The three modules of ADP/ATP carrier cooperate in receptor recruitment and translocation into mitochondria (original) (raw)
Abstract
The ADP/ATP carrier (AAC) is a major representative of mitochondrial preproteins lacking an N-terminal presequence. AAC contains targeting information in each of its three modules, which has led to a search for the dominant targeting region. An alternative, not yet tested model would be that several distinct targeting signals function simultaneously in import of the preprotein. We report that the three AAC modules cooperate in binding to the receptor Tom70 such that three Tom70 dimers are recruited to one preprotein. The modules are transferred to the import pore in a stepwise manner and cooperate again in the accumulation of AAC in the general import pore complex. AAC can cross the outer membrane with an internal segment first, i.e. in a loop formation. Each module of AAC is required for dimerization in the inner membrane. We propose a new concept for import of the hydrophobic carrier proteins into mitochondria where multiple signals cooperate in receptor recruitment, outer membrane translocation via loop formation and assembly in the inner membrane.
Keywords: ADP/ATP carrier/mitochondria/protein sorting/Saccharomyces cerevisiae
Introduction
Many mitochondrial proteins are synthesized in the cytosol with N-terminal targeting signals (presequences) that are proteolytically removed following their arrival in the mitochondrial matrix (Schatz and Dobberstein, 1996; Neupert, 1997; Jensen and Johnson, 1999; Voos et al., 1999). Typically, cleavable preproteins are recognized first by the receptors Tom20 and Tom22 of the translocase of the outer membrane (TOM). Tom22 forms part of the general import pore (GIP) complex, which contains the channel protein Tom40 as well as Tom5, Tom6 and Tom7. Preproteins are driven across Tom40 through their engagement with the TIM23 translocase complex of the inner membrane (TIM) in a membrane potential (Δψ)-dependent reaction. Preproteins are imported into the matrix with the aid of the Tim44–heat shock protein 70 motor system.
The mitochondrial inner membrane is rich in proteins that have been imported from the cytosol. A number of these proteins also contain N-terminal targeting signals (cleavable or non-cleavable) and therefore follow a similar pathway to that of matrix-targeted preproteins. At the level of the TIM23 complex, these proteins are directed into the inner membrane by hydrophobic sorting signals (Schatz and Dobberstein, 1996; Neupert, 1997; Voos et al., 1999). Another major set of inner membrane targeted preproteins, however, lack such N-terminal targeting signals. Examples of these are the metabolite carriers that form a large family of homologous proteins with the ADP/ATP carrier (AAC) as the most abundant protein. Detailed in vitro import studies of this protein revealed that unlike preproteins containing N-terminal targeting signals, AAC could be arrested at distinct stages along its import pathway (Pfanner and Neupert, 1987; Pfanner et al., 1987a). Addition of the AAC preprotein (stage I) to isolated mitochondria in the absence of ATP leads to its arrest at the outer membrane (termed stage II) where it is bound to the receptor Tom70 (Hines et al., 1990; Steger et al., 1990; Ryan et al., 1999). AAC can then be chased across the outer membrane by the addition of ATP. In the absence of a Δψ, it is arrested at the inner face of the TOM machinery (stage III), while in the presence of a Δψ it is inserted into the inner membrane (stage IV) where it assembles into its dimeric form (stage V). AAC engages with a number of components of the import machinery that are unique to this pathway. Upon its partial translocation across the outer membrane, AAC associates with the intermembrane space (IMS)-located small Tim proteins Tim9 and Tim10 (Koehler et al., 1998; Adam et al., 1999). In the presence of a membrane potential, AAC inserts into an inner membrane complex that consists of another small Tim protein, Tim12, along with the membrane-integrated subunits Tim22, Tim54 and Tim18 (Sirrenberg et al., 1996; Kerscher et al., 1997, 2000; Koehler et al., 1998, 2000). This translocase is termed the TIM22 complex.
Like other carrier proteins, the AAC monomer consists of three separate modules, where each consists of a pair of membrane-spanning segments connected by a matrix-exposed loop. In various studies, mitochondrial targeting signals have been found in several regions within carrier proteins. Taken together, the studies indicate that each of the three modules contains targeting information (Pfanner et al., 1987b; Liu et al., 1988; Smagula and Douglas, 1988; Brix et al., 1999; Endres et al., 1999). While Smagula and Douglas (1988) emphasized the importance of the N-terminal module for import of AAC into mitochondria, the two C-terminal modules were also found to be imported into mitochondria (Pfanner et al., 1987b). Endres et al. (1999) reported that although a mitochondrial targeting signal is found in each module, only the third module alone was able to insert into the inner membrane. Schleiff and McBride (2000) reported that the second module of the homologous uncoupling protein was crucial for import of the carrier protein.
So far, the models concerning targeting of preproteins into mitochondria have been derived from the findings made with presequence-containing preproteins, i.e. that one region of the preprotein contains the major targeting information. Additional targeting signals may have an auxiliary role and function in a consecutive manner. Indeed, in several non-cleavable preproteins, one internal region with major targeting function has been identified (Fölsch et al., 1996; Diekert et al., 1999). These results promoted the search for the dominant signal in the carrier proteins; however, different studies surprisingly led to the identification of different signal regions as described above. Taking into account that the import efficiency of carrier constructs with a reduced number of modules is lower than that of the full-length preprotein (Pfanner et al., 1987b), an alternative hypothesis would be that the presence of multiple signals is a functional hallmark of the import of carrier preproteins. One may speculate that the import of these hydrophobic proteins is not directed by one major targeting region, but that several signals of equal importance cooperate in protein import. For this report, we attempted to test this hypothesis. By using AAC constructs with one, two or three modules, we analyzed the distinct import stages of receptor binding, outer membrane translocation and assembly in the inner membrane, and found that each stage indeed involved the combined action of several AAC modules. These results strongly support the proposed hypothesis of carrier import into mitochondria and imply a novel mechanism whereby several signals within one preprotein that are separate on the linear sequence are simultaneously active at a given import stage.
Results
AAC accumulated on the mitochondrial surface interacts with multiple molecules of the receptor Tom70
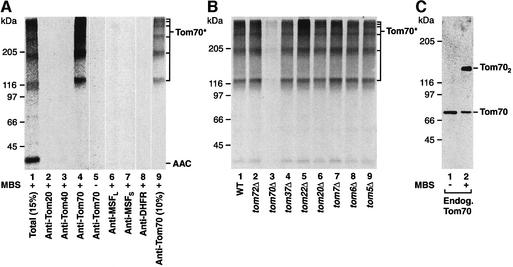
At low levels of ATP, the precursor of AAC can be arrested on the surface of the outer mitochondrial membrane, where it is bound specifically to the receptor Tom70 (stage II) (Pfanner et al., 1987a; Steger et al., 1990; Söllner et al., 1992; Ryan et al., 1999). We used chemical cross-linking to analyze the receptor-bound state of AAC. The preprotein of yeast AAC was synthesized in rabbit reticulocyte lysates in the presence of [35S]methionine/cysteine. Mitochondria, isolated from wild-type yeast cells, and reticulocyte lysate containing preprotein were depleted of ATP prior to mixing. After incubation at 25°C, mitochondria were re-isolated and treated with the cross-linking reagent _m_-maleimidobenzoyl-_N_-hydroxysuccinimide ester (MBS). A ladder of high molecular weight cross-linked species was detected by the use of a gradient SDS–polyacrylamide gel followed by digital autoradiography (Figure 1A, lane 1). All cross-linked species were immunoprecipitated under denaturing conditions with antibodies against Tom70, but not with antibodies against Tom20 or Tom40 (Figure 1A, lanes 2–4). Without cross-linking, no radiolabeled band could be immunoprecipitated (Figure 1A, lane 5).
Fig. 1. Cross-linking of AAC preprotein on the mitochondrial surface yields multiple products with Tom70. (A) [35S]AAC in reticulocyte lysate was incubated with yeast wild-type mitochondria in the absence of ATP at 25°C for 20 min. For sample 8, reticulocyte lysate containing unlabeled AAC–DHFR was also added. Mitochondria were re-isolated and, where indicated, treated with the cross-linking reagent MBS (1 mM) prior to immunoprecipitation with the indicated antibodies (under stringent conditions). The total cross-linked forms are shown in lane 1 (representing 15% of total used per immunoprecipitation). Lane 9 shows a lower exposure (10%) of lane 4. Tom70* indicates cross-links between AAC and Tom70. MSFL and MSFS are large and small subunits of MSF, respectively. (B) [35S]AAC was incubated with wild-type (WT) or tomΔ mitochondria in the absence of ATP at 25°C for 20 min. Mitochondria were re-isolated and subjected to cross-linking using MBS prior to immunoprecipitation with anti-Tom70. Samples were subjected to SDS–PAGE and phosphoimage analysis. (C) Wild-type mitochondria were left untreated or cross-linked with MBS (1 mM) and subjected to SDS–PAGE and immunodecoration with anti-Tom70. Tom702, dimer of Tom70.
Upon a lower exposure of the autoradiograph, a ladder consisting of six separate Tom70–AAC cross-links was discernible (Figure 1A, lane 9). Since only radiochemical amounts of AAC precursor are bound to mitochondria [much less than the number of import sites (Palmisano et al., 1998)], it can not be expected that more than a single AAC precursor molecule participates in formation of the individual cross-links. Indeed, when [35S]AAC was mixed with unlabeled AAC carrying dihydrofolate reductase (DHFR) as tag, none of the [35S]cross-links was precipitated by antibodies directed against DHFR (Figure 1A, lane 8). The ladder exhibited a migration pattern that would be consistent with each higher molecular weight cross-link formed containing sequential, additional molecules of Tom70. To determine whether any other Tom protein was involved in formation of the cross-links, mitochondria were isolated from yeast strains lacking genes encoding individual components of the TOM machinery: Tom70 (Hines et al., 1990; Steger et al., 1990); its homolog Tom72, which is expressed at a low level (Bömer et al., 1996); Tom37 (Gratzer et al., 1995); Tom22 (van Wilpe et al., 1999); Tom20 (Moczko et al., 1994; Dekker et al., 1998); Tom7; Tom6 (Hönlinger et al., 1996); and Tom5 (Dietmeier et al., 1997). The AAC preprotein was incubated with the different tomΔ mitochondria in the absence of ATP. Figure 1B shows that with the exception of tom70Δ mitochondria (lane 3), any tomΔ mitochondria formed the ladder of AAC cross-links (lanes 2 and 4–9) in a manner comparable with wild-type mitochondria (lane 1). Since none of the cross-links was recognized by antibodies against the essential protein Tom40 (Figure 1A, lane 3), we conclude that the stage II intermediate of AAC is found in a high molecular weight complex containing no other Tom protein besides Tom70.
Preproteins directed to Tom70 were found to bind to the mitochondrial import stimulation factor (MSF) of reticulocyte lysates, while preproteins targeted to Tom20 can bind to cytosolic homologs of heat shock protein 70 (Hsc70) (Komiya et al., 1997). Neither antibodies against the subunits of MSF nor those against cytosolic Hsc70s precipitated any of the cross-links (Figure 1A, lanes 6 and 7; data not shown), excluding the possibility that MSF or Hsc70 was present in the AAC–Tom70 cross-linking products. Thus, AAC accumulated on the mitochondrial surface selectively interacts with Tom70, suggesting that the AAC precursor contacts multiple Tom70 molecules.
To address whether Tom70 is found naturally as a large oligomer, we incubated isolated mitochondria with cross-linker in the absence of preprotein, but otherwise identical conditions, and analyzed the cross-linking profile of endogenous Tom70 by immunoblot analysis. Cross-linking led to the formation of an ∼140 kDa band (Figure 1C, lane 2) that corresponds to the previously described dimeric form of Tom70 (Söllner et al., 1992; Millar and Shore, 1994). The lack of other higher molecular weight Tom70 cross-links in mitochondria lacking bound preprotein suggests that the binding of AAC at stage II leads to the recruitment of extra Tom70 molecules to the preprotein.
The three modules of AAC cooperate in recruiting multiple molecules of Tom70
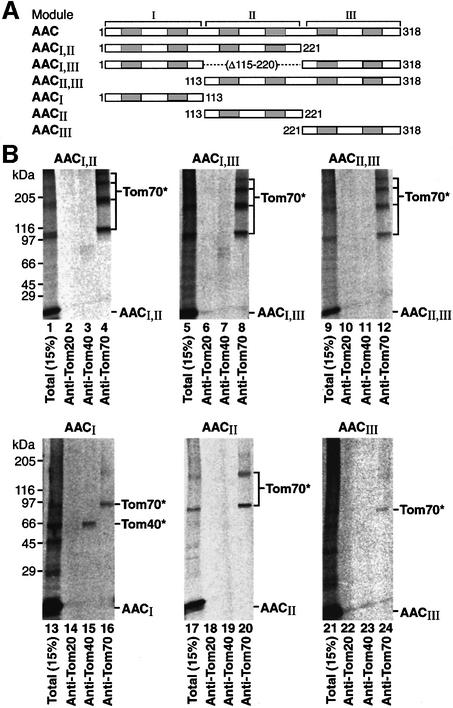
This finding of multiple cross-linking products of AAC with Tom70 is in contrast to the single cross-links of presequence-containing preproteins to their receptors Tom20 and Tom22 (Alconada et al., 1995; Hönlinger et al., 1995; Dietmeier et al., 1997; Rapaport et al., 1997). We asked which of the three modules of AAC contributed to the interaction with Tom70. We constructed various mutants of AAC containing different numbers of its modules (Figure 2A), accumulated them on the mitochondrial surface and performed cross-linking. Each AAC construct containing two modules was cross-linked specifically to Tom70, but not to Tom40 or Tom20 (Figure 2B, lanes 1–12). This indicates that no individual AAC module is essential for the formation of a stage II arrest. Interestingly, four Tom70-containing cross-linked bands were observed with each of the two-module constructs instead of the six bands seen with the full-length AAC. Each individual module could also be directed to mitochondria and cross-linked to Tom70 (Figure 2B, lanes 16, 20 and 24). However, the intensity of cross-linked bands was strongly reduced in comparison with AAC constructs containing more than one module. The first module of AAC was additionally cross-linked to Tom40 (Figure 2B, lane 15), indicating that its association and arrest with Tom70 is not as strong as with other modules. The efficiency of cross-linking of module II to Tom70 was better than that of modules I or III and represented the only one that yielded a significant second cross-link to Tom70 (Figure 2B, lane 20). However, every combination of two modules yielded a cross-linking efficiency that was at least an order of magnitude higher than that of module II (Figure 2B, lanes 4, 8 and 12 versus lane 20; note that a longer exposure of the autoradiographs was used for the single modules than for the two-module constructs in order to visualize the cross-links). This means that the combination of two modules, even of modules I and III with the weak individual interaction with Tom70, dramatically enhances the yield of cross-linking to Tom70.
Fig. 2. Each module of AAC contributes to the yield of cross-linking to Tom70. (A) Schematic diagram of AAC constructs consisting of one or two of the three modules of AAC. (B) The [35S]AAC constructs were bound to yeast wild-type mitochondria, cross-linked and immunoprecipitated with anti-Tom70 as described in the legend to Figure 1A. Tom70* and Tom40* are cross-links between AAC constructs and Tom70 or Tom40, respectively.
When all three modules of AAC were present, the efficiency of cross-linking was again increased by an order of magnitude compared with the constructs with two modules [note that a very low exposure of the cross-links of full-length AAC was necessary to visualize the six distinct cross-links (Figure 1A, lane 9)]. A double AAC construct, containing six modules, enhanced the formation of higher molecular weight cross-links even further (not shown). Considering the low probability of independent multiple cross-links (multiplication of the individual cross-linking probabilities), the efficient increase in the number of cross-links with the number of AAC modules can not be explained by a random association of the modules with several Tom70 molecules. These results indicate rather that the modules of AAC cooperate in receptor binding by recruiting multiple Tom70 molecules: two modules recruit two Tom70 dimers and three modules recruit three Tom70 dimers into a large Tom70–preprotein complex.
The AAC modules cooperate in the accumulation in the GIP complex
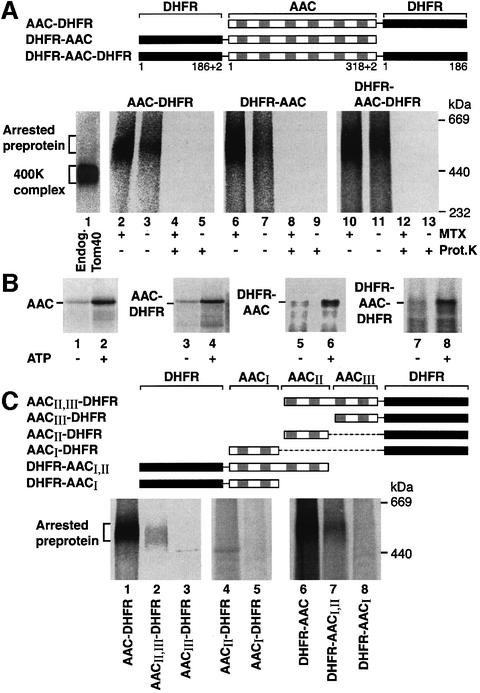
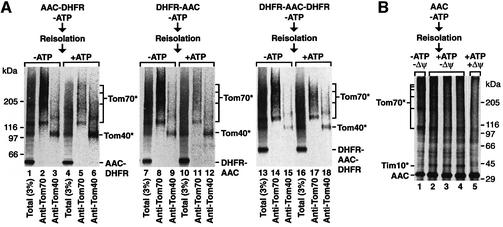
The fusion protein AAC–DHFR is selectively arrested in the outer membrane translocase at the 400 kDa GIP complex when the folded state of DHFR is stabilized by methotrexate (MTX) (Ryan et al., 1999). This suggests that, like the many preproteins with N-terminal targeting signals, AAC inserts into the GIP via its free N-terminus first. We wondered whether other means of AAC insertion into the GIP were conceivable and constructed a fusion protein containing DHFR at the N-terminus of AAC (Figure 3A). DHFR–AAC and, for comparison, AAC– DHFR were synthesized and radiolabeled in reticulocyte lysate. DHFR was stabilized by MTX, then the preproteins were incubated with isolated yeast wild-type mitochondria. The mitochondria were re-isolated, lysed in digitonin and subjected to blue native electrophoresis (BN-PAGE). Both DHFR–AAC and AAC–DHFR were found in a distinct high molecular mass region of 460–500 kDa on the native gel with comparable efficiency (Figure 3A, lanes 2 and 6). In the absence of preproteins, the GIP complex migrates at 400–440 kDa (Figure 3A, lane 1) (Dekker et al., 1998; van Wilpe et al., 1999). The size of each fusion protein of ∼55 kDa adds up to the sum of 460–500 kDa (Ryan et al., 1999). When the mitochondria carrying AAC constructs were treated with proteinase K, the GIP-accumulated preprotein forms were fully accessible to the protease (Figure 3A, lanes 4, 5, 8 and 9), demonstrating that they were not completely imported, but still exposed on the mitochondrial surface. In the absence of MTX (and presence of ATP), the accumulation of the fusion proteins in the GIP complex was strongly reduced (Figure 3A, lanes 3 and 7) and they were fully imported to a protease-protected location like authentic AAC (Figure 3B, lanes 2, 4 and 6). We conclude that neither a free N-terminus nor a free C-terminus is needed to initiate accumulation of AAC in the GIP complex.
Fig. 3. Cooperation of AAC modules in the accumulation in the GIP complex. (A) Schematic diagram of the N- and/or C-terminal fusions of DHFR to AAC. The radiolabeled fusion constructs were incubated in the presence or absence of 20 µM MTX with mitochondria isolated from wild-type S.cerevisiae. Following incubation at 25°C for 20 min, samples were halved and one half was treated with proteinase K (Prot. K). Mitochondria were re-isolated and subjected to BN-PAGE and phosphoimage analysis. Western transfer of a BN-PAGE followed by immunodecoration with anti-Tom40 indicates the mobility of the 400 kDa complex (lane 1). (B) The radiolabeled AAC constructs were incubated with mitochondria at 25°C for 15 min in the presence of Δψ and in the absence or presence of ATP as indicated, followed by treatment with proteinase K and analysis by SDS–PAGE. (C) Schematic diagram of N- and C-terminal deletions of AAC fused to DHFR. The indicated radiolabeled constructs were imported in the presence of 20 µM MTX as described in (A). The samples were not treated with proteinase K.
Fölsch et al. (1998) showed that the mitochondrial import machinery was able to translocate preproteins not only with the N-terminus first, but also with the C-terminus first. Therefore, we asked whether both AAC termini were dispensable at the same time for GIP accumulation by fusing DHFR to AAC at both the N- and C-terminus. Upon stabilization of the DHFR moieties by MTX, the fusion protein DHFR–AAC–DHFR was arrested in the GIP complex in a protease-accessible location (Figure 3A, lanes 10 and 12). In the absence of MTX and presence of ATP, DHFR–AAC–DHFR was imported into mitochondria (Figure 3B, lane 8). Although the efficiency of GIP accumulation of DHFR–AAC– DHFR was not as high as that of AAC–DHFR or DHFR–AAC, the characteristics of accumulation were comparable between all three AAC constructs, indicating that AAC can accumulate in the GIP complex despite both termini being blocked.
Endres et al. (1999) reported that the third module of AAC is important for accumulation in the TOM–GIP complex by determining the protease protection of constructs in the absence of a Δψ, yet did not directly address whether the preprotein constructs were associated with the complex. We used the BN-PAGE assay to monitor directly the accumulation of AAC constructs with different numbers of modules in the GIP complex. We found that removal of the third module of AAC from DHFR–AAC strongly reduced the efficiency of accumulation of the preprotein at the GIP complex (Figure 3C, lane 7). However, a similar effect was observed when module I was removed (Figure 3C, lane 2). In fact, none of the single modules alone was able to accumulate in the GIP complex to a significant amount that was stable enough to be observed via BN-PAGE (Figure 3C, lanes 3–5 and 8). Thus, it seems that at least two modules of AAC are required for its stable interaction with the GIP complex, and all three modules together strongly enhance the association. We conclude that the modules of AAC cooperate in the accumulation in the GIP complex.
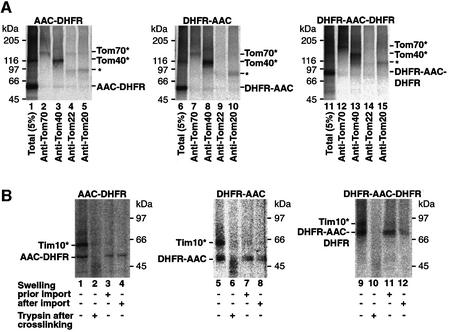
To obtain independent evidence that the AAC fusion proteins were indeed accumulated at the GIP in the presence of MTX, we performed cross-linking. The major cross-linking product of GIP-accumulated DHFR–AAC contained Tom40 (Figure 4A, lane 8), similar to the situation with AAC–DHFR (Figure 4A, lane 3) (Ryan et al., 1999), demonstrating that both fusion proteins were accumulated at the import pore. Additionally, a weaker cross-link to Tom70 was observed (Figure 4A, lanes 2 and 7) [cross-links of very low efficiency to Tom20 and Tom22 were also observed (Figure 4A, indicated by asterisks)]. The double fusion protein DHFR–AAC– DHFR yielded a qualitatively similar cross-linking pattern (Figure 4A, lanes 12 and 13), although the efficiency of cross-linking to Tom40 was reduced and that to Tom70 was enhanced compared with the single fusion proteins, indicating that two folded DHFR moieties reduced the efficiency of insertion into the import pore. Thus, both BN-PAGE and cross-linking indicate that the preprotein constructs are accumulated in the GIP of the outer membrane.
Fig. 4. Translocation of AAC across the outer membrane can be initiated while both termini are still located on the cytosolic side. (A) The GIP-accumulated AAC fusion constructs are in proximity to Tom70 and Tom40. [35S]AAC fusion proteins in reticulocyte lysate were incubated with isolated wild-type mitochondria in the presence of 20 µM MTX and subjected to cross-linking with MBS and immunoprecipitation. The total cross-linked forms are shown in lanes 1, 6 and 11 (5% of total used per immunoprecipitation). Tom70* and Tom40* are cross-links between the AAC construct and Tom70 or Tom40, respectively. The single asterisk indicates weak cross-links to Tom22/Tom20. (B) The GIP-arrested AAC fusion constructs can contact Tim10. Radiolabeled AAC–DHFR, DHFR–AAC or DHFR–AAC–DHFR was mixed with intact mitochondria (lanes 1, 2, 4–6, 8–10 and 12) or mitochondria that were pre-swollen to release intermembrane space content (lanes 3, 7 and 11) in the presence of MTX but in the absence of Δψ. Following incubation for 20 min at 25°C, mitochondria were re-isolated and some samples were subjected to swelling (lanes 4, 8 and 12). All samples were subjected to cross-linking with EGS (0.4 mM), followed by trypsin treatment where indicated (samples in lanes 2, 6 and 10) and immunoprecipitation with anti-Tim10 (all samples). Tim10*, cross-link between AAC construct and Tim10.
AAC can be translocated across the outer membrane in a loop form
We then asked whether the MTX-arrested preproteins not only associated with the GIP, but in fact traversed the outer membrane channel and became associated with the intermembrane space-located Tim9–Tim10 complex. Such an association was found by cross-linking MTX-arrested AAC–DHFR to Tim10 (Ryan et al., 1999), in agreement with the view of an N-terminal translocation. However, if arrested DHFR–AAC or DHFR–AAC–DHFR could also be cross-linked to Tim10, then a completely new concept of AAC translocation that involves a loop formation would be suggested. We therefore arrested AAC–DHFR, DHFR–AAC and DHFR–AAC–DHFR at the GIP complex and performed cross-linking with ethylene glycol-bis(succinimidylsuccinate) (EGS) and immunoprecipitation using antibodies against Tim10. In all cases, including even that of DHFR–AAC–DHFR, a cross-link to Tim10 was observed (Figure 4B, lanes 1, 5 and 9). Since an inherent characteristic of cross-linking experiments at organellar membranes is that only a fraction of the preproteins are cross-linked (Alconada, 1995; Rapaport et al., 1997), it is essential to demonstrate that the cross-linked species are indeed spanning across the outer mitochondrial membranes. To exclude the possibility that a population of preproteins was translocated completely across the outer membrane and then became bound to Tim10 (i.e. that the MTX arrest did not work), we arrested the preproteins as before, performed cross-linking and then degraded outer membrane-exposed preprotein with trypsin. Following immunoprecipitation, no Tim10 cross-links were visible (Figure 4B, lanes 2, 6 and 10). Thus, the preproteins that become cross-linked to Tim10 are not proteolytically protected, yet expose domains on the outer membrane surface. A further possibility is that the outer membranes of a fraction of mitochondria are broken before or during the import reaction and thus Tim10 could leak out and bind to a preprotein accumulated on the outer face of the mitochondria or preproteins could get direct access to the IMS-located Tim10. We thus performed a mitochondrial swelling either before or after preprotein arrest in order to break the outer membranes and determine whether cross-linking of AAC constructs to Tim10 would be increased. In both cases, however, no Tim10 cross-links were observed with any three of the constructs (Figure 4B, lanes 3, 4, 7, 8, 11 and 12). We conclude that the Tim10 cross-linking is specific to preproteins arrested at the GIP complex in a membrane-spanning manner. Since the DHFR fusions to the N- and C-termini of full-length AAC all result in GIP arrest and are accessible to Tim10 in the IMS, these results indicate that AAC can translocate across the GIP in a loop form.
A stepwise transfer of AAC from Tom70 to the GIP
We wondered whether the transfer of AAC from the Tom70 multimers to the components of the GIP occurs en bloc or is a stepwise process. In order to address this, we utilized the various DHFR-coupled forms of AAC that become arrested at the GIP complex before being translocated across the membrane and therefore can be analyzed at a transitionary stage. We incubated radiolabeled forms of AAC–DHFR, DHFR–AAC and DHFR– AAC–DHFR with mitochondria in the absence of ATP, thereby leading to their arrest at Tom70. Mitochondria were re-isolated and incubated in the presence or absence of ATP prior to cross-linking and immunoprecipiation with antibodies against Tom70 and Tom40. In the absence of ATP, four distinct cross-links of each of the preproteins to Tom70 were visible (Figure 5A, lanes 2, 8 and 14) instead of the six observed with AAC alone, indicating that the DHFR fusions may mask further binding sites to an additional Tom70 dimer. Some cross-linking of the preproteins to Tom40 was also observed (Figure 5A, lanes 3, 9 and 15). When the preproteins were chased to the GIP in the presence of ATP, most higher molecular weight Tom70 cross-links were no longer present, while a single cross-link to Tom70 (125–145 kDa) was still found (Figure 5A, lanes 5, 11 and 17) and the efficiency of cross-linking to Tom40 was increased (Figure 5A, lanes 6, 12 and 18). Since the presence of the DHFR domains may delay transfer of the preproteins, we performed a similar chase experiment with authentic AAC. The multiple high molecular weight cross-links of AAC to Tom70 upon accumulation in the absence of ATP (Figure 5B, lane 1) rapidly disappeared during the chase reaction in the presence of ATP, while the lower molecular weight cross-link persisted for a longer time (Figure 5B, lanes 2–4). Concomitantly, AAC was cross-linked to Tim10, indicating its translocation through the GIP (Figure 5B, lanes 2–4). Upon a complete chase reaction in the presence of both ATP and a Δψ, AAC was fully imported and significant cross-links to neither Tom70 nor to Tim10 were observed (Figure 5B, lane 5). We suggest that the transfer of preproteins from Tom70 to the GIP is not en bloc, but occurs via a stepwise release of AAC from the multiple contacts with Tom70.
Fig. 5. Stepwise transfer of AAC modules from Tom70 to the GIP. (A) Radiolabeled AAC–DHFR, DHFR–AAC or DHFR–AAC–DHFR were incubated with wild-type mitochondria at 25°C for 20 min in the presence of 20 µM MTX but in the absence of ATP. Samples were halved and re-isolated mitochondria were resuspended in import buffer containing 20 µM MTX in the absence or presence of ATP and incubated at 25°C for 10 min. The mitochondria were re-isolated and incubated with MBS, followed by immunoprecipitation with anti-Tom70 and anti-Tom40. The samples were subjected to gradient SDS–PAGE and cross-linked bands were detected by phosphoimaging (indicated by asterisks). The total cross-linking patterns are shown in lanes 1, 4, 7, 10, 13 and 16 (representing 3% of the total used per immunoprecipitation). (B) [35S]AAC was incubated with wild-type mitochondria in the absence of ATP (sample 1). The mitochondria were re-isolated and a chase reaction was performed (samples 2–5). The mitochondria were incubated by a stepwise increase of the temperature from 4°C [2.5 min (sample 2) to 10 min (sample 3)] to 12°C [an additional 2.5 min (sample 4)] and finally 25°C [10 min at each temperature (sample 5)] in import buffer containing ATP in the absence or presence of a Δψ. Cross-linking was performed with MBS and was analyzed by SDS–PAGE and autoradiography.
Each module is required for assembly of AAC in the inner membrane
It is unknown which of the AAC modules is required for the dimerization in the inner membrane. The assembly of in vitro imported AAC with pre-existing AAC can be monitored directly by following its dimerization by BN-PAGE (Palmisano et al., 1998; Ryan et al., 1999). In the presence of a Δψ, AAC assembles into a dimer and is protected from externally added protease (Figure 6, lane 2). When mitochondria were swollen and treated with protease, the AAC dimer was still intact, although it migrated somewhat faster in the gel (Figure 6, lane 4). Such protease treatment leads to the shaving of a short exposed region of at least one of the AAC monomers (Rassow and Pfanner, 1991). We asked whether a construct containing only modules I and III was sufficient for insertion into the inner membrane and dimerization. Radiolabeled AACI,III was incubated with mitochondria in the absence or presence of a Δψ, and analyzed by BN-PAGE and digital autoradiography. In both cases, only the monomeric form of the construct was observed (Figure 6, lanes 5 and 6). When mitochondria were swollen and protease treated following import in the absence of a Δψ, much of the preprotein was lost, suggesting that it was located in the IMS (Figure 6, lane 7). In the presence of a Δψ, the preprotein was partially protected, suggesting that it was inserted in the inner membrane in a monomeric form, but not assembled (Figure 6, lane 8). These results indicate that module II is crucial for dimer formation of AAC. We similarly analyzed the AAC constructs lacking module I (AACII,III) or module III (AACI,II) and did not observe any dimerization upon import in the presence of a Δψ (not shown). We then constructed AAC preproteins that retained five of the six transmembrane segments: AACΔ1–87 lacked the N-terminal portion but retained the second hydrophobic segment of module I, while AACΔ248–318 lacked the C-terminal portion, yet retained the first hydrophobic segment of module III. Neither of the two AAC constructs was able to assemble to a dimeric form (Figure 6, lanes 14, 16, 22 and 24), demonstrating that modules I and III are also crucial for dimerization of AAC. Finally, we removed the IMS-exposed terminal segments of AAC, the N-terminal 26 residues or the C-terminal 13 residues. Both constructs were imported into the inner membrane and assembled to the dimeric forms (Figure 6, lanes 10, 12, 18 and 20). We conclude that all three modules of AAC except the extreme termini are required for assembly of the protein to the dimeric form.
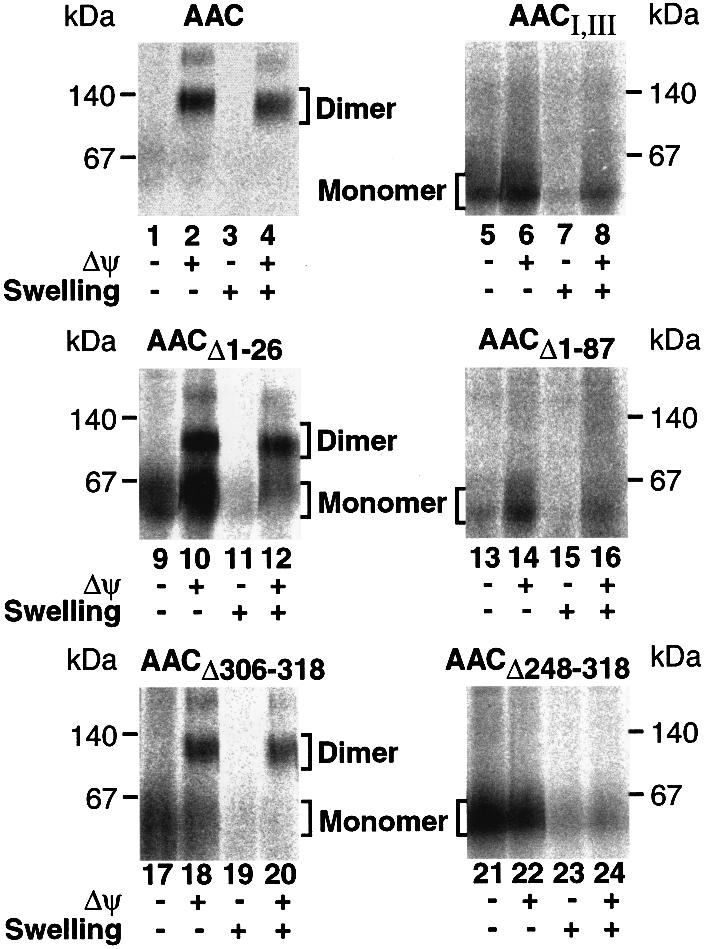
Fig. 6. Each module of AAC is required for assembly to the dimeric form in the inner membrane. The various [35S]AAC constructs were incubated with wild-type mitochondria in the absence or presence of a Δψ. Samples were split and mitochondria from one half were swollen. All samples were subjected to proteinase K treatment prior to BN-PAGE and phosphoimage analysis.
Discussion
The ADP/ATP carrier is the most abundant representative of a large number of hydrophobic proteins of the mitochondrial inner membrane that consist of three modules. In various attempts to define the major targeting region of these presequence-deficient proteins, individual modules have been identified. Surprisingly, however, different studies led to the assignment of distinct modules as the dominant signal region (Pfanner et al., 1987b; Liu et al., 1988; Smagula and Douglas, 1988; Endres et al., 1999; Schleiff and McBride, 2000). The results of this study lead to a new mechanistic concept of import of carrier proteins where the three modules do not function as independent units, but cooperate in targeting and membrane translocation of the preprotein.
We show that all three modules of AAC can act concomitantly in binding to the receptor Tom70. In the absence of preproteins, Tom70 forms a dimer in the outer membrane (Söllner et al., 1992; Millar and Shore, 1994). A full-length AAC recruits three dimers of Tom70, as demonstrated by efficient cross-linking of six Tom70 molecules to one AAC preprotein. The presence of any other Tom protein or of the cytosolic import factors MSF or Hsc70 in the cross-links was excluded. Interestingly, AAC constructs consisting of any combination of two modules can be cross-linked to four Tom70 molecules each, indicating a recruitment of two Tom70 dimers. Each single module contains targeting information sufficient for binding to Tom70 (Brix et al., 1999; this study); however, the efficiency is low. Although module II alone still shows the best yield of binding to Tom70, any combination of two modules, including a construct consisting of modules I and III, interacts with Tom70 with an efficiency that is one order of magnitude higher. Moreover, the full-length construct with three modules is one order of magnitude more efficient in cross-linking to Tom70 than the two-module constructs. We conclude that no single module represents the targeting-determining region of AAC, but rather all three modules are of roughly equal importance and cooperate in receptor recruitment. Since the cytosolic transport factor MSF (Komiya et al., 1997) has already been released at this import stage, Tom70 is the only binding partner that is responsible for preventing aggregation and misfolding of the carrier proteins that contain multiple hydrophobic segments. The recruitment of three receptor dimers to a single preprotein, i.e. one Tom70 dimer for each module, will provide an effective way of keeping the bound preprotein in a non-aggregated form. We propose that the receptor Tom70 serves two functions at the same time: as specific receptor and as chaperone for strongly hydrophobic preproteins such as the carrier proteins with six membrane-spanning segments.
The subsequent transfer of AAC from Tom70 to the GIP, in particular the channel protein Tom40, does not occur en bloc, but in a stepwise manner. This suggests a pathway where individual modules are released from Tom70 and transferred to Tom40 while another module is still bound to the receptor. Such a stepwise transfer of AAC modules from the receptor to the import pore would ensure that several regions of the preprotein are in contact with import components, and thus aggregation or misfolding of the preprotein would also be prevented during the transfer reaction. The stable accumulation of AAC in the GIP complex requires the involvement of at least two modules and is strongly enhanced when all three modules are present, indicating that the three modules cooperate not only in the binding to the receptor, but also in the accumulation in the GIP. Endres et al. (1999) proposed that module III plays the dominant role in the import process by interacting with the TOM complex with high affinity and directing translocation across the outer membrane; however, they did not analyze the interaction with the TOM complex directly but deduced it from the protease accessibility of the preprotein. We indeed find that removal of module III strongly impairs the stable accumulation of an AAC construct in the GIP complex, yet a comparable impairment is observed when only module I is removed. Similar to the situation with binding to Tom70, the efficiency of accumulation in the GIP complex differs by at least an order of magnitude between AAC constructs containing three modules, two modules or only one module, without a prominent role of an individual module.
By blocking the N- or C-terminus of AAC with a tightly folded domain, we demonstrate that neither terminus is required for the initiation of translocation. In both cases, the AAC construct can be cross-linked to Tim10, thereby demonstrating that part of it has traversed the outer membrane channel and stretches into the intermembrane space. Moreover, when both termini are blocked simultaneously, the resulting preprotein construct can still be cross-linked to Tim10. We carefully controlled that the cross-linked preprotein indeed spanned the outer membrane and excluded the possibility that the contact with Tim10 was due to a hypothetical leakage of Tim10 from broken outer membranes. To our knowledge, these results are the first experimental demonstration that an interior region of a mitochondrial preprotein can be translocated across the outer membrane prior to translocation of any terminal region, i.e. the preprotein inserts into the outer membrane in a loop formation. The effective diameter of the Tom40 translocation channel of the outer membrane was determined to be ∼2.2 nm (Hill et al., 1998; Künkele et al., 1998; Schwartz and Matouschek, 1999), i.e. it is indeed sufficiently wide to permit translocation of two polypeptide segments in α-helical conformation. Finally, we found that the assembly of AAC to the dimer in the inner membrane also involved all three modules.
We propose the concept that the import of carrier proteins into mitochondria is not directed by a single signal-containing region, but that the three modules function in a concerted manner. Although the analysis of transport intermediates does not exclude the existence of additional import mechanisms, it is evident that the import of AAC differs significantly from that of a typical cleavable preprotein, which is directed into mitochondria by the N-terminal presequence and translocates in a linear fashion. Our results suggest that the AAC modules cooperate at several stages of the import process: targeting to the receptor Tom70; translocation through the GIP in a loop form; and assembly in the inner membrane. This mode of import will help to shield the preprotein from improper interactions and thus permits an efficient translocation of such a hydrophobic preprotein.
Materials and methods
Plasmid construction and in vitro synthesis of precursor proteins
Yeast AAC2 was amplified by PCR with Vent polymerase (NEB) and cloned into pGEM-4Z (Promega) to yield pGEM-AAC. Mouse DHFR lacking its stop codon was amplified and a three-way ligation with AAC2 and pGEM-4Z yielded pGEM-DHFR-AAC. The _Bam_HI site linking the DHFR and AAC open reading frames encoded the amino acids G and S. The plasmid pGEM-DHFR-AAC-DHFR was constructed by digestion of pGEM-DHFR-AAC and pGEM-AAC-DHFR (Ryan et al., 1999), followed by ligation. Inverse PCR of pGEM-AAC and pGEM-AAC- DHFR yielded the plasmids pGEM-AACI,III, pGEM-AACII,III, pGEM-AACIII, pGEM-AACI-DHFR and pGEM-AACI,II-DHFR encoding AAC constructs 1–114(Δ115–220)221–318, 113–318, 221–318, 1–114–DHFR and 1–222–DHFR, respectively. Similarly, pGEM- AACI,II-DHFR was used for the generation of pGEM-AACII-DHFR (encoding 113–222–DHFR).
Constructs that were not cloned into a plasmid were amplified by PCR for in vitro transcription. C-terminal deletion constructs were amplified using the upstream vector primer and primers complementary to a region of AAC plus an artificial TAA stop codon. The constructs AACΔ306–318, AACΔ248–318, AACI,II and AACI were obtained using pGEM-AAC as template. Similarly, AACII, DHFR–AACI,II(1–221) and DHFR– AACI(1–113) were amplified using pGEM-AACII,III and pGEM-DHFR-AAC. For N-terminal deletions, we used the downstream vector primer and a primer containing the SP6 polymerase-binding site, followed by a start codon ATG and the sequence encoding the first 6–7 amino acids of the desired AAC construct. The constructs AACΔ1–26, AACΔ1–87, AACII,III(129–318)–DHFR and AACIII(231–318)–DHFR were obtained using pGEM-AAC and pGEM-AAC-DHFR as templates. Mitochondrial preproteins were synthesized by in vitro transcription using SP6 RNA polymerase (Stratagene) followed by in vitro translation in the presence of [35S]methionine/cysteine using rabbit reticulocyte lysate (Amersham).
Import of preproteins into isolated mitochondria
The Saccharomyces cerevisiae strains used in this study are shown in Table I. Yeast cells were grown on YP medium [1% (w/v) yeast extract, 2% (w/v) bactopeptone] with 3% (w/v) glycerol or 2% (w/v) glucose in the case of tom22Δ cells. Mitochondria were isolated (Ryan et al., 1999), resuspended in SEM buffer (250 mM sucrose, 1 mM EDTA, 10 mM MOPS–KOH pH 7.2) and stored at –80°C. For in vitro import, yeast mitochondria (25–50 µg of protein) were incubated in import buffer [3% (w/v) fatty acid-free bovine serum albumin (BSA), 80 mM KCl, 5 mM MgCl2, 10 mM MOPS–KOH pH 7.2]. The energetic conditions were varied by the addition of 2 mM NADH (Roche) for the maintenance of Δψ or by the addition of 8 µM antimycin A, 20 µM oligomycin and 1 µM valinomycin (Sigma) to dissipate Δψ. To provide external ATP, 2 mM ATP was added. For the regeneration of ATP levels, 100 µg/ml creatine kinase and 5 mM creatine phosphate (Roche) were added in addition to ATP. To deplete external ATP levels, 25 U/ml apyrase (Fluka) were added separately to the rabbit reticulocyte lysate containing 35S-labeled preproteins and to the mitochondria in import buffer. The apyrase-containing samples were incubated for 10 min at 25°C. Where indicated, MTX (20 µM) was added to the import buffer. The import reactions were started by the addition of rabbit reticulocyte lysate containing 35S-labeled preprotein [2.5–10% (v/v) of the import reaction] to the mitochondria in import buffer pre-treated according to the energetic requirements and incubated at 25°C. Mitochondria suspended in 1 vol. of SEM were swollen by resuspension in 9 vols of EM (1 mM EDTA, 10 mM MOPS–KOH pH 7.2), followed by a 15 min incubation on ice either before or after the import of 35S-labeled preproteins. Where indicated, samples were treated with either proteinase K (50 µg/ml) or trypsin (20 µg/ml) on ice for 15 min.
Table I. Saccharomyces cerevisiae strains used in this study.
| Strain | Genotype | Reference |
|---|---|---|
| PK82 (WT) | his4-713 lys2 ura3-52 Δtrp1 leu2-3,112 | Gambill et al. (1993) |
| YPH499 (WT) | ade2-101 his3-Δ200 leu2-Δ1 ura3-52 trp1-Δ63 lys2-801 | Sikorski and Hieter (1989) |
| KD30 (tom72Δ) | ade2-101 his3-Δ200 leu2-Δ1 ura3-52 trp1-Δ63 lys2-801 tom72::LEU2 | Bömer et al. (1996) |
| MM208 (tom70Δ) | ade2-101 his3-Δ200 leu2-Δ1 ura3-52 trp1-Δ63 lys2-801 tom70::HIS3 | Moczko et al. (1994) |
| MR100 (tom37Δ) | ade2-101 his3-Δ200 leu2-Δ1 ura3-52 trp1-Δ63 lys2-801 tom37::LEU2 | Ryan et al. (1999) |
| OL201 (tom22Δ) | _his3-Δ200 leu2-Δ1ura3-52 trp1-Δ63 tom22::HIS3 rho_0 | van Wilpe et al. (1999) |
| MM112-C (tom20Δ) | ade2-101 his3-Δ200 leu2-Δ1 ura3-52 trp1-Δ63 lys2-801 tom20::URA3 Yep13(LEU2)-TOM22 | Moczko et al. (1994); |
| Hönlinger et al. (1995); | ||
| Dekker et al. (1998) | ||
| AH101 (tom7Δ) | ade2-101 his3-Δ200 leu2-Δ1 ura3-52trp1-Δ63 lys2-801 tom7::TRP1 | Hönlinger et al. (1996) |
| MM307 (tom6Δ) | ade2-101 his3-Δ200 leu2-Δ1 ura3-52 trp1-Δ63 lys2-801 tom6::URA3 | Hönlinger et al. (1996) |
| KD56 (tom5Δ) | ade2-101 his3-Δ200 leu2-Δ1 ura3-52 trp1-Δ63 lys2-801 tom5::HIS3 | Dietmeier et al. (1997) |
Cross-linking and immunoprecipitation
Mitochondria were re-isolated by centrifugation through an S500EM (500 mM sucrose, 1 mM EDTA, 10 mM MOPS–KOH pH 7.2) sucrose cushion, resuspended in SEM and incubated with 0.4–1 mM EGS or MBS (Pierce) for 30 min on ice. The cross-linking reaction was quenched with 50 mM Tris–HCl pH 7.4. Mitochondria were resuspended in lysis buffer (150 mM NaCl, 10 mM Tris pH 7.6, 0.5% Triton X-100) containing 1% SDS, incubated for 5 min at 95°C, diluted with 20 vols of lysis buffer and subjected to immunoprecipitation using antibodies covalently attached to protein A–protein G beads. The antigens were eluted with 100 mM glycine pH 2.5. Antibodies specific for cytosolic Hsc70s were obtained from Santa Cruz Biotechnology (K-19).
Miscellaneous
Standard procedures were used for western transfer, immunodecoration, BN-PAGE, glycine– and tricine–SDS–PAGE (Ryan et al., 1999). To analyze high molecular weight cross-links, 4–10% tricine linear gradient gels with a 3.5% stacking gel were used.
Acknowledgments
Acknowledgements
We thank Drs K.Mihara and T.Komiya for antibodies against MSF, Drs W.Voos and K.Truscott for experimental advice and discussion, and Hanne Müller for expert technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 388 and the Fonds der Chemischen Industrie.
References
- Adam A., Endres,M., Sirrenberg,C., Lottspeich,F., Neupert,W. and Brunner,M. (1999) Tim9, a new component of the TIM22⋅54 translocase in mitochondria. EMBO J., 18, 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alconada A., Gärtner,F., Hönlinger,A., Kübrich,M. and Pfanner,N. (1995) Mitochondrial receptor complex from Neurospora crassa and Saccharomyces cerevisiae. Methods Enzymol., 260, 263–286. [DOI] [PubMed] [Google Scholar]
- Bömer U., Pfanner,N. and Dietmeier,K. (1996) Identification of a third yeast mitochondrial Tom protein with tetratricopeptide repeats. FEBS Lett., 382, 153–158. [DOI] [PubMed] [Google Scholar]
- Brix J., Rüdiger,S., Bukau,B., Schneider-Mergener,J. and Pfanner,N. (1999) Distribution of binding sequences for the mitochondrial import receptors Tom20, Tom22 and Tom70 in a presequence-carrying preprotein and a non-cleavable preprotein. J. Biol. Chem., 274, 16522–16530. [DOI] [PubMed] [Google Scholar]
- Dekker P.J.T., Ryan,M.T., Brix,J., Müller,H., Hönlinger,A. and Pfanner,N. (1998) Preprotein translocase of the outer mitochondrial membrane: molecular dissection and assembly of the general import pore complex. Mol. Cell. Biol., 18, 6515–6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekert K., Kispal,G., Guiard,B. and Lill,R. (1999) An internal targeting signal directing proteins into the mitochondrial intermembrane space. Proc. Natl Acad. Sci. USA, 96, 11752–11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietmeier K., Hönlinger,A., Bömer,U., Dekker,P.J.T., Eckerskorn,C., Lottspeich,F., Kübrich,M. and Pfanner,N. (1997) Tom5 functionally links mitochondrial preprotein receptors to the general import pore. Nature, 388, 195–200. [DOI] [PubMed] [Google Scholar]
- Endres M., Neupert,W. and Brunner,M. (1999) Transport of the ADP/ATP carrier of mitochondria from the TOM complex to the TIM22⋅54 complex. EMBO J., 18, 3214–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fölsch H., Guiard,B., Neupert,W. and Stuart,R.A. (1996) Internal targeting signal of the BCS1 protein: a novel mechanism of import into mitochondria. EMBO J., 15, 479–487. [PMC free article] [PubMed] [Google Scholar]
- Fölsch H., Gaume,B., Brunner,M., Neupert,W. and Stuart,R.A. (1998) C- to N-terminal translocation of preproteins into mitochondria. EMBO J., 17, 6508–6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambill B.D., Voos,W., Kang,P.J., Miao,B., Langer,T., Craig,E.A. and Pfanner,N. (1993) A dual role for mitochondrial heat shock protein 70 in membrane translocation of preproteins. J. Cell Biol., 123, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzer S. et al. (1995) Mas37p, a novel receptor subunit for protein import into mitochondria. J. Cell Biol., 129, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K., Model,K., Ryan,M.T., Dietmeier,K., Martin,F., Wagner,R. and Pfanner,N. (1998) Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature, 395, 516–521. [DOI] [PubMed] [Google Scholar]
- Hines V., Brandt,A., Griffiths,G., Horstmann,H., Brütsch,H. and Schatz,G. (1990) Protein import into yeast mitochondria is accelerated by the outer membrane protein MAS70. EMBO J., 9, 3191–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hönlinger A. et al. (1995) The mitochondrial receptor complex: Mom22 is essential for cell viability and directly interacts with preproteins. Mol. Cell. Biol., 15, 3382–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hönlinger A., Bömer,U., Alconada,A., Eckerskorn,C., Lottspeich,F., Dietmeier,K. and Pfanner,N. (1996) Tom7 modulates the dynamics of the mitochondrial outer membrane translocase and plays a pathway-related role in protein import. EMBO J., 15, 2125–2137. [PMC free article] [PubMed] [Google Scholar]
- Jensen R.E. and Johnson,A.E. (1999) Protein translocation: is hsp70 pulling my chain? Curr. Biol., 9, R779–R782. [DOI] [PubMed] [Google Scholar]
- Kerscher O., Holder,J., Srinivasan,M., Leung,R.S. and Jensen,R.E. (1997) The Tim54p–Tim22p complex mediates insertion of proteins into the mitochondrial inner membrane. J. Cell Biol., 139, 1663–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher O., Sepuri,N.B. and Jensen,R.E. (2000) Tim18p is a new component of the Tim54p–Tim22p translocon in the mitochondrial inner membrane. Mol. Biol. Cell, 11, 103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler C.M., Merchant,S., Oppliger,W., Schmid,K., Jarosch,E., Dolfini,L., Junne,T., Schatz,G. and Tokatlidis,K. (1998) Tim9p, an essential partner subunit of Tim10p for the import of mitochondrial carrier proteins. EMBO J., 17, 6477–6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler C.M., Murphy,M.P., Bally,N.A., Leuenberger,D., Oppliger,W., Dolfini,L., Junne,T., Schatz,G. and Or,E. (2000) Tim18p, a new subunit of the TIM22 complex that mediates insertion of imported proteins into the yeast mitochondrial inner membrane. Mol. Cell. Biol., 20, 1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya T., Rospert,S., Schatz,G. and Mihara,K. (1997) Binding of mitochondrial precursor proteins to the cytoplasmic domains of the import receptors Tom70 and Tom20 is determined by cytoplasmic chaperones. EMBO J., 16, 4267–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künkele K.P. et al. (1998) The preprotein translocation channel of the outer membrane of mitochondria. Cell, 93, 1009–1019. [DOI] [PubMed] [Google Scholar]
- Liu X., Bell,A.W., Freeman,K.B. and Shore,G.C. (1988) Topogenesis of mitochondrial inner membrane uncoupling protein. Rerouting transmembrane segments to the soluble matrix compartment. J. Cell Biol., 107, 503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar D.G. and Shore,G.C. (1994) Mitochondrial Mas70p signal anchor sequence: mutations in the transmembrane domain that disrupt dimerization but not targeting or membrane insertion. J. Biol. Chem., 269, 12229–12232. [PubMed] [Google Scholar]
- Moczko M., Ehmann,B., Gärtner,F., Hönlinger,A., Schäfer,E. and Pfanner,N. (1994) Deletion of the receptor MOM19 strongly impairs import of cleavable preproteins into Saccharomyces cerevisiae mitochondria. J. Biol. Chem., 269, 9045–9051. [PubMed] [Google Scholar]
- Neupert W. (1997) Protein import into mitochondria. Annu. Rev. Biochem., 66, 863–917. [DOI] [PubMed] [Google Scholar]
- Palmisano A., Zara,V., Hönlinger,A., Vozza,A., Dekker,P.J.T., Pfanner,N. and Palmieri,F. (1998) Targeting and assembly of the oxoglutarate carrier: general principles for biogenesis of carrier proteins of the mitochondrial inner membrane. Biochem. J., 333, 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N. and Neupert,W. (1987) Distinct steps in the import of ADP/ATP carrier into mitochondria. J. Biol. Chem., 262, 7528–7536. [PubMed] [Google Scholar]
- Pfanner N., Tropschug,M. and Neupert,W. (1987a) Mitochondrial protein import: nucleoside triphosphates are involved in conferring import-competence to precursors. Cell, 49, 815–823. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Hoeben,P., Tropschug,M. and Neupert,W. (1987b) The carboxy-terminal two-thirds of the ADP/ATP carrier polypeptide contains sufficient information to direct translocation into mitochondria. J. Biol. Chem., 262, 14851–14854. [PubMed] [Google Scholar]
- Rapaport D., Neupert,W. and Lill,R. (1997) Mitochondrial protein import. Tom40 plays a major role in targeting and translocation of preproteins by forming a specific binding site for the presequence. J. Biol. Chem., 272, 18725–18731. [DOI] [PubMed] [Google Scholar]
- Rassow J. and Pfanner,N. (1991) Mitochondrial preproteins en route from the outer membrane to the inner membrane are exposed to the intermembrane space. FEBS Lett., 293, 85–88. [DOI] [PubMed] [Google Scholar]
- Ryan M.T., Müller,H. and Pfanner,N. (1999) Functional staging of ADP/ATP carrier translocation across the outer mitochondrial membrane. J. Biol. Chem., 274, 20619–20627. [DOI] [PubMed] [Google Scholar]
- Schatz G. and Dobberstein,B. (1996) Common principles of protein translocation across membranes. Science, 271, 1519–1526. [DOI] [PubMed] [Google Scholar]
- Schleiff E. and McBride,H. (2000) The central matrix loop drives import of uncoupling protein 1 into mitochondria. J. Cell Sci., 113, 2267–2272. [DOI] [PubMed] [Google Scholar]
- Schwartz M.P. and Matouschek,A. (1999) The dimensions of the protein import channels in the outer and inner mitochondrial membranes. Proc. Natl Acad. Sci. USA, 96, 13086–13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R.S. and Hieter,P. (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics, 122, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirrenberg C., Bauer,M.F., Guiard,B., Neupert,W. and Brunner,M. (1996) Import of carrier proteins into the mitochondrial inner membrane mediated by Tim22. Nature, 384, 582–585. [DOI] [PubMed] [Google Scholar]
- Smagula C. and Douglas,M.G. (1988) ADP–ATP carrier of Saccharomyces cerevisiae contains a mitochondrial import signal between amino acids 72 and 111. J. Cell Biochem., 36, 323–327. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow,J., Wiedmann,M., Schlossmann,J., Keil,P., Neupert,W. and Pfanner,N. (1992) Mapping of the protein import machinery in the mitochondrial outer membrane by crosslinking of translocation intermediates. Nature, 355, 84–87. [DOI] [PubMed] [Google Scholar]
- Steger H.F., Söllner,T., Kiebler,M., Dietmeier,K.A., Pfaller,R., Trülzsch,K.S., Tropschug,M., Neupert,W. and Pfanner,N. (1990) Import of the ADP/ATP carrier into mitochondria: two receptors act in parallel. J. Cell Biol., 111, 2353–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wilpe S. et al. (1999) Tom22 is a multifunctional organizer of the mitochondrial preprotein translocase. Nature, 401, 485–489. [DOI] [PubMed] [Google Scholar]
- Voos W., Martin,H., Krimmer,T. and Pfanner,N. (1999) Mechanisms of protein translocation into mitochondria. Biochim. Biophys. Acta, 1422, 235–254. [DOI] [PubMed] [Google Scholar]