HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo (original) (raw)
Abstract
Microtubules are cylindrical cytoskeletal structures found in almost all eukaryotic cell types which are involved in a great variety of cellular processes. Reversible acetylation on the ε-amino group of α-tubulin Lys40 marks stabilized microtubule structures and may contribute to regulating microtubule dynamics. Yet, the enzymes catalysing this acetylation/deacetylation have remained unidentified until recently. Here we report that β-tubulin interacts with histone deacetylase-6 (HDAC-6) in a yeast two-hybrid assay and in vitro. We find that HDAC-6 is a micro tubule-associated protein capable of deacetylating α-tubulin in vivo and in vitro. HDAC-6’s microtubule binding and deacetylation functions both depend on the hdac domains. Overexpression of HDAC-6 in mammalian cells leads to tubulin hypoacetylation. In contrast, inhibition of HDAC-6 function by two independent mechanisms—pharmacological (HDAC inhibitors) or genetic (targeted inactivation of HDAC-6 in embryonic stem cells)—leads to hyperacetylation of tubulin and microtubules. Taken together, our data provide evidence that HDAC-6 might act as a dual deacetylase for tubulin and histones, and suggest the possibility that acetylated non-histone proteins might represent novel targets for pharmacological therapy by HDAC inhibitors.
Keywords: chromatin/cytoskeleton/deacetylase/HDAC (histone deacetylase)/tubulin
Introduction
In eukaryotes, the DNA in the cell nucleus is bound by histones and other chromosomal proteins to form a highly organized and compact structure called chromatin. A large body of evidence has demonstrated that chromatin plays a critical role in regulating gene expression. A number of post-translational protein modifications such as acetylation, methylation, ubiquitylation or phosphorylation of histones and other nuclear proteins create a code that orchestrates the organization and function of chromatin (Jenuwein and Allis, 2001). These modifications are interdependent and are under the control of signalling pathways yet to be fully deciphered. Histone acetylation has been shown to affect nucleosome stability and generally is considered to create a chromatin environment conducive to gene activation (Cheung et al., 2000). In addition to histones, many other proteins are also acetylated (reviewed by Polevoda and Sherman, 2002). For example, several transcription factors such as p53, E2F1 or c-Jun have been found to be acetylated. In this case, acetylation/deacetylation regulates, for example, the ability of these factors to bind to DNA (E2F1 or p53) or to interact with other proteins (c-Jun). Likewise, some cytoplasmic proteins such as α-tubulin are also known to be acetylated (Piperno et al., 1987). Acetylated tubulin is one of the charateristics of stabilized microtubules. The acetylases and deacetylases for non-histone substrates are mostly unknown.
The enzymes responsible for adding or removing acetyl groups on histones were only identified a few years ago. Acetylation of histones is catalysed by proteins that had been studied initially in other contexts, e.g. as tran scriptional coactivators, and which are now called histone acetyltransferases (HATs) (Brown et al., 2000; Marmorstein and Roth, 2001). Several of these HATs, however, are also able to acetylate non-histone proteins, and the list of new substrates continues to grow. Conversely, histone deacetylases (HDACs) are proteins that catalyse the removal of acetyl groups from histones and thereby lead to gene repression (Narlikar et al., 2002). Similarly to the HATs, HDACs may also deacetylate non-histone proteins. In cells, HDACs are often part of large multiprotein complexes that are recruited to promoter sequences through their interaction with specific DNA-binding transcription factors. HDACs have attracted a lot of attention not only for their role in transcriptional control but also because their pharmacological inhibition was found to have pleiotropic effects, such as induction of cell differentiation, arrest of cell growth (Richon et al., 2000) or prevention of tumour development in animal models. In addition, HDAC activity was found to be elevated in a number of tumours, potentially leading to deregulation of tumour suppressor genes (Marks et al., 2001). Thus, HDACs are considered as valuable targets for cancer treatment, and encouraging results have already been obtained with haematological malignancies (reviewed in Melnick and Licht, 2002). So far, >10 proteins with HDAC activity have been identified in mammalian cells. Based on sequence conservation, they fall into three classes. Class I has homology to the yeast global transcriptional regulator Rpd3 and comprises HDAC-1, HDAC-2, HDAC-3 and HDAC-8. Class II is composed of large proteins such as HDAC-4, HDAC-5, HDAC-6, HDAC-7, HDAC-9 and HDAC-10, all of which show homology to yeast Hda1. Finally, class III proteins have catalytic domains similar to that found in the yeast NAD+-dependent deacetylase Sir2 (Gray and Ekstrom, 2001; Khochbin et al., 2001; Kuzmichev and Reinberg, 2001).
Among the class II members, HDAC-6 is unique in that it has two hdac domains and also a C-terminal zinc finger domain that binds ubiquitin (Seigneurin-Berny et al., 2001). HDAC-10 appears to be the closest relative of HDAC-6, but it has only an incomplete second hdac domain (Fischer et al., 2002; Guardiola and Yao, 2002; Kao et al., 2002; Tong et al., 2002). Although several of the class II HDACs have been shown to play a role in transcriptional repression, some like HDAC-4, -5 and -6 have been found to shuttle between the nucleus and the cytoplasm (McKinsey et al., 2000a; Verdel et al., 2000). For HDAC-4 and -5, this is regulated by association with 14-3-3 proteins (Grozinger and Schreiber, 2000; McKinsey et al., 2000b) and, for HDAC-6, which is predominantly cytoplasmic, partial translocation into the nucleus was observed when cell proliferation was inhibited (Verdel et al., 2000). In addition, HDAC-6 has not been not found in any known HDAC-containing repression complexes. Thus, HDAC-6 might be functionally distinct from other known HDACs, and in particular might deacetylate substrates other than histones.
Here, we report the identification of β-tubulin as a protein interacting with HDAC-6. We show by a variety of assays that HDAC-6 is a microtubule-associated protein that can deacetylate α-tubulin in vitro. Pharmacological inhibition of HDAC-6 activity in culture cells leads to an increase in tubulin acetylation, and transient HDAC-6 overexpression reduces acetylation. Furthermore, using disruption of the HDAC-6 gene in embryonic stem (ES) cells, we provide compelling in vivo evidence that HDAC-6 regulates the status of tubulin acetylation. In contrast, global acetylation of histone H3 and H4 is not altered in these cells. Moreover, HDAC-6 was found to be a non-essential protein in ES cells.
Results
Identification of β-tubulin as a protein interacting specifically with HDAC-6
We sought to identify proteins that interact specifically with HDAC-6 and which might help explain the cellular role and regulation of this enzyme. To this end, we set up a yeast two-hybrid screen (Fields and Song, 1989; Gyuris et al., 1993) and prepared a LexA–HDAC-6 fusion protein, which was used as bait for screening an activation domain-tagged cDNA library derived from the human HeLa cell line. For the screen, we used either wild-type HDAC-6 or a version of this protein in which the histidine at positions 216 and 611 in each hdac domain had been mutated to alanine (HDAC-6-H216A/H611A). We reasoned that this double mutation might lead to stabilization of the HDAC–substrate interaction (Finnin et al., 1999) and thus might be advantageous for the identification of specific interactors. In transient transfection assays, these mutations in both hdac domains reduced the repression activity observed upon artificial recruitment of HDAC-6 to the promoter of a reporter plasmid, indicating that the enzyme is functionally inactivated (data not shown). From a screen of ∼2 × 106 colonies, 11 cDNA clones were identified that allowed growth of the yeast strain on selective medium. Four of these cDNAs were found to be multiple isolates of β-tubulin 2 or of its C-terminal portion (amino acids 323–446). However, in this assay, β-tubulin was found to interact equally with the wild-type or the mutated HDAC-6 bait (data not shown and see below). In addition, several clones encoding ubiquitin were also isolated in this screen, confirming recent results (Seigneurin-Berny et al., 2001). When retransformed into the parental yeast strain, containing the LexA– HDAC-6 fusion, these tubulin cDNAs all allowed growth on selective medium and also efficiently stimulated expression of a LexA-lacZ reporter in an HDAC-6-dependent manner (data not shown).
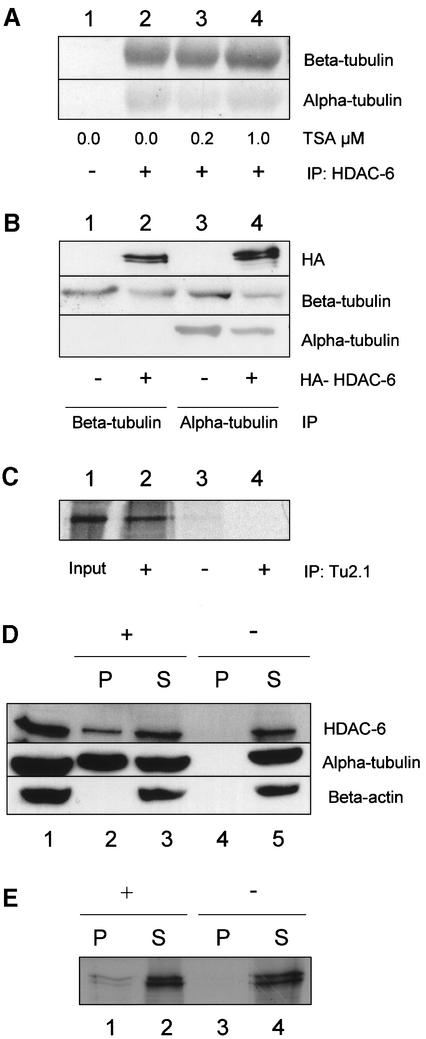
To test whether HDAC-6 and β-tubulin can also interact in mammalian cells, a co-immunoprecipitation assay was performed. First, cell extracts were prepared from NIH-3T3 cells and were immunoprecipitated with an antibody against HDAC-6 (Verdel et al., 2000). The precipitated material was separated by SDS–PAGE and the presence of tubulin was detected by western blotting with specific antibodies. As shown in Figure 1A, immunoprecipitation of HDAC-6 led to co-precipitation of β-tubulin and also of α-tubulin (lanes 2–4). Notably, treatment of the cells with increasing amounts of trichostatin A (TSA), a specific HDAC inhibitor, did not influence the interaction between HDAC-6 and tubulin (Figure 1A, lanes 3 and 4). Next, HEK 293T cells were transfected with a construct encoding an HDAC-6 protein tagged at the N-terminus with the haemagglutinin (HA) epitope. Subsequently, cell extracts were prepared and immunoprecipitated with antibodies against β- (Figure 1B, lanes 1 and 2) or α-tubulin (Figure 1B, lanes 3 and 4). In each case, HDAC-6 was found to co-immunoprecipitate with tubulin (Figure 1B, lanes 2 and 4). When β-tubulin was immunoprecipitated with antibody TU2.1, only HDAC-6, but not α-tubulin, was found in the precipitate (lane 2), indicating that this antibody disrupts the α/β-tubulin heterodimer. In contrast, immunoprecipitation of α-tubulin with antibody DM1A led to co-immunopre cipitation of HDAC-6 and also β-tubulin (lane 4). These observations suggest that the interaction between α-tubulin and HDAC-6 might be mediated by β-tubulin.
Fig. 1. HDAC-6 interacts with β-tubulin and microtubules in vivo and in vitro. (A) Co-immunoprecipitation assay. NIH-3T3 cells were treated for 6 h with TSA at the indicated concentration in order to inhibit HDAC activity. After cell extract preparation, HDAC-6 was immunoprecipitated with an anti-mouse HDAC-6-specific antibody (lanes 2–4) or with a control antibody (lane 1). The precipitate was analysed by SDS–PAGE and blotted onto nitrocellulose membranes. The filters were probed with antibodies against β-tubulin (TU 2.1, upper panel) or α-tubulin (DM1A, lower panel). (B) Co-immunoprecipitation assay in 293T cells. In this case, cells were transfected with an expression vector encoding HA-mHDAC-6 (lanes 2 and 4) or an empty expression vector (lanes 1 and 3). Two days after transfection, cell extracts were prepared and β- or α-tubulin was immunoprecipitated with specific antibodies (lanes 1 and 2 with TU2.1, lanes 3 and 4 with DM1A). The precipitates were analysed by western blotting: the filters were probed with antibodies against HA (upper panel), β-tubulin (TU2.1, middle panel) or α-tubulin (DM1A, lower panel). (C) In vitro interaction between HDAC-6 and tubulin. Purified bovine tubulin (Cytoskeleton, Inc.) was incubated with in vitro translated 35S-labelled mHDAC-6 (lanes 2 and 3) or with a control reticulocyte lysate (lane 4). β-tubulin was immunoprecipitated with the TU2.1 antibody (lanes 2 and 4), and the presence of HDAC-6 protein was detected by fluorography. Lane 1 contains 10% of the 35S-labelled mHDAC6 input. (D) HDAC-6 is part of the microtubule-associated proteins in NIH-3T3 cells. Microtubules were purified from NIH-3T3 cell lysate (lane 1) in the presence (lanes 2 and 3) or absence (lanes 4 and 5) of paclitaxel (taxol) and GTP. Microtubules were then pelleted by centrifugation. The proteins present in the pellet (lanes 2 and 4) and supernatant fractions (lanes 3 and 5) were analysed by western blotting using antibodies to detect mHDAC-6 (upper panel), α-tubulin (middle panel) and β-actin (lower panel). (E) Interaction between HDAC-6 and microtubules in vitro. Purified bovine tubulin (Cytoskeleton, Inc.) was assembled into microtubules in the presence (lanes 1 and 2) or absence (lanes 3 and 4) of paclitaxel (taxol) and GTP. Subsequently, in vitro translated 35S-labelled mHDAC6 was added and binding was allowed to proceed. Microtubules were then pelleted by centrifugation. The proteins present in the pellet (lanes 1 and 3) and supernatant (lanes 2 and 4) fractions were analysed by SDS–PAGE, and the presence of HDAC-6 was detected by fluorography.
HDAC-6 interacts with purified tubulin and microtubules in vitro
To test whether HDAC-6 interacts with tubulins directly, we used in vitro translated, radioactively labelled HDAC-6 and purified bovine tubulins for an in vitro interaction assay. For this, tubulin was pre-incubated with HDAC-6 and subsequently immunoprecipitated with an anti- β-tubulin antibody. The co-precipitation of HDAC-6 was detected by fluorography. As shown in Figure 1C, HDAC-6 bound purified tubulin (lane 2), whereas in the sample immunoprecipitated with a control antibody (lane 3), no HDAC-6 was detected.
Next, to test whether interaction could also be observed with the polymerized form of tubulin, microtubules were purified from NIH-3T3 cells (Tian et al., 2000) and the pellet fraction, which contains microtubules and their associated proteins (MAPs), was tested for the presence of HDAC-6. As seen in Figure 1D, HDAC-6 was found to associate with the polymerized microtubules (lane 2). In contrast, actin, a common potential contaminant, was not present in the pellet, showing the specificity of the MAP purification and therefore of the HDAC-6 interaction with tubulin. When GTP and the microtubules stabilizer paclitaxel (taxol) were omitted from the purification, no HDAC-6 was found in the pellet (lane 4), further demonstrating that the interaction is specific.
Finally, microtubules were assembled in vitro from purified dimeric tubulin, and radiolabelled HDAC-6 was added to the reaction mixture. Subsequently, polymerized microtubules were separated from tubulin by centrifugation. Again, HDAC-6 was found in the pellet fraction only when microtubules were stabilized by the addition of paclitaxel (Figure 1E, lane 1). Thus, HDAC-6 appears to interact with β-tubulin and also with microtubules.
Domain(s) of HDAC-6 required for interaction with β-tubulin
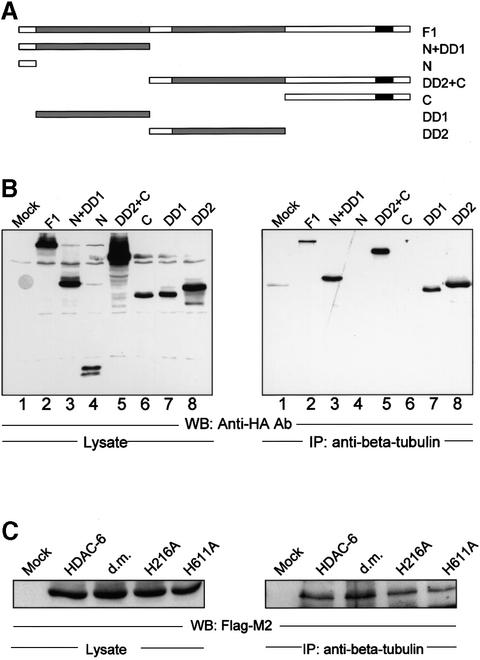
To define which domain(s) in HDAC-6 is required for interaction with β-tubulin, a series of HA-tagged HDAC-6 deletion mutants (Figure 2A; Seigneurin-Berny et al., 2001) were transfected into HEK 293T cells and association with tubulin was detected by a co-immunoprecipitation assay, as in Figure 1B. As shown in Figure 2B, the full-length protein (lane 2) and deletion mutants containing at least one hdac domain (lanes 3, 5, 7 and 8) were highly enriched in immunoprecipitates. In contrast, neither the N- nor the C-terminal portion of HDAC-6, which lack a hdac domain, was co-precipitated. These results demonstrate that the hdac domain is both necessary and sufficient for tubulin binding.
Fig. 2. HDAC-6 interacts with β-tubulin via its HDAC domains. (A) Schematic representation of the N-terminally HA-tagged HDAC-6 deletion constructs used. (B) Co-immunoprecipitation assay. 293T cells were transfected with the HDAC-6 expression vectors (lanes 2–8), and cellular extracts were prepared. HDAC-6 expression was measured by western blot with the anti-HA antibody (left panel). Association with β-tubulin was measured by performing an immunoprecipitation with an anti-β-tubulin antibody, followed by analysis of the precipitate by western blotting with the anti-HA antibody (right panel). (C) 293T cells were transfected with Flag-tagged HDAC-6 constructs and cell extracts were subjected to immunoprecipitation by anti-β-tubulin antibody. In the mutants proteins, the histidine at position 216 or 611 were mutated to alanine. d. m., double mutant protein.
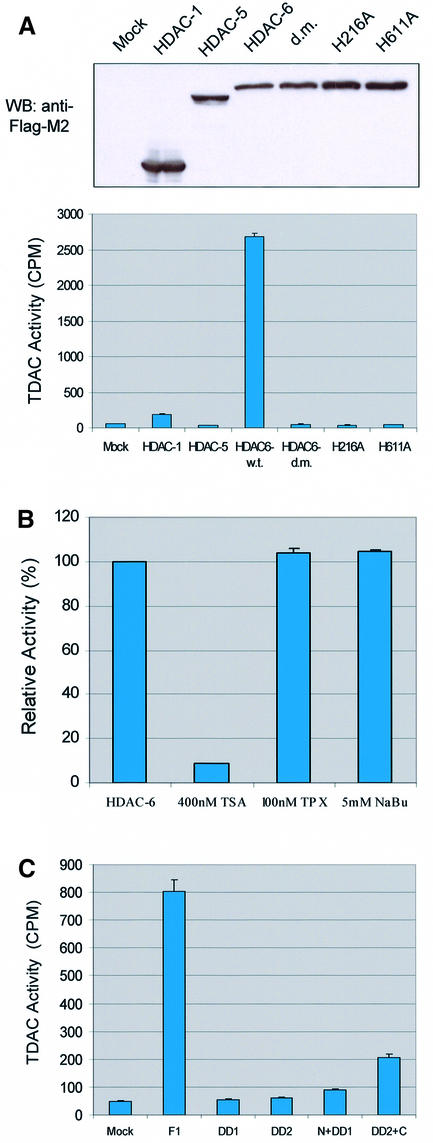
We then made use of HDAC-6 mutants that have point mutations in either or both of the hdac domains (Grozinger et al., 1999). Interestingly, both the single and double mutants could be co-precipitated as efficiently as the wild-type enzyme (Figure 2C). This indicates that the binding between HDAC-6 and β-tubulin, while being mediated by the hdac domain, does not depend on its catalytically active centre.
Partial co-localization between HDAC-6 and microtubules in NIH-3T3 cells
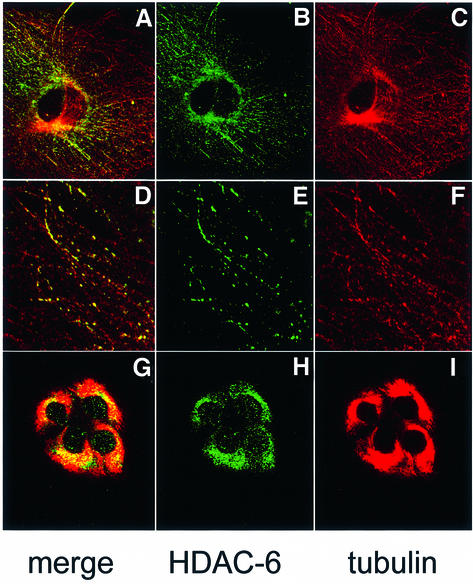
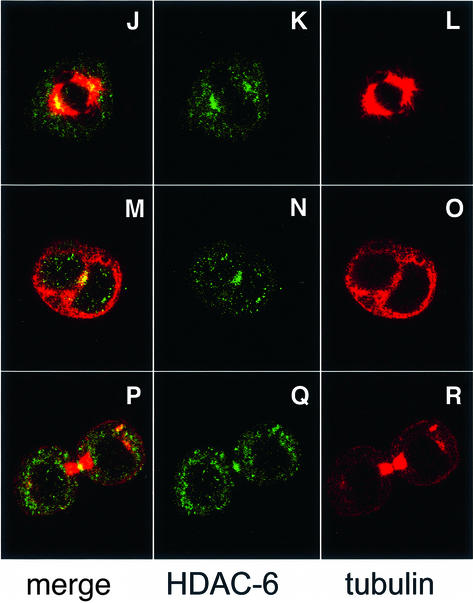
The biochemical interaction between HDAC-6 and β-tubulin or microtubules prompted us to test whether some HDAC-6 co-localizes in mammalian cells with the microtubule network. For this, NIH-3T3 cells were fixed and microtubules as well as HDAC-6 were revealed by immunostaining followed by confocal microscopy. As seen in Figure 3A–F, HDAC-6 shows a predominantly cytoplasmic distribution with a distinct punctuated pattern and an accumulation in particular around the nucleus, in agreement with earlier reports (Verdel et al., 2000). This distribution partly coincides with the microtubule network and, when examined at high magnification, a fraction of the microtubules appear decorated by HDAC-6 (Figure 3D–F). Furthermore, treatment of the cells with the microtubule stabilizing agent paclitaxel (taxol) led to re-organization of the microtubules and to concomitant changes in the distribution of HDAC-6 (Figure 3G–I). In contrast, the localization of HDAC-6 remained unaffected in cells treated with latrunculin B, a drug inducing the disassembly of the actin cytoskeleton (data not shown). This pattern of HDAC-6 localization strongly suggests that a major and perhaps primary role of this deacetylase is associated with the microtubule network and not with the control of histone acetylation. We next examined the localization of HDAC-6 during cell mitosis and found that it does not co-localize with DNA (see Supplementary figure S1 available at The EMBO Journal Online) but with the mitotic spindle in metaphase (Figure 3J–L) and also with γ-tubulin, a centrosomal marker (Supplementary figure S1). Interestingly, during cytokinesis, HDAC-6 is enriched in the centre of the midbody (Figure 3M–R). Together, these data suggest that HDAC-6 might play a role in mitosis by affecting microtubules.
Fig. 3. Partial co-localization of HDAC-6 and microtubules in NIH-3T3 cells. (A–F) Exponentially growing NIH-3T3 cells were fixed with cold methanol and double-stained for endogenous HDAC-6 (green) and β-tubulin (red). Analysis was performed by confocal microscopy, and individual stainings were merged digitally. In (G–I), cells were treated with 10 µM paclitaxel for 4 h to stabilize microtubules, and HDAC-6 and tubulin were immunolocalized as above. In (J–R), mitotic cells were examined: (J–L) metaphase; (M–O) anaphase; (P–R) telophase.
HDAC-6 inhibition leads to increased tubulin acetylation in mammalian cells
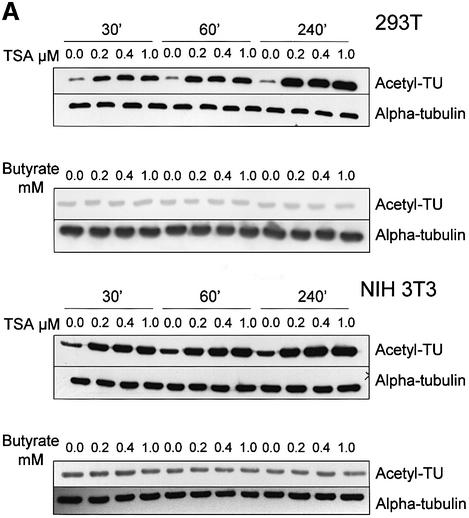
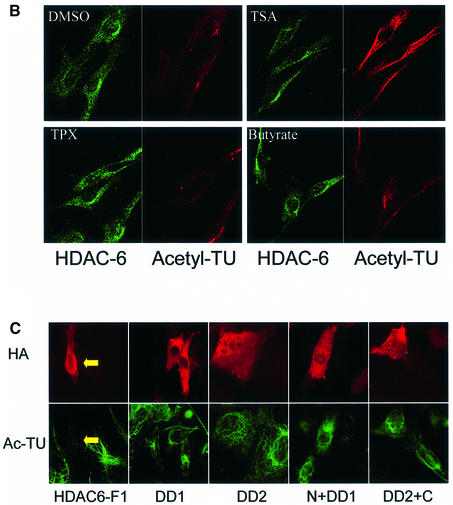
The cytoplasmic localization of HDAC-6 together with its interaction with microtubules suggested the possibility that this protein might deacetylate tubulin and/or microtubules. To examine this, we first treated cells with the deacetylase inhibitors TSA, which inhibits all known HDACs, or sodium butyrate, which inhibits HDACs with the exception of HDAC-6 (Guardiola and Yao, 2002). Cell lysates were then prepared and used to measure the level of tubulin acetylation with an antibody that specifically recognizes acetylated tubulin, TU6-11 (LeDizet and Piperno, 1991). As shown in Figure 4A, treatment of 293T or NIH-3T3 cells with TSA led to an increase in tubulin acetylation, already after 30 min. In contrast, treatment of the cells with sodium butyrate failed to influence the level of tubulin acetylation, suggesting that HDAC-6 might indeed be involved. The total amount of α-tubulin, measured by antibody DM1A, was found to be unaffected by either inhibitor. Histone acetylation was, as expected, increased significantly by both inhibitors (Supplementary figure S2). In agreement with these observations, immunostaining of cells treated with TSA showed a strong increase in tubulin acetylation, whereas trapoxin B (TPX) or butyrate did not change the level of tubulin acetylation (Figure 4B). To demonstrate a link between HDAC-6 expression and tubulin deacetylation more directly, 3T3 cells were transiently transfected with expression vectors encoding different HDAC-6 proteins, and tubulin acetylation was monitored by immunostaining. As shown in Figure 4C, in cells transfected with wild-type HDAC-6, tubulin acetylation became undetectable, while it was clearly visible in untransfected cells. In contrast, expression of the different HDAC-6 mutants failed to alter tubulin acetylation. Interestingly, mutants that encode a single hdac domain (e.g. N + DD1) and had been found to still interact well with β-tubulin (see Figure 2) also failed to deacetylate tubulin. This suggests that HDAC-6 enzymatic activity requires the presence of two intact hdac domains (see also below).
Fig. 4. Pharmacological inhibition of HDAC-6 activity leads to increased tubulin acetylation in vivo. (A) Exponentially growing 293T or NIH-3T3 cells were treated for 30 min, 1 h or 4 h with different concentrations of the HDAC inhibitors TSA or sodium butyrate, as indicated. Cell extracts were prepared and analysed by SDS–PAGE followed by western blotting. The membranes were probed with antibodies recognizing α-tubulin, either indiscriminately (DM1A) or only when acetylated (TU6-11). (B) NIH-3T3 cells were treated for 4 h with the indicated chemicals and then fixed. Immunostaining was used to visualize the level of HDAC-6 (green) and of tubulin acetylation (red). (C) Balb/c 3T3 cells were transfected with the indicated expression vectors encoding HDAC-6 proteins tagged at the N-terminus with the HA epitope. Expression of HDAC-6 deletions was monitored with an anti-HA antibody (red), and the state of tubulin acetylation was detected with the TU6-11 antibody (green).
HDAC-6 deacetylates α-tubulin Lys40 in vitro
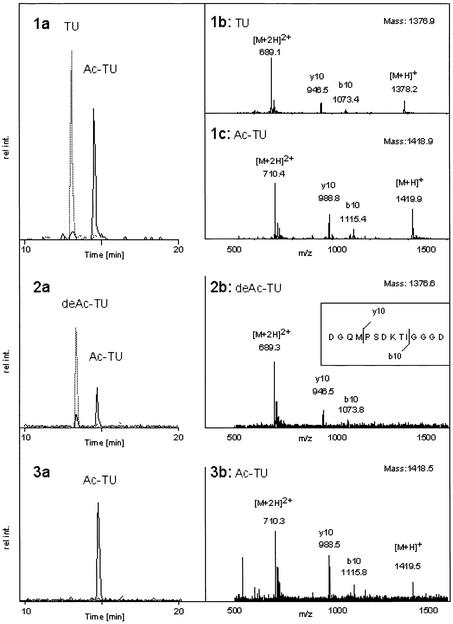
Although treatment of cells with TSA led to a rapid increase in tubulin acetylation (see Figure 4A), the data presented so far do not demonstrate unambiguously a direct involvement of HDAC-6 in tubulin deacetylation. To test this, an in vitro deacetylation assay was used. For this, two peptides corresponding to amino acids 33–46 of human α-tubulin were synthesized with or without acetylation of the lysine residue at position 40. As shown in Figure 5 (1a–c), a mixture of the two synthetic peptides could be well resolved by HPLC and mass spectrometric analysis. Additional peptide fragmentation allowed unambiguous identification of the peptides even in a complex mixture (1b and 1c). The acetylated peptide was next used for an in vitro deacetylation assay performed with HDAC-6 protein. As shown in Figure 5 (2a and b), incubation of the acetylated peptide with HDAC-6 led to efficient peptide deacetylation. For this, the hdac domains were necessary, as the HDAC-6 protein mutated in both hdac domains (HDAC-6-H216A/H611A) completely failed to deacetylate the peptide (3a and b). Likewise, the mock-transfected cell extracts did not show any tubulin deacetylase activity (data not shown). To obtain more quantitative results, a radioactive assay was used. HDAC-1,- 5 and -6, as well as mutated versions of the latter protein were used to deacetylate in vitro a radioactively acetylated peptide derived from α-tubulin. As shown in Figure 6A, HDAC-6, but not HDAC-1 or HDAC-5, efficiently deacetylated the peptide. In addition, the HDAC-6 proteins with mutations in the hdac domains were found to be completely inactive. In contrast, when an acetylated peptide derived from histone H4 was used, all proteins except the mutated versions of HDAC-6 and HDAC-5 were capable of efficient deacetylation, in agreement with earlier results (Supplementary figure S3). Similarly to histone deacetylation activity, tubulin deacetylation by HDAC6 was sensitive to TSA but not to either TPX or butyrate (Figure 6B). Furthermore, the deletion constructs from Figure 2 were assayed for TDAC activity (Figure 6C). Consistent with our previous in vivo data (Figure 4C), only the full-length wild-type HDAC-6 could deacetylate tubulin, whereas the deletions were not active for tubulin deacetylation even if they contained an intact hdac domain and still bound tubulin.
Fig. 5. In vitro deacetylation of a tubulin-derived peptide by HDAC-6. A peptide derived from α-tubulin was chemically synthesized as an unacetylated version (TU) or acetylated on Lys40 (Ac-TU). (1a) HPLC separation of a 1:1 mixture of the two peptides. The extracted ion current (XIC) of 689 (grey) and 710 (black) is shown, corresponding to the doubly charged ions of TU and Ac-TU, respectively. (1b) MS of TU with a mass of 1376.9 Da. The Ac-TU has a mass of 1418.9 Da as shown in (1c). Both peptides are partially fragmenting, generating the y10 as well as the b10 ion (insert 2b). (2a) The acetylated peptide was incubated with HDAC-6 and the reaction mix was analysed as described for the standard peptide mixture. The deacetylated TU peptide formed had a mass of 1376.6 Da as shown in (2b). The specific fragment ions y10 and b10 are indicated and explained in the inset of (2b). (3a) Analysis of the products of the incubation of the acetylated peptide with the doubly mutated protein HDAC-6-H216A/H611A shows only the acetylated peptide. This peptide had a mass of 1418.5 Da, and the two specific fragment ions y10 and b10 could be detected again (3b).
Fig. 6. Tubulin deacetylase activity is specific to HDAC-6. (A) 293T cells were transfected with expression vectors encoding FLAG-tagged versions of the indicated proteins. Two days later, cell extracts were prepared and immunoprecipitated with the anti-FLAG antibody M2. Upper panel: expression levels of the different HDACs were verified by western blotting using the anti-FLAG antibody. Lower panel: tubulin deacetylase activity (TDAC) was assayed by incubating an [3H]acetyl-labelled peptide from human α-tubulin (amino acids 33–46) and measuring the release of radioactivity. (B) TDAC activity was assayed as above, with immunoprecipitated HDAC-6 alone or in the presence of TSA, TPX or sodium butyrate (NaBu), as indicated. (C) HDAC-6 deletion constructs were transfected into 293T cells, and immunoprecipitated material was used for TDAC assay, as in (A).
HDAC-6 controls tubulin acetylation in vivo
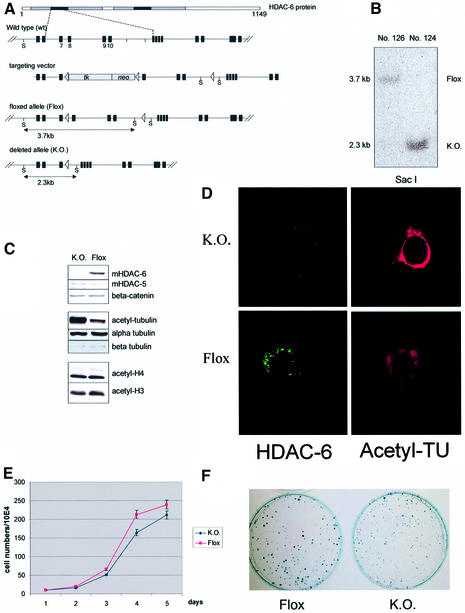
To test directly the role of HDAC-6 in the control of tubulin acetylation in vivo, we made use of mouse ES cells in which the HDAC-6 gene is flanked by lox P sites. These cells are derived from line E14, which has a male genotype. Since the HDAC-6 gene is on the X chromosome, treatment of a targeted ES cell clone with Cre recombinase would lead to a knockout allele. Therefore, a targeted ES cell clone, in which exons 7–10 in the first hdac domain are flanked by loxP sites and contain a floxed NeoR TK cassette (see Figure 7A; details to be published elsewhere) was used for transient transfection of a Cre recombinase expression vector. After selection against thymidine kinase (tk) resistance, clone 126 was identified as a floxed clone having the tk-Neo cassette deleted but retaining all HDAC-6 exons, while clone 124 was found to be knockout (Figure 7A and B). Cells from these two clones were used to examine the status of tubulin and histone acetylation in vivo. Cells were grown under non-differentiating conditions and cell extracts were examined by western blotting. As seen in Figure 7C, the knockout cells indeed completely lack HDAC-6 protein, but have normal levels of HDAC-5 or β-catenin. The absence of HDAC-6 resulted in elevated α-tubulin acetylation without change in the protein level of α- or β-tubulin. Strikingly, the global acetylation level of histone H3 or H4 did not appear to be affected by the complete absence of this HDAC. Furthermore, the increase in tubulin acetylation was confirmed further by immunostaining of the ES cells (Figure 7D). Wild-type or HDAC-6-deficient ES cells did not show any obvious morphological difference and both could differentiate in vitro equally well into embryoid bodies (Supplementary figure S4), indicating that in ES cells at least, HDAC-6 is not essential. In culture, the ES cells lacking HDAC-6 proliferated marginally more slowly than the floxed cells (Figure 7E), and in a colony formation assay they formed equivalent numbers of colonies, which were, however, somewhat smaller than those from the control cells (Figure 7F).
Fig. 7. Increased tubulin acetylation, but not histone acetylation in HDAC-6-deficient ES cells. (A) Scheme of the floxed mouse HDAC-6 locus and of the deleted (KO) allele (details to be published elsewhere). The position of _Sac_I (S) restriction sites is indicated, as well as the size of the resulting fragments. At the top, a scheme of the HDAC-6 protein is presented showing the two hdac domains (grey) and their core region (black). Cre-mediated recombination between loxP sites 1 and 2 leads to the floxed allele. Cre-mediated recombination between loxP sites 1 and 3 leads to the knockout allele; in this case, exons 7–10 of HDAC-6 are removed and no functional HDAC-6 is made. (B) Southern blot analysis of DNA from a floxed ES cell clone (No. 126) as well as from a knockout clone (No. 124). The position of the floxed or knockout allele is indicated. (C) Analysis of protein expression in floxed or knockout ES cells. Protein extracts were prepared from ES cell clones No. 126 and 124 and analysed by SDS–PAGE followed by western blotting with the indicated antibodies. (D) Immunostaining of HDAC-6 (green) and acetylated tubulin (red) in floxed or knockout ES cells, as indicated. (E) Growth curves of HDAC-6 floxed and knockout ES cell lines. Equal numbers of cells (1 × 105) were seeded in triplicate and aliquots were counted daily during a period of 5 days. (F) Colony formation assay with floxed and HDAC-6 knockout ES cells. A total of 2 × 103 cells were seeded and cultivated for 8 days in triplicate. Cells were then fixed and stained with Methylene Blue. One representative plate of each genotype is shown.
Discussion
HDAC-6 is a tubulin deacetylase
Post-translational modifications play a critical role in the regulation of protein structure and function. In particular, phosphorylation/dephosphorylation by protein kinases and phosphatases form the mechanistic basis for signal transduction cascades transmitting signals from the cell membrane to the nucleus. So far, protein acetylation/deacetylation has been discovered predominantly in histone tails and thereby implicated in regulation of chromatin structure and gene transcription. However, the list of non-histone proteins that are acetylated is growing continually, and there the role of aceytylation awaits to be understood. In this report, we have identified β-tubulin as a protein interacting specifically with HDAC-6. We found that, within HDAC-6, the hdac domains are necessary and sufficient for interaction with the C-terminal portion of β-tubulin. HDAC-6 was known to be a predominantly cytoplasmic protein (Verdel et al., 2000) and, by immunostaining of cells, we observed some co-localization of HDAC-6 with the microtubule network. Furthermore, treatment of cells with agents stabilizing (taxol) or disrupting (nocodazole; data not shown) microtubules led to significant redistribution of HDAC-6, indicating that association of these two proteins is not fortuitous. In contrast, disruption of the actin microfilament network by latrunculin B had no effect on the localization of HDAC-6 (data not shown). We found that treatment of cells with the HDAC inhibitor TSA led to an increase in tubulin acetylation, detectable both in cell extracts and by immunostaining of cells. In contrast, HDAC inhibitors such as TPX and sodium butyrate, which are known not to affect HDAC-6 activity, failed to alter the pattern of tubulin acetylation in cells. Furthermore, transient expression of HDAC-6 was found to reduce dramatically the level of tubulin acetylation in 3T3 cells. For this, the presence of both hdac domains was needed. By in vitro experiments, we could show that HDAC-6, but not HDAC-1 or HDAC-5, deacetylates Lys40 of α-tubulin. Finally, we took advantage of ES cells in which the HDAC-6 gene had been disrupted by gene targeting. In these cells, which completely lack HDAC-6, tubulin acetylation was found to be dramatically increased while global acetylation of histone H3 and H4 was unaffected. In addition, in vivo, during development and differentiation of the mouse mammary gland, expression of HDAC-6 showed an inverse pattern to that of acetyl-tubulin (N.Li, unpublished result). Together, these data suggest that the primary function of HDAC-6 is to deacetylate α-tubulin, and thereby to influence the stability of the microtubule network. Consistent with this, Hubbert et al. (2002) very recently found that 3T3 cells stably overexpressing HDAC-6 have an increased motility, probably due to the deacetylation of microtubules. The denomination HDAC therefore appears to be a misnomer that merely reflects the initial identification of HDAC-6 through its homology with ‘true’ HDACs such as HDAC-1.
Mechanism of deacetylation by HDAC-6
The interaction between HDAC-6 and β-tubulin might be used as one way of regulating the substrate specificity of HDAC-6. By binding to β-tubulin, HDAC-6 is recruited to either tubulin dimers or microtubules and can deacetylate Lys40 of α-tubulin. The binding between HDAC-6 and tubulin can be uncoupled from the deacetylation reaction since a point mutation in the active centre abolished the enzymatic activity but did not impair the interaction with β-tubulin. Recently a conserved ER (Esa1-Rpd3) motif was identified near the active centre of the hdac domain and also in the HAT domain (Adachi et al., 2002). Mutation and structure analysis of this motif in both Esa1 and Rpd3 suggested that it might be involved in the process of substrate (histone) binding. Our observations also suggest that the tubulin recognition domain is distinct from the active centre of HDAC-6. Further detailed mutational analysis of the hdac domain will be required to verify this hypothesis.
The catalytic mechanism for the hdac domain has been proposed by Finnin et al. (1999) after resolving the structure of an HDAC core domain from a hyperthermophilic bacterium. Except for HDAC-6 and HDAC-10, all HDACs have only one catalytic domain and are inhibited similarly by, for example, TSA and TPX. In contrast, HDAC-6 has two catalytic domains and HDAC-10 has one complete and one incomplete catalytic domain (Fischer et al., 2002; Guardiola and Yao, 2002; Tong et al., 2002). Both of these enzymes are resistant to inhibition by TPX and sodium butyrate. Here we found that in vitro and in vivo deacetylation of tubulin or histone by HDAC-6 requires the presence of two intact hdac domains. A single mutation in one hdac domain was sufficient to eliminate the entire activity, irrespective of the substrate. In vivo, the deacetylases often are found in large repression complexes in heterodimer or homodimer forms. This suggests that in vivo, the active deacetylase complexes may need more than one hdac domain, and mutation in one of the hdac domains may be sufficient to impair the activity of the whole complex. Additional experiments will be needed to test this hypothesis further.
HDAC-6, a dual- or multi-specificity deacetylase?
HDAC-6 was identified by searching for mammalian homologues of yeast histone deacetylase HDA1 (Grozinger et al., 1999; Verdel and Khochbin, 1999). In vitro, HDAC-6 acts, as shown here, on a tubulin peptide and also on microtubules (Hubbert et al., 2002) as well as on a histone H4-derived peptide substrate (Supplementary figure S3) or on purified core histones (Grozinger et al., 1999). Although in HDAC-6-deficient ES cells the acetylation of histone H3 and H4 did not change significantly (Figure 7), it cannot be ruled out that in certain cell types, or possibly in response to specific signals, HDAC-6 participates in deacetylating histones, and potentially other nuclear proteins, in vivo. We observed that, in transient transfection assays, artificial recruitment of HDAC-6 to promoter DNA represses the transcription of reporter plasmids, suggesting that the presence of HDAC-6 in the nucleus does have an impact on gene activity (data not shown). In addition, Verdel et al. (2000) showed that the subcellular localization of HDAC-6 is controlled by specific signals. A potent nuclear export signal at the N-terminus is essential for maintaining HDAC-6 in the cytoplasm: block of CRM1 activity by leptomycin B led to redistribution and nuclear localization of HDAC-6. Also, arrest of proliferation and subsequent differentiation of a mouse melanoma cell line was found to be associated with translocation of a fraction of the HDAC-6 protein into the nucleus (Verdel et al., 2000). Other class II HDACs also shuttle in and out of the nucleus to deacetylate histones, and their subcellular distribution is influenced in part by their interaction with 14-3-3 proteins (Grozinger and Schreiber, 2000; McKinsey et al., 2000b). Thus, the substrate specificity of HDAC-6 may be controlled at several levels: first, regulated subcellular localization determines the availability or unavailability of the potential targets; secondly, targeting to the substrate (tubulin) is achieved by selective interaction with the hdac domain; and finally, the active centre provides the direct substrate specificity.
HDAC-6 is not essential in ES cells
Here, we found that disruption of the HDAC-6 gene did not obviously alter the morphology of ES cells (data not shown). Yet, proliferation of HDAC-6-deficient ES cells was slightly slower than control cells, at least under the non-differentiating culture conditions used. Moreover, the ES cells lacking HDAC-6 could differentiate in vitro into embryoid bodies as efficiently as wild-type cells. Interestingly, when tested in a colony formation assay, knockout ES cells formed similar numbers of colonies, which, however, were smaller than colonies from wild-type cells (Figure 7F); at present, it is not clear whether this is a direct consequence of the tubulin hyperacetyl ation. In the case of HDAC-1-deficient ES cells, it was also found that in vitro differentiation was normal. However, in this case, proliferation was significantly impaired and this was correlated with an increase in expression of the cyclin-dependent kinase inhibitors p21 and p27 (Lagger et al., 2002). Also, acetylation of histone H3 and H4 was elevated in the mutant cells. In addition, HDAC-1 was found to be essential for early embryonic development: mutant embryos die before E10.5, probably as a consequence of the proliferation defect. Clearly, definition of the precise biological role(s) of the different HDACs in higher eukaryotes will require analysis of their function by gene targeting in the mouse, and these experiments are in progress.
The dramatically increased level of tubulin acetylation in HDAC-6-deficient ES cells, without a major observable phenotype, raises the question of the in vivo role of acetylated microtubules and of the contribution of the tubulin deacetylase/acetylase to cell function. Acetylated tubulin is not required for cell survival since it is missing from some eukaryotic cells even though the Lys40 in α-tubulin is highly conserved. Moreover, attempts have been made to determine the in vivo function of acetyl ated tubulin by either dominantly overexpressing, in Chlamydomonas, a tubulin mutant in which Lys40 was replaced by another amino acid (Kozminski et al., 1993), or by exchanging, in Tetrahymena thermophila, the wild-type tubulin gene with a mutated version (Gaertig et al., 1995). In both cases, the mutants had no detectable tubulin acetylation but were indistinguishable from the wild-type cells. Preliminary experiments suggested that in 3T3 cells overexpressing HDAC-6, taxol still could stabilize the microtubule structure in the absence of tubulin acetylation (C.Caron, unpublished results). These data suggest that tubulin acetylation might be redundant for those organisms or that it is an effect secondary to tubulin stabilization. Clearly, important questions remain open about the role of tubulin acetylation in multicellular organisms.
Non-histone substrates as targets of HDAC inhibitors
HDAC inhibitors have been shown to be potent inducers of growth arrest, differentiation and apoptotic cell death of transformed cells in vitro and in vivo. The overexpression of several HDACs and the HDAC-dependent aberrant transcriptional repression of tumour suppressor genes have been implicated as an important oncogenic mechanism in different types of cancers such as myeloid leukaemia and lymphoma (Melnick and Licht, 2002). Recent genome-wide studies in yeast have demonstrated that each HDAC has a distinct specificity, both on the histone tails and in the chromatin context (Peterson, 2002). In addition, the finding that HDACs also have non-histone substrates, e.g. tubulin, as shown here and by Hubbert et al. (2002), suggests that some of the effects ascribed to HDAC inhibition may reflect the function of these newly identified substrates. Therefore, it will be desirable to develop inhibitors that are HDAC specific and possibly also substrate specific. Tubulin-binding agents constitute an important class of antimitotics and are widely used for the treatment of solid tumours and haematopoietic malignancies. For example, docetaxel and paclitaxel act to promote tubulin polymerization and the formation of stable microtubules, which are resistant to disassembly by physiological stimuli: cells exposed to these agents exhibit an accumulation of disorganized microtubule arrays. Since, as shown here, inhibition of HDAC-6 leads to tubulin hyperacetylation, which is a marker for stable microtubules, it might be very interesting to investigate the collaboration of these two different kinds of chemicals.
Materials and methods
Co-immunoprecipitation
For co-immunoprecipitation, ∼500 µg of extracts from either NIH-3T3 cells or HEK 293T cells transfected by Fugene (Roche) were incubated overnight with the primary antibody at 4°C with gentle agitation. Rabbit IgG or mouse IgG was used as control. After this, 20 µl of protein A–Sepharose slurry were added and samples were incubated for 1 h at 4°C with gentle agitation. Beads were washed three times with IP buffer (20 mM HEPES pH 7.4, 0.5 mM EDTA, 150 mM NaCl and 0.1% Triton X-100) and subsequently resuspended and boiled in 20 µl of loading buffer for SDS–PAGE.
Immunofluoresence and immunoblotting
For tubulin and HDAC-6 staining, cells were fixed in methanol at –20°C for 10 min and stained with mHDAC-6 polyclonal antibody (Verdel et al., 2000), TU2.1 for α-tubulin or TU6-11 for acetylated tubulin (Sigma). The DNA was counterstained by propidium iodide (PI) or 4′,6-diamidino-2-phenylindole (DAPI).
Protein lysates were resolved by electrophoresis on 8 or 12% SDS–polyacrylamide gels and subsequently blotted to nitrocellulose membranes (Bio-Rad). Antibodies used were: TU2.1, TU6-11, Flag-M2 and DM1A (Sigma), mHDAC-5 and mHDAC-6 (Verdel et al., 2000), acetylated H3 and acetylated H4 (Upstate).
Translation in vitro and binding assay
Mouse HDAC-6 was translated in vitro by using plasmid pcDNA3.1-HA-mHDAC-6 F1 (Seigneurin-Berny et al., 2001) and the TNT T7 Coupled Reticulocyte Lysate system (Promega). [35S]methionine was included to label translated protein. For the binding assay, 5 µl of the final translation reaction mixture was incubated with 5 µg of purified bovine tubulins (Cytoskeleton Inc., Denver, CO) in PIPES buffer [80 mM PIPES pH 6.8, 1 mM MgCl2, 1 mM EGTA, 1 mM dithiothreitol (DTT), 5% glycerol, 1% Triton X-100] at 4°C for 4 h. Tubulins were then immunoprecipitated with anti-β-tubulin antibody TU2.1 (Sigma). Fluorography of 35S was performed to detect co-precipitated HDAC-6.
Microtubules and MAP preparation
Microtubules were assembled and purified from NIH-3T3 cells as described previously (Tian et al., 2000). Briefly, NIH3-T3 cells were swollen in 9 vols of ice-cold swelling solution and homogenized in 2 vols of swelling solution containing Complete® proteinase inhibitor mixture (Roche). After adding PIPES pH 6.8 to 0.1 M, the homogenate was centrifuged in a TLS-55 rotor (Beckman) at 50 000 r.p.m. for 90 min at 2°C. Paclitaxel and GTP were added to the supernatant at a final concentration of 20 µM and 0.5 mM, respectively. The cytosolic extract was warmed briefly to 37°C (3–5 min) and then chilled for 15 min on ice. The cytosolic extract was transferred to a chilled centrifuge tube underlaid with ice-cold PEG buffer containing 10% (w/v) sucrose, 20 µM paclitaxel, and 0.5 mM GTP. Microtubules were pelleted through the sucrose cushion by centrifugation at 29 000 r.p.m. in a TLS-55 rotor for 30 min at 2°C. Microtuble proteins were washed once by PEG buffer with paclitaxel and GTP, and analysed by SDS–PAGE and immunoblotting.
In vitro microtubule binding assays
Taxol-stabilized microtubules were assembled from dimeric tubulin (Cytoskeleton Inc.) in G-PEM buffer (80 mM PIPES pH 6.8, 1 mM MgCl2, 1 mM EGTA and 1 mM GTP) as instructed by the manufacturer. The microtubule binding experiments were performed as described (Tian et al., 2000). Briefly, 50 µl of in vitro translated, [35S]methionine-labelled mouse HDAC-6 were diluted in 200 µl of PB (80 mM K-PIPES pH 6.9, 1 mM EGTA, 1 mM MgCl2) containing Complete® proteinase inhibitor mixture (Roche). The mixture was spun at 50 000 g for 1 h at 4°C in a TLS-55 rotor. A 100 µl aliquot of supernatant was incubated with or without 50 µg of microtubules for 30 min at 37°C in the presence of 10 µM paclitaxel and 1 mM GTP. The microtubule and mHDAC-6 mixtures were layered over 2.5 ml of 15% sucrose in PB buffer in the presence of 10 µM paclitaxel and 1 mM GTP, and spun for 30 min at 30 000 g. Both supernatants (above the sucrose) and pellets were analysed for the presence of mHDAC-6 by autoradiography. The gels were then stained with Coomassie Blue to confirm that an equal amount of microtubules was loaded on the gel in each sample.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We wish to thank W.Krek and P.Caroni for critical comments on the manuscript, A.Hergovich and Dr Serebriski (Fox Chase Cancer Centre) for technical help and providing reagents, P.Kopp for help with ES cell work, Drs Grozinger (Harvard University), Marshall (University of California, San Francisco) and Seto (H.Lee Moffitt Cancer Centre) for plasmids, and A.Matus and laboratory members for discussions. This work was supported by the Novartis Research Foundation.
References
- Adachi N., Kimura,A. and Horikoshi,M. (2002) A conserved motif common to the histone acetyltransferase Esa1 and the histone deacetylase Rpd3. J. Biol. Chem., 277, 35688–35695. [DOI] [PubMed] [Google Scholar]
- Brown C.E., Lechner,T., Howe,L. and Workman,J.L. (2000) The many HATs of transcription coactivators. Trends Biochem. Sci., 25, 15–19. [DOI] [PubMed] [Google Scholar]
- Cheung W.L., Briggs,S.D. and Allis,C.D. (2000) Acetylation and chromosomal functions. Curr. Opin. Cell Biol., 12, 326–333. [DOI] [PubMed] [Google Scholar]
- Fields S. and Song,O. (1989) A novel genetic system to detect protein–protein interactions. Nature, 340, 245–246. [DOI] [PubMed] [Google Scholar]
- Finnin M.S., Donigian,J.R., Cohen,A., Richon,V.M., Rifkind,R.A., Marks,P.A., Breslow,R. and Pavletich,N.P. (1999) Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature, 401, 188–193. [DOI] [PubMed] [Google Scholar]
- Fischer D.D. et al. (2002) Isolation and characterization of a novel class II histone deacetylase, HDAC10. J. Biol. Chem., 277, 6656–6666. [DOI] [PubMed] [Google Scholar]
- Gaertig J., Cruz,M.A., Bowen,J., Gu,L., Pennock,D.G. and Gorovsky, M.A. (1995) Acetylation of lysine 40 in α-tubulin is not essential in Tetrahymena thermophila. J. Cell Biol., 129, 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S.G. and Ekstrom,T.J. (2001) The human histone deacetylase family. Exp. Cell Res., 262, 75–83. [DOI] [PubMed] [Google Scholar]
- Grozinger C.M. and Schreiber,S.L. (2000) Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc. Natl Acad. Sci. USA, 97, 7835–7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozinger C.M., Hassig,C.A. and Schreiber,S.L. (1999) Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc. Natl Acad. Sci. USA, 96, 4868–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola A.R. and Yao,T.P. (2002) Molecular cloning and characterization of a novel histone deacetylase HDAC10. J. Biol. Chem., 277, 3350–3356. [DOI] [PubMed] [Google Scholar]
- Gyuris J., Golemis,E., Chertkov,H. and Brent,R. (1993) Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell, 75, 791–803. [DOI] [PubMed] [Google Scholar]
- Hubbert C., Guardiola,A., Shao,R., Kawaguchi,Y., Ito,A., Nixon,A., Yoshida,M., Wang,X.F. and Yao,T.P. (2002) HDAC6 is a microtubule-associated deacetylase. Nature, 417, 455–458. [DOI] [PubMed] [Google Scholar]
- Jenuwein T. and Allis,C.D. (2001) Translating the histone code. Science, 293, 1074–1080. [DOI] [PubMed] [Google Scholar]
- Kao H.Y., Lee,C.H., Komarov,A., Han,C.C. and Evans,R.M. (2002) Isolation and characterization of mammalian HDAC10, a novel histone deacetylase. J. Biol. Chem., 277, 187–193. [DOI] [PubMed] [Google Scholar]
- Khochbin S., Verdel,A., Lemercier,C. and Seigneurin-Berny,D. (2001) Functional significance of histone deacetylase diversity. Curr. Opin. Genet. Dev., 11, 162–166. [DOI] [PubMed] [Google Scholar]
- Kozminski K.G., Diener,D.R. and Rosenbaum,J.L. (1993) High level expression of nonacetylatable α-tubulin in Chlamydomonas reinhardtii. Cell Motil. Cytoskeleton, 25, 158–170. [DOI] [PubMed] [Google Scholar]
- Krieg J., Hartmann,S., Vicentini,A., Glasner,W., Hess,D. and Hofsteenge,J. (1998) Recognition signal for C-mannosylation of Trp-7 in RNase 2 consists of sequence Trp-x-x-Trp. Mol. Biol. Cell, 9, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmichev A. and Reinberg,D. (2001) Role of histone deacetylase complexes in the regulation of chromatin metabolism. Curr. Top. Microbiol. Immunol., 254, 35–58. [DOI] [PubMed] [Google Scholar]
- Lagger G. et al. (2002) Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J., 21, 2672–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDizet M. and Piperno,G. (1991) Detection of acetylated α-tubulin by specific antibodies. Methods Enzymol., 196, 264–274. [DOI] [PubMed] [Google Scholar]
- Marks P., Rifkind,R.A., Richon,V.M., Breslow,R., Miller,T. and Kelly,W.K. (2001) Histone deacetylases and cancer: causes and therapies. Nat. Rev. Cancer, 1, 194–202. [DOI] [PubMed] [Google Scholar]
- Marmorstein R. and Roth,S.Y. (2001) Histone acetyltransferases: function, structure and catalysis. Curr. Opin. Genet. Dev., 11, 155–161. [DOI] [PubMed] [Google Scholar]
- McKinsey T.A., Zhang,C.L., Lu,J. and Olson,E.N. (2000a) Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature, 408, 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey T.A., Zhang,C.L. and Olson,E.N. (2000b) Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc. Natl Acad. Sci. USA, 97, 14400–14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means G.D., Toy,D.Y., Baum,P.R. and Derry,J.M. (2000) A transcript map of a 2-Mb BAC contig in the proximal portion of the mouse X chromosome and regional mapping of the scurfy mutation. Genomics, 65, 213–223. [DOI] [PubMed] [Google Scholar]
- Melnick A. and Licht,J.D. (2002) Histone deacetylases as therapeutic targets in hematologic malignancies. Curr. Opin. Hematol., 9, 322–332. [DOI] [PubMed] [Google Scholar]
- Narlikar G.J., Fan,H.Y. and Kingston,R.E. (2002) Cooperation between complexes that regulate chromatin structure and transcription. Cell, 108, 475–487. [DOI] [PubMed] [Google Scholar]
- Peterson C.L. (2002) HDAC’s at work. Everyone doing their part. Mol. Cell, 9, 921–922. [DOI] [PubMed] [Google Scholar]
- Piperno G., LeDizet,M. and Chang,X.J. (1987) Microtubules containing acetylated α-tubulin in mammalian cells in culture. J. Cell Biol., 104, 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polevoda B. and Sherman,F. (2002) The diversity of acetylated proteins. Genome Biol., 3, reviews0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richon V.M., Sandhoff,T.W., Rifkind,R.A. and Marks,P.A. (2000) Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc. Natl Acad. Sci. USA, 97, 10014–10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneurin-Berny D., Verdel,A., Curtet,S., Lemercier,C., Garin,J., Rousseaux,S. and Khochbin,S. (2001) Identification of components of the murine histone deacetylase 6 complex: link between acetylation and ubiquitination signaling pathways. Mol. Cell. Biol., 21, 8035–8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Nelson,D.L. and Stewart,D.M. (2000) Cdc42-interacting protein 4 mediates binding of the Wiskott–Aldrich syndrome protein to microtubules. J. Biol. Chem., 275, 7854–7861. [DOI] [PubMed] [Google Scholar]
- Tong J.J., Liu,J., Bertos,N.R. and Yang,X.J. (2002) Identification of HDAC10, a novel class II human histone deacetylase containing a leucine-rich domain. Nucleic Acids Res., 30, 1114–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdel A. and Khochbin,S. (1999) Identification of a new family of higher eukaryotic histone deacetylases. Coordinate expression of differentiation-dependent chromatin modifiers. J. Biol. Chem., 274, 2440–2445. [DOI] [PubMed] [Google Scholar]
- Verdel A., Curtet,S., Brocard,M.P., Rousseaux,S., Lemercier,C., Yoshida,M. and Khochbin,S. (2000) Active maintenance of mHDA2/mHDAC6 histone-deacetylase in the cytoplasm. Curr. Biol., 10, 747–749. [DOI] [PubMed] [Google Scholar]