Reversible Merger of Membranes at the Early Stage of Influenza Hemagglutinin-mediated Fusion (original) (raw)
Abstract
Fusion mediated by influenza hemagglutinin (HA), a prototype fusion protein, is commonly detected as lipid and content mixing between fusing cells. Decreasing the surface density of fusion-competent HA inhibited these advanced fusion phenotypes and allowed us to identify an early stage of fusion at physiological temperature. Although lipid flow between membranes was restricted, the contacting membrane monolayers were apparently transiently connected, as detected by the transformation of this fusion intermediate into complete fusion after treatments known to destabilize hemifusion diaphragms. These reversible connections disappeared within 10–20 min after application of low pH, indicating that after the energy released by HA refolding dissipated, the final low pH conformation of HA did not support membrane merger. Although the dynamic character and the lack of lipid mixing at 37°C distinguish the newly identified fusion intermediate from the intermediate arrested at 4°C described previously, both intermediates apparently belong to the same family of restricted hemifusion (RH) structures. Because the formation of transient RH structures at physiological temperatures was as fast as fusion pore opening and required less HA, we hypothesize that fusion starts with the formation of multiple RH sites, only a few of which then evolve to become expanding fusion pores.
INTRODUCTION
Influenza virus enters its host cell by fusing the viral envelope with the endosome membrane. This fusion reaction is triggered by acidification of endosome content and mediated by viral glycoprotein hemagglutinin (HA) (Wiley and Skehel, 1987; White, 1996). As HA-mediated membrane fusion became a paradigm of the ubiquitous protein-mediated fusion, numerous experimental approaches were applied to study low pH–triggered conformational change in HA (Carr and Kim, 1993; Bullough et al., 1994; Krumbiegel et al., 1994; Carr et al., 1997; Gray and Tamm, 1998; Chen et al., 1999; Epand et al., 1999; Korte et al., 1999) and membrane rearrangements in fusion (Stegmann et al., 1990; Melikyan et al., 1993, 1995b, 1997; Tse et al., 1993; Kemble et al., 1994; Zimmerberg et al., 1994; Blumenthal et al., 1995; Chernomordik et al., 1997, 1998).
At physiological temperature and high surface density of low pH–activated HA, membrane fusion is first detected as a fast formation of a small fusion pore connecting aqueous volumes initially separated by the membranes. Within a few seconds, fusion pore opening is followed by mixing of membrane lipids and irreversible expansion of the pore (Tse et al., 1993; Zimmerberg et al., 1994; Blumenthal et al., 1996). Replacing the transmembrane domain of HA with a lipid anchor (Kemble et al., 1994; Melikyan et al., 1995b) or decreasing either the surface density of activated wild-type HA or the temperature (Melikyan et al., 1997; Chernomordik et al., 1998) allowed identification of a less advanced fusion phenotype, hemifusion, which is characterized by merger of only contacting monolayers of the membranes with no opening of a fusion pore. Experimentally, this hemifusion state is detected as lipid mixing in the absence of content mixing.
Fusion is even more impaired at 4°C (Stegmann et al., 1990; Schoch et al., 1992; Chernomordik et al., 1998). Fusion between HA-expressing cells (HA-cells) and RBC triggered by application of low pH at 4°C is reversibly blocked upstream of both lipid mixing and fusion pore opening. Increasing the temperature to 37°C allows the completion of the fusion reaction. At the 4°C-arrested stage (also referred to as the frozen intermediate of fusion), contacting monolayers of the membranes are apparently connected but lipid redistribution through the hemifusion site is restricted by the low pH–activated HA trimers and observed only after application of treatments that destabilize hemifusion diaphragm (Chernomordik et al., 1998). Hereafter, membrane merger with this set of properties will be referred to as restricted hemifusion (RH).
Characterization of the 4°C-arrested stage as a possible long-living RH, and a recent finding of an RH phenotype at room temperature for a mutant HA (Melikyan et al., 1999), strengthened the hypothesis that complete fusion proceeds through a transient RH intermediate (Kemble et al., 1994; Melikyan et al., 1995b, 1999; Chernomordik et al., 1998). However, it remained possible that for wild-type HA, RH forms only at 4°C and is not a part of a normal fusion pathway.
The membrane contact area is large enough to allow formation of multiple fusion sites (Zimmerberg et al., 1994), which complicates the detection of hypothetical RH structures. Less advanced fusion phenotypes in the emerging hierarchy of fusion phenotypes—from RH (neither fusion pore nor lipid mixing), to unrestricted hemifusion (UH; lipid mixing in the absence of a fusion pore), to complete fusion (expanding fusion pore and lipid mixing)—are undetectable in the presence of more advanced phenotypes. For instance, formation of a single expanding fusion pore between an HA-cell and an RBC defines this contact zone as a complete fusion phenotype even if there are hundreds of hemifusion and/or RH sites present.
In this work, to study early fusion intermediates at physiological temperature, we slowed down the formation of advanced fusion intermediates by decreasing the number of activated HA molecules. We identified a new fusion intermediate that apparently belongs to the same family of RH intermediates as frozen intermediate of fusion (Chernomordik et al., 1998). However, in contrast to frozen intermediate of fusion, which maintains RH structure only at 4°C, the newly identified intermediate demonstrated RH phenotype even at 37°C. In addition, RH at physiological temperature was found to be a reversible membrane intermediate that disappeared within minutes after application of low pH. Our results suggest that multiple RH intermediates are formed and present in the contact zone before an opening of the first fusion pore. Formation and stabilization of transient RH intermediates, and their progression to more advanced fusion stages to allow lipid and content mixing, all use the energy released by conformational changes in HA.
MATERIALS AND METHODS
Preparation of Cells and Fusion Experiments
X31 HA-cells (HA300a cells expressing X:31 HA), GPI-HA cells (BHA-PI cells expressing the glycosylphosphatidylinositol [GPI]-anchored ectodomain of X:31 HA [Kemble et al., 1993]), and Japan HA-cells (HAb2 cells expressing A/Japan/305/57 HA [Doxsey et al., 1985]) were grown as described. Human RBC, freshly isolated from whole blood, were labeled with fluorescent lipid PKH26 (Sigma Chemical, St. Louis, MO) and, in some experiments, with an aqueous dye, 6-carboxyfluorescein, as described by Chernomordik et al. (1997).
If not stated otherwise, HAb2 cells were treated with 5 μg/ml trypsin (Fluka, Buchs, Switzerland) for 10 min at room temperature to cleave HA0 into its fusion-competent HA1-S-S-HA2 form. For HA300a cells, trypsin (5 μg/ml) was supplemented with neuraminidase (0.5 U/ml; Sigma) to improve binding of RBC. The enzymes were applied together for 10 min at room temperature. To terminate the reaction, HA-cells were washed twice with complete medium containing 10% FBS. After two washings with PBS, cells were incubated for 10 min with a 1-ml suspension of RBC (0.01% hematocrit). HA-cells with 0–2 bound RBC per cell were washed three times with PBS to remove unbound RBC and then used. When measuring RBC binding to cells, several areas of the dish were selected. We screened at least 200 cells to find the average number of RBC bound to each HA-cell.
To trigger fusion, HA-cells with bound RBC were incubated in PBS titrated with citrate to acidic pH. The low pH pulse was ended by replacing the acidic solution with PBS. The final extents of lipid and content mixing were assayed at room temperature by fluorescence microscopy as the ratio of dye-redistributed bound RBC to total bound RBC (Chernomordik et al., 1998). In each experiment, we verified that the extents of lipid and content mixing reached the final levels and did not increase further with time after low pH application. In most experiments, final fusion extents were achieved within 20 min after low pH application. Fusion completion after application of chlorpromazine (CPZ) or hypotonic osmotic shock (HOS) was much faster and reached the final lipid- and content- mixing extents within 5 min.
In some experiments, cells with bound RBC were treated with neuraminidase, proteinase K, or thermolysin (Sigma). Washing cells twice with complete medium terminated the reactions.
Because fusion extents and kinetics varied from day to day, apparently as a result of variation in the level of HA expression, we routinely started the experiments by choosing the precise conditions of the low pH treatment. Each experiment presented here was repeated at least three times, and all functional dependencies reported were observed in each experiment. The data presented were averaged from the same set of experiments.
Application of Exogenous Lipids, CPZ, and HOS
Stock solutions of lauroyl lysophosphatidylcholine (LPC; Avanti Polar Lipids, Birmingham, AL) and oleic acid (Sigma) were freshly prepared as a 0.5% (wt/wt) aqueous dispersion and 25 mM ethanolic solution, respectively. The cell medium bathing the plastic-attached HA-cells with bound RBC was replaced with 0.5 ml of PBS supplemented with LPC or oleic acid (Chernomordik et al., 1997). If not stated otherwise, low pH medium (used to trigger fusion) and “normal” pH medium (used to terminate the low pH treatment) were supplemented with the same concentration of lipid.
CPZ (Sigma) was prepared as a 0.25–0.5 mM solution in PBS. HA-cells with bound RBC were treated with low pH medium, returned to neutral pH, and exposed to CPZ-containing solution for 20–60 s.
To induce swelling of cells (HOS), we placed HA-cells with bound RBC into hypotonic medium (PBS diluted with water, 1:3), as described by Melikyan et al. (1995b)
Cell Surface ELISA
The percentage of HA trimers that undergo a low pH–induced conformational change was evaluated by cell surface ELISA (CELISA) performed with LC89 antibody (kindly provided by Dr. Stephen Wharton, Division of Virology, National Institute for Medical Research, London, United Kingdom). This antibody recognizes the low pH conformation but not the initial conformation of HA (Daniels et al., 1983). The X31 HA-cells were treated with different pH media at different temperatures and fixed for 10 min at room temperature in 4% (wt/vol) paraformaldehyde. After four washes with Ca- and Mg-free PBS, cells were incubated for 5 min in 5% FBS in PBS, and the surface HA was reacted with a 1:100 dilution of the LC89 antibodies (mouse immunoglobulin G) for 1 h at room temperature followed by three washes and a 5-min incubation in 5% FBS in Ca- and Mg-free PBS. The cells were then incubated for 60 min at room temperature in the same medium supplemented with a 1:1000 dilution of sheep anti-mouse immunoglobulin G conjugated with HRP (Amersham, Piscataway, NJ). After four washes, tetramethylbenzidine microwell peroxidase substrate 1-component (Kirkegaard & Perry Laboratories, Gaithersburg, MD) was used for detection as recommended by the manufacturer. The absorbance at 450 nm was measured on a microplate reader (THERMOmax, Molecular Devices; Sunnyvale, CA). After subtracting nonspecific binding, the relative level of low pH conformation of HA was compared with the maximal extent observed in the experiment in which the cells were incubated at pH 4.8 for 5 min at 37°C. The latter value was taken as 100%.
RESULTS
Experimental Approach
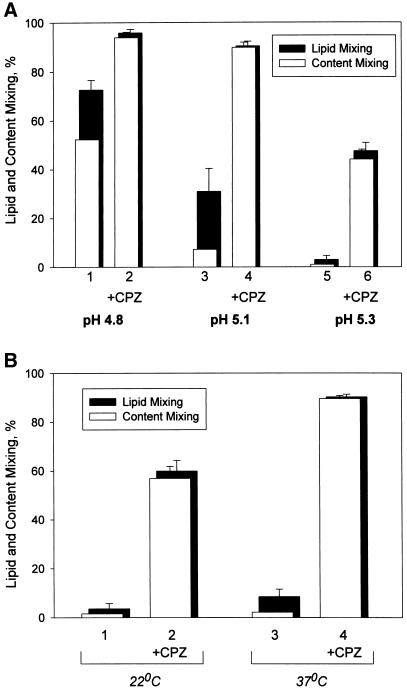
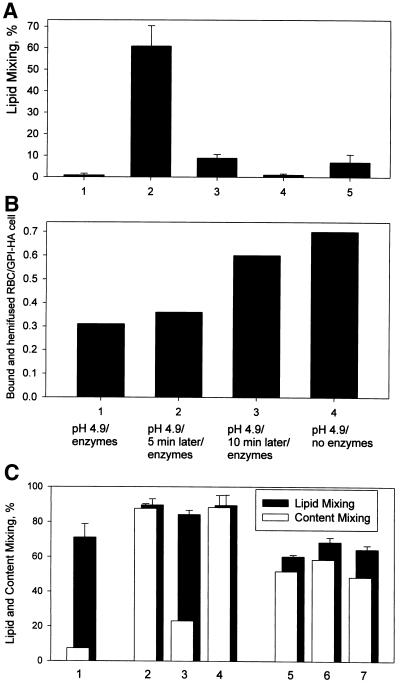
Open and closed bars in Figure 1A represent the percentages of RBC and X31 HA-cell pairs with content and lipid dyes, respectively, transferred from labeled RBC to HA-cells. Lipid mixing in the absence of content mixing (UH) is seen here as the difference between lipid mixing and content mixing extents, i.e., the closed band at the top of each open bar. For the same 2-min duration of low pH application at 37°C, increasing the pH of the fusion-triggering medium from 4.8 to 5.1 caused a fivefold decrease in the number of activated HA-cells, as assayed by CELISA. This decrease lowered the extents of both lipid and content mixing and shifted the prevailing fusion phenotype from mostly complete fusion to UH (Figure 1A, bars 1 and 3) (Chernomordik et al., 1998). Because some fusion pores are too small to allow the transfer of our aqueous dye carboxyfluorescein, some of the cell contacts scored here as UH probably have small pores detectable by electrophysiological technique (Tse et al., 1993; Zimmerberg et al., 1994). However, distinguishing between UH and a situation with a small nonexpanding fusion pore is not crucial for the present study. The decrease in the number of low pH–activated HA molecules inhibits fusion pore formation much more strongly than lipid mixing (Chernomordik et al., 1998), and we will focus here on the situation in which even lipid mixing is profoundly inhibited.
Figure 1.
Fusion intermediate detected at decreased numbers of low pH–activated HA molecules allows neither content nor lipid mixing before CPZ application. (A) Decreasing the number of low pH-activated HA molecules shifts the fusion phenotype from complete fusion to UH and then to RH. Fusion of X31 HA-cells with bound PKH26- and carboxyfluorescein-labeled RBC was triggered at 37°C by a 2-min application of pH 4.8 (bars 1 and 2), pH 5.1 (bars 3 and 4), and pH 5.3 (bars 5 and 6). As determined by CELISA, these low pH pulses activated 97 ± 0.2%, 20 ± 0.1%, and 6.4 ± 0.7% of HA for pH 4.8, 5.1, and 5.3, respectively (mean ± SE of quadruplicate determinations). In the experiments indicated by bars 2, 4, and 6, immediately after the end of a low pH pulse cells were exposed to a 1-min application of 0.25 mM CPZ. The extent of fusion was assayed by fluorescence microscopy at neutral pH 20 min after the end of low pH application as lipid dye (PKH26; closed bars) and aqueous dye (carboxyfluorescein; open bars) redistribution. Bars indicate mean ± SE, n > 3. The percentage of RBC/HA-cell contacts containing RH intermediates at different pH can be estimated as the difference between the extents of lipid mixing with and without CPZ application. (B) RH formation at 22°C (bars 1 and 2) and 37°C (bars 3 and 4). Lipid and content mixing (closed and open bars) in fusion of X31 HA-cells with bound PKH26- and carboxyfluorescein-labeled RBC, triggered by a 5-min pulse of pH 5.3, were assayed as in A. In the experiments indicated by bars 2 and 4, the low pH pulse was followed by a 1-min pulse of 0.25 mM CPZ applied at 22°C.
At pH 5.3 (2 min, 37°C), the number of low pH–activated HA-cells decreased to 6% of the total number of HA. Under these conditions, neither content nor even lipid mixing was observed (Figure 1A, bars 5). We hypothesized that some of the membrane contacts with no lipid mixing could contain local hemifusion connections that either somehow restricted lipid flow or were too short-lived to allow measurable lipid exchange. To detect such hypothetical RH intermediates, we used indirect experimental approaches based on transforming RH into either UH or complete fusion, both of which can be detected easily as lipid mixing or lipid and content mixing. These approaches (short-term application of HOS or CPZ and partial proteolysis) were developed previously for UH and 4°C-arrested intermediate (Melikyan et al., 1995b, 1997; Chernomordik et al., 1998). HOS stresses and breaks the hemifusion diaphragm (Melikyan et al., 1995b), and CPZ is thought to preferentially partition to and destabilize the inner membrane monolayers, which form the diaphragm (Melikyan et al., 1997). Partial proteolysis apparently cleaves some of the HA molecules, which restrict lipid flow through RH, and thus allow the detection of this intermediate (Chernomordik et al., 1998).
As shown below, we indeed found transient fusion intermediates with a set of properties expected from the hypothetical RH intermediates. Although the exact structure of these intermediates remains uncertain, we refer to them below as transient RH.
Low pH- and HA-dependent Formation of RH at Physiological Temperature
In the experiment presented in Figure 1A (bars 6), X31 HA-cells with bound RBC were first treated with a 2-min pulse of pH 5.3 and then, already at neutral pH, with a 1-min CPZ pulse. CPZ application caused a fast and significant increase in the extent of both lipid and content mixing (bars 6) compared with those observed after the low pH pulse without CPZ application (bars 5). The extent of lipid mixing observed after a 1-min CPZ pulse did not change for CPZ concentrations in the range from 0.2 to 0.5 mM used in this study (our unpublished results). CPZ pulse also promoted fusion when applied after treating cells with pH 4.8 and 5.1 (bars 2 versus 1 and bars 4 versus 3).
CPZ promotion of lipid and content mixing was also observed at 22°C (Figure 1B). CPZ pulse application at the same temperature (22°C) gave a higher extent of fusion if applied after low pH incubation at 37 versus 22°C. The percentages of activated HA at 22 and 37°C measured by CELISA were very close (8.2 ± 1.5% versus 6.4 ± 0.7%, respectively, mean ± SE of quadruplicate determinations). The difference between the number of CPZ-detected fusion intermediates developed at 37 and 22°C for the same number of low pH–activated HA-cells might reflect the difference in HA mobility (Junankar and Cherry, 1986) and/or in the rate of HA refolding beyond an early conformational change assayed with LC89 antibody (White and Wilson, 1987).
A significant increase in the extent of lipid mixing in the experiments in which application of mildly acidic pH was followed by a CPZ pulse was also observed for RBC fusion to Japan HA-cells and upon replacing a CPZ pulse with a short-term application of HOS (PBS diluted with water, pH 7.4) (Figure 2A). In this experiment, Japan HA-cells with bound RBC were incubated at pH 5.35 for different times. Immediately after the end of a low pH pulse, we treated cells with CPZ or HOS. After these treatments, some of the RBC/HA-cell contacts, which did not allow PKH26 transfer in the control experiment (neither CPZ nor HOS applied), demonstrated lipid mixing.
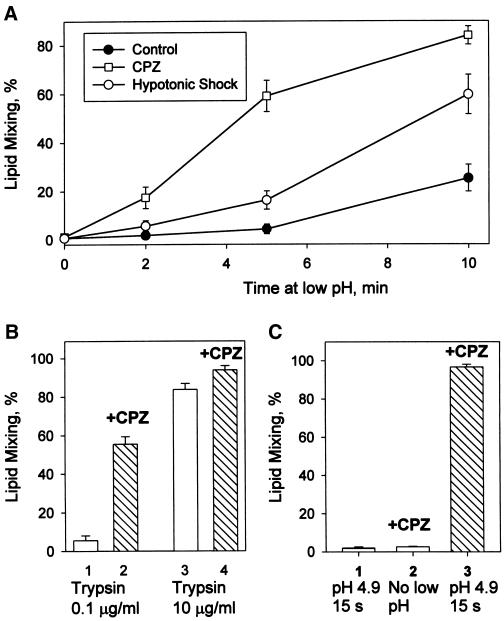
Figure 2.
RH detection at suboptimal fusion conditions such as moderately acidic pH (A), low surface density of trypsin-cleaved fusion-competent HA (B), and short pH applications (C). (A) At moderately acidic pH, an increase in the number of RH intermediates outpaces an increase in lipid mixing. Fusion of Japan HA-cells with bound RBC was triggered at room temperature by applying pH 5.35 for different times (2, 5, and 10 min). Closed circles represent the final extents of lipid mixing observed after these pH pulses; squares and open circles represent final lipid mixing extents observed when low pH pulses were followed immediately by CPZ (0.5 mM, 1 min) or HOS, respectively. Points indicate mean ± SE, n > 3. (B) RH formation at 37°C and pH 4.9 for cells with decreased numbers of trypsin-cleaved HA molecules. Lipid mixing extents observed for X31 HA-cells pretreated with 0.5 U/ml neuraminidase and either 0.1 μg/ml (bars 1 and 2) or 10 μg/ml (bars 3 and 4) trypsin (10 min, 22°C). Fusion was triggered by a 2-min pulse of pH 4.9 followed (bars 2 and 4) or not followed (bars 1 and 3) by a CPZ pulse (0.25 mM, 1 min). Bars indicate mean ± SE, n > 3. (C) Fast formation of RH intermediates after treating X31 HA-cells with bound RBC with a 15-s pulse of pH 4.9 at 37°C. RH was detected with a 20-s pulse of 0.5 mM CPZ applied immediately after the end of low pH application. Bar 1, lipid mixing extent observed in the control experiment with no CPZ pulse; bar 2, the control experiment in which CPZ was applied to RBC/HA-cell pairs, which were not treated with low pH; bar 3, the cells were treated with low pH and, already at neutral pH, with CPZ. Bars indicate mean ± SE, n > 3.
Our results suggested the existence of RH intermediates that allow neither lipid nor content mixing but were transformed into measurable fusion by CPZ or HOS application. The percentage of RBC/HA-cell contacts that developed lipid mixing only after CPZ pulse can be as high as 50–80% (for instance, subtracting bars 3 from bars 4 in Figure 1A indicates that ∼80% of all contacts contained RH intermediates). CPZ application was always more effective than HOS in promoting lipid mixing, suggesting that some RH intermediates were not detected with HOS. CPZ was also more efficient than HOS in transforming GPI-HA–mediated hemifusion into complete fusion (our unpublished results).
RH intermediates were formed by the low pH–activated HA molecules. No PKH26 redistribution was observed in the negative controls, in which either CPZ or HOS was applied to X31 or Japan RBC/HA-cell pairs not exposed to acidic pH or exposed to acidic pH but without previous trypsinization to cleave HA0 into the fusion-competent HA1-S-S-HA2 form (our unpublished results).
The inability of uncleaved HA0 to undergo a low pH–dependent conformational change and mediate fusion (White et al., 1981) was also used in the following experiment. We decreased the trypsin concentration to decrease the percentage of cleaved, and therefore, fusion-competent, HA molecules (Clague et al., 1991). At decreased surface density of cleaved HA, even a 2-min application of the “optimal” pH 4.9 medium at 37°C yielded mostly RH intermediates (Figure 2B).
To summarize, inhibition of lipid and content mixing by decreasing either the efficiency of HA activation (by application of less acidic pH) or the total number of fusion-competent HA-cells allowed us to detect low pH–triggered, HA-dependent formation of RH intermediates at physiological temperature. Because the properties of RH intermediates formed by X31 and Japan HA were very similar, most of the results will be presented for only one of the two strains of HA.
Time Course of RH Formation and Dissociation
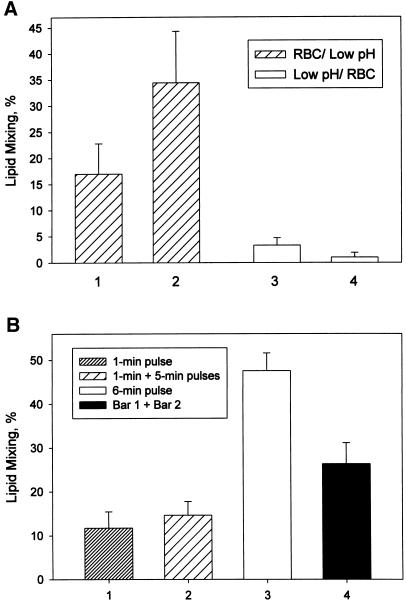
At physiological temperature, RH is a dynamic intermediate. RH formation developed within 35 s after decreasing pH. These intermediates were detected in the experiments in which X31 RBC/HA-cell pairs were exposed to a short, 15-s pulse of pH 4.9 at 37°C followed immediately by a 20-s CPZ pulse (Figure 2C).
Most importantly, RH formed at physiological temperature is a transient, reversible intermediate. The longer the time interval between low pH and CPZ pulses, the lower the extent of the lipid mixing observed. Although the number of bound RBC stayed constant throughout the experiment (our unpublished results), the promotion of lipid mixing by CPZ was decreased significantly if CPZ was applied 15 min after the end of the low pH pulse (Figure 3A). A qualitatively similar time course of RH dissociation was detected for cells expressing either X31 or Japan HA and also in the experiments in which the CPZ pulse was replaced with HOS. Similar results (transient state at which lipid and content mixing can be promoted by CPZ or HOS application) were also obtained for GPI-HA cells (our unpublished results).
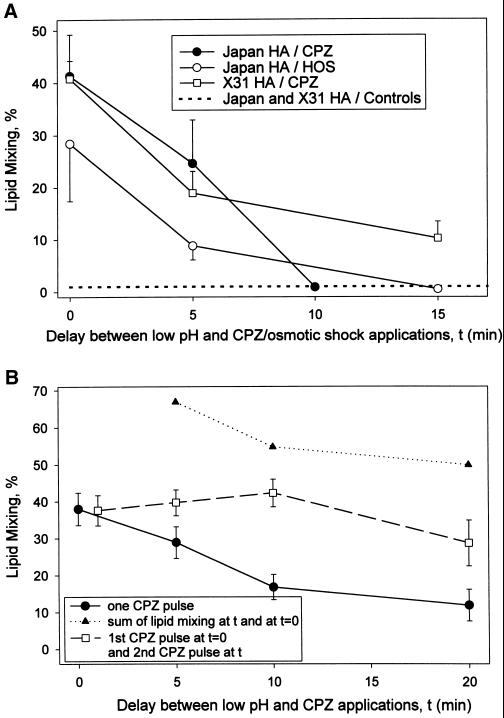
Figure 3.
Time course of RH formation and dissociation. (A) Dissociation of RH with time after low pH application. Japan HA-cells (circles) and X31 HA-cells (squares) with bound RBC were treated at room temperature with a 2-min pulse of pH 5.3 and 5.35, respectively, followed, after different time intervals, by a CPZ pulse (0.5 mM, 1 min; closed circles and squares) or HOS (open circles). Time 0 corresponds to the end of the low pH pulse. Lipid mixing extents were determined at least 40 min after a low pH pulse. In the control experiments, in which the same low pH application was followed by neither CPZ nor HOS, lipid mixing extents were <1% for both Japan and X31 cells (dashed line). Points indicate mean ± SE, n > 3. (B) Application of the second CPZ pulse does not reveal any additional, de novo formed RH intermediates that were not already detected by the first CPZ pulse applied immediately after a low pH pulse. Fusion of X31 HA-cells with bound RBC was triggered by a 2-min pulse of pH 5.3 at room temperature. This low pH pulse was followed, after different time intervals, with a 30-s CPZ pulse (0.5 mM; closed circles). Squares represent the results of the experiments in which cells were treated with the first CPZ pulse (0.5 mM, 30 s) immediately after the end of a low pH pulse and then, after different time intervals, with a second, identical CPZ pulse. Lipid mixing extents were determined at least 40 min after a low pH pulse. In the control experiments, in which neither CPZ nor HOS followed the same low pH application, lipid mixing extents were <1%. Points indicate mean ± SE, n > 3. Triangles represent the sum of the lipid mixing extents measured in the experiment shown by closed circles after a single CPZ pulse was applied at time 0 and at the given times.
The decrease in the number of RH intermediates detected by CPZ or HOS with time after low pH pulse (Figure 3) may reflect a gradual dissociation of RH intermediates, with only a fraction of the intermediates detected immediately after a low pH pulse still present 15 min later. Alternatively, the lifetime of each RH intermediate can be much shorter than the tens of minutes within which RH became undetectable. In this scenario, the time of RH disappearance characterizes the gradual decrease in the number of de novo forming, very short-lived RH intermediates present at the moment of CPZ or HOS application. If this latter scenario is correct, application of the second CPZ pulse before the disappearance of RH would detect additional RH sites. In this case, the extent of lipid mixing after the application of two CPZ pulses consecutively (the first one immediately after low pH pulse [time 0] and the second pulse at time t) should correspond to the sum of the extents of lipid mixing after single CPZ pulses applied in the separate experiments at time 0 and time t (triangles in Figure 3B). The lack of any increase in the total extent of X31 RBC/HA-cell fusion after the second CPZ pulse (Figure 3B) indicated that all RH intermediates were already present at the time of the first CPZ pulse. We conclude that all RH intermediates detected by a CPZ pulse 20 min after the end of a low pH treatment were already present immediately after it, at the time of the first CPZ pulse application. Thus, the lifetime of RH intermediates can be estimated from the time course of RH disappearance and is ∼10–20 min. The validity of the experimental design with application of two subsequent CPZ pulses was justified by the following control experiment. The extent of lipid mixing, observed when low pH application to X31 HA-cells with bound RBC was immediately followed by CPZ pulse, was not altered if the low pH pulse was preceded by an additional CPZ pulse (our unpublished results).
To summarize, for a few minutes after acidification, the membranes are tied together by a reversible RH connection.
Effects of 4°C and Exogenous Lipids on Transient RH
The lifetime of RH intermediates was greatly increased at 4°C (Figure 4A). We first formed transient RH by applying a low pH pulse at 22°C and then decreased the temperature to 4°C. Cells were incubated on ice for different times, returned to 22°C, and immediately treated with CPZ to assay for RH. CPZ application after 20 or 90 min (bars 2 and 3, respectively) of incubation of the cells at 4°C gave similar extents of lipid mixing to that observed when CPZ was applied at 22°C immediately after the low pH pulse (bar 1). Thus, there was no RH inactivation during 90 min of incubation at 4°C (compare with the profound inactivation of RH intermediates after 20 min at 22°C [bar 4]). In the experiment presented in Figure 4A, bar 5, we applied CPZ at 4°C to determine whether RH intermediates were present before increasing the temperature. Although less RH was detected when CPZ was applied at 4°C than at 22°C, perhaps reflecting less efficient partition of CPZ into membranes at 4°C, RH intermediates were present at 4°C rather than reappearing from some pre-RH structures upon increasing temperature. This finding and the lack of inactivation of RH at 4°C indicated that the properties of the RH intermediates were conserved during cell incubation at 4°C. Similar results were obtained for Japan HA-cells (our unpublished results).
Figure 4.
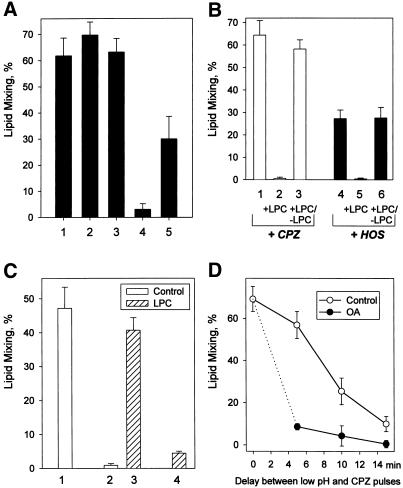
Effects of 4°C and membrane lipid composition on the properties of transient RH intermediates. (A) Dissociation of RH intermediates is blocked at 4°C. Fusion of X31 HA-cells with bound RBC was triggered by a 2-min pulse of pH 5.2 at 22°C. Bars 1 and 4, a low pH pulse was followed by a 1-min pulse of 0.5 mM CPZ applied immediately after a low pH pulse (1) or 20 min later (4). Bars 2 and 3, immediately after the end of a low pH pulse, the temperature was decreased to 4°C. After 20-min (2) or 90-min (3) incubation at 4°C, the temperature was returned to 22°C, and a CPZ pulse (0.5 mM, 1 min) was applied. Lipid mixing extents were determined at least 20 min after a CPZ pulse. As for bar 2, after the end of a low pH pulse the cells were incubated at 4°C for 20 min. Then, still at 4°C, a 1-min pulse of CPZ was applied. After incubating cells for another 10 min at 4°C, the temperature was increased to 22°C, and lipid mixing extents were assayed. Without CPZ application, lipid mixing observed after this low pH treatment at 22°C was only 3 ± 1.4%. Bars indicate mean ± SE, n > 3. (B) LPC reversibly suppresses RH intermediates as detected with CPZ (bars 1–3) or HOS (bars 4–6). Fusion of X31 HA-cells with bound RBC was triggered at 37°C by a 2-min pulse of pH 5.3. Immediately after the pulse, the cells were treated with either CPZ (0.25 mM, 1 min) or HOS (bars 1 and 4, respectively). In the experiments indicated by bars 2, 3, 5, and 6, after the pulse low pH medium was replaced with LPC-supplemented PBS (pH 7.4, 150 μM LPC). One minute later, a CPZ pulse (bar 2) or HOS (bar 5) was applied, still in the presence of LPC. In the experiments indicated by bars 3 and 6, LPC was washed out before CPZ (bar 3) and HOS (bar 6) application. Bars represent the mean extents of lipid mixing ± SE, n > 3. No measurable lipid mixing was observed after this low pH treatment with neither CPZ nor HOS applied. (C) LPC blocks inactivation of the HA machinery responsible for the formation of transient RH intermediates. Fusion of Japan HA-cells with bound RBC was triggered at room temperature by a 2-min pulse of pH 5.3. Bar 1, low pH pulse followed by a CPZ pulse (0.5 mM, 1 min). Bar 2, low pH pulse followed by a 10-min incubation at neutral pH and then a CPZ pulse. Bar 3, low pH pulse followed by a 10-min incubation atneutral pH in the presence of 80 μM LPC; LPC was then washed out, and the cells were treated with a CPZ pulse (0.5 mM, 1 min). Bar 4, low pH pulse followed by a 10-min incubation at neutral pH, still in the presence of LPC; LPC was then washed out, and the cells were incubated for another 10 min in the absence of LPC and finally treated with a CPZ pulse. Bars indicate mean ± SE, n > 3. (D) RH dissociation proceeds faster in the presence of oleic acid (OA). Fusion of Japan HA-cells with bound RBC was triggered by a 1-min pulse of pH 5.3 at room temperature. After the pulse, low pH medium was replaced with PBS (pH 7.4) supplemented (●) or not (○) with 10 μM oleic acid. Then, after different time intervals, the cells were treated with CPZ (0.25 mM, 1 min). Bars represent the mean extents of lipid mixing ± SE, n > 3.
Membrane fusion, and in particular HA-mediated fusion, may be modulated by altering membrane lipid composition (Chernomordik et al., 1995, 1997). The effects of different lipids on fusion correlate with their dynamic molecular shape. Inverted cone-shaped LPC inhibits, and cone-shaped oleic acid promotes, membrane merger in complete fusion and UH. RH formation was also inhibited by LPC present during a low pH pulse (our unpublished results). Even if LPC was added after the low pH pulse, at the time when RH intermediates were already formed, neither CPZ nor HOS promoted lipid mixing as long as LPC was present (Figure 4B).
In the experiment presented in Figure 4C we formed transient RH by treating Japan HA-cells with bound RBC with a pH 5.3 pulse at 22°C. In the LPC-free PBS, the number of RH intermediates decreased dramatically within 10 min after a low pH pulse, indicating rapid inactivation of the HA machinery responsible for RH formation (compare bars 2 and 1). However, if low pH pulse was followed by a 10-min incubation with LPC, the percentage of RH detected immediately upon LPC removal (bar 3) was almost the same as that seen right after low pH pulse in the control experiment with no LPC added (bar 1). Within 10 min after washing LPC out, we detected almost no RH intermediates (bar 4). Thus, removal of LPC resulted in the formation and subsequent inactivation of the RH intermediates with a rate similar to that in control experiments with no LPC added. These results indicated that although there were no RH intermediates in the presence of LPC, the machinery responsible for their formation remained intact and ready to form RH after LPC was washed out.
Although LPC slowed down inactivation of the HA machinery supporting RH, cone-shaped oleic acid accelerated RH disappearance after low pH pulse (Figure 4D). Similar results (Figure 4, C and D) were also obtained for X31 HA-cells (our unpublished results).
In brief, LPC prevents the formation of transient RH and dissociates or shrinks already formed RH intermediates. In parallel, LPC slowed down, and oleic acid accelerated, the disappearance of the structures responsible for RH development.
HA Stabilizes RH and Restricts Lipid Flow
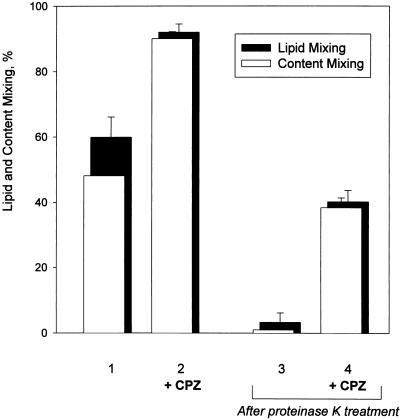
The role of HA in stabilization of the transient RH intermediates and in lipid flow restriction was probed by studying the sensitivity of these intermediates to proteolysis with thermolysin or proteinase K, the enzymes known to cleave low pH conformations of HA (Wiley and Skehel, 1987). In the experiment presented in Figure 5A, we formed transient RH connections (bar 2) and, immediately after the end of the low pH pulse, treated the cells with 40 μg/ml thermolysin for 1 min. After this treatment, almost no lipid mixing was observed either with or without CPZ application (bars 5 and 4, respectively). In contrast, partial cleaving of the activated HA molecules with very mild thermolysin treatment (2 μg/ml, 10 s) led to a statistically significant increase in lipid dye redistribution between cells (bar 3) compared with that observed in the control experiment with neither thermolysin nor CPZ applied (bar 1). Because neither CPZ nor HOS was needed to cause this lipid mixing, we conclude that cleaving a fraction of activated HA molecules facilitated lipid flow through the preexisting RH.
Figure 5.
Stability of transient RH (A) and UH (B, C) upon proteolysis of low pH form of HA (A, B) and in the presence of LPC (C). (A) Although mild treatment of transient RH intermediates with thermolysin facilitated lipid mixing, harsher treatment caused complete dissociation of RH. Fusion of X31 HA-cells with bound RBC was triggered by a 90-s pulse of pH 5.2 at room temperature. Bar 1, lipid mixing extent observed in the control experiment with neither CPZ nor thermolysin applied. Bar 2, low pH application was followed by a 1-min pulse of 0.25 mM CPZ. Bar 3, immediately after the end of the low pH pulse, cells were treated with thermolysin (2 μg/ml, 10 s) and no CPZ was applied. Bars 4 and 5, harsher treatment with thermolysin (40 μg/ml, 1 min) followed or not followed (bar 5 and 4, respectively) by the CPZ pulse. Bars represent the mean extents of lipid mixing ± SE, n > 3. (B) The development of HA-independent binding between PKH26- and carboxyfluorescein-labeled RBC and GPI-HA cells at later stages of UH. Cells were incubated at pH 4.9 for 5 min at 37°C. Immediately after the pulse (bar 1), or after 5 or 10 min of incubation at neutral pH (bars 2 and 3, respectively), the cells were treated for 20 min with proteinase K (0.1 mg/ml) and neuraminidase (1 U/ml). Then, we screened at least 200 cells to find the average number of carboxyfluorescein-labeled RBC bound to each PKH26-labeled GPI-HA cell. In the control experiment, neither proteinase K nor neuraminidase was applied (bar 4). The number of hemifused (i.e., PKH26-labeled) GPI-HA cells achieved the maximal level (66 ± 6%) within 5 min of low pH application and remained constant throughout the experiment. (C) Hemifusionconnections can be reversibly suppressed by LPC only at an early stage of the UH. Fusion of X31 HA-cells with bound PKH26- and carboxyfluorescein-labeled RBC was triggered at 37°C by a 2-min pulse of pH 5.2. After the pulse, the cells were incubated in PBS (pH 7.4) for 5 min, still at 37°C. During this time, the percentage of PKH26-labeled (closed bars) and carboxyfluorescein-labeled (open bars) HA-cells reached the maximum (“final”) levels (bars 1). After this 5-min incubation (bars 2–4), or after longer, 35-min incubation intervals (bars 5–7), the cells were treated with a 1-min pulse of 0.25 mM CPZ at 22°C (bars 2 and 5) or with CPZ in the presence of 150 μM LPC (bars 3 and 6). In the experiments indicated by bars 4 and 7, the cells were incubated with LPC for 2 min, LPC was washed out, and a CPZ pulse was applied. Bars represent the mean extents of lipid mixing ± SE, n > 3.
In the control experiments, treating cells with thermolysin or proteinase K before low pH application did not affect the fusion observed after subsequent application of a low pH pulse followed or not followed by CPZ. One may hypothesize that lipid mixing observed after mild proteolysis of the cells with the RH intermediates with thermolysin or proteinase K was mediated by the peptide digestion products. However, the lack of lipid mixing after harsher treatments with the same proteases, and thus the significantly higher concentrations of these peptide fragments, argues against this possibility.
Disappearance of RH and facilitation of lipid mixing after harsh and very mild proteolysis, respectively, of the activated HA molecules were also observed for Japan and HA-cells with proteinase K treatment instead of thermolysin (our unpublished results).
These experiments indicated that low pH forms of HA both stabilize transient RH intermediates and restrict lipid mixing through them.
HA-independent End State in Unrestricted Hemifusion
Although stabilization of RH intermediates requires the continuous presence of low pH forms of HA, membrane merger in UH reaches an HA-independent long-lived state. We detected this end-state hemifusion as a shift in the mechanism of RBC binding to HA-cells. Initial binding between RBC and HA-cells is mediated by HA1-receptor connection and can be inhibited by neuraminidase treatment (Chernomordik et al., 1997). After low pH application, this binding is complemented by additional, neuraminidase-insensitive binding mechanisms that are still dependent on HA. This HA-dependent binding may involve insertion of the HA fusion peptide into RBC and membrane merger. To study UH, we shifted to cells expressing GPI-HA (Kemble et al., 1994). Within 5 min after low pH application to RBC/GPI-HA cell complexes, almost half of the population of RBC, which had already been hemifused to GPI-HA cells, as shown by membrane dye redistribution, could be released by cleaving HA-dependent connections with proteinase K and neuraminidase (Figure 5B). However, when we increased the time interval between the low pH pulse and enzyme application to 10 min, the binding between hemifused RBC and GPI-HA cells became almost insensitive to proteinase K and neuraminidase. Application of a CPZ pulse to RBC/GPI-HA cell pairs at this stage yielded the same extent of complete fusion as that observed before enzymatic treatment (our unpublished results). These results document the existence of an end-state HA-independent hemifusion. Because under our conditions GPI-HA–mediated lipid mixing reaches its final extent (i.e., the maximal percentage of PKH26-redistributed cells) during 5 min of low pH application, the onset of measurable lipid mixing in UH precedes the establishment of end-state hemifusion. Similar results were obtained for UH mediated by wild-type X31 HA (our unpublished results).
As shown above (Figure 4B), RH connections formed at physiological temperature were reversibly dissociated by adding LPC. A similar effect of LPC, i.e., reversible inhibition of CPZ-induced transformation of hemifusion into complete fusion, was reported for GPI-HA–mediated UH (Melikyan et al., 1997). In the experiment presented in Figure 5C, we tested whether this inhibition can be observed at different stages of wild-type HA-mediated UH. We applied LPC and then CPZ to X31 RBC/HA-cell pairs 5 min after a 2-min pulse of pH 5.2 at a stage at which RBC binding and hemifusion were still HA-dependent (bars 2–4) and 30 min after the low pH pulse at the HA-independent end-state hemifusion (bars 5–7). At the HA-dependent stage, as described by Melikyan et al. (1997), LPC inhibited CPZ-induced transformation from UH to complete fusion, measured as an increase in the percentage of RBC, which transferred both PKH26 and carboxyfluorescein to HA-cells (bars 3 versus bars 4). In contrast, for the end-state hemifusion, LPC had no effect on CPZ-induced promotion of complete fusion (bars 6 versus bars 7). Similar results were obtained for UH mediated by GPI-HA (our unpublished results).
Thus, early UH intermediate, although already allowed lipid mixing, was stabilized by low pH–activated HA and inhibited by LPC. Once formed, these hemifusion intermediates progressed to HA-independent end-state hemifusion, which was not suppressible with LPC. The number of RBC bound in an HA-independent manner and the extent of complete fusion observed after CPZ application to RBC/GPI-HA cell pairs did not decrease with time for up to 2 h after low pH application (our unpublished results).
Switching the Fusion Phenotype to and from RH
Cells committed to complete fusion can be redirected to transient RH by cleaving some of the low pH–activated HA trimers with proteinase K (Figure 6). We committed X31 HA-cells with bound RBC to complete fusion by applying a 5-min pulse of pH 4.9 but blocked fusion at the 4°C-arrested stage. Then, cells were returned to neutral pH medium and treated with proteinase K, still at 4°C. The temperature was increased to 37°C, and fusion extent was assayed with or without CPZ application. Proteinase K treatment, which strongly inhibited complete fusion, shifted the prevailing fusion phenotype toward RH intermediates, detectable only by CPZ application. At longer incubations with proteinase K, CPZ treatment did not cause measurable fusion (see above). Thus, cleaving low pH–activated HA gradually shifted the fusion phenotype from complete fusion to UH (Chernomordik et al., 1998), then to transient RH, and finally to no fusion at all.
Figure 6.
Conversion of the fusion phenotype from complete fusion to transient RH. Cells committed to complete fusion but blocked at the 4°C-arrested stage could still be redirected to transient RH by cleaving some activated HA molecules. Fusion of X31 HA cells with bound PKH26- and carboxyfluorescein-labeled RBC was triggered by the application of pH 4.9 for 5 min at 4°C. Cells were incubated in neutral pH, still at 4°C, for 5 min and then warmed to 37°C and treated (bars 2 and 4) or not treated (bars 1 and 3) with 0.25 mM CPZ for 1 min. Twenty minutes later, fusion was assayed as lipid and content mixing (closed and open bars). In the experiments indicated by bars 3 and 4, in the time interval between the end of the low pH application and warming, cells were treated with proteinase K (50 μg/ml, 5 min, 4°C). Each bar indicates the mean ± SE, n > 3.
RH Requires Concerted Action of Multiple Trimers in the Contact Zone
Establishment or stabilization of HA conformations, which form transient RH, required the presence of the target membrane at the time of low pH application. When X31 HA-cells were treated by a 2-min pH 5.3 pulse at 22°C, cooled to 4°C, and then incubated with RBC, subsequent application of CPZ to X31 RBC/HA-cell pairs did not promote any redistribution of PKH26 (Figure 7A). Thus, no RH intermediates were developed under these conditions. In contrast, the identical pH pulse applied to X31 HA-cells with bound RBC, followed by decreasing the temperature to 4°C, resulted in the formation of transient RH intermediates, as indicated by CPZ-promoted lipid mixing. Thus, formation of transient RH requires HA structures that do not develop in the absence of cell contacts.
Figure 7.
Formation of transient RH intermediates requires the cooperative action of HA trimers activated in the presence of the target membrane. (A) RH formation requires HA structures that do not develop in the absence of cell contacts. Bars 1 and 2, fusion of X31 HA-cells with bound RBC was triggered by a 2-min pulse of pH 5.3 at 22°C followed immediately (bar 1) or after 30 min of incubation at 4°C (bar 2) by a 1-min application of 0.25 mM CPZ applied at 22°C. Bars 3 and 4, X31 HA-cells were treated with the same pulse of low pH in the absence of RBC. Immediately after the end of the low pH pulse, the temperature was decreased to 4°C and RBC were added immediately (bar 3) or 10 min later (bar 4). After a 30-min incubation at 4°C, unbound RBC were washed out, the temperature was returned to 22°C, and 0.25 mM CPZ was applied for 1 min. Bars represent the mean extents of lipid mixing ± SE, n > 3. (B) RH formation requires the concerted action of multiple HA trimers. The same number of HA molecules formed more RH intermediates if these HA molecules were activated at the same time rather than in portions. Fusion of X31 HA-cells with bound RBC was triggered at room temperature by a 1-min (bar 1) or 6-min (bar 3) pulse of pH 5.3 followed by a 1-min application of 0.5 mM CPZ. Bar 2, after a 1-min application of pH 5.3, cells were incubated at neutral pH for 10 min and then treated with a 5-min pulse of pH 5.3 followed immediately by CPZ (0.5 mM, 1 min). Bar 4 represents the sum of bars 1 and 2. No measurable lipid mixing was observed after each of these low pH pulses with no CPZ applied or after a 1-min pulse of pH 5.3 followed by a 15-min incubation and then by CPZ pulse. Bars 1–3 represent the mean extents of lipid mixing ± SE, n > 3.
Low pH application results in almost simultaneous activation of multiple HA molecules. RH formation can be mediated either by individual HA trimers or by concerted action of multiple trimers. In the former case, the formation of transient RH intermediates should depend only on the total number of activated HA molecules rather than on their simultaneous presence in the contact zones. To address this question, we compared the efficiency of RH formation in response to a 6-min pH 5.3 pulse with that obtained after applying 1-min and 5-min pulses of pH 5.3 separated by a 10-min interval (Figure 7B). Judging from the fusogenic activity, a significant part of HA molecules activated by a 6-min pulse was already activated after the first 1 min at low pH. When a 6-min pulse was divided into two pulses, transient RH intermediates induced by the first 1-min pulse were almost completely inactivated by the time of the second pulse application. The sum of the RH extents assayed by CPZ application after a 1-min low pH pulse, and, in a separate experiment, after a 5-min low pH pulse, was lower than that observed after a single 6-min pulse. Thus, the same total number of HA molecules formed more RH intermediates if these HA molecules were activated at the same time rather than in portions. This finding suggested that the formation of transient RH intermediates involves cooperative action of multiple HA trimers.
DISCUSSION
Transient RH: Structure and Properties
We describe here an early and transient intermediate of wild-type HA-mediated fusion at physiological temperature. This intermediate is formed and supported by low pH–activated HA and can be transformed into complete fusion by application of either CPZ or HOS, the treatments known to break hemifusion diaphragm (Melikyan et al., 1995b, 1997; Chernomordik et al., 1998). We interpret this intermediate as a hemifusion connection between the contacting monolayers of the membranes. Could the identified fusion intermediate correspond to a very tight proteinaceous transmembrane connection that develops before actual merger of membrane bilayers? If so, 1) LPC, an inhibitor of membrane hemifusion (Chernomordik, 1996; Chernomordik et al., 1997), should prevent this contact and dissociate it, if already formed; 2) CPZ and HOS treatment must destabilize this contact state and transform it into complete fusion; and 3) mild proteolysis with thermolysin or proteinase K must transform some of these contacts into UH. The existence of a fusion intermediate other than RH combining all these properties seems improbable. Thus, the transient transmembrane connection detected at low surface densities of low pH–activated HA is most likely a member of the family of RH intermediates, all long-lived at 4°C, but differing in the fusion phenotype at 37°C. Depending on the number of activated HA molecules, upon the temperature increase different RH intermediates proceed to complete fusion, or UH, or, in case of transient RH, dissociate with time. Transient RH intermediates require fewer activated HA molecules than UH, which in turn requires fewer HA molecules than opening of a fusion pore (Chernomordik et al., 1998).
As a flickering fusion pore (Melikyan et al., 1995a, 1999) that can repeatedly open and close during ∼10 min of electrophysiological recording (G. Melikyan, personal communication), RH is a dynamic intermediate. During the 10-min lifetime of this intermediate, the membranes either remain connected or dissociate and remerge with high enough frequency to make each site detectable during a 30-s CPZ pulse. The possibility of reversible dissociation of RH connections was substantiated by the experiments in which LPC either trapped normally flickering RH in a dissociated state or dissociated normally stable RH intermediates. LPC might shrink or dissociate hemifusion connections by increasing their elastic energy, because inverted cone-shaped LPC does not support the curvature of the lipid monolayer required in a stalk-like local connection between membranes (Chernomordik et al., 1995, 1997). Alternatively, shrinking of the connections can be explained by compression of the outer membrane monolayers with added exogenous lipid (Melikyan et al., 1997).
The dynamic character of the transient RH is in contrast to the properties of UH. It had been unknown whether HA-mediated UH is a stable structure or a transient intermediate allowing lipid flow. We found here that although UH starts with an early stage that is stabilized by HA and still can be suppressed by LPC, all hemifused cells, which demonstrated lipid mixing, eventually reach stable and HA-independent end-state hemifusion.
Mechanism of RH
All approaches used to emphasize transient RH over more advanced fusion phenotypes (shorter low pH pulses, less acidic pH, lower surface density of the fusion-competent HA) decreased the number of activated HA molecules. It appears improbable that different “suboptimal” activations result in the same suboptimal conformation of HA specifically required for RH. In addition, low pH conformations of HA, which are capable of mediating complete fusion at 37°C, can also support RH, as demonstrated by the shift from complete fusion to the transient RH phenotype upon cleaving of some of the activated HA molecules. Thus, the transition from RH to UH and complete fusion apparently reflects an increase in the number of activated HA molecules rather than some specific change in their conformation. In particular, our finding that HA conformations, which form and support RH, develop only in the presence of the target membrane indicate that these conformations differ from the stabilized activated HA conformations induced by HA preincubation at mildly acidic pH in the absence of the target membrane (Korte et al., 1999).
RH formation apparently involves multiple HA trimers (Figure 7B). Assuming that three to six activated HA trimers can mediate lipid mixing and fusion pore opening (Blumenthal et al., 1996; Danieli et al., 1996; Bentz, 2000; but see Gunther-Ausborn et al., 2000), RH formation may require just two trimers. Alternatively, an identical fusion machine might generate different fusion phenotypes with different probabilities. In this case, increasing the number of activated HA molecules increases the number of these fusion machines and thus allows realization of less probable and more advanced fusion phenotypes. At present, we cannot distinguish between these two interpretations.
The disappearance of RH intermediates after HA proteolysis indicates that the energy price of a membrane merger in early fusion intermediates is compensated for by the energy coming from HA refolding. This means that the membrane merger at RH and subsequent membrane rearrangements leading to opening of an expanding fusion pore are energy-intensive stages, which argues against the hypothesis that just bringing membrane lipid bilayers into close contact is sufficient to allow spontaneous fusion of membrane lipid bilayers. The recent finding that a point mutation within the transmembrane domain of HA results in the RH phenotype at physiological temperature (Melikyan et al., 1999) corroborates the conclusion that the transition from RH to a fusion pore is an HA-dependent process.
Even without proteolysis, RH formed at physiological temperature disappeared rather rapidly. LPC, an inhibitor of hemifusion, and oleic acid, a promoter of hemifusion, slow down and accelerate the inactivation of RH, respectively. Thus, we hypothesize that membrane merger mediates this inactivation. At an early stage of the low pH–induced conformational change, the fusion peptide of HA inserts into one of the fusing membranes (for review, see Gaudin et al., 1995). The energy released in the further refolding of HA can then be applied to the membranes through the fusion peptide (Weissenhorn et al., 1997; Kozlov and Chernomordik, 1998). Membrane merger allows relocation of the fusion peptide from the membrane in which it was initially inserted to another membrane by a “membranous” path without peptide dissociation from the membrane. Fusion peptide relocation through the RH site before fusion completion discharges the loaded spring of HA (Carr and Kim, 1993) and, thus, inactivates HA and dissociates HA-supported RH. The absence of RH inactivation at 4°C can indicate that this temperature inhibits fusion peptide relocation. In addition, or alternatively, the energy released in the low pH–induced HA refolding might drive dimpling of the membranes toward each other (Weissenhorn et al., 1997; Kozlov and Chernomordik, 1998). In this case, inverted cone-shaped LPC and cone-shaped oleic acid in the outer membrane monolayers can decrease and increase, respectively, the energy of the stressed membrane dimple developing in the fusion site, thus inhibiting or accelerating dissipation of this energy and dissociation of RH.
Regardless of the mechanism of RH dissociation, the limited lifetime of this intermediate indicates that just the presence of the stable lowest energy HA conformation (Bullough et al., 1994) in the membrane contact zone is not sufficient to mediate membrane merger or even support it when already formed. This conclusion is consistent with the recent finding that a large polypeptide fragment of the stable low pH form of HA2 does not induce lipid mixing between liposomes at neutral pH (Epand et al., 1999). Significant fusogenic activity of the same polypeptide at low pH is apparently mediated by low pH–dependent interactions between the polypeptide trimers (Kim et al., 1998), suggesting that the energy for fusion can come not only from the conformational change of individual HA trimers but also from their subsequent interactions. As soon as this energy temporarily stored either in an intermediate HA conformation or in membranes somehow primed for fusion (e.g., in the form of a strongly curved membrane dimple [Kozlov and Chernomordik, 1998]) dissipates, RH dissociates.
Transient RH can be a key intermediate in the physiologically relevant pathway leading to viral envelope fusion with an endosome membrane at the early stage of viral infection. RH intermediates were detected within 35 s after decreasing the pH, and thus RH formation is as fast as fusion pore opening (Tse et al., 1993; Zimmerberg et al., 1994; Chernomordik et al., 1998). Importantly, fewer low pH–activated HA molecules are required to form RH than to open a fusion pore. The number of activated HA molecules at the virus within the endosome may increase rather slowly, because acidification of the endosome content to pH 5 takes up to 50 min (Murphy et al., 1984; Roederer et al., 1987). Thus, the conditions for RH formation should develop earlier than those for fusion pore opening, suggesting that multiple RH intermediates are present in the membrane contact zone at the time of an opening of the first fusion pore.
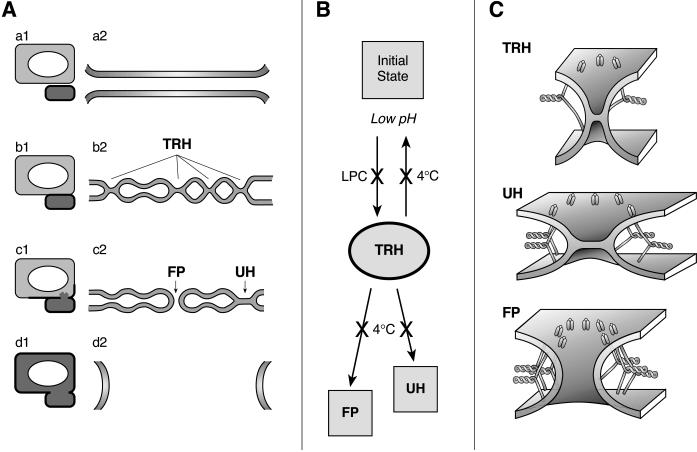
To conclude, our results indicate that after low pH application, the membrane contact zone contains a shifting distribution of multiple membrane merger sites, with their phenotypes dependent on the local surface density of activated HA, membrane lipid composition, and time after low pH application. The emerging pathway is summarized in Figure 8. Because RH formation requires fewer activated HA molecules than UH, and, in particular, complete fusion, we hypothesize that at an early stage most of the merger sites represent the newly identified transient RH intermediates (Figure 8, TRH). Although RH intermediates are stabilized at 4°C (Figure 8B), at physiological temperature most of these intermediates dissociate with time and do not lead to successful fusion events. However, the sites with local density of activated HA, either higher from the beginning or increased because of additional HA molecules coming by lateral motion to an already existing RH site, can evolve to allow lipid and content mixing. If the local density of activated HA molecules is sufficient to achieve UH (Figure 8, Ac2 and C), rearrangements may involve expansion of the hemifusion diaphragm to an HA-independent, LPC-insensitive state. At still higher surface densities of HA, activated HA molecules open a fusion pore (Figure 8, FP) and thus complete the fusion reaction.
Figure 8.
The place of the transient RH intermediates (TRH) in the pathway of the HA-mediated fusion. (A) In the scheme of the fusion site, the top and bottom membranes represent sections of an HA-cell and RBC, respectively. a1 and a2, b1 and b2, c1 and c2, and d1 and d2 illustrate the hypothetical pathway of complete fusion from the initial state (a1 and a2) to the onset of lipid and content mixing (c1 and c2) to fusion completion (d1 and d2), shown at the level of the fusing cells (1) and for the enlarged contact zones (2). (B) Formation of TRH intermediates is blocked in the presence of LPC. In contrast, 4°C stabilizes TRH by blocking both its dissociation and its evolution toward fusion pore (FP) or unrestricted hemifusion (UH). (C) The structures of the proposed fusion intermediates (TRH, UH, and FP) are shown. Each of the low pH–activated HA molecules is depicted as a loaded spring anchored to the membranes by the transmembrane domain pointing up and the fusion peptide pointing down.
ACKNOWLEDGMENTS
We greatly appreciate Dr. Joshua Zimmerberg for his advice and numerous stimulating discussions. We thank Drs. Yuri A. Chizmadzhev, Michael Kozlov, Kamran Melikov, and Gregory Melikyan for many helpful discussions and critical review of the manuscript and Dr. Stephen A. Wharton for the kind gift of the LC89 antibodies.
Abbreviations used:
CELISA
cell surface ELISA
CPZ
chlorpromazine
GPI
glycosylphosphatidylinositol
GPI-HA
HA ectodomain linked to GPI
HA
influenza virus hemagglutinin
HA-cell
HA-expressing cell
HOS
hypotonic osmotic shock
LPC
lysophosphatidylcholine
RH
restricted hemifusion
UH
unrestricted hemifusion
Note added in proof.
We note a work presenting electron micrographs of intermembrane contact sites in HA-mediated cell-cell fusion, which may relate to the transient RH intermediates described here. That work is: Frolov, V.A., Cho, M.-S., Bronk, P., Reese, T.S., and Zimmerberg, J. (2000). Multiple local contact sites are induced by GPI-linked influenza hemagglutinin during hemifusion and flickering pore formation. Traffic. (in press).
REFERENCES
- Benz J. Minimal aggregate size and minimal fusion unit for the first fusion pore of influenza hemagglutinin-mediated membrane fusion. Biophys J. 2000;78:227–245. doi: 10.1016/S0006-3495(00)76587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal R, Pak CC, Raviv Y, Krumbiegel M, Bergelson LD, Morris SJ, Lowy RJ. Transient domains induced by influenza hemagglutinin during membrane fusion. Mol Membr Biol. 1995;12:135–142. doi: 10.3109/09687689509038509. [DOI] [PubMed] [Google Scholar]
- Blumenthal R, Sarkar DP, Durell S, Howard DE, Morris SJ. Dilation of the influenza hemagglutinin fusion pore revealed by the kinetics of individual cell-cell fusion events. J Cell Biol. 1996;135:63–71. doi: 10.1083/jcb.135.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullough PA, Hughson FM, Skehel JJ, Wiley DC. Structure of influenza hemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- Carr CM, Chaudhry C, Kim PS. Influenza hemagglutinin is spring-loaded by a metastable native conformation. Proc Natl Acad Sci USA. 1997;94:14306–14313. doi: 10.1073/pnas.94.26.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CM, Kim PS. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993;73:823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- Chen J, Skehel JJ, Wiley DC. N- and C-terminal. residues combine in the fusion-pH influenza hemagglutinin HA(2) subunit to form an N cap that terminates the triple-stranded coiled coil. Proc Natl Acad Sci USA. 1999;96:8967–8972. doi: 10.1073/pnas.96.16.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L. Non-bilayer lipids and biological fusion intermediates. Chem Phys Lipids. 1996;81:203–213. doi: 10.1016/0009-3084(96)02583-2. [DOI] [PubMed] [Google Scholar]
- Chernomordik L, Kozlov M, Zimmerberg J. Lipids in biological membrane fusion. J Membr Biol. 1995;146:1–14. doi: 10.1007/BF00232676. [DOI] [PubMed] [Google Scholar]
- Chernomordik LV, Frolov VA, Leikina E, Bronk P, Zimmerberg J. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J Cell Biol. 1998;140:1369–1382. doi: 10.1083/jcb.140.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik LV, Leikina E, Frolov V, Bronk P, Zimmerberg J. An early stage of membrane fusion mediated by the low pH conformation of influenza hemagglutinin depends upon membrane lipids. J Cell Biol. 1997;136:81–94. doi: 10.1083/jcb.136.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague MJ, Schoch C, Blumenthal R. Delay time for influenza virus hemagglutinin-induced membrane fusion depends on hemagglutinin surface density. J Virol. 1991;65:2402–2407. doi: 10.1128/jvi.65.5.2402-2407.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danieli T, Pelletier SL, Henis YI, White JM. Membrane fusion mediated by the influenza virus hemagglutinin requires the concerted action of at least three hemagglutinin trimers. J Cell Biol. 1996;133:559–569. doi: 10.1083/jcb.133.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RS, Douglas AR, Skehel JJ, Wiley DC. Analyses of the antigenicity of influenza hemagglutinin at the pH optimum for virus-mediated membrane fusion. J Gen Virol. 1983;64:1657–1662. doi: 10.1099/0022-1317-64-8-1657. [DOI] [PubMed] [Google Scholar]
- Doxsey SJ, Sambrook J, Helenius A, White J. An efficient method for introducing macromolecules into living cells. J Cell Biol. 1985;101:19–27. doi: 10.1083/jcb.101.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epand RF, Macosko JC, Russell CJ, Shin YK, Epand RM. The ectodomain of HA2 of influenza virus promotes rapid pH dependent membrane fusion. J Mol Biol. 1999;286:489–503. doi: 10.1006/jmbi.1998.2500. [DOI] [PubMed] [Google Scholar]
- Gaudin Y, Ruigrok RWH, Brunner J. Low-pH induced conformational changes in viral fusion proteins: implications for the fusion mechanism. J Gen Virol. 1995;76:1541–1556. doi: 10.1099/0022-1317-76-7-1541. [DOI] [PubMed] [Google Scholar]
- Gray C, Tamm LK. pH-induced conformational changes of membrane-bound influenza hemagglutinin and its effect on target lipid bilayers. Protein Sci. 1998;7:2359–2373. doi: 10.1002/pro.5560071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther-Ausborn S, Schoen P, Bartoldus I, Wilschut J, Stegmann T. Role of hemagglutinin surface density in the initial stages of influenza virus fusion: lack of evidence for cooperativity. J Virol. 2000;74:2714–2720. doi: 10.1128/jvi.74.6.2714-2720.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junankar PR, Cherry RJ. Temperature and pH dependence of the hemolytic activity of influenza virus and of the rotational mobility of the spike glycoproteins. Biochim Biophys Acta. 1986;854:198–206. doi: 10.1016/0005-2736(86)90111-2. [DOI] [PubMed] [Google Scholar]
- Kemble GW, Danieli T, White JM. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell. 1994;76:383–391. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- Kemble GW, Henis YI, White JM. GPI- and transmembrane-anchored influenza hemagglutinin differ in structure and receptor binding activity. J Cell Biol. 1993;122:1253–1265. doi: 10.1083/jcb.122.6.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Macosko JC, Shin YK. The mechanism for low-pH-induced clustering of phospholipid vesicles carrying the HA2 ectodomain of influenza hemagglutinin. Biochemistry. 1998;37:137–144. doi: 10.1021/bi971982w. [DOI] [PubMed] [Google Scholar]
- Korte T, Ludwig K, Booy FP, Blumenthal R, Herrmann A. Conformational intermediates and fusion activity of influenza virus hemagglutinin. J Virol. 1999;73:4567–4574. doi: 10.1128/jvi.73.6.4567-4574.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov MM, Chernomordik LV. A mechanism of protein-mediated fusion: coupling between refolding of the influenza hemagglutinin and lipid rearrangements. Biophys J. 1998;75:1384–1396. doi: 10.1016/S0006-3495(98)74056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbiegel M, Herrmann A, Blumenthal R. Kinetics of the low pH-induced conformational changes and fusogenic activity of influenza hemagglutinin. Biophys J. 1994;67:2355–2360. doi: 10.1016/S0006-3495(94)80721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikyan GB, Brener SA, Ok DC, Cohen FS. Inner but not outer membrane leaflets control the transition from glycosylphosphatidylinositol-anchored influenza hemagglutinin-induced hemifusion to full fusion. J Cell Biol. 1997;136:995–1005. doi: 10.1083/jcb.136.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikyan GB, Lin S, Roth MG, Cohen FS. Amino acid sequence requirements of the transmembrane and cytoplasmic domains of influenza virus hemagglutinin for viable membrane fusion. Mol Biol Cell. 1999;10:1821–1836. doi: 10.1091/mbc.10.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikyan GB, Niles WD, Peeples ME, Cohen FS. Influenza hemagglutinin-mediated fusion pores connecting cells to planar membranes: flickering to final expansion. J Gen Physiol. 1993;102:1131–1149. doi: 10.1085/jgp.102.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikyan GB, Niles WD, Ratinov VA, Karhanek M, Zimmerberg J, Cohen FS. Comparison of transient and successful fusion pores connecting influenza hemagglutinin expressing cells to planar membranes. J Gen Physiol. 1995a;106:803–819. doi: 10.1085/jgp.106.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikyan GB, White JM, Cohen FS. GPI-anchored influenza hemagglutinin induces hemifusion to both red blood cell and planar bilayer membranes. J Cell Biol. 1995b;131:679–691. doi: 10.1083/jcb.131.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RF, Powers S, Cantor CR. Endosome pH measured in single cells by dual fluorescence flow cytometry: rapid acidification of insulin to pH 6. J Cell Biol. 1984;98:1757–1762. doi: 10.1083/jcb.98.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer M, Bowser R, Murphy RF. Kinetics and temperature dependence of exposure of endocytosed material to proteolytic enzymes and low pH: evidence for a maturation model for the formation of lysosomes. J Cell Physiol. 1987;131:200–209. doi: 10.1002/jcp.1041310209. [DOI] [PubMed] [Google Scholar]
- Schoch C, Blumenthal R, Clague MJ. A long-lived state for influenza virus-erythrocyte complexes committed to fusion at neutral pH. FEBS Lett. 1992;311:221–225. doi: 10.1016/0014-5793(92)81107-w. [DOI] [PubMed] [Google Scholar]
- Stegmann T, White JM, Helenius A. Intermediates in influenza induced membrane fusion. EMBO J. 1990;9:4231–4241. doi: 10.1002/j.1460-2075.1990.tb07871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse FW, Iwata A, Almers W. Membrane flux through the pore formed by a fusogenic viral envelope protein during cell fusion. J Cell Biol. 1993;121:543–552. doi: 10.1083/jcb.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- White J. Membrane fusion: the influenza paradigm. Cold Spring Harbor Symp. 1996;60:581–588. doi: 10.1101/sqb.1995.060.01.062. [DOI] [PubMed] [Google Scholar]
- White J, Matlin K, Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981;89:674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM, Wilson IA. Anti-peptide antibodies detect steps in a protein conformational change: low-pH activation of the influenza virus hemagglutinin. J Cell Biol. 1987;105:2887–2896. doi: 10.1083/jcb.105.6.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley DC, Skehel JJ. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J, Blumenthal R, Sarkar DP, Curran M, Morris SJ. Restricted movement of lipid and aqueous dyes through pores formed by influenza hemagglutinin during cell fusion. J Cell Biol. 1994;127:1885–1894. doi: 10.1083/jcb.127.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]