Furin Processing and Proteolytic Activation of Semliki Forest Virus (original) (raw)
Abstract
The alphavirus Semliki Forest virus (SFV) infects cells via a low-pH-dependent membrane fusion reaction mediated by the E1 envelope protein. Fusion is regulated by the interaction of E1 with the receptor-binding protein E2. E2 is synthesized as a precursor termed “p62,” which forms a stable heterodimer with E1 and is processed late in the secretory pathway by a cellular furin-like protease. Once processing to E2 occurs, the E1/E2 heterodimer is destabilized so that it is more readily dissociated by exposure to low pH, allowing fusion and infection. We have used FD11 cells, a furin-deficient CHO cell line, to characterize the processing of p62 and its role in the control of virus fusion and infection. p62 was not cleaved in FD11 cells and cleavage was restored in FD11 cell transfectants expressing human furin. Studies of unprocessed virus produced in FD11 cells (wt/p62) demonstrated that the p62 protein was efficiently cleaved by purified furin in vitro, without requiring prior exposure to low pH. wt/p62 virus particles were also processed during their endocytic uptake in furin-containing cells, resulting in more efficient virus infection. wt/p62 virus was compared with mutant L, in which p62 cleavage was blocked by mutation of the furin-recognition motif. wt/p62 and mutant L had similar fusion properties, requiring a much lower pH than control virus to trigger fusion and fusogenic E1 conformational changes. However, the in vivo infectivity of mutant L was more strongly inhibited than that of wt/p62, due to additional effects of the mutation on virus-cell binding.
Many cellular proteins, including pro-parathyroid hormone, insulin pro-receptor, and extracellular matrix protein, are synthesized as proproteins that are functionally matured by limited proteolytic processing by cellular endoproteases (5, 10, 30, 43). These posttranslational modifications are crucial for normal cellular functions. Viruses also take advantage of this processing machinery to successfully produce infectious progeny viruses. The infectivity and tropism of enveloped viruses are determined by their surface glycoproteins, which are often synthesized as precursors that are matured by endoproteolytic cleavage. For many viruses, this cleavage is vital to viral infectivity and pathogenicity (26). For instance, the human immunodeficiency virus (HIV) gp160 protein (35), the influenza virus HA0 hemagglutinin (46), and the respiratory syncytial virus F protein (16) all must be cleaved for the virus to be infectious. In each of these examples, the C-terminal product of the cleavage reaction contains the viral fusion peptide and a transmembrane domain and interacts with target membranes to mediate virus fusion. This is in contrast to the class II virus fusion proteins of the flaviviruses and alphaviruses, in which endoproteolytic cleavage acts to process not the fusion protein itself but an interacting companion protein. Cleavage of this interacting protein is required in order to activate virus fusion and infection under physiological conditions (23, 31, 32, 48).
These endoproteolytic processing events are ascribed to the action of members of a large family of calcium-dependent serine endoproteases termed “subtilisin-like proprotein convertases” (SPCs) (reviewed in reference 4). There are seven mammalian SPCs identified to date: SPC1 (also termed furin/PACE), SPC2, SPC3 (PC1/PC3), SPC4 (PACE4), SPC5 (PC4), SPC6 (PC5/PC6), and SPC7 (PC7/LPC/PC8). Different cells are believed to express different combinations of SPCs. For example, while SPC2, SPC3, and the soluble form of SPC6 are expressed exclusively in neuronal cells, SPC5 is only found in testicular germ cells. Furin, SPC4, and SPC7 have a broad tissue distribution and similar localization in the trans-Golgi network (TGN). While the unique expression patterns of SPCs suggest that these enzymes carry out specialized functions, since all cells express more than one SPC, SPCs may also have functional redundancies.
Among the SPCs, furin is the most studied and best characterized. Furin traffics between the TGN and the cell surface, and its cleavage activity has been detected within the TGN, the endocytic pathway and the cell surface (reviewed in references 38 and 40). Furin cleaves after the minimal recognition motif -R-X-X-R- in a Ca2+-dependent reaction. Many precursor proteins harbor this recognition sequence at their cleavage sites, making furin the most obvious candidate for proprotein proteolytic maturation in the secretory pathway. Furin involvement has been demonstrated for the proteolytic cleavage of a variety of cellular proteins, bacterial toxins, and viral membrane proteins. However, the exact requirement for furin in proprotein cleavage is largely undefined. In some cases, furin has been shown to be essential, as in cleavage of the prM protein of the flavivirus tick-borne encephalitis (TBE) virus (48). In other cases, furin functions redundantly with other SPCs or even non-SPC family endoproteases. For instance, furin, SPC4, and a calcium-independent endoprotease are all implicated in the processing of HIV gp160 (3, 20, 22, 39)
A furin-deficient cell line, FD11, was isolated by ethyl methanesulfonate (EMS) mutagenesis of CHO cells and selection for resistance to a mutant of Bacillus anthracis toxin protective antigen (18). FD11 cells are resistant to a variety of toxins containing the furin recognition motif, and transfection of FD11 cells with a human furin construct restores susceptibility to these toxins. Complementation studies with a known furin-deficient cell line formally demonstrated that FD11 cells are deficient in furin, and subsequent studies confirmed the absence of furin transcripts in FD11 cells (17). The FD11 cell system has been widely used in studies of proteolytic cleavage activation of a variety of bacterial toxins (1, 17, 18).
The alphavirus Semliki Forest virus (SFV) infects cells via low-pH-dependent membrane fusion in endocytic organelles (reviewed in references 24 and 49). The SFV membrane contains heterodimers of E1 and E2, transmembrane glycoproteins of about 50 kDa each. E1 acts as the membrane fusion protein and interacts with target membranes via a hydrophobic fusion peptide, while the companion protein E2 assists in E1 folding and transport and binds to the plasma membrane receptor. E2 is derived from a precursor protein termed p62 by proteolytic cleavage in a post-TGN compartment (7). The cleavage takes place after an -R-H-R-R- motif. Cleavage is blocked by mutation of the last arginine residue in this motif to either leucine (termed mutant L [mL]) (31) or phenylalanine (23). Extensive studies have demonstrated that such mutant SFV envelope proteins are unable to carry out membrane fusion at the normal pH threshold of 6.2 and require a pH as low as 5.0 or below to trigger fusion (23, 32, 44). This dramatic acid shift in the pH threshold for fusion is due to a shift in the pH needed to release the E1-E2 dimer interaction, a critical early step required for subsequent conformational changes in E1 (44). Virus mutants such as mL bud efficiently, but have markedly decreased infectivity and require a more acidic pH to trigger virus fusion (44). This phenotype is reversed by cleavage of mL p62 with exogenous trypsin. Thus, p62 processing acts as an important regulatory mechanism for SFV fusion activity. The sequence motif at the cleavage site and the observed inhibition of cleavage by an antibody to the active site region of furin (45) suggest a possible function for furin in p62 cleavage.
In order to address the role of furin in authentic p62 processing, we evaluated p62 cleavage in vivo in FD11 furin-deficient cells and in vitro by using purified proteases. We also compared the fusion and infectivity profiles of uncleaved p62-containing wild type (wt) virus with those of mL in which cleavage is blocked by mutation. Our results indicate that although other SPCs can cleave p62 in vitro, within CHO cells furin was required for cleavage, presumably due to intracellular protease localization or activity. The p62 forms of both wt and mL showed a similar pH dependence for virus-membrane fusion and E1 conformational changes. Interestingly, however, the wt/p62 virus was less impaired in infectivity than mL. This difference was due to the susceptibility of wt p62 protein to cleavage during uptake in furin-containing cells and to the inhibitory effect of the mL mutation on virus-cell binding.
MATERIALS AND METHODS
Cells and viruses.
BHK-21 cells were maintained at 37°C in Dulbecco's modified Eagle's medium (DMEM) containing 5% fetal bovine serum, 100 U of penicillin, and 100 μg of streptomycin per ml (P/S), and 10% tryptose phosphate broth. The furin-deficient CHO cell line FD11, its parental cell line (CHO-K1), and FD11 cells transfected with the human furin gene (FD11+) were kindly provided by Stephen H. Leppla at National Institutes of Health (18). All three lines were cultured at 37°C in α-minimal essential medium supplemented with P/S and 10% fetal bovine serum. The FD11+ cell culture medium contained 400 μg of G418 per ml.
wt SFV was a well-characterized, plaque-purified isolate (15). To prepare a stock of unprocessed, p62-containing wt SFV (wt/p62), FD11 cells were infected with wt SFV at 1 PFU per cell for 1.5 h at 37°C and then washed and further incubated for 20 h at 37°C in MEM containing 40 mg of proline per liter and 10 mM HEPES (pH 7.5). The medium was collected, and cell debris was removed by centrifugation at 10,000 rpm for 30 min at 4°C in a Sorvall SS34 rotor. To prepare radiolabeled wt/p62 virus, FD11 cells were infected with wt SFV at 50 PFU per cell for 3.5 h and then labeled with 100 μCi of [35S]methionine-cysteine per ml for 20 h at 37°C in methionine-deficient DMEM containing P/S, 40 mg of proline per liter, and 1% fetal bovine serum (previously dialyzed against Hanks balanced salt solution [pH 8.0]). Viruses were harvested and gradient purified as previously described (11).
mL was a previously described SFV mutant with an arginine-to-leucine substitution in the furin recognition motif (-R-H-R-R- to -R-H-R-L-) that completely blocks p62 cleavage (44). An mL virus stock was prepared by in vitro transcription of the mL infectious clone (kindly provided by Peter Liljeström) and RNA electroporation of BHK cells as previously described (33, 44). The stock was activated by trypsin cleavage, and the titer was determined on BHK cells by using a modified plaque assay with a trypsin-containing overlay (27, 44). Radiolabeled unprocessed mL was prepared by infecting BHK cells with activated mL at 50 PFU per cell for 3.5 h at 37°C. Cells were then labeled as described above, but in DMEM deficient for methionine and cysteine, and the radiolabeled virus was purified as described above.
In vitro processing of p62.
Trypsin cleavage of radiolabeled or stock p62-containing wt or mL was performed by treatment with trypsin (type XIII, crystallized; Sigma Chemical Corp.) at 15 μg/ml for 30 min on ice. The reaction was terminated by treatment with 100 μg of soybean trypsin inhibitor (STI; Sigma) per ml for 10 min on ice. Cleaved viruses were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) or used directly for infection.
The expression and purification of soluble forms of human furin/hSPC1 and hSPC7 were performed as described previously (13). In vitro cleavage with furin was performed using 1 nM furin in cleavage buffer (100 mM HEPES [pH 7.5], 1 mM CaCl2, with the addition of 1 mM β-mercaptoethanol or 0.5% Triton X-100 as indicated) and incubating the samples for 2 h at 37°C. hSPC7 cleavage was similarly assayed, but with 1 nM hSPC7 in cleavage buffer 7 (20 mM Bis-Tris [pH 6.5], 1 mM CaCl2). Where indicated, virus samples were pretreated at pH 6.0 for 10 min at 37°C by addition of a calibrated amount of 20 mM morpholineethanesulfonic acid (MES) or 20 mM Bis-Tris (pH 5.8) and then returned to neutral pH by dilution in the relevant cleavage buffer. For the experiment in Fig. 2B, infected cells were radiolabeled as described below and lysed in furin cleavage buffer containing 1% Triton X-100 and 1 mM phenylmethylsulfonyl fluoride (PMSF).
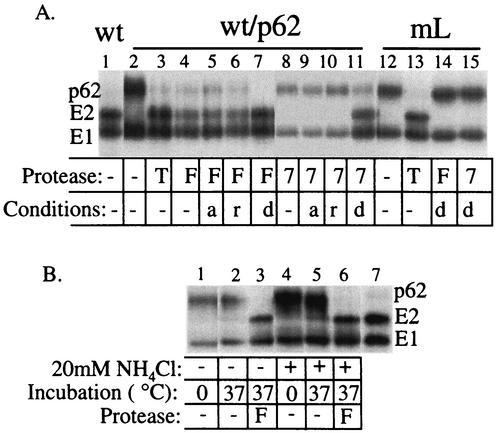
FIG. 2.
In vitro cleavage of wt/p62 and mL by furin, hSPC7, and trypsin. (A) [35S]methionine-cysteine-labeled wt SFV and mL were prepared in either BHK cells (wt, mL) or FD11 cells (wt/p62). These purified viruses were digested as indicated with 15 μg of trypsin per ml on ice for 30 min (T) or with 1 nM furin (F) or 1 nM hSPC7 (indicated as 7) for 2 h at 37°C. As indicated, protease digestion was performed in the presence of 1 mM β-mercaptoethanol (r) or 0.5% Triton X-100 (d), or with viruses that were pretreated at pH 6.0 and neutralized (a). The absence of protease or other treatments is indicated by −. Following digestion, the samples were acid precipitated and analyzed by SDS-PAGE. The data shown are representative of three separate experiments. (B) FD11 cells were infected with wt SFV as in Fig. 1 and radiolabeled for 2 h in the presence of 20 mM NH4Cl during and 30 min before the pulse or in the absence of NH4Cl. Cell lysates were incubated at 0 or 37°C for 2 h in the presence or absence of furin and analyzed by immunoprecipitation as in Fig. 1. Lane 7 is a purified wt virus control.
Virus cleavage during endocytic uptake.
Radiolabeled viruses were bound to the indicated cells in 35-mm-diameter plates in binding medium (RPMI without bicarbonate buffer, but containing 10 mM HEPES [pH 7.0] and 0.2% bovine serum albumin [BSA]) on ice for 1 h while shaking. The plates were then shifted to a 37°C water bath and incubated for 15 or 30 min to allow endocytic uptake of virus by the cells. Controls were maintained on ice. At the end of the incubation, the cells were placed on ice, washed with binding medium to remove any nonbound virus, lysed, immunoprecipitated with a polyclonal antibody against the SFV envelope proteins, and analyzed by SDS-PAGE (25).
Pulse-chase analysis.
Radiolabeling of virus-infected cells and immunoprecipitation of virus envelope proteins were performed as previously described (25). In brief, cells were infected with wt virus at 100 PFU per cell, pulse-labeled with 50 μCi of [35S]methionine-cysteine per ml for 20 min, and then chased in the absence of label for the indicated time. Media and cell lysates were immunoprecipitated with a polyclonal antibody against the SFV envelope proteins. Samples were analyzed by SDS-PAGE and fluorography.
Virus-cell binding, fusion, and infection assays.
[35S]methionine-cysteine-labeled viruses were incubated with BHK cells on ice in binding medium at the indicated pH. Cells were scraped in the binding medium and washed twice in the cold at the indicated pH. Cell-associated radioactivity was measured by liquid scintillation counting (41).
To quantitate virus-cell fusion, serial dilutions of virus were bound to BHK cells on ice as described above and treated with media at the indicated pH at 37°C for 3 min to trigger fusion. Alternatively, an infectious center assay was performed by infecting cells with serial dilutions of virus for 90 min at 37°C. Cells were then incubated at 28°C for 18 h in the presence of 20 mM NH4Cl to inhibit secondary infection. Infected cells were quantitated by immunofluorescence with an antibody to the SFV envelope proteins (34).
E1 homotrimer assay.
E1 homotrimer formation was assayed essentially as previously described (14). In brief, [35S]methionine-cysteine-labeled virus was mixed with 0.8 mM complete liposomes containing cholesterol, sphingomyelin, phosphatidylcholine, and phosphatidylethanolamine and treated at the indicated pH for 5 min at 37°C. Samples were placed on ice, adjusted to neutral pH, and solubilized for 3 min in SDS sample buffer at 30°C to preserve the E1 homotrimer. Samples were analyzed by SDS-PAGE, and homotrimer formation was quantitated by phosphorimager analysis.
RESULTS
The role of furin in p62 processing in vivo.
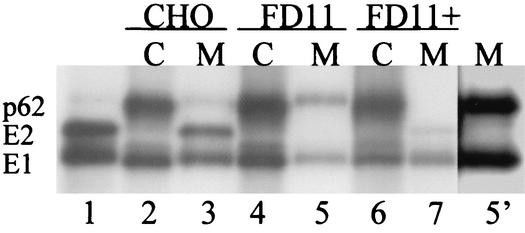
To characterize the role of furin in p62 processing, we made use of FD11 cells, a furin-deficient CHO cell line (18). FD11 cells, parental wt CHO cells, and FD11 cells transfected with furin were infected with wt SFV at high multiplicity and incubated for 4 h at 37°C. The expression and processing of the envelope proteins were then characterized by pulse-labeling with [35S]methionine-cysteine, chase in the absence of radiolabel, immunoprecipitation of the envelope proteins in the media and cell lysates, and analysis by SDS-PAGE (Fig. 1). As expected, all three cell lines were efficiently infected with SFV, as demonstrated by the robust expression of p62 and E1 detected immediately after a 20-min pulse-label (lanes 2, 4, and 6). Following a 4-h chase, the virus envelope proteins released in the medium of the wt CHO cells contained primarily E2 and E1, with a small amount of unprocessed p62 (lane 3). As expected, these proteins comigrated with E1 and E2 obtained from gradient-purified wt SFV prepared in BHK cells (lane 1). In contrast, the viral envelope proteins released from FD11 cells contained unprocessed p62 and E1 (lane 5), and E2 was not detected even in overexposed samples (lane 5′). This is in keeping with our finding that p62 cleavage was also inhibited in LoVo cells, another furin-deficient cell line (data not shown), and with previous work showing a block in Sindbis (SIN) virus processing in the furin-deficient CHO cell line RPE.40 (36, 53). Transfection of FD11 cells with furin restored efficient p62 processing (lane 7). Although cleavage was restored, virus production in these pulse-chase experiments was not as efficient in FD11+ cells as in the parental CHO cells, presumably due to the pleiotropic effects of the G418 selection required to maintain furin expression. We therefore used the parental CHO cell line with FD11 cells for comparative studies of virus growth kinetics in the presence and absence of furin, as presented below (Fig. 4). Together, our results indicate that furin is required for p62 cleavage during SFV maturation in CHO cells and that the wt virus propagated in FD11 cells (which we will refer to as wt/p62) appears to be exclusively in the p62 form.
FIG. 1.
Expression and processing of wt SFV membrane proteins in CHO, FD11, and FD11+ cells. wt CHO cells, furin-deficient FD11 cells, and FD11+ cells (permanently expressing furin) were infected with wt SFV at a multiplicity of 100 PFU per cell for 2 h. The incubation was continued for 4 h postinfection, and the cells were pulse-labeled with [35S]methionine-cysteine for 20 min and chased for 0 or 4 h in the absence of label. The media were collected, and the cells were lysed. The samples were immunoprecipitated with a polyclonal antibody against the SFV envelope proteins and analyzed by SDS-PAGE and fluorography. Shown are the cell lysate samples (C)at 0 h of chase (lanes 2, 4, 6) and the medium samples (M) after 4 h of chase (lanes 3, 5, and 7). Lane 1 is purified radiolabeled SFV, and lane 5′ is an overexposure of lane 5. The data are a representative example of two experiments.
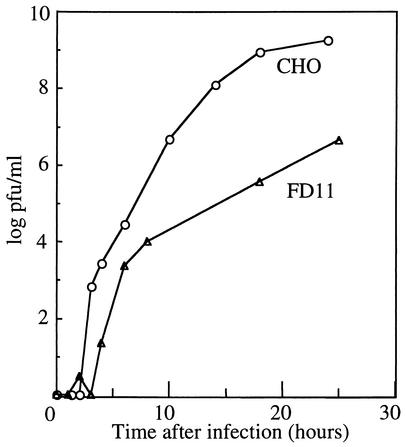
FIG. 4.
Growth kinetics of SFV on CHO and FD11 cells. CHO and FD11 cells were infected with wt SFV at a multiplicity of 0.01 PFU/cell. Cells were incubated in MEM containing P/S, 10 mM HEPES (pH 7.0), 0.2% BSA, and 40 μg of l-proline per ml for 1.5 h at 37°C, washed twice with this medium, and incubated for the indicated times after infection. Progeny virus released into the medium was quantitated by plaque assay on furin-containing BHK cells.
Requirements for in vitro cleavage of p62.
Previous results with the flavivirus TBE virus demonstrated that proteolytic maturation of the prM protein was mediated by furin and required prior exposure of the virus to mildly acidic pH (21, 48). TBE virus buds into the endoplasmic reticulum, and thus during transit through the secretory pathway, the virus particles would naturally be exposed to the low pH of the TGN. In contrast, alphaviruses such as SFV bud from the plasma membrane, and p62 processing normally occurs prior to budding (7). Prior work suggested that the p62 cleavage site is accessible to cleavage in the absence of preexposure to low pH (23). However, p62 cleavage in this study was tested with chymotrypsin and a mutant p62 containing a chymotrypsin site at the normal furin processing site (-R-H-R-F-). To characterize cleavage in the context of wt virus, we used FD11 cells to produce wt/p62 virus and assayed p62 cleavage in vitro by using a variety of proteases and treatment conditions (Fig. 2A). Native wt/p62 protein in virus particles was efficiently cleaved by very mild trypsin digestion on ice (lane 3) or by purified soluble furin after incubation at 37°C (lane 4). The efficiency of furin cleavage was not altered by pretreatment of virus at pH 6.0 (lane 5) or with reducing agent (lane 6), both treatments known to alter the conformation of the SFV envelope proteins (14, 25). The efficiency of furin cleavage was somewhat increased when the digestion was carried out in reaction buffer containing 0.5% Triton X-100 (lane 7), presumably reflecting an increase in the accessibility of the cleavage site upon disruption of the highly ordered virus structure. To further test the role of low-pH exposure in furin cleavage, virus-infected cells were pretreated and radiolabeled in the presence of 20 mM NH4Cl to neutralize the acidic pH of the TGN (48). We then incubated the cell lysates at 37°C in the presence or absence of added furin (Fig. 2B). No cleavage was observed in the absence of incubation at 37°C (lanes 1 and 4). A small amount of cleavage was observed in lysates incubated at 37°C in the absence of added furin (lanes 2 and 5), in keeping with cleavage by a cellular protease other than furin present in the FD11 cells (see below). In samples incubated in the presence of purified furin, p62 from the control and NH4Cl-treated cells was efficiently and comparably cleaved (lanes 3 and 6). p62 was also fully susceptible to in vitro furin cleavage when tested in lysates of cells treated with brefeldin A to disrupt transport to the TGN and thus block low-pH exposure (data not shown). Together these data indicate that furin processing of p62 differs from that of the prM protein of TBE virus, which requires prior exposure to a pH of <6.7 (48).
Most cells express more than one species of SPC, and redundant functions of those SPCs inside the cell have been suggested (1, 20). FD11 cells are deficient in both furin and SPC4 expression (16, 19), while LoVo cells lack furin but contain SPCs 4, 6, and 7 (summarized in reference 9). As discussed above, both cell lines are defective in p62 processing. Since SPC7 is also located in the TGN and shares some substrates with furin, we tested the ability of purified SPC7 to cleave p62 in vitro. In keeping with its known recognition motif, SPC7 cleaved p62 to produce a product that comigrated with authentic E2. However, cleavage only occurred if 0.5% Triton X-100 was included in the reaction buffer (Fig. 2A, lane 11), but not for p62 in intact virus particles with or without acid or mercaptoethanol treatment (Fig. 2A, lanes 8, 9, and 10), suggesting that the accessibility of the cleavage site is problematic for this enzyme.
To determine if the in vitro cleavage of wt/p62 by purified furin and SPC7 occurred at the correct site, mL was similarly digested. Neither furin nor SPC7 was able to cleave mL, even in optimal, detergent-containing buffer (Fig. 2A, lanes 14 and 15) or after treatment with pH 6.0 buffer or mercaptoethanol (data not shown), implying that wt/p62 protein cleavage was taking place specifically after the -R-H-R-R- motif. Trypsin cleavage, as previously reported (44), occurred efficiently for both wt and mL (Fig. 2A, lanes 3 and 13), suggesting that the R residues in the cleavage motif serve as a trypsin site. Together these results indicate that wt/p62 protein is efficiently cleaved in vitro by purified furin without a detectable requirement for low-pH exposure. The absence of cleavage by SPC7 in vivo and its properties in vitro suggest that this enzyme normally does not process p62 due to its relative activity or subcellular localization.
Cellular processing of exogenous virus.
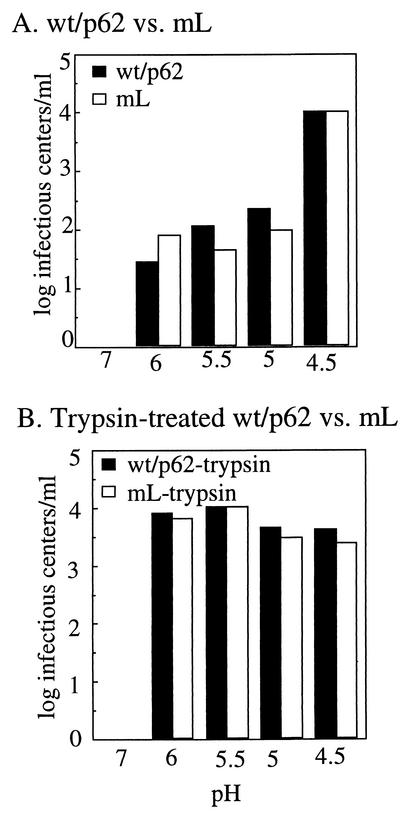
We tested the infectivity of wt/p62 and mL on BHK cells. Previous studies demonstrated that mL infectivity on BHK cells was dramatically increased by trypsin preactivation of p62 and that mL plaque formation required a trypsin-containing overlay to permit secondary infection (44). We treated wt/p62 and mL with trypsin and tested the level of activation by infectious center assay on BHK cells. mL had titers of 6.3 × 102 infectious centers (IC)/ml without trypsin and 9.3 × 106 IC/ml with trypsin. wt/p62 had titers of 2.0 × 105 IC/ml without trypsin and 8.9 × 106 IC/ml with trypsin. Thus, the primary infection of mL on BHK cells was increased about 15,000-fold by trypsin activation, in keeping with the prior results. In contrast, while wt/p62 infectivity on BHK cells was increased by activation with trypsin, the increase was about 45-fold, much less than that of mL. This result suggested that mL might have additional effects on virus infectivity aside from the role of p62 cleavage in activating virus fusion, as further investigated below. In addition, however, we evaluated the possibility that furin-containing cells might cleave wt/p62 to result in more efficient primary infection.
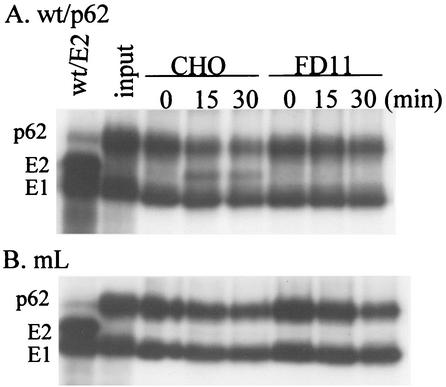
Furin is known to cycle between the TGN and the plasma membrane and is enzymatically active in various cellular compartments, including the TGN, cell surface, and endosome (38). The results in Fig. 2 indicated that in vitro, furin was able to cleave p62 on intact virus particles. We therefore tested whether cellular furin was able to cleave p62 when virus particles were exogenously added to cells (Fig. 3). Radiolabeled wt/p62 and mL viruses were bound to CHO or FD11 cells on ice. The cultures were incubated at 37°C to permit endocytic uptake of virus. A significant amount of wt/p62 protein was cleaved after uptake by CHO cells for 15 or 30 min, but not in samples maintained on ice (Fig. 3A). No cleavage was observed after wt/p62 uptake by FD11 cells, in keeping with the lack of furin in these cells (Fig. 3A). Cleavage of wt/p62 protein was at the furin recognition motif, since no processing of mL p62 was observed in either CHO or FD11 cells (Fig. 3B). Cellular cleavage of wt/p62, although incomplete, resulted in more efficient infection, as demonstrated by a 10-fold increase in wt/p62 infection on CHO versus FD11 cells (Table 1). This is in agreement with the finding that trypsin cleavage of only 10% of the p62 in mL virus particles resulted in a significant increase in virus infectivity (44). Little difference in infection efficiency was observed between CHO and FD11 cells infected either with wt/E2 virus, in which p62 is already cleaved, or with mL virus, in which p62 is not a furin substrate (Table 1). Together, these data indicate that cellular furin can cleave exogenous viral p62, resulting in increased viral infection.
FIG. 3.
p62 cleavage during virus uptake by CHO and FD11 cells. Radiolabeled wt/p62 (A) and mL (B) viruses were prepared as described in the legend to Fig. 2A and prebound to CHO or FD11 cells on ice for 60 min. The cells were then incubated at 37°C for the indicated times to allow endocytic uptake. The cells were lysed, and the samples were immunoprecipitated with antibody to the envelope proteins and analyzed by SDS-PAGE. The first lane in each panel shows the migration pattern of a radiolabeled wt/E2 virus control, while the second lane shows the input virus added to the cells. The results shown are representative of three independent experiments.
TABLE 1.
Effect of endogenous cellular furin on virus infectiona
| Virus | Cell type | Titer (IC/ml) |
|---|---|---|
| wt/p62 | FD11 | 4.0 × 104 |
| CHO | 3.8 × 105 | |
| wt/E2 | FD11 | 5.3 × 108 |
| CHO | 6.5 × 108 | |
| mL | FD11 | 3.7 × 102 |
| CHO | 8.3 × 102 |
Virus growth kinetics.
The FD11 cells provided a system to address the role of p62 cleavage on virus infectivity in the absence of any mutagenesis of the wt protein sequence. We first compared the growth kinetics of wt virus in the parental CHO cells and FD11 cells. Cells were infected with wt/E2 virus at a low multiplicity (0.01 PFU per cell). Progeny virus production was assayed by plaque titration on BHK indicator cells. As discussed above, since BHK cells contain furin, they permit relatively efficient infection and plaque formation of p62-containing virus due to p62 cleavage during virus uptake at 37°C. During the first 8 h of infection, levels of production of progeny virus were similar in FD11 cells and the parental CHO line, with a titer of about 103 PFU/ml (Fig. 4). This result suggests that primary infection and budding of wt/E2 in CHO and FD11 cells were about equally effective. After 24 h of infection, progeny virus production reached a maximum titer of ∼109 PFU/ml in CHO cells but only ∼107 PFU/ml in FD11 cells. This decrease in virus yield was expected because of decreased efficiency of secondary infection of FD11 cells by unprocessed, p62-containing progeny viruses (44). However, although the absence of furin cleavage had a strong effect, the observed 100-fold difference in virus yield between CHO and FD11 cells appeared to be less than that predicted from previous mL results. We therefore considered the possibility that the intact wt cleavage sequence might increase the efficiency of secondary infection via effects on binding to the cellular attachment factor heparan sulfate.
Virus-cell binding.
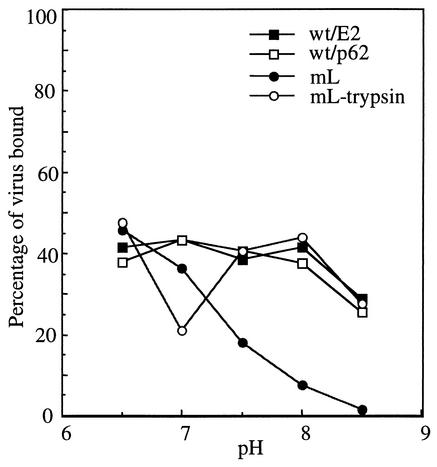
The intact alphavirus furin recognition sequence can mediate virus attachment to cell surface heparan sulfate (28, 47). The infectivity of mL would thus be predicted to be decreased both by the lack of p62 cleavage and by the loss of the furin cleavage motif and its effects on cell binding. Indeed, decreased binding of mL to cells was observed at neutral pH (44). To evaluate potential differences in virus binding, we carried out binding studies with BHK cells and radiolabeled wt/E2 virus, wt/p62 virus, mL virus, and mL virus pretreated with trypsin (Fig. 5). Viruses were bound to cells on ice in medium ranging from pH 6.5 to 8.5. wt/E2 virus exhibited a maximum binding efficiency of ∼40% and was relatively unaffected by the pH of the medium. The binding properties of wt/p62 virus were indistinguishable from those of wt/E2 virus. In contrast, the binding efficiency of mL, although similar to wt at pH 6.5, decreased markedly at a pH above 7.0, with essentially no binding at pH 8.5 (in agreement with reference 44). Activation of mL by trypsin cleavage restored its binding to a pattern similar to that of wt virus. These data therefore suggest that wt/p62 virus showed a less stringent defect in secondary infection due to its relatively efficient virus binding profile.
FIG. 5.
The pH dependence of binding of wt and mL virus to BHK cells. Radiolabeled virus preparations of wt/E2, wt/p62, and mL were prepared as described in the legend to Fig. 2A. mL was also precleaved with trypsin as described in the legend to Fig. 2A. Viruses were then incubated with 35-mm-diameter plates of BHK cells at the indicated pH on ice with shaking for 90 min. Unbound viruses were removed by washing at the indicated pH, and the bound radioactivity was quantitated by scintillation counting and expressed as a percent of the input radioactivity. The data shown are the average of two experiments.
Membrane fusion activity of wt/p62 and mL.
In order to directly analyze the fusion phenotype of wt/p62 virus, we monitored the pH-triggered fusion of prebound virus with the plasma membrane of BHK cells, a reaction that results in virus infection (50). This assay has the advantage that it has a very wide sensitivity range, since serial dilutions of virus are assayed and individual virus-infected cells are scored. Previous studies showed that mL fusion, although inefficient at the normal SFV fusion threshold of pH 6.2, can be triggered at pH values of 5.0 to 4.5 (44). wt/p62 or mL was prebound to BHK cells in the cold under optimal binding conditions for both viruses (pH 6.5). The cells were then pulsed for 3 min at 37°C with media of the indicated pH to trigger direct fusion of virus with the plasma membrane. Following fusion, the cells were returned to neutral pH and cultured in the presence of NH4Cl to prevent secondary infection. Infected cells were quantitated by immunofluorescence. As expected, inefficient fusion of mL was observed at pH 6.0 to 5.0, and fusion was increased about 100-fold by treatment at pH 4.5 (Fig. 6A). A comparable pattern of low-pH-triggered fusion was observed for wt/p62 virus. Pretreatment of mL and wt/p62 virus with trypsin to cleave p62 restored the normal wt SFV membrane fusion threshold of ∼pH 6.0 (Fig. 6B). Thus, wt/p62 produced in furin-deficient cells showed a membrane fusion phenotype comparable to that of mL, both requiring treatment at ∼pH 4.5 to trigger maximal fusion.
FIG. 6.
pH dependence of fusion of wt and mL viruses. wt/p62 and mL virus stocks were produced by growth in FD11 or BHK cells, respectively. In panel B, these viruses were preactivated by trypsin cleavage as described in the legend to Fig. 2A. To test the pH dependence of virus fusion, serial dilutions of the indicated virus stocks were prebound to BHK cells on ice for 90 min and then treated with media at the indicated pH in the presence of 20 mM NH4Cl at 37°C for 3 min to trigger virus-plasma membrane fusion. Cells were incubated at 28°C for 18 h in the presence of 20 mM NH4Cl, and virus-infected cells were scored by immunofluorescence. The highest titer for each virus was normalized to 104 infectious centers per ml.
E1 homotrimer formation in wt/p62 and mL.
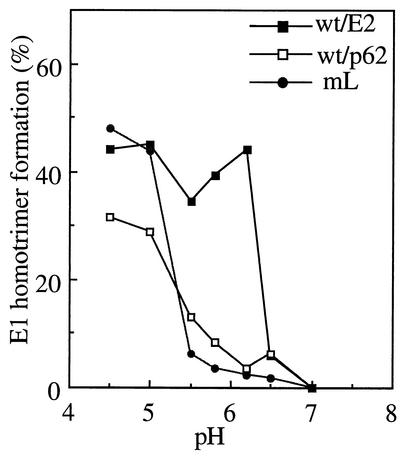
The p62/E1 dimer is more resistant to dissociation by acid pH than the E2/E1 dimer (51). This affects the overall fusion reaction, since an acid shift in dimer dissociation causes the pH threshold for fusogenic conformational changes in E1 to be similarly shifted to a more acidic pH (15, 44, 47). A key conformational change in E1 during the fusion reaction is the formation of a highly stable E1 homotrimer (14, 52). We assayed E1 homotrimer formation in wt/p62 virus to determine if the altered virus-membrane fusion threshold is the result of alterations in E1 conformational changes. Radiolabeled viruses were treated at the indicated pH for 5 min at 37°C in the presence of target liposomes, and homotrimer formation was quantitated by SDS-PAGE and phosphorimager analysis (Fig. 7). Efficient E1 homotrimer formation was observed with wt/E2 viruses at pH 6.2, the pH threshold for membrane fusion. In contrast, both mL and wt/p62 viruses showed a markedly more acidic pH requirement for E1 homotrimer formation, with maximal homotrimer formation observed at pH 4.5. This is in agreement with the observation of a more acidic pH threshold for the dissociation of the heterodimer in wt/p62 compared to wt/E2 (data not shown). Thus, the more acidic pH dependence of both wt/p62 and mL was due to the more acidic pH required for the conformational changes in their E1 proteins.
FIG. 7.
pH dependence of E1 homotrimer formation in wt/E2, wt/p62, and mL viruses. Radiolabeled wt/E2, wt/p62, and mL viruses were prepared as described in the legend to Fig. 2A. Viruses were treated at the indicated pH for 5 min at 37°C in the presence of 0.8 mM complete liposomes and then placed on ice and adjusted to neutral pH. Samples were mixed with SDS sample buffer, incubated at 30°C for 3 min, and then analyzed by SDS-PAGE. E1 homotrimer formation was quantitated by phosphorimager analysis and expressed as the percentage of the total E1 (monomer plus homotrimer) in each sample. The data shown are the average of two experiments for wt/p62 and mL and a representative example of three experiments for wt/E2.
DISCUSSION
We have here shown that processing of the SFV p62 protein was blocked in furin-deficient FD11 cells and restored by furin transfection. The FD11 cells thus provided a system to produce and characterize virus particles containing unprocessed p62 with the wt sequence. In vitro studies with purified enzymes demonstrated that furin efficiently cleaved p62 in native virus, while cleavage by SPC7 was dependent on detergent disruption of virus particles. Furin was also capable of processing p62 during cellular uptake of uncleaved virus, resulting in more efficient infection. While initial wt virus infection was similar in both CHO and FD11 cells, the final virus yield was ∼100-fold less from FD11 cells than that from CHO cells. The wt/p62 virus showed a very similar pH threshold for fusion and E1 homotrimer formation to that of mL containing a blocking mutation in the cleavage site. Since even small amounts of p62 cleavage increase virus fusion and infectivity (44), the comparable fusion properties of wt/p62 and mL confirm that processing of p62 was negligible in FD11 cells. Unlike wt/p62 virus, however, mL also showed decreased binding efficiency to cell surface receptors, particularly at basic pH.
Our data comparing mL and wt/p62 demonstrate that the extent of the viral growth defect in the absence of p62 cleavage was affected by both the fusion defect of the uncleaved virus and the binding effect of the intact furin recognition site. This is in agreement with studies demonstrating the binding properties of SIN mutants with impaired p62 processing. Those mutants with an intact furin recognition motif bound efficiently to BHK cells, while those with mutations or deletions in this motif were highly impaired for binding (28, 47). A cleavage mutant containing second site-resuscitating mutations showed both efficient binding due to its uncleaved furin site, and increased fusion and E1 homotrimer formation at the wt pH range (47). Interestingly, in vitro trypsin cleavage of p62 restores the binding capacity of the mL SFV mutant (Fig. 5) (44). Cryoelectron microscopy and computer reconstruction studies demonstrate that p62 cleavage causes a localized structural rearrangement in the projecting domain of SFV particles (12). Presumably this conformational change exposes previously hidden sites on mL E2 that are then available to mediate efficient virus-receptor binding.
What is the role of p62 in the function of E1? P62 rapidly associates with E1 in the endoplasmic reticulum and assists in its folding (2, 6, 37). Transport of the p62/E1 dimer through the exocytic pathway then occurs, and it has been hypothesized that p62 acts to protect E1 from prematurely triggering its fusion activity in the mildly acidic pH of the TGN (2). This is an appealing model given the evidence that the unprocessed dimer is more resistant to low pH (51). Unlike the prM protein of the flavivirus TBE virus (48), p62 cleavage does not require prior exposure to acidic pH. The pH of the TGN is ∼5.9 (8), and while this pH is low enough to trigger dissociation of the E2/E1 dimer and membrane fusion, it does not dissociate the unprocessed dimer (51) or cause any apparent conformational change. While the pH of the post-TGN compartment in which p62 is processed by furin is unknown, furin is active at neutral pH, and presumably cleavage would take place in an environment in which no further protection from exocytic acidity is necessary.
Of the seven known mammalian SPCs, furin (SPC1), SPC4, and the recently identified SPC7 appear to have redundant functions. Previous studies of anthrax toxin showed that both furin and SPC4 played a role in the proteolytic activation of the toxin protective antigen (18, 19). Studies of HIV gp160 cleavage demonstrated that processing involved furin, SPC4, and possibly other endoproteases (20, 22). These results are in accordance with the similarities in the substrate specificity and intracellular localization of furin, SPC4, and SPC7 and with their widespread expression pattern. Our data indicate that SFV p62 is not processed in the furin-deficient cell lines FD11 and LoVo, and previous studies demonstrated that cleavage of the prM protein of TBE virus is also blocked in LoVo cells (48). Thus cleavage is blocked in spite of the presence of SPCs 4 and 7 in LoVo cells (9). In vitro, we found that cleavage of viral wt/p62 by SPC7 required the presence of nonionic detergent in the reaction, suggesting that cleavage by this enzyme has more stringent accessibility requirements than furin. Taken together, the available data indicate that substrate selection among SPCs probably involves the intracellular localization of the protease and its steric constraints at the cleavage site, as well as the expression level of the protease.
Given that the in vitro studies indicated that furin was able to process p62 on intact virus particles, we tested whether exogenous p62 virus could be processed during its binding or endocytic uptake by furin-containing cells. Viral p62 was cleaved specifically in furin-containing cells, in a reaction that required 37°C incubation and the presence of an intact furin recognition motif and led to increased virus infectivity. Previous studies showed that the toxin proaerolysin can be activated by furin cleavage and that substantial cleavage occurred either during a 1-h incubation at 4°C or following ATP depletion, both conditions that prevent endocytosis (1). For wt/p62, no detectable cleavage was observed after incubation with cells for 90 min on ice, while a significant fraction of p62 was cleaved during a 15-min incubation at 37°C. Studies with an antibody to the furin active site show that endocytosed antibody can inhibit p62 processing in virus-infected cells, thus demonstrating the accessibility of furin to the endocytic pathway (45). Although we cannot exclude the possibility that cleavage of exogenous p62 virus takes place at the cell surface, taken together, the data are most consistent with furin processing within an endocytic compartment. Interestingly, the ability of cellular furin to cleave viral p62 may explain why the infectivity of SFV produced in RPE.40, a furin-deficient CHO line, was increased only about sixfold by trypsin cleavage (53), since the indicator cells used to assay infection contained furin and hence would be able to process p62. The full extent of the fusion defect in wt/p62 would only be observed when assayed under conditions that prevent p62 cleavage, such as in vitro virus-plasma membrane or virus-liposome fusion (47) or infection experiments on furin-deficient cells. Thus, our studies point out the complex effects of furin cleavage: it controls the pH dependence of virus fusion by regulating E1's dimer interactions and conformational changes, it affects the binding efficiency of virus to cells via both conformational changes in E2 and binding effects of the furin recognition motif, and it permits activation of fusion during virus uptake by furin-expressing cells.
The mechanism by which p62 processing destabilizes the E1/E2 heterodimer is as yet unclear, although at least a local conformational change is involved, as observed in the cryoelectron microscopy studies discussed above (12). The structure of the E1 ectodomain (29) and the general features of how it fits into the virus particle (29, 42, 54) have been defined. However, the exact protein regions that are involved in the p62-E1 interaction and their alteration upon cleavage are unknown. The FD11 cells provide a system to select for virus mutants that can grow more efficiently in the absence of furin processing. Such mutants could provide important information on the mechanism by which processing activates the activity of such class II virus fusion proteins.
Acknowledgments
We are grateful to Stephen Leppla for providing the FD11, FD11+, and parental CHO cells and for helpful advice on their properties. We thank Peter Liljeström for providing the mL construct and for helpful advice. We thank the members of our laboratory for helpful discussions and suggestions and for critical reading of the manuscript.
This work was supported by a grant to M.K. from the Public Health Service (R01 GM52929), by the Jack K. and Helen B. Lazar fellowship in Cell Biology, and by Cancer Center Core Support grant NIH/NCI P30-CA13330. This work was also supported by the Canadian Institues of Health research (CIHR) to R.D. R.D. is a fellow of the Fonds de la Recherche en Santé du Québec (FRSQ). M.F. holds a studentship from the Fonds pour la Formation de Chercheurs et d'Aide à la Recherche (FCAR-FRSQ).
REFERENCES
- 1.Abrami, L., M. Fivaz, E. Decroly, N. G. Seidah, F. Jean, G. Thomas, S. H. Leppla, J. T. Buckley, and F. G. van der Goot. 1998. The pore-forming toxin proaerolysin is activated by furin. J. Biol. Chem. 273**:**32656-32661. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, H., B.-U. Barth, M. Ekström, and H. Garoff. 1997. Oligomerization-dependent folding of the membrane fusion protein of Semliki Forest virus. J. Virol. 71**:**9654-9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendjennat, M., B. Bahbouhi, and E. Bahraoui. 2001. Purification and characterization of a Ca2+-independent endoprotease activity from peripheral blood lymphocytes: involvement in HIV-1 gp160 maturation. Biochemistry 40**:**4800-4810. [DOI] [PubMed] [Google Scholar]
- 4.Bergeron, F., R. Leduc, and R. Day. 2000. Subtilase-like pro-protein convertases: from molecular specificity to therapeutic applications. J. Mol. Endocrinol. 24**:**1-22. [DOI] [PubMed] [Google Scholar]
- 5.Bravo, D. A., J. B. Gleason, R. I. Sanchez, R. A. Roth, and R. S. Fuller. 1994. Accurate and efficient cleavage of the human insulin proreceptor by the human proprotein-processing protease furin. Characterization and kinetic parameters using the purified, secreted soluble protease expressed by a recombinant baculovirus. J. Biol. Chem. 269**:**25830-25837. [PubMed] [Google Scholar]
- 6.Carleton, M., H. Lee, M. Mulvey, and D. T. Brown. 1997. Role of glycoprotein PE2 in formation and maturation of the Sindbis virus spike. J. Virol. 71**:**1558-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.deCurtis, I., and K. Simons. 1988. Dissection of Semliki Forest virus glycoprotein delivery from the trans-Golgi network to the cell surface in permeabilized BHK cells. Proc. Natl. Acad. Sci. USA 85**:**8052-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demaurex, N., W. Furuya, S. D'Souza, J. S. Bonifacino, and S. Grinstein. 1998. Mechanism of acidification of the trans-Golgi network (TGN). In situ measurements of pH using retrieval of TGN38 and furin from the cell surface. J. Biol. Chem. 273**:**2044-2051. [DOI] [PubMed] [Google Scholar]
- 9.Dubois, C. M., F. Blanchette, M. H. Laprise, R. Leduc, F. Grondin, and N. G. Seidah. 2001. Evidence that furin is an authentic transforming growth factor-β1-converting enzyme. Am. J. Pathol. 158**:**305-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois, C. M., M. H. Laprise, F. Blanchette, L. E. Gentry, and R. Leduc. 1995. Processing of transforming growth factor beta 1 precursor by human furin convertase. J. Biol. Chem. 270**:**10618-10624. [DOI] [PubMed] [Google Scholar]
- 11.Duffus, W. A., P. Levy-Mintz, M. R. Klimjack, and M. Kielian. 1995. Mutations in the putative fusion peptide of Semliki Forest virus affect spike protein oligomerization and virus assembly. J. Virol. 69**:**2471-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferlenghi, I., B. Gowen, F. D. Haas, E. J. Mancini, H. Garoff, M. Sjoberg, and S. D. Fuller. 1998. The first step: maturation of the Semliki Forest virus spike occurs through a dramatic localized conformational change. J. Mol. Biol. 283**:**71-81. [DOI] [PubMed] [Google Scholar]
- 13.Fugere, M., P. C. Limperis, V. Beaulieu-Audy, F. Gagnon, P. Lavigne, K. Klarskov, R. Leduc, and R. Day. 2002. Inhibitory potency and specificity of subtilase-like pro-protein convertase (SPC) prodomains. J. Biol. Chem. 277**:**7648-7656. [DOI] [PubMed] [Google Scholar]
- 14.Gibbons, D. L., A. Ahn, P. K. Chatterjee, and M. Kielian. 2000. Formation and characterization of the trimeric form of the fusion protein of Semliki Forest virus. J. Virol. 74**:**7772-7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glomb-Reinmund, S., and M. Kielian. 1998. fus-1, a pH shift mutant of Semliki Forest virus, acts by altering spike subunit interactions via a mutation in the E2 subunit. J. Virol. 72**:**4281-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Reyes, L., M. B. Ruiz-Arguello, B. Garcia-Barreno, L. Calder, J. A. Lopez, J. P. Albar, J. J. Skehel, D. C. Wiley, and J. A. Melero. 2001. Cleavage of the human respiratory syncytial virus fusion protein at two distinct sites is required for activation of membrane fusion. Proc. Natl. Acad. Sci. USA 98**:**9859-9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon, V. M., R. Benz, K. Fujii, S. H. Leppla, and R. K. Tweten. 1997. Clostridium septicum alpha-toxin is proteolytically activated by furin. Infect. Immun. 65**:**4130-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon, V. M., K. R. Klimpel, N. Arora, M. A. Henderson, and S. H. Leppla. 1995. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect. Immun. 63**:**82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon, V. M., A. Rehemtulla, and S. H. Leppla. 1997. A role for PACE4 in the proteolytic activation of anthrax toxin protective antigen. Infect. Immun. 65**:**3370-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu, M., J. Rappaport, and S. H. Leppla. 1995. Furin is important but not essential for the proteolytic maturation of gp160 of HIV-1. FEBS Lett. 365**:**95-97. [DOI] [PubMed] [Google Scholar]
- 21.Heinz, F. X., and S. L. Allison. 2000. Structures and mechanisms in flavivirus fusion. Adv. Virus Res. 55**:**231-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inocencio, N. M., J. F. Sucic, J. M. Moehring, M. J. Spence, and T. J. Moehring. 1997. Endoprotease activities other than furin and PACE4 with a role in processing of HIV-I gp160 glycoproteins in CHO-K1 cells. J. Biol. Chem. 272**:**1344-1348. [DOI] [PubMed] [Google Scholar]
- 23.Jain, S. K., S. DeCandido, and M. Kielian. 1991. Processing of the p62 envelope precursor protein of Semliki Forest virus. J. Biol. Chem. 266**:**5756-5761. [PubMed] [Google Scholar]
- 24.Kielian, M. 1995. Membrane fusion and the alphavirus life cycle. Adv. Virus Res. 45**:**113-151. [DOI] [PubMed] [Google Scholar]
- 25.Kielian, M., S. Jungerwirth, K. U. Sayad, and S. DeCandido. 1990. Biosynthesis, maturation, and acid activation of the Semliki Forest virus fusion protein. J. Virol. 64**:**4614-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klenk, H. D., and W. Garten. 1994. Host cell proteases controlling virus pathogenicity. Trends Microbiol. 2**:**39-43. [DOI] [PubMed] [Google Scholar]
- 27.Klenk, H. D., R. Rott, M. Orlich, and J. Blodorn. 1975. Activation of influenza A viruses by trypsin treatment. Virology 68**:**426-439. [DOI] [PubMed] [Google Scholar]
- 28.Klimstra, W. B., H. W. Heidner, and R. E. Johnston. 1999. The furin protease cleavage recognition sequence of Sindbis virus PE2 can mediate virion attachment to cell surface heparan sulfate. J. Virol. 73**:**6299-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lescar, J., A. Roussel, M. W. Wien, J. Navaza, S. D. Fuller, G. Wengler, and F. A. Rey. 2001. The fusion glycoprotein shell of Semliki Forest virus: an icosahedral assembly primed for fusogenic activation at endosomal pH. Cell 105**:**137-148. [DOI] [PubMed] [Google Scholar]
- 30.Liu, B., N. Amizuka, D. Goltzman, and S. A. Rabbani. 1995. Inhibition of processing of parathyroid hormone-related peptide by anti-sense furin: effect in vitro and in vivo on rat Leydig (H-500) tumor cells. Int. J. Cancer 63**:**276-281. [DOI] [PubMed] [Google Scholar]
- 31.Lobigs, M., and H. Garoff. 1990. Fusion function of the Semliki Forest virus spike is activated by proteolytic cleavage of the envelope glycoprotein precursor p62. J. Virol. 64**:**1233-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lobigs, M., J. M. Wahlberg, and H. Garoff. 1990. Spike protein oligomerization control of Semliki Forest virus fusion. J. Virol. 64**:**5214-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu, Y. E., C. H. Eng, S. G. Shome, and M. Kielian. 2001. In vivo generation and characterization of a soluble form of the Semliki Forest virus fusion protein. J. Virol. 75**:**8329-8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marquardt, M. T., and M. Kielian. 1996. Cholesterol-depleted cells that are relatively permissive for Semliki Forest virus infection. Virology 224**:**198-205. [DOI] [PubMed] [Google Scholar]
- 35.McCune, J. M., L. B. Rabin, M. B. Feinberg, M. Lieberman, J. C. Kosek, G. R. Reyes, and I. L. Weissman. 1988. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell 53**:**55-67. [DOI] [PubMed] [Google Scholar]
- 36.Moehring, J. M., N. M. Inocencio, B. J. Robertson, and T. J. Moehring. 1993. Expression of mouse furin in a Chinese hamster cell resistant to Pseudomonas exotoxin A and viruses complements the genetic lesion. J. Biol. Chem. 268**:**2590-2594. [PubMed] [Google Scholar]
- 37.Molinari, M., and A. Helenius. 2000. Chaperone selection during glycoprotein translocation into the endoplasmic reticulum. Science 288**:**331-333. [DOI] [PubMed] [Google Scholar]
- 38.Molloy, S. S., E. D. Anderson, F. Jean, and G. Thomas. 1999. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 9**:**28-35. [DOI] [PubMed] [Google Scholar]
- 39.Moulard, M., and E. Decroly. 2000. Maturation of HIV envelope glycoprotein precursors by cellular endoproteases. Biochim. Biophys. Acta 1469**:**121-132. [DOI] [PubMed] [Google Scholar]
- 40.Nakayama, K. 1997. Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem. J. 327**:**625-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phalen, T., and M. Kielian. 1991. Cholesterol is required for infection by Semliki Forest virus. J. Cell Biol. 112**:**615-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pletnev, S. V., W. Zhang, S. Mukhopadhyay, B. R. Fisher, R. Hernandez, D. T. Brown, T. S. Baker, M. G. Rossmann, and R. J. Kuhn. 2001. Locations of carbohydrate sites on alphavirus glycoproteins show that E1 forms an icosahedral scaffold. Cell 105**:**127-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raghunath, M., E. A. Putnam, T. Ritty, D. Hamstra, E. S. Park, M. Tschodrich-Rotter, R. Peters, A. Rehemtulla, and D. M. Milewicz. 1999. Carboxy-terminal conversion of profibrillin to fibrillin at a basic site by PACE/furin-like activity required for incorporation in the matrix. J. Cell Sci. 112**:**1093-1100. [DOI] [PubMed] [Google Scholar]
- 44.Salminen, A., J. M. Wahlberg, M. Lobigs, P. Liljeström, and H. Garoff. 1992. Membrane fusion process of Semliki Forest virus. II. Cleavage-dependent reorganization of the spike protein complex controls virus entry. J. Cell Biol. 116**:**349-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sariola, M., J. Saraste, and E. Kuismanen. 1995. Communication of post-Golgi elements with early endocytic pathway: regulation of endoproteolytic cleavage of Semliki Forest virus p62 precursor. J. Cell Sci. 108**:**2465-2475. [DOI] [PubMed] [Google Scholar]
- 46.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69**:**531-569. [DOI] [PubMed] [Google Scholar]
- 47.Smit, J. M., W. B. Klimstra, K. D. Ryman, R. Bittman, R. E. Johnston, and J. Wilschut. 2001. PE2 cleavage mutants of Sindbis virus: correlation between viral infectivity and pH-dependent membrane fusion activation of the spike heterodimer. J. Virol. 75**:**11196-11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stadler, K., S. L. Allison, J. Schalich, and F. X. Heinz. 1997. Proteolytic activation of tick-borne encephalitis virus by furin. J. Virol. 71**:**8475-8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strauss, J. H., and E. G. Strauss. 1994. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 58**:**491-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vashishtha, M., T. Phalen, M. T. Marquardt, J. S. Ryu, A. C. Ng, and M. Kielian. 1998. A single point mutation controls the cholesterol dependence of Semliki Forest virus entry and exit. J. Cell Biol. 140**:**91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wahlberg, J. M., W. A. M. Boere, and H. Garoff. 1989. The heterodimeric association between the membrane proteins of Semliki Forest virus changes its sensitivity to low pH during virus maturation. J. Virol. 63**:**4991-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wahlberg, J. M., R. Bron, J. Wilschut, and H. Garoff. 1992. Membrane fusion of Semliki Forest virus involves homotrimers of the fusion protein. J. Virol. 66**:**7309-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watson, D. G., J. M. Moehring, and T. J. Moehring. 1991. A mutant CHO-K1 strain with resistance to Pseudomonas exotoxin A and alphaviruses fails to cleave Sindbis virus glycoprotein PE2. J. Virol. 65**:**2332-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, W., S. Mukhopadhyay, S. V. Pletnev, T. S. Baker, R. J. Kuhn, and M. G. Rossmann. 2002. Placement of the structural proteins in Sindbis virus. J. Virol. 76**:**11645-11658. [DOI] [PMC free article] [PubMed] [Google Scholar]