EEA1, a Tethering Protein of the Early Sorting Endosome, Shows a Polarized Distribution in Hippocampal Neurons, Epithelial Cells, and Fibroblasts (original) (raw)
Abstract
EEA1 is an early endosomal Rab5 effector protein that has been implicated in the docking of incoming endocytic vesicles before fusion with early endosomes. Because of the presence of complex endosomal pathways in polarized and nonpolarized cells, we have examined the distribution of EEA1 in diverse cell types. Ultrastructural analysis demonstrates that EEA1 is present on a subdomain of the early sorting endosome but not on clathrin-coated vesicles, consistent with a role in providing directionality to early endosomal fusion. Furthermore, EEA1 is associated with filamentous material that extends from the cytoplasmic surface of the endosomal domain, which is also consistent with a tethering/docking role for EEA1. In polarized cells (Madin-Darby canine kidney cells and hippocampal neurons), EEA1 is present on a subset of “basolateral-type” endosomal compartments, suggesting that EEA1 regulates specific endocytic pathways. In both epithelial cells and fibroblastic cells, EEA1 and a transfected apical endosomal marker, endotubin, label distinct endosomal populations. Hence, there are at least two distinct sets of early endosomes in polarized and nonpolarized mammalian cells. EEA1 could provide specificity and directionality to fusion events occurring in a subset of these endosomes in polarized and nonpolarized cells.
INTRODUCTION
Animal cells are continuously internalizing proteins and lipids of their plasma membrane via endocytosis. The internalized surface components enter a complex and dynamic membrane system, the early endosome, which plays a vital role in sorting endocytosed proteins to different destinations in the cell (Gruenberg and Maxfield, 1995). It is now clear that the early endosome comprises at least two functionally distinct compartments or subdomains (Ghosh et al., 1994; Ghosh and Maxfield, 1995; Gruenberg and Maxfield, 1995;Ullrich et al., 1996; Zacchi et al., 1998). Markers first enter the early sorting endosome, a complex organelle with tubular and multivesicular domains, where membrane proteins destined for degradation are sorted away from those proteins, such as the transferrin receptor, that are recycled back to the plasma membrane. Recycling proteins can then enter a second subcompartment, termed the recycling endosome, which has a tubular morphology and in many cell types is located in the pericentriolar area of the cell (Yamashiro et al., 1984; Dunn et al., 1989; Ghosh and Maxfield, 1995). Further complexity is added to this picture by the finding that fibroblasts, generally regarded as nonpolarized cells, may contain two sets of early endosomes analogous to those in polarized cells (Wilson and Colton, 1997). In these studies, endotubin, a membrane protein of the apical early endosomal compartment in neonatal rat intestine (Wilson et al., 1987), was heterologously expressed in normal rat kidney (NRK) cells and shown to associate with an apparently unique early endosomal compartment. This compartment was distinct from transferrin-containing early endosomes and was relatively insensitive to brefeldin A (BFA) (Wilson and Colton, 1997).
EEA1, a 162-kDa autoantigen associated with subacute cutaneous systemic lupus erythematosus, has been shown to be specifically associated with the cytoplasmic face of the early endosome membrane (Mu et al., 1995). Unlike many other endosomal proteins, EEA1 has not been detected on other membrane compartments and appears to be one of the most specific early endosomal markers known to date (Mu_et al._, 1995). EEA1 contains coiled-coil regions and a zinc finger–like domain (Mu et al., 1995; Stenmark et al., 1996). This cysteine-rich motif, termed the FYVE finger, interacts with phosphatidylinositol-3-phosphate (Patki et al., 1997; Gaullier et al., 1999) and has been shown to be required for endosomal targeting of EEA1 (Stenmark et al., 1996). This motif is found in several other proteins, including Vps27p, Fab1p, and Vac1p, proteins shown to be involved in membrane traffic in yeast (Mu et al., 1995; Stenmark_et al._, 1996). Recent work has shown that EEA1 interacts with the GTP-bound form of Rab5 and that these two proteins are sufficient to mediate early endosomal fusion in vitro (Simonsen_et al._, 1998; Mills et al., 1998; Christoforidis_et al._, 1999). EEA1 may act as a docking protein that confers targeting specificity before the SNARE-dependent early endosomal fusion event (Christoforidis et al., 1999).
Because of the important role of EEA1 in the tethering and/or docking of endosomal vesicles, it is critical that its subcellular location be carefully defined. In the present study, we have examined the distribution of EEA1 in fibroblastic cells and polarized cells by immunoelectron microscopy, immunofluorescence, and subcellular fractionation. We show that EEA1 is concentrated on the sorting domain of the early endosome but is not present on clathrin-coated vesicles and that it associates with putative tethering filaments, consistent with a role in targeting and docking. In addition, our results suggest that EEA1 is predominantly associated with a putative “basolateral-type” early endosome in polarized neuronal cells, because EEA1 is located on early endosomes of the cell body and dendrites but is undetectable on presynaptic early endosomes. Further evidence for the association of EEA1 with basolateral-type endosomes was the finding that in Madin-Darby canine kidney (MDCK) cells that have been transfected with the apical endosomal protein endotubin, there is little colocalization of endotubin and EEA1. These findings illustrate the existence of cognate apical and basolateral early endosomal subpopulations in fibroblasts and polarized cells and demonstrate molecular differences between these endosomal compartments.
MATERIALS AND METHODS
Antibodies
Autoimmune sera against EEA1 were from two patients (designated patients 1 and 2) with subacute cutaneous systemic lupus erythematosus identified at the Monash Clinical Immunology Laboratory. Both antisera recognized a single 180-kDa band on Western blots that corresponded to EEA1, as shown by Western blotting of EEA1 fusion proteins (Mu et al., 1995; our unpublished results). Antibodies against the lumenal domain of rat synaptotagmin I were raised by immunizing rabbits with the peptide MVSASHPEALAAPVTTVATLVP coupled to keyhole limpet hemocyanin (Calbiochem, San Diego, CA) as described (Kreis, 1986). Antibodies were affinity purified before use (Zerial et al., 1992). The specificity of the antibodies obtained was determined by Western blotting and indirect immunofluorescence. On Western blot, the antibody reacted with a brain-specific protein of ∼65 kDa. The reaction could be inhibited by adding the specific peptide used for immunization and antibody purification. When the antibody was used for immunofluorescence studies on cultured neurons from the rat hippocampus, colocalization with synaptophysin was observed. The staining was inhibited by the addition of the specific peptide. No staining was observed in nonneuronal cells or in hippocampal neurons fixed before synaptogenesis had occurred. When added to the medium, antibody was internalized by mature neurons and internalization was inhibited when depolarization was blocked. The internalized antibody could be detected only in nerve terminals and varicosities, indicating that the antibody indeed recognized the lumenal domain of synaptotagmin, which is exposed during the exocytosis of synaptic vesicles (Matteoli et al., 1992).
Antibody against endotubin was derived from cell culture supernatant (Wilson et al., 1987). mAbs against Rab5 were generously provided by Dr. Marino Zerial (European Molecular Biology Laboratory, Heidelberg, Germany). HRP-conjugated goat antibodies against rabbit and mouse immunoglobulin G (IgG) and rhodamine- and FITC-labeled donkey antibodies against mouse and rabbit IgG were purchased from Jackson Immunoresearch (West Grove, PA). FITC-labeled goat antibodies against human IgG were from Sigma Chemical (St. Louis, MO).
Cell Culture
Rat hippocampal neurons were cultured according to published techniques (De Hoop et al., 1998). In all experiments, cells were cultured for 10 d or more until fully differentiated. MDCK cells, baby hamster kidney (BHK) cells, A431 cells, and NRK cells were cultured as described previously (Parton et al., 1994; Wilson and Colton, 1997). For internalization of synaptotagmin antibodies, coverslips with cultured neurons were transferred from normal N2 medium (Bottenstein and Sato, 1979) to preheated N2 medium containing heat-inactivated (30 min at 56°C) affinity-purified antibodies against the lumenal domain of synaptotagmin I and 55 mM KCl to enhance depolarization. Incubations lasted 30 min at 37°C, and then cells were fixed and processed for indirect immunofluorescence. To treat cells with BFA, A431 cells were incubated for 30 min with 5 μg/ml BFA at 37°C before fixation.
Cell Transfections
Endotubin was expressed in NRK cells by transient expression with the use of lipofectamine transfection. Briefly, the cells were split to 50% confluence the day before transfection. One microgram of DNA in 50 μl of OPTI-MEM (Life Technologies–BRL, Gaithersburg, MD) was mixed with 2 μl of lipofectamine in 50 μl of OPTI-MEM and incubated at room temperature for 30 min. The coverslips were washed twice with OPTI-MEM and transferred to a 24-well plate, and 400 μl of OPTI-MEM was added. One hundred microliters of the DNA/lipofectamine mixture was then added to each coverslip. The cells were incubated at 37°C for 6 h before washing with growth medium. For double transfections, 0.5 μg of each construct was used. Cells were analyzed by immunofluorescence after either 24 or 48 h. For expression of endotubin in MDCK cells, cells were transfected by calcium phosphate precipitation followed by selection in 400 μg/ml G418. Resistant colonies were pooled and cultured in the presence of G418.
Light and Electron Microscopy
Cells on glass coverslips were fixed with 3% paraformaldehyde, permeabilized with 0.1% Triton X-100, and labeled as described previously (de Hoop et al., 1994). Samples were viewed with either a Zeiss (Thornwood, NY) Axiovert or an Olympus (Tokyo, Japan) AX-70 microscope. For fluid-phase uptake experiments, MDCK cells were plated on nitrocellulose filters and cultured for 4 d to obtain a tight monolayer. Ovalbumin–Texas Red (10 mg/ml; Molecular Probes, Eugene, OR) in serum-free medium was added to the apical or basolateral chamber and incubated for 15 min at 37°C. Monolayers were fixed and labeled as described above. Imaging was performed with the use of a_Leica_ TCS 4D laser scanning confocal microscope (Arizona Research Laboratory, Division of Biotechnology, University of Arizona, Tucson) with the use of a 100× oil-immersion objective (numerical aperture 1.3).
For immunoelectron microscopic localization of EEA1 on frozen sections, BHK cells were incubated with 5 nm BSA–gold (OD520∼30) in the medium for 10 min at 37°C. They were then fixed with 8% paraformaldehyde in 100 mM phosphate buffer and processed for frozen sectioning (Griffiths, 1993). A431 cells were labeled with cholera toxin–binding subunit (CT-B)–gold (14 nm) at 4°C (Parton et al., 1994) and then warmed to 37°C for 10 min to label the early endosomes. Thawed frozen sections were labeled with antibodies to EEA1 followed by 10 nm protein A–gold (University of Utrecht, The Netherlands).
MDCK cells were perforated and labeled exactly as described previously (Ikonen et al., 1996). Briefly, MDCK cells grown on filters were perforated with the use of nitrocellulose filters applied to the apical surface. The opened cells were then labeled with anti-EEA1 followed by 5 or 10 nm protein A–gold and embedded in Epon after a tannic acid en bloc stain. Cells were cut perpendicular to the filter support. Sections were examined on a Zeiss EM10 microscope (European Molecular Biology Laboratory) or on a JEOL 1010 microscope (Center for Microscopy and Microanalysis, University of Queensland).
Subcellular Fractionation and Western Blotting
Synaptosomes were prepared from three mouse brains as described by Dunkley et al. (1988). The brain homogenate was centrifuged for 10 min at 1000 × g, and the supernatant was loaded onto a discontinuous Ficoll (Pharmacia LKB, Bromma, Sweden) gradient in isotonic sucrose. The synaptosomes that band at the 15–23% Ficoll interface were pooled, diluted with an equal volume of PBS, and pelleted by a 10-min centrifugation at 15,800 × g. The protein concentrations in the total brain homogenate, the 1000 × g pellet plus the corresponding supernatant, and the synaptosomal fraction were determined with the use of the Micro BCA assay (Pierce, Rockford, IL) according to the instructions of the supplier. The protein concentrations were verified by SDS-PAGE and staining with Coomassie Brilliant Blue R (Sigma). The fractions (25 μg of the synaptosomal material, 50 μg of the other fractions) were analyzed by Western blotting with the use of the following antibodies: mAb against synaptophysin-38 (dilution, 1:1000; Boehringer Mannheim, Mannheim, Germany); mAb against transferrin receptor (dilution, 1:1000; Zymed [San Francisco, CA], obtained from Wak Chemie, Bad Homburg, Germany); human polyclonal antibodies against EEA1 (dilution, 1:1000); and a rabbit polyclonal antibody against MAP2 (very generously provided by Dr. Diez-Guerra, Centro de Biologia Molecular, Madrid, Spain) at a 1:5000 dilution. The bound antibodies were detected with ECL according to the instructions of the supplier (Amersham International, Buckinghamshire, United Kingdom). The fractions were blotted twice, at least two different exposures of each autoradiogram were scanned on a Scanmaker III (Microtek, Rotterdam, The Netherlands), and the scans were quantitated on a Power Macintosh 6100/60 computer with Adobe (Mountain View, CA) Photoshop 3.0 and NIH Image 1.6 (developed at the U.S. National Institutes of Health, Bethesda, MD). Results are presented as percentages of the total amount of protein ± SD.
RESULTS
EEA1 Labels a Subdomain of the Early Endosome
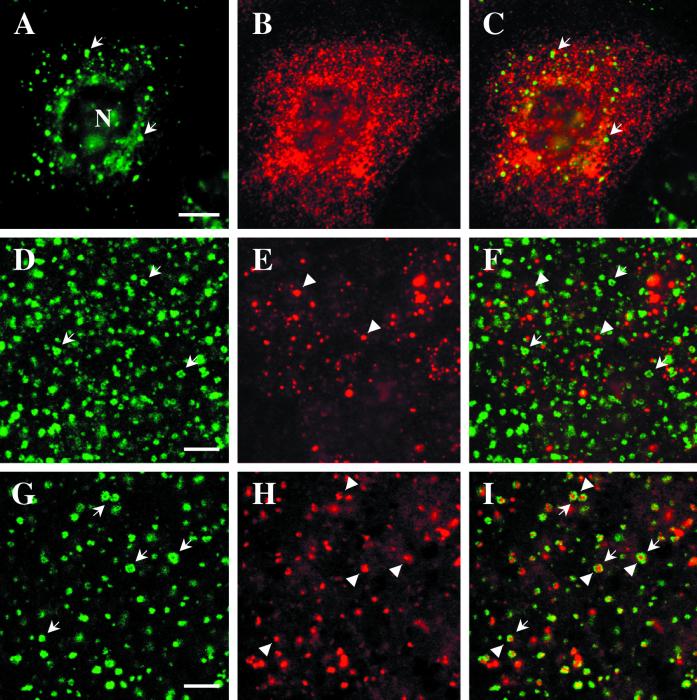
We first examined the distribution of EEA1 in fibroblasts. Previous studies showed that EEA1 associates with the early endosome, as judged by colocalization with internalized markers and with Rab5 (Mu_et al._, 1995; D'Arrigo et al., 1997). Because recent studies have shown a highly complex early endosomal organization consisting of at least two functionally distinct domains (see INTRODUCTION), we were interested in determining the domain of the early endosome with which EEA1 associates. In all cell types studied, EEA1 labeled distinct puncta dispersed throughout the cells (Figure1; see also Figures 7 and 8). We then examined the distribution of EEA1 (Figure 1, A and C) with respect to transferrin receptors (Figure 1, B and D) in A431 cells. In control cells, the two markers showed good, although incomplete, colocalization (Figure 1, A and B). To analyze the distribution of the two markers in more detail, the cells were treated with BFA, which causes tubulation of the endosomal system (Tooze and Hollinshead, 1992; Cid-Arregui_et al._, 1995). Under these conditions, the transferrin receptor is present within an extensive tubular endosomal network (Figure 1D) known to be organized by microtubules (Tooze and Hollinshead, 1992). In contrast, the EEA1 labeling appeared largely unaffected by BFA treatment (Figure 1C), remaining as punctate structures throughout the cell. Close examination of the labeled cells, however, suggested that the transferrin receptor–positive tubules appeared to interconnect the EEA1-positive puncta (Figure 1, C and D). These conditions demonstrate convincingly that EEA1 shows high specificity for one particular domain of the early endosomal system and is not distributed over the entire early endosomal compartment. Although the morphology of the EEA1-positive structures was indistinguishable from that of control cells, the number of EEA1-positive puncta decreased after BFA treatment (mean, 26 ± 5 puncta in control cells, 15 ± 5 in BFA-treated cells).
Figure 1.
EEA1 is associated with the sorting endosomes of A431 and CHO cells. (A–D) Control or BFA-treated A431 cells were labeled for the transferrin receptor (B and D) and for EEA1 (A and C). EEA1 and transferrin receptor colocalize partially in the untreated cells (A and B, arrows), although some transferrin-positive structures are negative for EEA1 (B, arrowhead). After BFA treatment, the transferrin receptor–containing elements are converted into an extensive tubular system that runs throughout the cell (D). The EEA1 labeling remains as discrete puncta (C). The only colocalization with transferrin receptor–positive elements is at foci from which tubules appear to emanate (C and D, arrows). (E–H) CHO cells were incubated with FITC–transferrin for 30 min at 37°C and then labeled for EEA1. (E and G) FITC–transferrin; (F and H) EEA1 labeling. EEA1 is distributed throughout the CHO cells (F), and some colocalization with peripheral transferrin (G) is evident (F, small arrows). However, there is no colocalization with internalized transferrin labeling the pericentriolar recycling endosome (E and F, large arrows). In G and H, cells were incubated with transferrin as in E and then further incubated for 20 min at 37°C in the presence or absence of 5 μg/ml BFA. In the absence of BFA, the bulk of the FITC–transferrin labeling was lost, but in the presence of BFA, the transferrin remains within the pericentriolar region (G, arrowheads). EEA1 shows a redistribution toward the pericentriolar region but negligible overlap with pericentriolar transferrin (H). Bars, 10 μm.
Figure 7.
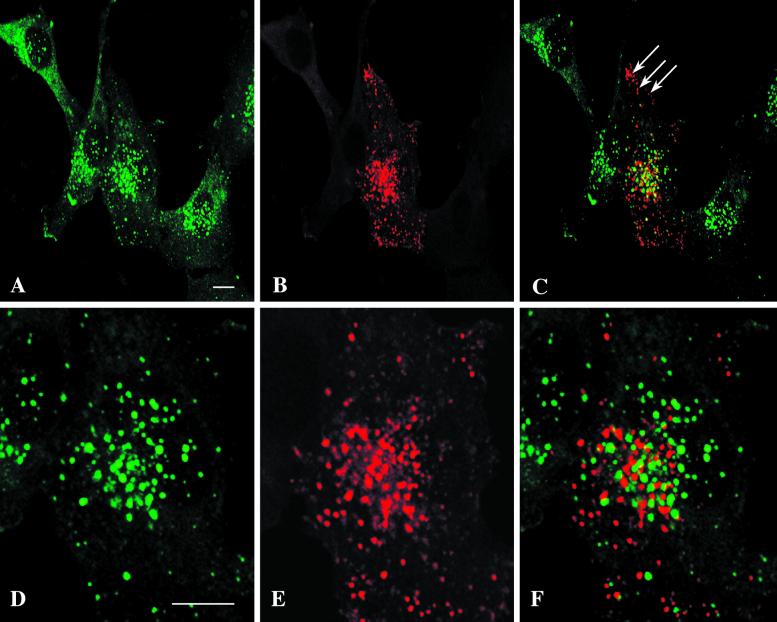
EEA1 labels a subset of endosomes in MDCK cells. MDCK cells that had been transfected with the apical endosomal marker endotubin were incubated with cycloheximide for 1 h at 37°C, fixed, and labeled with antibodies against EEA1 (A, arrows) and endotubin (B). The endotubin is present in a fine reticular network, whereas the EEA1 is present in punctate structures. There is little colocalization of the two markers (C, merged image). (D–I) Fluid-phase uptake in MDCK cells grown on filters. MDCK cells grown on filters were incubated with 10 mg/ml ovalbumin–Texas Red for 15 min at 37°C in the apical (D–F) or basolateral (G–I) chamber. The cells were fixed and labeled with anti-EEA1. For visualization of both EEA1 and ovalbumin, panels D–F show composites of three stacked images. EEA1 labeling (arrows in D, F, G, and I) is present in distinct ring-shaped structures. After apical uptake of fluid-phase marker (arrowheads in E and F), there is no colocalization of tracer and EEA1 labeling (F, merged image). Uptake of ovalbumin–Texas Red from the basolateral side (G–I) results in extensive colocalization of the markers, and the ovalbumin–Texas Red (arrowheads in H and I) fills the EEA1-positive rings (arrows in G and I) The images shown in panels G–I are from a single optical section. Bars, 5 μm.
Figure 8.
EEA1 and expressed endotubin label distinct endosomal populations in NRK cells. NRK cells were transfected with endotubin and after 24 h were labeled for EEA1 (A and D) and endotubin (B and E). The two markers label distinct endosomal elements, as shown in the overlays (C and F). This is particularly evident in peripheral areas of the cell (C, arrows), which contain endotubin-positive elements and no EEA1. The EEA1 and endotubin staining in the central area of the transfected cell in panels A–C is shown in panels D and E, respectively. Note the general lack of colocalization despite the close proximity of labeled elements. Bars, 10 μ m.
EEA1 Does Not Associate with the Recycling Endosome
Next, we examined the distribution of EEA1 with respect to the recycling endosome. For these studies, we used Chinese hamster ovary (CHO) cells, in which the recycling endosome is well characterized and shown to be highly concentrated in the pericentriolar region (Yamashiro_et al._, 1984; Ghosh and Maxfield, 1995; Marsh et al., 1995). FITC-labeled transferrin was internalized continuously for 30 min, conditions under which the main compartment labeled is the pericentriolar recycling endosome (Ullrich et al., 1996). EEA1 puncta were distributed throughout the cell (Figure 1F), with no enrichment in the pericentriolar region labeled with internalized transferrin (Figure 1E). Furthermore, double labeling with antibodies against Rab11, a marker of the recycling endosome, and EEA1 showed no significant colocalization (our unpublished results). This suggests that EEA1 is not highly enriched on the recycling endosome. We also examined the effect of BFA on the recycling endosome in these cells. FITC–transferrin was first internalized into the recycling endosome, and then the cells were further incubated in the presence or absence of 5 μg/ml BFA. Cells incubated in the absence of BFA showed a general decrease in transferrin labeling (our unpublished resuls), but BFA-treated cells showed a retention of FITC–transferrin in the recycling endosome consistent with a BFA-sensitive exocytosis of transferrin from this compartment (Figure 1G). In these cells, there was a slight increase in the EEA1 associated with the pericentriolar region (Figure 1H), but there was still negligible colocalization of the two markers. These results suggest that EEA1 is a marker of the early sorting endosome.
Ultrastructural Examination of EEA1 Distribution
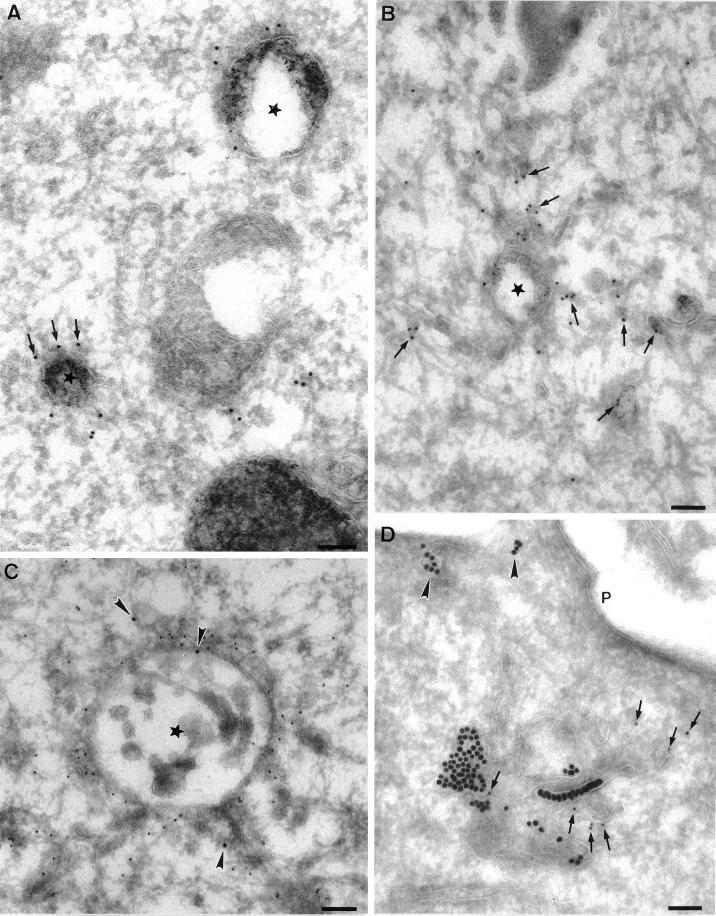
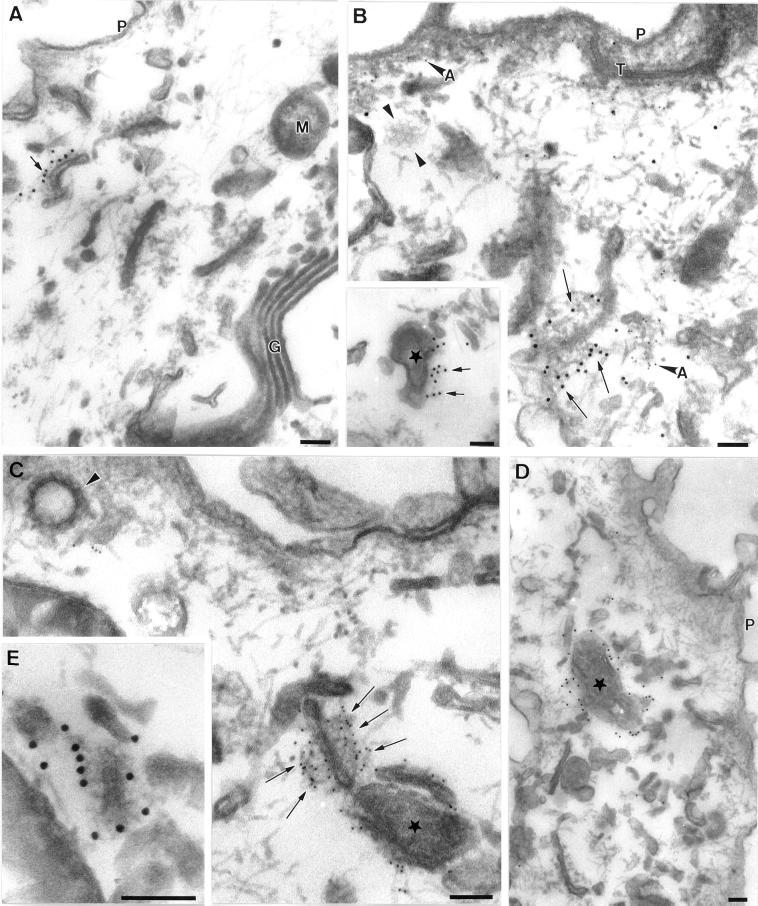
Because EEA1 was associated with a distinct early endosomal subpopulation, we next examined the morphology of the EEA1-labeled endosomes by immunoelectron microscopy. BHK cells were incubated with 5 nm BSA–gold as a fluid-phase marker for 10 min at 37°C (Figure2, A and B) and then immunolabeled with EEA1 with the use of 10 nm gold. EEA1 was predominantly associated with the cytoplasmic face of multivesicular/ring-shaped endosomes labeled with internalized gold (Figure 2A). In addition, EEA1 labeling could occasionally be observed in association with tubular elements in close proximity to the vacuolar domain (Figure 2B; also see Figure 3). These results suggest that EEA1 is predominantly associated with the endosomal sorting vacuoles, consistent with the immunofluorescent analysis of BFA-treated A431 cells (Figure 1).
Figure 2.
Immunoelectron microscopic localization of EEA1 in BHK and A431 cells. BHK (A and B) or A431 cells (C and D) were incubated with endocytic markers (BHK cells with 5 nm BSA–gold for 10 min at 37°C; A431 cells with 14 nm CT-B–gold) and then processed for frozen sectioning. Sections were labeled with antibodies to EEA1 followed by 10 nm protein A–gold. EEA1 labeling is predominantly associated with endosomal vacuoles (asterisks). (A) Labeling of two early endosomal vacuoles containing internalized 5 nm BSA–gold. Note that the labeling is clearly located at some distance from the membrane of the endosome in the cytoplasm (arrows). A putative late endosomal structure filled with internal membranes at the lower edge of the figure is unlabeled by EEA1. (B) Labeling for EEA1, which is in the area of the BSA–gold-labeled endosomal vacuole, but labeling is also associated with the tubular elements surrounding (and presumably emanating from) the endosomal vacuole (arrows). (C and D) CT-B–gold-labeled endosomes in A431 cells. (C) An extracted cell in which EEA1 labeling of the vacuole and associated membranes is particularly high. Arrowheads indicate internalized CT-B–gold. (D) An endosome with particularly high CT-B–gold labeling. EEA1 (arrows) is associated with the CT-B–gold-labeled structure. Also note the labeling of surface caveolae by the CT-B–gold (arrowheads). P, plasma membrane. Bars, 100 nm.
Figure 3.
Immunoelectron microscopic localization of EEA1 in MDCK cells. MDCK cells grown on polycarbonate filters were perforated with the use of nitrocellulose to remove parts of the apical surface. Cells were labeled for EEA1, fixed, and processed for Epon embedding with the use of tannic acid to increase staining of cytoplasmic coat proteins. All images show areas underlying the apical plasma membrane. Labeling for EEA1 (arrows) is associated with both tubular structures (A, B, C, and E) and vesicular elements (asterisks in C, D, and B inset) of the endocytic system. Mitochondria (M), Golgi (G), and other membranes are completely unlabeled. In addition, the plasma membrane (P) and clathrin-coated pits and vesicles (arrowheads in B and C; note clathrin lattice in B) are invariably completely negative for EEA1. EEA1 labeling is apparent on filamentous material surrounding the membrane compartments, particularly in B, C, and E. This filamentous material does not contain actin, as shown by double labeling for actin (A in panel B indicates anti-actin 5 nm gold labeling; arrows indicate 10 nm anti-EEA1 labeling). T, tight junction. Bars, 100 nm.
In view of the finding that membrane markers such as ricin preferentially localize to a distinct transferrin-negative endosomal compartment (Wilson and Colton, 1997), we also examined whether a membrane-bound marker of caveolae would be internalized into EEA1-positive elements. A431 cells were surface labeled with CT-B adsorbed to 14 nm gold at 8°C. This protocol specifically labels caveolae, with negligible labeling of clathrin-coated pits (Parton_et al._, 1994). The cells were then further incubated for 10 min at 37°C to allow gold internalization. CT-B–gold was found within surface caveolae and within EEA1-positive endosomes (Figure 2, C and D), indicating that EEA1-labeled endosomes receive internalized macromolecules via both clathrin- and caveolae-mediated endocytosis.
A notable finding in these studies was that EEA1 was often localized within the cytosol at some distance from the cytoplasmic face of the endosomal membrane (Figure 2). A similar observation was made in our previous studies with a less active antibody (Mu et al., 1995). Therefore, we sought to address the nature of the EEA1–endosome interaction. Because EEA1 labeling is consistently higher in extracted cells (Figure 2C), we used permeabilized cells and preembedding labeling. With this technique, the cell cytoskeleton is well visualized and the labeling efficiency is higher than with frozen sections (Ikonen_et al._, 1996). MDCK cells were permeabilized with the use of an apical rip-off technique before incubation with EEA1 antibodies and protein A–gold. Tannic acid was used to produce heavy staining of proteinaceous cytoplasmic material such as filaments and cytoplasmic coats (see, for example, the clathrin lattice in Figure 3B).
Heavy labeling for EEA1 was associated with the cytoplasmic face of a discrete subset of intracellular membranes (Figure3) comprising both tubular (Figure 3, A, B, C, and E) and vesicular (Figure 3, B–D) profiles. The tannic acid stain was not compatible with peroxidase labeling, but in parallel experiments the labeled elements were labeled by HRP internalized for 10 min (our unpublished results). The high labeling efficiency obtained with this technique demonstrated convincingly that clathrin-coated vesicles were devoid of EEA1 labeling (Figure 3, B and C). The absence of labeling of clathrin-coated vesicles is consistent with a role for EEA1 as a targeting molecule that provides directionality in clathrin-coated vesicle–to–endosome transport. The high contrast produced by the tannic acid stain allowed us to visualize extensive filamentous elements extending from the surface of the endosomes, which were heavily labeled by EEA1 antibodies (Figure 3). The filamentous material extended over 50 nm from the cytoplasmic surface of the endosome. Although the filaments resembled short actin filaments, they were not labeled with anti-actin antibodies; however, actin was seen in close proximity to labeled endosomes (Figure 3B). In view of the labeling of these elements and the postulated role of EEA1 in vesicle tethering/docking before fusion, we speculate that this material may represent an EEA1-containing tethering complex. The specific localization of EEA1 to the endosome and its absence from clathrin-coated pits/vesicles supports the view that this tethering complex is important in directional transport to the endosome.
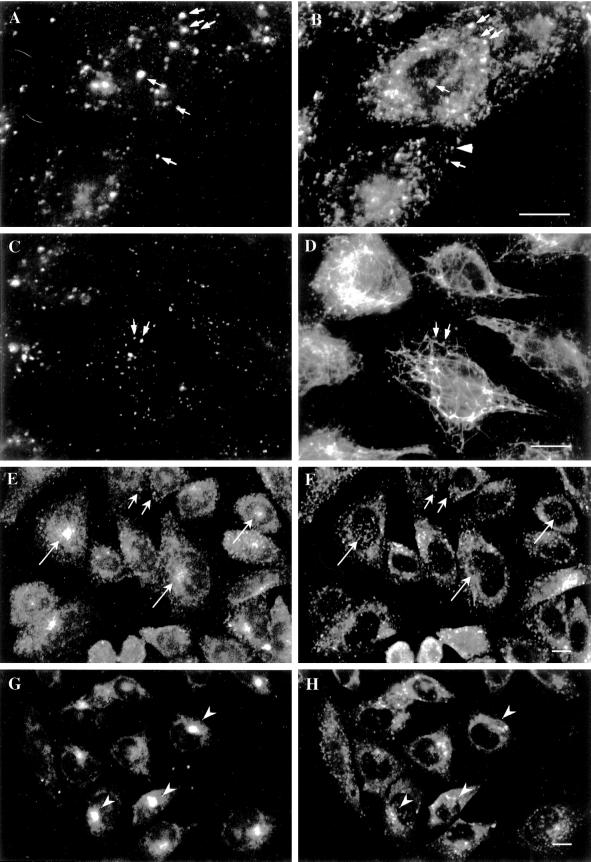
EEA1 Is Associated with Somatodendritic but Not Presynaptic Early Endosomes of Cultured Rat Hippocampal Neurons
Endosomal populations in neurons have been shown to differ between somatodendrites and axons, with transferrin-containing endosomes concentrated in the cell body and dendrites. Because EEA1 labels transferrin-containing endosomes, we next determined if EEA1 was present on a subset of endosomes in neurons. We examined the distribution of EEA1 in polarized hippocampal neurons in which the axonal and somatodendritic endosomal populations are well separated (Parton and Dotti, 1993). Previous studies have shown that the axonal and somatodendritic domains of polarized rat hippocampal neurons possess distinct endocytic circuits (Parton et al., 1992) but that they share common components of the endocytic machinery, such as the small GTPase Rab5 (de Hoop et al., 1994).
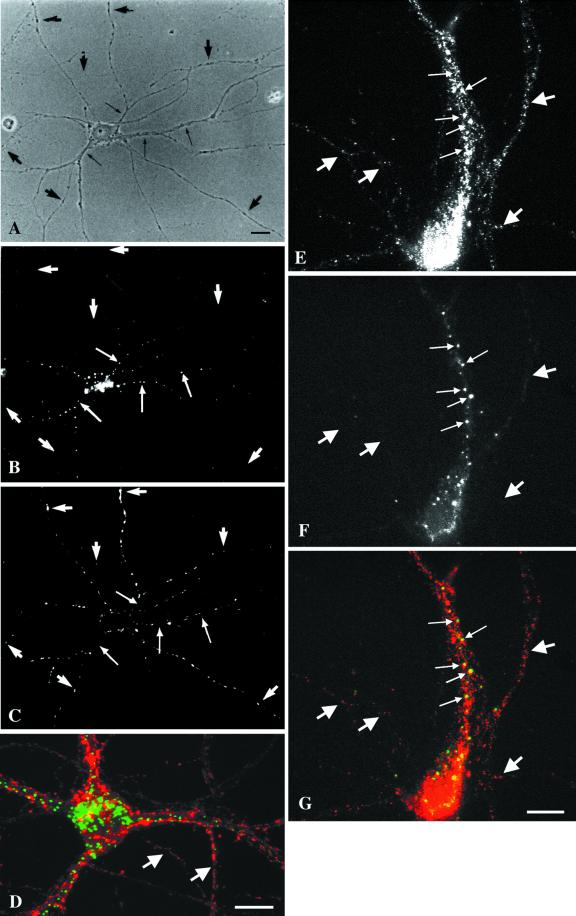
We first examined whether, as in fibroblasts, EEA1 is restricted to the early endosomal system. Double-labeling experiments showed that EEA1 colocalized with the early endosomal marker internalized rhodamine transferrin but not with the late endosomal/lysosomal marker LAMP1 (our unpublished results). We then examined the distribution of EEA1 with respect to the two populations of early endosomes in the axons and in the somatodendritic domain. Axons were distinguished from dendrites by their characteristic uniform shape and smaller diameter and by the absence of the dendritic marker protein MAP2 (Figure4). EEA1 staining, as determined by two different antisera, was observed only in the MAP2-positive somatodendritic domain (Figure 4). To further examine the distribution of EEA1 in these cells, we incubated differentiated neurons with antibodies against the extracellular domain of synaptotagmin. These antibodies are endocytosed exclusively into synaptic vesicles that reside in the axon terminals (Matteoli et al., 1992). Indeed, the internalized anti-synaptotagmin antibodies could be detected only in small dots present in thin, uniform neurites that run along the dendrites and the cell body (Figure5, A–D). This staining pattern colocalized with staining obtained with the use of antibodies against synaptophysin that mark clusters of synaptic vesicles in the axon terminals (our unpublished results). The internalized anti-synaptotagmin antibodies showed no colocalization with antibodies to EEA1 (Figure 5, A–D). To further demonstrate that EEA1 is undetectable on bona fide presynaptic endosomes, mature neurons were double labeled for Rab5 and EEA1 (Figure 5, E–G). Previous studies have shown that Rab5 is associated with both presynaptic and somatodendritic endosomes in rat hippocampal neurons (de Hoop et al., 1994). Consistent with these results, EEA1 and Rab5 colocalized on large punctate structures within dendrites (Figure 5, small arrows). In contrast, Rab5, but not EEA1, was detected within the axonal domains.
Figure 4.
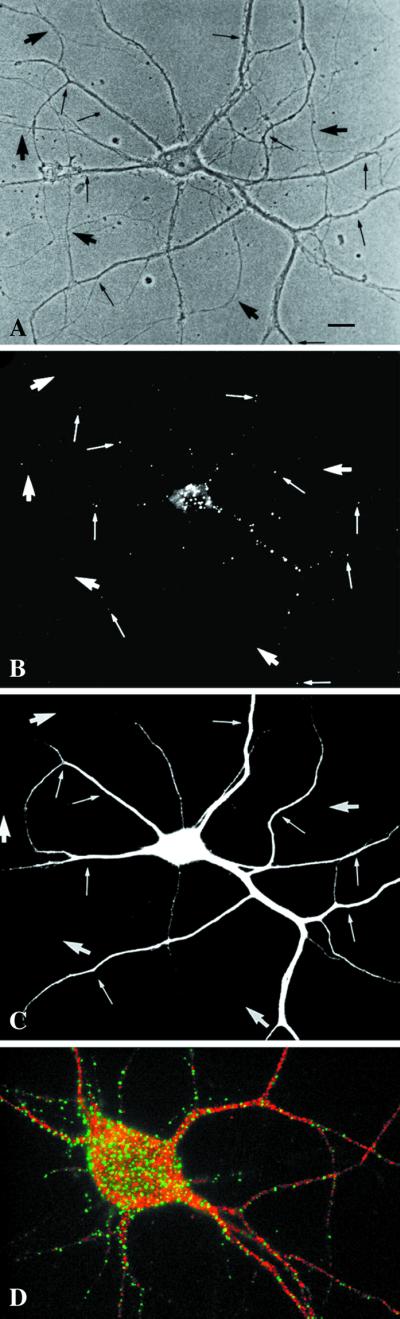
EEA1 is restricted to somatodendritic endosomes in neurons. (A–C) A mature hippocampal neuron by phase contrast (A) and stained for EEA1 (B) and MAP2 (C). The EEA1-positive endosomes (B) can be detected only in the dendrites (small arrows) identified by the presence of MAP2 (C). The large arrows mark the axons, visible in the phase-contrast image (A), that are MAP2 negative and that show no EEA1-positive staining. (D) An overlaid image of a different neuron showing that EEA1 (green) is present in MAP2-positive processes (red). Bar, 10 μm.
Figure 5.
EEA1 is undetectable in axonal endosomes but colocalizes with Rab5 in somatodendritic endosomes. (A–D) Nerve terminals and varicosities of mature hippocampal neurons were labeled by a 30-min incubation with rabbit antibodies against synaptotagmin. (A–C) A mature hippocampal neuron by phase contrast (A) and stained for EEA1 (B) and internalized synaptotagmin (C). Dot-like synaptotagmin-positive structures are present all along the thin axons (C, large arrows). EEA1 labeling is present in processes that do not contain synaptotagmin labeling (small arrows). (D) A merged image of a different neuron showing the labeling pattern of EEA1 (green) and synaptotagmin (red). There is no colocalization of the two markers (arrows indicate EEA1-negative, synaptotagmin-positive processes). (E–G) Mature neurons were labeled for Rab5 (E) and for EEA1 (F); the overlay of Rab5 (red) and EEA1 (green) is shown in G. Small arrows indicate Rab5- and EEA1-positive somatodendritic endosomes; large arrows indicate axons containing Rab5-positive, EEA1-negative endosomes. Bars, 10 μm.
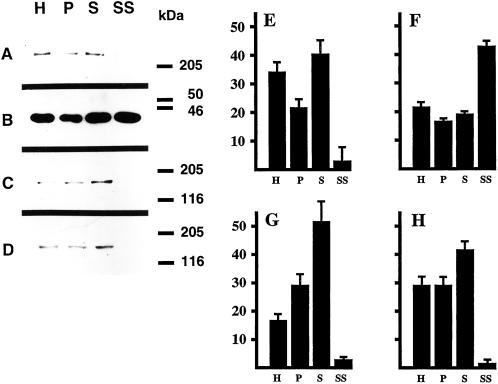
These results suggest that EEA1 is present in a subset of early endosomes. We next used a subcellular fractionation approach as an independent method to determine the relative levels of EEA1 in presynaptic membranes. Synaptosomes were prepared from rat brain, and the relative levels of a presynaptic marker (synaptophysin), a postsynaptic marker (MAP2; Dotti et al., 1988), and EEA1 (detected with two different antisera) were determined during the purification. Under these purification conditions, the resealed synaptosomes retain peripheral membrane components (de Hoop et al., 1994) and remain functional (Takei et al., 1996). As shown in Figure 6, purification of synaptosomes was accompanied by enrichment of synaptophysin but depletion of MAP2. EEA1 also showed a comparable depletion to the somatodendritic marker, MAP2, consistent with the absence of EEA1 from presynaptic endosomal membranes. These results suggest that EEA1 is a polarized component of the early endosomal machinery in rat hippocampal neurons.
Figure 6.
EEA1 is depleted from purified synaptosomes. Synaptosomes (pinched-off nerve terminals) were isolated from rat brain, and fractions from the brain homogenate (H), 10-min 100 ×g pellet (P), 10-min 1000 × g supernatant (S), and the synaptosomes (SS) were analyzed by Western blotting (A–D) with the use of antibodies against MAP2 (A and E), synaptophysin (B and F), EEA1 (human antiserum from patient 1; C and G), and EEA1 (human antiserum from patient 2; D and H). Sizes of molecular mass markers are indicated on the right. Note that 50 μg of protein was loaded in lanes H, S, and P, whereas only 25 μg of protein was loaded in lane SS. Blots were quantified as described in MATERIALS AND METHODS (E–H). The total amount of material (based on an equal amount of protein) loaded in all four lanes is considered to be 100%. Bars indicate the relative amount present in each fraction.
EEA1 Distribution in Epithelia
The polarized distribution of EEA1 in neurons and the postulated similarity between somatodendritic sorting and basolateral sorting in epithelia raise the possibility that EEA1 is a marker of basolateral/somatodendritic or cognate basolateral endosomes. We investigated this in MDCK cells, in which the apical and basolateral endosomes are well characterized. We used endotubin, an apical endosomal marker from developing intestine (Wilson and Colton, 1997), as a marker of the apical endosomes. When endotubin is expressed in MDCK cells, it is targeted to an apical early endosomal compartment that is distinct from transferrin-containing endosomes and is labeled only by apically internalized ricin (Gokay and Wilson, 2000). To determine the relationship between EEA1 and apical endosomes in epithelial cells, MDCK cells that had been stably transfected with the cDNA encoding endotubin were incubated with cycloheximide to deplete newly synthesized endotubin from the biosynthetic pathway. The cells were then fixed and labeled for immunofluorescence to determine the distribution of these markers. As shown in Figure7, in MDCK cells endotubin is seen in a fine, tubular-vesicular pattern, whereas EEA1 is present in large ring-like structures. Merging of the two images showed that there is little colocalization of EEA1 and endotubin, indicating that in this model system the two markers are associated with distinct domains/compartments of the endosomal system.
To further analyze the relationship between the apical and basolateral endosomes and EEA1, we incubated filter-grown MDCK cells with fluid-phase markers on either the apical or basolateral surface for 15 min followed by fixation and labeling for EEA1. These uptake conditions allow marking of distinct populations or subdomains of putative apical and basolateral sorting endosomes (Bomsel et al., 1989). As shown in Figure 7, the fluid-phase marker filled the EEA1-positive endosomes when internalization was from the basolateral surface. However, there was no colocalization of EEA1 and the marker taken up from the apical surface. Thus, EEA1 labels the basolateral sorting endosome but is not detectable on the comparable apical compartment. These results underscore the functional differences between these two endosomal populations and suggest that EEA1 could provide specificity to polarized endocytic transport processes.
EEA1 and Endotubin Are Associated with Distinct Endosomal Populations in Fibroblasts
In fibroblasts, EEA1 labels transferrin-positive early sorting endosomes. We next investigated whether it is also associated with the putative cognate apical endosomal compartment that is labeled by expressed endotubin (Wilson and Colton, 1997). For these experiments, endotubin was expressed in NRK cells. As in other cells, the endogenous EEA1 labeled a distinct population of uniformly sized punctate or ring-shaped elements (Figure8, A and D). Expressed endotubin showed a generally similar pattern of labeling to EEA1, with labeling of punctate structures throughout the cells (in marked contrast to the morphology of the endotubin-containing structures in polarized MDCK cells [Figure 7]). However, EEA1 and endotubin showed negligible colocalization (Figure 8). A consistent difference in the distribution of the endotubin and EEA1-labeled elements was the association of endotubin with EEA1-negative peripheral elements within cellular projections (Figure 8C, arrows). These results suggest that EEA1 and endotubin are targeted to distinct populations of endosomal elements and that apical and basolateral cognate endosomal populations exist in fibroblastic cells that can be discriminated by the presence of EEA1.
DISCUSSION
The early endosomal system of mammalian cells is organized into morphologically distinct elements and subdomains. As the machinery underlying vesicular traffic in the endocytic system is dissected, it becomes increasingly important to assign specific components of the molecular machinery to distinct transport steps or compartments. This is particularly evident in polarized cells, in which the endocytic system must receive and deliver material from two distinct surface domains. In the present study, we have investigated the subcellular distribution of one of these proteins, EEA1, in fibroblasts and in two model polarized cell systems, rat hippocampal neurons and MDCK epithelial cells. Our results show that EEA1 labels the sorting endosome of fibroblasts and associates with filamentous material, which we postulate represents the tethering complex required for the association of vesicles with the early sorting endosome before fusion. We also propose that EEA1 represents a specific marker of the somatodendritic sorting endosomes in neurons as well as the analogous compartment in fibroblasts and epithelial cells. The complexity of the endosomal system is emphasized by the demonstration that EEA1 and endotubin, an apical endosomal marker, label distinct early endosomal populations or subdomains in both epithelial cells and fibroblasts.
EEA1 Localization and Function
EEA1 represents one of the best-characterized early endosomal markers. EEA1 is a member of a family of conserved proteins possessing homology in a zinc finger–like domain, the FYVE finger. The FYVE finger domain is conserved in other proteins involved in membrane traffic, including the yeast proteins Fab1p (Yamamoto et al., 1995), Vac1p, and Vps27p (Piper et al., 1995). This domain of EEA1 possesses the region of the molecule mediating early endosome association, and this association requires the presence of phosphatidylinositol-3-phosphate (Stenmark et al., 1996), with which EEA1 shows a specific interaction (Simonsen et al., 1998). EEA1 is also a vital effector of the small GTPase Rab5 (Christoforidis et al., 1999), and the coordinated action of these two proteins is required for early endosome fusion. EEA1 was recently shown to be the only Rab5 effector that could confer minimal fusion activity in a reconstituted endosomal fusion assay and was suggested to play a docking/tethering role before SNARE-dependent fusion (Christoforidis et al., 1999; reviewed by Pfeffer, 1999). It has also been suggested that EEA1 may confer directionality on heterotypic fusion of coated vesicles with early endosomes.
The hypothesis that EEA1 acts as a tethering protein before fusion and confers unidirectional specificity to the early endosomal fusion event is entirely consistent with, and extended by, the results presented here. First, we have shown that EEA1 associates with a subdomain of the early endosomal system. Second, we have shown that EEA1-positive filamentous material extends some distance (>50 nm) from the early endosomal membrane. These filaments are not apparently composed of cytoskeletal elements, and it is interesting to speculate that this represents a tethering complex containing EEA1 that extends from the endosomal surface. This organization is intriguing in terms of the postulated size and shape of the EEA1 molecule, which may form an extended dimer of up to 80 nm in length (Callaghan et al., 1999). Our results suggest that these filaments may extend away from the endosomal membrane to capture incoming vesicles before docking and SNARE-dependent fusion. Such a model was recently proposed based on the size and shape of other tethering proteins (Pfeffer, 1999). Third, we have also been able to convincingly demonstrate that clathrin-coated vesicles lack EEA1 under conditions in which endosomal labeling is optimized through a preembedding labeling scheme. This further reinforces the idea that EEA1 could confer directionality and specificity to the heterotypic fusion event in which clathrin-coated vesicles fuse with early endosomes.
Many studies have suggested that early endosomes comprise sorting and recycling compartments (Gruenberg and Maxfield, 1995; Mukherjee_et al._, 1997). We now show that EEA1 is specifically associated with the sorting early endosome. This restricted distribution suggests that EEA1 can be used to define this early endosomal compartment and presumably represents the subdomain of the early endosomal system with which coated vesicles fuse. Ultrastructural analyses also revealed that EEA1 is mainly associated with the periphery of the vacuole-like multivesicular domain of the sorting endosome, both with the vacuolar domain itself and to a limited extent with nearby tubules. The distribution of EEA1 overlaps that of Rab5 but is more restricted (Figure 5F), raising the question of the precise distribution of the EEA1-binding lipid, phosphatidylinositol-3-phosphate, in the endosomal system. Surprisingly, upon BFA treatment of A431 cells the endosomal system became highly tubulated but the EEA1 subdomain showed no dramatic change in morphology. This treatment clearly identified the domain-restricted localization of EEA1. This is in contrast to the effects of the proton ATPase inhibitor bafilomycin A, which causes a tubulation of the EEA1-positive domain (D'Arrigo et al., 1997; Gu et al., 1997), and the phosphatidylinositol-3-phosphate kinase inhibitor wortmannin, which causes dissociation of EEA1 and subsequent tubulation of the early endosomal system (Patki et al., 1997). Although BFA had no affect on EEA1 tubulation, it did cause a change in the size and number of EEA1-positive elements, possibly reflecting changes in fusion events. In CHO cells, this was accompanied by a shift in distribution of EEA1 toward the pericentriolar region and an apparent decrease in transferrin exit from this compartment. Further work will be required to define the molecular events accompanying BFA treatment. However, our results clearly show that EEA1 associates with filamentous material associated with a subdomain of the early endosomal system.
Early Endosomes in Polarized Neurons
The organization of the early endosomal system of polarized cells is an area of active research and of some controversy. Some cells, such as neurons, have clearly separated early endosomal populations (Parton_et al._, 1992; Parton and Dotti, 1993; de Hoop et al., 1994). In other polarized cells, such as epithelia, the two domains are less well separated; morphological evidence has been presented for separate apical and basolateral endosome populations (Bomsel et al., 1989, 1990; Parton et al., 1989;Fujita et al., 1990; Van Deurs et al., 1990) supported by biochemical data (Bomsel et al., 1989) and in vitro fusion studies (Bomsel et al., 1990), but strong morphological evidence for an alternative single compartment serving both domains has also accumulated (Odorizzi et al., 1996;Futter et al., 1998; Gibson et al., 1998).
We first examined neurons as a model polarized system. The endosomal compartments of the somatodendritic region and axons of neurons have been thought to be both functionally and structurally distinct. The somatodendritic endosomes are enriched in transferrin receptors and presumably have a “housekeeping” role (Parton and Dotti, 1993), whereas the presynaptic endosomes contain recycling membrane proteins and may play a unique role in generating synaptic vesicles (de Hoop_et al._, 1994; Takei et al., 1996). However, despite the postulated distinct functions, previous studies have shown no clear differences in the molecular machinery of the sorting endosomes in these two domains. Rab5, one of the best-characterized early endosomal proteins, shows a functional association with both somatodendritic and axonal early endosomes (de Hoop et al., 1994). The two populations are also similar morphologically with multivesicular and tubular domains, although the tubular domain is far more extensive in the somatodendritic region (Parton et al., 1992). The demonstration of differences in sensitivity to BFA, however, suggests that their molecular compositions may be distinct (Cameron_et al._, 1991). We have now shown that EEA1 is associated exclusively with endosomes of the somatodendritic domains of mature rat hippocampal neurons and not with axonal endosomes marked by internalized antibodies to synaptotagmin. This finding provides strong evidence for the idea that these endosomal compartments are differentially regulated and raises the question of the nature of the Rab5 effector associated with the EEA1-negative presynaptic endosomes.
EEA1 and Endotubin Label Distinct Compartments/Endosomal Subdomains in Epithelia and Fibroblasts
In view of the controversy regarding polarized endosomal compartments in epithelial cells, the need for defined molecular markers of polarized endosomal subpopulations becomes increasingly evident. Here we show that EEA1 and the apical endosomal marker protein endotubin label morphologically distinct compartments in polarized epithelial cells and that fluid-phase tracers internalized basolaterally, but not apically, label the EEA1 endosome. In these cells, the endotubin-positive endosomes are accessible to apically but not basolaterally internalized ricin (Gokay and Wilson, 2000). The identification of differences in BFA sensitivity of endosomal compartments in MDCK cells and the accessibility of endotubin-positive endosomes to only apical membrane markers strongly suggest that apical and basolateral endosomes are structurally, and presumably functionally, distinct. This is supported by recent studies in the hepatocyte cell line WIF-B showing that wortmannin affected apical endosomal compartments but not basolateral endocytosis or transcytosis (Tuma et al., 1999).
We have used EEA1 and endotubin as markers to determine whether cognate endosomal populations exist in fibroblast-like cells. Fibroblasts have generally been considered nonpolarized cells compared with the classic models of cell polarity, as exemplified by epithelial cells and neurons, but recent evidence has suggested the existence of distinct pathways of exocytic transport in fibroblasts (Musch et al., 1996; Yoshimori et al., 1996) analogous to the apical and basolateral pathways of epithelia (Simons and Fuller, 1985;Rodriguez-Boulan and Nelson, 1989) or to the axonal and somatodendritic pathways in neurons (Dotti and Simons, 1990; Dotti et al., 1991). This polarity may extend to the endocytic pathways, because endotubin labels a distinct population of endosomal elements that are transferrin receptor negative, show a relative resistance to BFA treatment, and are labeled by a membrane-associated internalized probe but not fluid-phase markers (Wilson and Colton, 1997). In this study, we expressed endotubin in NRK cells and now show that the expressed endotubin and the EEA1 label distinct endosomal elements. These results suggest that EEA1 and endotubin represent markers for basolateral/somatodendritic and apical/axonal cognate sorting endosomes in fibroblasts and strongly strengthen the evidence for two sets of sorting endosomes in both polarized cells and fibroblast-like cells.
What might be the function of the apical and basolateral cognate endosomal populations in fibroblasts? The EEA1-positive transferrin-containing sorting endosome appears to be the principal sorting compartment involved in dissociating ligands from receptors and sorting receptors to different destinations, the so-called housekeeping functions of all cells. The axonal/apical endosomes may be more specialized. In neurons, the major function of these endosomes may be synaptic vesicle formation, and recent evidence has been provided for a novel process for synaptic vesicle formation from both the plasma membrane and the endosomal compartment in presynaptic terminals (Takei_et al._, 1996). In epithelia, a similar compartment may be involved in the regulated insertion of water channels into the apical plasma membrane (Lencer et al., 1990). The apical cognate pathway in nonpolarized cells may have similar properties. Recent results have demonstrated that D1 and D2 dopamine receptors are internalized into distinct early endocytic compartments, which may serve to segregate signaling pathways (Vickery and von Zastrow, 1999). One striking difference in the pattern of EEA1 and endotubin staining in NRK cells was the association of endotubin with EEA1-negative endosomal elements in the most peripheral areas of the cell, often including projections from the cell surface (Figure 8). This specialized distribution may reflect a specific role of these endocytic elements.
Although our results support the idea that EEA1 and endotubin label distinct, disconnected compartments, we cannot completely exclude the possibility that the two endosomal populations are connected and that the two markers label distinct domains. However, by analogy to the neuronal situation, in which axonal and somatodendritic early endosomes are separated by vast distances, we favor the view that these compartments are largely distinct. This is supported by the differential sensitivity of these compartments to BFA (Wilson and Colton, 1997).
The present studies define new markers that can be used for the molecular characterization of the endocytic pathways of mammalian cells. This will provide a framework for assigning the unique Rab proteins, SNARE proteins, FYVE finger proteins, and other machinery to distinct endosomal populations and for characterizing the apical/axonal and basolateral/somatodendritic endosomal populations in epithelia, neurons, and fibroblast-like cells in terms of both their composition and function. Moreover, our results suggest that EEA1 is a key component of the cellular machinery that is ideally placed to provide directionality and specificity to specific membrane fusion events in polarized and nonpolarized cells.
ACKNOWLEDGMENTS
We are very grateful to Elina Ikonen for assistance with permeabilization experiments, Agnès Hémar for the generous gift of rhodamine-labeled transferrin, Liane Meyn for the preparation of primary cultures of rat hippocampal neurons, and Inken Huttner for technical help. We also thank David James, Espen Stang, Harald Stenmark, Nia Bryant, and Judy Callaghan for comments on the manuscript, and Harald Stenmark and Marino Zerial for numerous discussions and for providing reagents. This work was supported by a grant to R.G.P. from the Australian Research Council and by National Institutes of Health grant DK43329 to J.M.W. The Center for Molecular and Cellular Biology is a Special Research Center of the Australian Research Council.
REFERENCES
- Bomsel M, Parton RG, Kuznetsov A, Schroer TA, Gruenberg J. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell. 1990;62:719–731. doi: 10.1016/0092-8674(90)90117-w. [DOI] [PubMed] [Google Scholar]
- Bomsel M, Prydz K, Parton RG, Gruenberg J, Simons K. Endocytosis in filter-grown Madin-Darby canine kidney cells. J Cell Biol. 1989;109:3243–3258. doi: 10.1083/jcb.109.6.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad USA. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan J, Simonsen A, Gaullier JM, Toh BH, Stenmark H. The endosome fusion regulator early-endosomal autoantigen 1 (EEA1) is a dimer. Biochem J. 1999;338:539–543. [PMC free article] [PubMed] [Google Scholar]
- Cameron PL, Südhof TC, Jahn R, de Camilli P. Colocalization of synaptophysin with transferrin receptors: implications for receptor biogenesis. J Cell Biol. 1991;115:151–164. doi: 10.1083/jcb.115.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- Cid-Arregui A, Parton RG, Simons K, Dotti CG. Nocodazole-dependent transport, and brefeldin A-sensitive processing and sorting, of newly-synthesized integral membrane proteins in cultured neurons. J Neurosci. 1995;15:4259–4269. doi: 10.1523/JNEUROSCI.15-06-04259.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arrigo A, Bucci C, Toh BH, Stenmark H. Microtubules are involved in bafilomycin A1-induced tubulation and Rab5-dependent vacuolation of early endosomes. Eur J Cell Biol. 1997;72:95–103. [PubMed] [Google Scholar]
- de Hoop M, Huber L, Stenmark H, Williamson E, Zerial M, Parton RG, Dotti CG. The involvement of the small GTP-binding protein rab5a in neuronal endocytosis. Neuron. 1994;13:1–20. doi: 10.1016/0896-6273(94)90456-1. [DOI] [PubMed] [Google Scholar]
- de Hoop M, Meyn L, Dotti CG. Culturing hippocampal neurons and astrocytes from fetal rodent brain. In: Celis JE, editor. Cell Biology: A Laboratory Handbook. Vol. 1. San Diego: Academic Press; 1998. pp. 154–163. [Google Scholar]
- Dotti C, Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990;62:63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Parton RG, Simons K. Polarized sorting of glypiated proteins in hippocampal neurons. Nature. 1991;349:158–161. doi: 10.1038/349158a0. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley PR, Heath JW, Harrison SM, Jarvie PE, Glenfield PJ, Rostas JAP. A rapid Percoll gradient procedure for isolation of synaptosomes directly from an S1 subfraction: homogeneity and morphology of subcellular fractions. Brain Res. 1988;441:59–71. doi: 10.1016/0006-8993(88)91383-2. [DOI] [PubMed] [Google Scholar]
- Dunn KW, McGraw E, Maxfield FR. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Reinhart F, Neutra M. Convergence of apical and basolateral endocytic pathways at apical late endosomes in absorptive cells of suckling rat ileum in vivo. J Cell Sci. 1990;97:385–394. doi: 10.1242/jcs.97.2.385. [DOI] [PubMed] [Google Scholar]
- Futter CE, Gibson A, Allchin EH, Maxwell S, Ruddock LJ, Odorizzi G, Domingo D, Trowbridge IS, Hopkins CR. In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules. J Cell Biol. 1998;141:611–623. doi: 10.1083/jcb.141.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaullier JM, Simonsen A, D'Arrigo A, Bremnes B, Stenmark H. FYVE finger proteins as effectors of phosphatidylinositol 3-phosphate. Chem Phys Lipids. 1999;98:87–94. doi: 10.1016/s0009-3084(99)00021-3. [DOI] [PubMed] [Google Scholar]
- Ghosh RN, Gelman DL, Maxfield FR. Quantification of low density lipoprotein and transferrin endocytic sorting HEp2 cells using confocal microscopy. J Cell Sci. 1994;107:2177–2189. doi: 10.1242/jcs.107.8.2177. [DOI] [PubMed] [Google Scholar]
- Ghosh RN, Maxfield FR. Evidence for nonvectorial, retrograde transferrin trafficking in the early endosomes of HEp2 cells. J Cell Biol. 1995;128:549–561. doi: 10.1083/jcb.128.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A, Futter CE, Maxwell S, Allchin EH, Shipman M, Kraehenbuhl JP, Domingo D, Odorizzi G, Trowbridge IS, Hopkins CR. Sorting mechanisms regulating membrane protein traffic in the apical transcytotic pathway of polarized MDCK cells. J Cell Biol. 1998;143:81–94. doi: 10.1083/jcb.143.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokay KE, Wilson JM. Targeting of an apical endosomal protein to endosomes in MDCK cells. Traffic. 2000;1:354–365. doi: 10.1034/j.1600-0854.2000.010408.x. [DOI] [PubMed] [Google Scholar]
- Griffiths G. Fine Structure Immunocytochemistry. Berlin: Springer-Verlag; 1993. [Google Scholar]
- Gruenberg J, Maxfield F. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Gu F, Aniento F, Parton RG, Gruenberg J. Functional dissection of COP-I subunits regulating multivesicular endosome biogenesis. J Cell Biol. 1997;139:1183–1195. doi: 10.1083/jcb.139.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E, Parton RG, Lafont F, Simons K. Analysis of the role of p200-containing vesicles in post-Golgi traffic. Mol Biol Cell. 1996;7:961–974. doi: 10.1091/mbc.7.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis TE. Microinjected antibodies against the cytoplasmic domain of vesicular stomatitis virus glycoprotein blocks its transport to the cell surface. EMBO J. 1986;5:931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer WI, Verkman AS, Arnaout MA, Ausiello DA, Brown D. Endocytic vesicles from renal papilla which retrieve the vasopressin-sensitive water channel do not contain a functional H+ATPase. J Cell Biol. 1990;111:379–389. doi: 10.1083/jcb.111.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh EW, Leopold PL, Jones NL, Maxfield FR. Oligomerized transferrin receptors are selectively retained by a lumenal sorting signal in a long-lived endocytic recycling compartment. J Cell Biol. 1995;129:1509–1522. doi: 10.1083/jcb.129.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli M, Takei K, Peris MS, Südhof TC, De Camilli P. Exo-endocytic recycling of synaptic vesicles in developing processes of cultured hippocampal neurons. J Cell Biol. 1992;117:849–861. doi: 10.1083/jcb.117.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills IG, Jones AT, Clague MJ. Involvement of the endosomal autoantigen EEA1 in homotypic fusion of early endosomes. Curr Biol. 1998;8:881–884. doi: 10.1016/s0960-9822(07)00351-x. [DOI] [PubMed] [Google Scholar]
- Mu FT, Callaghan JM, Steele-Mortimer O, Stenmark H, Parton RG, Campbell PL, McCluskey J, Yeo JP, Tock EP, Toh BH. EEA1, an early endosome-associated protein: EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J Biol Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Musch A, Xu H, Shields D, Rodriguez-Boulan E. Transport of vesicular stomatitis virus G protein to the cell surface is signal mediated in polarized and nonpolarized cells. J Cell Biol. 1996;133:543–558. doi: 10.1083/jcb.133.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Pearse A, Domingo D, Trowbridge IS, Hopkins CR. Apical and basolateral endosomes of MDCK cells are interconnected and contain a polarized sorting mechanism. J Cell Biol. 1996;135:139–152. doi: 10.1083/jcb.135.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Dotti CG. Cell biology of neuronal endocytosis. J Neurosci Res. 1993;36:1–9. doi: 10.1002/jnr.490360102. [DOI] [PubMed] [Google Scholar]
- Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Prydz K, Bomsel M, Simons K, Griffiths G. Meeting of the apical and basolateral endocytic pathways of the Madin-Darby canine kidney cell in late endosomes. J Cell Biol. 1989;109:3259–3272. doi: 10.1083/jcb.109.6.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Simons K, Dotti CG. Axonal and dendritic endocytic pathways in cultured neurons. J Cell Biol. 1992;119:123–137. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki V, Virbasius J, Lane WS, Toh BH, Shpetner HS, Corvera S. Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1997;94:7326–7330. doi: 10.1073/pnas.94.14.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. Transport-vesicle targeting: tethers before SNAREs. Nat Cell Biol. 1999;1:17–22. doi: 10.1038/8967. [DOI] [PubMed] [Google Scholar]
- Piper RC, Cooper AA, Yang H, Stevens TH. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J Cell Biol. 1995;131:603–617. doi: 10.1083/jcb.131.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Simons K, Fuller SD. Surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Aasland R, Toh BH, D'Arrigo A. Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger. J Biol Chem. 1996;271:24048–24054. doi: 10.1074/jbc.271.39.24048. [DOI] [PubMed] [Google Scholar]
- Takei K, Mundigl O, Daniell L, De Camilli P. The sv cycle: a single vesicle budding step involving clathrin and dynamin. J Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J, Hollinshead M. In AtT20 and HeLa cells brefeldin A induces the fusion of tubular endosomes and changes their distribution and some of their endocytic properties. J Cell Biol. 1992;118:813–830. doi: 10.1083/jcb.118.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma PL, Finnegan CM, Yi JH, Hubbard AL. Evidence for apical endocytosis in polarized hepatic cells: phosphoinositide 3-kinase inhibitors lead to the lysosomal accumulation of resident apical plasma membrane proteins. J Cell Biol. 1999;145:1089–1102. doi: 10.1083/jcb.145.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich, O., Reinsch, S., Urbé, S., Zerial, M., and Parton, R.G. (1996). Rab11 regulates recycling through the pericentriolar recycling endosome. 135, 913–924. [DOI] [PMC free article] [PubMed]
- Van Deurs B, Hansen SH, Petersen OW, Melby EL, Sandvig K. Endocytosis, intracellular transport and transcytosis of the toxic protein ricin by a polarized epithelium. Eur J Cell Biol. 1990;51:96–109. [PubMed] [Google Scholar]
- Vickery RG, von Zastrow M. Distinct dynamin-dependent and -independent mechanisms target structurally homologous dopamine receptors to different endocytic membranes. J Cell Biol. 1999;144:31–43. doi: 10.1083/jcb.144.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Colton TL. Targeting of an intestinal apical endosomal protein to endosomes in nonpolarized cells. J Cell Biol. 1997;136:319–330. doi: 10.1083/jcb.136.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Whitney JA, Neutra MR. Identification of an endosomal antigen specific to absorptive cells of suckling rat ileum. J Cell Biol. 1987;105:691–703. doi: 10.1083/jcb.105.2.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, DeWald DB, Boronenkov IV, Anderson RA, Emr SD, Koshland D. Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol Biol Cell. 1995;6:525–539. doi: 10.1091/mbc.6.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro DJ, Tycko B, Fluss SR, Maxfield FR. Segregation of transferrin to mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984;37:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- Yoshimori T, Keller P, Roth MG, Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchi P, Stenmark H, Parton RG, Orioli D, Lim F, Giner A, Mellman I, Zerial M, Murphy C. Rab17 regulates membrane trafficking through apical recycling endosomes in polarized epithelial cells. J Cell Biol. 1998;140:1039–1053. doi: 10.1083/jcb.140.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, Parton R, Chavrier P, Frank R. Localization of rab family members in animal cells. Methods Enzymol. 1992;219:398–407. doi: 10.1016/0076-6879(92)19039-9. [DOI] [PubMed] [Google Scholar]