Physical State of the Extracellular Matrix Regulates the Structure and Molecular Composition of Cell-Matrix Adhesions (original) (raw)
Abstract
This study establishes that the physical state of the extracellular matrix can regulate integrin-mediated cytoskeletal assembly and tyrosine phosphorylation to generate two distinct types of cell-matrix adhesions. In primary fibroblasts, α5β1 integrin associates mainly with fibronectin fibrils and forms adhesions structurally distinct from focal contacts, independent of actomyosin-mediated cell contractility. These “fibrillar adhesions” are enriched in tensin, but contain low levels of the typical focal contact components paxillin, vinculin, and tyrosine-phosphorylated proteins. However, when the fibronectin is covalently linked to the substrate, α5β1 integrin forms highly tyrosine-phosphorylated, “classical” focal contacts containing high levels of paxillin and vinculin. These experiments indicate that the physical state of the matrix, not just its molecular composition, is a critical factor in defining cytoskeletal organization and phosphorylation at adhesion sites. We propose that molecular organization of adhesion sites is controlled by at least two mechanisms: 1) specific integrins associate with their ligands in transmembrane complexes with appropriate cytoplasmic anchor proteins (e.g., fibronectin–α5β1 integrin–tensin complexes), and 2) physical properties (e.g., rigidity) of the extracellular matrix regulate local tension at adhesion sites and activate local tyrosine phosphorylation, recruiting a variety of plaque molecules to these sites. These mechanisms generate structurally and functionally distinct types of matrix adhesions in fibroblasts.
INTRODUCTION
The association of cells with the extracellular matrix (ECM) initiates the assembly of specific cell-matrix adhesion sites. These sites are involved in physical attachment of cells to external surfaces, which is essential for cell migration and tissue formation as well as for activation of adhesion-mediated signaling events. Key mediators of both matrix attachment and signaling responses are the integrins, which are heterodimeric transmembrane receptors for ECM components (Hynes, 1992; Clark and Brugge, 1995). Following association with their ligands, integrins induce reorganization of the actin cytoskeleton and associated proteins, resulting in the formation of cell-matrix adhesion sites.
The best-known class of matrix adhesions in cultured cells are the focal contacts (FCs), which can be visualized by electron microscopy or interference reflection microscopy (Abercrombie and Dunn, 1975; Izzard and Lochner, 1976; Jockusch et al., 1995). These sites contain a multitude of anchor and cytoskeletal molecules such as vinculin, paxillin, and talin (Burridge et al., 1992; Jockusch et al., 1995; Yamada and Geiger, 1997) as well as signal transduction molecules, such as focal adhesion kinase (FAK), C-terminus Src kinase (csk), protein kinase C, and others (for review, see Yamada and Miyamoto, 1995). Recent studies have shown that the assembly and tyrosine phosphorylation of FCs depend on actomysin contractility, which in turn is regulated by cytoplasmic factors such as Rho, caldesmon, or microtubular integrity (Jockusch et al., 1995; Bershadsky et al., 1996; Chrzanowska-Wodnicka and Burridge, 1996; Craig and Johnson, 1996; Gilmore and Burridge, 1996; Burridge et al., 1997; Pelham and Wang, 1997; Helfman et al., 1999; Zamir et al., 1999).
The specific type of integrin present in matrix adhesions can vary, depending on the nature of the underlying ECM. The dominant integrin in mature FCs is αvβ3 (Dejana et al., 1988; Singer et al., 1988; Fath et al., 1989). In addition, however, fibroblasts can form a distinct class of adhesive contacts in which cell surface integrins bind to fibronectin fibrils in fibrillar adhesions (Chen and Singer, 1982; Chen et al., 1985; Singer et al., 1988). Moreover, it has been shown that fibronectin uniformly adsorbed on the culture substrate can be cleared from under the FC and reorganized into fibrils (Avnur and Geiger, 1981). Thus, the process of classical FC assembly may reflect only one type of association of integrins with the ECM, and different cells can display different patterns of matrix adhesions. For example, FC and fibronectin fibrils appear to be colocalized in NIL-8 cells (Hynes and Destree, 1978), yet FN is absent from beneath the FC of epithelial and other fibroblastic cells (Chen and Singer, 1980; Fox et al. 1980; Avnur and Geiger, 1981).
This notion of distinct types of matrix adhesions was recently corroborated by digital microscopic observations of fibroblasts double-labeled for pairs of adhesion-associated molecules (Zamir et al., 1999). This study established that FCs and fibrillar adhesions differ in their cytoskeletal association and in the composition of the submembrane plaque (Zamir et al., 1999). However, the mechanism responsible for the differential assembly of the two types of matrix adhesions was unclear.
In the present study, we investigated the assembly of fibrillar adhesions and FCs and then identified a crucial physical regulator governing the choice between these two distinct types of matrix adhesion. We first characterized the differential distribution of selected integrins and cytoskeletal components in each type of adhesion in human fibroblasts cultured on fibronectin. We show here that the α5β1 fibronectin receptor is excluded from the core of FC and is mainly associated with their periphery as well as with fibronectin-associated fibrils. In contrast, the αvβ3 integrin is confined to FCs. We hypothesize that the fibrillar distribution of α5β1 integrin might be dependent on its ability to reorganize fibronectin into fibrils. To test this hypothesis, we cultured cells on fibronectin that was covalently linked to the substrate. This immobilization of fibronectin did not have any major effect on its concentration or apparent conformation on the substrate, yet it dramatically changed integrin localization, altered the composition of cytoskeletal molecules in adhesion sites, and inhibited cell motility. These results indicate that the physical state of the ECM, not just its composition, plays a critical role in the regulation of differential assembly of adhesion sites.
MATERIALS AND METHODS
Cells, Chemical Reagents, and Antibodies
Primary human foreskin fibroblasts were kindly provided by Susan S. Yamada (National Institute of Craniofacial and Dental Research, National Institutes of Health, Bethesda, Maryland). Rat anti-human α5 integrin (mAb 11), rat anti-human β1 integrin (mAb 13), and mouse anti-human activated β1 integrin (12G10) monoclonal antibodies were previously described (Akiyama et al., 1989; Miyamoto et al., 1995a; Mould et al., 1995, 1996; Humphries, 1996). Mouse anti-αv integrin was obtained from the American Type Culture Collection (Manassas, VA). Anti-human fibronectin antibodies were either rat monoclonal (11E5 and 16G3; Nagai et al., 1991) or a rabbit polyclonal (R745; unpublished results). Mouse anti-human vinculin monoclonal antibody was kindly provided by V. Koteliansky (Biogen, Boston, MA), and rabbit anti-vinculin polyclonal antibody (R694) was prepared against purified chicken vinculin (Geiger, 1979). Mouse monoclonal antibodies to FAK, paxillin, and tensin were purchased from Transduction Laboratories (Lexington, KY). Polyclonal rabbit anti-phosphotyrosine antibody (PT40) was kindly provided by Israel Pecht and Arie Licht (Weizmann Institute, Rehovot, Israel). Rhodamine-labeled phalloidin was purchased from Molecular Probes (Eugene, OR). Fluorescein- or rhodamine-conjugated goat F(ab′)2 anti-mouse or anti-rabbit immunoglobulin G were from Biosource International (Camerillo, CA), and Cy3-conjugated goat anti-mouse immunoglobulin G was from Jackson Laboratories (West Grove, PA). Poly-l-lysine was purchased from Sigma Chemical Co. (St. Louis, MO).
ECM Coating of Coverslips
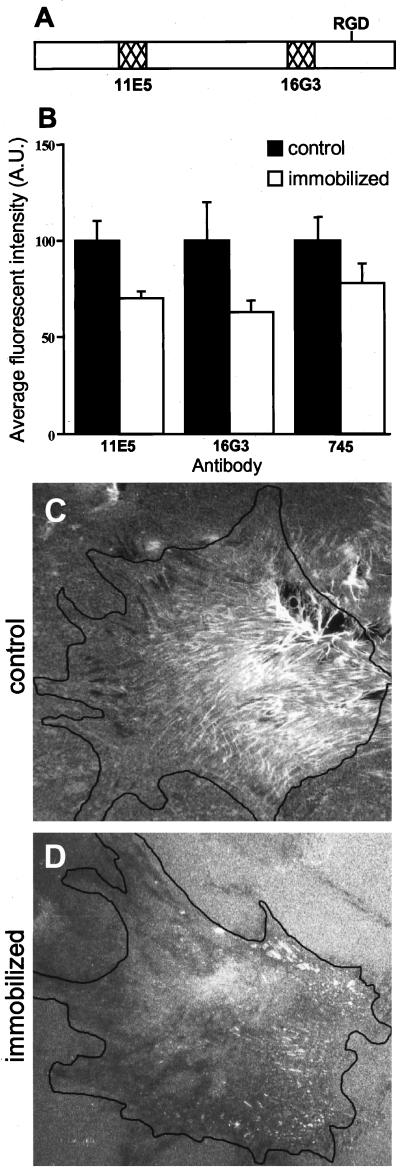
Coverslips were coated with 50 μg/ml poly-l-lysine in PBS for 20 min, washed with water, and incubated with either PBS (control) or 1% glutaraldehyde (Fluka, Ronkonkoma, NY) for 15 min. The coverslips were then extensively washed and incubated with 100 μl of FN (at 10 μg/ml in PBS) for 30 min. The coverslips were washed three times with PBS and blocked with 1 M ethanolamine, pH 7.0 (Fluka, Ronkonkoma, NY) for 20 min and then washed with PBS. Human foreskin fibroblasts (2 × 105 cells) were plated on 18-mm coverslips in Dulbecco's modified Eagle's medium supplemented with 0.5% FCS and incubated at 37°C in a humidified incubator for 16 h in an atmosphere of 10% CO2 and 90% air. To examine the possible effect of the glutaraldehyde fixation on the conformation or the density of fibronectin on the glass, coverslips coated with either immobilized or control fibronectin were washed with PBS, fixed for 20 min in PBS containing 3% parformaldehyde, and immunofluorescently labeled using either 11E5, 16G3, or R745 anti-fibronectin antibodies. Digital images were acquired using a digital microscopic system (see below).
Indirect Immuofluorescence
Cultured cells were fixed and permeabilized for 3 min in PBS containing 0.5% Triton X-100, 4% formaldehyde, and 5% sucrose and then were fixed further with 4% formaldehyde and 5% sucrose in PBS for 20 min. The cells were then incubated for 1 h with the primary antibodies in PBS, washed, and further incubated with the appropriate secondary antibodies for 1 h. After extensive washes, coverslips were mounted in Gel/Mount (Biomeda, Foster City, CA) containing 1 mg/ml _p_-phenylenediamine (Fluka) to inhibit photobleaching. Cells were examined and photographed using a Zeiss (Oberkochen, Germany) Axiophot photomicroscope.
Digital Fluorescence Ratio Imaging Analysis of the Molecular Composition of Cell-Matrix Adhesion Sites
The system for computerized microscopy and fluorescence ratio imaging was described in detail elsewhere (Kam et al., 1995; Zamir et al., 1999). Briefly, images of double-stained cells were acquired using an Axioscope microscope (Zeiss) equipped with a charged-coupled device (CCD) camera (model C220; Photometrics, Tucson, AZ) with Texas Instruments (Dallas, TX) 1024 × 1024 pixels chip readout generating 12-bit digital data. In the present study, cells were examined with a 100×/1.3NA plan-Neofluar objective (Zeiss), resulting in a pixel length of 0.118 μm. Correction for nonhomogenous illumination and pixel-to-pixel variations in CCD sensitivities as well as aligning the Cy3 image and the FITC image, were routinely performed. The images were then high-pass filtered with a box size of 4.7 × 4.7 μm and thresholded to eliminate the background fluorescence. Ratio images (Cy3/FITC) were then calculated and presented in a spectral, log scale, color look-up table that ranged from blue for low Cy3/FITC ratios (≤0.1) to red for high Cy3/FITC ratios (≥10). To utilize optimally this range of two orders of magnitude and to compensate for the differences in antibody binding and photon yields of different secondary antibodies, all the ratios were normalized linearly by a constant that shifted their average toward a ratio value of 1.
Measurements of Cell Migration Rates
The migration rates of human foreskin fibroblasts on the different substrates were measured as previously described (Savagner et al., 1997). Briefly, cells were plated on ECM-coated coverslips, and 16 h later the migration of single cells was recorded for 5 h using an Opton inverted microscope (Zeiss) equipped with a CCD camera (Hamamatsu Photonics, Hamamatsu City, Japan). In each experiment 18–22 cells were examined (repeated twice). Statistical analysis was done with Instat software (GraphPad Software, San Diego, CA).
RESULTS
Primary Human Fibroblasts Generate Two Distinct Types of Cell-Matrix Adhesion Sites
Integrins mediate the specific association of cells with the ECM and the assembly of cytoplasmic cytoskeletal and signaling complexes (for reviews, see Burridge et al., 1997; Yamada and Geiger, 1997). In the present study we examined the effects of altering the physical properties of the ECM on the distribution of the α5β1 integrin, its ligand fibronectin, and various anchor and cytoskeletal molecules. As shown in Figure 1, A and B, paxillin was localized predominantly in FCs at the periphery of cells, with very low labeling along fibronectin fibrils. Similar distribution patterns were observed for other cytoskeletal or FC-associated molecules, including vinculin, FAK, and α-actinin (our unpublished observations). We did not detect fibronectin in classical FC (Figure 1, A and B), in agreement with previous studies (Chen and Singer, 1980; Avnur and Geiger, 1981). In striking contrast, tensin was localized primarily along fibronectin fibrils, with only faint staining in FCs (Figure 1, C and D). Thus, the cytoskeletal complex associated with fibronectin fibrils appears to be distinctly different from that present in FCs. The segregation of these molecules into the two different types of adhesions was, however, incomplete: variable but significant levels of tensin were detected in FCs (Figure 1C). Quantitative information on the molecular properties of the two classes of cell-matrix adhesions was obtained by digital microscopy, followed by fluorescence ratio image analysis (Zamir et al., 1999). Double immunofluorescence microscopy indicated that the fibronectin receptor (visualized with anti-α5 integrin monoclonal antibody) was predominantly associated with fibrillar structures (Figure 2), whereas the staining for the vitronectin receptor (using anti-αv integrin monoclonal antibody) was associated with classical FC structures (Figure 2). Some α5 integrin was identified at the periphery of FCs, often forming “needle eye” patterns (Figure 2). The distribution of β3 integrin was similar to that of the αv subunit, whereas activated β1 integrin identified by the monoclonal antibody 12G10 (Mould et al., 1995) colocalized with the α5 subunit (our unpublished results). Thus, the two different integrins α5β1 and αvβ3 are sorted into two distinct types of cell-matrix adhesions.
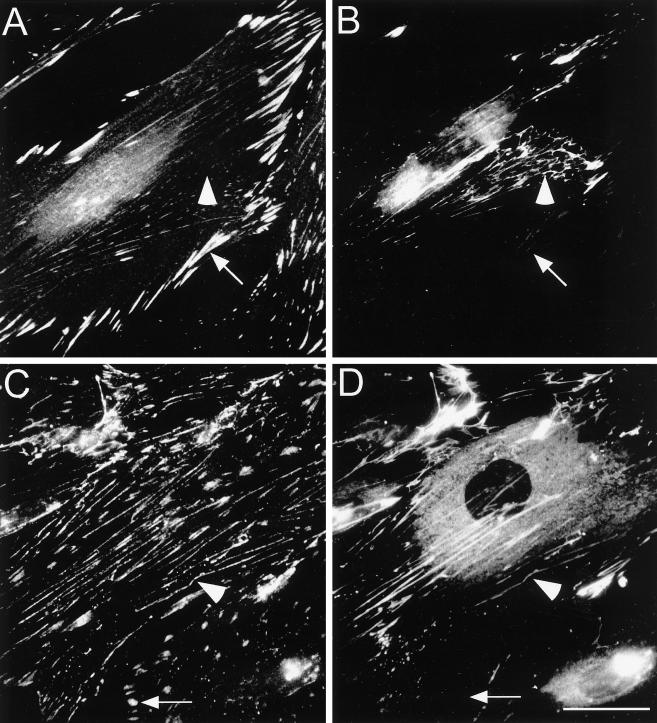
Figure 1.
Distribution of fibronectin, paxillin and tensin in primary human fibroblasts. Cells were cultured for 16 h on coverslips. Double immunofluorescence staining was then performed with antibodies to paxillin (A) and fibronectin (B) or with tensin (C) and fibronectin (D). Note the localization of paxillin and tensin in FCs (arrows) and tensin along fibronectin fibrils (arrowheads). Bar, 20 μm.
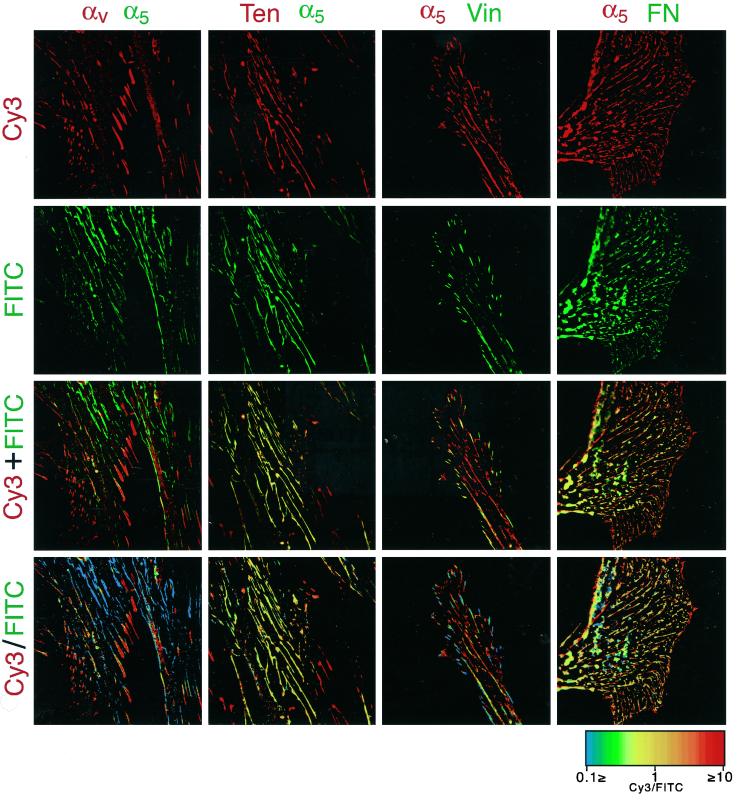
Figure 2.
Ratio imaging of α5 integrin with other components of cell-matrix adhesions. Primary human fibroblasts were cultured on coverslips for 16 h. Double immunofluorescence staining was then performed with antibodies to α5 integrin compared with αv integrin (mouse anti-αv integrin monoclonal antibody followed by Cy3-conjugated goat anti-mouse antibody, and rat anti-α5 integrin monoclonal antibody followed by FITC-conjugated goat anti-rat antibody; first column of panels, left), tensin (mouse anti-tensin monoclonal antibody followed by Cy3-conjugated goat anti-mouse antibody with rat anti-α5 integrin monoclonal antibody followed by FITC-conjugated goat anti-rat antibody; second column), vinculin (rabbit anti-vinculin followed by FITC-conjugated goat anti-rabbit antibody with rat anti-α5 integrin monoclonal antibody followed by Cy3-cojugated goat anti-rat antibody; third column), or fibronectin (rat anti-α5 integrin monoclonal antibody followed by Cy3-cojugated goat anti-rat antibody with FITC-conjugated goat anti-fibronectin antibody; right column). Cy3 and FITC images are presented in the top and second row, respectively. Superimposed images of Cy3 and FITC double-labeled images are shown in the third row. Ratio image analyses of the Cy3 and FITC-double labeled images were performed as described in MATERIALS AND METHODS, and the resulting images are shown in the bottom row. Spectrum color scale indicates the value of the ratios. Note the colocalization of α5 integrin with tensin and fibronectin in fibrillar adhesions and the sorting of α5 integrin from αv integrin and vinculin into distinct structures, as reflected by the ratio images of these components.
Next, we compared the distribution of the α5 integrin to that of tensin and vinculin in adhesion sites. As shown in Figure 1C, tensin was mainly associated with the α5 integrin- and fibronectin-containing fibrillar structures, whereas the distribution of vinculin and α5 integrin were largely mutually exclusive (Figure 2). Double immunolabeling also indicated that the α5 integrin and its ligand fibronectin largely colocalized at most sites.
It has been previously established that FCs and actin stress fibers are interdependent structures, because FCs are the major membrane anchors of the actin cytoskeleton and the integrity of the actin cytoskeleton is required to maintain the structure of FCs (Burridge and Fath, 1989; Burridge et al., 1990; Volberg et al., 1994; Bershadsky et al., 1996). To examine for differential interactions of the actin cytoskeleton with fibrillar adhesions and FCs, we performed double immunofluorescence staining for the different integrins and F-actin. As shown in Figure 3, fibrillar adhesions localize along thin actin filaments that appear structurally distinct from the thicker actin stress fibers (Figure 3, A′ and B′), whereas classical FCs are associated with the termini of actin stress fibers (Geiger, 1979; Burridge and Fath, 1989; Burridge et al., 1990).
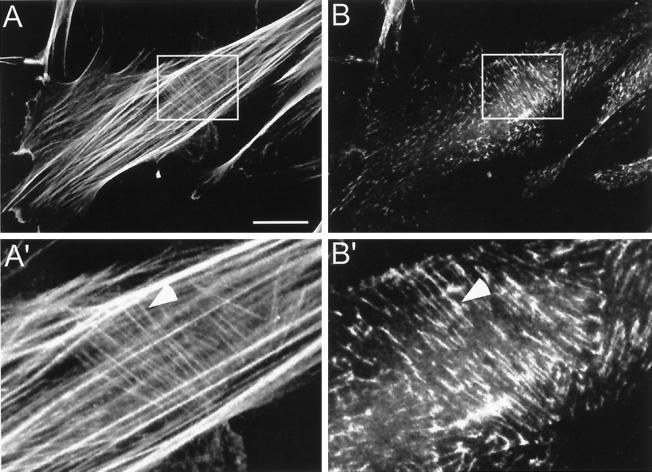
Figure 3.
F-actin localization at cell-matrix adhesions. Primary human fibroblasts were cultured on coverslips for 16 h. Double immunofluorescence staining was then performed for (A) F-actin (rhodamine-conjugated phalloidin) and (B) α5 integrin (rat anti-α5 integrin monoclonal antibody followed by FITC-conjugated goat anti-rat antibody). (A′) and (B′) High-power magnification of the regions defined by rectangles in (A) and (B), respectively. Arrowheads indicate the location of fibrillar adhesions and thin actin filaments. Bar, 15 μm.
The maintenance of actin stress fibers and FCs depends on the contractility of the actin–myosin cytoskeleton (Bershadsky et al., 1996; Chrzanowska-Wodnicka and Burridge, 1996; Helfman et al., 1999), and their integrity is impaired by inhibitors of myosin light chain kinase or of Rho kinases, such as H-7 or ML-7, which impair cellular contractility (Volberg et al., 1994; Zhong et al., 1997). In a previous study we showed that although actomyosin contractility is required to maintain both actin stress fibers and FCs, it may not be essential for the maintenance of fibrillar adhesions (Zamir et al., 1999).
The results of immunofluorescence staining for different integrins and adhesion-associated molecules further distinguish the two different cell-matrix adhesions as summarized in Table 1. These data indicate that FCs contain predominantly the αvβ3 integrin and a specific set of cytoskeletal molecules and that their maintenance depends on actomyosin contractility. Fibrillar adhesions, on the other hand, contain α5β1 integrin, its ligand fibronectin, and tensin as the major cytoskeletal component, and they are less sensitive to inhibition of actomyosin. Moreover, confocal microscopic analyses confirmed that FCs were localized only at the ventral cell surface, whereas fibrillar adhesions were observed on both the ventral and dorsal aspects of the plasma membrane (our unpublished results).
Table 1.
Summary of immunofluorescence comparisons of the molecular composition of cell-matrix adhesions of primary human fibroblasts
| Component | Focal contacts | Fibrillar adhesions |
|---|---|---|
| Integrins | ||
| α5,β1* | +/− | +++ |
| αv,β3 | +++ | − |
| Cytoskeletal components | ||
| Vinculin | +++ | +/− |
| Paxillin | +++ | +/− |
| α-Actinin | +++ | +/− |
| Tensin | +++ | +++ |
| Talin | +++ | +/− |
| F-Actin | +++ | +/− |
| FAK/pTyr | ||
| FAK | +++ | − |
| Phosphotyrosine | +++ | +/− |
Fibrillar Adhesions Contain Low Levels of Phosphotyrosine
One of the characteristics of FC is their high level of tyrosine phosphorylation (Burridge et al., 1992). This feature is attributed to the association of several tyrosine kinases and their substrates with FCs (for review, see Yamada and Geiger, 1997). As described above, potentially highly phosphorylated molecules such as FAK and paxillin were present in FCs but were absent from fibrillar adhesions. We therefore compared the phosphotyrosine (pTyr) levels in the two types of adhesions. Figure 4 shows that fibrillar adhesions (labeled by α5 integrin) contained very low levels of pTyr. In contrast, FCs (labeled by αv integrin) were highly phosphorylated, as expected. Previous studies established that cell adhesion to fibronectin stimulates tyrosine phosphorylation of several adhesion-associated molecules, such as FAK and paxillin (Burridge et al., 1992; Hanks et al., 1992). However, quantitative analyses indicated that the levels of pTyr are very low along fibronectin fibrils (Figure 4). FCs, on the other hand, usually contained high levels of pTyr, yet almost no fibronectin (see also Figure 1).
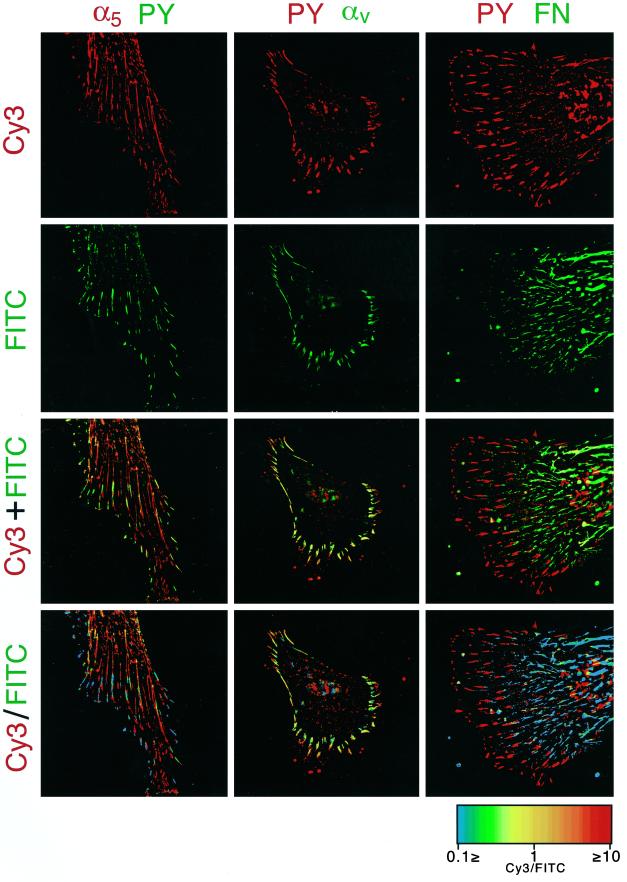
Figure 4.
Ratio imaging of tyrosine phosphorylation in cell-matrix adhesions. Primary human fibroblasts were cultured on coverslips for 16 h. Double immunofluorescence staining was then performed with antibodies to pTyr with α5 integrin (rat anti-α5 integrin monoclonal antibody followed by Cy3-conjugated goat anti-rat antibody compared with mouse anti-pTyr monoclonal antibody followed by FITC-conjugated goat anti-mouse antibody; first column of panels, left), αv integrin (mouse anti-αv integrin monoclonal antibody followed by FITC-conjugated goat anti-mouse antibody paired with rabbit anti-pTyr antibody followed by Cy3-cojugated goat anti-rabbit antibody; second column), or fibronectin (rabbit anti-pTyr antibody followed by Cy3-cojugated goat anti-rabbit antibody with FITC-conjugated goat anti-fibronectin antibody; right column). Cy3 and FITC-labeled images are presented in the top and second row, respectively. Superimposed images of Cy3 and FITC-double labeled images are shown in the third row. Ratio image analysis of the Cy3 and FITC-double labeled images was performed as described in MATERIALS AND METHODS, and the resulting images are shown in the bottom row. Spectrum color scale indicates the value of the ratios. Note the colocalization of αv integrin with pTyr in FCs, whereas the relative amounts of pTyr in fibrillar adhesions (containing α5 integrin and fibronectin) are low, as reflected by the ratio images of these components.
Effects of Fibronectin Immobilization on the Assembly of Matrix Adhesions
In view of the observation that the formation of fibrillar adhesions involves mobilization and reorganization of fibronectin, we tested the hypothesis that matrix mobilization is responsible for the segregation of FCs and fibrillar adhesions. To determine whether the covalent immobilization of fibronectin had major effects on its levels or conformation, we performed a quantitative immunofluorescence microscopic assay using a polyclonal and two different monoclonal anti-fibronectin antibodies. The monoclonal antibodies interact with distinct epitopes located at either N-terminal or C-terminal regions of the 37-kDa cell-binding domain of fibronectin (Figure 5A; Nagai et al., 1991). The rabbit polyclonal anti-fibronectin is an adhesion-inhibitory antibody (unpublished data). As shown in Figure 5B, all three antibodies interact with routinely adsorbed versus covalently immobilized fibronectin to a comparable extent (differences not exceeding 30%), indicating that the different epitopes on fibronectin remain available for interactions, and are not blocked by the covalent linkage. These minimal changes contrast with the large differences in specific epitope exposure that can exist between soluble and substrate-adsorbed fibronectin molecules (Garcia et al., 1999). Interestingly, the small differences detected in this study occurred similarly with all of the antibodies tested (Figure 5B), pointing to small differences in the total amount of surface-bound fibronectin after the treatments involved in immobilization, without gross conformational changes affecting only certain epitopes of the molecule.
Figure 5.
Effects of covalent immobilization on the quantity and epitope exposure of fibronectin and on cell spreading. Coverslips coated with immobilized or nonimmobilized (control) fibronectin were fixed and immunolabeled for fibronectin using monoclonal antibodies 11E5 or 16G3 or the polyclonal antibody R745. (A) Diagram of the 37-kDa fibronectin cell-binding domain showing locations of the epitopes of monoclonal antibodies 11E5 and 16G3 compared with the RGD site. (B) Bar graph showing the average immunofluorescence labeling in arbitrary units (A.U.) of immobilized or nonimmobilized (control) fibronectin. Primary human fibroblasts were plated for 16 h on control, nonimmobilized fibronectin (C) or immobilized fibronectin as described in MATERIALS AND METHODS (D). Immunofluorescence staining was then performed for fibronectin (FITC-conjugated goat anti-fibronectin; C and D). Note that cells plated on immobilized fibronectin did not form fibrillar adhesions.
To determine the effect of fibronectin immobilization on matrix adhesion, human fibroblasts were plated on immobilized or control (noncovalently adsorbed) fibronectin and then cultured for 16 h in medium containing 0.5% FCS. The cells were fixed and immunolabeled for fibronectin. As shown in Figure 5D, very little fibronectin rearrangement into fibrils occurred when the cells were plated on the immobilized fibronectin for 16 h. Nevertheless, fibronectin immobilization had no apparent effect on the extent of cell spreading compared with cells plated on nonimmobilized fibronectin (Figure 5, C and D).
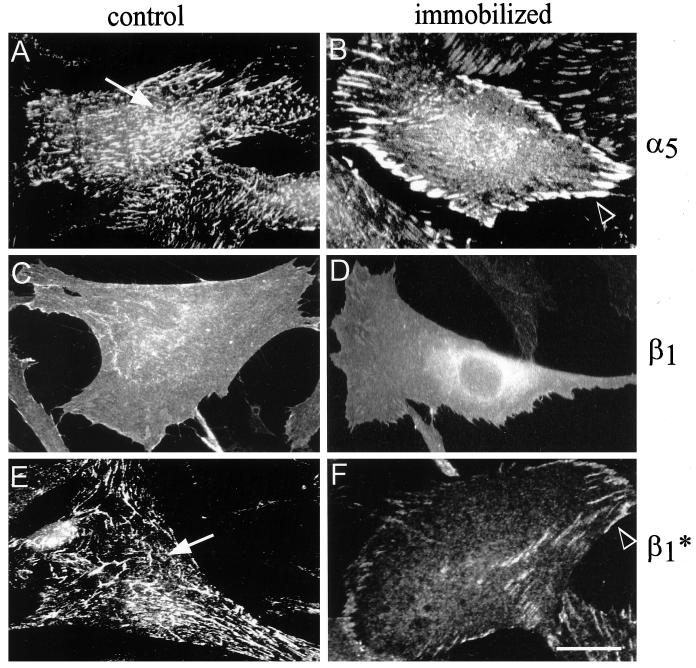
Fibronectin immobilization had a major effect on integrin distribution. Thus, in cells plated on immobilized fibronectin, α5β1 integrin was associated with classical FCs, with only a very small number of fibrillar adhesions (Figure 6, B and F). In contrast, when the cells were plated on nonimmobilized fibronectin, the ligand-associated α5β1 integrin was predominantly localized along fibronectin fibrils (Figure 6, A and E). Staining for total β1 integrins revealed a relatively diffuse distribution of this integrin in cells, irrespective of whether they were cultured on regular or immobilized fibronectin (Figure 6, C and D). The distribution of tensin in cells cultured on the different substrates was similar to that of the fibronectin-associated α5β1 integrin. In cells cultured on either regular or immobilized fibronectin, the αvβ3 integrin was associated with typical FCs; it was colocalized with α5β1 integrin only on immobilized fibronectin (our unpublished results).
Figure 6.
α5β1 integrin localizes to FCs of cells cultured on immobilized fibronectin. Primary human fibroblasts were cultured for 16 h on control, nonimmobilized fibronectin (A, C, and E) or on immobilized fibronectin (B, D, and F). Immunofluorescence staining was then performed for α5 integrin (rat anti-α5 integrin monoclonal antibody followed by FITC-conjugated goat anti-rat antibody; A and B), the total population of β1 integrin molecules (rat anti-β1 integrin monoclonal antibody followed by FITC-conjugated goat anti-rat antibody; C and D), or activated β1 integrin (β1*) molecules (mouse anti-activated β1 integrin monoclonal antibody followed by Cy3-conjugated goat anti-mouse antibody; E and F). Activated α5β1 integrins localize predominantly in fibrillar adhesions of cells cultured on control fibronectin (arrows), but in the FCs of cells plated on immobilized fibronectin (arrowheads). Bar, 15 μm.
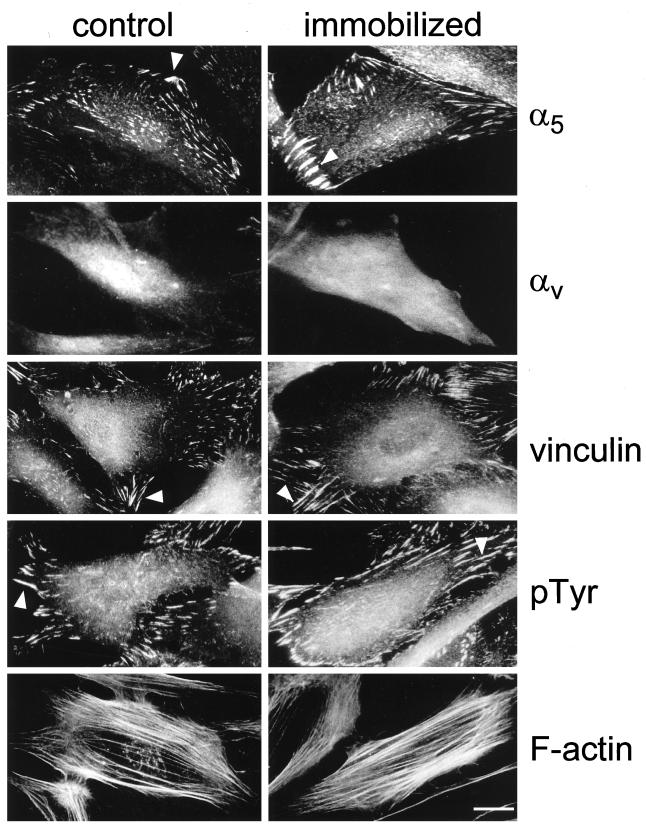
Next, the molecular composition of putative FCs containing the α5β1 integrin was examined. As shown in Figure 7, cells plated on immobilized fibronectin (in the presence of inhibitory anti-αv integrin monoclonal antibody to prevent potential αv interactions) formed FCs containing α5β1 integrin, vinculin, and other cytoskeletal components, including F-actin bundles and high levels of pTyr. Moreover, when the cells were cultured on an immobilized anti-α5 monoclonal antibody, they spread and organized similar vinculin- and phosphotyrosine-containing FCs that were devoid of αv integrins (unpublished results). It should be emphasized that cells cultured on poly-l-lysine alone in the absence of fibronectin spread poorly and formed virtually no FCs (unpublished results). These data directly demonstrate that the α5β1 integrin can generate typical, highly phosphorylated FCs when associated with an immobilized, nondeformable matrix.
Figure 7.
The molecular composition of FCs of cells plated on immobilized fibronectin in the presence of anti-αv integrin inhibitory antibody. Primary human fibroblasts were cultured on control, nonimmobilized fibronectin (left) or on immobilized fibronectin (right) for 16 h, both in the presence of inhibitory anti-αv integrin monoclonal antibody. The following immunofluorescence stainings were then performed: α5 integrin (rat anti-α5 integrin monoclonal antibody followed by FITC-conjugated goat anti-rat antibody; top row), αv integrin (mouse anti-αv integrin monoclonal antibody followed by Cy3-conjugated goat anti-mouse antibody; second row), vinculin (rabbit anti-vinculin antibody followed by Cy3-conjugated goat anti-rabbit antibody; third row), pTyr (rabbit anti-pTyr antibody followed by Cy3-conjugated goat anti-rabbit antibody; fourth row), and F-actin (rhodamine-conjugated phalloidin; bottom row). Bar, 10 μm.
Effect of Fibronectin Immobilization on Cell Migration
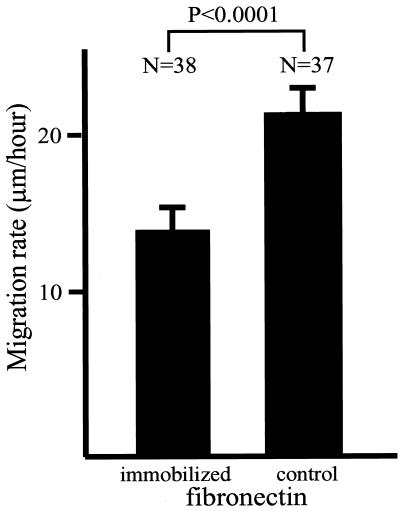
Integrin–ECM interactions participate in the regulation of cell migration (Schmidt et al., 1993). A recent study demonstrated that migration rates decrease when cells are plated on a less flexible substrate (Pelham and Wang, 1997). To test for functional effects on cell migration rates when cells interact by different adhesions with the two types of fibronectin substrate, cells were plated for 16 h on immobilized or control (adsorbed) fibronectin, and migration rates were recorded by time-lapse video microscopy. As shown in Figure 8, cells plated on immobilized fibronectin displayed a substantial reduction in migration rates compared with cells plated on the control fibronectin-coated substrate.
Figure 8.
Migration rates are reduced when cells are plated on immobilized fibronectin compared with cells plated on control, nonimmobilized fibronectin. Primary human fibroblasts were plated on coverslips coated with fibronectin substrates as described in MATERIALS AND METHODS. After 16 h, the migration of single cells was recorded for 5 h. Eighteen to 22 cells were examined in each experiment, and each experiment was performed two times. The graph shows the average and SD of data pooled from two independent experiments, and the difference between the migration rates of cells plated on the different substrates is statistically significant (p < 0.0001).
DISCUSSION
The main objective of the present study was to elucidate the roles of ECM molecular composition and physical properties (i.e., rigidity) in the assembly of matrix adhesions. Previous studies showed that integrin-mediated cytoskeletal assembly and signaling are hierarchical responses regulated by a combination of integrin occupancy and clustering (Miya-moto et al., 1995a,b). These studies also indicated that integrins have the potential to form various types of cytoskeletal assemblies.
In the present study, we tested how a change in the physical properties of an ECM protein affects the assembly of different types of adhesion complexes. When we examined matrix adhesions in cells growing on regular fibronectin (coated on the culture dish), we found that two different integrins are associated with two distinct types of adhesions: 1) The αvβ3 integrin is associated with “classical” FCs, which display a high level of tyrosine phosphorylation, are enriched with paxillin, vinculin, α-actinin, and FAK and localize at the termini of actin stress fibers. 2) The α5β1 integrin is localized mainly along fibronectin fibrils and forms fibrillar adhesions that contain relatively high levels of tensin as a major cytoskeletal component and low levels of tyrosine phosphorylation, vinculin, and paxillin. Fibrillar adhesions are clearly distinct from the previously described “apical plaques” (Katoh et al., 1996), because they contain little or no paxillin and vinculin.
Part of this diversity in cytoskeletal complexes in adhesion sites could theoretically be attributed to molecular heterogeneity in the ECM itself, consisting of varying mixtures of proteins such as vitronectin, fibronectin, laminin, and collagen. Nonhomogenous matrices might induce a nonhomogenous distribution of the respective integrin receptors (Dejana et al., 1988; Dogic et al. 1998) and possibly differential interactions with integrin-associated cytoskeletal systems and/or signaling networks.
The question addressed here, however, was whether structural and molecular diversity of adhesion sites could be attributed to other properties of the ECM beside its molecular composition. Our working hypothesis was that the differential assembly of focal and fibrillar contacts depends on an active reorganization of the ECM and specifically by the mobilization of substrate-bound fibronectin and its assembly into fibrils during the formation of fibrillar adhesions.
This hypothesis implies that cellular forces applied to newly formed adhesions may drive the α5β1 integrin associated with a “soft” or “deformable” fibronectin matrix out of the “classic FCs,” where the cells attach to ECM that is tightly bound to the external surface (e.g., vitronectin). To test this hypothesis, we directly examined whether association of the α5β1 integrin with covalently immobilized, nondeformable fibronectin would result in the assembly of adhesion sites with different morphology and molecular composition. We confirmed that cells plated on control, nonimmobilized fibronectin reorganized the planar matrix to form fibrils enriched in activated α5β1 integrins. On the other hand, when cells were cultured on immobilized fibronectin, a restricted localization of activated α5β1 integrins to FCs and a marked reduction in fibrillar adhesion formation were observed. The morphology and molecular composition of FCs, associated with this relocated α5β1 integrin on immobilized fibronectin, was similar to that of FCs associated with αvβ3 integrin on vitronectin substrates. This result indicates that the physical state of the ECM, not only its molecular composition, is a critical factor in the sorting of integrins and the assembly of characteristic associated cytoskeletal structures and their tyrosine phosphorylation.
It should be noted that the specific types of integrins and the specific ECM molecules with which they interact may lead to heterogeneity of cytoskeletal assemblies. In the present study, we demonstrated that the localization of tensin is tightly linked to activated α5β1 integrin and its association with fibronectin (Figure 9). Tensin colocalized with ligand-occupied α5β1 integrins predominantly in fibrillar adhesions associated with fibronectin fibrils as well as with FCs containing predominantly αvβ3 integrin. This localization indicates that it may associate with this integrin directly, or indirectly via other components of FCs. The possibility of a direct link between tensin and the β1 integrin cytoplasmic tail as well as between tensin and vinculin have been previously suggested (Lin, S., and Lin, D.C. The American Society for Cell Biology Annual Meeting, 1996. Abstract 2259). However, fibrillar adhesions are rich in tensin, but contain very little vinculin. This observation may indicate that the molecular interactions that mediate tensin–vinculin association are not available within fibrillar adhesions. Interestingly, we also observed in fibrillar adhesions very low levels of additional molecules previously identified as putative direct ligands for the β1 integrin cytoplasmic tail (e.g., α-actinin, FAK, and paxillin [Pavalko et al., 1991; Otey et al., 1993; Schaller et al., 1995]), even though these sites contained the activated, ligand-occupied form of this integrin, as identified by an activated integrin epitope–specific antibody. These cytoskeletal components became colocalized with α5β1 integrin in FCs of cells cultured on an immobilized fibronectin substrate. This finding indicates that the changes in the physical state of the ECM may radically alter the organization, composition, and signaling activity of integrin-mediated adhesions (Figure 9). One possible mechanism that may be involved in the delivery of critical assembly signals is actomyosin-mediated cytoskeletal contractility (Bershadsky et al., 1996; Chicurel et al., 1998; Helfman et al., 1999).
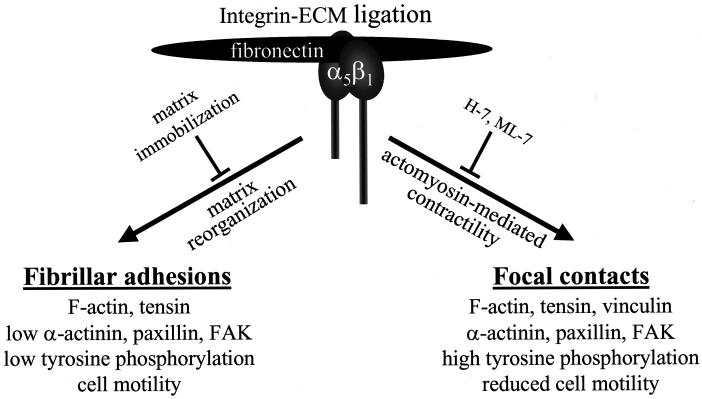
Figure 9.
Summary diagram: integrin-mediated cytoskeletal assembly and cell migration are both controlled by the physical status of the ECM. Binding of the α5β1 integrin to its ligand fibronectin results in the formation of fibrillar adhesions that contain tensin as a major cytoskeletal component and that are associated with actin filaments. Matrix immobilization prevents matrix reorganization and formation of fibrillar adhesions. When the same integrin binds to an immobilized fibronectin ligand, it forms typical, highly phosphorylated FCs that associate with the termini of actin stress fibers, accompanied by reduction of cell migration rates. The latter FC type of adhesion depends on actomyosin-mediated contractility that can be inhibited by H-7 or ML-7. In contrast, fibrillar adhesions are not disrupted by these inhibitors.
FC assembly depends on the formation of tension, regulated both by intrinsic cytoskeletal contractility and the properties of the extracellular substrate (Burridge et al., 1997; Pelham and Wang, 1997). In contrast, fibrillar adhesions are maintained even when cell contractility is inhibited with specific drugs (Zamir et al., 1999). Recent studies indicate that the generation of tension may be a cardinal factor in the induction of integrin-mediated tyrosine phosphorylation (Bershadsky et al., 1996; Schmidt et al., 1998, Helfman et al., 1999), and may also regulate the strength of integrin–cytoskeleton linkage (Choquet et al., 1997). Here, we provide evidence that the α5β1 integrin can be associated with two types of adhesions, depending on the degree of matrix “deformability” or “rigidity.” Thus, it can form highly phosphorylated adhesion sites (FCs) when associated with immobilized fibronectin or typical fibrillar adhesions when the underlying fibronectin can be mobilized to form fibrils.
Possible interrelationship between the two types of adhesion sites may exist. Some α5β1 integrin is localized at the periphery of FCs (e.g., Figure 2, first panel, left), suggesting that different integrins may be associated with subregions within single adhesion sites. Immunofluorescence staining provides only static views of the molecular organization of the adhesion sites. However, the variability in the molecular composition of cell-matrix adhesions, observed in this study and in our previous study (Zamir et al., 1999), indicates that the assembly of FCs and the formation of FAs is a highly dynamic process. In a recent study we have addressed this aspect by expressing in cells fusion proteins of GFP and various anchor proteins. This study showed that fibrillar adhesions assemble in FCs before they undergo centripetal translocation toward the cell center (Zamir et al., 2000).
The physical properties of the ECM may thus provide important regulatory signals governing the shape and molecular composition of adhesion sites in a variety of physiological states. For example, the involvement of fibronectin in wound healing processes is well documented (Herard et al., 1996; Nakamura et al., 1997). At early stages of the wound healing process (up to day 5 after an injury), fibroblasts migrate into the injured tissue and assemble fibronectin fibrils, without developing large actin bundles (Welch et al., 1990). These stages may involve interactions of the cells with a “soft” matrix, resulting in increased migration rates and limited formation of actin bundles. At later stages (from day 7 after an injury), actin bundles are formed and contraction of the granulation tissue occurs (Welch et al., 1990). Some pathological states (e.g., Dupuytren's disease) may involve extensive tissue contraction associated with the formation of adhesion sites that contain both fibronectin and actin filaments (Tomasek and Haaksma, 1991). Thus, the two types of adhesion sites studied here in cultured fibroblasts may represent different types of adhesions involved in physiological or pathological processes in vivo. This study demonstrates that integrins can provide cells with a mechanism to explore and respond to the physical state of the ECM.
ACKNOWLEDGMENTS
This study was supported by the Israel Science Foundation and The Minerva Foundation (to B.G.). B.G. holds the E. Neter Chair in Cell and Tumor Biology, and Z.K. the Israel Pollak Chair in Biophysics.
Abbreviations used:
ECM
extracellular matrix
FAK
focal adhesion kinase
FC
focal contact
FN
fibronectin
pTyr
phosphotyrosine
REFERENCES
- Abercrombie M, Dunn GA. Adhesions of fibroblasts to substratum during contact inhibition observed by interference reflection microscopy. Exp Cell Res. 1975;92:57–62. doi: 10.1016/0014-4827(75)90636-9. [DOI] [PubMed] [Google Scholar]
- Akiyama SK, Yamada SS, Chen W-T, Yamada KM. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989;109:863–875. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avnur Z, Geiger B. The removal of extracellular fibronectin from areas of cell-substrate contact. Cell. 1981;25:121–132. doi: 10.1016/0092-8674(81)90236-1. [DOI] [PubMed] [Google Scholar]
- Bershadsky A, Chausovsky A, Becker E, Lyubimova A, Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr Biol. 1996;6:1279–1289. doi: 10.1016/s0960-9822(02)70714-8. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M, Zhong CL. Focal adhesion assembly. Trends Cell Biol. 1997;7:342–347. doi: 10.1016/S0962-8924(97)01127-6. [DOI] [PubMed] [Google Scholar]
- Burridge K, Fath K. Focal contacts: transmembrane links between the extracellular matrix and the cytoskeleton. Bioessays. 1989;10:104–108. doi: 10.1002/bies.950100403. [DOI] [PubMed] [Google Scholar]
- Burridge K, Nuckolls G, Otey C, Pavalko F, Simon K, Turner C. Actin-membrane interaction in focal adhesions. Cell Differ Dev. 1990;32:337–342. doi: 10.1016/0922-3371(90)90048-2. [DOI] [PubMed] [Google Scholar]
- Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-T, Singer SJ. Fibronectin is not present in the focal adhesions formed between normal cultured fibroblasts and their substrata. Proc Natl Acad Sci USA. 1980;77:7318–7322. doi: 10.1073/pnas.77.12.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-T, Singer SJ. Immunoelectron microscopic studies of the sites of cell-substratum and cell-cell contacts in cultured fibroblasts. J Cell Biol. 1982;95:205–222. doi: 10.1083/jcb.95.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-T, Hasegawa E, Hasegawa T, Weinstock C, Yamada KM. Development of cell surface linkage complexes in cultured fibroblasts. J Cell Biol. 1985;100:1103–1114. doi: 10.1083/jcb.100.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicurel ME, Chen CS, Ingber DE. Cellular control lies in the balance of forces. Curr Opin Cell Biol. 1998;10:232–239. doi: 10.1016/s0955-0674(98)80145-2. [DOI] [PubMed] [Google Scholar]
- Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkage. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Craig SW, Johnson RP. Assembly of focal adhesions: progress, paradigms, and portents. Curr Opin Cell Biol. 1996;8:74–85. doi: 10.1016/s0955-0674(96)80051-2. [DOI] [PubMed] [Google Scholar]
- Dejana E, Colella S, Conforti G, Abbadini M, Gaboli M, Marchisio PC. Fibronectin and vitronectin regulate the organization of their respective Arg-Gly-Asp adhesion receptors in cultured human endothelial cells. J Cell Biol. 1988;107:1215–1223. doi: 10.1083/jcb.107.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogic D, Rousselle P, Aumailley M. Cell adhesion to laminin 1 or 5 induces isoform-specific clustering of integrins and other focal adhesion components. J Cell Sci. 1998;111:793–802. doi: 10.1242/jcs.111.6.793. [DOI] [PubMed] [Google Scholar]
- Fath KR, Edgell CJ, Burridge K. The distribution of distinct integrins in focal contacts is determined by the substratum composition. J Cell Biol. 1989;92:67–75. doi: 10.1242/jcs.92.1.67. [DOI] [PubMed] [Google Scholar]
- Fox CH, Cottler-Fox MH, Yamada KM. The distribution of fibronectin in attachment sites of chick fibroblasts. Exp Cell Res. 1980;130:477–481. doi: 10.1016/0014-4827(80)90031-2. [DOI] [PubMed] [Google Scholar]
- Garcia AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10:785–798. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. A 130K protein from chicken gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979;18:193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- Gilmore AP, Burridge K. Molecular mechanisms for focal adhesion assembly through regulation of protein-protein interactions. Structure. 1996;4:647–651. doi: 10.1016/s0969-2126(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Calalb MB, Harper MC, Patel SK. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci USA. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfman DM, Levy ET, Berthier C, Shtutman M, Riveline D, Grosheva I, Lachish-Zalait A, Elbaum M, Bershadsky AD. Caldesmon inhibits nonmuscle cell contractility and interferes with the formation of focal adhesions. Mol Biol Cell. 1999;10:3097–3112. doi: 10.1091/mbc.10.10.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herard AL, Pierrot D, Hinnrasky J, Kaplan H, Sheppard D, Puchelle E, Zahm JM. Fibronectin and its alpha 5 beta 1-integrin receptor are involved in the wound-repair process of airway epithelium. Am J Physiol. 1996;271:726–733. doi: 10.1152/ajplung.1996.271.5.L726. [DOI] [PubMed] [Google Scholar]
- Humphries MJ. Integrin activation: the link between ligand binding and signal transduction. Curr Opin Cell Biol. 1996;8:632–640. doi: 10.1016/s0955-0674(96)80104-9. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Destree AT. Relationships between fibronectin (LETS protein) and actin. Cell. 1978;15:875–886. doi: 10.1016/0092-8674(78)90272-6. [DOI] [PubMed] [Google Scholar]
- Izzard C, Lochner LR. Cell-to-substrate contacts in living fibroblasts: an interference reflexion study with an evaluation of the technique. J Cell Sci. 1976;21:129–159. doi: 10.1242/jcs.21.1.129. [DOI] [PubMed] [Google Scholar]
- Jockusch BM, Bubeck P, Giehl K, Kroemker M, Moschner J, Rothkegel M, Rudiger M, Schluter K, Stanke G, Winkler J. The molecular architecture of focal adhesions. Annu Rev Cell Dev Biol. 1995;11:379–416. doi: 10.1146/annurev.cb.11.110195.002115. [DOI] [PubMed] [Google Scholar]
- Kam Z, Volberg T, Geiger B. Mapping of adherence junction components using microscopic resonance energy-transfer imaging. J Cell Sci. 1995;108:1051–1062. doi: 10.1242/jcs.108.3.1051. [DOI] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Masuda M, Fujiwara F. Mutually exclusive distribution of the focal adhesion associated proteins and the erythrocyte membrane skeleton proteins in the human fibroblast plasma membrane undercoat. Cell Struct Funct. 1996;21:27–39. doi: 10.1247/csf.21.27. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Akiyama SK, Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995a;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995b;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould AP, Akiyama SK, Humphries MJ. The inhibitory anti-beta1 integrin monoclonal antibody 13 recognizes an epitope that is attenuated by ligand occupancy. Evidence for allosteric inhibition of integrin function. J Biol Chem. 1996;271:20365–20374. doi: 10.1074/jbc.271.34.20365. [DOI] [PubMed] [Google Scholar]
- Mould AP, Garratt AN, Askari JA, Akiyama SK, Humphries MJ. Identification of a novel anti-integrin monoclonal antibody that recognizes a ligand-induced binding site epitope on the beta 1 subunit. FEBS Lett. 1995;363:118–122. doi: 10.1016/0014-5793(95)00301-o. [DOI] [PubMed] [Google Scholar]
- Nagai T, Yamakawa N, Aota S, Yamada SS, Akiyama SK, Olden K, Yamada KM. Monoclonal antibody characterization of two distant sites required for function of the central cell-binding domain of fibronectin in cell adhesion, cell migration, and matrix assembly. J Cell Biol. 1991;114:1295–1305. doi: 10.1083/jcb.114.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Sato N, Chikama T, Hasegawa Y, Nishida T. Fibronectin facilitates corneal epithelial wound healing in diabetic rats. Exp Eye Res. 1997;64:355–359. doi: 10.1006/exer.1996.0216. [DOI] [PubMed] [Google Scholar]
- Otey CA, Vasquez GB, Burridge K, Erickson BW. Mapping of the alpha-actinin binding site within the beta 1 integrin cytoplasmic domain. J Biol Chem. 1993;268:21193–21197. [PubMed] [Google Scholar]
- Pelham RJ, Wang YL. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko FM, Otey CA, Simon KO, Burridge K. Alpha-actinin: a direct link between actin and integrins. Biochem Soc Trans. 1991;19:1065–1069. doi: 10.1042/bst0191065. [DOI] [PubMed] [Google Scholar]
- Savagner P, Yamada KM, Thiery JP. The zinc-finger protein Slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol. 1997;137:1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Otey CA, Hildebrand JD, Parsons JT. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CE, Horwitz AF, Lauffenburger DA, Sheetz MP. Integrin-cytoskeletal interactions in migrating fibroblasts are dynamic, asymmetric, and regulated. J Cell Biol. 1993;123:977–991. doi: 10.1083/jcb.123.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Pommerenke H, Dyrr F, Nebe B, Rychly J. Mechanical stressing of integrin receptors induces enhanced tyrosine phosphorylation of cytoskeletally anchored proteins. J Biol Chem. 1998;273:5081–5085. doi: 10.1074/jbc.273.9.5081. [DOI] [PubMed] [Google Scholar]
- Singer II, Scott SD, Kawaka W, Kazazis DM, Gailit J, Ruoslahti E. Cell surface distribution of fibronectin and vitronectin receptors depends on substrate composition and extracellular matrix accumulation. J Cell Biol. 1988;106:2171–2182. doi: 10.1083/jcb.106.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Haaksma CJ. Fibronectin filaments and actin microfilaments are organized into a fibronexus in Dupuytren's diseased tissue. Anat Rec. 1991;230:175–182. doi: 10.1002/ar.1092300205. [DOI] [PubMed] [Google Scholar]
- Volberg T, Geiger B, Citi S, Bershadsky AD. Effects of protein-kinase inhibitor H-7 on the contractility, integrity, and membrane anchorage of the microfilament system. Cell Motil Cytoskel. 1994;29:321–338. doi: 10.1002/cm.970290405. [DOI] [PubMed] [Google Scholar]
- Welch MP, Odland GF, Clark RA. Temporal relationships of F-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J Cell Biol. 1990;110:133–145. doi: 10.1083/jcb.110.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, Geiger B. Molecular interactions in cell adhesion complexes. Curr Opin Cell Biol. 1997;9:76–85. doi: 10.1016/s0955-0674(97)80155-x. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Miyamoto S. Integrin transmembrane signaling and cytoskeletal control. Curr Opin Cell Biol. 1995;7:681–689. doi: 10.1016/0955-0674(95)80110-3. [DOI] [PubMed] [Google Scholar]
- Zamir E, Katz B-Z, Aota S-I, Yamada KM, Geiger B, Kam Z. Molecular diversity of cell-matrix adhesions. J Cell Sci. 1999;112:1655–1669. doi: 10.1242/jcs.112.11.1655. [DOI] [PubMed] [Google Scholar]
- Zamir, E., et al. (2000). Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat. Cell Biol. (in press). [DOI] [PubMed]
- Zhong C, Kinch MS, Burridge K. Rho-stimulated contractility contributes to the fibroblastic phenotype of Ras-transformed epithelial cells. Mol Biol Cell. 1997;8:2329–2344. doi: 10.1091/mbc.8.11.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]