Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation (original) (raw)
Abstract
Congenital progressive hydronephrosis (cph) is a spontaneous recessive mutation that causes severe hydronephrosis and obstructive nephropathy in affected mice. The mutation has been mapped to the distal end of mouse chromosome 15, but the mutated gene has not been found. Here, we describe the identification of a single base pair change in aquaporin-2 (Aqp2) in cph mutants through genetic linkage mapping. The C-T change led to the substitution of a Ser (S256) by a Leu in the cytoplasmic tail of the Aqp2 protein, preventing its phosphorylation at S256 and the subsequent accumulation of Aqp2 on the apical membrane of the collecting duct principal cells. The interference with normal trafficking of Aqp2 by this mutation resulted in a severe urine concentration defect. cph homozygotes demonstrated polydipsia and produced a copious amount of hypotonic urine. The urine concentration defect could not be corrected by [deamino-Cys1,d-Arg8]-vasopressin (DDAVP, a vasopressin analog), characteristic of nephrogenic diabetes insipidus. The nephrogenic diabetes insipidus symptoms and the absence of developmental defects in the pyeloureteral peristaltic machinery in the mutants before the onset of hydronephrosis suggest that the congenital obstructive nephropathy is most likely a result of the polyuria. This study has revealed the genetic basis for the classical cph mutation and has provided direct genetic evidence that S256 in Aqp2 is indispensable for the apical accumulation, but not the general glycosylation or membrane association, of Aqp2.
Keywords: nephrogenic diabetes insipitus, obstructive nephropathy, polarized trafficking
Congenital obstructive nephropathy is the most frequent cause of renal failure in infants and children (1). Antenatal screening detects fetal hydronephrosis in 1 of 100 births, with at least 20% being clinically significant (2). If left untreated, congenital obstructive nephropathy can cause severe renal failure and death (1). Despite the impact of this devastating disease, the causes in most congenital obstructive cases in humans are not yet known. Besides environmental influence, there are clearly genetic determinants in congenital obstructive nephropathy (3, 4) (OMIM, Online Mendelian Inheritance in Man). Congenital obstructive nephropathy has been described in a number of animal models with spontaneous mutations or targeted genetic modifications (5). The genetic defects in most, if not all, of the naturally occurring mutants are still undetermined, let alone the molecular and cellular mechanism by which the genetic defects cause congenital obstructive nephropathy.
Congenital progressive hydronephrosis (cph) was first discovered as a spontaneous, autosomal recessive trait in C57BL/6J mice at The Jackson Laboratory in the 1970s (6, 7). Because these mutants were thought to have renal cysts, they were named juvenile polycystic kidney until a later study by Horton et al. (7) found that the cystic dysplasia was caused by progressive hydronephrosis resulting from urinary tract obstruction. Horton et al. (7) also mapped cph to the distal part of the long arm of mouse chromosome 15. A rough chromosomal location of 57.8 cM was assigned to the cph locus by Mouse Genome Informatics (MGI), largely based on the genetic mapping results from Horton et al. (7) and the relative positions of the three markers used: caracul (Ca), underwhite (uw), and belted (bt).
Here, we describe the identification of the cph mutation as a single base change in codon 256 of aquaporin-2 (Aqp2). This mutation causes a Ser to Leu substitution, loss of Aqp2 phosphorylation at amino acid 256, and the absence of apical accumulation of the protein. We have demonstrated that the mutants have no response to a vasopressin analog and produce large quantities of hypotonic urine, characteristic of patients with nephrogenic diabetes insipidus (NDI) (8). The polyuria caused by the Aqp2 mutation in cph mutants likely overwhelms the pyeloureteral peristaltic machinery, resulting in the observed hydronephrosis, obstructive nephropathy, renal failure, and death. This study provides direct genetic evidence that phosphorylation of Aqp2 at S256 is essential for its apical membrane accumulation and water reabsorption function in vivo.
Results
The cph Mutants Have Apparent Congenital Functional Obstruction of the Urinary Tract.
The cph mutants appeared grossly normal at birth and made up 27.9% of the pups born in heterozygous intercrosses, very close to the 25% expected for an autosomal recessive mutation following Mendelian inheritance. However, the cph mutants grew slowly and showed a significant size and weight difference from postnatal day (P) 8 onward (Fig. 1A and B). About 90% of the cph mutants died between 2 and 4 weeks of age. By 2 weeks, most mutants also had visibly enlarged abdomens and appeared lethargic. Around 10% of the cph homozygotes survived past weaning, with the oldest cph homozygote living for 10 months. The adult cph homozygotes are either infertile or have modestly reduced fertility.
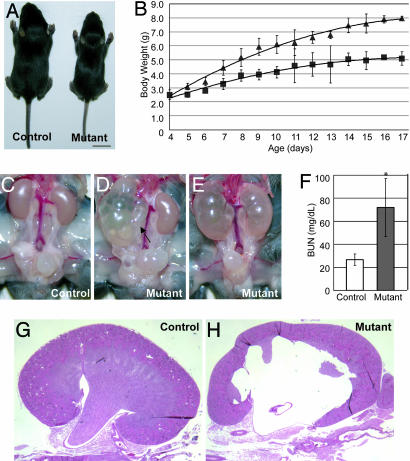
Fig. 1.
The cph mutants have apparent congenital obstruction at multiple levels. (A) At 2 weeks of age, the mutants are significantly smaller than their control littermates. (Scale bar: 1 cm.) (B) The mutants grow slower than the controls. ▴, controls; ■, mutants. (C_–_E) The mutants have unilateral or bilateral hydronephrosis and hydroureter (arrow). (F) The blood urea nitrogen (BUN) level in the mutants (P5–P16) is 70.0 ± 25.0 mg/dl (n = 35), significantly (P < 0.001) higher than that of the controls (P5–P16, 26.8 ± 5.0 mg/dl; n = 29). (G and H) Kidney sections from P14 control and mutant littermates stained with hematoxylin/eosin.
Although younger mutants (<P14) had unilateral or bilateral hydronephrosis of various degrees, older mutants (>P14) almost always had severe bilateral hydronephrosis (Fig. 1 C_–_E). This observation is consistent with the findings in the Horton et al. (7) study but argues against the hypothesis that this is a model of polycystic kidney disease (6). However, the finding of hydroureter (arrow in Fig. 1D) and apparent obstruction at the ureterovesical junction (UVJ) in ≈25% of the mutants suggests that these mutants are unlikely to have complete physical blockage at the ureteropelvic junction (UPJ) level as proposed (7). Furthermore, we have not found any correlation between the volume of the bladder and the genotypes of the mice dissected, suggesting that the defect in the mutants is likely in a location proximal to the bladder along the urinary tract. Interestingly, ≈66.3% of the mutants had more severe defects in the right kidney at the time of examination, whereas only 22.8% had more severe defects in the left and 10.9% had both kidneys equally affected.
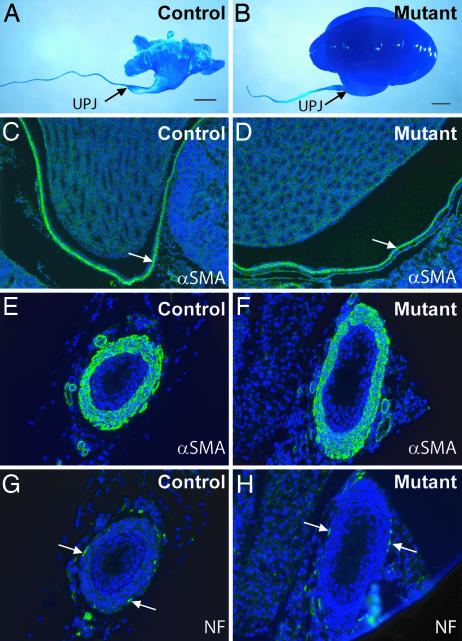
Blood urea nitrogen (BUN) levels in the mutants were significantly elevated (Fig. 1F), suggesting renal failure as a result of obstructive nephropathy. The mutant kidneys had pathological changes, characteristic of obstructive nephropathy, ranging from parenchymal atrophy, erosion of the renal pelvis, expansion of the pelvicocaliceal space, and dilatation of the collecting ducts (Fig. 1 G and H and data not shown). Molding polymers injected into the pelvicocaliceal space were able to travel along the urinary path to the bladder in both the controls and mutants, although the mutant urinary path is distorted by the hydronephrosis, especially in the pelvicocaliceal space (Fig. 2A and B). This result argues against the existence of physical blockage from the kidney to the bladder and suggests that hydronephrosis is likely caused by a functional obstruction, namely urine retention in the urinary tract as a result of a functionally, but not structurally, impaired downward urine transport. Morphological examination of newborn mutants and their control littermates did not reveal any significant changes in the smooth muscle cells that are important for contractability of the pyeloureteral complex (Fig. 2 C_–_F) or in the innervating nerves (Fig. 2 G and H) that modulate pyeloureteric peristalsis.
Fig. 2.
The cph mutants do not have complete physical obstruction or gross developmental abnormalities in the smooth muscles and nerves along the urinary tract. (A and B) Resin moldings of the pelvicocaliceal space and ureteric lumen at P5. (Scale bars: 1 mm.) (C_–_F) αSMA staining (green) of the smooth muscles in the pelvic wall (arrows in C and D) and ureteric sections (E and F) at P6. (G and H) Nerve fibers (arrows) on the cross sections of the ureters from P6 mice were revealed by the antineurofilament antibody (NF, green). The slides were counterstained with DAPI to reveal the nuclei (blue).
Genetic Linkage Mapping of the cph Locus.
Because the cph mutation is in a pure C57BL/6J background, we outcrossed cph heterozygotes to three inbred strains (DBA/2J, AKR/J, and MOLD/RkJ) to bring in different genetic backgrounds for testing segregation and linkage. By backcrossing aphenotypic F1s to confirmed cph heterozygotes, we identified heterozygous F1 mice based on their ability to produce mutants. Mapping was done primarily with F2s derived from intercrossing F1 heterozygotes. Previous genetic mapping efforts using three classical genetic markers on chromosome 15: Ca, uw, and bt suggested that the likely arrangement of the markers is _uw_-_bt_-_Ca_-cph. The cph locus was tentatively assigned by MGI (Mouse Genome Informatics; www.informatics.jax.org) to mouse chromosome 15 at 57.8 cM distal to the Hoxc complex, based largely on these results (7). Due to the relatively low number of informative recombinations in the original study and the repositioning of reference markers after the sequencing of the mouse genome, we began mapping with markers covering the distal ≈40 Mbp of mouse chromosome 15, from marker D15Mit63 (≈65.5 Mbp) to the end of the chromosome (≈104 Mbp). Both known microsatellite markers found in public databases and novel ones discovered through our computational analyses were used. After screening 618 mice representing 1,050 informative meioses, we localized the mutation to a 0.7-Mbp chromosomal interval proximal (not distal) to the Hoxc complex and between the classical markers bt and Ca. This interval is defined by newly discovered microsatellite markers C15LD6 and C15LD5 at positions 99104970 and 99804700 (Ensembl build 34), respectively (Fig. 3A and Fig. 7, which is published as supporting information on the PNAS web site). This region is syntenic to human chromosome 12q13.12.
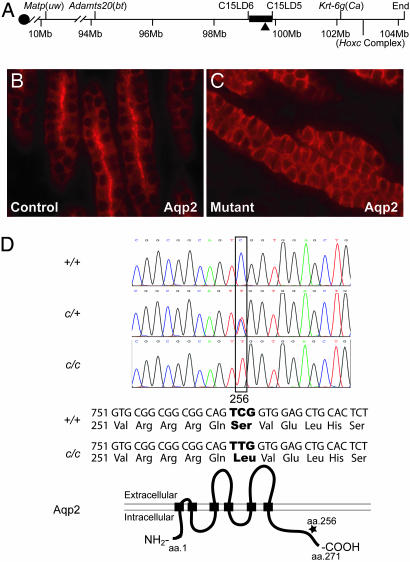
Fig. 3.
Genetic linkage mapping and positional cloning of cph. (A) Physical map of relevant portions of mouse chromosome 15. Our genetic mapping efforts locate the cph locus to the chromosomal interval of ≈0.7 Mbp, defined by the microsatellite markers C15LD6 and C15LD5. The black triangle indicates the chromosomal location of Aqp2. (B and C) Immunostaining of the collecting ducts in the outer medulla shows the apical accumulation of Aqp2 in the controls (B) but a diffuse staining with no apical accumulation in the mutant (C). (D) Sequence of the fourth exon of Aqp2 reveals the C-T substitution at nucleotide 767 in the homozygous mutants, whereas the heterozygotes have both C and T represented at position 767. This substitution results in a Ser to Leu change at amino acid 256 in the cytoplasmic tail of the Aqp2 protein. +, WT allele; c, cph mutant allele.
Identification of the cph Mutation.
There are 25 known and predicted genes in the interval between C15LD6 and C15LD5 (Fig. 7, based on NCBI Genome Mapview and Ensembl mouse genome server). At least five of these genes (Gpd1, ACCN2, Racgap1, Aqp5, and Prph1) were given low priority because their mutant alleles produce distinctively different phenotypes from those observed in cph mutants (MGI; www.informatics.jax.org). After careful review of the remaining genes, one of these genes, Aqp2, was given high priority for further analysis due to its known expression in the kidney, its physiological function, and the observations that mutations in Aqp2 or its upstream regulator, arginine vasopressin receptor 2 (AVPR2), cause NDI and kidney defects reminiscent of obstructive nephropathy (9–11). To find a possible link between Aqp2 and the cph mutation, we investigated the expression and distribution of Aqp2 in kidney sections from control and mutant littermates. Although Aqp2 was predominantly distributed on the apical membrane in the principal cells of the collecting duct in the control samples, especially in the cortex and outer medulla, its apical accumulation was completely lost at all levels of the collecting duct in all mutants studied (Fig. 3 B and C). There was also an apparent overall increase in Aqp2 levels in mutant collecting ducts. Furthermore, we examined the distribution of Aqp2 in the principal cells of the Pax3Cre-Cnb1 mutants we have previously studied that also have severe congenital obstructive nephropathy (12). The distribution of Aqp2 was not changed in the Pax3Cre-Cnb1 mutants despite overt collecting duct dilatation (data not shown), suggesting that the absence of Aqp2 apical accumulation was not simply a secondary result of the congenital obstructive nephropathy.
These findings prompted us to sequence all coding sequences and exon/intron junctions of the Aqp2 gene of the wild-type (WT), cph heterozygous, and cph homozygous mice. A mutation was found in nucleotide 767 in the fourth exon of Aqp2 in the mutants (Fig. 3D). The C in codon 256 was changed to a T in the mutants, leading to a Ser to Leu substitution in the protein. This Ser is highly conserved in all vertebrate species analyzed (Table 1, which is published as supporting information on the PNAS web site). This mutation correlated perfectly with cph genotype and phenotype: all homozygous mutant samples sequenced were homozygous for the mutation, all WT mice in the same colony as well as all of the WT inbred mice from C57BL/6J, DBA/2J, AKR/J, and MOLD/RkJ lacked it, and all confirmed cph heterozygotes had both C and T in this position. These findings strongly suggest that the Aqp2-S256L mutation is associated with the cph defects.
The S256L Mutation Affects the Expression of Aqp2 and Its Phosphorylation at S256.
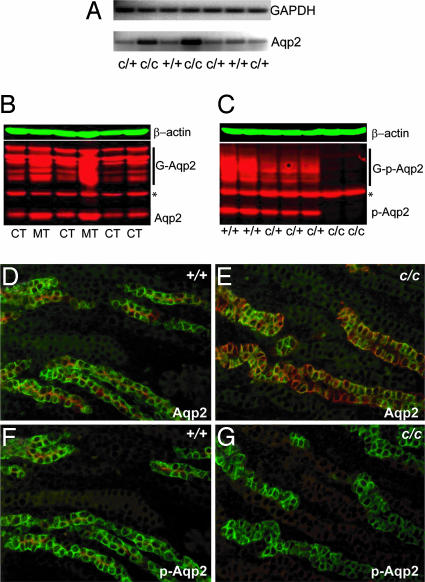
Previous studies have indicated that phosphorylation of Aqp2-S256 is a key regulatory event in Aqp2 trafficking (13, 14, 18–21). However, point mutations at S256 have not yet been reported in human patients or animal models before this study. To better understand the effects of the S256L mutation at the cellular and molecular levels in vivo, we examined the expression and phosphorylation of Aqp2 in kidney samples from control and mutant mice. RT-PCR using total RNA from whole kidneys demonstrated a clear increase in Aqp2 transcripts in the mutant kidneys (Fig. 4A), likely as the result of a futile feed-back mechanism. Aqp2 protein levels were also increased in the mutants as shown in Western blotting assays (Fig. 4B). This finding is consistent with the apparent increase of Aqp2 signal in mutant kidney sections immunostained with the anti-Aqp2 antibody (Fig. 3C). This increase pertained to both the unglycosylated and glycosylated forms of Aqp2, suggesting that the S256L mutation does not affect the overall glycosylation pattern of Aqp2. As expected, Aqp2 phosphorylation at S256 was completely absent in the cph homozygotes, as shown by probing kidney extracts (Fig. 4C) and kidney sections (Fig. 4 D_–_G) with an antibody that specifically recognizes the S256-phosphorylated form of Aqp2 (14).
Fig. 4.
Absence of S256 phosphorylation and increased Aqp2 expression in the cph mutants. (A) RT-PCR of total RNA from whole kidneys revealed an increase of Aqp2 transcripts in the mutants (c/c). (B) Western blot of total Aqp2 protein from control and mutant kidneys. G-Aqp2 indicates the various forms of glycosylated Aqp2. ∗, a band that cross-reacts with the secondary antibody alone and is deemed unrelated to Aqp2. Aqp2 protein levels in the mutant samples are elevated. (C) Western blot of proteins from whole kidney extracts probed with an Aqp2-S256 phosphorylation-specific antibody. The mutant samples (c/c) lost the S256 phosphorylation on Aqp2. (D_–_G) Immunostaining of the outer medullary collecting ducts. The Aqp2-S256 phospho-specific antibody (pAqp2) revealed apical distribution of the S256-phosphorylated Aqp2 (red) in the WT principal cells labeled by Dolichos biflorus agglutinin (green) (F), closely resembling the Aqp2 distribution shown by the nonphospho-specific Aqp2 antibody staining (red) on adjacent sections (D). The mutant (c/c), however, has a complete absence of phospho-specific Aqp2 staining (G), but apparently overexpresses the Aqp2-S256L that lacks the S256 phosphorylation (E).
cph Heterozygotes Have an Intermediate Phenotype in Aqp2 Distribution and Aqp2-S256L Seems to Retain Membrane Association Without S256 Phosphorylation.
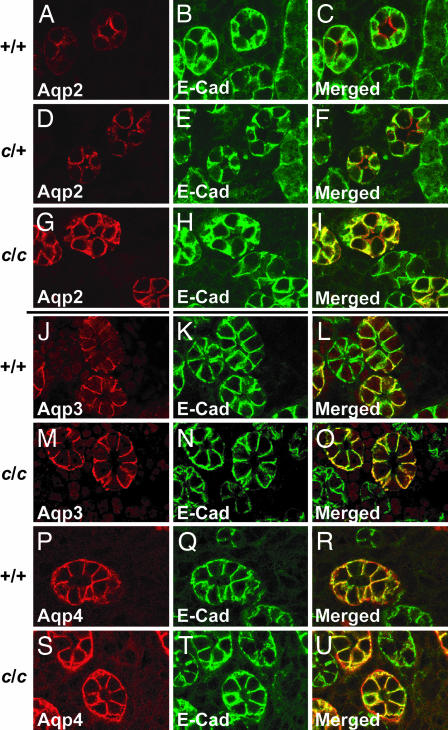
The identification of Aqp2-S256L in the cph mutants opens the opportunity to study the consequence of the loss of Aqp2 phosphorylation in vivo. Aqp2-S256L seems to retain its ability to localize to the plasma membrane as revealed by confocal images of overlapping staining of Aqp2-S256L and E-Cadherin (expressed on the basolateral membrane in the collecting duct principal cells) in the cph mutants (Fig. 5A_–_I). These observations suggest that the phosphorylation of S256 is critical for apical accumulation but not membrane association of Aqp2. Whereas the overall pattern of Aqp2 expression in the heterozygotes is similar to that of the WT mice, the heterozygotes tend to have a higher degree of basolateral distribution of Aqp2 compared with the WTs, apparently an indication of misrouting of mutant tetramers or chimeric tetramers with both WT and mutant subunits (Fig. 5 A_–_I).
Fig. 5.
Aqp2-S256L has membrane association but lacks apical accumulation. Paraffin sections of the outer medulla from P1 mice of WT (A_–_C), heterozygous (D_–_F), and homozygous (G_–_I) littermates were stained with an Aqp2 antibody (A, D, and G) or an anti-E-Cadherin (E-Cad) antibody (B, E, and H). C, F, and I are merged images of the first two channels. E-Cadherin is expressed on the basolateral membrane whereas Aqp2 accumulates on the apical membrane of the principal cells in the WT. There is very little overlap between the Aqp2 staining and the E-Cadherin staining. In the cph homozygotes, however, Aqp2-S256L does not accumulate on the apical membrane but shows a prominent basolateral distribution as revealed by the yellow signal produced by the overlapping red Aqp2 and green E-Cadherin staining on basolateral membranes. Although the overall pattern of Aqp2 expression in the heterozygotes is similar to that of the WT mice, the heterozygotes show a higher degree of basolateral distribution of Aqp2 compared with the WT (A_–_I). In cph mutants, both Aqp3 and Aqp4 are expressed on the basolateral membrane and at levels similar to those seen in the WT mice (J_–_U).
Besides the vasopressin-regulated Aqp2, two other non-vasopressin-sensitive aquaporins, Aqp3 and Aqp4, are expressed in the principal cells of the collecting ducts. Although Aqp2 is predominantly expressed on the apical membrane, Aqp3 and Aqp4 are exclusively located on the basolateral membranes (15). Confocal images of immunostained kidney sections from WT and cph mutant mice showed that the expression level and the basolateral localization of Aqp3 and Aqp4 are not changed in the mutants (Fig. 5 J_–_R), suggesting that the observed loss of Aqp2 apical accumulation is not caused by general alteration of cell polarity. These results also suggest that the three aquaporins in the collecting ducts are regulated independently without any existing mechanism of compensatory coordination.
cph Mutants Have both NDI Symptoms and Obstructive Nephropathy.
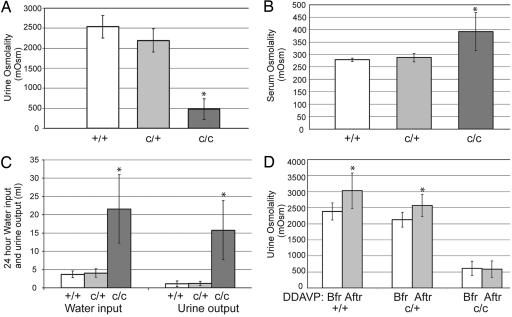
The discovery of the Aqp2-S256L mutation suggests the possibility that the congenital progressive hydronephrosis in cph homozygotes could be a result of NDI symptoms, especially polyuria (16). To study this possibility further, we first showed that the cph homozygotes failed to concentrate urine as effectively as their WT and heterozygous littermates (Fig. 6A). The failure of urine concentration directly led to higher serum osmolality, a sign of dehydration, in the homozygotes (Fig. 6B). We then subjected these mice to 24-h urine collections in metabolic cages. As expected, the homozygotes had a much higher urine output than their WT and heterozygous littermates (Fig. 6C). Although the administration of [deamino-Cys1,d-Arg8]-vasopressin (DDAVP), a synthetic agonist for arginine vasopressin receptor 2 (AVPR2), was effective in stimulating urine concentration in the WT and heterozygous mice, it had no effects on the homozygotes (Fig. 6D). These findings are consistent with the hypothesis that the Aqp2-S256L mutation in the cph mutants interrupts the water reabsorption function of Aqp2 and leads to polyuria as well as obstructive nephropathy. Although the water reabsorption defect in the homozygotes is prominent, there were no significant differences in excretion of key electrolytes, such as Na and K, as indicated by urinary sodium/creatinine and potassium/creatinine ratios (data not shown).
Fig. 6.
The obstructive nephropathy is likely induced by polyuria in the cph mutants. (A) cph mutants have defects in urine concentration. The urine osmolality in adult mice is significantly lower in the cph homozygotes (480.8 ± 258.0; n = 10) versus WT (2535.9 ± 283.6; n = 8) and heterozygotes (2192.3 ± 289.8; n = 16). ∗, P < 0.001 compared with the other two groups. All measurements are in mOsm/kg unless otherwise stated. (B) Serum osmolality is significantly increased in the homozygotes (391.9 ± 76.7; n = 27) compared with the WT (278.8 ± 6.7; n = 6) and heterozygous mice (287.6 ± 16.4; n = 20). ∗, P < 0.001 compared with the other two groups. (C) cph homozygotes have increased water input (21.6 ± 9.4 ml; n = 11) and urine output (15.8 ± 8.1 ml) than those of the WT (3.7 ± 0.9 ml water intake and 1.1 ± 0.8 ml urine output, n = 9) and heterozygous (4.0 ± 1.1 ml of water intake and 1.2 ± 0.6 ml of urine output; n = 11) littermates. ∗, P < 0.001 compared with the other two groups. (D) DDAVP injection promotes urine concentration in the WT and heterozygotes but not in the cph homozygotes. The postinjection urine osmolality is increased from 2,385.3 ± 265.7 (n = 4) and 2,127.3 ± 228.2 (n = 11) to 3,030.0 ± 554.7 and 2,571.4 ± 343.3 for +/+ and c/+ mice respectively. ∗, P < 0.05 compared with measurements taken before injection. The osmolality of the post injection urine of the mutant is not significantly different from that measured before the injection (from 614.7 ± 222.5 to 584.3 ± 260.3; n = 11). Bfr, before DDAVP injection; Aftr, after DDAVP injection.
Discussion
The nature of the cph mutation had been elusive for over three decades since its discovery in the early 1970s (6). In this study, we used genetic linkage mapping to localize cph to a small chromosomal interval and identified an S256L mutation in Aqp2 that correlates with the genotypes and phenotypes of the cph mice.
Although some cases of congenital hydronephrosis are caused by physical obstruction of the urinary tract, others are likely due to functionally impaired transport of urine from the kidney through the lower urinary tract to the outside environment. Besides neuronal and muscular abnormalities affecting the pyeloureteral peristaltic machinery and urine transfer (5), polyuria, as a result of defective urine concentrating ability, can also overwhelm the urine transport system, causing temporary or irreversible hydronephrosis (16, 17). The absence of physical blockage and developmental defects of the urinary tract (Fig. 2) are not surprising, considering the late onset of Aqp2 expression in embryogenesis and its principal cell-restricted distribution within the urinary system. The observed hydronephrosis in the cph mutants is apparently a result of the inability of the urine transport system to handle the dramatically increased urine production in the mutants (Fig. 6).
Although original characterization of the cph mutants as a model for polycystic kidney disease was corrected by the study of Horton et al. (7), our discovery of the Aqp2-S256L mutation and the finding of NDI defects in these mutants indicate that the obstructive nephropathy is accompanied by, and most likely secondary to, the defective urine concentrating ability in these mice, analogous to the observation of hydronephrosis in NDI patients with poorly controlled polyuria (16). These studies also underscore the fact that kidney diseases in many cases have ambiguous pathological presentations and require an understanding of the primary molecular and cellular defects to uncover the true nature of the condition. Hydronephrosis is a common prenatal diagnosis (1) that frequently resolves spontaneously. Because urine output is highly variable and influenced by multiple factors, it is conceivable that temporary polyuria may contribute substantially to these spontaneously resolved hydronephrosis cases.
Although the phosphorylation of Aqp2-S256 has been shown to affect Aqp2 trafficking (14, 18–21), point mutation of S256 has not been reported in human patients or animals before this study (8). As expected, the S256L mutation in Aqp2 disrupts phosphorylation at S256 (Fig. 4C) and prevents the apical accumulation of Aqp2 in the principal cells of the collecting duct (Fig. 3 B and C). In contrast to the intracellular vesicle retention observed in Aqp2-S256A mutants transfected into cultured cells (19), Aqp2-S256L seems to still be able to localize to the cell membrane, although the polarized transport to the apical surface of the cell is disturbed (Fig. 5 A_–_I). These observations suggest that the phosphorylation of S256 is critical for apical accumulation but not membrane association of Aqp2 in vivo. Although the expression level of Aqp2-S256L is increased in the cph homozygotes, the glycosylation pattern of the mutant protein appeared unchanged (Fig. 4B), suggesting that the phosphorylation of S256 may not be required for the glycosylation of Aqp2. Dileucine motifs have been indicated as a basolateral trafficking signal (22). Recent studies have also suggested the involvement of single leucine residues in basolateral trafficking (23), although the requirement of optimal context has not been ruled out. Nevertheless, the possibility exists that L256 in the mutant Aqp2 contributes to the shift from apical to basolateral distribution due to the loss of S256 phosphorylation.
Impaired routing of WT AQP2 after tetramerization with mutant AQP2 has been indicated as the molecular basis for the autosomal dominant form of NDI (24, 25). In autosomal recessive NDI, the absence of symptoms in heterozygotes may be due to the lack of interaction between mutant and WT Aqp2 subunits or the ability of the apical sorting signal in the WT subunit to override the aberrant sorting signals (26) in interacting mutant subunits. Interestingly, whereas the overall pattern of Aqp2 expression in the heterozygotes is similar to that of the WT mice, the heterozygotes tend to have a higher degree of basolateral distribution of Aqp2 compared with the WTs (Fig. 5 A_–_I). Although the ability of Aqp2-S256L to form tetramers with WT Aqp2 subunits has not been directly investigated, previous in vitro studies have indicated that changes in S256 did not affect tetramerization (27). The recessive inheritance of cph suggests that there are enough functional Aqp2 tetramers on the apical membrane of the principal cells in the heterozygotes for water reabsorption. In addition to the WT homotetramers, the chimeric tetramers consisting of both WT and mutant subunits may also reach the apical membrane, guided by the WT subunits.
The cph mutants represent a special class of NDI caused by protein trafficking defects in which most of the Aqp2 proteins are misrouted to the basolateral membrane rather than being sequestered intracellularly. Investigation of factors controlling the polarized trafficking of Aqp2 may yield strategies for reversing the misrouting and restoring the apical accumulation of Aqp2. The cph mutants would be a nice model for testing such strategies that may have potential therapeutic benefits for NDI as well as implications for other trafficking related diseases. On the other hand, whereas surgical models have been instrumental in the study of pathological changes in obstructive nephropathy, the abrupt onset of the obstruction differs dramatically from those seen in most congenital obstructive nephropathy cases. The cph mutants have naturally occurring hydronephrosis that is 100% penetrant, making them an excellent model for the study of congenital obstructive nephropathy, especially those caused by functional but not physical obstruction.
Materials and Methods
Mice and Microsatellite Markers.
We intercrossed cph heterozygotes, obtained from The Jackson Laboratory, to generate phenotypically normal mice that are considered controls (either heterozygotes or WT) and mice having kidney defects that are considered mutants (homozygotes). The cph heterozygotes have no detectable abnormality and, before the revelation of the mutation, can be identified only by their ability to produce cph homozygous mutants in matings with confirmed cph heterozygotes. DBA/2J, AKR/J, and Mold/RkJ inbred strains were also obtained from The Jackson Laboratory for genetic mapping. To identify polymorphic microsatellite markers for the genetic mapping of cph, we have used the following databases: MGI, the Ensembl Mouse Genome Server, and the Mouse Genetic Map Database from Massachusetts Institute of Technology. In addition, we developed a computer program to scan the mouse genome for DNA fragments consisting of at least 15 copies of repeating units of 2–5 nt. By blasting the DNA fragment containing the repeats and unique flanking sequences identified by our computational analyses against the strain-specific genomic fragments within the Celera Discovery System, we were able to identify unpublished polymorphic microsatellite markers.
Histological analyses, immunostaining, and corrosive casting were performed as described (12, 28). Antibodies used were as follows: Aqp2 (polyclonal 1:500; BD Biosciences); neurofilament (monoclonal 1:100; Developmental Hybridoma Studies Bank, University of Iowa); αSMA (monoclonal 1:300; Sigma); Aqp2S256 phosphorylation specific (polyclonal 1:400; from S. Nielsen); E-Cadherin (monoclonal 1:200; BD Transduction Laboratories); Aqp3 (polyclonal 1:150; Chemicon); Aqp4 (polyclonal 1:400; Chemicon); Alexa Fluor 488- or Alexa Fluor 566-conjugated goat anti-mouse or goat anti-rabbit (1:1,000; Molecular Probes). FITC-labeled dolichos biflorus agglutinin (Vector Laboratories) was used at 1:100. Images in Fig. 5 were taken with a Zeiss LSM510 confocal microscope.
Serological Analysis and Urinalysis.
Mice were housed in metabolic cages (Nalge) and given free access to water and powdered chow (Rodent Diet 5001; LabDiet). Mice were allowed to acclimatize in the cages for 24 h before DDAVP (Sigma) injection at 0.4 μg/kg i.p. Urine samples were collected for the 24 h before injection from the metabolic cage and at 2 h after DDAVP injection by spontaneous voiding or bladder massage. A vapor pressure osmometer (Wescor, Logan, UT) was used to determine urine osmolality. Urine and serum chemistry was analyzed by the Renal Chemistry Laboratory at Washington University School of Medicine.
Western Blotting and RT-PCR.
Kidney protein extracts were prepared as described (29), separated by SDS/PAGE, transferred to Immobilon-P membranes (Millipore), and probed by the anti-Aqp2 antibody and an anti-β-actin antibody (JLA20; Developmental Hybridoma Studies Bank, University of Iowa). After incubation with Alexa Fluor 680-conjugated goat anti-rabbit IgG (Molecular Probes) and IRDye 800CW-conjugated goat anti-mouse IgG (Rockland, Gilbertsville, PA) secondary antibodies, the antibody complexes were visualized by the Odyssey Infrared Imaging System (LI-COR). RT-PCR primers for Aqp2 were as follows: 5′-CACATCAACCCTGCTGTGAC-3′ and 5′-CAGCTGCATGGTCAGGAAGAG-3′, 228 bp product). Cycling conditions were 45°C for 30 min, 95°C for 5 min, then 30 cycles of 95°C for 20 s, 58°C for 35 s, and 72°C for 45 s.
Supplementary Material
Supporting Information
Acknowledgments
We thank Drs. J. H. Miner, C. Moulson, E. Eicher, H. Liapis, and J. Mandell for helpful information and suggestions; Dr. S. Nielsen (Water and Salt Research Center, University of Aarhus, Aarhus, Denmark) for the Aqp2-S256 phosphorylation-specific antibody; and D. Martin for running the urine and serum chemistry assays. F.C. is supported in part by institutional funds from the Department of Internal Medicine/Renal Division at Washington University School of Medicine, a March of Dimes Basil O'Conner Award, and National Institutes of Health (NIH) Grants R21DK64816 and R01DK067386. P.A.K is supported by a fellowship from the NIH.
Abbreviations
cph
congenital progressive hydronephrosis
Aqp2
_Aqp2_aquaporin-2
NDI
nephrogenic diabetes insipitus
DDAVP
[deamino-Cys1,d-Arg8]-vasopressin
P_n_
postnatal day n.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Chevalier R. L. Pediatr. Nephrol. 1999;13:612–619. doi: 10.1007/s004670050756. [DOI] [PubMed] [Google Scholar]
- 2.Grasso M., Gitlin J. eMedicine. 2001 www.emedicine.com/med/topic3074.htm.
- 3.Feather S. A., Malcolm S., Woolf A. S., Wright V., Blaydon D., Reid C. J., Flinter F. A., Proesmans W., Devriendt K., Carter J., et al. Am. J. Hum. Genet. 2000;66:1420–1425. doi: 10.1086/302864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chertin B., Puri P. J. Urol. 2003;169:1804–1808. doi: 10.1097/01.ju.0000058428.00284.d5. [DOI] [PubMed] [Google Scholar]
- 5.Peters C. A. Prenatal Diagn. 2001;21:917–923. doi: 10.1002/pd.211. [DOI] [PubMed] [Google Scholar]
- 6.Fox S., Eicher E. Mouse News Letter. 1978;58:47. [Google Scholar]
- 7.Horton C. E., Jr., Davisson M. T., Jacobs J. B., Bernstein G. T., Retik A. B., Mandell J. J. Urol. 1988;140:1310–1315. doi: 10.1016/s0022-5347(17)42033-7. [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara T. M., Bichet D. G. J. Am. Soc. Nephrol. 2005;16:2836–2846. doi: 10.1681/ASN.2005040371. [DOI] [PubMed] [Google Scholar]
- 9.Yang B., Gillespie A., Carlson E. J., Epstein C. J., Verkman A. S. J. Biol. Chem. 2001;276:2775–2779. doi: 10.1074/jbc.M008216200. [DOI] [PubMed] [Google Scholar]
- 10.Yun J., Schoneberg T., Liu J., Schulz A., Ecelbarger C. A., Promeneur D., Nielsen S., Sheng H., Grinberg A., Deng C., Wess J. J. Clin. Invest. 2000;106:1361–1371. doi: 10.1172/JCI9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lloyd D. J., Hall F. W., Tarantino L. M., Gekakis N. PLoS Genet. 2005;1:e20. doi: 10.1371/journal.pgen.0010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang C. P., McDill B. W., Neilson J. R., Joist H. E., Epstein J. A., Crabtree G. R., Chen F. J. Clin. Invest. 2004;113:1051–1058. doi: 10.1172/JCI20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noda Y., Sasaki S. Biol. Cell. 2005;97:885–892. doi: 10.1042/BC20040120. [DOI] [PubMed] [Google Scholar]
- 14.Nejsum L. N., Zelenina M., Aperia A., Frokiaer J., Nielsen S. Am. J. Physiol. Renal Physiol. 2005;288:F930–F938. doi: 10.1152/ajprenal.00291.2004. [DOI] [PubMed] [Google Scholar]
- 15.King L. S., Kozono D., Agre P. Nat. Rev. Mol. Cell Biol. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- 16.Zatuchni J., Armento D. F., Menzel P. H. Am. J. Med. Sci. 1964;247:445–450. doi: 10.1097/00000441-196404000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Laycock J. F., Barrett M. C., Woodrow D. F. Int. J. Exp. Pathol. 1991;72:581–587. [PMC free article] [PubMed] [Google Scholar]
- 18.Fushimi K., Sasaki S., Marumo F. J. Biol. Chem. 1997;272:14800–14804. doi: 10.1074/jbc.272.23.14800. [DOI] [PubMed] [Google Scholar]
- 19.Katsura T., Gustafson C. E., Ausiello D. A., Brown D. Am. J. Physiol. 1997;272:F817–F822. [PubMed] [Google Scholar]
- 20.van Balkom B. W., Savelkoul P. J., Markovich D., Hofman E., Nielsen S., van der Sluijs P., Deen P. M. J. Biol. Chem. 2002;277:41473–41479. doi: 10.1074/jbc.M207525200. [DOI] [PubMed] [Google Scholar]
- 21.de Mattia F., Savelkoul P. J., Kamsteeg E. J., Konings I. B., van der Sluijs P., Mallmann R., Oksche A., Deen P. M. J. Am. Soc. Nephrol. 2005;16:2872–2880. doi: 10.1681/ASN.2005010104. [DOI] [PubMed] [Google Scholar]
- 22.Campo C., Mason A., Maouyo D., Olsen O., Yoo D., Welling P. A. Rev. Physiol. Biochem. Pharmacol. 2005;153:47–99. doi: 10.1007/s10254-004-0037-1. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Boulan E., Kreitzer G., Musch A. Nat. Rev. Mol. Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 24.Kamsteeg E. J., Bichet D. G., Konings I. B., Nivet H., Lonergan M., Arthus M. F., van Os C. H., Deen P. M. J. Cell Biol. 2003;163:1099–1109. doi: 10.1083/jcb.200309017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamsteeg E. J., Wormhoudt T. A., Rijss J. P., van Os C. H., Deen P. M. EMBO J. 1999;18:2394–2400. doi: 10.1093/emboj/18.9.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Mattia F., Savelkoul P. J., Bichet D. G., Kamsteeg E. J., Konings I. B., Marr N., Arthus M. F., Lonergan M., van Os C. H., van der Sluijs P., et al. Hum. Mol. Genet. 2004;13:3045–3056. doi: 10.1093/hmg/ddh339. [DOI] [PubMed] [Google Scholar]
- 27.Kamsteeg E. J., Heijnen I., van Os C. H., Deen P. M. J. Cell Biol. 2000;151:919–930. doi: 10.1083/jcb.151.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graef I. A., Chen F., Chen L., Kuo A., Crabtree G. R. Cell. 2001;105:863–875. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- 29.Wang W., Li C., Kwon T. H., Knepper M. A., Frokiaer J., Nielsen S. Am J. Physiol. Renal Physiol. 2002;283:F1313–25. doi: 10.1152/ajprenal.00040.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information