Lysyl Oxidase Inhibits Ras-Mediated Transformation by Preventing Activation of NF-κB (original) (raw)
Abstract
Lysyl oxidase (LO), which catalyzes the oxidation of lysine residues, was previously shown to have anti-oncogenic activity on _ras_-transformed cells. Since oncogenic Ras mediates transformation, in part, through the activation of the transcription factor nuclear factor-κB (NF-κB), we tested here the effects of LO on NF-κB activity. Expression of LO in _ras_-transformed NIH 3T3 cells led to decreased NF-κB binding and activity, as well as the expression of the NF-κB target gene c-myc. Importantly, ectopic expression of LO led to a dramatic decrease in colony formation by _ras_-transformed NIH 3T3 cells, a finding comparable to the expression of the IκBα dominant-negative mutant, which could be rescued by p65/p50 NF-κB subunit expression. LO was unable to directly inhibit the activity of ectopically expressed p65 and c-Rel NF-κB subunits, suggesting that LO affected an upstream signaling pathway(s) induced by Ras. Consistent with this hypothesis, LO expression decreased both the rate of IκBα turnover and the activities of IKKα and IKKβ. Moreover, the ectopic expression of a constitutively active version of either kinase reversed the negative effects of LO. Ras can induce NF-κB via both the phosphatidylinositol 3-kinase (PI3K)/Akt and Raf/MEK pathways. LO potently downregulated the PI3K and Akt kinases, while partially inhibiting MEK kinase activity. Expression of a constitutively activated, myristylated Akt or PDK1 was able to counteract the effect of LO on NF-κB, whereas constitutively activated Raf was only partially effective. Importantly, LO blocked membrane localization of Akt and PDK1 in Ras-transformed cells. Overall, these results strongly argue that the anti-oncogenic effects of LO on _ras_-mediated transformation are due to its ability to inhibit signaling pathways that lead to activation of NF-κB.
Lysyl oxidase (LO; protein-6-oxidase [EC 1.4.3.13]) is the key enzyme that controls collagen and elastin maturation (53, 62). LO catalyzes the oxidative deamination of peptidyl lysine and hydrolysine to peptidyl-α-aminoadipic-δ-semialdehyde in elastin and collagen chains. The consequent aldehydes lead to a spontaneous condensation forming inter- and intrachain cross-links. This posttranslational modification of extracellular matrix molecules plays a very important role in collagen and elastin structural aspects and possibly in triggering still-unknown signal transduction pathways. Several reports have suggested a clear association between organ fibrosis and increased LO activity (10, 31, 64). The most intriguing aspect regarding LO activity relates to its putative cell phenotype control and tumor suppressor activity. LO was identified as a “ras recision gene” (rrg), and levels of LO are decreased in cells transformed by ras or _ras_-dependent oncogenes (12, 34, 39). Furthermore, Friedman and coworkers showed that ras_-transfected NIH 3T3 cells induced to revert by beta or gamma interferon would return to their transformed phenotype upon transfection with an antisense LO vector, and retransformation did not affect p21_ras levels (12, 34). In many naturally occurring and oncogene-induced tumors, LO is downregulated, and LO was induced concomitantly with reversion (12, 24, 26, 27, 34, 39).
Recently, nuclear factor-κB (NF-κB)/Rel factors have been strongly implicated in the regulation of cell growth and neoplastic transformation (25, 54). For example, our laboratory demonstrated a direct role of NF-κB/Rel in the ability of transformed hepatocytes and breast cancer cell lines to grow in soft agar (3, 59). NF-κB is a family of dimeric transcription factors with subunits that contain an approximately 300-amino-acid NH2-terminal stretch, termed the Rel homology domain, that shares homology with the v-Rel oncoprotein (4). The mammalian members of the NF-κB/Rel family are p65 (RelA), RelB, c-Rel, p50 (NF-κB1), and p52 (NF-κB2). Only p65, RelB, and c-Rel have carboxy-terminal transactivating domains, and classical NF-κB is composed of p50 and p65 subunits (5, 71). NF-κB is ubiquitously expressed; however, in untransformed non-B cells, it is sequestered in the cytoplasm with specific inhibitory proteins termed IκBs, of which IκBα is the best characterized (73). The IκB kinase complex consists of two IκB kinases, IKKα and IKKβ, and a 48-kDa essential component, alternatively termed IKK-associated protein 1 (IKKAP1), NF-κB essential modulator (NEMO), or IKKγ (47, 60). Activation of the IκB kinase complex is mediated via phosphorylation of either IKKα and/or IKKβ (17, 46, 55, 77). IκBα is then recruited in the IκB kinase complex, with which it is phosphorylated by the functional IKKα-IKKβ heterodimer at serine residues at positions 32 and 36. This phosphorylation is followed by ubiquitination and rapid degradation through the proteasome pathway (8, 11, 17), allowing for translocation of the released NF-κB to the nucleus (4).
Several oncogenes are able to promote the degradation of IκBα and to activate NF-κB/Rel, including ras and HER2/neu (3, 52). Our laboratory recently showed that in rat liver epithelial cells oncogenic ras mediates NF-κB activation via two distinct pathways, phosphatidylinositol 3-kinase (PI3K) and Raf/MEK, leading to the activation of IKKα and IKKβ, respectively (3). Since the transformation mediated by ras has been related, in part, to its ability to activate NF-κB (3, 21, 22, 29, 44), we tested here the hypothesis that LO overexpression leads to the inhibition of _ras_-induced NF-κB. We demonstrate that ectopic LO expression in _ras_-transformed NIH 3T3 cells inhibits NF-κB binding and activity and leads to reversion of the transformed phenotype, effects comparable to the effects of IκBα. Inhibition of NF-κB was mediated by decreasing IκBα turnover and IKK activity via potent and moderate inhibition of the PI3K/Akt and Raf/MEK signaling pathways, respectively. Overall, these findings suggest that the tumor suppressor effects of LO are due to its ability to inhibit NF-κB activation by Ras, suggesting a potential clinical role for LO in the treatment of _ras_-induced cancers.
MATERIALS AND METHODS
Plasmids.
The LO expression vector (pSV40-LO) (69) was kindly provided by P. Trackman (Boston University School of Dentistry, Boston, Mass.). The constitutively activated forms (pRCβ-actin-IKKα SS/EE and pCMVneo-IKKβ SS/EE) of IKKα and IKKβ have been described previously (46). The human serine-to-alanine 32/36 dominant-negative (dn) mutant version of IκBα clone, pRCβ-actin-IκBα (16) was provided by M. Karin (University of California, San Diego). The thymidine kinase promoter-driven luciferase reporter plasmid controlled by six reiterated κB sites (70) was a gift from G. Rawadi (Hoechst-Marion-Roussel, Romainville, France). The pSG5-V12 H-ras, pSG5-V12C40 H-ras, pSG5-V12S35 H-ras, and pSG5-V12G37 H-ras expression vectors have been described previously (58). M-Akt (myristylated membrane kinase) was cloned in a cytomegalovirus (CMV) promoter-driven expression vector and was kindly provided by Z. Luo (Boston University Medical School, Boston, Mass.). The SR-αΔp85 and pZIP-NeoSV-ΔRaf-22W expression vectors have been described previously (38, 51). Vectors directing expression of either p65, p50, or c-Rel have been described elsewhere (2, 8). Both M-PDK1 (myristylated membrane kinase), cloned into pSR-α and then subcloned into the pBJ5 vector, and the pEGFP-C1-PDK1 constructs (35) were kindly provided by J. Chung (Korea Advanced Institute of Science and Technology, Taejon, Republic of Korea). The pEGFP-AKT (76) expression vector was a gift of J. Downward (Imperial Cancer Research Fund, London, United Kingdom).
Cell culture and treatment conditions.
The _ras_-transformed cell line (RAS) was isolated from a clonal population of NIH 3T3 cells (WT) stably expressing the oncoprotein c-Ha-Ras (kindly provided by D. Faller, Boston University Medical School). WT and RAS NIH 3T3 cells were maintained in Ham F-12 nutrient mixture medium (Gibco-BRL, Gaithersburg, Md.) supplemented with 2 mM l-glutamine, 50 U of penicillin/ml, and 50 μg of streptomycin/ml (all from Sigma Chemical Co., St. Louis, Mo.), 10% heat-inactivated fetal bovine serum (FBS; Gibco-BRL), and 1 M dextrose (Mallinckrodt Baker, Inc., Phillipsburg, N.J.). To prevent protein synthesis, cells were treated with 50 μg of cycloheximide (Sigma)/ml for the indicated periods of time. To inhibit MEK activity, 100 μM of the inhibitor PD98059 (Calbiochem, La Jolla, Calif.) was used as indicated.
Transient transfections and luciferase assays.
For transient transfections, NIH 3T3 cells were plated in P100 or P35 culture dishes. Cells were transfected overnight, in triplicate, with the indicated expression vectors by using Fugene 6 transfection reagent (Roche Molecular Biochemicals, Indianapolis, Ind.) in Ham medium plus 0.5% FBS (serum starvation conditions). For luciferase assays, 1 μg of NF-κB-dependent luciferase reporter plasmid and 0.5 μg of pSV40 β-galactosidase (β-Gal) reporter gene were cotransfected with the indicated DNAs. Cells were stimulated with addition of FBS to a final concentration of 10%, and total cell extracts were prepared after 72 h. A time course analysis indicated that cotransfection with an LO expression vector required a 72-h period since no effect on NF-κB activation was seen at 24 and 48 h posttransfection. The resulting extracts were normalized for total protein content and β-Gal expression, as previously described (2), and used in a luciferase activity assay according to the manufacturer's instructions (Promega kit). Results are expressed as the fold induction of luciferase activity calculated under stimulation conditions (10% FBS) compared to starvation conditions (0.5% FBS). The results are expressed as the mean ± the standard deviation (SD).
Gel mobility and supershift assays.
For electrophoretic mobility shift assay (EMSA), the NF-κB binding site 5′-AAGTCCGGGTTTTCCCCAACC-3′ (with the core NF-κB binding site underlined) upstream of the c_-myc_ promoter, termed URE, was used as a probe (20, 28). The DNA was labeled by using the Klenow fragment of Escherichia coli DNA polymerase I (Life Technologies, Inc., Gaithersburg, Md.) and [α-32P]dCTP (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). Samples of nuclear extracts (2 to 3 μg), prepared as previously described (28), were incubated in sample buffer [0.4 μg of poly(dI-dC), 0.1% Triton X-100, 0.5% glycerol, 0.8 mM dithiothreitol, 2 mM HEPES (pH 7.5)] and adjusted to 100 mM KCl in a final volume of 25 μl. Then, 32P-labeled URE probe (40,000 cpm, ∼2 ng) was added to the mixture, followed by incubation for 30 min at room temperature. For supershift experiments, 2 μg of antibodies to p65 (SC-372), p50 (SC-114), and RelB (SC-226X), purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.), or anti-c-Rel or anti-p52 (a generous gift of N. Rice; National Cancer Institute, National Institutes of Health, Frederick, Md.) was added, and the mixture was incubated overnight at 4°C prior to the addition of the labeled probe. Complexes were separated in 4.5% acrylamide gels as described previously (28). Specific bands were quantified by densitometric analysis by using the Kodak Zoom digital camera model DC120 system and two-dimensional image analysis software.
Immunoblotting and antibodies.
Whole-cell extracts were prepared in radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate [SDS], 1% sodium sarcosyl, 1 mM dithiothreitol, 0.25 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin/ml, 10 μg of aprotinin/ml, 1 μg of pepstatin/ml). The protein concentration was measured by Bradford assay by using the Bio-Rad reagent (Bio-Rad, Hercules, Calif.) according to the manufacturer's directions. Samples (20 to 50 μg of protein) were resolved by SDS-10% polyacrylamide gel electrophoresis (PAGE) under reducing conditions, transferred onto polyvinylidene difluoride membranes (Bio-Rad), and stained with Ponceau red to verify equal loading. The membranes were incubated overnight at 4°C in TBS-T (50 mM Tris-HCl [pH 7.6], 200 mM NaCl, 0.1% Tween 20) with 2% bovine serum albumin. Proteins were detected by incubation with the specific antibody in TBS-T with 2% bovine serum albumin. After an extensive washing in TBS-T, a 1:1,000 dilution of horseradish peroxidase-conjugated protein G (Bio-Rad) was added for 1 h, and specific protein bands were visualized by using an enhanced chemiluminescence detection system (NEN Life Science Products, Boston, Mass.) according to the manufacturer's instructions. The antibody to IκBα (SC-371) was purchased from Santa Cruz Biotechnology. The antibodies reagents for Akt (no. 9272), Akt-Ser473P (no. 9271), and Erk-1/Erk-2 (no. 9102) were obtained from New England Biolabs (Beverly, Mass.). The antibodies to β-actin (A-5441) and P-Erk-1/P-Erk-2 (V803A) were from Sigma and Promega, respectively. The polyclonal antibody to c-Myc was kindly provided by S. Hann (Vanderbilt University, Nashville, Tenn.). Polyclonal anti-LO antibody (41) was generated in rabbits against rat LO peptide sequences 181 to 196 (YDTYERPRSGSRHRPG) and 293 to 309 (DEFSHYDLLDASTQRRV). The resulting anti-peptide antibody was affinity purified and directed against both of these sequences, which are highly conserved in rat, human, mouse, and chick LO.
IKK kinase assay.
Kinase assays for IKKs were performed as previously described (59). Briefly, immunoprecipitated IKKα or IKKβ proteins were subjected to an IKK kinase assay in kinase buffer C (20 mM HEPES [pH 7.7], 2 mM MgCl2, 10 μM ATP, 3 μCi of [γ-32P]ATP, 10 mM β-glycerophosphate, 10 mM NaF, 10 mM _p_-nitrophenyl phosphate, 300 μM Na3VO4, 1 mM benzamidine, 1 mM dithiothreitol, 2 μM phenylmethylsulfonyl fluoride, 1 μg of leupeptin/ml, 10 μg of aprotinin/ml, 1 μg of pepstatin/ml) at 30°C for 45 min in the presence of WT IκBα-glutathione _S_-transferase (GST) protein as substrate. The reaction was stopped by addition of 4× SDS-PAGE sample buffer, subjected to SDS-PAGE analysis, and visualized by autoradiography.
Akt kinase assay.
The Akt kinase assay was performed according to the directions of the Akt kinase assay kit (New England Biolabs). Briefly, samples (100 μg) of whole-cell extracts were immunoprecipitated overnight with an agarose-conjugated anti-Akt antibody (New England Biolabs) at 4°C. The immunoprecipitated protein was resuspended in kinase buffer, and the assay performed at 30°C for 45 min by using 1 μg of GSK3α-GST fusion protein as substrate in the presence of 10 μM ATP. The resulting products were resolved in a SDS-10% PAGE and subjected to immunoblotting, as described above, with a phospho-specific GSK-3α antibody. As a loading control, samples of the extracts used for kinase assay (20 μg) were resolved by gel electrophoresis and subjected to immunoblotting with the Akt antibody.
Focus formation assay.
After transfection with the indicated expression vectors, cells were plated, in triplicate, at 104 cells/ml in top plugs consisting of complete Ham F-12 nutrient mixture medium and 0.4% SeaPlaque agarose (FMC Bioproducts, Rockland, Maine). Plates were subsequently incubated for 14 days in a humidified incubator at 37°C, and colonies stained with 0.5 ml of 0.0005% crystal violet solution for 1 h and counted by using a dissecting microscope (×50 magnification). Three random fields were counted from each triplicate samples, and average values presented ± the SD.
Fluorescence microscopy.
Cells were cotransfected with vectors expressing either green fluorescent protein (GFP)-tagged Akt or PDK1 protein, and either LO expression vector or its empty vector for 48 h. Cultures were then incubated overnight under serum starvation conditions. Localization of the GFP-tagged proteins was determined by using an Anxiovert 200 M fluorescent microscope (Carl Zeiss MicroImaging, Inc., Thornwood, N.Y.). Analyses and pictures were performed by using Axiovision (v.3.1 software; Carl Zeiss MicroImaging, Inc.). Alternatively, WT cells were stimulated with 200 nM insulin for 5 min to induce AKT and PDK1 membrane localization.
RESULTS
LO inhibits activation of NF-κB by Ras.
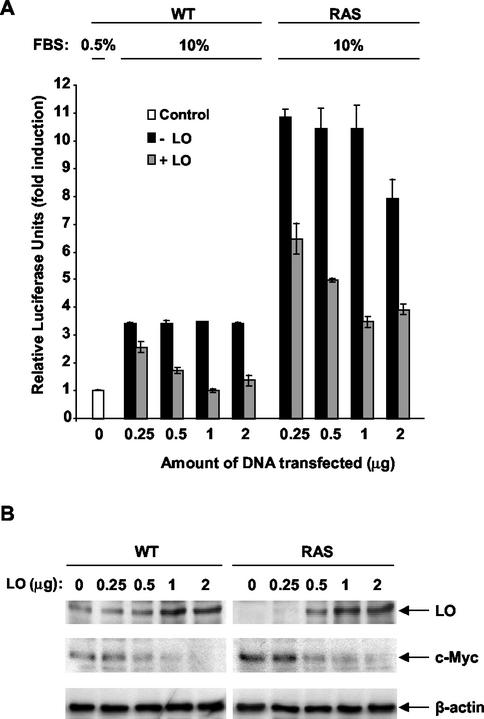
We first tested the possible effects of ectopic LO expression on NF-κB activity in _ras_-transformed NIH 3T3 cells. Increasing concentrations of LO expression vector or of its empty parental vector DNA were cotransfected with an NF-κB element-driven luciferase construct into normal (WT) and _ras_-transformed NIH 3T3 cells (RAS) under serum starvation conditions. Cells were then stimulated with the addition of FBS to a 10% final concentration for 72 h, and the luciferase activity was measured (Fig. 1A). In WT cells transfected with empty vector DNA, serum stimulation led to a 3.4-fold induction of luciferase activity, whereas a 10.8-fold NF-κB activation was seen in _ras_-transformed cells (Fig. 1A). Transfection of increasing concentrations of LO expression vector decreased luciferase activity in a dose-dependent manner up to 1 μg of DNA (Fig. 1A). Transfection of 1 μg of LO vector led to a total inhibition of serum-mediated activation of NF-κB in WT cells and to a 3.0-fold reduction in _ras_-transformed cells (Fig. 1A). Expression of LO was verified in cells transfected in the absence or in the presence of the LO expression vector (Fig. 1B). Basal levels of LO were higher in WT cells than in RAS cells (Fig. 1B), confirming that ras transformation leads to a drop of LO expression in NIH 3T3 cells, as described previously (12, 39). As expected, an increase in the quantity of LO expression vector from 0.5 to 2 μg led to enhanced levels of LO in both cell lines (Fig. 1B). The expression of c-myc, a well-known target gene of NF-κB (20), was also analyzed by using an anti-c-Myc antibody on the same membrane (Fig. 1B). Higher levels of c-Myc were found in RAS cells than in WT cells, a finding consistent with the higher NF-κB activity in these cells (Fig. 1B). Increased expression of LO in WT and RAS cells led to a dose-dependent decrease in c-Myc expression (Fig. 1B). Immunoblotting for β-actin levels confirmed equal sample loading.
FIG. 1.
LO inhibits NF-κB transcriptional activity. (A) Normal (WT) and _ras_-transformed NIH 3T3 fibroblasts (RAS) were transfected, in triplicate, with 1 μg of NF-κB-luciferase reporter plus 0.5 μg of SV40-β-Gal expression vector in the presence of 0, 0.25, 0.5, 1, or 2 μg of either LO expression vector (+LO) or empty parental vector (−LO) in Ham medium plus 0.5% FBS (Control). FBS was then added to a final concentration of 10% and, after 72 h, extracts were prepared and subjected to luciferase and β-Gal reporter assays. The values for luciferase were normalized to the β-Gal activity as a measure of transfection efficiency. The results are expressed as the mean of the fold induction normalized to the luciferase activity ± the SD as obtained in three different experiments. (B) The extracts described in panel A were subjected to immunoblot analysis with a specific LO antibody to check the expression of LO in transfected cells. Membranes were reprobed with anti-c-Myc antibody for the expression of the product of the NF-κB gene target c-myc and with an anti-β-actin antibody to confirm equal loading.
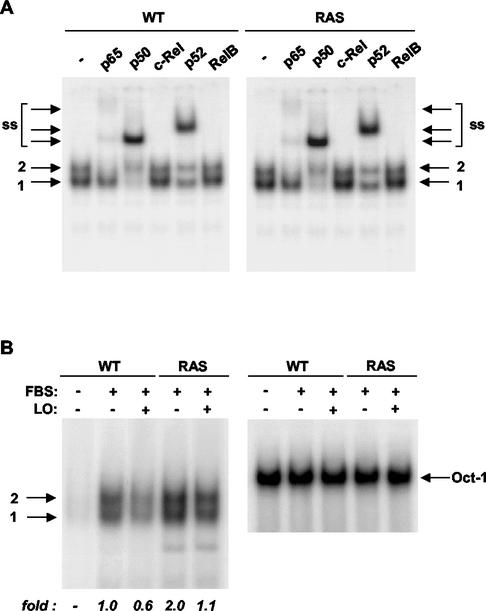
We then sought to determine whether LO is able to inhibit NF-κB binding. Nuclear extracts were prepared from serum-deprived and serum-stimulated WT and _ras_-transformed cells transfected with control parental or LO expression vector DNA for 48 h (Fig. 2). EMSA of nuclear extracts of serum-stimulated WT and RAS cells indicated the presence of two bands (Fig. 2A). Antibodies against the five known members of the NF-κB family were used to determine the nature of the NF-κB complexes present. In both WT and _ras_-transformed cell lines, the p65 antibody specifically supershifted the upper complex (Fig. 2A). The p50 and p52 antibodies were also able to supershift both the upper and the lower complexes but not to the same extents. The p50 antibody essentially completely supershifted the lower band and reduced the upper complex, whereas the p52 antibody only partially affected the migration of these complexes (Fig. 2A). In contrast, neither the c-Rel nor the RelB antibodies affected the migration of the two complexes (Fig. 2A). Thus, these results indicate that in both WT and RAS cells band 2 upper complexes are composed of p65/p50 and p65/p52 heterodimers, whereas band 1 seems to consist of p50 and p52 subunits apparently composed of p50 homodimers and p50/p52 heterodimers, and possibly p52 homodimers.
FIG. 2.
LO inhibits NF-κB binding activity. (A) Nuclear extracts from serum-stimulated WT or RAS cells were incubated overnight in the absence (−) or in the presence of 2 μg of antibody to either p65, p50, c-Rel, p52, or RelB, as indicated, and subjected to EMSA with the URE NF-κB element as probe. NF-κB band 1 contains both p50 and p52 subunits as homodimers and/or heterodimers, whereas band 2 probably contains both p65/p50 and p65/p52 heterodimer complexes. ss, supershift. (B) WT or RAS cells were transfected with either 1 μg of parental empty vector (−) or LO expression vector (+) in 0.5% FBS. At 48 h after the addition of FBS to a final concentration of 10%, nuclear extracts were prepared and analyzed for binding of NF-κB with the URE NF-κB element as probe or of Oct-1 as control for equal loading. The NF-κB bands 1 and 2 are noted. The films were subjected to densitometry, and the changes are expressed as the fold relative to stimulated WT cells.
The effects of LO on the levels of NF-κB DNA binding in WT and RAS cells were then analyzed (Fig. 2B). NF-κB complexes were barely detected in nuclear extracts of unstimulated WT cells (Fig. 2B). Serum stimulation for 48 h greatly increased the NF-κB binding activity (Fig. 2B). Expression of LO reduced the activation of NF-κB by ca. 40% (Fig. 2B). The NF-κB activity in _ras_-transformed cells stimulated by serum for 48 h was two times the level seen in WT cells, a finding consistent with the relative luciferase activity seen above in cells stimulated for 72 h (Fig. 1). The _ras_-induced NF-κB binding activity was similarly diminished by approximately one-half upon LO expression (Fig. 2B). Equal loading was verified by analysis of Oct-1 binding levels. Thus, LO expression reduces NF-κB binding and activity in both WT and _ras_-transformed NIH 3T3 cells.
LO-induced reduction in anchorage-independent growth of RAS cells can be rescued by p65/p50.
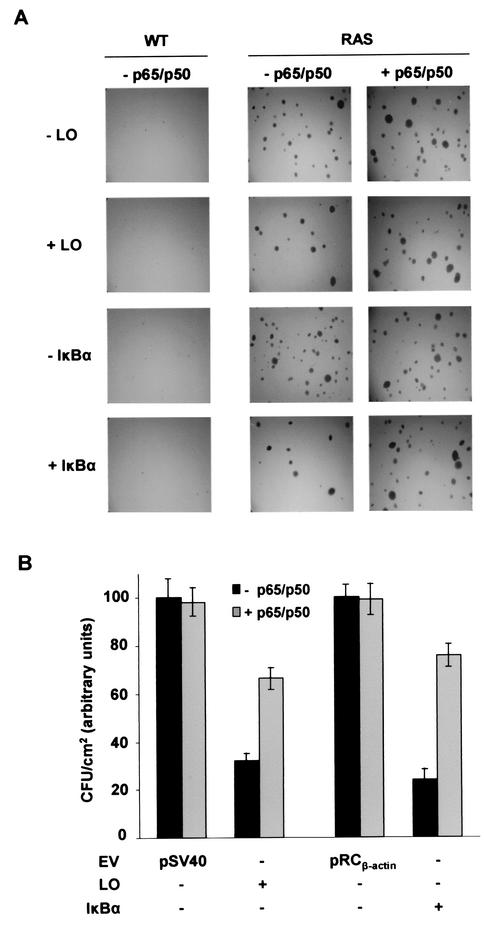
We next tested the effects of LO and inhibition of NF-κB on transformed phenotype of RAS cells by measuring growth in a soft agar assay. Specifically, the effects of transfection of LO and a serine-to-alanine 32/36 double mutant IκBα (dn IκBα) on colony formation were compared. After 2 weeks, control RAS cells gave rise to about 250 to 350 CFU/cm2, whereas no foci were seen with WT cells, as expected (Fig. 3A). Expression of either LO or dn IκBα significantly reduced colony formation of RAS cells by approximately the same extent, i.e., 70 to 78% (Fig. 3B). Interestingly, ectopic p65/p50 NF-κB subunit expression partially reverted the ability of LO and dn IκBα to reduce colony formation from 70 to 33% and 78 to 25%, respectively (Fig. 3), whereas they did not increase the number in foci in RAS cells (Fig. 3A). These findings strongly suggest that NF-κB is a crucial mediator of the _ras_-induced transformation phenotype and that inhibition of NF-κB is largely responsible for the ability of LO to block anchorage-independent growth of _ras_-transformed cells.
FIG. 3.
LO-mediated inhibition of oncogenic Ras focus-forming activity can be overridden by ectopic NF-κB expression. WT and RAS cells were transfected with 1 μg of LO (+LO) or S32/36A dn mutant IκBα (+IκBα) expression vectors or their parental empty vectors (EV; pSV40 and pRCβ-actin, respectively) in 0.5% FBS. Alternatively, cells were cotransfected either with 1 μg of p65 and p50 expression vectors (+p65/p50) or 2 μg of their empty vector pMT2 (−p65/p50). After 24 h, cells were plated in soft agar as described in Materials and Methods. (A) After 2 weeks, the colonies were stained with 0.0005% crystal violet and photographed by using a digital camera coupled to a dissection microscope. (B) The numbers of foci in panel A were determined, and the results are reported for RAS cells as the mean CFU/square centimeter ± the SD of triplicates obtained in two separate experiments.
LO does not directly inhibit NF-κB activity.
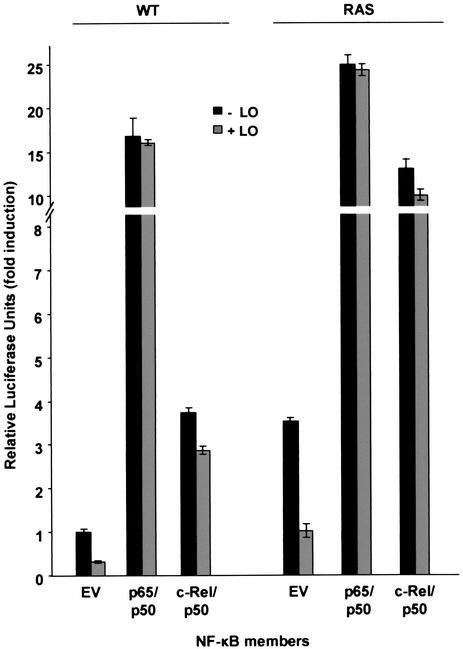
To determine whether LO inhibits NF-κB activation or activity, WT and _ras_-transformed NIH 3T3 cells were cotransfected with the LO expression vector DNA or the empty parental vector DNA in the presence of vectors expressing p50, either p65 or c-Rel, and the NF-κB element driven luciferase reporter (Fig. 4). As seen above, the basal level of NF-κB activity was 3.5-fold higher in _ras_-transformed cells than in WT cells and LO decreased NF-κB activity in both WT and RAS cells by approximately the same extent. Overexpression of p65/p50 increased the luciferase activity in WT and RAS cells, and the addition of LO was no longer able to reduce NF-κB activity (Fig. 4). As expected, ectopic expression of p50 alone had no effect on NF-κB activity (data not shown). The expression of c-Rel/p50, which resulted in a smaller increase in NF-κB activity, was also resistant to the inhibitory effects of LO (Fig. 4). Thus, the ability of LO to inhibit NF-κB activity was counteracted by the overexpression of transacting NF-κB members, indicating that LO probably inhibits an upstream signaling pathway(s) leading to NF-κB induction.
FIG. 4.
The overexpression of p65 and c-Rel counteracts the inhibitory effects of LO on NF-κB activation. WT and RAS cells were transfected with 1 μg of NF-κB-luciferase reporter plus 0.5 μg of SV40-β-Gal expression vector in the presence of 1 μg of either LO expression vector (+LO) or parental empty vector (−LO) in medium plus 0.5% FBS. Cotransfections of 1 μg of p65, p50, and c-Rel expression vectors or parental empty vector pMT2 (EV) were performed as indicated. At 72 h after the addition of FBS to a final concentration of 10%, extracts were prepared and subjected to luciferase reporter assays. Empty pMT2 expression vector was added to control the total amount of DNA transfected. The values for luciferase were normalized to the β-Gal activity. The results are expressed as the fold induction of luciferase activity relative to the WT cells transfected with pMT2 vector DNA and are the mean ± the SD of triplicates obtained in three separate experiments.
LO increases IκBα half-life.
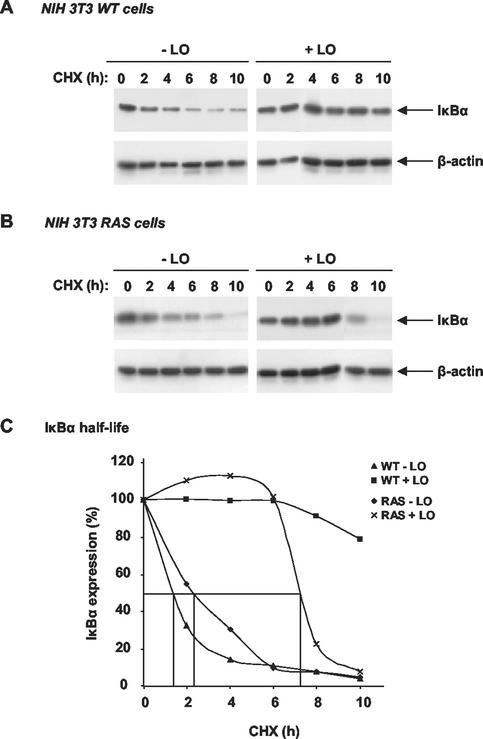
To begin to investigate the involvement of LO on upstream signaling pathways of NF-κB activation, we measured the effects of LO on IκBα half-life. WT and _ras_-transformed NIH 3T3 cells were transfected either with LO expression vector or its parental empty vector DNA. After 72 h, cycloheximide was added, and the expression levels of IκBα evaluated by immunoblot analysis of total cell extracts prepared at the indicated times from WT (Fig. 5A) and _ras_-transformed NIH 3T3 cells (Fig. 5B). A progressive decay in IκBα levels was noted as a function of length of the cycloheximide treatment in both cell lines transfected with the empty vector (Fig. 5A and B). Densitometry normalized to the levels of the control β-actin expression indicated that the half-lives of decay of IκBα were approximately 79 and 138 min in WT and RAS cells, respectively (Fig. 5C). In cells expressing LO, an increase in the IκBα half-life was noted (Fig. 5A and B). Densitometric analyses indicated that the half-life of IκBα was about 439 min in RAS cells expressing LO (Fig. 5C), whereas a half-life of decay of greater than 10 h was seen in WT cells (Fig. 5C). Extrapolation of the curve for these cells estimated a half-life of approximately 16 h in WT cells expressing LO. Consistent with these changes, a marked decrease in the phosphorylation of IκBα was noticed in the presence of LO (data not shown). These findings indicate that the inhibitory effects of LO are the result of its ability to promote an increase in IκBα stability.
FIG. 5.
LO enhances IκBα half-life. WT (A) and RAS (B) cells were transfected with LO expression vector (+LO) or parental empty vector (−LO) in medium plus 0.5% FBS. FBS was then added to a final concentration of 10% for 72 h in the absence (0) or presence of 50 μg of cycloheximide (CHX)/ml for the indicated times. Total cell extracts were subjected to immunoblot analysis for IκBα and then β-actin. (C) The percentage of IκBα expression, normalized for β-actin level, was determined relative to time zero.
LO inhibits IKK activation.
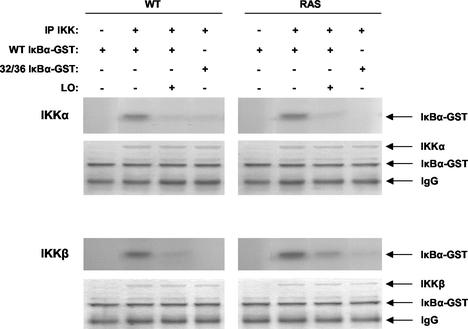
Given the observed roles of the two upstream IKKs in targeting IκBα for phosphorylation and decay in _ras_-transformed cells (3), kinase assays were carried out to measure the effect of LO on their activities. As substrates, we used GST fusion proteins of either WT IκBα or Ser32/36A double-mutant IκBα, which cannot be phosphorylated by the IKK kinases, as a negative control (Fig. 6). WT and _ras_-transformed NIH 3T3 cells were transiently transfected with LO expression vector or its parental empty vector DNA. After 72 h, extracts were prepared, immunoprecipitated with antibodies against either IKKα or IKKβ, and subjected to kinase assays. Both IKKα and IKKβ activities were detected in WT and RAS cells transfected with the empty vector, with a somewhat higher activation level of IKKβ in _ras_-transformed cells (Fig. 6). Expression of LO inhibited both IKKα and IKKβ activities in both cell lines (Fig. 6). The measurement of IKKα and IKKβ activities appeared to be specific since essentially no activity was detected with the Ser32/36A mutant IκBα-GST protein as a substrate (Fig. 6). The presence of equal amounts of IKK and GST proteins was verified by Coomassie blue staining of the gel. An anti-rabbit immunoglobulin G was used as control to confirm IKK immunoprecipitation by their respective antibodies. These results indicate that LO expression leads to the inhibition of both IKKα and IKKβ kinases.
FIG. 6.
LO inhibits IKK activity. Cells were transfected as described above in Fig. 5. After immunoprecipitation with antibodies to IKKα or IKKβ or with an anti-rabbit immunoglobulin G as a control (IP IKK), extracts were subjected to kinase assays with either WT IκBα-GST or the serine-to-alanine 32/36 double mutant nonphosphorylatable IκBα-GST version, as indicated. The gel was stained with Coomassie blue to verify equal loading and IKK immunoprecipitation (bottom of each panel).
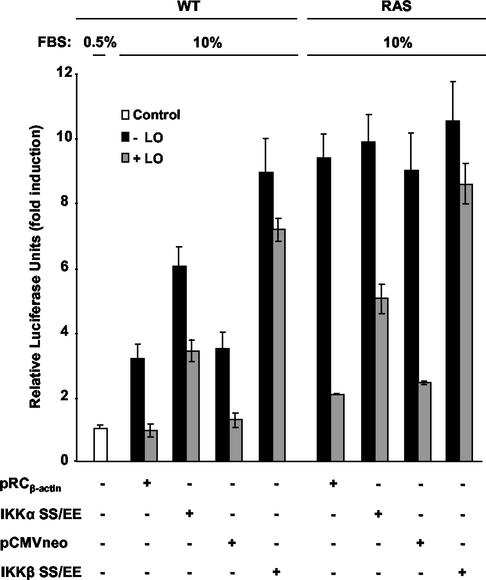
To address the roles of IKKα or IKKβ, we next sought to determine whether their constitutive expression could reestablish NF-κB activation in the presence of LO. WT and RAS cells were cotransfected with LO or its empty vector in the presence of constitutively activated forms of IKKα (IKKα SS/EE) or IKKβ (IKKβ SS/EE) or their empty vectors pRCβ-actin and pCMVneo, respectively. Expression of LO led to the expected decreases in activation of NF-κB activity in the WT and RAS cell lines, i.e., 2.7- to 3.4-fold and 3.7- to 4.5-fold decreases, respectively (Fig. 7). Overexpression of constitutively active forms of IKKα or IKKβ enhanced NF-κB basal activity in WT cells from 3.2- to 6.0-fold and 3.5- to 8.9-fold, respectively, while having little effect on NF-κB activity in RAS cells, a finding consistent with the activation of the kinases seen above in the untreated cells (Fig. 7). Constitutively active IKKα partially reestablished NF-κB activation in the presence of LO in WT and RAS cells, whereas IKKβ SS/EE expression vector led to an almost complete rescue from LO-mediated inhibition of NF-κB activity in both WT and RAS cell lines (Fig. 7). Taken together, these results indicate that LO is able to inhibit activation of both IKKα and IKKβ by serum and Ras. Moreover, constitutively activated forms of IKKα and IKKβ are able to counteract the inhibitory effects of LO on NF-κB activation, indicating that LO targets IKKs, which likely provide a mechanism leading to the increase of IκBα half-life and inhibition of NF-κB seen above.
FIG. 7.
Constitutively active IKK counteracts LO inhibition of NF-κB induction. WT and RAS cells were transfected, in triplicate, with 1 μg of NF-κB-luciferase reporter plus 0.5 μg of SV40-β-Gal expression vector in the presence of 1 μg of either LO expression vector (+LO) or parental empty vector (−LO) in medium plus 0.5% FBS (Control), in the absence or presence of 1 μg of constitutively active forms of IKKα or IKKβ or their parental empty vector (pRCβ-actin and pCMVneo, respectively). At 72 h after addition of FBS to a final concentration of 10%, extracts were prepared and subjected to luciferase reporter assays. The values for luciferase were normalized according to the β-Gal activity. The results are expressed as the mean fold induction of luciferase activity ± the SD obtained in three separate experiments.
LO partially inhibits the Raf/MEK pathway activated by Ras.
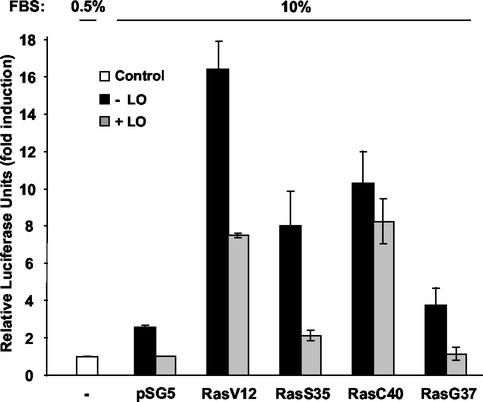
The oncoprotein Ras has been previously described to activate NF-κB through the induction of two distinct upstream signaling pathways: PI3K/Akt and Raf/MEK (3). To further evaluate which _ras_-activated pathways are affected by the expression of LO, we employed vectors expressing ras mutants known to selectively activate only one pathway. RasV12S35, RasV12C40, and RasV12G37 mutants have been shown to interact only with PI3K (p110α), Raf, and Ral.GDS, respectively (3, 58). WT cells were also transfected with a vector expressing the constitutively activated form of Ras, RasV12, which is capable of interacting with all of the known Ras effectors or parental vector pSG5. The expression of RasV12 led to a 16.4-fold induction in NF-κB activity, compared to the 2.6-fold induction seen with parental pSG5 DNA (Fig. 8). Expression of LO significantly decreased this induction to 7.5-fold (Fig. 8). As expected, expression of the RasV12S35 and RasV12C40 mutant Ras proteins induced NF-κB activation to a lower extent, 8.0- and 10.3-fold, respectively (Fig. 8). Coexpression of LO was able to effectively decrease luciferase activity induced by PI3K, i.e., from 8.0- to 2.1-fold (Fig. 8), but only able to slightly decrease luciferase activity induced via Raf, i.e., from 10.3- to 8.3-fold (Fig. 8). Finally, expression of the RasV12G37 mutant had only a marginal effect on NF-κB activity compared to the parental pSG5 vector (3.7- versus 2.6-fold). Together, these results demonstrate that LO is able to potently inhibit ras activation of NF-κB by the PI3K/Akt pathway, while only partially reducing activation via the Raf/MEK pathway.
FIG. 8.
LO inhibits oncogenic Ras activation of NF-κB principally by the PI3K pathway. WT cells were transfected, in triplicate, with 1 μg of NF-κB-luciferase reporter plus 0.5 μg of SV40-β-Gal expression vector in the presence of 1 μg of either LO expression vector (+LO) or parental empty vector (−LO) in medium plus 0.5% FBS (Control) and in the presence of 1 μg of either V12 H-ras, V12S35 H-ras, V12C40 H-ras, V12G37 H-ras or parental empty vector (pSG5), as indicated. The values for luciferase were normalized to β-Gal activity. The results are expressed as the mean fold induction of luciferase activity ± the SD obtained in three separate experiments.
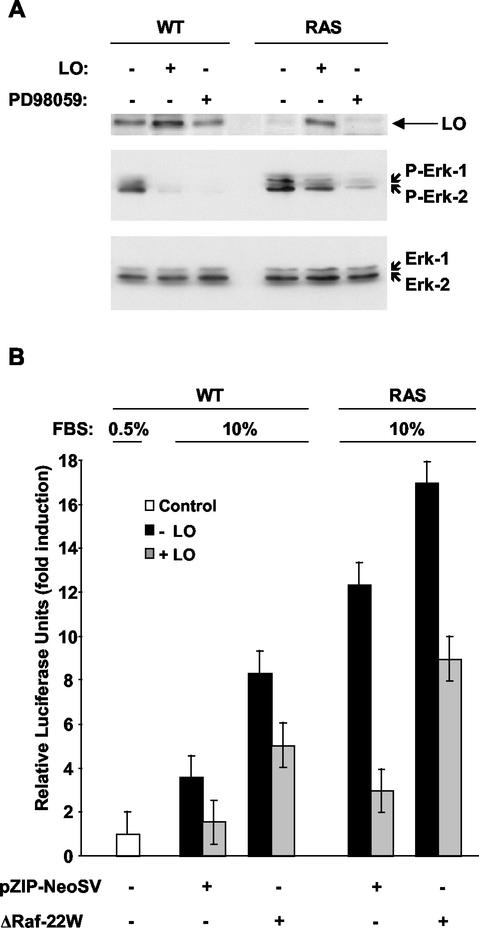
We next sought to determine whether LO can affect the Raf/MEK pathway activated by ras. WT and RAS cells were transfected with an empty vector or the vector expressing LO and then treated for 1 h with a 100 μM concentration of the MEK selective inhibitor PD98059 or the same volume of vehicle dimethyl sulfoxide, and whole-cell extracts were prepared. MEK activity was assessed by immunoblot analysis of the serine-phosphorylated form of the downstream MEK targets Erk-1 and Erk-2 (Fig. 9A). An increase of MEK activity was noted in cells expressing Ras, compared to the WT cells, a finding consistent with the ability of Ras to induce MEK activity in NIH 3T3 cells. As expected, incubation with PD98059 markedly decreased the phosphorylation of Erk-1 and Erk-2 in both cell lines (Fig. 9A). Upon expression of LO, the phosphorylated forms of Erk-1 and Erk-2 were barely detectable in WT cells, while in RAS cells the MEK activity was partially inhibited, although to a lower extent than that caused by PD98059 treatment (Fig. 9A). Equal loading was confirmed by probing the blot with an antibody that recognizes total Erk-1 and Erk-2 (Fig. 9A, lower panel). LO expression was also confirmed by probing the membrane with a specific antibody to LO (Fig. 9A, upper panel).
FIG. 9.
LO partially inhibits MEK activation. (A) WT and RAS cells were transfected with 1 μg of LO or parental empty expression vector as described above in Fig. 5. At 71 h after the addition of FBS to a final concentration of 10%, 100 μM PD98059 or the equivalent carrier dimethyl sulfoxide was added for 1 h, and whole-cell extracts were prepared. Samples (50 μg) were analyzed by immunoblotting with anti-threonine/tyrosine phosphorylated Erk1/2 specific antibodies. LO expression was confirmed by immunoblotting with a specific LO antibody (upper panel). Membranes were stripped and reprobed with anti-Erk1/2 mitogen-activated protein kinase antibodies to confirm equal loading (lower panel). (B) WT and RAS cells were transfected, in triplicate, with 1 μg of NF-κB-luciferase reporter plus 0.5 μg of SV40-β-Gal expression vector in the absence or presence of 1 μg of a constitutively active form of Raf (ΔRaf-22W) or parental empty vector, as indicated. The values for luciferase were normalized according to the β-Gal activity. The results are expressed as the mean fold induction of luciferase activity ± the SD obtained in three separate experiments.
To further investigate the ability of LO to inhibit the Raf/MEK pathway activated by Ras, the vector expressing a constitutively active form of Raf, ΔRaf-22W, obtained after truncation of the NH2-terminal region of Raf, or its counterpart, empty pZIP-NeoSV vector DNA, was cotransfected with the vector expressing LO or its parental vector in WT and RAS cells (Fig. 9B). Expression of the constitutively active form of Raf ΔRaf-22W induced luciferase activity in both WT and RAS cells, suggesting that this pathway is not fully activated in the RAS cells (Fig. 9B). LO was able to partially inhibit NF-κB activation mediated by ΔRaf-22W, suggesting that LO only partially affects _ras_-activated MEK in NIH 3T3 cells.
LO inhibits PI3K/Akt pathway activation by Ras.
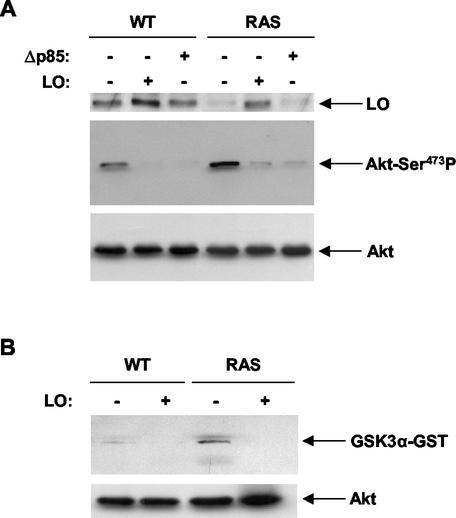
To evaluate the ability of LO to inhibit the PI3K pathway, we compared its effects with a vector expressing Δp85, a dn version of the p85 subunit of PI3K. Whole-cell extracts were prepared from cells transfected with LO or Δp85 expression vectors or empty vector DNA, and the PI3K activity was assayed (Fig. 10A). Immunoblot analysis of the phosphorylated form of the downstream PI3K target Akt was performed with an antibody specifically recognizing the phosphorylated serine 473 of Akt. An increase of PI3K activity was noted in cells expressing Ras compared to the WT cells (Fig. 10A), a finding consistent with the known ability of Ras to induce PI3K activity (36). As expected, Δp85 expression markedly decreased the phosphorylation of Akt in both cell lines (Fig. 10A). Expression of LO caused a similar marked inhibition of PI3K activity (Fig. 10A). To verify equal loading, the same membrane was probed with an antibody against total Akt (Fig. 10A). Furthermore, the membrane was probed with a specific antibody against LO in order to verify LO expression in transfected WT and RAS cells (Fig. 10A). As expected, basal levels of LO were higher in untransfected WT than in RAS cells, and the LO expression vector led to an increase of LO expression in transfected WT and RAS cells. Thus, LO inhibits PI3K activity to a similar extent as the dn Δp85 subunit.
FIG. 10.
LO inhibits the PI3K/Akt pathway activated by Ras. (A) WT and RAS cells were transfected as described above in Fig. 5 with a dn version of PI3K (Δp85) or the LO expression vector or their parental empty vectors. Samples of whole-cell extracts (50 μg) were subjected to immunoblotting with anti-Ser473 phosphorylated Akt specific antibody. LO expression was confirmed by immunoblotting with a specific LO antibody (upper panel). Membranes were stripped and reprobed with anti-Akt antibody to confirm the equal loading (lower panel). (B) Cells were transfected with LO (+) or parental empty vector (−) as in panel A, and whole-cell extracts were isolated with lysis buffer. Samples containing 100 μg of protein were subjected to kinase assay by using 1 μg of GSK3α-GST protein as a substrate. Phosphorylated GSK3α was identified by immunoblotting. Samples of whole-cell extracts (40 μg) were analyzed by immunoblotting with anti-Akt antibody to confirm equal loading (lower panel).
To determine whether LO is also able to inhibit Akt activity, whole-cell extracts were prepared from cells transfected with LO expression vector or its parental empty vector DNA and subjected to kinase assays with the GST fusion protein of the Akt downstream target GSK3α (Fig. 10B). Cells expressing Ras displayed an increased amount of Akt activity compared to WT cells, as expected. In both RAS and WT cells, a decrease in Akt activity was noted upon expression of LO (Fig. 10B). Taken together, these results indicate that LO blocks the activation of the PI3K/Akt pathway by Ras.
Ectopic expression of myristylated forms of Akt or PDK1 can overcome the inhibition of NF-κB activity by LO.
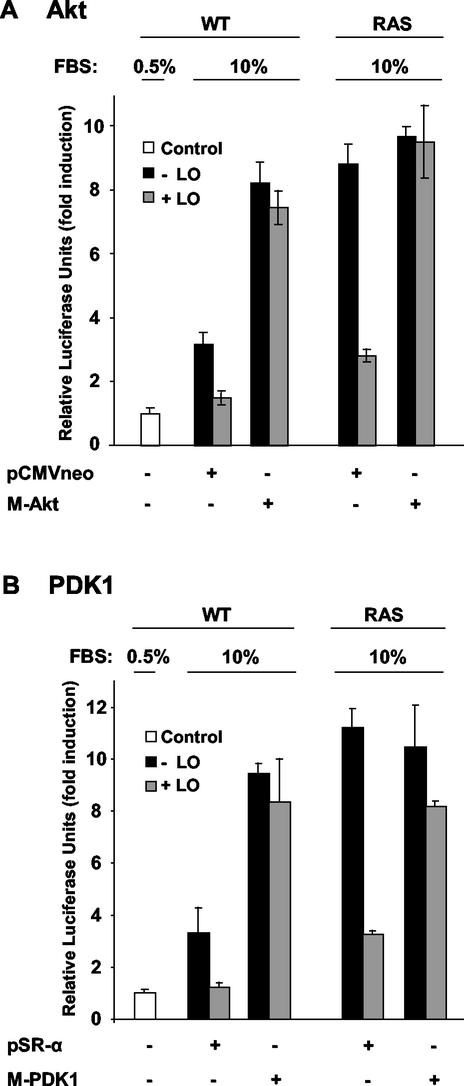
Akt kinase activity has been shown to be regulated by the phosphoinositide-dependent kinase-1 (PDK1), which is recruited to the membrane by PI3K-induced formation of phosphatidylinositol 3,4,5-triphosphate (PtdIns-3,4,5-P3) and phosphatidylinositol 3,4-biphosphate (PtdIns-3,4-P2) (72). To be activated, Akt needs to form a plasma membrane-bound complex with PDK1 (72). To confirm the inhibitory effects of LO on the PI3K/Akt pathway, we sought to determine whether expression of a constitutively activated myristylated (membrane bound) version of either Akt (M-Akt) or M-PDK1 could counteract the effects of LO on NF-κB activation. WT and RAS cells were transiently transfected with LO expression vector or its parental empty vector DNA in the presence of a vector expressing M-Akt (Fig. 11A) or M-PDK1 (Fig. 11B) or their empty vector DNAs. As expected, transfection of the empty vector did not affect the levels of NF-κB activation. Serum stimulation led to a 3.2-fold increase and to a 8.8-fold induction of luciferase activity in WT and RAS cells, respectively, and the expression of LO reduced it to a 1.5- and to a 2.8-fold induction of luciferase activity, respectively (Fig. 11A). Expression of the M-Akt led to an increase from a 3.2- to a 8.2-fold induction of luciferase activity in WT cells, whereas its expression did not affect NF-κB activation in RAS cells, a finding consistent with its activated state in these cells as seen above. LO was unable to inhibit NF-κB activation in either WT or RAS cells in the presence of M-Akt (Fig. 11A), indicating that the constitutive activation of Akt via forced membrane localization counteracts the inhibitory effects of LO on NF-κB activation. Similarly, the expression of M-PDK1 gave essentially identical results (Fig. 11B). Overall, these findings indicate that the membrane association of both Akt and PDK1 prevents LO inhibitory effects, suggesting LO may affect Akt/PDK1 complex formation at the plasma membrane level.
FIG. 11.
Membrane-bound M-Akt and M-PDK1 counteract LO inhibition of Ras-induced NF-κB activity. WT and RAS cells were transfected, in triplicate, with 1 μg of NF-κB-luciferase reporter plus 0.5 μg of SV40-β-Gal expression vector in the absence or presence of 1 μg of vector expressing the constitutively active form of either M-Akt (A) or M-PDK1 (B). Extracts were subjected to luciferase and β-Gal reporter assays, and the values for luciferase activity are presented normalized to the β-Gal activity. The results, which are expressed as the fold induction of luciferase activity, are the mean ± the SD obtained in three separate experiments.
LO prevents membrane localization of Akt and PDK1 induced by Ras.
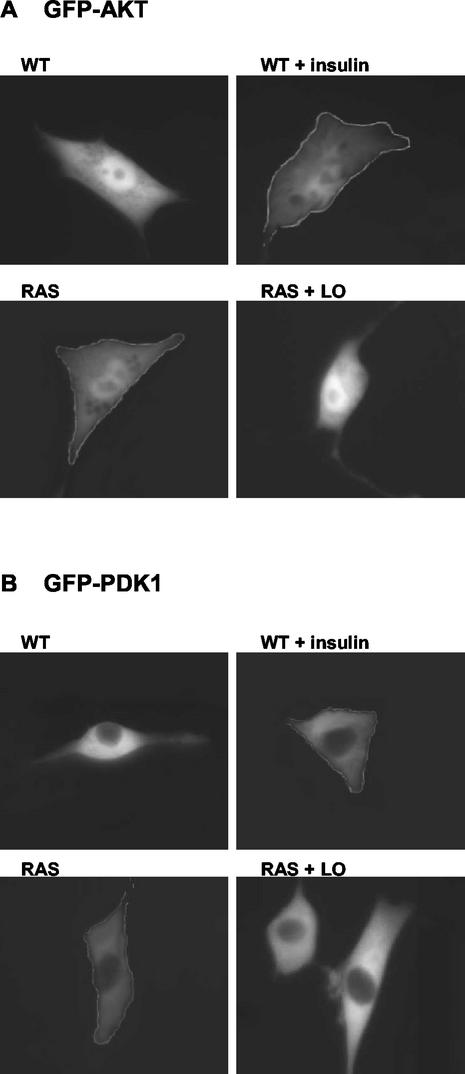
We next sought to test directly whether LO affects the membrane localization of Akt and/or its partner PDK1. WT and RAS cells were transiently transfected with GFP-tagged Akt (Fig. 12A) or PDK1 (Fig. 12B). In addition, for RAS cells, LO or its empty vector was cotransfected as indicated. Cultures were incubated overnight under serum starvation conditions. As a control for Akt and PDK1 membrane localization, transfected WT cells were stimulated with 200 nM insulin for 5 min. Fluorescence microscopy was used to visualize GFP-Akt or GFP-PDK1. In serum-deprived WT cells, GFP-Akt was found in the cytoplasm and nuclei, and insulin stimulation led to a substantial increase in Akt membrane localization (Fig. 12A). In contrast, RAS cells showed constitutive membrane-bound Akt, a result comparable to that found in insulin-stimulated WT cells. Ectopic expression of LO caused dissociation of Akt from the membrane (Fig. 12A). GFP-PDK1 was seen exclusively in the cytoplasm of WT cells, whereas it was localized to the membrane in RAS cells (Fig. 12B). Insulin stimulation of WT cells led to membrane translocation of GFP-PDK1, a result comparable to that found constitutively in serum-deprived RAS cells (Fig. 12B). Importantly, LO expression inhibited membrane localization of PDK1 in RAS cells (Fig. 12B). Thus, the membrane localization of Akt and PDK1 that is constitutively induced by Ras in NIH 3T3 cells is inhibited by LO. Together, these results suggest that the LO-mediated inhibition of Ras-induced NF-κB activation and transformation results from its ability to release the PDK1/Akt complex from the plasma membrane.
FIG. 12.
LO prevents constitutive membrane localization of Akt and PDK1 induced by Ras. WT and RAS cells were transfected with 2 μg of GFP-tagged Akt (A) or GFP-tagged PDK1 (B) constructs. RAS cells were cotransfected with LO expression vector (RAS + LO) or its empty vector DNA (RAS). Cells were incubated overnight under serum starvation conditions. Where indicated, WT cells were stimulated with 200 nM insulin for 5 min as a control for Akt (A) and PDK1 (B) membrane localization (WT + insulin). Akt and PDK1 cellular localization was visualized by fluorescence microscopy as described in the Materials and Methods.
DISCUSSION
We show here for the first time that LO blocks the induction of NF-κB binding and transcriptional activity by oncogenic Ras in NIH 3T3 cells by inhibiting the PI3K/Akt and Raf/MEK signaling pathways and thereby reverting transformed phenotype. LO decreased the phosphorylation and turnover of IκBα and expression of the NF-κB target c-myc gene. Ras activated both the IKKα and IKKβ kinases in the NIH 3T3 cells, as we had observed previously in rat liver epithelial cells (3). LO inhibited both activities but had an apparently stronger effect on IKKα. Two distinct pathways of activation of NF-κB by Ras have been demonstrated, i.e., the PI3K/Akt and Raf/MEK pathways (3, 33, 36, 58). LO was found to strongly inhibit Ras-mediated activation of the PI3K and Akt kinases, while having more modest inhibitory effects on the MEK kinases. LO was found to block Ras-mediated membrane localization of both Akt and PDK1. Furthermore, we showed that LO and the NF-κB super-repressor IκBα decreased growth in soft agar of _ras_-transformed NIH 3T3 cells to nearly the same extent; the slight difference may be attributed to the incomplete inhibition of the Raf/MEK signaling pathway by LO. Importantly, ectopic expression of p65/p50 ablated the ability of LO to inhibit the transformed phenotype of the Ras cells. Overall, these results strongly argue that the anti-oncogenic effects of LO on _ras_-mediated transformation are due to its ability to inhibit NF-κB activation.
The ras recision gene (rrg) was identified as a cDNA species that was dramatically reduced upon transformation of mouse NIH 3T3 cells by LTR-c-Ha-ras and was reexpressed upon interferon-mediated reversion of the transformants (12). The demonstration that mouse rrg and rat LO cDNA sequences were identical provided a direct molecular link between LO and transformation (34). More recently, Giampuzzi et al. have shown that downregulation of LO induces transformation of NRK-49F cells characterized by a constitutive activation of Ras (24). Altogether, these data strongly support the hypothesis that LO is a tumor suppressor gene, which is able to downregulate oncogenic Ras activity. Our results provide the first evidence that the anti-oncogenic effects of LO are mediated by its ability to inhibit the activation of NF-κB. NF-κB has been shown to play an important role in the development of cancer and metastasis (54). Many primary human tumors and derived cell lines display either increased NF-κB nuclear levels or reporter activity compared to nontransformed tissue or cell lines, including breast cancer, cutaneous T-cell lymphoma, adult T-cell leukemias, acute lymphoblastic leukemia, and pancreatic adenocarcinomas (6, 19, 52, 59, 66, 75). In parallel, both the important contributions of aberrant Ras activation in the promotion of a large spectrum of tumors and the ability of Ras to activate NF-κB have been extensively documented (1, 7, 21, 22, 44). Interestingly, several studies have shown that the PI3K pathway is crucial for transformation by Ras (58, 61). Baldwin's group has recently shown that the major oncogenic pathway for Ras is mediated via activation of NF-κB through Akt, and not through Raf (43, 50). Furthermore, both NF-κB and Akt have been associated with promotion of cell survival (reviewed in references 18, 54, and 65). The ability of LO to potently inhibit the PI3K/Akt pathway may then explain its ability to very effectively inhibit a transformed phenotype, as evidenced by anchorage-independent growth. Interestingly, Krzyzosiak et al. have reported that, after treatment with azatyrosine, _ras_-transformed and Her-2/_neu_-transformed NIH 3T3 cells convert to a flat, revertant cell phenotype and rrg is reexpressed, suggesting that LO has tumor suppressor effects on Her-2/_neu_-induced tumor as well (39). We have recently shown that Her-2/neu also activates NF-κB through the activation of the PI3K/Akt signaling pathway (52). Thus, LO represents a potentially important therapeutic inhibitor of NF-κB via its ability to prevent activation of the PI3K/Akt/NF-κB pathway by Ras and possibly other oncogenes such as Her-2/neu.
The regulation of LO expression is only poorly understood. Kuivaniemi et al. first observed deficient LO production in a variety of malignantly transformed cells (40). The loss of LO expression has been noted during progression of prostate and colorectal cancers (14, 56). Conversely, increased LO expression was found in spontaneous revertants of H-_ras_-transformed rat fibroblasts (26). In the present study, reduced LO was similarly observed in the _ras_-transformed cells. Interestingly, inhibitors of PI3K and MEK kinases had no effect on LO levels (Fig. 8 and 9), indicating these signaling pathways do not play a role in the regulation of LO expression. Interestingly, Tan et al. have shown that the LO gene is a transcriptional target of the anti-oncogenic transcription factor IRF-1, indicating that interferon induces the expression of LO through the Stat-1/IRF-1 pathway (68), although this finding has been somewhat controversial and is apparently dependent upon the cell type used (13, 57). More recently, the stimulation of LO expression by platelet-derived growth factor has been shown to be dependent upon the PKC-MEK-MAPK signaling pathway (63). Work is in progress to further elucidate the factors induced by Ras-mediated transformation that lead to inhibition of LO expression in NIH 3T3 cells.
Although LO has many known extracellular and cellular functions, little information was available on the molecular mechanism(s) of its action to inhibit ras signaling pathways. LO is secreted as a precursor, which is processed extracellularly to active enzyme (69). The LO enzyme can modify lysines on extracellular elastin and collagen proteins, which permits the covalent cross-linking that facilitates their deposition into an insoluble matrix; this processing occurs in proximity to the cell membrane (32). More recent studies have also been shown that LO can localize to the nuclei (15, 41, 49) and regulate transcription (23). In addition, LO has also been found in association with fine filamentous structures in the cytoplasm of fibroblasts, a finding consistent with localization with cytoskeletal proteins (32, 74). Thus, various workers have speculated that LO inhibits Ras signaling by altering or preventing either the recruitment or the association of Ras, Raf, PI3K, PDK1, and/or Akt to the inner surface of the plasma membrane, which is critical for their activation (9, 30, 37, 45, 67) or subsequent signaling steps, or by stimulating an antagonist, such as the 14-3-3 protein (42) or the plasma membrane protein SHK-1 (48), which were recently shown to inhibit the recruitment of Raf and Akt to the plasma membrane, respectively. We show here that myristylation of either Akt or PDK1, which leads to their constitutive binding to the plasma membrane, counteracts the inhibitory effects of LO, in a PI3K-independent manner. Moreover, we demonstrated that LO is able to block the membrane recruitment of GFP-tagged Akt and PDK1 that is constitutively induced by Ras in NIH 3T3 cells. Overall, our findings strongly suggest that LO inhibition of Ras transformation is mediated by its ability to block the membrane recruitment of Akt and PDK1, indicating that LO inhibits PI3K and the formation of the second messengers PtdIns-3,4,5-P3 and PdtIns-3,4-P2. In turn, LO leads to inhibition of the activation of NF-κB, a crucial factor mediating transformation by Ras.
Acknowledgments
We thank D. Faller, P. Trackman, M. Karin, G. Rawadi, Z. Luo, S. Hann, N. Rice, J. Downward, and J. Chung for generously providing cell lines, clones, and antibodies and M. Jeay for assistance in preparation of the manuscript. We gratefully acknowledge Marcello Arsura for helpful comments and suggestions and K. Symes and M. Sherman for the use of the dissecting and fluorescence microscopes, respectively.
This work was supported by grants from the NIH (PO1 HL 13262 [H.M.K. and G.E.S.]), the Association pour la Recherche sur le Cancer (S.J.), and the Philippe Foundation (S.J.).
REFERENCES
- 1.Abrams, S. I., P. H. Hand, K. Y. Tsang, and J. Schlom. 1996. Mutant ras epitopes as targets for cancer vaccines. Semin. Oncol. 23**:**118-134. [PubMed] [Google Scholar]
- 2.Arsura, M., M. J. FitzGerald, N. Fausto, and G. E. Sonenshein. 1997. Nuclear factor-κB/Rel blocks transforming growth factor β1-induced apoptosis of murine hepatocyte cell lines. Cell Growth Differ. 8**:**1049-1059. [PubMed] [Google Scholar]
- 3.Arsura, M., F. Mercurio, A. L. Oliver, S. S. Thorgeirsson, and G. E. Sonenshein. 2000. Role of the IκB kinase complex in oncogenic Ras- and Raf-mediated transformation of rat liver epithelial cells. Mol. Cell. Biol. 20**:**5381-5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeuerle, P. A., and T. Henkel. 1994. Function and activation of NF-κB in the immune system. Annu. Rev. Immunol. 12**:**141-179. [DOI] [PubMed] [Google Scholar]
- 5.Ballard, D. W., E. P. Dixon, N. J. Peffer, H. Bogerd, S. Doerre, B. Stein, and W. C. Greene. 1992. The 65-kDa subunit of human NF-κB functions as a potent transcriptional activator and a target for v-Rel-mediated repression. Proc. Natl. Acad. Sci. USA 89**:**1875-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bargou, R. C., F. Emmerich, D. Krappmann, K. Bommert, M. Y. Mapara, W. Arnold, H. D. Royer, E. Grinstein, A. Greiner, C. Scheidereit, and B. Dorken. 1997. Constitutive nuclear factor-κB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. J. Clin. Investig. 100**:**2961-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bos, J. L. 1989. ras oncogenes in human cancer: a review. Cancer Res. 49**:**4682-4689. [PubMed] [Google Scholar]
- 8.Brown, K., S. Gerstberger, L. Carlson, G. Franzoso, and U. Siebenlist. 1995. Control of IκBα proteolysis by site-specific, signal-induced phosphorylation. Science 267**:**1485-1488. [DOI] [PubMed] [Google Scholar]
- 9.Burgering, B. M., and P. J. Coffer. 1995. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376**:**599-602. [DOI] [PubMed] [Google Scholar]
- 10.Chanoki, M., M. Ishii, H. Kobayashi, H. Fushida, N. Yashiro, T. Hamada, and A. Ooshima. 1995. Increased expression of lysyl oxidase in skin with scleroderma. Br. J. Dermatol. 133**:**710-715. [DOI] [PubMed] [Google Scholar]
- 11.Chen, Z. J., L. Parent, and T. Maniatis. 1996. Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell 84**:**853-862. [DOI] [PubMed] [Google Scholar]
- 12.Contente, S., K. Kenyon, D. Rimoldi, and R. M. Friedman. 1990. Expression of gene rrg is associated with reversion of NIH 3T3 transformed by LTR-c-H-ras. Science 249**:**796-798. [DOI] [PubMed] [Google Scholar]
- 13.Contente, S., K. Kenyon, P. Sriraman, S. Subramanyan, and R. M. Friedman. 1999. Epigenetic inhibition of lysyl oxidase transcription after transformation by ras oncogene. Mol. Cell. Biochem. 194**:**79-91. [DOI] [PubMed] [Google Scholar]
- 14.Csiszar, K., S. F. Fong, A. Ujfalusi, S. A. Krawetz, E. P. Salvati, J. W. Mackenzie, and C. D. Boyd. 2002. Somatic mutations of the lysyl oxidase gene on chromosome 5q23.1 in colorectal tumors. Int. J. Cancer 97**:**636-642. [DOI] [PubMed] [Google Scholar]
- 15.Di Donato, A., J. C. Lacal, M. Di Duca, M. Giampuzzi, G. Ghiggeri, and R. Gusmano. 1997. Micro-injection of recombinant lysyl oxidase blocks oncogenic p21-Ha-Ras and progesterone effects on Xenopus laevis oocyte maturation. FEBS Lett. 419**:**63-68. [DOI] [PubMed] [Google Scholar]
- 16.Di Donato, J. A., F. Mercurio, C. Rosette, J. Wu-Li, H. Suyang, S. Ghosh, and M. Karin. 1996. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol. Cell. Biol. 16**:**1295-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Donato, J. A., M. Hayakawa, D. M. Rothwarf, E. Zandi, and M. Karin. 1997. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388**:**548-554. [DOI] [PubMed] [Google Scholar]
- 18.Downward, J. 1998. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 10**:**262-267. [DOI] [PubMed] [Google Scholar]
- 19.Duffey, D. C., Z. Chen, G. Dong, F. G. Ondrey, J. S. Wolf, K. Brown, U. Siebenlist, and C. Van Waes. 1999. Expression of a dominant-negative mutant inhibitor-κBα of nuclear factor-κB in human head and neck squamous cell carcinoma inhibits survival, proinflammatory cytokine expression, and tumor growth in vivo. Cancer Res. 59**:**3468-3474. [PubMed] [Google Scholar]
- 20.Duyao, M. P., A. J. Buckler, and G. E. Sonenshein. 1990. Interaction of an NF-κB-like factor with a site upstream of the c-myc promoter. Proc. Natl. Acad. Sci. USA 87**:**4727-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finco, T. S., J. K. Westwick, J. L. Norris, A. A. Beg, C. J. Der, and A. S. Baldwin, Jr. 1997. Oncogenic Ha-Ras-induced signaling activates NF-κB transcriptional activity, which is required for cellular transformation. J. Biol. Chem. 272**:**24113-24116. [DOI] [PubMed] [Google Scholar]
- 22.Folgueira, L., A. Algeciras, W. S. MacMorran, G. D. Bren, and C. V. Paya. 1996. The Ras-Raf pathway is activated in human immunodeficiency virus-infected monocytes and participates in the activation of NF-κB. J. Virol. 70**:**2332-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giampuzzi, M., G. Botti, M. Di Duca, L. Arata, G. Ghiggeri, R. Gusmano, R. Ravazzolo, and A. Di Donato. 2000. Lysyl oxidase activates the transcription activity of human collagen III promoter: possible involvement of Ku antigen. J. Biol. Chem. 275**:**36341-36349. [DOI] [PubMed] [Google Scholar]
- 24.Giampuzzi, M., G. Botti, M. Cilli, R. Gusmano, A. Borel, P. Sommer, and A. Di Donato. 2001. Down-regulation of lysyl oxidase-induced tumorigenic transformation in NRK-49F cells characterized by constitutive activation of ras proto-oncogene. J. Biol. Chem. 276**:**29226-29232. [DOI] [PubMed] [Google Scholar]
- 25.Gilmore, T. D., M. Koedood, K. A. Piffat, and D. W. White. 1996. Rel/NF-κB/IκB proteins and cancer. Oncogene 13**:**1367-1378. [PubMed] [Google Scholar]
- 26.Hajnal, A., R. Klemenz, and R. Schafer. 1993. Up-regulation of lysyl oxidase in spontaneous revertants of H-_ras_-transformed rat fibroblasts. Cancer Res. 53**:**4670-4675. [PubMed] [Google Scholar]
- 27.Hamalainen, E. R., R. Kemppainen, H. Kuivaniemi, G. Tromp, A. Vaheri, T. Pihlajaniemi, and K. I. Kivirikko. 1995. Quantitative polymerase chain reaction of lysyl oxidase mRNA in malignantly transformed human cell lines demonstrates that their low lysyl oxidase activity is due to low quantities of its mRNA and low levels of transcription of the respective gene. J. Biol. Chem. 270**:**21590-21593. [DOI] [PubMed] [Google Scholar]
- 28.Jeay, S., G. E. Sonenshein, M. C. Postel-Vinay, and E. Baixeras. 2000. Growth hormone prevents apoptosis through activation of nuclear factor-κB in interleukin-3-dependent Ba/F3 cell line. Mol. Endocrinol. 14**:**650-661. [DOI] [PubMed] [Google Scholar]
- 29.Jo, H., R. Zhang, H. Zhang, T. A. McKinsey, J. Shao, R. D. Beauchamp, D. W. Ballard, and P. Liang. 2000. NF-κB is required for H-ras oncogene induced abnormal cell proliferation and tumorigenesis. Oncogene 19**:**841-849. [DOI] [PubMed] [Google Scholar]
- 30.Johnston, S. R., and L. R. Kelland. 2001. Farnesyl transferase inhibitors: a novel therapy for breast cancer. Endocr. Relat. Cancer 8**:**227-235. [DOI] [PubMed] [Google Scholar]
- 31.Jourdan-Le Saux, C., C. Gleyzal, J. M. Garnier, M. Peraldi, P. Sommer, and J. A. Grimaud. 1994. Lysyl oxidase cDNA of myofibroblast from mouse fibrotic liver. Biochem. Biophys. Res. Commun. 199**:**587-592. [DOI] [PubMed] [Google Scholar]
- 32.Kagan, H. M., C. A. Vaccaro, R. E. Bronson, S. S. Tang, and J. S. Brody. 1986. Ultrastructural immunolocalization of lysyl oxidase in vascular connective tissue. J. Cell Biol. 103**:**1121-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy, S. G., A. J. Wagner, S. D. Conzen, J. Jordan, A. Bellacosa, P. N. Tsichlis, and N. Hay. 1997. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 11**:**701-713. [DOI] [PubMed] [Google Scholar]
- 34.Kenyon, K., S. Contente, P. C. Trackman, J. Tang, H. M. Kagan, and R. M. Friedman. 1991. Lysyl oxidase and rrg messenger RNA. Science **253:**802. [DOI] [PubMed]
- 35.Kim, S., K. Jee, D. Kim, H. Koh, and J. Chung. 2001. Cyclic AMP inhibits Akt activity by blocking the membrane localization of PDK1. J. Biol. Chem. 276**:**12864-12870. [DOI] [PubMed] [Google Scholar]
- 36.Kodaki, T., R. Woscholski, B. Hallberg, P. Rodriguez-Viciana, J. Downward, and P. J. Parker. 1994. The activation of phosphatidylinositol 3-kinase by Ras. Curr. Biol. 4**:**798-806. [DOI] [PubMed] [Google Scholar]
- 37.Kohn, A. D., F. Takeuchi, and R. A. Roth. 1996. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J. Biol. Chem. 271**:**21920-21926. [DOI] [PubMed] [Google Scholar]
- 38.Kotani, K., K. Yonezawa, K. Hara, H. Ueda, Y. Kitamura, H. Sakaue, A. Ando, A. Chavanieu, B. Calas, and F. Grigorescu. 1994. Involvement of phosphoinositide 3-kinase in insulin- or IGF-1-induced membrane ruffling. EMBO J. 13**:**2313-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krzyzosiak, W. J., N. Shindo-Okada, H. Teshima, K. Nakajima, and S. Nishimura. 1992. Isolation of genes specifically expressed in flat revertant cells derived from activated _ras_-transformed NIH 3T3 cells by treatment with azatyrosine. Proc. Natl. Acad. Sci. USA 89**:**4879-4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuivaniemi, H., R. M. Korhonen, A. Vaheri, and K. I. Kivirikko. 1986. Deficient production of lysyl oxidase in cultures of malignantly transformed human cells. FEBS Lett. 195**:**261-264. [DOI] [PubMed] [Google Scholar]
- 41.Li, W., K. Nellaiappan, T. Strassmaier, L. Graham, K. M. Thomas, and H. M. Kagan. 1997. Localization and activity of lysyl oxidase within nuclei of fibrogenic cells. Proc. Natl. Acad. Sci. USA 94**:**12817-12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Light, Y., H. Paterson, and R. Marais. 2002. 14-3-3 antagonizes Ras-mediated Raf-1 recruitment to the plasma membrane to maintain signaling fidelity. Mol. Cell. Biol. 22**:**4984-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madrid, L. V., C. Y. Wang, D. C. Guttridge, A. J. Schottelius, A. S. Baldwin, Jr., and M. W. Mayo. 2000. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-κB. Mol. Cell. Biol. 20**:**1626-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayo, M. W., C. Y. Wang, P. C. Cogswell, K. S. Rogers-Graham, S. W. Lowe, C. J. Der, and A. S. Baldwin, Jr. 1997. Requirement of NF-κB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science 278**:**1812-1815. [DOI] [PubMed] [Google Scholar]
- 45.McCormick, F. 1989. ras GTPase activating protein: signal transmitter and signal terminator. Cell 56**:**5-8. [DOI] [PubMed] [Google Scholar]
- 46.Mercurio, F., H. Zhu, B. W. Murray, A. Shevchenko, B. L. Bennett, J. Li, D. B. Young, M. Barbosa, M. Mann, A. Manning, and A. Rao. 1997. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278**:**860-866. [DOI] [PubMed] [Google Scholar]
- 47.Mercurio, F., B. W. Murray, A. Shevchenko, B. L. Bennett, D. B. Young, J. W. Li, G. Pascual, A. Motiwala, H. Zhu, M. Mann, and A. M. Manning. 1999. IκB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol. Cell. Biol. 19**:**1526-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moniakis, J., S. Funamoto, M. Fukuzawa, J. Meisenhelder, T. Araki, T. Abe, R. Meili, T. Hunter, J. Williams, and R. A. Firtel. 2001. An SH2-domain-containing kinase negatively regulates the phosphatidylinositol-3 kinase pathway. Genes Dev. 15**:**687-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nellaiappan, K., A. Risitano, G. Liu, G. Nicklas, and H. M. Kagan. 2000. Fully processed lysyl oxidase catalyst translocates from the extracellular space into nuclei of aortic smooth-muscle cells. J. Cell. Biochem. 79**:**576-582. [DOI] [PubMed] [Google Scholar]
- 50.Norris, J. L., and A. S. Baldwin, Jr. 1999. Oncogenic Ras enhances NF-κB transcriptional activity through Raf-dependent and Raf-independent mitogen-activated protein kinase signaling pathways. J. Biol. Chem. 274**:**13841-13846. [DOI] [PubMed] [Google Scholar]
- 51.Oldham, S. M., G. J. Clark, L. M. Gangarosa, R. J. Coffey, Jr., and C. J. Der. 1996. Activation of the Raf-1/MAP kinase cascade is not sufficient for Ras transformation of RIE-1 epithelial cells. Proc. Natl. Acad. Sci. USA 93**:**6924-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pianetti, S., M. Arsura, R. Romieu-Mourez, R. J. Coffey, and G. E. Sonenshein. 2001. Her-2/neu overexpression induces NF-κB via a PI3-kinase/Akt pathway involving calpain-mediated degradation of IκBα that can be inhibited by the tumor suppressor PTEN. Oncogene 20**:**1287-1299. [DOI] [PubMed] [Google Scholar]
- 53.Pinnell, S. R., and G. R. Martin. 1968. The cross-linking of collagen and elastin: enzymatic conversion of lysine in peptide linkage to α-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc. Natl. Acad. Sci. USA 61**:**708-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rayet, B., and C. Gelinas. 1999. Aberrant rel/nfkb genes and activity in human cancer. Oncogene 18**:**6938-6947. [DOI] [PubMed] [Google Scholar]
- 55.Regnier, C. H., H. Y. Song, X. Gao, D. V. Goeddel, Z. Cao, and M. Rothe. 1997. Identification and characterization of an IκB kinase. Cell 90**:**373-383. [DOI] [PubMed] [Google Scholar]
- 56.Ren, C., G. Yang, T. L. Timme, T. M. Wheeler, and T. C. Thompson. 1998. Reduced lysyl oxidase messenger RNA levels in experimental and human prostate cancer. Cancer Res. 58**:**1285-1290. [PubMed] [Google Scholar]
- 57.Reynaud, C., C. Gleyzal, C. Jourdan-Le Saux, and P. Sommer. 1999. Comparative functional study of the lysyl oxidase promoter in fibroblasts, Ras-transformed fibroblasts, myofibroblasts and smooth muscle cells. Cell. Mol. Biol. 45**:**1237-1247. [PubMed] [Google Scholar]
- 58.Rodriguez-Viciana, P., P. H. Warne, A. Khwaja, B. M. Marte, D. Pappin, P. Das, M. D. Waterfield, A. Ridley, and J. Downward. 1997. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell 89**:**457-467. [DOI] [PubMed] [Google Scholar]
- 59.Romieu-Mourez, R., E. Landesman-Bollag, D. C. Seldin, A. M. Traish, F. Mercurio, and G. E. Sonenshein. 2001. Roles of IKK kinases and protein kinase CK2 in activation of nuclear factor-κB in breast cancer. Cancer Res. 61**:**3810-3818. [PubMed] [Google Scholar]
- 60.Rothwarf, D. M., E. Zandi, G. Natoli, and M. Karin. 1998. IKKγ is an essential regulatory subunit of the IκB kinase complex. Nature 395**:**297-300. [DOI] [PubMed] [Google Scholar]
- 61.Sheng, H., J. Shao, and R. N. DuBois. 2001. Akt/PKB activity is required for Ha-Ras-mediated transformation of intestinal epithelial cells. J. Biol. Chem. 276**:**14498-14504. [DOI] [PubMed] [Google Scholar]
- 62.Smith-Mungo, L. I., and H. M. Kagan. 1998. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. 16**:**387-398. [DOI] [PubMed] [Google Scholar]
- 63.Smith-Mungo, L. I., and H. M. Kagan. 2002. PKC-MEK-MAPK-dependent signal transduction pathway mediates the stimulation of lysyl oxidase expression by serum and PDGF in rat aortic smooth muscle cells. J. Cell. Biochem. 85**:**775-784. [DOI] [PubMed] [Google Scholar]
- 64.Sommer, P., C. Gleyzal, M. Raccurt, M. Delbourg, M. Serrar, P. Joazeiro, S. Peyrol, H. M. Kagan, P. C. Trackman, and J. A. Grimaud. 1993. Transient expression of lysyl oxidase by liver myofibroblasts in murine schistosomiasis. Lab. Investig. 69**:**460-470. [PubMed] [Google Scholar]
- 65.Sonenshein, G. E. 1997. Rel/NF-κB transcription factors and the control of apoptosis. Semin. Cancer Biol. 8**:**113-119. [DOI] [PubMed] [Google Scholar]
- 66.Sovak, M. A., R. E. Bellas, D. W. Kim, G. J. Zanieski, A. E. Rogers, A. M. Traish, and G. E. Sonenshein. 1997. Aberrant nuclear factor-κB/Rel expression and the pathogenesis of breast cancer. J. Clin. Investig. 100**:**2952-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stokoe, D., S. G. Macdonald, K. Cadwallader, M. Symons, and J. F. Hancock. 1994. Activation of Raf as a result of recruitment to the plasma membrane. Science 264**:**1463-1467. [DOI] [PubMed] [Google Scholar]
- 68.Tan, R. S., T. Taniguchi, and H. Harada. 1996. Identification of the lysyl oxidase gene as target of the anti-oncogenic transcription factor, IRF-1, and its possible role in tumor suppression. Cancer Res. 56**:**2417-2421. [PubMed] [Google Scholar]
- 69.Trackman, P. C., D. Bedell-Hogan, J. Tang, and H. M. Kagan. 1992. Post-translational glycosylation and proteolytic processing of a lysyl oxidase precursor. J. Biol. Chem. 267**:**8666-8671. [PubMed] [Google Scholar]
- 70.Traenckner, E. B., H. L. Pahl, T. Henkel, K. N. Schmidt, S. Wilk, and P. A. Baeuerle. 1995. Phosphorylation of human IκBα on serines 32 and 36 controls IκBα proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 14**:**2876-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Urban, M. B., R. Schreck, and P. A. Baeuerle. 1991. NF-κB contacts DNA by a heterodimer of the p50 and p65 subunit. EMBO J. 10**:**1817-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vanhaesebroeck, B., and D. R. Alessi. 2000. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 346**:**561-576. [PMC free article] [PubMed] [Google Scholar]
- 73.Verma, I. M., J. K. Stevenson, E. M. Schwarz, D. Van Antwerp, and S. Miyamoto. 1995. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 9**:**2723-2735. [DOI] [PubMed] [Google Scholar]
- 74.Wakasaki, H., and A. Ooshima. 1990. Immunohistochemical localization of lysyl oxidase with monoclonal antibodies. Lab. Investig. 63**:**377-384. [PubMed] [Google Scholar]
- 75.Wang, W., J. L. Abbruzzese, D. B. Evans, L. Larry, K. R. Cleary, and P. J. Chiao. 1999. The nuclear factor-κB RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin. Cancer Res. 5**:**119-127. [PubMed] [Google Scholar]
- 76.Watton, S. J., and J. Downward. 1999. Akt/PKB localisation and 3′ phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr. Biol. 9**:**433-436. [DOI] [PubMed] [Google Scholar]
- 77.Woronicz, J. D., X. Gao, Z. Cao, M. Rothe, and D. V. Goeddel. 1997. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science 278**:**866-869. [DOI] [PubMed] [Google Scholar]