Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis (original) (raw)
Abstract
Metastasis is a frequent and lethal complication of cancer. Vascular endothelial growth factor-C (VEGF-C) is a recently described lymphangiogenic factor. Increased expression of VEGF-C in primary tumours correlates with dissemination of tumour cells to regional lymph nodes. However, a direct role for VEGF-C in tumour lymphangiogenesis and subsequent metastasis has yet to be demonstrated. Here we report the establishment of transgenic mice in which VEGF-C expression, driven by the rat insulin promoter (Rip), is targeted to β-cells of the endocrine pancreas. In contrast to wild-type mice, which lack peri-insular lymphatics, RipVEGF-C transgenics develop an extensive network of lymphatics around the islets of Langerhans. These mice were crossed with Rip1Tag2 mice, which develop pancreatic β-cell tumours that are neither lymphangiogenic nor metastatic. Double-transgenic mice formed tumours surrounded by well developed lymphatics, which frequently contained tumour cell masses of β-cell origin. These mice frequently developed pancreatic lymph node metastases. Our findings demonstrate that VEGF-C-induced lymphangiogenesis mediates tumour cell dissemination and the formation of lymph node metastases.
Keywords: islet of Langerhans/lymphangiogenesis/tumour metastasis/VEGF-C
Introduction
The metastatic spread of tumour cells is responsible for the majority of cancer deaths. Tumour cell dissemination is mediated by a number of mechanisms, including local tissue invasion, lymphatic spread, haematogenous spread, or direct seeding of body cavities or surfaces. Clinical and pathological observations have long suggested that, for many tumours, the most common pathway of initial dissemination is via lymphatics, with patterns of spread via afferent vessels following routes of natural drainage (reviewed by Fidler, 1997; Cotran et al., 1999; Sleeman, 2000). However, the lymphatic system has traditionally been overshadowed by the greater emphasis placed on the blood vascular system. This has been due in part to the absence of suitable markers that distinguish lymphatic from blood vascular endothelium, and to the lack of identification of lymphatic-specific growth factors.
In recent years, these limitations have been relieved by the discovery of a small number of potential lymphatic-specific markers (reviewed by Jackson, 2001). These include: LYVE-1, a lymphatic endothelial receptor for the extracellular matrix/lymphatic fluid mucopolysaccharide hyaluronan (Banerji et al., 1999); Prox-1, a homeobox gene product involved in regulating early lymphatic development (Wigle and Oliver, 1999); podoplanin, a glomerular podocyte membrane mucoprotein (Breiteneder-Geleff et al., 1999); and the vascular endothelial growth factor receptor-3 (VEGFR-3), a transmembrane tyrosine kinase receptor for the lymphatic endothelial growth factors vascular endothelial growth factor-C (VEGF-C) and VEGF-D (reviewed in Veikkola et al., 2000). Targeted inactivation of the VEGFR-3 gene has revealed that it also plays an important role in the development of the early blood vascular system, prior to the emergence of lymphatic vessels (Dumont et al., 1998).
VEGF-C, the first ligand to be discovered for VEGFR-3 (Joukov et al., 1996; Lee et al., 1996), is a member of the VEGF family of polypeptide growth factors, which comprises VEGF-A, -B, -C, -D and orf virus VEGFs (or VEGF-E) (reviewed in Eriksson and Alitalo, 1999; Ferrara, 1999). VEGF-C is produced in a pre-pro-peptide form that is proteolytically processed to a mature homodimer of ∼40 kDa (Joukov et al., 1997). Proteolytic processing increases the affinity of VEGF-C for VEGFR-3 some 400-fold, and also enables it to bind to and activate VEGFR-2 (Joukov et al., 1997). Based on its expression profile and its binding to VEGFR-3, VEGF-C has been implicated in the development of the lymphatic system (Kukk et al., 1996; Lymboussaki et al., 1999). In addition, transgenic overexpression of VEGF-C using the keratin 14 promoter induces lymphatic vessel enlargement/dilatation in the skin (Jeltsch et al., 1997), and recombinant VEGF-C induces lymphangiogenesis in the chick chorioallantoic membrane (Oh et al., 1997). The capacity of VEGF-C to bind to and activate VEGFR-2 may partially explain why it also stimulates angiogenesis under certain experimental conditions (Cao et al., 1998; Witzenbichler et al., 1998).
In contrast to VEGF-A, whose crucial role in tumour angiogenesis is well established (Ferrara, 1999), very little is known about the function of VEGF-C in tumour formation and/or progression. Nonetheless, a correlation between VEGF-C expression, tumour lymphangiogenesis and the formation of metastases in regional lymph nodes has recently been described. Thus, levels of VEGF-C in primary tumours are significantly correlated with lymph node metastases in a variety of cancers, including thyroid, prostate, gastric, colorectal and lung (Bunone et al., 1999; Tsurusaki et al., 1999; Yonemura et al., 1999; Akagi et al., 2000; Niki et al., 2000; Ohta et al., 2000). One study has described a strong correlation between lymphatic vessel density and VEGF-C expression (Ohta et al., 1999). However, in this study, no correlation was observed between lymphatic vessel density and lymph node metastases. Despite the continuing accumulation of correlative clinical data, a functional role for VEGF-C in tumour lymphangiogenesis and/or lymphatic enlargement, and its role in tumour cell dissemination, have yet to be demonstrated directly.
In order to test the hypothesis that VEGF-C-induced lymphangiogenesis can promote tumour metastasis, we generated transgenic mouse lines in which VEGF-C expression, driven by the rat insulin promoter, is targeted to β-cells of the islets of Langerhans, and tested their capacity to form metastases by crossing them with the Rip1Tag2 transgenic line, a transgenic mouse model of non-metastatic β-cell carcinogenesis (Hanahan, 1985).
Results
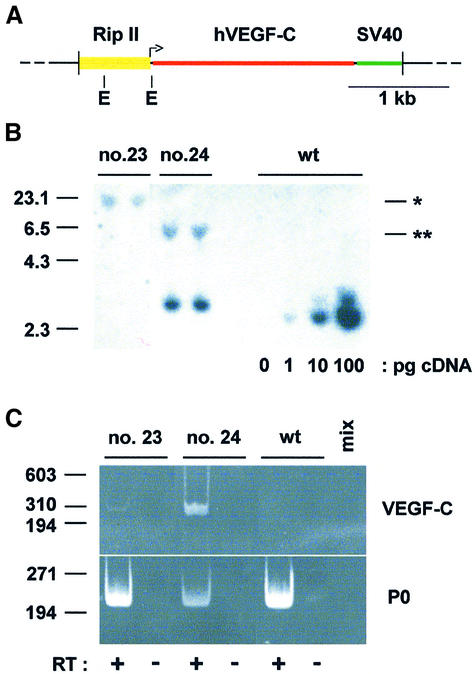
A full-length human VEGF-C cDNA was cloned between the rat insulin II gene promoter (Rip) and the SV40 small T antigen intron and polyadenylation signal (Figure 1A). Two founder (F0) mice (designated nos 23 and 24) were identified, which were capable of germline transmission. RipVEGF-C transgenic mice were viable, similar in size to wild-type littermates, normoglycaemic and fertile. Mice derived from F0 no. 23 had a single copy of the transgene, whereas those derived from F0 no. 24 had four to six copies integrated in a head-to-tail array (Figure 1B and data not shown). Pancreas-specific expression of the VEGF-C transgene was confirmed by reverse transcription–polymerase chain reaction (RT– PCR) screening of several organs, using oligonucleotide primers designed to amplify VEGF-C cDNA of human but not of mouse origin (Figure 1C and data not shown). In contrast to wild-type littermates (Figure 2A), immunohistochemical analysis of the pancreata of RipVEGF-C transgenic mice revealed strong VEGF-C staining in the majority of islet cells (Figure 2B), as would be expected from the relative abundance of insulin-expressing β-cells in islets (∼80%).
Fig. 1. Molecular characterization of RipVEGF-C transgenic mice. (A) The transgene was constructed by cloning the complete human VEGF-C cDNA (nucleotides 1–1997; DDBJ/EMBL/GenBank accession No. X94216) between the ∼695 bp _Bam_HI–_Xba_I fragment of Rip (Hanahan, 1985) and the SV40 small T antigen intron and polyadenylation signal. The L-shaped arrow indicates insulin gene transcription initiation. E, _Eco_RI restriction sites. (B) Ten micrograms of genomic DNA from two RipVEGF-C transgenic mice from families 23 and 24 or from a wild-type littermate (wt) (the latter spiked with the RipVEGF-C transcriptional unit as indicated in picograms) were digested with _Eco_RI and analysed by Southern blotting using the SV40 moiety of the transgene as a probe. Single or double asterisks indicate the 3′ end of the insertion site in family 23 and 24, respectively. Markers on the left indicate kilobases. (C) Reverse transcription products from oligo dT-primed total RNAs from pancreata of RipVEGF-C transgenic mice from families 23 and 24 or from wild-type mice (wt) were analysed by PCR using hVEGF-C specific primers or acidic ribosomal phosphoprotein P0 (P0) primers. Where indicated, RT was omitted, or PCR mix alone (mix) was analysed. Markers on the left indicate base pairs.
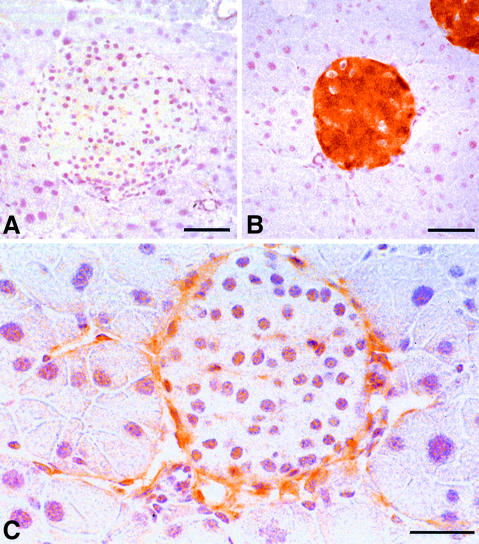
Fig. 2. Expression of VEGF-C and VEGFR-3 in RipVEGF-C transgenic mice. Immunohistochemistry for (A and B) VEGF-C and (C) VEGFR-3. (A) Ten-month-old female wild-type-littermate; (B and C) 11-month-old female RipVEGF-C mouse, family 24. (B) and (C) are serial sections. Bar: (A and B) 50 µm; (C) 30 µm.
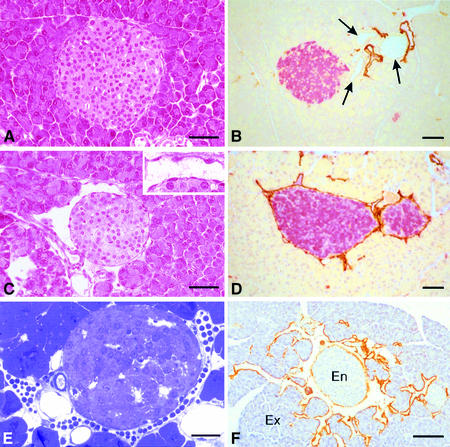
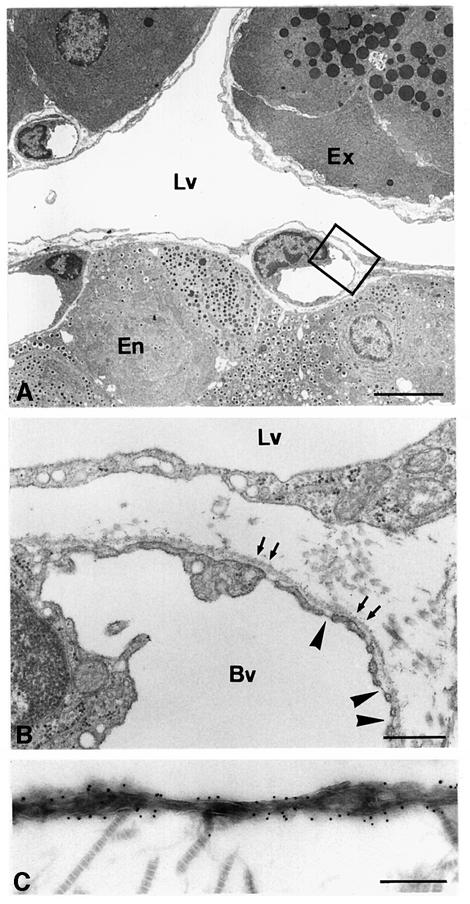
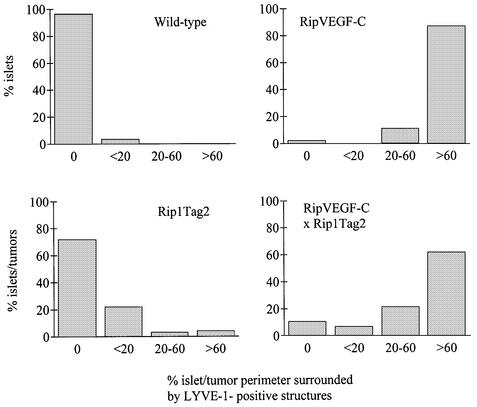
Striking morphological differences were observed between transgenic and wild-type pancreata. Clearly demarcated spaces, lined by a single layer of flattened cells, which frequently contained lymphocytes rather than red blood cells, were observed around the majority of transgenic islets (Figure 3C and E). These morphological features were observed in all RipVEGF-C mice killed between E15.5 and 18 months. These structures, which were never observed around islets of wild-type mice (Figure 3A), were identified as lymphatic vessels on the basis of three separate criteria. First, they were lined by endothelial cells that were immunoreactive for VEGFR-3 (Figure 2C). Weak VEGFR-3 immunostaining was also observed within some islets of both wild-type and transgenic mice (Figure 2C and data not shown). Secondly, as revealed by transmission electron microscopy (TEM), the basement membrane of these vessels was either absent or discontinuous, and the endothelial cells were devoid of the characteristic fenestrations found in contiguous capillary blood vessels—both hallmarks of lymphatic endothelium (Figure 4A and B). Thirdly, as revealed by immunohistochemistry and immuno-EM, they stained with an antibody to the novel lymphatic endothelial marker LYVE-1 (Banerji et al., 1999; Prevo et al., 2001) on both their luminal and abluminal surfaces (Figure 3D, F and 4C), similar to lymphatic vessels in normal wild-type mouse tissues (Prevo et al., 2001). A quantitative analysis of lymphatic vessel density in RipVEGF-C mice, based on LYVE-1 immunoreactivity, revealed that 98% of islets were in intimate association with LYVE-1-positive lymphatics (Figures 3D, F and 5; Table I), which contrasts with the finding that only 4% of islets had closely apposed lymphatics in wild-type littermates (Figures 3B and 5; Table I). In some RipVEGF-C mice, peri-insular lymphatics were particularly well developed, and extended between the lobules of exocrine acini (Figure 3F). Previous studies in the rodent pancreas have revealed the absence of an association between lymphatics and islets of Langerhans (Bertelli et al., 1993; Navas et al., 1995; Ji and Kato, 1997; reviewed by O’Morchoe, 1997), which is consistent with our LYVE-1 and VEGFR-3 immunohistochemical data. From these data we conclude that RipVEGF-C mice form lymphatics de novo (lymphangiogenesis). In contrast, mice expressing VEGF-C in the skin under the control of the keratin 14 promoter display an increase both in the size and number of lymphatic vessels (Jeltsch et al., 1997; T.Veikkola, unpublished data).
Fig. 3. Histological analysis of wild-type and RipVEGF-C mice. (A and C) Haematoxylin and eosin staining; (B, D and F) immunohistochemistry for LYVE-1 (DAB brown) and insulin (B and D) (fast red); (E) semithin section stained with methylene blue. (A) Twelve-month-old male wild-type littermate; (B) 2-month-old male wild-type littermate; (C) 12-month-old male RipVEGF-C mouse, family 24; (D) 2-month-old male RipVEGF-C mouse, family 24; (E) 11-month-old male RipVEGF-C mouse, family 24; (F) 10-month-old male RipVEGF-C mouse, family 24. Arrows in (B) indicate ducts; En, endocrine; Ex, exocrine. Bar: (A–D) 50 µm; (E) 25 µm; (F) 100 µm.
Fig. 4. Ultrastructural analysis of RipVEGF-C transgenic mouse pancreas. (A and B) TEM of the pancreas from a 2-month-old male RipVEGF-C transgenic mouse from family 24. (B) is a higher magnification of the area delimited in (A). Arrows in (B) indicate the basement membrane surrounding a capillary blood vessel (Bv). Note the absence of a basement membrane along the basal surface of the lymphatic endothelium. Arrowheads indicate endothelial fenestrations in the capillary blood vessel. Note the absence of fenestrations in the lymphatic endothelium. (C) Immunoelectron microscopy analysis of LYVE-1 distribution on lymphatic endothelium in the same animal as shown in (A) and (B). Cross-striated collagen fibrils are on the abluminal side of the vessel. Lv, lymphatic vessel; Ex, exocrine tissue; En, endocrine tissue. Bar: (A) 5 µm; (B) 500 nm; (C) 250 nm.
Fig. 5. Quantitation of lymphatic vessel density and disposition in wild-type, single- (RipVEGF-C and Rip1Tag2) and double- (RipVEGF-C/Rip1Tag2) transgenic mice. Lymphatic vessels were identified by LYVE-1 immunoreactivity, and the per cent islet/insulinoma perimeter surrounded by LYVE-1-positive structures was determined. For wild type, 185 islets were analysed from five mice; for RipVEGF-C, 99 islets were analysed from five mice; for Rip1Tag2, 232 islets were analysed from seven mice; for RipVEGF-C/Rip1Tag2, 322 islets were analysed from eight mice.
Table I. Blood vascular and lymphatic phenotypes in wild-type versus RipVEGF-C mice.
| | Wild type | RipVEGF-C | | | ------------------------------ | ------------ | ----------- | | MECA-32-positive | 13.2 ± 3.6 | 13.6 ± 4.5 | | vascular profiles/10 000 µm2 | (n = 30) | (n = 30) | | % islet volume occupied by | 13.0 ± 3.4 | 12.9 ± 4.4 | | MECA-32-positive blood vessels | (n = 30) | (n = 30) | | % islets with tightly apposed | 3.6 | 98 | | LYVE-1-positive lymphatics | (n = 185) | (n = 99) |
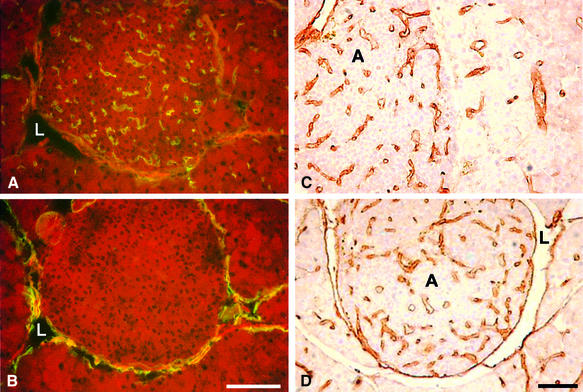
Vascular density and the percentage islet volume occupied by blood vessels were determined by immunohistochemistry with the vascular endothelial-specific antibody MECA-32 (Hallmann et al., 1995). In contrast to LYVE-1, which stains lymphatic vessels (Banerji et al., 1999), MECA-32 only stains blood vessels (Figure 6A and B). No differences were observed between wild-type and RipVEGF-C transgenic islets (Table I). Similar results were obtained using the pan-endothelial marker CD31 (data not shown), indicating that forced expression of VEGF-C did not promote vascular angiogenesis.
Fig. 6. MECA-32 and CD31 immunohistochemistry in wild-type and transgenic islets. (A) MECA-32 and (B) LYVE-1 immunolabelling in consecutive frozen sections of a RipVEGF-C islet. CD31 immunolabelling of adenomas from 14-week-old (C) Rip1Tag2 and (D) double-transgenic RipVEGF-C × Rip1Tag2 mice. L, lymphatic vessel; A, adenoma. Bar: 100 µm.
No alterations were observed in the normal proportion or spatial distribution of α, β, δ or PP cells in the islets of RipVEGF-C mice when compared with wild-type littermates, as assessed by immunohistochemical analysis for glucagon, insulin, somatostatin and pancreatic polypeptide, respectively (data not shown).
We thus conclude that increased expression of VEGF-C in the β-cells of RipVEGF-C transgenic mice specifically promotes lymphangiogenesis, which is observed around but not within the islets of Langerhans, and which does not affect islet architecture or cellular composition.
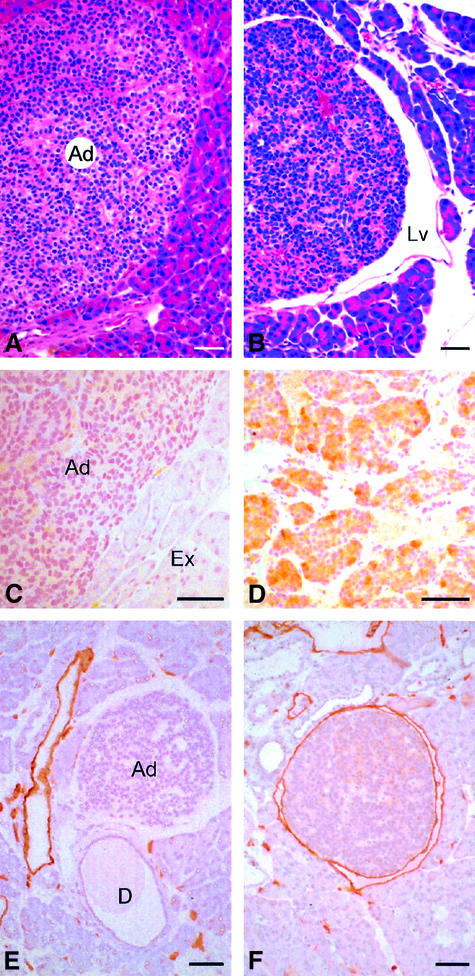
To assess the role of VEGF-C-induced lymphangiogenesis in tumour metastasis, we crossed RipVEGF-C mice with Rip1Tag2 mice, a well characterized transgenic model of non-metastatic β-cell carcinogenesis (Hanahan, 1985; Perl et al., 1998, 1999) that displays morphological features typical of human pancreatic β-cell tumours (Holm et al., 1988). Of note, β-cell tumours that develop in Rip1Tag2 mice are capable of local invasion, but do not induce extensive lymphangiogenesis (Figure 7A and E). When RipVEGF-C mice were crossed with Rip1Tag2 mice, the resulting double-transgenic animals displayed a modest but significant increase in tumour incidence (defined as the number of tumours per pancreas having a size >0.5 mm) compared with single Rip1Tag2 transgenic animals, yet neither tumour volume (expressed in cubic millimetres) nor the ratio of benign adenoma to invasive carcinoma was altered (Table II). RipVEGF-C × Rip1Tag2 double-transgenic tumours consisted almost exclusively of insulin-producing β cells (data not shown), similar to what has been reported for Rip1Tag2 single-transgenic tumours (Hanahan, 1985). Detailed immunohistochemical analyses revealed heterogeneous VEGF-C immunoreactivity in the majority of double-transgenic tumour cells, whereas VEGF-C immunoreactivity was absent from Rip1Tag2 tumours (Figure 7C and D). Furthermore, there was an increase in the number of VEGFR-3 immunoreactive peri-tumoural lymphatic vessels in double-transgenic mice compared with single-transgenic mice (data not shown). The extent of this new lymphatic vessel formation was demonstrated dramatically by LYVE-1 immunostaining, which revealed that insulinomas were either partly surrounded by or fully enclosed within such vessels (Figure 7F). Indeed, quantitative analyses confirmed that 62% of insulinomas/islets in double-transgenic animals had closely apposed lymphatics encompassing most or all of the insulinoma/islet circumference (Figures 5, 7B and F). In contrast, only 29% of insulinomas/islets in the Rip1Tag2 mice had associated lymphatics (Figures 5, 7A and E). The lower levels of lymphangiogenesis in the latter tumours correlate with the finding that endogenous VEGF-C was not upregulated during tumourigenesis in Rip1Tag2 single-transgenic mice (Figure 7C and data not shown). In small adenomas of double-transgenic mice, LYVE-1-positive lymphatics surrounded the tumour tissue without penetrating it (Figure 7F). In contrast, in large adenomas and carcinomas, in addition to surrounding the tumours, lymphatics (positive for LYVE-1 and VEGFR-3) were infrequently observed in the peripheral rim of the tumour, but never extended into the body of the tumour itself (data not shown).
Fig. 7. Histological analysis of RipVEGF-C/Rip1Tag2 double-transgenic mice. (A and B) Haematoxylin and eosin staining; (C and D) anti-VEGF-C immunohistochemistry; (E and F) anti-LYVE-1 immunohistochemistry. (A, C and E) Rip1Tag2 single-transgenic mice. (B, D and F) RipVEGF-C/Rip1Tag2 double-transgenic mice. Ad, adenoma; Lv, lymphatic vessel; Ex, exocrine tissue; D, duct. The white space around the adenoma in (E) is artefactual. All mice were killed at 14 weeks; double-transgenic mice are from RipVEGF-C family 23. Bar: 50 µm.
Table II. Tumour phenotypes and metastasis in Rip1Tag2 versus RipVEGF-C × Rip1Tag2 mice.
| | Rip1Tag2 | RipVEGF-C × Rip1Tag2 | | | ---------------------- | -------------------- | ----------- | | Tumour incidencea | 5.4 ± 1.4 | 12.3 ± 2.9 | | (per mouse) | (n = 27) | (n = 33) | | Tumour volumeb | 56.5 ± 35.8 | 38.3 ± 29.1 | | (per mouse) | (n = 27) | (n = 33) | | Adenoma | 36% | 46% | | | (n = 17) | (n = 27) | | | Carcinoma | 64% | 54% | | | (n = 17) | (n = 27) | | | Lymph node metastasesc | 0% | 37% | | | (0/17) | (10/27) | |
It has recently been reported that VEGFR-3, although absent from mature blood vessels, is induced in angiogenic tumour blood vessel endothelium (Partanen et al., 1999; Valtola et al., 1999). We have also observed VEGFR-3-positive intra-tumoural vessels in our system, although a large degree of heterogeneity was observed within individual islet cell tumours: some regions were positive, while other regions in the same tumour were devoid of immunoreactivity (data not shown). Since we have never observed LYVE-1-positive vessels within normal or tumourigenic islets (Figures 3B, D, F, 6B, 7E and F), we are confident that the VEGFR-3-positive intra-islet or intra-tumoural staining we have seen is confined to vessels of the blood vascular system. VEGFR-3-positive blood vessels were observed within tumours in both Rip1Tag2 and RipVEGF-C × Rip1Tag2 double-transgenic mice (data not shown).
Since VEGF-C can be both lymphangiogenic and angiogenic, our next objective was to compare blood vessel density in Rip1Tag2 mice versus RipVEGF-C × Rip1Tag2 double transgenics. Blood vessel density is frequently used as a measure of tumour-induced angiogenesis (reviewed by Weidner, 1998). Using the pan-endothelial marker CD31 (Figure 6C and D), we found no differences between the two genotypes (Table III). Similarly, no differences were observed when vessel density was determined in the peripheral or central parts of the tumour (Table III). These CD31-positive structures are blood vessels, since we have never observed LYVE-1-positive vessels within tumours (see above). We thus conclude that VEGF-C does not induce angiogenesis in double-transgenic RipVEGF-C × Rip1Tag2 mice.
Table III. Vascular phenotype in Rip1Tag2 versus RipVEGF-C × Rip1Tag2 mice.
| CD31-positive vascular profiles/10 000 µm2 | Rip1Tag2 | RipVEGF-C × Rip1Tag2 |
|---|---|---|
| Tumour periphery | 6.6 ± 2.8 (n = 30) | 4.8 ± 2.4 (n = 30) |
| Tumour centre | 3.9 ± 2.5 (n = 30) | 3.7 ± 2.3 (n = 30) |
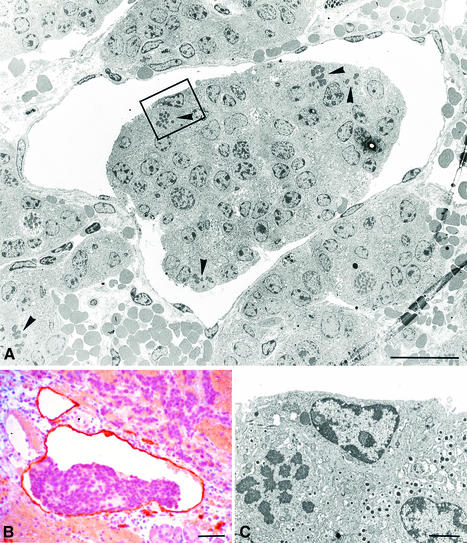
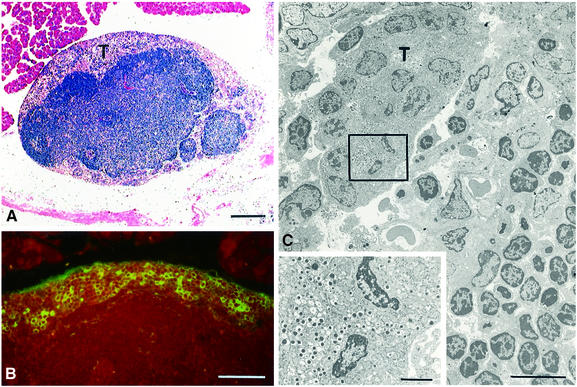
Further examination of pancreata from RipVEGF-C × Rip1Tag2 double transgenics aged between 12 and 15 weeks revealed aggregates of tumour cells within the lumen of lymphatic vessels in every case (Figure 8 and data not shown). These intra-lymphatic tumour cells, which were very rarely seen in Rip1Tag2 single-transgenic mice, were clearly derived from primary β-cell tumours, as established by their ultrastructural characteristics and insulin immunoreactivity (Figure 8C and data not shown). A proportion of the cells within these aggregates contained mitotic figures (Figure 8A and C), indicating that they were actively dividing. As before, the lymphatic nature of these vessels was confirmed by both LYVE-1 and VEGFR-3 immunoreactivity (Figure 8B and data not shown).
Fig. 8. Intra-lymphatic tumour cell masses in RipVEGF-C/Rip1Tag2 double-transgenic mice. (A) TEM showing a tumour cell mass within an endothelial-lined lymphatic space. (B) Anti-LYVE-1 immunohistochemistry. (C) Enlargement of the area delimited in (A) showing a mitotic figure and endocrine secretory granules. Mice were killed at 14 weeks and were generated from RipVEGF-C family 23. Arrowheads in (A) indicate mitotic figures. Bars: (A) 20 µm; (B) 50 µm; (C) 2 µm.
We reasoned that intravascular tumour cells, by following routes of natural drainage, would reach the marginal sinus of pancreatic mesenteric lymph nodes, thus forming metastases at these sites. We therefore analysed regional lymph nodes in double-transgenic mice for the presence of tumour cells. Tumour cells were indeed observed in regional mesenteric lymph nodes in 37% of RipVEGF-C × Rip1Tag2 mice (Table II; Figure 9A). In relatively early metastases, which contained a thin rim of tumour cells confined to the periphery of affected lymph nodes, tumour cells displayed ultrastructural features of β-cells or were insulin immunoreactive (Figure 9B and C), thus confirming their metastatic nature and their derivation from primary β-cell tumours. No PP-, somatostatin- or glucagon-immunoreactive cells could be detected in metastases (data not shown). At later stages of metastatic growth, lymph node tissue was almost completely replaced by tumour cells (data not shown). These advanced metastases displayed morphological heterogeneity, including an altered nucleus/cytoplasm ratio and nuclear atypia, both hallmarks of the cellular anaplasia that accompanies the later phases of tumour progression (data not shown). Consistent with these observations, insulin immunoreactivity was only retained by a minority of tumour cells in these metastases (data not shown). At the time points analysed, no overt metastases were observed in distant lymph nodes or in other organs of RipVEGF-C × Rip1Tag2 double-transgenic mice.
Fig. 9. Lymph node metastases in RipVEGF-C/Rip1Tag2 double-transgenic mice. (A) Haematoxylin and eosin staining of a lymph node containing a peripheral ring of tumour cells (T). (B) Immunofluorescent antibody staining for insulin. (C) TEM showing tumour cells (T) surrounded by lymphocytes. The inset is an enlargement of the delimited area, and shows the presence of endocrine secretory granules in tumour cells. Mice were killed at 14 weeks and were generated from RipVEGF-C family 23. Bar: (A) 200 µm; (B) 50 µm; (C) 10 µm; inset = 2 µm.
Peri-tumoural lymphatics, intra-lymphatic tumour cell aggregates and lymph node metastases have been observed in double-transgenic mice derived from the two founder families (Nos 23 and 24). Thus, we can firmly exclude a transgene integration effect as the reason for the metastatic phenotype.
Discussion
In the present study we have generated transgenic mice to investigate the consequences of VEGF-C overexpression on tumour cell dissemination in a well defined model of β-cell carcinogenesis. Two lines were generated, which express VEGF-C under the control of Rip. Although in post-natal life this promoter specifically directs transgene expression to pancreatic β-cells, during embryogenesis it is transiently active in cells of the neural tube and neural crest (Alpert et al., 1988). In almost all (98%) islets of Langerhans analysed in RipVEGF-C transgenic mice, extensive lymphatic channel formation was observed around (but not within) the islets, the anatomical units in which the transgene is expressed. In contrast, in wild-type littermates, islets are very rarely in direct contact with LYVE-1-positive vessels, indicating that in RipVEGF-C mice lymphatics have formed de novo (lymphangiogenesis). The latter result is consistent with the lymphangiogenic effects of VEGF-C observed in transgenic mice which express VEGF-C in the skin under the control of the keratin 14 promoter (T.Veikkola, unpublished data) and in the differentiated avian chorioallantoic membrane (Oh et al., 1997). K14-VEGF-C mice also display an increase in the size of lymphatic vessels (Jeltsch et al., 1997). The parameters that dictate whether VEGF-C will induce lymphangiogenesis or lymphatic enlargement/dilatation are not known. However, it has been well documented that the activity of many cytokines is context dependent, and it will therefore be important in the future to determine what (additional) factors are involved in determining this selectivity.
To assess the role of VEGF-C-induced lymphangiogenesis in tumour metastasis, we crossed RipVEGF-C mice with Rip1Tag2 mice, a well characterized transgenic model of β-cell carcinogenesis (Hanahan, 1985; Perl et al., 1998, 1999). Of note, β-cell tumours that develop in Rip1Tag2 mice are capable of local invasion but are not metastatic. In our model, increased VEGF-C expression in double transgenics resulted in the de novo formation of lymphatics in intimate association with β-cell tumours. Concomitant with lymphangiogenesis, all of these mice exhibited tumour cell aggregates within lymphatic vessels. This was associated with the formation of metastases in the draining regional mesenteric lymph nodes of the pancreata in 37% of mice. While intra-lymphatic tumour cell masses were occasionally seen in Rip1Tag2 mice, lymph node metastases have never been observed in these animals.
Although the metastatic dissemination of tumour cells to regional lymph nodes is a common feature of many human cancers, it is not clear whether tumours utilize existing lymphatic channels or whether tumour dissemination requires the de novo formation of lymphatics (lymphangiogenesis) (Saaristo et al., 2000; reviewed by Pepper, 2001). Our findings suggest that Rip1Tag2 tumour cells are intrinsically metastatic, but are unable to realize their metastatic potential because they lack access to appropriate portals and routes to distant sites. When such routes are provided, in this instance via the lymphangiogenesis induced by ectopically expressed VEGF-C, then metastases are observed. The finding that increased peri-tumoural lymphatic density results in metastasis in only 37% of the mice that develop islet tumours suggests that additional, rate-limiting steps must also exist.
RipVEGF-C × Rip1Tag2 double-transgenic tumours were similar to Rip1Tag2 single-transgenic tumours in terms of tumour volume, transition from adenoma to carcinoma and the relative distribution of pancreatic hormone immunoreactive cells. Interestingly, however, tumour incidence was increased in RipVEGF-C × Rip1Tag2 mice. Although we have no explanation for this increase, we speculate that this could be due to the formation of local intra-pancreatic metastases, which occur as a consequence of tumour cell dissemination through the extensive peri-tumoural lymphatic network induced by VEGF-C. Alternatively, trophic and/or mitogenic signals released by lymphatic endothelial cells in the nearby parenchyma of islets/tumours might have a positive effect on islet/tumour cell survival and/or proliferation, thus increasing the fraction of islets that form primary tumours.
In addition to its capacity to induce lymphangiogenesis and an increase in the size of lymphatic vessels, VEGF-C has also been demonstrated to be angiogenic under certain circumstances (Cao et al., 1998; Witzenbichler et al., 1998). However, we found no evidence for increased angiogenesis in RipVEGF-C or RipVEGF-C × Rip1Tag2 double-transgenic mice, as determined by blood vessel density. Thus, there was no increase in the number of MECA-32- or CD31-positive blood vascular profiles within RipVEGF-C islets when compared with wild-type littermate controls, nor could we detect alterations in the percentage of islet volume occupied by these vessels (demonstrating that vessel size was likewise unaltered). Similarly, there was no difference in vessel density between Rip1Tag2 versus RipVEGF-C × Rip1Tag2 mice, either in the centre of the tumour or in the tumour periphery. We have recently generated Rip1Tag2 × RipVEGF-A double-transgenic mice (manuscript in preparation). Extensive phenotypic characterization of these animals has revealed that VEGF-A has an exclusively angiogenic effect during tumour progression. This observation points to the fact that in the setting of the pancreas, exquisite selectivity with regard to angiogenesis and lymphangiogenesis can be achieved with VEGF-A and VEGF-C, respectively, despite the fact that both cytokines can activate VEGFR-2. Although currently there is no explanation for this selectivity, this may depend on the extent of VEGF-C proteolytic processing or on the repertoire of VEGFRs expressed (including heterodimers between different VEGFRs).
VEGFR-3 is downregulated in blood vascular endothelium during development, and becomes restricted almost exclusively to lymphatic endothelium and fenestrated blood vascular endothelium in post-natal life (Kaipainen et al., 1995; Kukk et al., 1996; Jussila et al., 1998; Partanen et al., 2000). It has been shown that VEGFR-3 is re-expressed in the blood vascular endothelium of several types of tumours (Partanen et al., 1999; Valtola et al., 1999). Although we observed a variable degree of VEGFR-3 staining on blood vascular endothelium in Rip1Tag2 tumours, this was not increased in the tumours of double-transgenic RipVEGF-C × Rip1Tag2 mice.
It has long been argued that lymphatic vessels may be lost and/or collapsed in expanding primary tumours because of high endogenous tumour interstitial pressure (Leu et al., 2000). However, their presence can be demonstrated in the peri-tumoural stromal component intimately associated with several types of tumours. In our system, LYVE-1-positive lymphatics were occasionally observed in the periphery of large adenomas, but were never seen within islets of Langerhans or β-cell tumours. The reasons for this selective localization of lymphatic vessels around but not within normal and tumorigenic islets of Langerhans is not known.
A correlation between VEGF-C expression, tumour lymphangiogenesis and the formation of metastases in regional lymph nodes has recently been described (Bunone et al., 1999; Ohta et al., 1999, 2000; Tsurusaki et al., 1999; Yonemura et al., 1999; Akagi et al., 2000; Niki et al., 2000). Although these findings provide no information on the mechanisms of tumour cell dissemination, they raise the possibility that VEGF-C may increase metastasis by increasing the number and size of lymphatic vessels, or alternatively by altering the functional properties of existing lymphatics. For example, it is possible that lymphatic endothelial cells locally release paracrine factors, which influence primary tumour cell invasiveness, possibly through altered tumour cell–extracellular matrix adhesion. Alternatively, VEGF-C may facilitate tumour cell intravasation by altering the functional properties of pre-existing or newly formed lymphatic endothelium (e.g. tumour cell–endothelial cell adhesion). Future studies in this area will need to be conducted on cultured lymphatic endothelial cells. Although to date all in vitro studies have been performed on large-vessel lymphatic endothelial cells (reviewed by Pepper, 2001), it will be important in the future to work with primary endothelial cells from lymphatic capillaries. These cells would be useful (i) for the identification of VEGF-C-induced phenotypic alterations (e.g. molecules involved in adhesive interactions with tumour cells) and (ii) for application of techniques of differential gene expression to identify molecular differences between blood and lymphatic capillary endothelial cells. The utility of these techniques in identifying gene expression profiles in normal versus tumour endothelial cells has recently been demonstrated (St Croix et al., 2000).
Tumour cell metastasis to regional lymph nodes is an early event in metastatic tumour spread, and is frequently used as a prognostic factor to predict disease outcome or to determine therapeutic strategies. Our observations provide the first direct evidence for a causal role for VEGF-C-mediated lymphangiogenesis in the dissemination of tumour cells. They also provide a relevant animal model in which the mechanisms of lymphangiogenesis and lymphatic tumour metastasis can be dissected, and in which potential inhibitors of these processes can be tested.
Materials and methods
Transgenic mice
RipVEGF-C transgenic mice were generated according to standard procedures (Hogan et al., 1994). The transgene was constructed by cloning the human VEGF-C cDNA between the ∼695 bp _Bam_HI–_Xba_I fragment of Rip (Hanahan, 1985) and the SV40 small T antigen intron and polyadenylation signal. Genotypes were confirmed by Southern blotting and PCR analysis. Ten micrograms of genomic DNA from mouse tails were digested with _Eco_RI, run in 0.7% agarose gels and analysed by Southern blotting using the ∼450 bp SV40 moiety of the RipVEGF-C transgene as a probe. Routine PCR screening of RipVEGF-C heterozygotes was performed using a pair of primers specific for the human VEGF-C cDNA (forward: 5′-TCCGGACTCGACCTCTCGGAC; reverse 5′-CCCCACATCTATACACACCTCC), starting from standard tail or finger genomic DNA preparations. PCR cycles were: 94°C, 3 min (1×); 94°C, 1 min, 60°C, 1 min, 72°C, 1 min (30×); 72°C, 3 min (1×). PCR products were analysed on 6% polyacrylamide gels.
Expression of the RipVEGF-C transgene was assessed by RT–PCR using primers specific for the human VEGF-C cDNA, and by immunohistochemical analysis as described below. Total RNA purified from mouse pancreas using TRIZOL Reagent (Gibco-BRL, Life Technologies) was reverse transcribed using oligo-dT (Boehringer) and Superscript II (Life Technologies). RT products were subjected to PCR analysis using a pair of primers specific for human VEGF-C (see above) or a pair of primers for the acidic ribosomal phosphoprotein P0 (Pepper and Mandriota, 1998). Where indicated, RT was omitted. PCR cycles were: 94°C, 3 min (1×); 94°C, 1 min, 60°C, 1 min, 72°C, 1 min (30× for VEGF-C; 25–30× for P0); 72°C, 3 min (1×). Equal volumes of PCR products were analysed on 6% polyacrylamide gels.
The generation and phenotypic characterization of Rip1Tag2 mice have been described previously (Hanahan, 1985). Glucose (5% w/v) was added to the drinking water of all tumour-bearing mice, beginning at 10 weeks of age, to counteract hypoglycaemia, which results from insulinoma development.
Histopathological analyses
For light microscopy, pancreata were either frozen or fixed in Bouin’s fixative or 4% paraformaldehyde, and embedded in paraffin. Antibodies used for immunohistochemistry were as follows: mouse monoclonal anti-human insulin (Storch et al., 1985); rabbit polyclonal anti-human VEGF-C (Joukov et al., 1997); rabbit polyclonal anti-human VEGFR-3 (Pajusola et al., 1993); rat monoclonal anti-mouse VEGFR-3 (Kubo et al., 2000); MECA-32 (Hallmann et al., 1995); rat monoclonal anti-mouse CD31 (clone MEC13.3; PharMingen); rabbit polyclonal anti-mouse LYVE-1 ectodomain. The latter was generated by immunizing rabbits with the mouse LYVE-1 ectodomain fused with human IgFc followed by pre-absorption of the antiserum on human Ig–Sepharose (Prevo et al., 2001). MECA-32 was applied to cryostat sections. All other antibodies were applied to paraffin-embedded material following antigen retrieval (microwave), with the exception of MEC13.3, for which no antigen retrieval was used. Immunoreactivity was visualized using either peroxidase–DAB or fast red- or fluorescein isothiocyanate-conjugated streptavidin or secondary antibodies. For CD31 immunostaining, antigen–antibody complexes were revealed by means of a rabbit anti-rat linker antibody (Dako) followed by anti-rabbit/HRP Envision system (Dako).
For determination of blood vessel density, the number of MECA-32- or CD31-positive vascular profiles per 10 000 µm2 of islet or adenoma tissue was determined using an in-house morphometric apparatus. For adenomas, three randomly selected fields (∼300 × 300 µm each) at the periphery and in the centre of each tumour were counted. The per cent islet volume occupied by MECA-32-positive vessels was determined using the point counting method (Weibel, 1973). Values are mean ± SD.
For electron microscopy, mice were either perfused with 1–2% glutaraldehyde in 100 mM phosphate buffer pH 7.4 and the pancreas isolated, or freshly isolated pancreatic tissue was minced and fixed by immersion in 2% glutaraldehyde for 2 h. After washing in 100 mM phosphate buffer, tissue fragments were post-fixed in 2% osmium tetroxide, dehydrated in graded ethanols and embedded in Epon 812. Thin sections were stained with uranyl acetate and lead citrate, and viewed in a Philips CM10 electron microscope. For immunoelectron microscopy, islets were dissected from perfused pancreatic tissue, infused with 2.3 M sucrose and processed for cryo-ultramicrotomy (Tokuyasu, 1980). Ultrathin cryosections were incubated using the protein A–gold technique (Roth et al., 1978) with LYVE-1 antibody (diluted 1/50) followed by protein A coupled to 10 nm gold particles.
Note added in proof
The reader is referred to the following two papers, which, using VEGF-C- and VEGF-D-transfected tumour cells in xenotransplantation models, obtained findings comparable to those described in the present paper:
Skobe,M., Hawighorst,T., Jackson,D.G., Prevo,R., Velasco,P., Riccardi,L., Alitalo,K., Claffey,K. and Detmar,M. (2001) Induction of tumour lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nature Med., 7, in press.
Stacker,S.A. et al. (2001) VEGF-D promotes metastatic spread of tumor cells by the lymphatics. Nature Med., 7, in press.
Acknowledgments
Acknowledgements
The project presented in this manuscript was conceived and started in Helsinki, and the work is the result of an equal contribution from the laboratories in Helsinki, Vienna and Geneva, together with a major contribution from the Oxford group. We thank Drs Hajime Kubo and Shin-Ishi Nishikawa for the anti-VEGFR-3 antibody, and Dr Jocelyn Holash for advice with CD31 immunohistochemistry. We also thank Danielle Ben Nasr, Mireille Quayzin, Marie Ebrahim Malek, Gorana Perrelet and Petra Wilgenbus for excellent technical assistance, and Nadine Dupont and Gerard Negro for photographic work. R.M., M.S.P. and L.O. are supported by grants from the Swiss National Science Foundation (Nos 31-43364.95 and 31-43366.95). A.C. and G.C. gratefully acknowledge support by Boehringer Ingelheim and by the Austrian Industrial Research Promotion Fund. K.A., L.J. and M.J. thank the Finnish Academy, the Sigrid Juselius Foundation, the University of Helsinki Hospital (TYH 8105), the State Technology Development Centre as well as the EU Biomed programs (BMH-CT96-0669 and 98-3380) for support. D.G.J. is supported by the UK Medical Research Council (MRC Human Immunology Unit) and a project grant (No. 00-311) from the Association for International Cancer Research.
REFERENCES
- Akagi K., Ikeda,Y., Miyazaki,M., Abe,T., Kinoshita,J., Maehara,Y. and Sugimachi,K. (2000) Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br. J. Cancer, 83, 887–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert S., Hanahan,D. and Teitelman,G. (1988) Hybrid insulin genes reveal a developmental lineage for pancreatic endocrine cells and imply a relationship with neurons. Cell, 53, 295–308. [DOI] [PubMed] [Google Scholar]
- Banerji S., Ni,J., Wang,S.X., Clasper,S., Su,J., Tammi,R., Jones,M. and Jackson,D.G. (1999) LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol., 144, 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelli E., Regoli,M. and Comparini,L. (1993) Histotopographic and ultrastructural study on the lymphatic network of the pancreas in the guinea pig. Acta Anat. (Basel), 147, 233–239. [DOI] [PubMed] [Google Scholar]
- Breiteneder-Geleff S. et al. (1999) Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am. J. Pathol., 154, 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunone G., Vigneri,P., Mariani,L., Buto,S., Collini,P., Pilotti,S., Pierotti,M.A. and Bongarzone,I. (1999) Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical–pathologic features. Am. J. Pathol., 155, 1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Linden,P., Farnebo,J., Cao,R., Eriksson,A., Kumar,V., Qi,J.H., Claesson-Welsh,L. and Alitalo,K. (1998). Vascular endothelial growth factor C induces angiogenesis in vivo. Proc. Natl Acad. Sci. USA, 95, 14389–14394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran R.S., Kumar,V. and Tucker,C. (1999) Neoplasia. In Robbins Pathologic Basis of Disease, 6th edn. W.B.Saunders Co., Philadelphia, PA, pp. 268–271.
- Dumont D.J., Jussila,L., Taipale,J., Lymboussaki,A., Mustonen,T., Pajusola,K., Breitman,M. and Alitalo,K. (1998) Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science, 282, 946–949. [DOI] [PubMed] [Google Scholar]
- Eriksson U. and Alitalo,K. (1999) Structure, expression and receptor-binding properties of novel vascular endothelial growth factors. Curr. Top. Microbiol. Immunol., 237, 41–57. [DOI] [PubMed] [Google Scholar]
- Ferrara N. (1999) Vascular endothelial growth factor: molecular and biological aspects. Curr. Top. Microbiol. Immunol., 237, 1–30. [DOI] [PubMed] [Google Scholar]
- Fidler I.J. (1997) Molecular biology of cancer: invasion and metastasis. In DeVita,V.T., Hellman,S. and Rosenberg,S.A. (eds), Cancer: Principles and Practice of Oncology, 5th edn. Lippincott-Raven, Philadelphia, PA, pp. 135–152.
- Hallmann R., Mayer,D.N., Berg,E.L., Broermann,R. and Butcher,E.C. (1995) Novel mouse endothelial cell surface marker is suppressed during differentiation of the blood brain barrier. Dev. Dyn., 202, 325–332. [DOI] [PubMed] [Google Scholar]
- Hanahan D. (1985) Heritable formation of pancreatic β-cell tumors in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature, 315, 115–122. [DOI] [PubMed] [Google Scholar]
- Hogan B., Beddington,R., Costantini,F. and Lacy,E. (1994) Manipulating the Mouse Embryo. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 497 pp.
- Holm R., Vardnell,I.M., Power,R.F., Bishop,A.E., Madsen,O.D., Alpert,S., Hanahan,D. and Polak,J.M. (1988) Ultrastructure and electron immunocytochemistry of insulin-producing B-cell tumors from transgenic mice: comparison with counterpart human tumors. Ultrastruct. Pathol., 12, 547–559. [DOI] [PubMed] [Google Scholar]
- Jackson D.G. (2001) New molecular markers for the study of tumour lymphangiogenesis. Anticancer Res., in press. [PubMed] [Google Scholar]
- Jeltsch M. et al. (1997) Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science, 276, 1423–1425. [DOI] [PubMed] [Google Scholar]
- Ji R.C. and Kato,S. (1997) Demonstration of the intralobular lymphatics in the guinea pig pancreas by an enzyme–histochemical method. J. Anat., 191, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukov V., Pajusola,K., Kaipainen,A., Chilov,D., Lahtinen,I., Kukk,E., Saksela,O., Kalkkinen,N. and Alitalo,K. (1996) A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J., 15, 290–298. [PMC free article] [PubMed] [Google Scholar]
- Joukov V., Sorsa,T., Kumar,V., Jeltsch,M., Claesson-Welsh,L., Cao,Y., Saksela,O., Kalkkinen,N. and Alitalo,K. (1997) Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J., 16, 3898–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jussila L. et al. (1998) Lymphatic endothelium and Kaposi’s sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res., 58, 1599–1604. [PubMed] [Google Scholar]
- Kaipainen A., Korhonen,J., Mustonen,T., van Hinsbergh,V.W.M., Fang,G.-H., Dumont,D., Breitman,M. and Alitalo,K. (1995) Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl Acad. Sci. USA, 92, 3566–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo H. et al. (2000) Involvement of vascular endothelial growth factor receptor-3 in maintenance of integrity of endothelial cell lining during tumor angiogenesis. Blood, 96, 546–553. [PubMed] [Google Scholar]
- Kukk E., Lymboussaki,A., Taira,S., Kaipainen,A., Jeltsch,M., Joukov,V. and Alitalo,K. (1996) VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development, 122, 3829–3827. [DOI] [PubMed] [Google Scholar]
- Lee J., Gray,A., Yuan,J., Luoh,S.M., Avraham,H. and Wood,W.I. (1996) Vascular endothelial growth factor-related protein: a ligand and specific activator of the tyrosine kinase receptor Flt4. Proc. Natl Acad. Sci. USA, 93, 1988–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu A.J., Berk,D.A., Lymboussaki,A., Alitalo,K. and Jain R.K. (2000) Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res., 60, 4324–4327. [PubMed] [Google Scholar]
- Lymboussaki A., Olofsson,B., Eriksson,U. and Alitalo,K. (1999) Vascular endothelial growth factor (VEGF) and VEGF-C show overlapping binding sites in embryonic endothelia and distinct sites in differentiated adult endothelia. Circ. Res., 85, 992–999. [DOI] [PubMed] [Google Scholar]
- Navas V., O’Morchoe,P.J. and O’Morchoe,C.C.C. (1995) Lymphatic system of the rat pancreas. Lymphology, 28, 4–20. [PubMed] [Google Scholar]
- Niki T., Iba,S., Tokunou,M., Yamada,T., Matsuno,Y. and Hirohashi,S. (2000) Expression of vascular endothelial growth factors A, B, C and D and their relationships to lymph node status in lung adenocarcinoma. Clin. Cancer Res., 6, 2431–2439. [PubMed] [Google Scholar]
- Oh S.J., Jeltsch,M.M., Birkenhager,R., McCarthy,J.E., Weich,H.A., Christ,B., Alitalo,K. and Wilting,J. (1997) VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev. Biol., 188, 96–109. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Shridhar,V., Bright,R.K., Kalemkerian,G.P., Du,W., Carbone,M., Watanabe,Y. and Pass,H.I. (1999) VEGF and VEGF type C play an important role in angiogenesis and lymphangiogenesis in human malignant mesothelioma tumors. Br. J. Cancer, 81, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y., Nozawa,H., Tanaka,Y., Oda,M. and Watanabe,Y. (2000) Increased vascular endothelial growth factor and vascular endothelial growth factor-C and decreased nm23 expression associated with microdissemination in the lymph nodes in stage I non-small cell lung cancer. J. Thorac. Cardiovasc. Surg., 119, 804–813. [DOI] [PubMed] [Google Scholar]
- O’Morchoe C.C.C. (1997) Lymphatic system of the pancreas. Microsc. Res. Tech., 37, 456–477. [DOI] [PubMed] [Google Scholar]
- Pajusola K., Aprelikova,O., Armstrong,E., Morris,S. and Alitalo,K. (1993) Two human FLT4 receptor tyrosine kinase isoforms with distinct carboxy terminal tails are produced by alternative processing of primary transcripts. Oncogene, 8, 2931–2937. [PubMed] [Google Scholar]
- Partanen T.A., Alitalo,K. and Miettinen,M. (1999) Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor-3 in 185 vascular tumors. Cancer, 86, 2406–2412. [PubMed] [Google Scholar]
- Partanen T.A., Arola,J., Saaristo,A., Jussila,L., Ora,A., Miettinen,M., Stacker,S.A., Achen,M.G. and Alitalo,K. (2000) VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J., 14, 2087–2096. [DOI] [PubMed] [Google Scholar]
- Pepper M.S. (2001) Lymphangiogenesis and tumor metastasis: myth or reality? Clin. Cancer Res., in press. [PubMed] [Google Scholar]
- Pepper M.S. and Mandriota,S.J. (1998) Regulation of vascular endothelial growth factor receptor-2 (Flk-1) expression in vascular endothelial cells. Exp. Cell Res., 241, 414–425. [DOI] [PubMed] [Google Scholar]
- Perl A.K., Wilgenbus,P., Dahl,U., Semb,H. and Christofori,G. (1998) A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature, 392, 190–193. [DOI] [PubMed] [Google Scholar]
- Perl A.K., Dahl,U., Wilgenbus,P., Cremer,H., Semb,H. and Christofori,G. (1999) Reduced expression of neural cell adhesion molecule (N-CAM) induces metastatic dissemination of pancreatic β tumor cells. Nature Med., 5, 286–291. [DOI] [PubMed] [Google Scholar]
- Prevo R., Banerji,S., Ferguson,D., Clasper,S. and Jackson,D. (2001) Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J. Biol. Chem., in press. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan,M. and Orci,L. (1978) Ultrastructural localization of intracellular antigens by the use of protein A–gold technique. J. Histochem. Cytochem., 26, 1074–1081. [DOI] [PubMed] [Google Scholar]
- Saaristo A., Karpanen,T. and Alitalo,K. (2000) Mechanisms of angiogenesis and lymphangiogenesis and their use in the inhibition of tumor growth and metastasis. Oncogene, 19, 6122–6129. [DOI] [PubMed] [Google Scholar]
- Sleeman J.P. (2000). The lymph node as a bridgehead in the metastatic dissemination of tumors. Recent Results Cancer Res., 157, 55–81. [DOI] [PubMed] [Google Scholar]
- St Croix B. et al. (2000) Genes expressed in human tumor endothelium. Science, 289, 1197–1202. [DOI] [PubMed] [Google Scholar]
- Storch M.J., Petersen,K.G., Licht,T. and Kerp,L. (1985) Recognition of human insulin and proinsulin by monoclonal antibodies. Diabetes, 34, 808–811. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K.T. (1980) Immunochemistry on ultrathin frozen sections. Histochem. J., 12, 381–403. [DOI] [PubMed] [Google Scholar]
- Tsurusaki T., Kanda,S., Sakai,H., Kanetake,H., Saito,Y., Alitalo,K. and Koji,T. (1999) Vascular endothelial growth factor-C expression in human prostatic carcinoma and its relationship to lymph node metastasis. Br. J. Cancer, 80, 309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtola R. et al. (1999) VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am. J. Pathol., 154, 1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veikkola T., Karkkainen,M., Claesson-Welsh,L. and Alitalo,K. (2000) Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res., 60, 203–212. [PubMed] [Google Scholar]
- Weibel E.R. (1973) Stereological techniques for electron microscope morphometry. In Hayat,M.A. (ed.), Principles and Techniques of Electron Microscopy, Biological Applications. Vol. 3. Van Nostrand Reinholdt, New York, NY, pp. 237–296.
- Weidner N. (1998) Tumoural vascularity as a prognostic factor in cancer patients: the evidence continues to grow. J. Pathol., 184, 119–122. [DOI] [PubMed] [Google Scholar]
- Wigle J.T. and Oliver,G. (1999) Prox1 function is required for the development of the murine lymphatic system. Cell, 98, 769–778. [DOI] [PubMed] [Google Scholar]
- Witzenbichler B. et al. (1998) Vascular endothelial growth factor-C (VEGF-C/VEGF-2) promotes angiogenesis in the setting of tissue ischemia. Am. J. Pathol., 153, 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura Y. et al. (1999) Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin. Cancer Res., 5, 1823–1829. [PubMed] [Google Scholar]