous, and some neoplasms remain dependent on sustained angiogenesis even at advanced stages.
In conclusion, this hypothesis could explain, in at least some cases, how tumor dormancy can be interrupted: A transient change in the microenvironment, such as that provided by local inflammation, would suffice for tumor cells with even low angiogenic potential to escape from dormancy and give rise to progressively growing lesions.
Materials and Methods
Cell Lines and Culture Conditions.
The human T lymphoblastic cell line MOLT-3 was purchased from American Type Culture Collection and grown in RPMI medium 1640 supplemented with 10% FCS and 1% l-glutamine (all from Life Technologies, Grand Island, NY). The Kaposi’s sarcoma-derived cell line KS-IMM (18) was grown in DMEM (Sigma) supplemented with 10% FCS and 1% l-glutamine.
Tumorigenicity Assay.
NOD/SCID mice were purchased from Charles River Breeding Laboratories. Procedures involving animals and their care conformed with institutional guidelines that comply with national and international laws and policies (European Economic Community Council Directive 86/609, OJ L 358, 12 December, 1987). Seven- to 9-week-old female mice were used for all experiments. For tumor establishment, exponentially growing MOLT-3 cells were washed and resuspended in serum-free RPMI medium 1640 at a concentration of 2 × 107 cells per ml. Subconfluent KS-IMM cells were dispersed with 0.1% trypsin/EDTA (Life Technologies, Rockville, MD) and washed once with medium containing 10% FCS to remove trypsin; the cells were then irradiated at 45 Gy, washed, and resuspended as above. The mice were injected s.c. with 5 × 106 cells per injection into both dorsolateral flanks; in some experimental groups, the mice received a total of 10 × 106 cells (5 × 106 tumor cells plus 5 × 106 irradiated KS-IMM cells) per injection in a 250-μl total volume. The resulting tumors were inspected twice weekly and measured by caliper; tumor volume was calculated with the use of the following formula: tumor volume (mm3) = L × l2 × 0.5, where L is the longest diameter, l is the shortest diameter, and 0.5 is a constant to calculate the volume of an ellipsoid. At the end of the experiment, the mice were killed by cervical dislocation. The tumors were harvested by dissection and either snap-frozen or fixed in formalin and embedded in paraffin for immunohistochemistry or other analyses.
Details about cytofluorimetric analysis, RNA extraction, and RT-PCR amplification, real-time PCR analysis, in vitro proliferation/apoptosis assays, transduction of MOLT-3 and KS-IMM cells with retroviral vectors, microarray analysis, and VEGF ELISA are provided in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Angiogenesis Assay.
Matrigel was used as described in ref. 17. Briefly, MOLT-3 or irradiated KS-IMM cells (1 × 106 cells in 150 μl) were added to liquid Matrigel at 4°C. The mixture (final volume 300 μl) was slowly injected s.c. into the flanks of NOD/SCID mice, where the Matrigel immediately polymerizes to form a solid gel. Six days later, pellets were recovered, weighed, and either snap-frozen for histologic and immunohistochemical analysis or minced and diluted in water, and the hemoglobin content measured with Drabkin reagent (Sigma). The OD values of each sample were normalized to 100 mg of recovered gel. In a set of experiments, recombinant human VEGF or bFGF (100 ng/ml; PeproTech, London) were added to Matrigel-MOLT-3 suspensions and inoculated into NOD/SCID mice (300 μl per flank) to investigate the effects of recombinant angiogenic factors on MOLT-3 angiogenesis and in vivo growth.
Immunohistochemistry.
Six-micrometer-thick sections were cut from frozen samples, and the sections were blocked with irrelevant serum, followed by incubation with primary antibody. Immunostaining was performed by using the avidin–biotin–peroxidase complex technique (Vector Laboratories) and 3–3′ diaminobenzidine as chromogen (DAKO). Sections were then lightly counterstained with Mayer’s hematoxylin. The primary antibodies used were a rat anti-mouse CD31 mAb (Clone MEC 13.3, Pharmingen; 1: 50 final dilution), which recognizes an epitope on the surface of endothelial cells, and a rabbit anti-cleaved caspase-3 mAb (Cell Signaling Technology, Beverly, MA). Parallel negative controls were run, replacing the primary antibody with PBS. Microvessel density was quantified by screening the CD31-stained sections for the areas of highest vascularity, as described in ref. 36. Five representative fields for each tumor were counted.
Statistical Methods.
Data were managed by the program statgraphics version 2.6 (Statgraphics, Rockville, MD). The nonparametric Wilcoxon’s test was used to compare quantitative variables and tumor growth. The χ2 for trends was used to compare outcomes between dormant and growing tumors.
Supplementary Material
Supporting Information
Acknowledgments
We thank P. Gallo for artwork preparation; A. Sebelin, E. Favaro, S. Mandruzzato, and L. Persano for technical support; A. Albini (Genoa, Italy) for providing the KS-IMM cell line; G. Parmiani and P. Dalerba (Milan, Italy) for providing the MICOL-29 cells; and M. Presta (Brescia, Italy) for providing the pZip-neo-FGF2 and pTET-VEGF plasmids and for critical reading of the manuscript. This work was supported, in part, by grants from the Italian Association for Research on Cancer (AIRC); the Italian Foundation for Research on Cancer (FIRC); Ministero dell’Istruzione, dell’Università e della Ricerca 60% and 40%; Ministero della Salute, Ricerca Finalizzata 2002; and the Investment Fund for Basic Research (FIRB), Fondazione Cassa di Risparmio di Padova e Rovigo, Ministry of Health (AIDS Project).
Abbreviations
bFGF
basic FGF
NOD
nonobese diabetic
SCID
severe combined immunodeficient.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Demicheli R., Terenziani M., Valagussa P., Moliterni A., Zambetti M., Bonadonna G. J. Natl. Cancer Inst. 1994;86:45–48. doi: 10.1093/jnci/86.1.45. [DOI] [PubMed] [Google Scholar]
- 2.Uhr J. W., Scheuermann R. H., Street N. E., Vitetta E. S. Nat. Med. 1997;3:505–509. doi: 10.1038/nm0597-505. [DOI] [PubMed] [Google Scholar]
- 3.Naumov G. N., MacDonald I. C., Chambers A. F., Groom A. C. Semin. Cancer Biol. 2001;11:271–276. doi: 10.1006/scbi.2001.0382. [DOI] [PubMed] [Google Scholar]
- 4.Hart I. R. J. Pathol. 1999;187:91–94. doi: 10.1002/(SICI)1096-9896(199901)187:1<91::AID-PATH234>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Gimbrone M. A., Jr, Leapman S. B., Cotran R. S., Folkman J. J. Exp. Med. 1972;136:261–276. doi: 10.1084/jem.136.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmgren L., O’Reilly M. S., Folkman J. Nat. Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 7.Rak J., Mitsuhashi Y., Bayko L., Filmus J., Shirasawa S., Sasazuki T., Kerbel R. S. Cancer Res. 1995;55:4575–4580. [PubMed] [Google Scholar]
- 8.Yoshiji H., Harris S. R., Thorgeirsson U. P. Cancer Res. 1997;57:3924–3928. [PubMed] [Google Scholar]
- 9.Bayko L., Rak J., Man S., Bicknell R., Ferrara N., Kerbel R. S. Angiogenesis. 1998;2:203–217. doi: 10.1023/A:1009275307663. [DOI] [PubMed] [Google Scholar]
- 10.Giavazzi R., Giuliani R., Coltrini D., Bani M. R., Ferri C., Sennino B., Tosatti M. P., Stoppacciaro A., Presta M. Cancer Res. 2001;61:309–317. [PubMed] [Google Scholar]
- 11.Carmeliet P., Jain R. K. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 12.Relf M., LeJeune S., Scott P. A., Fox S., Smith K., Leek R., Moghaddam A., Whitehouse R., Bicknell R., Harris A. L. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- 13.O’Reilly M. S., Holmgren L., Shing Y., Chen C., Rosenthal R. A., Cao Y., Moses M., Lane W. S., Sage E. H., Folkman J. Cold Spring Harbor Symp. Quant. Biol. 1994;59:471–482. doi: 10.1101/sqb.1994.059.01.052. [DOI] [PubMed] [Google Scholar]
- 14.Guba M., Cernaianu G., Koehl G., Geissler E. K., Jauch K. W., Anthuber M., Falk W., Steinbauer M. Cancer Res. 2001;61:5575–5579. [PubMed] [Google Scholar]
- 15.Hudson W. A., Li Q., Le C., Kersey J. H. Leukemia. 1998;12:2029–2033. doi: 10.1038/sj.leu.2401236. [DOI] [PubMed] [Google Scholar]
- 16.Fusetti L., Pruneri G., Gobbi A., Rabascio C., Carboni N., Peccatori F., Martinelli G., Bertolini F. Cancer Res. 2000;60:2527–2534. [PubMed] [Google Scholar]
- 17.Albini A., Fontanini G., Masiello L., Tacchetti C., Bigini D., Luzzi P., Noonan D. M., Stetler-Stevenson W. G. AIDS. 1994;8:1237–1244. doi: 10.1097/00002030-199409000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Albini A., Paglieri I., Orengo G., Carlone S., Aluigi M. G., DeMarchi R., Matteucci C., Mantovani A., Carozzi F., Donini S., Benelli R. AIDS. 1997;11:713–721. doi: 10.1097/00002030-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Albini A., Marchisone C., Del Grosso F., Benelli R., Masiello L., Tacchetti C., Bono M., Ferrantini M., Rozera C., Truini M., et al. Am. J. Pathol. 2000;156:1381–1393. doi: 10.1016/S0002-9440(10)65007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Indraccolo S., Morini M., Gola E., Carrozzino F., Habeler W., Minghelli S., Santi L., Chieco-Bianchi L., Cao Y., Albini A., Noonan D. M. Cancer Res. 2001;61:5441–5446. [PubMed] [Google Scholar]
- 21.Indraccolo S., Gola E., Rosato A., Minuzzo S., Habeler W., Tisato V., Roni V., Esposito G., Morini M., Albini A., et al. Gene Ther. 2002;9:867–878. doi: 10.1038/sj.gt.3301703. [DOI] [PubMed] [Google Scholar]
- 22.Folkman J. Cancer Biol. Ther. 2003;2:S127–S133. [PubMed] [Google Scholar]
- 23.Kobayashi H., Man S., MacDougall J. R., Graham C. H., Lu C., Kerbel R. S. Am. J. Pathol. 1994;144:776–786. [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Atayde A. R., Sallan S. E., Tedrow U., Connors S., Allred E., Folkman J. Am. J. Pathol. 1997;150:815–821. [PMC free article] [PubMed] [Google Scholar]
- 25.Fiedler W., Graeven U., Ergun S., Verago S., Kilic N., Stockschlader M., Hossfeld D. K. Blood. 1997;89:1870–1875. [PubMed] [Google Scholar]
- 26.Bellamy W. T., Richter L., Frutiger Y., Grogan T. M. Cancer Res. 1999;59:728–733. [PubMed] [Google Scholar]
- 27.Kerbel R., Folkman J. Nat. Rev. Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 28.Revesz L. Nature. 1956;178:1391–1392. doi: 10.1038/1781391a0. [DOI] [PubMed] [Google Scholar]
- 29.Seelig K. J., Revesz L. Br. J. Cancer. 1960;14:126–138. doi: 10.1038/bjc.1960.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters L. J., Hewitt H. B. Br. J. Radiol. 1974;47:739–740. [PubMed] [Google Scholar]
- 31.Olumi A. F., Grossfeld G. D., Hayward S. W., Carroll P. R., Tlsty T. D., Cunha G. R. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziegler H. W., Frizzera G., Bach F. H. J. Natl. Cancer Inst. 1982;68:15–18. [PubMed] [Google Scholar]
- 33.Orimo A., Gupta P. B., Sgroi D. C., Arenzana-Seisdedos F., Delaunay T., Naeem R., Carey V. J., Richardson A. L., Weinberg R. A. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 34.Boehm T., Folkman J., Browder T., O’Reilly M. S. Nature. 1997;390:404–407. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- 35.Benjamin L. E., Keshet E. Proc. Natl. Acad. Sci. USA. 1997;94:8761–8766. doi: 10.1073/pnas.94.16.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weidner N., Semple J. P., Welch W. R., Folkman J. N. Engl. J. Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information