Spatial dynamics of receptor-mediated endocytic trafficking in budding yeast revealed by using fluorescent α-factor derivatives (original) (raw)
Abstract
Much progress defining the order and timing of endocytic internalization events has come as a result of real-time, live-cell fluorescence microscopy. Although the availability of numerous endocytic mutants makes yeast an especially valuable organism for functional analysis of endocytic dynamics, a serious limitation has been the lack of a fluorescent cargo for receptor-mediated endocytosis. We have now synthesized biologically active fluorescent mating-pheromone derivatives and demonstrated that receptor-mediated endocytosis in budding yeast occurs via the clathrin- and actin-mediated endocytosis pathway. We found that endocytic proteins first assemble into patches on the plasma membrane, and then α-factor associates with the patches. Internalization occurs next, concomitant with actin assembly at patches. Additionally, endocytic vesicles move toward early endosomes on actin cables. Early endosomes also associate with actin cables, and they actively move toward endocytic sites to capture vesicles being released from the plasma membrane. Thus, endocytic vesicle formation and capture of the newly released vesicles by early endosomes occur in a highly concerted manner, mediated by the actin cytoskeleton.
Keywords: actin, cytoskeleton, endocytosis, endosome
In recent years, live-cell imaging of endocytic events has proved extremely powerful in yeast and other cell types for revealing mechanistic principles of endocytic internalization (1–7). In budding yeast, dynamics of at least two-dozen endocytic proteins have been analyzed by real-time analysis of GFP fusions, and effects of numerous mutants on pathway dynamics have been quantitatively analyzed. Although much is known about the dynamics and regulation of the endocytic machinery as a result of these studies, a full appreciation of the process depends on being able to analyze in real time the transit of an endocytic cargo through the pathway. This analysis is necessary so that functions such as cargo recruitment, concentration, internalization and trafficking can be attributed to specific steps in the assembly and dynamics of the endocytic machinery.
An ideal cargo for such studies would be a fluorescent molecule that could be introduced to the cell externally, bound to cell-surface receptors, and then taken up by the endocytic machinery, such that the full history of the molecule would be known, and its fate could be followed as a function of time. Such a cargo molecule could be used to define operationally the different compartments of the endocytic pathway. The lipophilic dye FM4–64 and Ste2-GFP, an integral membrane protein that is taken up by endocytosis, have previously been used to label endocytic compartments fluorescently in budding yeast. FM4–64 is introduced to cells externally and it is a good marker for bulk-phase endocytosis (3, 5). However, once inside the cell, FM4–64 is transported along bifurcating pathways, with some dye entering a recycling pathway and the rest traveling to the vacuole (8); therefore, FM4–64's utility for unambiguously labeling internal endocytic compartments to reveal spatiotemporal features of the downstream pathway is limited. Ste2p is a receptor for the peptide α-factor, which is a mating pheromone. Ste2-GFP has been used to mark endocytic compartments when expressed in cells at steady state (9). However, because Ste2-GFP is not introduced externally to cells and then tracked through the endocytic pathway over time, it is not possible to know the identity of compartments labeled by Ste2-GFP. This molecule is expected to label both endocytic and biosynthetic compartments, and potentially nonphysiological compartments if the GFP tag causes missorting of this integral membrane protein.
Results and Discussion
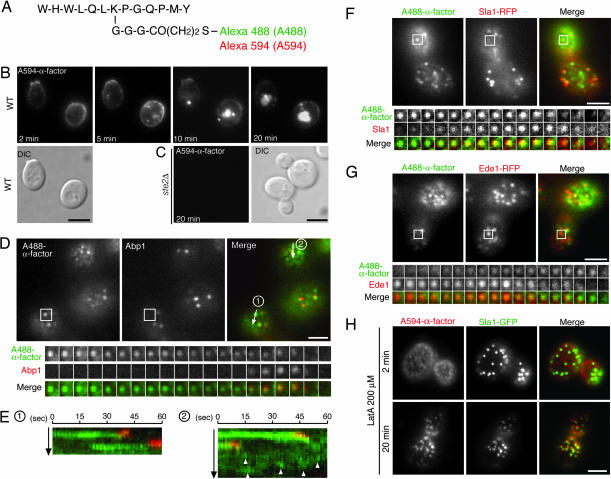
For our synthesis of fluorescent α-factor derivatives, Alexa Fluor-488 C5 or -594 C5 maleimide was conjugated to the ε-amine of lysine 7 of α-factor, via thiopropionyl-Gly3 as a flexible, hydrophilic linker (Fig. 1A). These labeled pheromones maintained biological activity, as assessed by induction of mating morphology in a cells, although the activities were ≈25- to 50-fold less than for wild-type α-factor (see Fig. 6_A_, which is published as supporting information on the PNAS web site). Ste2p receptor-dependent binding and internalization of Alexa Fluor-594 (A594)-α-factor indicated that A594-α-factor is specifically internalized by receptor-mediated endocytosis (Fig. 1 B and C). When added to cells, A594-α-factor (and A488-α-factor, data not shown) was first seen in internal endocytic compartments by 5 min (Fig. 1B; and see Movie 1, which is published as supporting information on the PNAS web site). By 10 min, A594-α-factor began to concentrate in the vacuole and in bright structures that often were proximal to the vacuole. By 20 min, the α-factor was mostly in the vacuole (Fig. 1B; Movie 1).
Fig. 1.
Structure and localization of fluorophor-conjugated α-factor. (A) Diagram of Alexa Fluor-488 (A488)- and Alexa Fluor-594 (A594)-α-factor. (B and C) Ste2p receptor-dependent binding and internalization of Alexa-α-factor. Alexa-α-factor was added to wild-type (B) or _ste2_Δ (C) cells and was followed through the endocytic pathway for the indicated times. (D, F, and G) A488-α-factor (TIRF optics) appeared in endocytic patches before Abp1p and Sla1p but after Ede1p (epifluorescence). Shown are single frames from the GFP and the RFP channels of the movie and a merged image (Upper) and time series of single patches from wild-type cells expressing the indicated fluorophor-tagged proteins (Lower). The time to acquire one image pair was 2 s. (E) Kymographs of time-lapse images collected at 2-s intervals. Arrows in D mark where the kymograph was generated. Numbers and the direction of the arrows in D correspond to those in E. Arrowheads (E Right) indicate independent A488-α-factor-labeled spots. (H) Localization of A594-α-factor and Sla1-GFP in cells treated with 200 μM LatA. After incubating cells expressing Sla1-GFP with 200 μM LatA at 25°C for 30 min, cells were incubated with A594-α-factor at 0°C for 30 min in minimal medium lacking glucose in the continued presence of 200 μM LatA. The images were acquired at 2 min and 20 min after washing out unbound Alexa-α-factor with glucose-containing medium and warming cells to 25°C in the continued presence of 200 μM LatA. (Scale bars, 2.5 μm.)
Recent studies showed that cortical actin patches are sites of bulk-phase, clathrin-mediated endocytic internalization (2, 3, 5). Therefore, it is reasonable to ask whether receptor-mediated endocytosis of α-factor also occurs via these patches. To reveal the spatiotemporal relationships between cargo molecules and endocytic proteins, we tagged Abp1p, a marker for actin assembly at endocytic sites, with monomeric red fluorescent protein (mRFP) and imaged the cells in real time as they endocytosed A488-α-factor. Using total internal reflection fluorescence (TIRF) microscopy, we observed A488-α-factor as fluorescent spots moving diffusely on the cell surface (Fig. 1D Left; and see Movie 2, which is published as supporting information on the PNAS web site). Two-color analyses revealed that Abp1p (viewed by epifluorescence) joined preexisting A488-α-factor spots (viewed by TIRF optics), and then both molecules disappeared concomitantly (Fig. 1D; Movie 2). We found that, within the plane of the plasma membrane, A488-α-factor spots have a highly motile state and a nonmotile state. As shown in kymographs, all A488-α-factor spots in the nonmotile state are eventually joined by Abp1p and then internalized (100%; n = 55) (Fig. 1E). In contrast, Abp1p never joined the highly motile A488-α-factor spots (n = 120) (Fig. 1E Right, arrowheads). Similar to Abp1p, the endocytic coat protein Sla1p (2, 3) also joined preexisting A488-α-factor spots and was cointernalized with them (100%; n = 47) (Fig. 1F; and see Movie 3, which is published as supporting information on the PNAS web site). Because TIRF was used to image A488-α-factor and epifluorescence to image Sla1p, A488α-factor occasionally disappeared before Sla1p. Our observations establish that cortical actin patches are sites of receptor-mediated α-factor internalization, an important conclusion, because Chang et al. (9) recently proposed that α-factor may be internalized by a pathway independent from the actin-dependent endocytosis pathway and because earlier immunoelectron microscopy studies failed to localize Ste2p to actin patches (10).
Ede1p, a ubiquitin-associated Eps15-like protein, has been reported to localize at cortical patches (11), but the timing of its association with patches has not been described. By comparing the temporal localization of Ede1-RFP and Sla1-GFP, we found that Ede1p, which has a wide range of lifetimes from ≈30–180 s, always appears before Sla1p and stays immotile at the cell surface throughout its lifetime (n = 100) (Fig. 6 B and C). This behavior is similar to what has been described for clathrin, although both clathrin and Sla1p persist after Ede1p disappears, and, in contrast to Ede1p, they both are internalized (see ref. 3 and Fig. 6_B_). Interestingly, we observed that Ede1p forms patches that are subsequently joined by A488-α-factor. Thus, endocytic sites form before α-factor recruitment (Fig. 1G; and see Movie 4, which is published as supporting information on the PNAS web site), consistent with recent findings in mammalian cells (7). The movement and disassembly of Sla1p patches are known to be inhibited by Latrunculin A (LatA) treatment (2). Treatment of cells with 200 μM LatA, which leads to the complete disassembly of cortical actin, blocked A594-α-factor internalization and caused it to accumulate in foci on the plasma membrane (Fig. 6_D_). This accumulation was time-dependent, such that α-factor was observed as dispersed, highly motile, faint spots at 2 min but coalesced into more prominent, nonmotile spots after 20 min (Figs. 1H and 6_D_). Interestingly, ≈90% of the Sla1p patches colocalized with the α-factor spots at 20 min (Fig. 1H). These observations further support the conclusion that α-factor first binds to randomly distributed receptors, and the receptor–ligand complexes subsequently become associated with the endocytic machinery.
Endocytic vesicles in yeast can be recognized as structures that are labeled by both GFP-tagged endocytic coat proteins and GFP-tagged actin-binding proteins that move off the plasma membrane when actin assembles at endocytic sites (2, 3). Here, we operationally define early endosomes as the internal structures that become dimly labeled by fluorescent α-factor 2–5 min after initiation of endocytic internalization, and late endosomes and/or prevacuolar compartments as structures brightly labeled by fluorescent α-factor by 5–10 min after internalization. It is instructive to compare the time-dependent localization of α-factor with localization of other markers for endocytic compartments. Ste2-GFP has been used previously as an endosome marker (9). Early endosomes could be detected by using fluorescent α-factor but not by using Ste2-GFP, presumably because of the high quantum yield of the Alexa dye and the low autofluorescence in the red wavelengths (see Fig. 7_A_, which is published as supporting information on the PNAS web site). However, late endosomal/prevacuolar compartments were readily labeled by both markers, possibly because the receptor and cargo become concentrated in these compartments. Many of these structures appear to be tethered to the vacuole (Fig. 7_A_ and ref. 9). We conclude that Ste2-GFP expressed at steady state mostly identifies vacuoles and late endosomes/prevacuolar compartments. In addition, Ste2-GFP labeled other compartments that we were not able to identify because they were never labeled by fluorescent α-factor. Endocytosed α-factor colocalized well with the lipophilic dye FM4–64 and partially with Snc1-GFP, an exocytic v-SNARE that is endocytosed and localizes to early endosomes (12) (Fig. 7 B and C). Snc1-GFP may also label exocytic compartments.
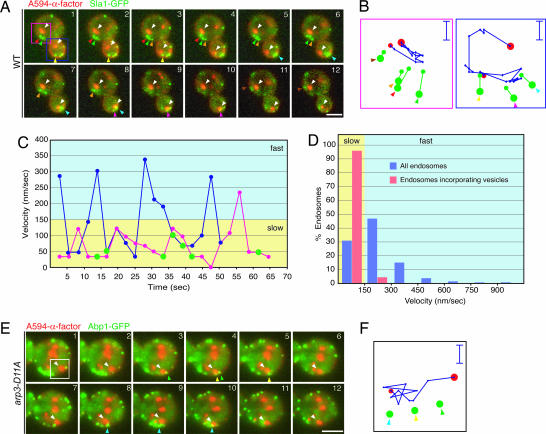
Using A594-α-factor as an early endosome marker and Sla1-GFP as an endocytic vesicle marker, we observed that, when endocytic vesicles start moving, they move in a directed manner toward early endosomes [Fig. 2A (mother cell) and B; and see Movie 5, which is published as supporting information on the PNAS web site]. Thus, endocytic vesicles carrying cargo for receptor-mediated endocytosis move in the manner described previously for bulk-phase endocytosis (5). Our analysis also revealed an unexpected feature of endosome movement. We found that early endosomes often move in a directed manner to sites of endocytic internalization just as internalization is occurring (Fig. 2 A (daughter cell) and B; Movie 5). Because of these two mechanisms, within 2–3 seconds of their release from the plasma membrane, newly formed endocytic vesicles merge with early endosomes. The remarkable efficiency with which endocytic vesicles and early endosomes find each other had not previously been appreciated.
Fig. 2.
Dynamic behavior of endosomes and endocytic vesicles. (A) Active movement of endosomes and endocytic vesicles toward each other. Endosomes labeled with A594-α-factor and endocytic vesicles labeled with Sla1-GFP were imaged in wild-type cells. Time to acquire one image pair was 2.8 s. (Scale bar, 2.5 μm.) (B) Tracking of endosomes and endocytic vesicles shown in A. Blue and red frames correspond to blue and red boxes in A. Red and green dots indicate endosomes and endocytic vesicles, respectively. Big and small dots denote the first and last positions, respectively, of endosomes or patches. (Scale bars, 0.5 μm.) (C) Two phases of endosome motility. Velocities of endosomes shown in A were plotted at 2.8-s intervals. The blue and red lines represent the velocities of the endosomes shown in the blue and red boxes, respectively, in A. Green circles indicate the points at which endocytic vesicles merge with endosomes. (D) Quantification of endosome velocity and the timing of patch internalization. Wild-type cells expressing Abp1-GFP were incubated with A594-α-factor, and internalization was induced 3 min before imaging. Endosome velocities were acquired at 0.5-s intervals, and the velocities were categorized according to velocity range. Blue bars indicate velocities of all endosomes (n = 1,268). Red bars indicate the velocities of endosomes that were merging with endocytic vesicles (n = 150). (E) Movements of endosomes in arp3-D11A cells. The time to acquire one image pair was 6.5 s. (Scale bars, 2.5 μm.) (F) Tracking of the endosome in the boxed area in E. Green dots are Abp1-GFP patches. (Scale bar, 0.5 μm.)
We performed in-depth, quantitative analysis of early endosome motility. Endosomes moved toward endocytic vesicles with a speed of >150 nm/s. However, they incorporated endocytic vesicles only when moving at a slower speed of <150 nm/s (Fig. 2 _B_ and _C_, green circles). We defined the movement of early endosomes as having a fast phase, >150 nm/s, and a slow phase, <150 nm/s. Although the durations of the fast or slow phases were different for individual endosomes, all endosomes examined (_n_ = 150) displayed similar behaviors. Quantification of early endosome velocity revealed that the slow phase accounts for ≈31% of all endosome movements and that >95% of endocytic vesicles incorporated into endosomes do so during the slow phase of endosome movement (n = 150) (Fig. 2D). These observations indicate that early endosome movement is highly coordinated with vesicle internalization. To further examine the coupling of these events, we used the arp3-D11A mutant of the Arp2/3 complex. The arp3-D11A mutant has severe defects in endocytosis and Abp1p patch internalization (Fig. 7 D and E) (13). Nevertheless, we still observed A594-α-factor staining in the endosomes and vacuoles of this mutant (Fig. 2E), indicating that endocytic trafficking is not completely blocked. Interestingly, we found that, in this mutant, early endosomes move actively toward Abp1p patches on or near the plasma membrane, apparently absorbing the patches (Fig. 2 E and F; and see Movie 6, which is published as supporting information on the PNAS web site), indicating that endosomes can compensate for the absence of directed movement by endocytic vesicles.
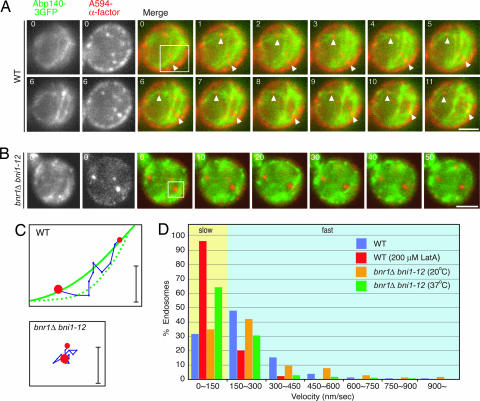
Actin cables are used as tracks for organelle segregation and secretion of exocytic vesicles in budding yeast (14). Thus, we determined whether actin cables mediate the directed movements of early endosomes. To test for associations between early endosomes and actin cables, we tagged Abp140p, which binds to F-actin and localizes to actin patches and cables (15), with three tandem copies of GFP (3GFP). Simultaneous imaging of early endosomes and actin cables revealed that ≈89% of early endosomes localize along actin cables, and move in association with the cables (n = 73) (Fig. 3A; and see Movie 7, which is published as supporting information on the PNAS web site). Quantification of endosome velocity revealed that early endosomes in wild-type cells move with an average speed of 213.46 ± 139.47 nm/s (n = 1,268), whereas endosomes in LatA-treated cells move with an average speed of 75.25 ± 46.84 nm/s (n = 485), indicating that endosome motility depends on the actin cytoskeleton. Formins are conserved proteins that nucleate actin assembly by associating with actin filament barbed ends (16). The yeast formins Bni1p and Bnr1p promote actin cable assembly (17, 18). To test how the loss of actin cables affects endosome motility, we expressed Abp140p-3GFP in _bni1–12 bnr1_Δ cells that display normal-looking actin cables at 20°C but that rapidly lose actin cables when switched to the nonpermissive temperature (19). The prominent Abp140-3GFP-labeled actin cables that normally align with the mother–daughter axis disappeared at 37°C, as reported in ref. 19 (Fig. 3B). Tracking and quantification of early endosome movements revealed that endosome velocity in this mutant is markedly decreased at 37°C (138.67 ± 119.32 nm/s, n = 987), whereas the velocity at 20°C is similar to that in wild-type cells (234.46 ± 167.11 nm/s, n = 456) (Fig. 3 C and D; and see Movie 8, which is published as supporting information on the PNAS web site). This result further supports the conclusion that endosomes move on actin cables.
Fig. 3.
Endosome motility along actin cables. (A) Localization and motility of endosomes on actin cables. Cells expressing Abp140–3GFP were incubated with A594-α-factor, and internalization was induced 3 min before imaging. Time to acquire one image pair was 1.0 s. (B) Localization and motility of endosomes on actin cables in _bni1–12 bnr1_Δ cells at the restrictive temperature. Cells were cultured for 1 h at 37°C and then labeled with A594-α-factor on ice for 2 h. Internalization was initiated as described in Materials and Methods. Endosome movement was imaged at room temperature. The time difference between each frame is 10 s. [Scale bars, 2.5 μm (A and B).] (C) Tracking of the endosome in the boxed area in A or B. The time difference between each position along the track is 1.0 s. (Scale bars, 0.5 μm.) (D) Quantification of endosome velocity in cells treated with 200 μM LatA for 30 min or at the permissive temperature and nonpermissive temperature in _bni1–12 bnr1_Δ cells.
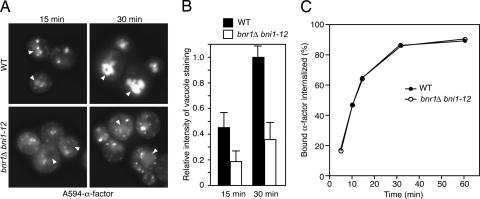
If actin cables are important for bringing endosomes and endocytic vesicles together, then the overall rate of α-factor transport downstream of internalization should be slowed in the absence of the cables. To test this prediction, we incubated wild-type or _bni1–12 bnr1_Δ cells in A594-α-factor at 37°C and followed its trafficking. As predicted, α-factor transport to vacuole was significantly delayed in the absence of actin cables (Fig. 4A and B). The _bni1–12 bnr1_Δ cells did not exhibit any defect in the binding or internalization of α-factor (Fig. 4C and data not shown). These results demonstrate that actin cables are important for efficient cargo transport from the endocytic vesicle to the vacuole.
Fig. 4.
Actin cables are necessary for efficient transport of α-factor from endocytic vesicles to the vacuole. (A) Cells were cultured at 37°C for 1 h, and 5 μM A594-α-factor was added for the indicated times. Arrowheads identify vacuoles. (B) Relative fluorescence intensity of vacuoles stained by A594-α-factor (n = 30 cells for each strain). The intensity of A594-α-factor in the vacuole was measured by using the program imagej v1.32. Values were relative to the fluorescence intensity in wild-type cells at 30 min. (C) Internalization of [35S]-labeled α-factor in wild-type cells or _bni1–12 bnr1_Δ cells at 37°C. Results are the mean of two experiments.
Two possible actin-dependent force generators that might drive early endosome movements are myosin V motor proteins (Myo2p and Myo4p) and yeast WASp (Las17p)-dependent actin polymerization. Previous reports suggested that late endosome motility may depend on the Arp2/3 complex activation activity of Las17p (9, 20). Such a mechanism is hard to reconcile with observations that Las17p remains on the plasma membrane during endocytic internalization and that neither Las17p, the Arp2/3 complex, nor actin tails have been seen on yeast endosomal compartments (2, 21, 22), even when genes encoding negative regulators of Las17p are deleted, causing abnormally large actin structures to assemble at the plasma membrane (3). In our analysis, the average early endosome velocity (213.95 ± 135.66 nm/s, n = 376) in las17_Δ_WCA cells was essentially the same as in wild-type cells (see Fig. 8 A, B, and I, which is published as supporting information on the PNAS web site). This finding is consistent with the result that the arp3-D11A mutant did not affect early endosome motility, even though it had a severe defect in endocytic vesicle internalization (Figs. 2E and 7 D and E). Myo2p and Myo4p have been shown to be required for various actin cable-dependent movements, including secretion of exocytic vesicles (14). The velocity of secretory-vesicle movement depends on the length of the Myo2p lever arm, an α-helical domain containing six IQ motifs. Deletion of all the IQ motifs (myo2–0IQ) resulted in a significant reduction in secretory-vesicle velocity (23). In our studies, myo2–0IQ cells did not exhibit a defect in the average early endosome velocity (217.51 ± 134.50 nm/s, n = 559) (Fig. 8 C, D, and I). Similarly, _myo4_Δ (217.13 ± 146.63 nm/s, n = 424) and the double mutant of myo2–0IQ and _myo4_Δ (215.53 ± 141.56 nm/s, n = 410) did not show a detectable defect in endosome motility (Fig. 8 E_–_I).
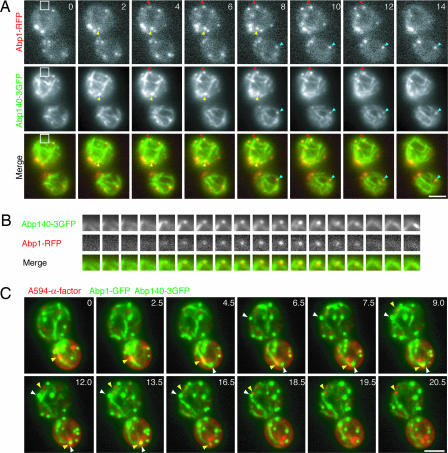
A previous study reported that retrograde linear endocytic-vesicle movement accounted for ≈20% of the total movements and were mediated by actin cables (5). By expressing Abp1-mRFP and Abp140–3GFP to visualize endocytic vesicles and actin cables respectively, we observed that ≈85% of endocytic vesicles associate with actin cables and that these vesicles invariably appeared to form in association with the cables (n = 87) (Fig. 5A and B; and see Movie 9, which is published as supporting information on the PNAS web site). The fact that both endocytic vesicles and endosomes associate with actin cables raised the possibility that they might find each other by this association. To test this possibility, we used Abp1-GFP as an endocytic vesicle marker and Abp140–3GFP as an actin cable marker. Abp140 localizes to both of actin patches and actin cables (15) and >98% of Abp140p spots (patches) colocalize with Abp1p patches (n = 140) (Fig. 5A). It was possible to distinguish endocytic vesicles from actin cables, even though both were labeled with GFP. By labeling endocytic vesicles, actin cables, and early endosomes, we observed that endosomes associated with actin cables move toward endocytic vesicles, which were also associated with the cables (Fig. 5C; and see Movie 10, which is published as supporting information on the PNAS web site). In total, our results suggest that actin cables increase the efficiency of targeting endocytic vesicles and early endosomes to each other.
Fig. 5.
Endosomes move along actin cables to endocytic vesicles. (A) Localization of 3GFP-tagged Abp140p and RFP-tagged Abp1p in living cells. Time to acquire one image pair was 2.0 s. Arrowheads indicate examples of colocalization. (B) Higher magnification view of the boxed area in A. Time series of single patch and cable from wild-type cells expressing Abp1-RFP and Abp140–3GFP. Time to acquire one image pair was 1 s. (C) Cells expressing Abp1-GFP and Abp140–3GFP were incubated with A594-α-factor and internalization was induced 5 min before imaging. Yellow arrowheads identify endosomes that move to endocytic vesicles (white arrowheads). (Scale bars, 2.5 μm.)
Materials and Methods
Yeast Strains, Growth Conditions, and Plasmids.
The yeast strains used in this study are listed in Table 1, which is published as supporting information on the PNAS web site. All strains were grown in standard rich media (YPD) or synthetic media (SM) supplemented with the appropriate amino acids. GFP and mRFP tags were integrated at the C terminus of each gene. The triple GFP tag was integrated at the C terminus of the ABP140 gene as follows: The 3GFP fragment was subcloned into BamHI- and NotI-digested pBlueScript II SK (pBS-3GFP), and the NotI–SacII fragment, which contains the Saccharomyces cerevisiae ADH1 terminator and the His3MX6 module, was amplified by PCR using pFA6a-GFP (S65T)-His3MX6 as a template and was inserted into NotI- and SacII-digested pBS-3GFP (pBS-3GFP-His-3). To create an integration plasmid, fragments of the ABP140 ORF (nt 1501–1884) and of a region extending 340-bp downstream of the ABP140 ORF were generated by PCR and cloned into the NotI or SacI site of pBS-3GFP-His-3, respectively. To integrate 3GFP at the C terminus of the ABP140 gene, the integration plasmid was linearized by HindIII and transformed into yeast.
Fluorescence Labeling of α-Factor and Endocytosis Assays.
3-Thiopropionyl-G3 was appended to the free ε-amine of K7 in otherwise fully protected α-factor by standard DCC/HOBT FMOC solid-phase chemistry, and Alexa Fluor-594 maleimide (Molecular Probes) was coupled to the purified peptide in NMM-HOAc buffer, pH 8.0. Peptides were purified by reverse-phase HPLC; structure and purity (>97%) were assessed by ESI-FTICR mass spectrometry (9.4T; Bruker).
For endocytosis assays, cells were grown to an OD600 of 0.2 in 1.25 ml of YPD, briefly centrifuged, and resuspended in 50 μl of synthetic media (SM) with 1% (wt/vol) BSA and 5 μM Alexa-α-factor. After incubation on ice for 2 h, cells were washed into ice-cold SM containing 1% BSA. Internalization was initiated by the addition of ice-cold SM containing 4% Glucose and amino acids and then transferring cells to a glass slide at room temperature. Alexa Fluor-594 α-factor imaging was done by using a rhodamine/Texas-red filter, and images were acquired with a digital charge-coupled device (CCD) camera (see below) by using the program metamorph (Universal Imaging). [35S]-labeled α-factor internalization assays were performed as described in ref. 4.
Fluorescence Microscopy.
Fluorescence microscopy was performed by using an Olympus IX81 microscope equipped with a ×100/NA1.4 or a ×100/NA 1.45 (Olympus) objective and Orca-ER cooled CCD camera (Hamamatsu). For TIRF illumination, the expanded beam (488 nm) of an argon krypton laser (Melles Griot) was used to excite Alexa Fluor-488. The beam was focused at an off-axis position in the back focal plane of the objective. Simultaneous imaging of red and green fluorescence was performed by using an Olympus IX81 microscope equipped with a ×100/NA 1.45 (Olympus) objective, Orca-ER cooled CCD camera (Hamamatsu), and an image splitter (Dual-View; Optical Insights) that divided the red and green components of the images with a 565-nm dichroic mirror and passed the red component through a 630/50-nm filter and the green component through a 530/30-nm filter.
Analysis of Endosome Motility.
Endosome motility and velocity is analyzed by using the program imagej v1.32. For the quantification of endosome velocity, the time-lapse images were acquired for a 0.5-s interval. To determine the velocity, the distance traveled by each endosome in 0.5 s was calculated based on pixel coordinates (1 pixel = 64.5 μM).
Supplementary Material
Supporting Information
Acknowledgments
We thank Anthony Bretscher (Cornell University, Ithaca, NY) for the bni1 and myo2 strains; Roger Tsien (University of California at San Diego, La Jolla, CA) for the mRFP plasmid; Hugh R. Pelham (Cambridge University, Cambridge, U.K.) for the Snc1-GFP plasmid; Benjamin S. Glick (University of Chicago) for the triple GFP plasmid; the members of the Drubin/Barnes laboratory for sharing materials and for helpful discussions; Georjana Barnes for helpful comments on the manuscript; and Kensaku Mizuno for encouragement. This work was supported by Postdoctoral Fellowship Grant PF-03-231-01-CSM from the American Cancer Society (to J.Y.T.) and National Institutes of Health Grant GM50399 (to D.G.D.).
Abbreviations
TIRF
total internal reflection fluorescence
LatA
Latrunculin A
mRFP
monomeric red fluorescent protein.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Merrifield C. J., Feldman M. E., Wan L., Almers W. Nat. Cell Biol. 2002;4:691–698. doi: 10.1038/ncb837. [DOI] [PubMed] [Google Scholar]
- 2.Kaksonen M., Sun Y., Drubin D. G. Cell. 2003;115:475–487. doi: 10.1016/s0092-8674(03)00883-3. [DOI] [PubMed] [Google Scholar]
- 3.Kaksonen M., Toret C. P., Drubin D. G. Cell. 2005;123:305–320. doi: 10.1016/j.cell.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Sekiya-Kawasaki M., Groen A. C., Cope M. J., Kaksonen M., Watson H. A., Zhang C., Shokat K. M., Wendland B., McDonald K. L., McCaffery J. M., Drubin D. G. J. Cell Biol. 2003;162:765–772. doi: 10.1083/jcb.200305077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huckaba T. M., Gay A. C., Pantalena L. F., Yang H. C., Pon L. A. J. Cell Biol. 2004;167:519–530. doi: 10.1083/jcb.200404173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newpher T. M., Smith R. P., Lemmon V., Lemmon S. K. Dev. Cell. 2005;9:87–98. doi: 10.1016/j.devcel.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich M., Boll W., Van Oijen A., Hariharan R., Chandran K., Nibert M. L., Kirchhausen T. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Wiederkehr A., Avaro S., Prescianotto-Baschong C., Haguenauer-Tsapis R., Riezman H. J. Cell Biol. 2000;149:397–410. doi: 10.1083/jcb.149.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang F. S., Han G. S., Carman G. M., Blumer K. J. J. Cell Biol. 2005;171:133–142. doi: 10.1083/jcb.200501086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulholland J., Konopka J., Singer-Kruger B., Zerial M., Botstein D. Mol. Biol. Cell. 1999;10:799–817. doi: 10.1091/mbc.10.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagny B., Wiederkehr A., Dumoulin P., Winsor B., Riezman H., Haguenauer-Tsapis R. J. Cell Sci. 2000;113:3309–3319. doi: 10.1242/jcs.113.18.3309. [DOI] [PubMed] [Google Scholar]
- 12.Lewis M. J., Nichols B. J., Prescianotto-Baschong C., Riezman H., Pelham H. R. Mol. Biol. Cell. 2000;11:23–38. doi: 10.1091/mbc.11.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin A. C., Xu X. P., Rouiller I., Kaksonen M., Sun Y., Belmont L., Volkmann N., Hanein D., Welch M., Drubin D. G. J. Cell Biol. 2005;168:315–328. doi: 10.1083/jcb.200408177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bretscher A. J. Cell Biol. 2003;160:811–816. doi: 10.1083/jcb.200301035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H. C., Pon L. A. Proc. Natl. Acad. Sci. USA. 2002;99:751–756. doi: 10.1073/pnas.022462899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zigmond S. H. Curr. Opin. Cell Biol. 2004;16:99–105. doi: 10.1016/j.ceb.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Pruyne D., Evangelista M., Yang C., Bi E., Zigmond S., Bretscher A., Boone C. Science. 2002;297:612–615. doi: 10.1126/science.1072309. [DOI] [PubMed] [Google Scholar]
- 18.Sagot I., Rodal A. A., Moseley J., Goode B. L., Pellman D. Nat. Cell Biol. 2002;4:626–631. doi: 10.1038/ncb834. [DOI] [PubMed] [Google Scholar]
- 19.Evangelista M., Pruyne D., Amberg D. C., Boone C., Bretscher A. Nat. Cell Biol. 2002;4:32–41. doi: 10.1038/ncb718. [DOI] [PubMed] [Google Scholar]
- 20.Chang F. S., Stefan C. J., Blumer K. J. Curr. Biol. 2003;13:455–463. doi: 10.1016/s0960-9822(03)00131-3. [DOI] [PubMed] [Google Scholar]
- 21.Sirotkin V., Beltzner C. C., Marchand J. B., Pollard T. D. J. Cell Biol. 2005;170:637–648. doi: 10.1083/jcb.200502053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonsdottir G. A., Li R. Curr. Biol. 2004;14:1604–1609. doi: 10.1016/j.cub.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 23.Schott D. H., Collins R. N., Bretscher A. J. Cell Biol. 2002;156:35–39. doi: 10.1083/jcb.200110086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information