Mechanism for down-regulation of CD28 by Nef (original) (raw)
Abstract
SIV and HIV Nef proteins disrupt T-cell receptor machinery by down-modulating cell surface expression of CD4 and expression or signaling of CD3–TCR. Nef also down-modulates class I major histocompatibility complex (MHC) surface expression. We show that SIV and HIV-1 Nefs down-modulate CD28, a major co-stimulatory receptor that mediates effective T-cell activation, by accelerating CD28 endocytosis. The effects of Nef on CD28, CD4, CD3 and class I MHC expression are all genetically separable, indicating that all are selected independently. In cells expressing a Nef–green fluorescent protein (GFP) fusion, CD28 co-localizes with the AP-2 clathrin adaptor and Nef–GFP. Mutations that disrupt Nef interaction with AP-2 disrupt CD28 down-regulation. Furthermore, HIV and SIV Nefs use overlapping but distinct target sites in the membrane-proximal region of the CD28 cytoplasmic domain. Thus, Nef probably induces CD28 endocytosis via the AP-2 pathway, and this involves a ternary complex containing Nef, AP-2 and CD28. The likely consequence of the concerted down-regulation of CD28, CD4 and/or CD3 by Nef is disruption of antigen-specific signaling machineries in infected T cells following a productive antigen recognition event.
Keywords: AP-2 clathrin adaptor/CD28/lentivirus/Nef/T-cell activation
Introduction
Nef, a multifunctional regulatory protein of human and simian immunodeficiency viruses (HIV and SIV), is required for optimal virulence in vivo (Kestler et al., 1991; Deacon et al., 1995; Alexander et al., 1999; Kirchhoff et al., 1999). Nef increases virion infectivity and the replication of SIV and HIV viruses in primary T cells and model cell lines (Spina et al., 1994; Alexander et al., 1997). Nef has multiple independent effects on normal T-cell function: it modulates signal transduction pathways in T cells; it disrupts the sorting of proteins important for antigen-specific responses in major histocompatibility complex (MHC) class II-restricted T cells; and it compromises the antiviral response of the host to HIV/SIV-infected cells (for recent reviews see Oldridge and Marsh, 1998; Collins and Baltimore, 1999; Piguet et al., 1999; Skowronski et al., 1999; Renkema and Saksela, 2000).
Nef disrupts several aspects of the T-cell receptor (TCR) machinery in CD4-positive T cells. It accelerates the endocytosis of the CD4 co-receptor (Garcia and Miller, 1991) by promoting the recruitment of CD4 to the AP-2 clathrin adaptor at the cell membrane (for recent reviews see Oldridge and Marsh, 1998; Piguet et al., 1999; Skowronski et al., 1999). Both HIV-1 and SIV Nefs interact directly with the AP-2 adaptor complex, and this interaction is mediated in HIV-1 Nef by a di-leucine sorting signal located in the C-terminal disordered loop of the Nef molecule (Bresnahan et al., 1998; Craig et al., 1998; Greenberg et al., 1998a), and in SIV Nef by two non-di-leucine-based elements located in the N-terminal loop of the molecule (Piguet et al., 1998; Lock et al., 1999). HIV-1 Nef also interacts directly with CD4 (Grzesiek et al., 1996; Hua and Cullen, 1997), possibly forming a ternary complex that stabilizes the normal interaction between the di-leucine-based sorting signal in the CD4 cytoplasmic domain and the AP-2 clathrin adaptor.
Nef proteins also disrupt normal TCR-initiated signaling by interfering with the CD3–TCR complex (Iafrate et al., 1997). HIV-1 Nef blocks a membrane-proximal event in the CD3–TCR signaling cascade (Luria et al., 1991; Iafrate et al., 1997), while SIV Nef interacts directly with the ζ-subunit of CD3 and down-regulates expression of the CD3–TCR complex at the cell surface by an as yet unknown pathway (Bell et al., 1998; Howe et al., 1998; Swigut et al., 2000). The observation that HIV-1 and SIV Nefs use different mechanisms to disrupt TCR signaling and to down-regulate CD4 expression is evidence that Nef-mediated disruption of antigen-specific signaling in infected T cells provides a distinct survival advantage for the virus. Additional effects of Nef involve the modulation of several effectors and signaling pathways in T cells such as PAK and PKC kinases (Lu et al., 1996; Smith et al., 1996), activation of NFAT1 (Manninen et al., 2000) and modulation of calcium signaling (Skowronski et al., 1993; Baur et al., 1994). The biological consequences and roles of these effects are not clear.
Nef down-regulates expression of class I MHC at the cell surface, and may thereby compromise immune surveillance of HIV/SIV-infected cells (Schwartz et al., 1996). The abnormally low class I MHC expression on the surface of HIV-1-infected cells increases the probability that these cells will not be detected and eliminated by cytotoxic T cells that recognize epitopes derived from viral proteins (Collins et al., 1998). Decreased class I MHC expression on the surface of Nef-expressing cells reflects the accelerated endocytosis of MHC (Schwartz et al., 1996) and sorting to a Golgi subcompartment that contains the AP-1 clathrin adaptor (Greenberg et al., 1998a; Le Gall et al., 1998). Nef-induced class I MHC down-regulation possibly involves an interaction between Nef and the _trans_-Golgi network (TGN) sorting protein PACS-1 (Piguet et al., 2000), but does not appear to require Nef’s interaction with AP-2 clathrin adaptor (Greenberg et al., 1998a; Lock et al., 1999; Le Gall et al., 2000). Notably, induction of CD4 endocytosis by Nef requires Nef’s ability to interact with the AP-2 (Greenberg et al., 1998a,b; Lock et al., 1999), but not with PACS-1. Thus, Nef probably exploits distinct sorting pathways to induce CD4 and class I MHC endocytosis.
Here we report that HIV-1 as well as SIV Nef proteins down-regulate cell surface expression of the CD28 molecule. The CD28-initiated co-stimulatory signal is critical for normal antigen-specific T-cell responses, and interference with the CD28 signaling pathway could result in suppression of the immune response and anergy (reviewed by Schwartz, 1992; Lenschow et al., 1996). CD28 down-regulation by Nef involves direct molecular interactions between Nef and CD28 molecules. Genetic and functional evidence suggests that the mechanism that Nef uses to down-regulate CD28 expression is similar to that used to down-regulate CD4, since in both instances Nef accelerates the rate of endocytosis via the AP-2 clathrin adaptor pathway. The likely consequence of the concerted disruption of antigen-specific signaling machineries in HIV-1-infected T cells by Nef is to uncouple T-cell activation from the antigen-specific interactions of T cells with antigen-presenting cells (APCs) and thus facilitate the spread of the infected T cells.
Results
Nef accelerates the rate of CD28 endocytosis
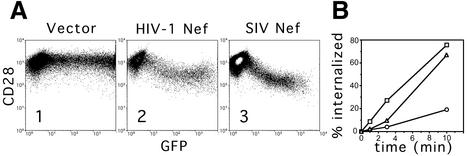
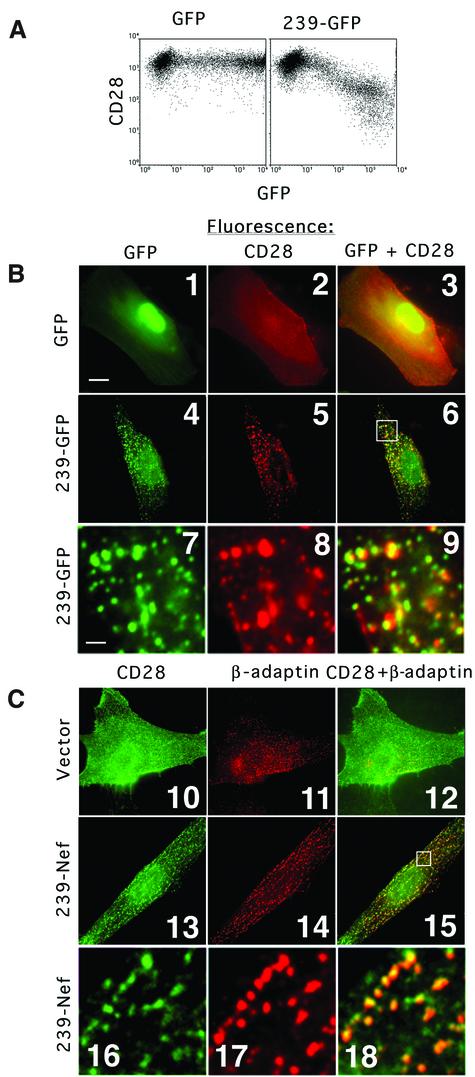
A transient expression assay in human Jurkat T cells was used to study the effect of HIV-1 and SIV Nef proteins on CD28 expression at the cell surface (Iafrate et al., 1997). Jurkat T cells were transfected with plasmids expressing Nef and a green fluorescent protein (GFP) reporter from the same bi-cistronic transcription unit, and CD28 cell surface expression and GFP expression were detected by flow cytometry. As shown Figure 1A, expression of SIV Nef from the pathogenic SIV mac239 strain resulted in a dose-dependent decrease in the steady-state CD28 surface expression, with a maximal 10-fold decrease in cells expressing the highest levels of GFP (compare panel 3 with panel 1). This result confirmed an earlier observation that the SIV mac239 nef allele can decrease CD28 surface expression (Bell et al., 1998). Expression of a natural HIV-1 Nef protein encoded by the NA7 allele (Mariani and Skowronski, 1993) also resulted in a similar decrease in the steady-state level of CD28 on the cell surface (panel 2).
Fig. 1. Nef down-regulates surface CD28 expression. (A) Dose–response analysis of surface CD28 antigen down-regulation by HIV-1 and SIV Nef proteins. Jurkat T cells were transfected with 10 µg of the bi-cistronic vectors expressing HIV-1 Nef (panel 2) or SIV Nef (panel 3) and GFP reporter, and a control vector expressing GFP alone (panel 1). Surface CD28 and GFP were detected simultaneously by two-color flow cytometry and are shown on the logarithmic scale on the ordinate and abscissa, respectively. (B) CD28 internalization is accelerated by HIV-1 and SIV Nef proteins. The percentage fraction of CD28 molecules internalized in Jurkat T cells expressing HIV-1 Nef (squares) or SIV Nef (triangles) together with GFP, or a control vector expressing GFP alone (circles), determined as described in Materials and methods, is shown as a function of time.
We next measured the rates of CD28 endocytosis in Jurkat T cells transiently expressing Nef and GFP, or GFP alone as a control. Cells were reacted with anti-CD28 monoclonal antibody (mAb) labeled with phycoerythrin (PE), and the rates of CD28 internalization in Nef-expressing cells and in control cells were determined for populations of cells showing identical levels of GFP fluorescence using a flow cytometry-based endocytosis assay (Greenberg et al., 1997). As shown in Figure 1B, expression of SIV or HIV-1 Nef proteins resulted in an ∼5-fold increase in the rate of CD28 internalization over that seen with cells expressing GFP alone. Thus, the accelerated endocytosis of CD28 is probably responsible for down-regulation of CD28 expression on the surface of Nef-expressing cells.
Mutations that abolish the interaction of Nef with the AP-2 clathrin adaptor disrupt CD28 down-regulation
To address how Nef induces CD28 endocytosis, we determined which surfaces of SIV mac239 Nef protein (239.Nef) are required for this effect. Previous studies have revealed that Nef uses different surfaces and mechanisms to induce endocytosis of CD4 and class I MHC, and that defined mutations in Nef disrupt molecular interactions selectively required for these different functions (Iafrate et al., 1997; Lock et al., 1999; Le Gall et al., 2000; Swigut et al., 2000). We tested the effects of these Nef mutations on down-regulation of CD28 in a transient expression assay in Jurkat T cells.
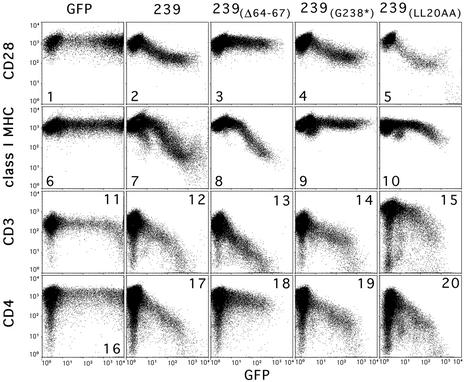
We first tested mutations that disrupt the interaction of SIV Nef with the AP-2 clathrin adaptor. As shown in Figure 2 and Table I, one such mutation in 239.Nef, namely deletion of amino acids Gln64–Asn67 [239(Δ64–67)], abolished CD28 down-regulation. This deletion disrupts the N-distal element in the N-terminal region of 239.Nef that mediates interaction with the AP-1/AP-2 clathrin adaptors and that is required for down-modulation of CD4 cell surface expression by Nef (Lock et al., 1999). Since HIV-1 Nef and SIV Nef are related proteins but use different surfaces to interact with the clathrin adaptors (Bresnahan et al., 1998; Lock et al., 1999; Skowronski et al., 1999; Swigut et al., 2000), we performed the same studies with HIV-1 Nef. HIV-1 Nef requires Leu164 and Leu165 to bind to clathrin adaptors (Bresnahan et al., 1998; Greenberg et al., 1998a). As shown in Table I, alanine substitutions for Leu164 and Leu165 [NA7(LL164AA)] disrupted the ability of HIV-1 Nef to down-regulate CD28 surface expression. Thus, both HIV-1 and SIV Nef are likely to induce CD28 endocytosis via a pathway involving the AP-2 clathrin adaptor.
Fig. 2. Effect of mutations in 239.Nef that disrupt CD4, CD3 and class I MHC down-regulation from the cell surface on CD28 down-regulation. Jurkat T cells were transfected with 10 µg of bi-cistronic plasmids expressing wild-type or mutant 239.Nef proteins and GFP reporter, or a control vector expressing GFP alone. Surface CD28, class I MHC, CD4 or CD3 and GFP were detected simultaneously by two-color flow cytometry and are shown in logarithmic scale as indicated.
Table I. Effect of mutations in SIV and HIV-1 Nef on their ability to down-regulate surface CD3, CD4 and class I MHC expression.
| Nef allele | Down-regulation of | ||
|---|---|---|---|
| CD4 | CD28 | MHC I | |
| SIV | |||
| 239.Nef | +++ | +++ | +++ |
| 239(Δ23–74) | – | – | ++++ |
| 239(Δ23–43) | +++ | ++ | +++ |
| 239(Δ42–45) | +++ | +++ | +++ |
| 239(Δ46–49) | +++ | +++ | +++ |
| 239(Δ54–57) | +++ | +++ | +++ |
| 239(Δ64–67) | – | – | +++ |
| 239(D155L) | +++ | +++ | – |
| 239(D204R) | – | – | +++ |
| 239(G238*) | +++ | +++ | – |
| 239(Δ96–103) | ++ | ++++ | + |
| HIV-1 | |||
| NL43 | +++ | +++ | +++ |
| NL43(WL57AA) | – | – | +++ |
| NL43(WLE57AAA) | – | – | +++ |
| NL43(E59A) | +++ | +++ | +++ |
| NL43(R106A) | ++ | ++ | ++ |
| NA7(LL164AA) | – | – | +++ |
CD4 and CD28 down-regulation are related functions of Nef
We then tested additional mutations that selectively disrupt other Nef functions. As shown in Figure 2 and summarized in Table I, mutations in the C-terminal region of 239.Nef that disrupt interactions required for the down-regulation of class I MHC and have some negative effect on the down-regulation of CD3 surface expression, such as a deletion of the C-terminal region of the 239(G238*), mutation of Asp155 to leucine [239(D155L)] or mutation of Leu20 and Leu21 to alanines [239(LL20AA)], had no detectable effect on CD28 down-regulation. In contrast, mutation of Asp204 to arginine in 239.Nef, which disrupts down-regulation of CD4 expression without affecting the ability of 239.Nef to interact with the AP-1/AP-2 clathrin adaptors [239(D204R); Lock et al., 1999; Iafrate et al., 2000], abolished 239.Nef’s ability to down-regulate CD28 surface expression. These observations suggest that induction of CD28 and CD4 endocytosis could share a common molecular interaction in addition to that with the AP-2 clathrin adaptor.
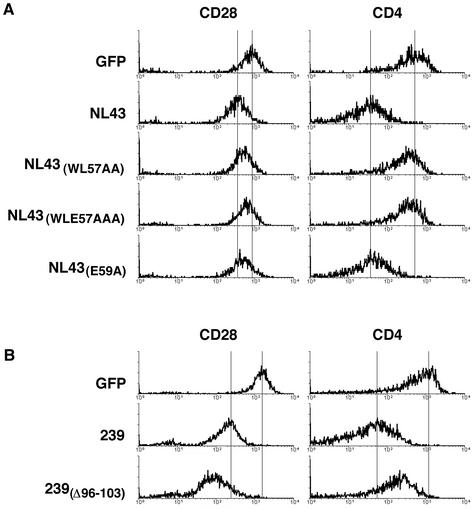
Nef also interacts directly with an element in the cytoplasmic domain of CD4. The putative surface of HIV-1 Nef required for this interaction was defined previously by solution NMR in the presence of CD4 cytoplasmic domain peptides, and is comprised of Trp57, Leu58, Glu59 and Arg106 (Grzesiek et al., 1996). We therefore tested the effects of substitutions at these residues on CD28 and CD4 down-regulation by HIV-1 Nef. As summarized in Table I and shown in Figure 3A, mutation of Glu59 or Arg106 alone had little detectable effect on the ability of Nef to down-regulate CD28 and CD4 expression, indicating that these residues contribute little to the interaction with CD4 and CD28. Triple alanine substitution of Trp57, Leu58 and Glu59 [NL43(WLE57AAA)] disrupted both CD4 and CD28 down-regulation. Also, a double alanine substitution of Trp57 and Leu58 had a similar effect [NL43(WL57AA); see Table I and Figure 3A]. Thus, HIV-1 Nef probably uses similar and overlapping surfaces to interact with CD4 and CD28, which probably contact determinants other than di-leucine residues in CD4 and CD28 cytoplasmic domains.
Fig. 3. (A) Mutations of the putative CD4-binding surface in HIV-1 Nef have similar effects on CD4 and CD28 down-regulation. Jurkat T cells were transfected with bi-cistronic vectors expressing wild-type HIV-1 Nef protein, or HIV-1 Nef with mutations of amino acid residues that form the putative CD4-binding site, and GFP reporter. Histograms of CD28 and CD4 cell surface expression on populations of cells with identical GFP fluorescence recorded by two-color flow cytometry are shown. (B) Mutation in SIV 239.Nef separates CD4 and CD28 down-regulation. Experiments were performed as described in (A).
Mutation separates the effect of SIV Nef on CD4 and CD28 expression
To assess whether the effects of Nef on CD4 and CD28 expression are separable functions, we screened a large number of previously characterized mutations in HIV-1 and SIV Nefs (data not shown). In this search, we found that deletion of amino acids 96–103 in 239.Nef enhances CD28 down-regulation but suppresses CD4 down-regulation [239(Δ96–103); Table I and Figure 3B). The separation of CD4 and CD28 down-regulation by the Δ96–103 deletion in SIV Nef is evidence that CD28 and CD4 down-regulation are genetically separable functions, even though both link to the AP-2 clathrin adaptor pathway.
Evidence for direct interaction of Nef with the CD28 cytoplasmic domain
To understand further the interactions between Nef, CD28 and the AP-2 clathrin adaptor, we studied the amino acid residues in CD28 required for constitutive and Nef-induced endocytosis.
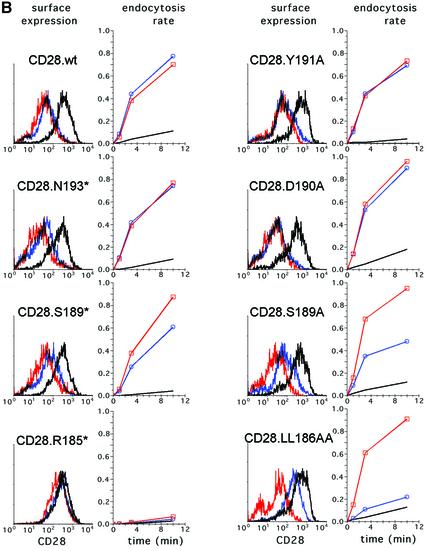
First, we tested the effect of deletions in the CD28 cytoplasmic domain on the rate of constitutive CD28 endocytosis. To permit an accurate and sensitive comparison of the wild-type and mutant CD28 proteins, we constructed a vector co-expressing CD28 and GFP, which is used as a marker of transfected cells, from the same bi-cistronic transcription unit. This design results in a constant ratio of CD28 and GFP, thus allowing reliable comparison of the properties of the wild-type and mutant CD28 proteins. HeLa cells were transiently transfected with bi-cistronic plasmids co-expressing wild-type or mutant forms of CD28 together with GFP. The rates of endocytosis of different forms of CD28 were determined for populations of cells showing identical GFP fluorescence. As summarized in Figure 4A, column 1, and shown in Figure 4B, deleting all but the first four membrane-proximal amino acids of the CD28 cytoplasmic domain (CD28.R185*) decreased the rate of constitutive CD28 endocytosis by ∼5-fold. A truncation at Asn193 (CD28.N193*) showed a wild-type level of constitutive endocytosis, suggesting that important sorting signals are located between Arg185 and Asn193. Next, the effects of amino acid substitutions for amino acid residues located proximal to Asn193, including two putative sorting signals located in this region, namely Tyr191 and Leu186 and Leu187, were tested. The Y191A substitution disrupted constitutive CD28 endocytosis to an extent similar to that seen with the R185* deletion (CD28.Y191A). In contrast, substitutions of Leu186 and Leu187 (CD28.LL186AA), or other amino acid residues in this membrane-proximal region, had little effect (CD28.D190A and CD28.S189A). Thus, Tyr191 is critical for the normal interaction of CD28 with the endocytic machinery in HeLa cells. These observations are consistent with previous data from experiments with T cells (Cefai et al., 1998).
Fig. 4. Summary of mutations in CD28 and their effects on CD28 endocytosis. (A) Alignment of amino acid sequences of a wild-type and mutant CD28 cytoplasmic domains is shown. Dots indicate amino acid identity with the wild-type protein, letters identify amino acid substitutions in the single letter code and the asterisks reflect stop codons. The nomenclature of mutant CD28 proteins is shown on the left. The relative ability of mutant CD28 molecules to undergo endocytosis in the absence or presence of HIV-1 or SIV Nef proteins is indicated on the right. (B) Effect of mutations in the CD28 cytoplasmic domain on steady-state cell surface expression and CD28 endocytosis. CD28-negative HeLa cells were co-transfected with plasmids expressing wild-type, or mutant CD28 proteins and GFP from the same bi-cistronic transcription unit, and a plasmid expressing HIV-1 Nef (NA7 allele) or SIV Nef (mac239), or an empty control vector. Histograms of CD28 expression on cells co-transfected with an empty control vector (black line), or on cells co-expressing HIV-1 Nef (red line) or SIV Nef (blue line) are shown for populations of cells with identical levels of GFP expression and therefore comparable CD28 expression rates (left panels). The percentage fraction of CD28 internalized from the surface cells expressing CD28 alone, or co-expressing CD28 and HIV-1 Nef, or SIV Nef, as a function of time is also shown (right panels). The internalization rates were measured for populations of cells with identical levels of GFP expression.
To assess whether the induction of CD28 endocytosis by Nef involves an interaction of CD28 with the sorting machinery, we determined the effect of the same set of mutations on CD28 endocytosis induced by HIV-1 Nef and SIV Nef. HeLa cells were co-transfected with bi-cistronic plasmids expressing wild-type or mutant CD28 molecules together with GFP and with a plasmid expressing HIV-1 Nef or SIV Nef proteins. The effect of Nef on cell surface expression and the rate of endocytosis of different forms of CD28 was determined for a population of cells with identical GFP expression. As summarized in Figure 4A, columns 2 and 3, and shown in Figure 4B, deletion of the cytoplasmic domain abolished the accelerated rate of CD28 endocytosis induced by either SIV or HIV-1 Nef (CD28.R185*). In contrast, deletions of sequences distal to Ser189 had no detectable effect on the rate of endocytosis seen with HIV-1 Nef, and only a minor negative effect on that seen with SIV Nef. Notably, the Y191A substitution, which disrupted constitutive CD28 endocytosis, had little effect on endocytosis induced by SIV and HIV-1 Nefs. Interestingly, mutations of Leu186 and Leu187 to alanines disrupted the effect of SIV Nef, but had only a marginal effect on HIV-1 Nef-induced or constitutive CD28 endocytosis. The observation that the amino acid residues required for the induction of CD28 endocytosis by Nef are different from those critical for constitutive CD28 endocytosis suggests that Nef modifies normal interactions between the CD28 cytoplasmic domain and the endocytic machinery. Furthermore, the observation that the effects of HIV-1 and SIV Nef require different amino acid residues in the CD28 cytoplasmic tail suggests that these two Nef molecules make direct but different contacts with CD28, and that these interactions are critical for the acceleration of the CD28 endocytosis rate.
Nef redistributes CD28 to the AP-2 clathrin adaptor and to endosomes
Evidence from genetic experiments predicted that Nef is likely to interact with the AP-2 clathrin adaptor to facilitate the recruitment of CD28 to the endocytic machinery in a manner similar to that proposed previously for CD4 (reviewed in Piguet et al., 1999; Skowronski et al., 1999). To test this model, we studied the cellular localization of CD28 in cells expressing 239.Nef. We used a chimeric protein comprised of 239.Nef joined at the C-terminal end to a strongly fluorescing variant of the GFP reporter (239–GFP) to visualize 239.Nef directly (Lock et al., 1999). As shown in Figure 5A, all Nef functions necessary for the down-regulation of CD28 surface expression are retained in this fusion protein.
Fig. 5. CD28 co-localizes with the 239–GFP fusion and AP-2 clathrin adaptor in IMR90 fibroblasts. (A) 239–GFP down-regulates CD28 expression on the cell surface. Jurkat T cells were transfected with 10 µg of plasmids expressing GFP alone (left panel) or 239–GFP (right panel), and CD28 expression on the cell surface and GFP expression were analyzed by two-color flow cytometry. (B) CD28 co-localizes with 239–GFP. IMR90 fibroblasts co-transfected with plasmids expressing CD28 and GFP (panels 1–3) or with plasmids expressing CD28 and 239–GFP (panels 4–9) were fixed, and CD28 was detected with mAb CD28.2 and visualized by indirect immunofluorescence (panels 2, 3, 5, 6, 8 and 9). GFP and 239–GFP were revealed by direct fluorescence (panels 1, 3, 4, 6, 7 and 9). Panels 7–9 are magnifications of a fragment of the cell shown in panels 4–6, respectively. The overlays of GFP and CD28 images were produced using Oncor imaging software (panels 3, 6 and 9). The bar in the lower left corner of panel 1 represents a distance of ∼20 µm in panels 1–6 and 10–15, and ∼2 µm in the remaining panels. (C) CD28 co-localizes with AP-2 clathrin adaptor in cells expressing 239.Nef. IMR90 fibroblasts were transfected with a plasmid expressing CD28 (panels 10–12), or CD28 and 239.Nef (panels 13–18). Cells were fixed and the CD28 pattern was revealed by indirect fluorescence with mAb CD28.2 (panels 10, 12, 13, 15, 16 and 18). Subsequently, cells were permeabilized and the β-adaptin subunit of AP-1 and AP-2 clathrin adaptors was revealed by indirect fluorescence with mAb 100/1 (panels 11, 12, 14, 15, 17 and 18). The overlays of CD28 and β-adaptin patterns are also shown (panels 12, 15 and 18). Panels 16–18 are magnifications of images shown in panels 13–15, respectively.
The relative distribution of CD28 and 239–GFP was studied in a CD28-negative human fibroblast line (IMR90) transiently expressing these proteins. As shown in Figure 5B, indirect immunofluorescence with a mAb specific for CD28 under conditions that block endocytosis revealed a uniform pattern of CD28 expression at the cell surface (panel 2). In contrast, in cells co-expressing CD28 and 239–GFP, CD28 was redistributed and displayed a characteristic punctate pattern at the cell surface (panel 5). Superposition of the 239–GFP and CD28 patterns revealed a large extent of co-localization (panels 6 and 9). In T cells and fibroblasts, Nef co-localizes with AP-2-containing clathrin coats (Greenberg et al., 1997, 1998a; Lock et al., 1999). To demonstrate directly that CD28 co-localizes with AP-2 coats in Nef-expressing cells, CD28 and AP-2 were visualized simultaneously by two-color fluorescent microscopy in IMR90 cells transiently co-expressing 239.Nef and CD28. As shown in Figure 5C, the CD28 fluorescent pattern co-localized with the AP-2 pattern in cells expressing 239.Nef (panels 15 and 18). In contrast, CD28 was distributed uniformly at the cell surface and was not detectable at AP-2 coats in the absence of 239-Nef (panels 10–12). The co-localization of CD28 with 239.Nef and AP-2 clathrin adaptor suggests that Nef redistributes CD28 to AP-2 coats at the plasma membrane.
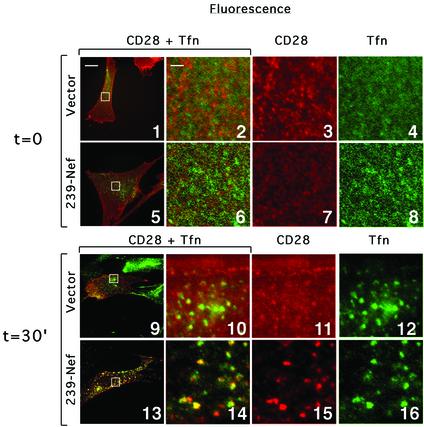
In Nef-expressing cells, class I MHC complexes internalized from the cell surface are redistributed to the TGN while the internalized CD4 is redistributed to endosomes and lysosomes (Schwartz et al., 1995; Greenberg et al., 1998b; Le Gall et al., 1998). Experiments were performed to determine which pathway is used with Nef-induced CD28 sorting from the plasma membrane. To visualize the internalized CD28, cells transiently co-expressing CD28 and 239.Nef, or expressing CD28 alone, were cultured in the presence of CD28.2 mAb (reacting with CD28). Fluorescein isothiocyanate (FITC)-labeled transferrin was also included in the culture medium to visualize the early compartments of the endocytic pathway. Cells were collected at various time points, fixed, permeabilized and the internalized CD28 was revealed by indirect fluorescence using Texas red (TxR)-labeled anti-mouse IgG antibody.
Two-color fluorescence microscopy revealed rapid internalization of CD28 in cells expressing 239.Nef. Figure 6 shows that after 30 min of incubation with CD28.2 mAb and transferrin, the majority of cell surface CD28 redistributed into a punctate pattern similar to the pattern of transferrin (panel 13). Close examination of magnified images revealed a large extent of co-localization of the two patterns (compare panels 14–16). In cells expressing 239.Nef, the redistribution of CD28 and transferrin into punctate co-localizing patterns could be detected even as early as after 2 min culture with CD28.2 mAb (data not shown). In contrast, control cells expressing CD28 alone had a fairly uniform distribution of CD28. Furthermore, this uniform distribution persisted for >30 min, indicating much slower internalization of CD28 in the absence of Nef (compare panels 9 and 11 with 13 and 15). In contrast, transferrin was redistributed rapidly into a punctate pattern in both the absence and presence of Nef, which shows that the effect of Nef on CD28 sorting was specific. Finally, the co-localization of internalized CD28 and transferrin in early endosomes indicates that they are sorted along similar pathways.
Fig. 6. Localization of CD28 molecules internalized from the surface of Nef-expressing cells. IMR90 fibroblasts expressing CD28 alone (panels 1–4 and 9–12) or co-expressing CD28 and 239–Nef (panels 5–8 and 13–16) were cultured for 30 min in the presence of CD28.2 mAb and FITC–transferrin (time = 30; panels 9–16). As a control to reveal the initial distribution of CD28 and transferrin receptor at the cell surface, cells were first fixed and then reacted with CD28.2 mAb and FITC–transferrin (time = 0; panels 1–8). The CD28.2 mAb was revealed with TxR-labeled goat anti-mouse IgG. The overlays of the two images are show in panels 1–2, 5–6, 9–10 and 13–14. Panels 2–4, 6–8, 10–12 and 14–16 are magnifications of boxed regions in panels 1, 5, 9 and 13, respectively. The bar in the upper left corner of panel 1 represents a distance of ∼20 µm in panels 1, 5, 9 and 13, and ∼2 µm in all remaining panels.
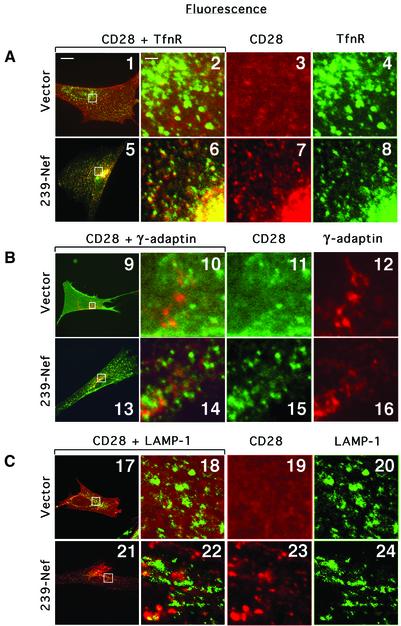
To characterize compartments containing internalized CD28 further, the steady-state distribution of CD28 in cells expressing 239.Nef was examined in co-localization experiments with: (i) transferrin receptor (TnfR), a marker of the early endosomes in the peripheral cytoplasm and recycling endosomes, which are usually concentrated in the perinuclear region adjacent to the Golgi; (ii) the γ-subunit of the AP-1 clathrin adaptor, which resides in the TGN; and (iii) LAMP-1, a lysosomal protein (Figure 7). The pattern of CD28 fluorescence closely overlapped the pattern of TfnR fluorescence both throughout the periphery of the cell and in the perinuclear area (panel 5). Analysis of the magnified images from the perinuclear region also consistently revealed close similarity of the CD28 and TfnR patterns (panels 6–8). The low-resolution fluorescent microscopy suggested that some overlap may exist between CD28 and γ-adaptin, as well as CD28 and LAMP-1 patterns, as all three proteins were found concentrated in the same general perinuclear region (panels 13 and 21). However, analysis of magnified images from the this area revealed that there was little co-localization between CD28 and γ-adaptin, or the LAMP-1 patterns (panels 14–16 and 22–24). These observations suggest that Nef induces redistribution of CD28 from the cell surface, and possibly traps its target in the endocytic and recycling compartments.
Fig. 7. Immunofluorescence microscopy analysis of the effect of Nef on the steady-state distribution of CD28 in IMR90 cells. IMR90 fibroblasts transiently transfected with plasmid expressing CD28 (panels 1–4, 9–12 and 17–20) or co-transfected with plasmids expressing CD28 and 239–Nef were fixed, permeabilized, and CD28 was detected together with TnfR and γ-adaptin or LAMP-1 by indirect fluorescence. CD28 was detected with CD28.2 mAb, which was revealed by TxR-labeled goat anti-mouse IgG antibody (A and C), or with FITC-labeled CD28.2 mAb (B). TfnR was detected with FITC-labeled anti-CD71 mAb, γ-adaptin with mAb 100/3 (Ahle et al., 1988) followed by TxR-labeled goat anti-mouse IgG antibody, and LAMP-1 with FITC-labeled anti-CD107a mAb. The overlays of the two images are shown in the two leftmost panels in each row. The three rightmost panels in each row are magnifications of the boxed region in the left panel. The bar in the upper left corner of panel 1 represents a distance of ∼20 µm in panels 1, 5, 9, 13, 17 and 21, and ∼2 µm in the remaining panels.
Discussion
We studied the mechanisms underlying the down-regulation of CD28 expression on the cell surface by HIV-1 and SIV Nef proteins. Genetic and functional evidence from our studies indicates that Nef induces CD28 endocytosis via an AP-2 clathrin adaptor-dependent pathway, and that this is mediated by direct interactions between Nef, AP-2 and CD28.
Nef probably makes direct contacts with the membrane-proximal region in the CD28 cytoplasmic domain. This is suggested by the observation that induction of CD28 endocytosis by SIV and HIV-1 Nef proteins requires partially overlapping but distinct sets of amino acid residues in the membrane-proximal region of the CD28 cytoplasmic domain. The use of different target sites in the CD28 cytoplasmic domain by HIV-1 and SIV Nef proteins argues against the possibility that they bind CD28 via a common bridging protein and implies that they contact the CD28 cytoplasmic domain directly.
Previous data indicate that Nef-induced endocytosis of CD4 and class I MHC requires intact native endocytic signals in the target molecules. Indeed, the Nef-induced endocytosis of CD4 requires the di-leucine sorting motif in the CD4 cytoplasmic domain (Aiken et al., 1994; Salghetti et al., 1995; Hua and Cullen, 1997), while that of class I MHC requires Tyr320 in the cytoplasmic domain of HLA A2 and B7 heavy chains (Greenberg et al., 1998b; Le Gall et al., 1998; Cohen et al., 1999). Both sequences are sorting signals required for the constitutive endocytosis of these molecules in the absence of Nef (Marsh and Pelchen-Matthews, 1996; M.Greenberg and J.Skowronski, unpublished data). Thus, it appears that for CD4 and class I MHC, Nef facilitates the interactions with AP-2, or with other components of the endocytic machinery that are normally required for constitutive endocytosis, rather than replacing these interactions with new ones.
Our data suggest that induction of CD28 endocytosis by Nef does not involve native endocytic signals in the CD28 cytoplasmic domain. Constitutive CD28 endocytosis requires Tyr191 and proceeds via a clathrin-dependent pathway (Cefai et al., 1998). Although Tyr191 is located in an amino acid sequence context similar to that of known tyrosine-based YppΦ sorting signals (where Φ is a bulky hydrophobic amino acid residue; reviewed in Kirchhausen, 1999), it is not likely to be a bona fide tyrosine-based sorting signal because CD28 with the N193* mutation, which disrupts this putative sorting signal, still internalizes well in the absence of Nef (CD28.N193*, see Figure 4A and B). Our data show that Tyr191 is not required for the effect of Nef, although both the HIV-1 and SIV Nef target sites in the CD28 cytoplasmic domain are located within a few amino acids from Tyr191. The close proximity of Nef target sites in the CD28 cytoplasmic domain to the likely normal site of interaction with the endocytic machinery is consistent with the possibility that CD28 contacts the endocytic machinery in a similar manner in the absence or presence of the viral protein, and Nef probably strengthens these normal interactions.
It is evident that the ability of Nef to down-regulate CD28 cell surface expression is genetically separable from other known functions of Nef such as CD4, CD3 and class I MHC down-regulation, and from the association with serine-threonine kinases. Hence, CD28 down-regulation is an independently selected function of Nef.
The benefit of reducing levels of CD28 on the surface of Nef-expressing T cells for SIV/HIV replication in the host has not been investigated yet, but can be speculated upon based on known functions of CD28 and on known modulating effects of Nef on signal transduction in T cells. During antigen presentation, T cells form tight conjugates with APCs. Cell–cell adhesion is mediated by multiple low-affinity interactions of several molecules expressed on the surface of T cells, including CD28, TCR, CD4 and leukocyte function antigen 1 (LFA1), with ligands presented on the surface of APCs, which include B7, MHC class II complexes and LFA1, as well as additional pairs of ligands (Grakoui et al., 1999; Hwang et al., 2000). The interactions between CD28 and B7, and between TCR/CD4 and class II MHC, are thought to make important contributions to the stability and maintenance of a tight and prolonged contact between T cells and APCs (Grakoui et al., 1999; Hwang et al., 2000). The concerted down-modulation of CD28 and CD4 by both HIV-1 and SIV Nefs, and of CD3 by SIV Nef, probably limits the adhesion of a Nef-expressing T cell to the APC following a productive antigen presentation event and promotes the disengagement of the activated T cell from the APC and its subsequent movement into circulation or to other APCs, thus facilitating the spread of the virus.
Another possible consequence of the co-ordinated down-modulation of CD3, CD4 and CD28 is a compromised duration and strength of antigen-specific signaling in the infected T cell. However, it should be noted that HIV-1 and SIV Nef proteins activate certain downstream effectors in signaling pathways that mediate T-cell activation. Specifically, previous studies have demonstrated that HIV-1 Nef promotes activation of thymic and peripheral T cells when expressed in these cells in transgenic mice, and that HIV-1 Nef decreases the threshold for antigen-specific stimulation in T-cell clones (Skowronski et al., 1993; Hanna et al., 1998; Schrager and Marsh, 1999). Moreover, Nef activates the nuclear factor of activated T cells 1 (NFAT 1; Manninen et al., 2000), stimulates interleukin-2 (IL-2) production in Epstein–Barr virus-immortalized T-cell clones and can overcome the requirement for exogenous IL-2 in these cells (Alexander et al., 1997). This indicates that Nef activates signaling events that conceivably can replace the normal signaling cascades that govern antigen-dependent T-cell activation. By down-regulating cell surface expression of CD28, CD4 and TCR, and simultaneously activating selected signaling pathways that bypass or synergize with the normal antigen-specific signaling events, Nef may promote the progression of the T-cell activation process while uncoupling it from antigen-dependent T cell interactions with the APC, thereby facilitating the spread of infected T cells.
Materials and methods
Plasmid construction
The oligo-directed site-specific mutagenesis of SIV mac239 nef was performed as described previously (Iafrate et al., 1997; Lock et al., 1999). Point mutations in CD28 cDNAs were introduced by oligonucleotide-directed mutagenesis, and cDNAs encoding CD28 molecules with progressive deletions in the cytoplasmic domain were constructed using PCR. All mutations were verified by DNA sequencing, and mutant alleles were subcloned into CMV-based pCG, or bi-cistronic pCGCG expression vector, as described previously (Tanaka and Herr, 1990; Lock et al., 1999). The pCGCG vector contains cDNA encoding GFP (Stauber et al., 1998) under translational control of the EMCV IRES element and was constructed in pCG as described previously (Lock et al., 1999). Genes expressing SIV mac239 Nef and GFP were kindly provided by R.C.Desrosiers and G.N.Pavlakis, respectively.
Cell lines and DNA transfection
Jurkat T cells expressing high levels of CD4 (JJK cells) were maintained in RPMI 1640 medium supplemented with 2 mM glutamine, 10 mM HEPES pH 7.4 and 10% fetal bovine serum (FBS). The cells were diluted to a concentration of 2 × 104 cells/ml upon reaching 2–5 × 105 cells/ml. IMR90 fibroblasts and HeLa cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS and subcultured 1:3 when subconfluent. Cells were transfected by electroporation at 200 V and 960 µF with 20 µg of DNA containing various amounts of appropriate expression vectors, as described previously (Iafrate et al., 1997; Greenberg et al., 1998b). Following transfection, cells were cultured for an additional 12–20 h prior to flow cytometry analysis, or biochemical analyses of Nef and CD28 expression. For microscopic analysis, fibroblasts were plated on coverslips and cultured for an additional 24–48 h prior to the analysis to allow time for cells to flatten.
Flow cytometry analysis
Flow cytometry analysis of CD28, CD4, CD3 or class I MHC molecules, and GFP reporter molecules in cells transfected with a bi-cistronic transcription unit expressing 239.Nef and GFP was performed on an Epics-Elite or a FACS Calibur flow cytometer as described previously (Greenberg et al., 1997; Lock et al., 1999). Briefly, aliquots of 2 × 105 cells were reacted with saturating amounts of PE-conjugated CD28.2 mAb, specific for CD28, mAb Leu-3A, specific for human CD4, HIT3A mAb, specific for the ε-subunit of the CD3–TCR complex (both from Becton Dickinson), or biotinylated B9.12.1 mAb, specific for the assembled class I MHC heavy chain–β2-microglobulin complex (Immunotech), followed by PE-conjugated streptavidin (Caltag), in phosphate-buffered saline (PBS) containing 1% FBS and 0.1% sodium azide (PBS-FA). Fluorescence of stained cells was detected by two-color flow cytometry on an Epics-Elite flow cytometer or on a FACS Calibur flow cytometer (Greenberg et al., 1998b).
CD28 endocytosis assays
Jurkat T cells were transfected with bi-cistronic plasmids expressing wild-type or mutant Nef proteins and GFP, and internalization of CD28 molecules from the cell surface was characterized by flow cytometry (Greenberg et al., 1997), as described below. HeLa cells were transiently co-transfected with pCGCG bi-cistronic plasmids co-expressing wild-type, or mutant CD28 proteins and GFP, together with pCG plasmid expressing Nef protein, or an empty control vector, and the rate of CD28 endocytosis was also determined by flow cytometry as described below. Briefly, following transfection, cells were cultured overnight and 5 × 106 cells were reacted with PE-conjugated CD28.2 mAb specific for CD28 (Becton Dickinson) for 10 min on ice in RPMI 1640 medium containing 0.2% bovine serum albumin (BSA), 10 mM HEPES pH 7.4. Following removal of excess unbound antibody, aliquots of 106 cells were incubated for the indicated amounts of time at 37°C. The reactions were terminated on ice and each sample was then divided into two aliquots and diluted 5-fold with PBS, or with RPMI 1640 adjusted to pH 2 (acid wash to remove mAb that had not been internalized). Total CD28 and internalized CD28 were determined by flow cytometry for cells showing identical levels of GFP expression. The percentage of CD28 internalized at time T(t) was calculated by subtracting the mean fluorescence of cells following acid wash at time T(0) (representing background fluorescence) from the mean fluorescence of cells following acid wash at time T(t) (representing fluorescence of the internalized anti-CD28 antibody) and dividing this by the mean fluorescence of cells prior to acid wash at time T(t) minus the background fluorescence T(0).
Fluorescent microscopy analysis
IMR90 fibroblasts, cultured on coverslips, were fixed with 3% paraformaldehyde for 20 min at room temperature and permeabilized in 0.1% NP-40 in PBS. Cells were then incubated in blocking solution, followed by incubation with mAb 100/1 (Sigma) specific for β-adaptin and then incubation with TxR-conjugated goat anti-mouse IgG (Amersham). Subsequently, cells were reacted with FITC-conjugated mAb CD28.2, as described previously (Greenberg et al., 1997). TfnR was detected with FITC-labeled anti-CD71 mAb (PharMingen), γ-adaptin was detected with mAb 100/3 (Ahle et al., 1988; Sigma) followed by TxR-labeled goat anti-mouse IgG antibody (Amersham), and LAMP-1 was detected with FITC-labeled anti-CD107a mAb (PharMingen). Coverslips were washed in PBS and mounted onto glass slides in glycerol-based mounting medium. Fluorescence microscopy images were taken with a Nikon Microphot-FXN microscope equipped with a CCD camera and processed using Oncor imaging software, as described previously (Greenberg et al., 1997), or with Zeiss Axioplan 2 and Open Lab Imaging software.
Acknowledgments
Acknowledgements
We thank Klara Velizon and Tamara Howard for excellent assistance with flow cytometry and fluorescent microscopy analyses. We also thank Michael Greenberg and other members of the laboratory for sharing reagents and for discussions, and Michael Greenberg, John Iafrate and Ajit Janardhan for editorial help. This work was supported by a grant from Public Health Service (AI-42561).
References
- Ahle S., Mann,A., Eichelsbacher,U. and Ungewickell,E. (1988) Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J., 7, 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken C., Konner,J., Landau,N.R., Lenburg,M.E. and Trono,D. (1994) Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell, 76, 853–864. [DOI] [PubMed] [Google Scholar]
- Alexander L., Du,Z., Rosenzweig,M., Jung,J.U. and Desrosiers,R.C. (1997) A role for natural simian immunodeficiency virus type 1 nef alleles in lymphocyte activation. J. Virol., 71, 6094–6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander L., Du,Z., Howe,A.Y., Czajak,S. and Desrosiers,R.C. (1999) Induction of AIDS in rhesus monkeys by a recombinant simian immunodeficiency virus expressing nef of human immunodeficiency virus type 1. J. Virol., 73, 5814–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur A.S., Sawai,E.T., Dazin,P., Fantl,W.J., Cheng-Mayer,C. and Peterlin,B.M. (1994) HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity, 1, 373–384. [DOI] [PubMed] [Google Scholar]
- Bell I., Ashman,C., Maughan,J., Hooker,E., Cook,F. and Reinhart,T.A. (1998) Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) ζ chain leads to TCR down-modulation. J. Gen. Virol., 79, 2717–2727. [DOI] [PubMed] [Google Scholar]
- Bresnahan P.A., Yonemoto,W., Ferrell,S., Williams-Herman,D., Geleziunas,R. and Greene,W.C. (1998) A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol., 8, 1235–1238. [DOI] [PubMed] [Google Scholar]
- Cefai D., Schneider,H., Matangkasombut,O., Kang,H., Brody,J. and Rudd,C.E. (1998) CD28 receptor endocytosis is targeted by mutations that disrupt phosphatidylinositol 3-kinase binding and costimulation. J. Immunol., 160, 2223–2230. [PubMed] [Google Scholar]
- Cohen G.B., Ghandi,R.T., Davis,D.M., Mandelboim,O., Chen,B.K., Strominger,J.L. and Baltimore,D. (1999) The selective down-regulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity, 10, 661–672. [DOI] [PubMed] [Google Scholar]
- Collins K.L. and Baltimore,D. (1999) HIV’s evasion of the cellular immune response. Immunol. Rev., 168, 65–74. [DOI] [PubMed] [Google Scholar]
- Collins K.L., Chen,B.K., Kalams,S.A., Walker,B.D. and Baltimore,D. (1998) HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature, 391, 397–401. [DOI] [PubMed] [Google Scholar]
- Craig H.M., Pandori,M.W. and Guatelli,J.C. (1998) Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc. Natl Acad. Sci. USA, 95, 11229–11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon N.J. et al. (1995) Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science, 270, 988–991. [DOI] [PubMed] [Google Scholar]
- Garcia J.V. and Miller,A.D. (1991) Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature, 350, 508–511. [DOI] [PubMed] [Google Scholar]
- Grakoui A., Bromley,S.K., Sumen,C., Davis,M.M., Shaw,A.S., Allen,P.M. and Dustin,M.L. (1999) The immunological synapse: a molecular machine controlling T cell activation. Science, 285, 221–227. [DOI] [PubMed] [Google Scholar]
- Greenberg M.E., Bronson,S., Lock,M., Neumann,M., Pavlakis,G.N. and Skowronski,J. (1997) Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J., 16, 6964–6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M., DeTulleo,L., Rapoport,I., Skowronski,J. and Kirchhausen,T. (1998a) A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol., 8, 1239–1242. [DOI] [PubMed] [Google Scholar]
- Greenberg M.E., Iafrate,A.J. and Skowronski,J. (1998b) The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J., 17, 2777–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesiek S., Stahl,S.J., Wingfield,P.T. and Bax,A. (1996) The CD4 determinant for downregulation by HIV-1 Nef directly binds to Nef. Mapping of the Nef binding surface by NMR. Biochemistry, 35, 10256–10261. [DOI] [PubMed] [Google Scholar]
- Hanna Z., Kay,D.G., Rebai,N., Guimond,A., Jothy,S. and Jolicoeur,P. (1998) Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell, 95, 163–175. [DOI] [PubMed] [Google Scholar]
- Howe A.Y., Jung,J.U. and Desrosiers,R.C. (1998) ζ chain of the T-cell receptor interacts with nef of simian immunodeficiency virus and human immunodeficiency virus type 2. J. Virol., 72, 9827–9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J. and Cullen,B.R. (1997) Human immunodeficiency virus types 1 and 2 and simian immunodeficiency virus Nef use distinct but overlapping target sites for downregulation of cell surface CD4. J. Virol., 71, 6742–6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I., Huang,J.-F., Kishimoto,H., Brunmark,A., Peterson,P.A., Jackson,M.R., Surh,C.D., Cai,Z. and Sprent,J. (2000) T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen presenting cells. J. Exp. Med., 191, 1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iafrate A.J., Bronson,S. and Skowronski,J. (1997) Separable functions of Nef disrupt two aspects of T cell receptor machinery: CD4 expression and CD3 signaling. EMBO J., 16, 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iafrate A.J., Carl,S., Bronson,S., Stahl-Henning,C., Swigut,T., Skowronski,J. and Kirchhoff,F. (2000) Disrupting surfaces of nef required for downregulation of CD4 and for enhancement of virion infectivity attenuates simian immunodeficiency virus replication in vivo. J. Virol., 74, 9836–9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler H.W., Ringler,D.J., Mori,K., Panicali,D.L., Sehgal,P.K., Daniel,M.D. and Desrosiers,R.C. (1991) Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell, 65, 651–662. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. (1999) Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol., 15, 705–732. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F. et al. (1999) The human immunodeficiency virus type 1 nef gene can to a large extent replace simian immunodeficiency virus nef in vivo. J. Virol., 73, 8371–8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall S., Erdtmann,L., Benichou,S., Berlioz-Torrent,C., Liu,L., Benarous,R., Heard,J.M. and Schwartz,O. (1998) Nef interacts with the µ subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity, 8, 483–495. [DOI] [PubMed] [Google Scholar]
- Le Gall S., Buseyne,F., Trocha,A., Walker,B.D., Heard,J.M. and Schwartz,O. (2000) Distinct trafficking pathways mediate Nef-induced and clathrin-dependent major histocompatibility complex class I down-regulation. J. Virol., 74, 9256–9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow D.J., Walunas,T.L. and Bluestone,J.A. (1996) CD28/B7 system of T cell costimulation. Annu. Rev. Immunol., 14, 233–258. [DOI] [PubMed] [Google Scholar]
- Lock M., Greenberg,M.E., Iafrate,A.J., Swigut,T., Muench,J., Kirchhoff,F., Shohdy,N. and Skowronski,J. (1999) Two elements target SIV Nef to the AP-2 clathrin adaptor complex, but only one is required for the induction of CD4 endocytosis. EMBO J., 18, 2722–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Wu,X., Plemenitas,A., Yu,H., Sawai,E.T., Abo,A. and Peterlin,B.M. (1996) CDC42 and Rac1 are implicated in the activation of the Nef-associated kinase and replication of HIV-1. Curr. Biol., 6, 1677–1684. [DOI] [PubMed] [Google Scholar]
- Luria S., Chambers,I. and Berg,P. (1991) Expression of the type 1 human immunodeficiency virus Nef protein in T cells prevents antigen receptor-mediated induction of interleukin 2 mRNA. Proc. Natl Acad. Sci. USA, 88, 5326–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manninen A., Renkema,G.H. and Saksela,K. (2000) Synergistic activation of NFAT by HIV-1 Nef and the Ras/MAPK pathway. J. Biol. Chem., 275, 16513–16517. [DOI] [PubMed] [Google Scholar]
- Mariani R. and Skowronski,J. (1993) CD4 down-regulation by nef alleles isolated from human immunodeficiency virus type 1-infected individuals. Proc. Natl Acad. Sci. USA, 90, 5549–5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M. and Pelchen-Matthews,A. (1996) Endocytotic and exocytotic regulation of CD4. Curr. Top. Microbiol. Immunol., 205, 107–153. [DOI] [PubMed] [Google Scholar]
- Oldridge J. and Marsh,M. (1998) Nef—an adaptor adaptor? Trends Cell Biol., 8, 302–305. [DOI] [PubMed] [Google Scholar]
- Piguet V., Chen,Y.L., Mangasarian,A., Foti,M., Carpentier,J.L. and Trono,D. (1998) Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the µ chain of adaptor complexes. EMBO J., 17, 2472–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet V., Schwartz,O., Le Gall,S. and Trono,D. (1999) The down regulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol. Rev., 168, 51–63. [DOI] [PubMed] [Google Scholar]
- Piguet V., Wan,L., Borel,C., Mangasarian,A., Demaurex,N., Thomas,G. and Trono,D. (2000) HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nature Cell Biol., 2, 163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkema H.G. and Saksela,K. (2000) Interactions of HIV-1 Nef with cellular signal transducing proteins. Frontiers Biosci., 5, D268–D283. [DOI] [PubMed] [Google Scholar]
- Salghetti S., Mariani,R. and Skowronski,J. (1995) Human immunodeficiency virus type 1 Nef and p56lck protein-tyrosine kinase interact with a common element in CD4 cytoplasmic tail. Proc. Natl Acad. Sci. USA, 92, 349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Martin,M., Agarraberes,F.A., Yin,L., Rapoport,I., Kirchhausen,T. and Rudd,C.E. (1999) Cytolytic T lymphocyte-associated antigen-4 and the TCRζ/CD3 complex, but not CD28, interacts with clathrin adaptor complexes AP-1 and AP-2. J. Immunol., 163, 1868–1879. [PubMed] [Google Scholar]
- Schrager J.A. and Marsh,J.W. (1999) HIV-1 Nef increases T cell activation in a stimulus-dependent manner. Proc. Natl Acad. Sci. USA, 96, 8167–8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O., Dautry-Varsat,A., Goud,B., Marechal,V., Subtil,A., Heard,J.M. and Danos,O. (1995) Human immunodeficiency virus type 1 Nef induces accumulation of CD4 in early endosomes. J. Virol., 69, 528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O., Marechal,V., LeGall,S., Lemonnier,F. and Heard,J.M. (1996) Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nature Med., 2, 338–342. [DOI] [PubMed] [Google Scholar]
- Schwartz R.H. (1992) Costimulation of T lymphocytes: the role of CD28, CTLA-4 and B7/BB1 in interleukin-2 production and immunotherapy. Cell, 71, 1065–1068. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., van Seventer,G.A., Horgan,K.J. and Shaw,S. (1990) Roles of adhesion molecules in T cell recognition: fundamental similarities between four integrins on resting human T cells (LFA-1, VLA-4, VLA-5, VLA-6) in expression, binding and costimulation. Immunol. Rev., 114, 109–143. [DOI] [PubMed] [Google Scholar]
- Skowronski J., Parks,D. and Mariani,R. (1993) Altered T cell activation and development in transgenic mice expressing the HIV-1 Nef gene. EMBO J., 12, 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronski J., Greenberg,M.E., Lock,M., Mariani,R., Salghetti,S., Swigut,T. and Iafrate,A.J. (1999) HIV and SIV Nef modulate signal transduction and protein sorting in T cells. Cold Spring Harb. Symp. Quant. Biol., 64, 453–463. [DOI] [PubMed] [Google Scholar]
- Smith B.L., Krushelnycky,B.W., Mochly-Rosen,D. and Berg,P. (1996) The HIV nef protein associates with protein kinase Cθ. J. Biol. Chem., 271, 16753–16757. [DOI] [PubMed] [Google Scholar]
- Spina C.A., Kwoh,T.J., Chowers,M.Y., Guatelli,J.C. and Richman,D.D. (1994) The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J. Exp. Med., 179, 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber R.H., Horie,K., Carney,P., Hudson,E.A., Tarasova,N.I., Gaitanaris,G.A. and Pavlakis,G.N. (1998) Development and applications of enhanced green fluorescent protein mutants. Biotechniques, 24, 462–471. [DOI] [PubMed] [Google Scholar]
- Swigut T., Iafrate,A.J., Muench,J., Kirchhoff,F. and Skowronski,J. (2000) Simian and human immunodeficiency virus Nef proteins use different surfaces to downregulate class I major histocompatibility antigen expression. J. Virol., 74, 5691–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M. and Herr,W. (1990) Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell, 60, 375–386. [DOI] [PubMed] [Google Scholar]