Regulation of Membrane Type-1 Matrix Metalloproteinase Activation by Proprotein Convertases (original) (raw)
Abstract
Membrane type-1 matrix metalloproteinase (MT1-MMP) is the prototypical member of a subgroup of membrane-anchored proteinases that belong to the matrix metalloproteinase family. Although synthesized as a zymogen, MT1-MMP plays an essential role in extracellular matrix remodeling after an undefined process that unmasks its catalytic domain. We now report the existence of a proprotein convertase–MT1-MMP axis that regulates the processing and functional activity of the metalloproteinase. Two sets of basic motifs in the propeptide region of MT1-MMP are identified that potentially can be recognized by the proprotein convertase family of subtilisin-like proteases. Processing of proMT1-MMP as well as the expression of its proteolytic activity were blocked by mutating these recognition motifs or by inhibiting the proprotein convertases furin and PC6 with the serpin-based inhibitor α1 antitrypsin Portland. Furthermore, both furin-dependent and furin-independent MT1-MMP processing pathways are identified that require tethering of the metalloproteinase to the cell surface. These findings demonstrate the existence of a proprotein convertase–MT1-MMP axis that can regulate extracellular matrix remodeling.
INTRODUCTION
Membrane type-1 matrix metalloproteinase (MT1-MMP) is a member of a family of membrane-anchored matrix metalloproteinases (MMPs) implicated in tissue-remodeling events that range from tumor invasion and angiogenesis to growth and development (Sato et al., 1994; Hiraoka et al., 1998; Belien et al., 1999; Holmbeck et al., 1999; Zhou et al., 2000). With the recent generation of MT1-MMP–deficient mice, it is now clear that this protease plays an essential role in the formation and maintenance of skeletal tissues (Holmbeck et al., 1999; Zhou et al., 2000). Current evidence indicates that MT1-MMP regulates matrix turnover by means of its ability to degrade matrix-associated molecules either directly or via the activation of downstream MMPs (Pei and Weiss, 1996; Ohuchi et al., 1997; Nagase and Woessner, 1999). Like all of the other known members of the human MMP gene family, MT1-MMP is synthesized as a zymogen that can be processed to its mature, catalytically active form after the removal of the regulatory propeptide domain (Nagase and Woessner, 1999). However, the mechanisms responsible for the processing of proMT1-MMP to its mature form have remained controversial, and the zymogen itself has been reported to express enzymic activity (Cao et al., 1996, 1998; Zucker et al., 1998).
Recently, the secreted MMP stromelysin-3 was shown to undergo intracellular activation after the proteolytic removal of its propeptide domain by furin, a member of the proprotein convertase family (Pei and Weiss, 1995). Activation of the prostromelysin-3 zymogen was controlled by a decapeptide insert located immediately upstream of the N terminus of the catalytically active enzyme that encodes a proprotein convertase recognition motif, RXRXKR (where R = Arg, K = Lys, and X = a nonbasic amino acid) (Pei and Weiss, 1995). Interestingly, MT1-MMP contains a similar 108RRKR motif upstream of the N terminus of its mature form (Sato et al., 1994; Nagase and Woessner, 1999). Because RX(K/R)R sequences can act as general recognition motifs for at least four members of the proprotein convertase family, i.e., furin, PACE4, PC6, and PC7 (Nakayama, 1997; Zhou et al., 1999), the MT1-MMP zymogen could present itself as a substrate for these processing enzymes.
In this report, we demonstrate that processing and activation of full-length MT1-MMP are controlled by proprotein convertases that recognize one of two potential recognition motifs in the enzyme's prodomain. Although a furin-dependent pathway efficiently processed membrane-anchored proMT1-MMP, a furin-independent route was also identified that required the MMP zymogen to be tethered to the cell surface. Importantly, inhibition of proMT1-MMP processing completely blocked the ability of MT1-MMP–expressing cells to display proteolytic activity. We conclude that cooperative interactions between proprotein convertases and membrane-anchored MMPs play an important role in regulating the remodeling of the extracellular matrix.
MATERIALS AND METHODS
Cell Culture
COS-1, HT-1080, and LoVo cells (all obtained from the American Type Culture Collection, Rockville, MD) were maintained in MEM (Life Technologies, Grand Island, NY), DMEM (Life Technologies), and F-12 nutrient mixture (Life Technologies), respectively. CHO-K1 and RPE.40 cells (kindly provided by T. Moehring, University of Vermont, Burlington, VT) were maintained in RPMI 1640 (Life Technologies). All media were supplemented with 10% FBS (Hyclone, Logan, UT), 4 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Plasmid Constructs and Transfection
Full-length MT1-MMP (Met1 to Val582), a soluble transmembrane deletion mutant of MT1-MMP (ΔMT1-MMP; Met1 to Ser538), and a cytosolic-domain deletion mutant of MT1-MMP (Met1 to Phe563) were generated as described (Pei and Weiss, 1996; Hiraoka et al., 1998) and inserted in the PCR3.1-uni expression vector (Invitrogen, Carlsbad, CA). Mutagenic primers for substitutions in MT1-MMP at Arg89, Arg108, Arg198, Lys110, and Arg111 or in ΔMT1-MMP at Glu240 were used as follows (nucleotides in boldface indicate the altered codons): 5′-ATC AAG GCC AAT GTT GCG GCC GCT GCC TAC GCC ATC TAG GGT-3′ and 5′-CTG GTG GCT GTG CAC GCT CTG GGC CAT GCC CTG-3′ to generate MT1-MMP Arg108-Arg-Lys-Arg → Ala-Ala-Ala-Ala and MT1-MMP Glu240 → Ala, respectively, with the Muta-Gene phagemid in vitro mutagenesis kit (version 2, Bio-Rad, Richmond, CA). The mutagenic primers 5′-G GCG CGC CCC CGA TGT GGT GTT CCA GAC A-3′ and 5′-GG AAC ACC ACA TCG GGG GCG GCG CAT GGC CTT CA-3′ were used in a sequential PCR-based method to generate MT1-MMP Arg89 → Ala. Primers encoding the FLAG epitope (5′-GAC TAC AAG GAC GAC GAT GAC AAG-3′) were inserted either between Phe34 and Ser35 in the MT1-MMP prodomain or after Val582 at the C terminus of MT1-MMP by PCR-based methods (see Figures 1 and 2 for schemes). Each MT1-MMP construct was cloned into PCR3.1-uni and characterized by sequencing. MT1-MMP expression vectors encoding both a FLAG epitope inserted between Arg-Arg-Lys-Arg111 and Tyr112 in the wild-type enzyme or between Ala-Ala-Ala-Ala111 and Tyr112 in the MT1-MMP mutant and a chimeric mutant wherein the transmembrane domain and cytosolic tail were replaced with that of the interleukin 2 receptor α-chain were provided by M. Seiki and Y. Itoh (University of Tokyo) (Nakahara et al., 1997). Expression vectors for α1 antitrypsin Portland (α1PDX), full-length furin, and soluble furin were constructed from cDNAs provided by G. Thomas (Oregon Health Sciences University, Portland, OR), A. Rehemtulla (University of Michigan), and R. Kaufman (University of Michigan, Ann Arbor, MI), respectively (Wesley et al., 1993; Jean et al., 1998). Cells were transfected with purified plasmid DNA by LipofectAMINE treatment (Life Technologies) as described (Pei and Weiss, 1996). After transfection, cells were incubated either alone or with the synthetic inhibitor BB-94 (5 μM final; British Biotechnology, Oxford, UK) (Botos et al., 1996), progelatinase A (see below), or soluble furin.
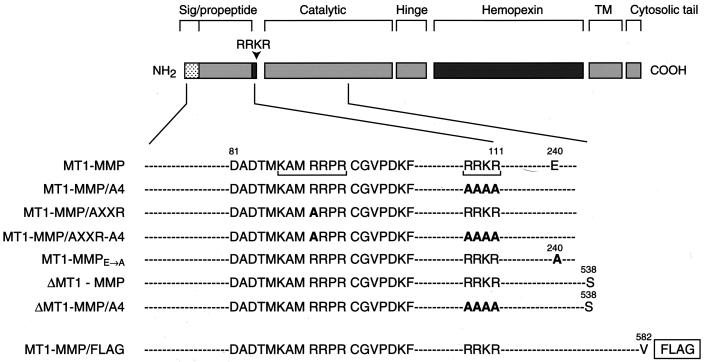
Figure 1.
A scheme of MT1-MMP mutants. MT1-MMP domains are depicted as shaded boxes. Amino acid substitutions were inserted either into the 108RRKR and 86KAMRRPR domains (each bracketed) alone as 108RRKR → AAAA (MT1-MMP/A4) or 86KAMRRPR → KAMARPR (MT1-MMP/AXXR) or into both domains as an MT1-MMP/AXXR-A4 mutant. Additional mutations included a 240E → A substitution in the catalytic domain to create an enzymically inactive mutant of MT1-MMP, a soluble transmembrane-deletion mutant with the C terminus truncated at S538 (ΔMT1-MMP), a soluble mutant with an RRKR → AAAA substitution (ΔMT1-MMP/A4), or an epitope-tagged variant of MT1-MMP with FLAG inserted at the C terminus of the wild-type proteinase (MT1-MMP/FLAG).
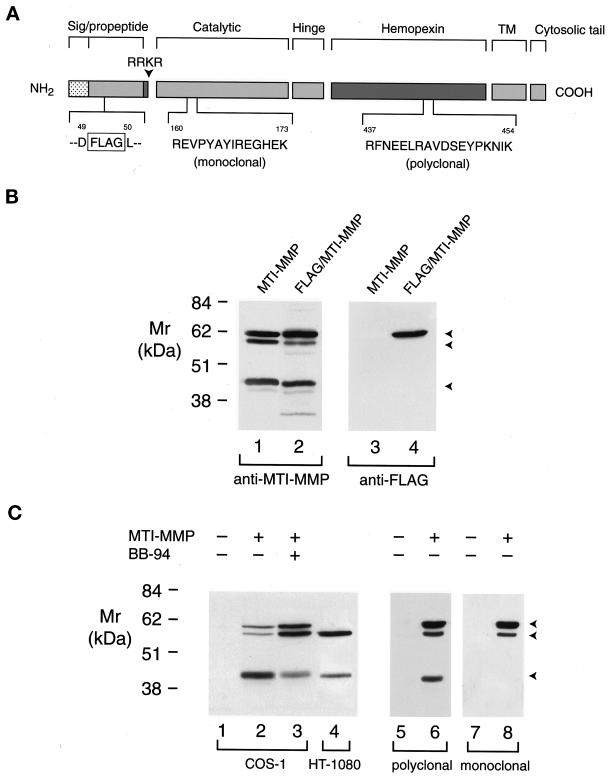
Figure 2.
Processing of proMT1-MMP in COS-1 and HT-1080 cells. (A) A scheme of MT1-MMP epitopes. Wild-type MT1-MMP and MT1-MMP containing a FLAG epitope inserted in the prodomain were detected with antibodies directed against a 14-amino acid residue (R160 to K173) in the catalytic domain, an 18-amino acid residue (R437 to K454) in the hemopexin domain, or a FLAG epitope inserted between residues D49 and L50 in the prodomain. (B) Western blot analysis of FLAG/MT1-MMP expression in COS-1 cells. COS-1 cells were transiently transfected with MT1-MMP or FLAG/MT1-MMP expression vectors. Triton X-114 extracts were then analyzed by immunoblotting with either hemopexin domain–specific polyclonal antisera (lanes 1 and 2) or anti-FLAG mAb (lanes 3 and 4). Although anti-MT1-MMP polyclonal antisera recognized both wild-type MT1-MMP (lane 1) and FLAG/MT1-MMP (lane 2), the anti-FLAG mAb recognized the ∼63-kDa pro form of FLAG/MT1-MMP alone (lane 4). The anti-FLAG mAb did not react with wild-type MT1-MMP (lane 3). (C) Western blot analysis of MT1-MMP in transiently transfected COS-1 cells or HT-1080 cells. Triton X-114 extracts of COS-1 cells transfected with control (lane 1) or wild-type MT1-MMP expression vectors and incubated in the absence or presence of 5 μM BB-94 (lanes 2 and 3, respectively) were compared with extracts of HT-1080 cells (lane 4) by immunoblotting with MT1-MMP hemopexin domain–specific polyclonal antisera. The three arrowheads indicate the positions of the putative ∼63-kDa pro form, ∼60-kDa mature form, and ∼45-kDa truncated form of MT1-MMP. COS-1 cell extracts pre-pared from cells transiently transfected with control (lanes 5 and 7) or MT1-MMP (lanes 6 and 8) expression vectors were analyzed by immunoblotting in a tandem manner with hemopexin domain–specific polyclonal antisera or anti-catalytic domain mAb, respectively.
Western Blotting and Zymography
Hydrophobic cell-associated proteins were solubilized and extracted into Triton X-114 (Sigma Chemical, St. Louis, MO) in the presence of a cocktail of proteinase inhibitors as described (Toth et al., 1997). Detergent extracts (normalized to equivalent cell numbers) were resolved by 10% PAGE under reducing conditions (Pei and Weiss, 1996). For Western blotting, proteins were transferred to nitrocellulose and probed with anti-MT1-MMP or anti-FLAG antibodies. MT1-MMP was immunodetected with one of the following: 1) a rabbit polyclonal antibody raised against a synthetic peptide in the hemopexin domain of MT1-MMP (Figure 2A; prepared at Research Genetics, Huntsville, AL) (Toth et al., 1997); 2) a mouse mAb directed against a synthetic peptide in the catalytic domain of MT1-MMP (Figure 2A; clone 114-2F2, Ab-1; Calbiochem, La Jolla, CA); or 3) a mouse mAb directed against a FLAG epitope inserted into either the MT1-MMP prodomain or the C terminus (Figures 1 and 2A; Eastman Kodak, Rochester, NY). After incubation of blots with the appropriate HRP-conjugated secondary antibody, the immune complexes were detected with the ECL system (Pierce, Rockford, IL). Densitometric analysis of ECL-developed immunoblots was performed with a Nucleovision Imaging workstation (Nucleotech, San Carlos, CA). The percentage processing of proMT1-MMP to mature MT1-MMP was calculated (in underexposed gels) with the use of the GelExport software (Nucleotech, San Carlos, CA).
Progelatinase A processing was assessed by gelatin zymography as described (Pei and Weiss, 1996). Cells were incubated with human progelatinase A (generated from COS-1 cells transfected with a progelatinase A expression vector) under serum-free conditions for 24 h at 37°C. Gelatin zymography of conditioned media was performed on 10% polyacrylamide gels that were cast in the presence of 2 mg/ml gelatin (Sigma Chemical).
Cell Surface Biotinylation
Cell monolayers were washed with ice-cold PBS (pH 8.0) and incubated with 0.5 mg/ml sulfo-NHS-biotin (Pierce) at 15°C for 40 min as described (Lehti et al., 1998). Cell lysates were incubated with strepavidin–Sepharose beads (Pierce) for 1 h at 4°C, and the complexes were resolved under reducing conditions by SDS-PAGE (10%). The captured biotinylated proteins were then transferred to nitrocellulose, probed with anti-MT1-MMP hemopexin domain–specific polyclonal antisera, and visualized with the use of the ECL system.
Immunofluorescence
After transfection with FLAG-tagged MT1-MMP expression vectors, CHO-K1 or RPE.40 cells were fixed with 3% paraformaldehyde, blocked with 5% goat serum and 3% BSA in Tris-buffered saline for 1 h at 25°C, and then reacted with either anti-FLAG M1 mAb (10 μg/ml; Sigma-Aldrich) in the presence of 1 mM CaCl2 as described (Itoh et al., 1999) or an anti-MT1-MMP mAb (3H7; Chenard et al., 1999) for 2 h at 25°C. Antibody-coated cells were visualized with Texas red–conjugated anti-mouse immunoglobulin G (Vector Laboratories, Burlingame, CA) by confocal microscopy with the use of a Bio-Rad MRC 600 laser scanning microscope with CoMos version 7.0a software (BioRad, Hercules, CA). In each micrograph, three sequential images were collected and combined in a Z series with a final resolution of ∼6 μm.
Subjacent Proteolysis
The ability of MT1-MMP–transfected cells to express subjacent proteolytic activity was determined by a modification of previously published protocols (Rice and Weiss, 1990; d'Ortho et al., 1998). In brief, gelatin (Sigma) was labeled with Texas red (Molecular Probes, Eugene, OR). Labtek slides (ICN Biochemicals, Costa Mesa, CA) were then coated with poly-l-lysine (Sigma) and incubated with gelatin for 2 h at 25°C. Gelatin and poly-l-lysine were then cross-linked with glutaraldehyde and unreacted aldehyde groups blocked with ammonium chloride. Wild-type and transfected cells were then seeded atop the gelatin-coated slides in 10% FBS for 16 h before analysis by phase-contrast or confocal laser microscopy.
Pulse-Chase Analysis
HT-1080 cells were treated with concanavalin A (20 μM) under serum-free conditions for 16 h. The monolayers were incubated in methionine-free DMEM that was supplemented with 500 μCi of [35S]methionine for 15 min at 37°C and chased as described (Toth et al., 1997). Equivalent cell lysates were then incubated with anti-MT1-MMP hemopexin domain–specific polyclonal antisera for 16 h at 4°C, followed by incubation with protein A–Sepharose beads (Pierce) for 5 h at 4°C. The beads were harvested by centrifugation, the reduced samples were resolved by 10% SDS-PAGE, and the radiolabeled proteins were visualized by autoradiography.
RESULTS
MT1-MMP Processing in COS-1 Cells
COS-1 cells transfected with MT1-MMP cDNA have been reported to generate a single immunoreactive product tentatively identified as the pro form of the enzyme (Sato et al., 1994; Cao et al., 1996, 1998; Zucker et al., 1998). However, after transient transfection with wild-type MT1-MMP cDNA, the hemopexin domain–specific polyclonal antisera detected three immunoreactive bands at ∼63, 60, and 45 kDa (Figure 2, A and B). To determine whether the 63-kDa band detected in MT1-MMP–transfected COS-1 cells is the pro form of the enzyme, a mutant MT1-MMP molecule was engineered with a FLAG epitope inserted in its prodomain (Figure 2A). As shown, both the wild-type and epitope-tagged forms of MT1-MMP were processed similarly in COS-1 cells (as determined with anti-MT1-MMP polyclonal antisera; Figure 2B). However, when extracts from epitope-tagged MT1-MMP COS cells were developed with the anti-FLAG mAb, only the 63-kDa species was detected (Figure 2B, lane 4).
With the identification of the ∼63-kDa species as the MT1-MMP zymogen, the ∼60- and 45-kDa forms of MT1-MMP were noted to comigrate with those identified in HT-1080 cells (Figure 2C, lanes 2 and 4), a cell line previously shown to process proMT1-MMP to both an ∼60-kDa mature form and an ∼45-kDa truncation product (Lohi et al., 1996; Lehti et al., 1998; Stanton et al., 1998). Consistent with this interpretation, a mAb directed at the catalytic domain of MT1-MMP identified the ∼63- and 60-kDa forms of the enzyme, but not the 45-kDa fragment (Figure 2C, lanes 5–8). Because the formation of the 45-kDa truncation product in COS-1 cells may arise from the autolytic cleavage of the mature ∼60-kDa enzyme (Stanton et al., 1998), MT1-MMP–transfected cells were incubated with the broad-spectrum MMP inhibitor BB-94. Under these conditions, the accumulation of the 45-kDa species decreased coincident with the accumulation of the 63- and 60-kDa products (Figure 2C, compare lanes 2 and 3).
MT1-MMP Processing Is Regulated by a Pair of Tetrabasic Motifs in the Prodomain
Given that MT1-MMP–transfected COS-1 cells process the zymogen to its prodomain-deleted form, we assessed the role of the tetrabasic motif, 108RRKR, which is positioned immediately upstream of the N terminus of the active enzyme. As described (Pei and Weiss, 1996), transmembrane-deleted MT1-MMP (i.e., ΔMT1-MMP) was processed efficiently to its mature form (see Figure 1 for schemes of mutants and Figure 3A, lanes 1–3), whereas processing was eliminated when the 108RRKR motif was mutated to 108AAAA (i.e., ΔMT1-MMP/A4). In contrast, a comparison of the processing of full-length MT1-MMP and MT1-MMP/A4 demonstrated that the 108RRKR → 108AAAA mutant continued to undergo partial processing (Figure 3A, compare lanes 6 and 7). In three paired experiments, the formation of mature MT1-MMP was inhibited by 83 ± 12% (mean ± SD) when cells were transfected with MT1-MMP/A4.
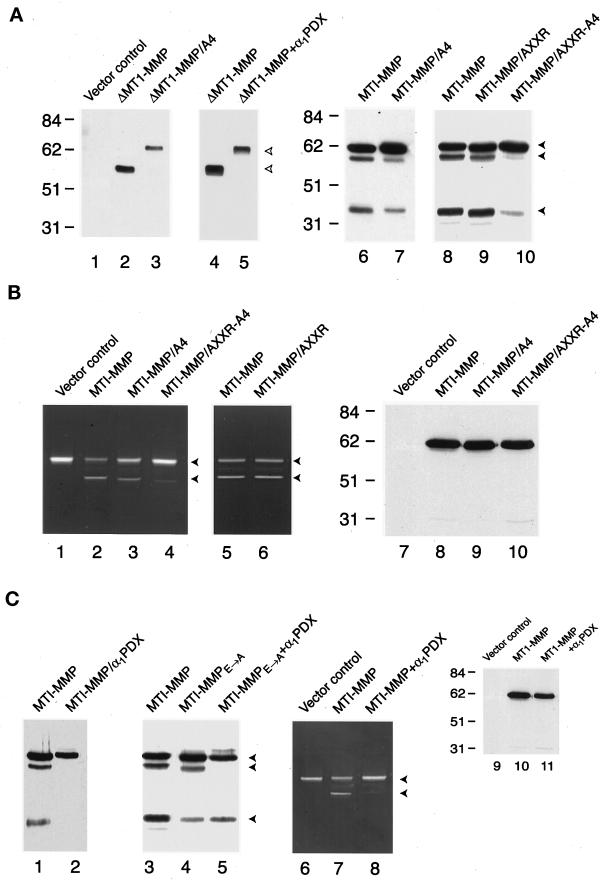
Figure 3.
Processing of membrane-anchored and soluble forms of MT1-MMP. (A) Western blot analysis of MT1-MMP variants harboring mutations in the 108RRKR and/or 86KAMRRPR basic motifs. COS-1 cells were transfected with control (lane 1), ΔMT1-MMP (lanes 2–5), or full-length MT1-MMP (lanes 6–10) expression vectors, and cell-free supernatants (lanes 1–5) or Triton X-114 extracts (lanes 6–10) were analyzed by Western blot analysis with hemopexin domain–specific polyclonal antisera. In lanes 2 and 3, cell-free supernatants of COS-1 cells expressing ΔMT1-MMP or ΔMT1-MMP/A4 were compared, whereas in lanes 4 and 5, the ability of α1PDX to inhibit ΔMT1-MMP processing was assessed. The upper bands in lanes 3 and 5 (marked with open arrowheads) are the pro forms of ΔMT1-MMP. In the case of full-length MT1-MMP (lanes 6–10), COS-1 cells expressing the MT1-MMP/A4 mutant (lane 7) or the MT1-MMP/AXXR-A4 mutant (lane 10) displayed a diminished ability to process the proenzyme relative to cells expressing wild-type MT1-MMP (lanes 6 or 8). The MT1-MMP/AXXR mutant (lane 9) was processed comparably to the MT1-MMP control (lane 8). The closed arrowheads mark the positions of pro, mature, and truncated MT1-MMP. (B) Progelatinase A activation and surface display of MT1-MMP in transiently transfected COS-1 cells. For zymography, COS-1 cells were transiently transfected with control (lane 1), MT1-MMP (lanes 2 and 5), MT1-MMP/A4 (lane 3), MT1-MMP/AXXR-A4 (lane 4), or MT1-MMP/AXXR (lane 6) expression vectors. The COS-1 cells were subsequently incubated with progelatinase A for 16 h, and processing was assessed by zymography (lanes 1–6). The upper and lower arrowheads mark the positions of the pro and fully processed forms of gelatinase A, respectively. In lanes 7–10, control-, MT1-MMP–, or MT1-MMP mutant–transfected COS-1 cells were surface biotinyl-ated, and Triton X-114 extracts were immunoblotted with hemopexin domain–specific polyclonal antisera. (C) Effect of α1PDX on MT1-MMP processing and activity. COS-1 cells were cotransfected with wild-type MT1-MMP and a control expression vector (lanes 1 and 3), MT1-MMPE → A and a control expression vector (lane 4), wild-type MT1-MMP and α1PDX (lane 2), or MT1-MMPE → A and α1PDX (lane 5) expression vectors, and Triton X-114 extracts were analyzed by immunoblotting. Progelatinase A activation was monitored by zymography after incubation with COS-1 cells transfected with the control expression vector (lane 6), with MT1-MMP and a control expression vector (lane 7), or with MT1-MMP and α1PDX expression vectors (lane 8). Cell surface biotinylation followed by Western blot analysis demonstrated that trafficking of proMT1-MMP (lane 10) was not affected by the α1PDX expression vector (lane 11).
The ability of COS-1 cells to process proMT1-MMP in the absence of the RRKR motif indicated that alternative cleavage sites exist in the prodomain. Indeed, although previously unrecognized, an overlapping “triplet” of minimal proprotein convertase recognition motifs is embedded in the 86KAMRRPR peptide as 86KXXR, 89RXXR, or 89RR (Figure 1) (Zhou et al., 1999). To simultaneously eliminate all three sites, an R89 → A substitution was inserted in either wild-type MT1-MMP (i.e., MT1-MMP/AXXR) or MT1-MMP/A4 (i.e., MT1-MMP/AXXR-A4). Significantly, whereas the MT1-MMP/AXXR mutant was processed normally, the double mutant (i.e., MT1-MMP/AXXR-A4) remained almost entirely in its unprocessed form (Figure 3A, lanes 8–10).
To determine the impact of blocking MT1-MMP processing on enzymic activity, COS-1 cells were transfected with the wild-type proteinase or mutants bearing basic motif substitutions and incubated with progelatinase A as a target substrate (Sato et al., 1994; Pei and Weiss, 1996). As expected, MT1-MMP–transfected COS-1 cells processed the progelatinase A zymogen to its mature form (Figure 3B, lanes 1 and 2). Under identical conditions, COS-1 cells transfected with MT1-MMP/A4 also processed progelatinase A, but less efficiently than the wild-type enzyme, as reflected in the consistent detection of higher gelatinolytic activity in the pro form band (Figure 3B, lanes 2 and 3). However, the ability of the AXXR-A4 mutant to process progelatinase A was almost completely inhibited, whereas the AXXR mutant functioned normally (Figure 3B, lanes 4–6). The inability of MT1-MMP/AXXR-A4–transfected COS-1 to process progelatinase A could not be attributed to altered trafficking of the mutant zymogen to the cell surface because extracellular biotinylation demonstrated an identical presentation of each MT1-MMP construct (Figure 3B, lanes 7–10). Although the mature form of wild-type MT1-MMP cannot be detected by cell surface biotinylation (the 111-amino acid propeptide contains a total of 13 Arg and/or Lys residues; Lehti et al., 1998), these data demonstrate that surface-displayed MT1-MMP/AXXR-A4 neither undergoes efficient processing nor displays catalytic activity.
α1PDX Blocks ProMT1-MMP Processing
Four members of the proprotein convertase family, i.e., furin, PACE4, PC6, and PC7, have been implicated in the processing of target molecules in the constitutive secretory pathway (Zhou et al., 1999). α1PDX is an engineered mutant of α1 proteinase inhibitor in which the active site loop has been altered to display an Arg-X-X-Arg motif that acts specifically as a bait region for the proprotein convertases furin and PC6 (Benjannet et al., 1997; Cui et al., 1998; Jean et al., 1998). To determine if these α1PDX-sensitive convertases participate in MT1-MMP maturation, processing was examined in COS-1 cells cotransfected with MT1-MMP. As shown in Figure 3, α1PDX inhibited processing of either ΔMT1-MMP or full-length MT1-MMP (Figure 3, A, lanes 4 and 5, and C, lanes 1 and 2). Similar results were obtained when the processing of a catalytically inactive form of MT1-MMP (i.e., MT1-MMPE→A) was examined in the absence or presence of α1PDX (Figure 3C, lanes 3–5). Finally, COS-1 cells cotransfected with MT1-MMP and α1PDX cDNAs were unable to process progelatinase A (Figure 3C, lanes 6–8) despite normal trafficking of proMT1-MMP to the cell surface (Figure 3C, lanes 9–11).
MT1-MMP Processing and Activity in Furin-deficient Cell Lines
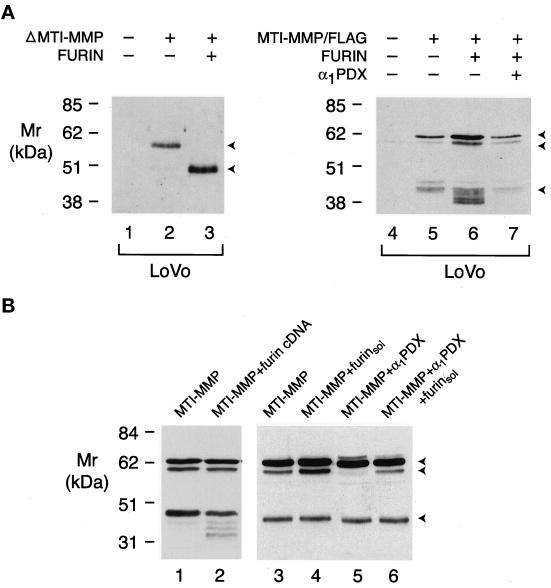
To determine if furin, a ubiquitously expressed proprotein convertase, plays a predominant role in the α1PDX-sensitive maturation of proMT1-MMP, the processing of the proteinase was examined in two furin-deficient cell lines, LoVo and RPE.40 (Inocencio et al., 1997; Zhou et al., 1999). Significantly, LoVo was unable to process soluble ΔMT1-MMP unless cotransfected with the furin cDNA (Figure 4A, lanes 1–3). To determine the ability of LoVo cells to process full-length MT1-MMP, the cells were transiently transfected with a C-terminal domain, epitope-tagged zymogen (i.e., MT1-MMP/FLAG), because LoVo cell extracts contained an immunoreactive product consistent with proMT1-MMP (our unpublished results). As observed for soluble MT1-MMP, full-length MT1-MMP was inefficiently processed unless the cells were cotransfected with a furin cDNA (Figure 4A, lanes 4–6). The ability of furin to mediate MT1-MMP processing was reversed when LoVo cells were cotransfected with the furin and α1PDX cDNAs (Figure 4A, compare lanes 6 and 7). Although furin was unable to enhance MT1-MMP processing in cotransfected COS-1 cells (Figure 4B, compare lanes 1 and 2), proMT1-MMP displayed on the cell surface could be cleaved after exposure to soluble furin (Figure 4B, lanes 3–6).
Figure 4.
Furin-dependent processing of MT1-MMP in LoVo and COS-1 cells. (A) Furin-dependent processing in LoVo cells. LoVo cells were transfected with a control expression vector alone (lane 1), ΔMT1-MMP and a control expression vector (lane 2), or ΔMT1-MMP and furin expression vectors (lane 3). The cell-free supernatants were then examined for soluble forms of ΔMT1-MMP by Western blotting. The two arrowheads to the right of lane 3 mark the positions of proΔMT1-MMP and processed ΔMT1-MMP. In lanes 4–7, LoVo cells were transfected with a control expression vector (lane 4), epitope-tagged MT1-MMP and a control expression vector (lane 5), epitope-tagged MT1-MMP and a furin expression vector (lane 6), or epitope-tagged MT1-MMP, furin, and α1PDX expression vectors (lane 7). Immunoblots were performed on Triton X-114 extracts as described above. (B) Furin-dependent processing of proMT1-MMP by soluble furin. COS-1 cells cotransfected with MT1-MMP and either a control expression vector (lane 1) or a furin expression vector (lane 2) displayed similar profiles of MT1-MMP products as assessed by immunoblotting. Processing of MT1-MMP in COS-1 cells (lane 3) or α1PDX-transfected cells (lane 5) was enhanced by the addition of soluble furin to the serum-free culture medium (lanes 4 and 6, respectively).
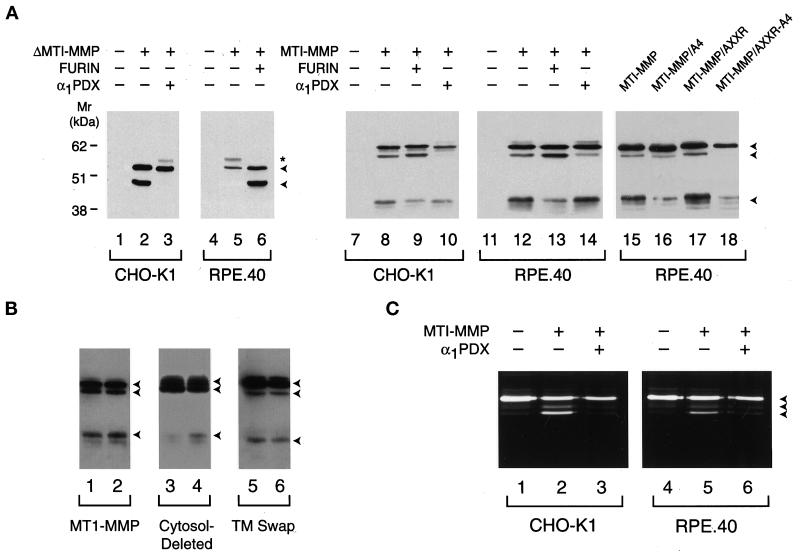
Furin-deficient LoVo cells were unable to efficiently process wild-type MT1-MMP, but a role for alternative convertases would depend on the repertoire of processing enzymes expressed by the host cell. Consequently, MT1-MMP processing was also examined in the wild-type cell line CHO-K1 and its furin-deficient strain RPE.40 (Inocencio et al., 1997) (Figure 5). As expected, both soluble ΔMT1-MMP (Figure 5A, lanes 1–3) and wild-type MT1-MMP (lanes 7–10) were processed to their mature forms via an α1PDX-inhibitable process in CHO-K1 cells. Furthermore, furin-deficient RPE.40 cells were unable to process ΔMT1-MMP unless cotransfected with a furin cDNA (Figure 5A, lanes 4–6). However, in contrast to LoVo cells, RPE.40 cells processed full-length MT1-MMP as efficiently as the furin-sufficient CHO-K1 cell line (Figure 5A, compare lanes 8 and 12). In three paired experiments, MT1-MMP processing in RPE.40 cells was 82 ± 6% (mean ± SD) of that observed in CHO-K1 cells.
Figure 5.
MT1-MMP processing and activation in CHO-K1 and RPE.40 cells. (A) Processing of soluble and full-length MT1-MMP. CHO-K1 (lanes 1–3) or RPE.40 (lanes 4–6) cells were transfected with a control expression vector (lanes 1 and 4), ΔMT1-MMP (lanes 2 and 5), ΔMT1-MMP and α1PDX (lane 3), or ΔMT1-MMP and furin (lane 6) expression vectors. Cell-free supernatants were subjected to immunoblot analysis with anti-hemopexin domain–specific polyclonal antisera. The arrowheads to the right of lane 6 mark the positions of the pro and mature forms of ΔMT1-MMP, whereas the asterisk marks the position of a minor glycosylated form of proΔMT1-MMP as determined by tunicamycin sensitivity (our unpublished results). In lanes 8–10 and 12–14, Western blotting of Triton X-114 extracts was performed on CHO-K1 or RPE.40 cells expressing full-length MT1-MMP that had been cotransfected with a control expression vector (lanes 8 and 12), a furin expression vector (lanes 9 and 13), or an α1PDX expression vector (lanes 10 and 14). Immunoblots of CHO-K1 or RPE.40 cells transfected with control expression vectors are shown in lanes 7 and 11. In lanes 15–18, RPE.40 cells were transfected with expression vectors for MT1-MMP (lane 15), MT1-MMP/A4 (lane 16), MT1-MMP/AXXR (lane 17), or MT1-MMP/AXXR-A4 (lane 18). (B) MT1-MMP processing in CHO-K1 and RPE.40 cells. Western blotting was performed on CHO-K1 and RPE.40 cell extracts that had been transfected with full-length MT1-MMP (lanes 1 and 2, respectively), cytosolic domain–deleted MT1-MMP (lanes 3 and 4), or a chimeric MT1-MMP variant containing the interleukin 2 receptor transmembrane domain and cytosolic tail (TM swap; lanes 5 and 6). The arrowheads to the right mark the positions of the pro, mature, and truncated forms of MT1-MMP. (C) Processing of progelatinase A by CHO-K1 or RPE.40 cells. CHO-K1 or RPE.40 cells transfected with control expression vectors (lanes 1 and 4), cotransfected with MT1-MMP and control expression vectors (lanes 2 and 5), or cotransfected with MT1-MMP and α1PDX expression vectors (lanes 3 and 6) were incubated with progelatinase A for 16 h, and the supernatants were analyzed by gelatin zymography. The pro, intermediate, and mature forms of gelatinase A are marked by the arrowheads to the right of lane 6.
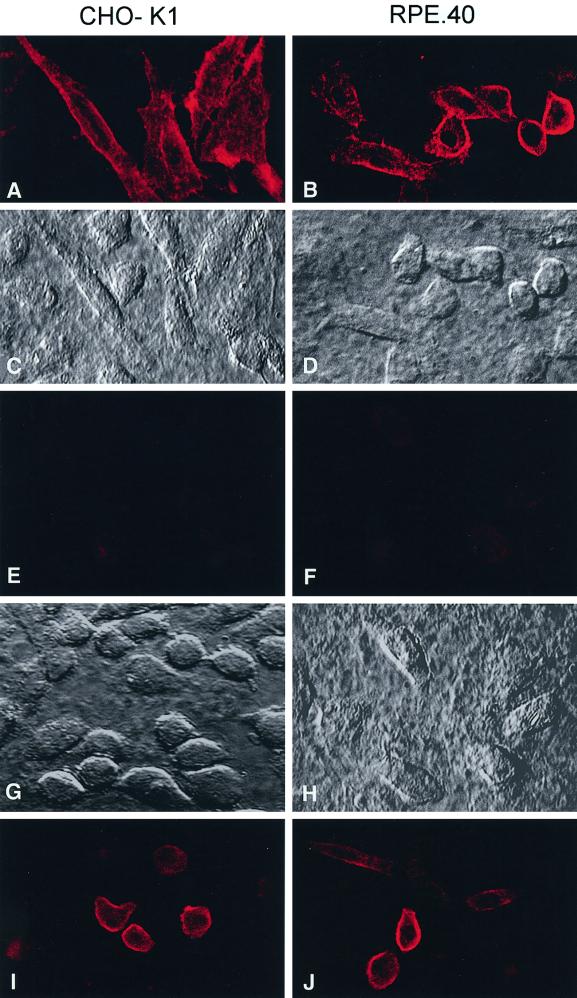
To determine whether the furin-independent MT1-MMP processing pathway operative in RPE.40 cells was dependent on the basic motifs identified previously in the prodomain, the cells were transfected with the MT1-MMP/A4, MT1-MMP/AXXR, or MT1-MMP/AXXR-A4 mutant. Compared with CHO-K1 cells, in which MT1-MMP/A4 processing was 16% of control values relative to wild-type MT1-MMP processing (n = 2), the furin-deficient RPE.40 cells were more efficient in processing the A4 mutant (i.e., 35% of control relative to wild-type MT1-MMP [n = 2]; Figure 5A, lanes 15 and 16). However, although the AXXR mutant was processed comparably to the wild-type enzyme in RPE.40 cells (lane 17), the AXXR/A4 mutant remained entirely in its pro form when expressed in the furin-deficient cell line (lane 18; 98% inhibition of processing relative to wild-type MT1-MMP in RPE.40 cells versus 100% inhibition in CHO-K1 cells [n = 2]). Because these data are consistent with a model wherein furin-independent cleavage occurred primarily downstream of 108RRKR in RPE.40 cells, an MT1-MMP construct was engineered with a FLAG epitope inserted at the C-terminal end of the basic motif (i.e., 108RRKR-FLAG). Transfected cells were then stained with a mAb (anti-FLAG M1) that specifically recognizes FLAG-tagged proteins only when the epitope is located at the extreme N terminus of the protein (Itoh et al., 1999). As shown in Figure 6, anti-FLAG M1 reacted with furin-sufficient CHO-K1 cells as well as furin-deficient RPE.40 cells transfected with the 108RRKR-FLAG construct (A–D). Consistent with the specificity of the antibody, when the FLAG epitope was inserted downstream of the MT1-MMP/A4 mutant (i.e., 108AAAA-FLAG), the M1 antibody did not stain either transfected CHO-K1 or RPE.40 cells (E and F). Negative staining of the 108AAAA-FLAG construct could not be attributed to either inefficient expression or surface localization because MT1-MMP was readily recognized by a mAb (3H7; Chenard et al., 1999) directed against the extracellular domain of the proteinase (I and J). Thus, as in furin-sufficient cells, MT1-MMP is processed by furin-deficient cells after cleavage on the C-terminal side of the 108RRKR motif.
Figure 6.
Maturation of MT1-MMP in CHO-K1 and RPE.40 cells. (A–D) CHO-K1 cells (left panels) or RPE.40 cells (right panels) were transfected with MT1-MMP expression vectors that contained a FLAG insert immediately downstream of 108RRKR. The cells were then fixed, and mature MT1-MMP was immunolocalized with anti-FLAG M1 mAb by confocal laser microscopy (A and B). Nomarski images of the fields are shown for CHO-K1 (C) and RPE.40 (D) cells. (E–H) CHO-K1 or RPE.40 cells were transfected with expression vectors for MT1-MMP/A4 that contained the FLAG insert downstream of 108AAAA. After fixation, anti-FLAG mAb did not stain either CHO-K1 cells (E) or RPE.40 cells (F). Nomarski images of each field are shown in G and H. Cell surface expression of the MT1-MMP/A4-FLAG construct was confirmed with the anti-MT1-MMP 3H7 mAb in CHO-K1 cells (I) and RPE.40 cells (J).
Given the selection of the 108RRKR motif as the primary cleavage site in furin-deficient RPE.40 cells, a potential role for other proprotein convertases in the processing event was assessed by cotransfecting these cells with wild-type MT1-MMP and α1PDX cDNAs. As shown in Figure 5A, α1PDX almost completely inhibited MT1-MMP processing to its mature form in RPE.40 cells (compare lanes 13 and 14). In three experiments, α1PDX inhibited MT1-MMP processing by 92 ± 7% in RPE.40 cells and by 99 ± 2% in CHO-K1 cells (mean ± SD). Because MT1-MMP processing would be expected to progress until the α1PDX concentration increased to a level sufficient to inhibit targeted proprotein convertases, the 45-kDa truncation product of MT1-MMP was detected occasionally (Figure 5A, lane 14).
The inability of RPE.40 cells to process soluble ΔMT1-MMP while maintaining the ability to mediate the activation of the full-length proteinase raised the possibility that differential processing is regulated by membrane anchoring per se and/or by signals embedded in the MT1-MMP cytosolic tail or transmembrane domains (Nakahara et al., 1997; Fernandez-Larrea et al., 1999; Urena et al., 1999). Thus, the processing of a cytosol tail–deleted form of MT1-MMP or a chimeric MT1-MMP molecule containing the transmembrane domain and tail of the interleukin 2 receptor was examined after transfection into CHO-K1 and furin-deficient RPE.40 cells. Significantly, the cytosolic tail–deleted mutant was processed comparably to the full-length enzyme in both the CHO-K1 and RPE.40 cell strains (Figure 5B). The transmembrane-substitution mutant was processed less efficiently than wild-type MT1-MMP, but both cell strains generated the mature form of the enzyme comparably (Figure 5B, lanes 5 and 6). Thus, membrane anchoring alone dictated the selection of a furin-independent pathway for the processing of MT1-MMP.
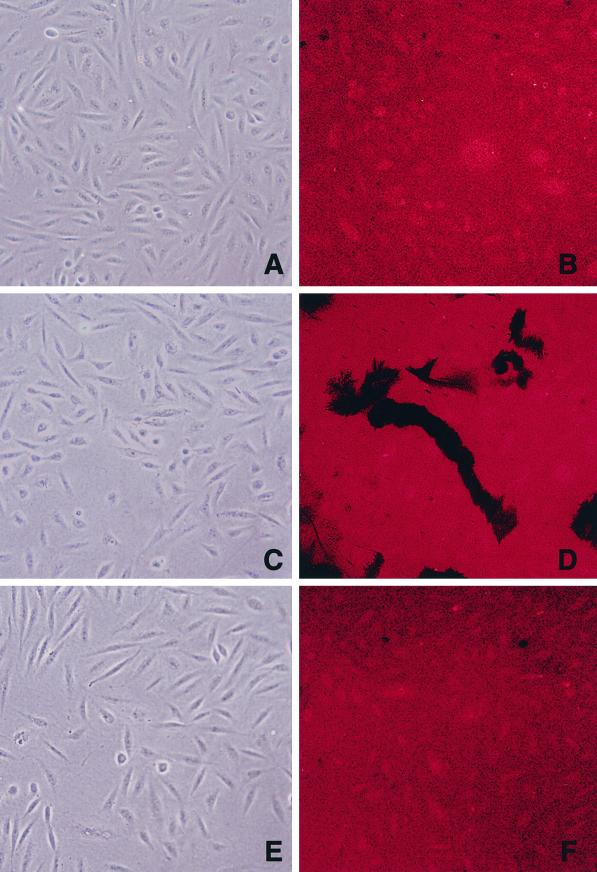
Because CHO-K1 cells processed full-length MT1-MMP by either furin-dependent or furin-independent pathways, the ability of α1PDX to regulate cell-associated proteolytic activity in this more complex system was assessed. As shown in Figure 5C, the activation of progelatinase A by either MT1-MMP–transfected CHO-K1 or RPE.40 cells could be blocked almost completely by α1PDX. Finally, because the processing of progelatinase A only indirectly monitors MT1-MMP activity (Nagase and Woessner, 1999), the ability of adherent MT1-MMP–transfected cells to degrade a fluorescently labeled subjacent substrate (i.e., gelatin) was determined in the absence or presence of α1PDX. As shown in Figure 7, MT1-MMP–transfected, but not control, CHO-K1 cells proteolyzed Texas red–labeled gelatin as they migrated across the coated surface, leaving dark chemokinetic “tracks” (A–D). In contrast, CHO-K1 cells cotransfected with MT1-MMP and α1PDX were unable to express proteolytic activity (E and F).
Figure 7.
Regulation of MT1-MMP–dependent subjacent proteolytic activity by α1PDX. Control-transfected (A and B), MT1-MMP–transfected (C and D), and MT1-MMP– and α1PDX–cotransfected (E and F) CHO-K1 cells were cultured on surfaces coated with Texas red–labeled gelatin. After 16 h of incubation, cells were visualized by phase-contrast microscopy (A, C, and E), and zones of gelatinolytic activity were identified by confocal laser microscopy (B, D, and F).
Proprotein Convertase-dependent Processing of MT1-MMP in HT-1080 Cells
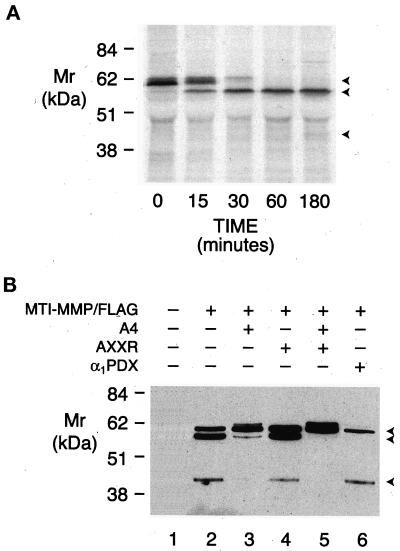
To determine whether endogenously expressed MT1-MMP is similarly processed by proprotein convertases, metalloproteinase activation was examined in HT-1080 cells. HT-1080 cells constitutively process proMT1-MMP on the C-terminal side of 108RRKR to generate mature 112Y-MT1-MMP (Strongin et al., 1995; Lehti et al., 1998). Consistent with these data, pulse-chase analysis of MT1-MMP processing in HT-1080 cells demonstrated that the mature enzyme could be detected within 15 min (Figure 8A). Although epitope-tagged MT1-MMP was also processed to the mature 60-kDa proteinase, both the A4 mutant and the AXXR-A4 mutant accumulated as their respective pro forms (Figure 8B, lanes 2, 4, and 5). Because other serine proteinases have been posited to participate in MT1-MMP processing (e.g., trypsin, plasmin, and urokinase; Will et al., 1996; Okumura et al., 1997; Kazes et al., 1998), the role of proprotein convertases was finally assessed by cotransfecting HT-1080 cells with MT1-MMP/FLAG and α1PDX. Significantly, in the presence of α1PDX, the mature form of epitope-tagged MT1-MMP was no longer detected (Figure 8B, lane 6). MT1-MMP processing was unaffected by serine (aprotinin, soybean trypsin inhibitor), cysteine (E-64), or aspartate (pepstatin A) proteinase inhibitors (our unpublished results). Based on the substrate specificity of α1PDX (Cui et al., 1998; Jean et al., 1998), we conclude that furin and/or PC6 regulate the processing of endogenously derived MT1-MMP.
Figure 8.
Processing of MT1-MMP in HT-1080 cells. (A) Pulse-chase analysis of MT1-MMP processing. HT-1080 cells were pulsed with [35S]methionine as described and chased for 0, 15, 30, 60, and 180 min. Immunoprecipitates of cell lysates were analyzed by SDS-PAGE/autoradiography. The arrowheads mark the positions of pro, mature, and truncated forms of MT1-MMP. (B) Processing of epitope-tagged MT1-MMP in HT-1080 cells. Triton X-114 extracts of HT-1080 cells expressing MT1-MMP/FLAG (lane 2), MT1-MMP/FLAG containing the A4 mutation (lane 3), MT1-MMP/FLAG containing the AXXR mutation (lane 4), MT1-MMP containing both the A4 and AXXR mutations (lane 5), or MT1-MMP/FLAG and α1PDX (lane 6) were analyzed by immunoblotting with anti-FLAG mAb. Glycosylated proMT1-MMP could be detected when processing was blocked by the A4 or AXXR-A4 mutations in lanes 3 and 5. Extracts of HT-1080 cells transfected with a control expression vector did not reveal anti-FLAG immunoreactive products (lane 1).
DISCUSSION
MT1-MMP has been implicated in the remodeling of the extracellular matrix in events ranging from growth and development to wound healing and tumor invasion (Okada et al., 1997; Hiraoka et al., 1998; Belien et al., 1999; Holmbeck et al., 1999; Zhou et al., 2000). Like other members of the MMP family, proMT1-MMP is organized into discrete domains, including a propeptide region that contains a canonical PRCG(V/N)PD sequence (Nagase and Woessner, 1999). In cooperation with other portions of the prodomain, the cysteinyl residue in this peptide is proposed to interact with the Zn2+ atom of the catalytic site to act as a “lock” to maintain proteinase latency (Nagase and Woessner, 1999). Given the ability of MT1-MMP to process other MMP zymogens to their active forms (e.g., progelatinase A and procollagenase-3), to directly cleave a range of extracellular matrix molecules (Pei and Weiss, 1996; Ohuchi et al., 1997), and to regulate collagen turnover in vivo (Holmbeck et al., 1999; Zhou et al., 2000), increased attention has focused on the means by which proMT1-MMP is itself converted to a catalytically active species (Strongin et al., 1995; Cao et al., 1996, 1998; Zucker et al., 1998).
Until recently, MMPs were thought to undergo activation extracellularly after either oxidative or proteolytic perturbation of propeptide domain–catalytic domain interactions (Weiss et al., 1985; Nagase and Woessner, 1999). However, a precedent for intracellular processing was established when the stromelysin-3 zymogen was reported to undergo constitutive activation (Pei and Weiss, 1995; Santavicca et al., 1996). Unlike other secreted MMPs, stromelysin-3 was cleaved immediately downstream of a tetrabasic motif (RXRXKR) that served as a recognition site for furin. With the subsequent discovery of RX(K/R)R sequences at the predicted C-terminal ends of the prodomains of MT1-, MT2-, MT3-, MT4-, and MT5-MMP (Nagase and Woessner, 1999; Pei, 1999), proprotein convertases have been posited to play a more prominent role in regulating MMP activation.
Processing of the MT1-MMP Zymogen
The detection of a single MT1-MMP species whose molecular mass appears consistent with that predicted for the proenzyme has been widely described in COS cells as well as in endothelial cells and tumor cell lines (Sato et al., 1994; Cao et al., 1996, 1998; Rajavashisth et al., 1999). However, the detected species was assumed to represent the pro form of MT1-MMP based solely on the predicted molecular mass for the unprocessed zymogen. Because mature MT1-MMP migrates anomalously relative to the migration expected from the loss of the 111-amino acid propeptide, positive identification of an immunoreactive band as either proMT1-MMP or mature MT1-MMP can only be made with prodomain-specific antibodies. In our study, MT1-MMP transfectants expressed at least three distinct products (we also note that a glycosylated pro form of MT1-MMP tended to accumulate under those conditions in which proteinase processing was inhibited [Maquoi et al., 1998]). By epitope tagging of the prodomain, COS-1 cells were shown to process the MT1-MMP zymogen to two propeptide-deleted forms. Because only the 60-kDa species was recognized by a mAb directed against the catalytic domain, the smaller product represents an inactive product of the mature enzyme (Lehti et al., 1998; Stanton et al., 1998). However, because the enzymically inactive 45-kDa species was formed 1) in the presence of a broad-spectrum MMP inhibitor, 2) after transfection with a catalytically inactive form of MT1-MMP, or 3) in the presence of α1PDX, routes other than the autocatalytic degradation of mature MT1-MMP are operative under these conditions.
A Role for Multibasic Motifs in MT1-MMP Processing
Based on earlier studies with stromelysin-3 (Pei and Weiss, 1995), the 108RRKR domain in full-length MT1-MMP was predicted to play a critical role in the activation process by acting as a recognition motif for proprotein convertases. Indeed, in the HT-1080 cell line, two groups have identified the N terminus of the 60-kDa mature form of MT1-MMP as Y112, which lies immediately downstream of the 108RRKR sequence (Strongin et al., 1995; Lehti et al., 1998). However, whereas the processing of the 108RRKR → AAAA mutant of soluble ΔMT1-MMP was blocked completely in transiently transfected COS cells, the A4 mutant of the full-length enzyme continued to undergo processing. Thus, despite an unequivocal role for the tetrabasic motif in regulating the maturation of the soluble mutant form of the enzyme, the processing of the full-length proteinase proceeded in a divergent manner.
Given that proprotein convertase can recognize alternative motifs within a framework (Arg/Lys)-(X)n-(Arg-Lys) (Zhou et al., 1999) wherein n = 0, 2, 4, or 6 residues, we noted that MT1-MMP displays an overlapping tandem of potential cleavage sites 22 amino acid residues upstream of the 108RRKR site at 86KXXRRXR (i.e., 86KXXR, 89RRXR, and 89RR). To eliminate these cleavage sites, an 89R → A mutation was inserted into either wild-type MT1-MMP or the A4 mutant. Although the 89R → A mutant of full-length MT1-MMP was processed normally, the partial processing of the A4 mutant was completely inhibited when the putative alternative sites within the 86KXXRRXR motif were eliminated. In agreement with these findings, the inability of the proMT1-MMP double mutant to undergo processing correlated with the enzyme's inability to activate progelatinase A. Although we have been unable to recover processed MT1-MMP from transiently transfected cells in sufficient quantities for microsequencing, together these data support a model wherein wild-type MT1-MMP is processed primarily, if not solely, at the 108RRKR↓ site to generate the active proteinase. Given that the secondary site within 86KXXRRXR was apparently used only when the primary 108RRKR motif was mutated, a physiological role for the alternative site has not been demonstrated in transfected cells. Nonetheless, primary and secondary cleavage sites for proprotein convertases have been posited in other target molecules, including Notch, bone morphogenic protein-4, and insulin growth factor-1 (Duguay et al., 1997; Cui et al., 1998; Logeat et al., 1998).
Proprotein Convertases as Processing Enzymes for MT1-MMP
The ability of basic motif mutations within the MT1-MMP prodomain to interfere with processing is consistent with a role for any proteinase that cleaves target molecules on the C-terminal side of the RRKR sequence. Indeed, others have proposed roles for plasmin, trypsin, and urokinase in MT1-MMP processing at or near the C-terminal end of the RRKR motif (Will et al., 1996; Okumura et al., 1997; Kazes et al., 1998). However, proteinase inhibitors directed against these enzymes did not affect MT1-MMP maturation or activity. Instead, MT1-MMP processing was blocked by the mutant α1 proteinase inhibitor α1PDX, which specifically inactivates furin and PC6 (Cui et al., 1998; Jean et al., 1998) (although higher concentrations of α1PDX may inhibit PACE4; Benjannet et al., 1997). Significantly, α1PDX inhibited both the processing of MT1-MMP to its mature form and the enzyme's proteolytic activity against either progelatinase A or a subjacent matrix of denatured collagen. Although we note that the inactive 45-kDa form of MT1-MMP was occasionally formed under these conditions, MT1-MMP proteolytic activity was nonetheless completely inhibited.
These findings are in contrast to those of Zucker and colleagues, who reported proMT1-MMP to act as a fully functional enzyme (Cao et al., 1996, 1998; Zucker et al., 1998). However, their conclusions were predicated, in part, on the following assumptions: 1) that COS-1 cells do not process the MT1-MMP zymogen; 2) that 108RRKR mutations would completely prevent MT1-MMP processing; and 3) that MT1-MMP processing would be blocked by the furin inhibitor α1PIPITT. Based on our findings that COS-1 cells can process wild-type MT1-MMP as well as its 108RRKR mutant, coupled with the recent demonstration that α1PIPITT is unable to efficiently inhibit proprotein convertase–dependent pathways (Vollenweider et al., 1996; Cui et al., 1998), we propose that the removal of the MT1-MMP prodomain is a prerequisite for the efficient display of proteolytic activity. Interestingly, although partial blockade of proMT1-MMP processing was reported recently with a chloromethylketone inhibitor (Maquoi et al., 1998; Kurschat et al., 1999), this agent is not specific for proprotein convertases and also exerts cytotoxic effects (Hallenberger et al., 1992; Okumura et al., 1997; Jean et al., 1998).
Inhibition of MT1-MMP processing by α1PDX alone does not allow one to definitively identify the target proprotein convertase(s). However, furin-deficient cell lines have proven to be a useful adjunct for discriminating between furin-dependent and furin-independent processing pathways (Pei and Weiss, 1995; Duguay et al., 1997; Inocencio et al., 1997; Logeat et al., 1998). As expected, neither of the well-characterized furin-deficient cell lines, LoVo or RPE.40, processed soluble ΔMT1-MMP to its mature form. LoVo cells have been reported to express PACE4 as well as PC7, and this furin-deficient cell line can process other RXKR-encrypted targets including human immunodeficiency virus-1, E-cadherin and gp-160 (Gu et al., 1995; Decroly et al., 1997; Zarkik et al., 1997; Posthaus et al., 1998). However, neither PACE4 nor PC7 appeared to play a major role in the processing of ΔMT1-MMP in LoVo cells. In turn, a role for furin as the major processing enzyme for MT1-MMP was further buttressed by the ability of furin-reconstituted LoVo cells to efficiently process MT1-MMP and the ability of extracellular soluble furin to cleave proMT1-MMP displayed on the cell surface.
The processing of proMT1-MMP by furin is consistent with the ability of this proprotein convertase to recognize RX(K/R)R, RXXR, KXXR, or RR motifs (Duguay et al., 1997; Nakayama, 1997; Cui et al., 1998; Zhou et al., 1999). Unexpectedly, however, MT1-MMP was processed after cleavage immediately downstream of the 108RRKR motif in the furin-deficient RPE.40 cell line by a predominately α1PDX-sensitive process. These findings demonstrate not only that furin-independent maturation pathways exist but also that soluble and membrane-anchored MT1-MMP were processed in distinct fashions. The α1PDX-inhibitable proprotein convertase responsible for wild-type MT1-MMP processing has not been identified. However, in contrast to LoVo cells, RPE.40 cells do express PC6 (as both the transmembrane-anchored and soluble forms, PC6B and P6CA, respectively; Duguay et al., 1997). Interestingly, PC6, like furin, can process RXKR-, RXXR-, and KXXR-containing molecules (Zarkik et al., 1997; Cui et al., 1998). Furthermore, because PC6 cannot be detected in LoVo cells (Vollenweider et al., 1996; Logeat et al., 1998), differences in the MT1-MMP processing pathways displayed between LoVo and RPE.40 cells are likely attributed to each cell line's distinct repertoire of processing enzymes. Whatever the relative roles of furin and PC6 in MT1-MMP processing, we note that α1PDX did not completely inhibit MT1-MMP maturation in RPE.40 or LoVo cells. Additional studies are needed to determine the role of PC7, an α1PDX-resistant proprotein convertase (Benjannet et al., 1997; Jean et al., 1998), as well as proprotein convertase–independent systems in MT1-MMP processing.
The ability of furin-deficient RPE.40 cells to process membrane-anchored, but not soluble, MT1-MMP by an α1PDX-sensitive process illustrates the complexities associated with attempts to extrapolate results obtained with transmembrane-deleted mutants to membrane-anchored wild-type proteins. Along these lines, recent studies have suggested that the cytosolic tail of MT1-MMP may contain signals critical for membrane trafficking (Nakahara et al., 1997; Fernandez-Larrea et al., 1999; Urena et al., 1999). Nonetheless, cytosolic tail–deletion mutants as well as transmembrane domain “swaps” failed to reveal roles for either domain. These results are consistent with studies demonstrating the ability of cells transfected with cytosolic domain–deleted MT1-MMP to express heightened invasive activity (Hiraoka et al., 1998; Hotary et al., 2000). We conclude that membrane association alone may increase the residence time of proMT1-MMP at cell surfaces where the likelihood of its cleavage by proprotein convertases is enhanced. Although the cellular sites at which MT1-MMP processing takes place have not been defined, both furin and PC6 can be expressed as membrane-anchored enzymes that potentially could cleave MT1-MMP intracellularly or at the cell surface (Malloy et al., 1994).
SUMMARY
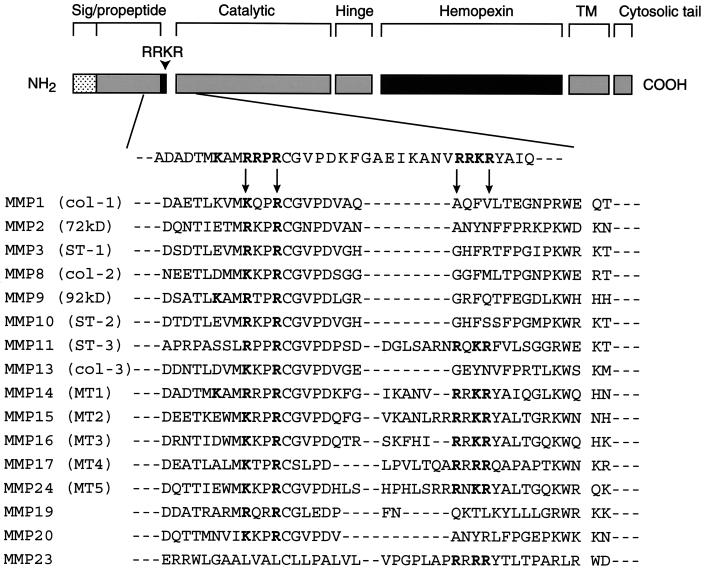
Previous efforts to identify MMP activation schemes have focused largely on the role of the plasminogen activator–plasminogen system (Nagase and Woessner, 1999). However, a link between these systems is based primarily on in vitro studies, whereas in vivo studies in plasminogen activator- or plasminogen-deleted animals clearly support the existence of plasmin-independent activation cascades (Hiraoka et al., 1998; Lund et al., 1999; Hotary et al., 2000). Significantly, RXKR motifs are found in at least six other human MMPs (i.e., MT2-, MT3-, MT4-, and MT5-MMP, stromelysin-3, and MMP-23) (Nagase et al., 1999; Pei, 1999). Furthermore, RXXR and KXXR motifs can be identified in the prodomains of multiple MMPs (Figure 9), and similar processing cascades may apply to the structurally related metalloprotease–disintegrin family (Schlondorff and Blobel, 1999). Along these lines, it is noteworthy that preliminary reports indicate that progelatinase A can be processed directly to its active form after furin-dependent cleavage at 69RQPR↓ (Cao et al., 1999). Because proprotein convertases can process target molecules in both intracellular and extracellular compartments (Malloy et al., 1994; Zhou et al., 1999), the proprotein convertase–metalloproteinase axis may play a major role in regulating complex arrays of proteolytic activities in vivo.
Figure 9.
Prodomain alignments of potential proprotein recognition motifs in soluble and membrane-anchored MMPs. An alignment of MMP prodomains demonstrates that RXKR motifs can be found in stromelysin-3, MT1-, MT2-, MT3-, MT4-, and MT5-MMP, and MMP-23. Either RXXR or KXXR motifs are found in all MMPs except for metalloelastase (MMP-12) and matrilysin (MMP-7) (sequences not shown).
ACKNOWLEDGMENTS
We thank Drs. B. Donohoe, E. Allen, and K. Hotary for help with the confocal laser microscopy and J. Lowe (Howard Hughes Medical Institute, University of Michigan), M. Seiki, and Y. Itoh (both University of Tokyo) for helpful discussions. The work was supported by the National Institutes of Health (5R01 CA71699) and Novartis.
REFERENCES
- Belien ATJ, Paganetti PA, Schwab ME. Membrane-type 1 matrix metalloproteinase (MT1-MMP) enables invasive migration of glioma cells in central nervous system white matter. J Cell Biol. 1999;144:373–384. doi: 10.1083/jcb.144.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjannet S, Savaria D, Laslop A, Munzer JS, Chretien M, Marcinkiewicz M, Seidah NG. α1-antitrypsin Portland inhibits processing of precursors mediated by proprotein convertases primarily within the constitutive secretory pathway. J Biol Chem. 1997;272:26210–26218. doi: 10.1074/jbc.272.42.26210. [DOI] [PubMed] [Google Scholar]
- Botos I, Scapozza L, Zhang D, Liotta LA, Meyer EF. Batimastat, a potent matrix metalloproteinase inhibitor, exhibits an unexpected mode of binding. Proc Natl Acad Sci USA. 1996;39:2749–2754. doi: 10.1073/pnas.93.7.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Drews M, Lee HM, Conner C, Bahou WF, Zucker S. The propeptide domain of membrane type 1 matrix metalloproteinase is required for binding of tissue inhibitor of metalloproteinases and for activation of pro-gelatinase A. J Biol Chem. 1998;273:34745–34752. doi: 10.1074/jbc.273.52.34745. [DOI] [PubMed] [Google Scholar]
- Cao J, Rehemtulla A, Bahou W, Zucker S. Membrane type matrix metalloproteinase 1 activates pro-gelatinase A without furin cleavage of the N-terminal domain. J Biol Chem. 1996;271:30174–30180. doi: 10.1074/jbc.271.47.30174. [DOI] [PubMed] [Google Scholar]
- Cao J, Rehemtulla A, Conner C, Drews M, Bahou W, Zucker S. Furin directly processes pro-gelatinase A within the secretory pathway of cells. Proc Am Assoc Cancer Res. 1999;40:3432A. [Google Scholar]
- Chenard M-P, Lutz Y, Mechine-Neuville A, Stoll I, Bellocq J-P, Rio M-C, Basset P. Presence of high levels of MT1-MMP protein in fibroblastic cells of human invasive carcinomas. Int J Cancer. 1999;82:208–212. doi: 10.1002/(sici)1097-0215(19990719)82:2<208::aid-ijc10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Cui Y, Jean F, Thomas G, Christian JL. BMP-4 is proteolytically activated by furin and/or PC6 during vertebrate embryonic development. EMBO J. 1998;17:4735–4743. doi: 10.1093/emboj/17.16.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Ortho M-P, Stanton H, Butler M, Atkinson SJ, Murphy G, Hembry RM. M.T1-M.M.P. on the cell surface causes focal degradation of gelatin films. FEBS Lett. 1998;421:159–164. doi: 10.1016/s0014-5793(97)01555-x. [DOI] [PubMed] [Google Scholar]
- Decroly E, Benjannet S, Savaria D, Seidah NG. Comparative functional role of PC7 and furin in the processing of the HIV envelope glycoprotein gp160. FEBS Lett. 1997;405:68–72. doi: 10.1016/s0014-5793(97)00156-7. [DOI] [PubMed] [Google Scholar]
- Duguay SJ, Milewski WM, Young BD, Nakayama K, Steiner DF. Processing of wild-type and mutant proinsulin-like growth factor-IA by subtilisin-related proprotein convertases. J Biol Chem. 1997;272:6663–6670. doi: 10.1074/jbc.272.10.6663. [DOI] [PubMed] [Google Scholar]
- Fernandez-Larrea J, Merlos-Suarez A, Urena JM, Baselga J, Arribas J. A role for a PDZ protein in the early secretory pathway for the targeting of proTGF-α to the cell surface. Mol Cell. 1999;3:423–433. doi: 10.1016/s1097-2765(00)80470-0. [DOI] [PubMed] [Google Scholar]
- Gu M, Rappaport J, Leppla SH. Furin is important but not essential for the proteolytic maturation of gp160 of HIV-1. FEBS Lett. 1995;365:95–97. doi: 10.1016/0014-5793(95)00447-h. [DOI] [PubMed] [Google Scholar]
- Hallenberger S, Bosch V, Angliker H, Shaw E, Klenk H-D, Garten W. Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160. Nature. 1992;360:358–361. doi: 10.1038/360358a0. [DOI] [PubMed] [Google Scholar]
- Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998;95:365–377. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- Holmbeck K, et al. M.T1-M.M.P-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Hotary, K., Allen, E., Punturieri, A., Yana, I., and Weiss, S.J. (2000). Regulation of cell invasion and morphogenesis in a 3-dimensional type 1 collagen matrix by membrane-type matrix metalloproteinase 1, 2 and 3. J. Cell Biol. (in press). [DOI] [PMC free article] [PubMed]
- Inocencio NM, Sucic JF, Moehring JM, Spence MJ, Moehring TJ. Endoprotease activities other than furin and PACE4 with a role in processing of HIV-I gp160 glycoproteins in CHO-K1 cells. J Biol Chem. 1997;272:1344–1348. doi: 10.1074/jbc.272.2.1344. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Kajita M, Kinoh H, Mori H, Okada A, Seiki M. Membrane type 4 matrix metalloproteinase (MT4-MMP, MMP-17) is a glycosylphosphatidylinositol-anchored proteinase. J Biol Chem. 1999;274:34260–34266. doi: 10.1074/jbc.274.48.34260. [DOI] [PubMed] [Google Scholar]
- Jean F, Stella K, Thomas L, Liu G, Xiang Y, Reason AJ, Thomas G. α1-antitrypsin Portland, a bioengineered serpin highly selective for furin: application as an antipathogenic agent. Proc Natl Acad Sci USA. 1998;95:7293–7298. doi: 10.1073/pnas.95.13.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazes I, Delarue F, Hagege J, Bouzhir-Sima L, Rondeau E, Sraer J-D, Nguyen G. Soluble latent membrane-type 1 matrix metalloprotease secreted by human mesangial cells is activated by urokinase. Kidney Int. 1998;54:1976–1984. doi: 10.1046/j.1523-1755.1998.00216.x. [DOI] [PubMed] [Google Scholar]
- Kurschat P, Zigrino P, Nischt R, Breitkopf K, Steurer P, Klein CE, Krieg T, Mauch C. Tissue inhibitor of matrix metalloproteinase-2 regulates matrix metalloproteinase-2 activation by modulation of membrane-type 1 matrix metalloproteinase activity in high and low invasive melanoma cell lines. J Biol Chem. 1999;274:21056–21062. doi: 10.1074/jbc.274.30.21056. [DOI] [PubMed] [Google Scholar]
- Lehti K, Lohi J, Valtanen H, Keski-Oja J. Proteolytic processing of membrane-type-1 matrix metalloproteinase is associated with gelatinase A activation at the cell surface. Biochem J. 1998;334:345–353. doi: 10.1042/bj3340345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah NG, Israel A. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci USA. 1998;95:8108–8112. doi: 10.1073/pnas.95.14.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohi J, Lehti K, Westermarck J, Kahari V-M, Keski-Oja J. Regulation of membrane-type matrix metalloproteinase-1 expression by growth factors and phorbol 12-myristate 13-acetate. Eur J Biochem. 1996;239:239–247. doi: 10.1111/j.1432-1033.1996.0239u.x. [DOI] [PubMed] [Google Scholar]
- Lund LR, Romer J, Bugge TH, Nielsen BS, Frandsen TL, Degen JL, Stephens RW, Dano K. Functional overlap between two classes of matrix-degrading proteases in wound healing. EMBO J. 1999;17:4645–4656. doi: 10.1093/emboj/18.17.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy SS, Thomas L, VanSlyke JK, Stenberg PE, Thomas G. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J. 1994;13:18–33. doi: 10.1002/j.1460-2075.1994.tb06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquoi E, Noel A, Frankenne F, Angliker H, Murphy G, Foidart J-M. Inhibition of matrix metalloproteinase 2 maturation and HT1080 invasiveness by a synthetic furin inhibitor. FEBS Lett. 1998;424:262–266. doi: 10.1016/s0014-5793(98)00187-2. [DOI] [PubMed] [Google Scholar]
- Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Howard L, Thompson EW, Sato H, Seiki M, Yeh Y, Chen W-T. Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc Natl Acad Sci USA. 1997;94:7959–7964. doi: 10.1073/pnas.94.15.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K. Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J. 1997;327:625–635. doi: 10.1042/bj3270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- Okada A, Tomasetto C, Lutz Y, Bellocq J-P, Rio M-C, Basset P. Expression of matrix metalloproteinases during rat skin wound healing: evidence that membrane type-1 matrix metalloproteinase is a stromal activator of pro-gelatinase A. J Cell Biol. 1997;137:67–77. doi: 10.1083/jcb.137.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura Y, Sato H, Seiki M, Kido H. Proteolytic activation of the precursor of membrane type 1 matrix metalloproteinase by human plasmin. FEBS Lett. 1997;402:181–184. doi: 10.1016/s0014-5793(96)01523-2. [DOI] [PubMed] [Google Scholar]
- Pei D. Identification and characterization of the fifth membrane-type matrix metalloproteinase MT5-MMP. J Biol Chem. 1999;274:8925–8932. doi: 10.1074/jbc.274.13.8925. [DOI] [PubMed] [Google Scholar]
- Pei D, Weiss SJ. Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature. 1995;375:244–247. doi: 10.1038/375244a0. [DOI] [PubMed] [Google Scholar]
- Pei D, Weiss SJ. Transmembrane-deletion mutants of the membrane-type matrix metalloproteinase-1 process progelatinase A and express intrinsic matrix-degrading activity. J Biol Chem. 1996;271:9135–9140. doi: 10.1074/jbc.271.15.9135. [DOI] [PubMed] [Google Scholar]
- Posthaus H, Dubois CM, Laprise M-H, Grondin F, Suter MM, Muller E. Proprotein cleavage of E-cadherin by furin in baculovirus over-expression system: potential role of other convertases in mammalian cells. FEBS Lett. 1998;438:306–310. doi: 10.1016/s0014-5793(98)01330-1. [DOI] [PubMed] [Google Scholar]
- Rajavashisth TB, et al. Inflammatory cytokines and oxidized low density lipoproteins increase endothelial cell expression of membrane type 1-matrix metalloproteinase. J Biol Chem. 1999;274:11924–11929. doi: 10.1074/jbc.274.17.11924. [DOI] [PubMed] [Google Scholar]
- Rice WG, Weiss SJ. Regulation of proteolysis at the neutrophil-substrate interface by secretory leukoprotease inhibitor. Science. 1990;249:178–181. doi: 10.1126/science.2371565. [DOI] [PubMed] [Google Scholar]
- Santavicca M, Noel A, Angliker H, Stoll I, Segain J-P, Anglard P, Chretien M, Seidah N, Basset P. Characterization of structural determinants and molecular mechanisms involved in pro-stromelysin-3 activation by 4-aminophenylmercuric acetate and furin-type convertases. Biochem J. 1996;315:953–958. doi: 10.1042/bj3150953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumor cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Schlondorff J, Blobel CP. Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J Cell Sci. 1999;112:3603–3617. doi: 10.1242/jcs.112.21.3603. [DOI] [PubMed] [Google Scholar]
- Stanton H, Gavrilovic J, Atkinson SJ, d'Ortho M-P, Yamada KM, Zardi L, Murphy G. The activation of proMMP-2 (gelatinase A) by HT1080 fibrosarcoma cells is promoted by culture on a fibronectin substrate and is concomitant with an increase in processing of M.T1-M.M.P. (M.M.P-14) to a 45 kDa form. J Cell Sci. 1998;111:2789–2798. doi: 10.1242/jcs.111.18.2789. [DOI] [PubMed] [Google Scholar]
- Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- Toth M, Gervasi DC, Fridman R. Phorbol ester-induced cell surface association of matrix metalloproteinase-9 in human MCF10A breast epithelial cells. Cancer Res. 1997;57:3159–3167. [PubMed] [Google Scholar]
- Urena JM, Merlos-Suarez A, Baselga J, Arribas J. The cytoplasmic carboxy-terminal amino acid determines the subcellular localization of proTGF-α and membrane type matrix metalloprotease (M.T1-M.M.P) J Cell Sci. 1999;112:773–784. doi: 10.1242/jcs.112.6.773. [DOI] [PubMed] [Google Scholar]
- Vollenweider F, Benjannet S, Decroly E, Savaria D, Lazure C, Thomas G, Cretien M, Seidah NG. Comparative cellular processing of the human immunodeficiency virus (HIV-1) envelope glycoprotein gp160 by the mammalian subtilisin/kexin-like convertases. Biochem J. 1996;314:521–532. doi: 10.1042/bj3140521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ, Peppin G, Ortiz X, Ragsdale C, Test ST. Oxidative autoactivation of latent collagenase by human neutrophils. Science. 1985;227:747–749. doi: 10.1126/science.2982211. [DOI] [PubMed] [Google Scholar]
- Wesley CL, Rehemtulla A, Kaufman RJ. PACE/furin can process the vitamin K-dependent pro-factor IX precursor within the secretory pathway. J Biol Chem. 1993;268:8458–8465. [PubMed] [Google Scholar]
- Will H, Atkinson SJ, Butler GS, Smith B, Murphy G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A. and initiates autoproteolytic activation. J Biol Chem. 1996;271:17119–17123. doi: 10.1074/jbc.271.29.17119. [DOI] [PubMed] [Google Scholar]
- Zarkik S, Decroly E, Wattiez R, Seidah NG, Burny A, Ruysschaert J-M. Comparative processing of bovine leukemia virus envelope glycoprotein gp72 by subtilisin/kexin-like mammalian convertases. FEBS Lett. 1997;406:205–210. doi: 10.1016/s0014-5793(97)00275-5. [DOI] [PubMed] [Google Scholar]
- Zhou A, Webb G, Zhu X, Steiner DF. Proteolytic processing in the secretory pathway. J Biol Chem. 1999;274:20745–20748. doi: 10.1074/jbc.274.30.20745. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker S, Drews M, Conner C, Foda HD, DeClerck YA, Langley KE, Bahou WF, Docherty AJP, Cao J. Tissue inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic domain of the cell surface receptor, membrane type 1-matrix metalloproteinase 1 (M.T1-M.M.P) J Biol Chem. 1998;273:1216–1222. doi: 10.1074/jbc.273.2.1216. [DOI] [PubMed] [Google Scholar]