Promotion of Tumor Invasion by Cooperation of Granulocytes and Macrophages Activated by Anti-tumor Antibodies (original) (raw)
Abstract
We investigated the potential role of anti-tumor antibodies and tumor antigens in the formation of immune complexes which promote matrix degradation and angiogenesis. B-cell deficient or B-cell depleted mice showed a reduction in tumor invasion and metastasis. In vitro invasion assays and in vivo models of metastasis showed that anti-sTn antibodies and sTn tumor antigens form complexes which induce granulocytes and macrophages together to mediate tumor invasion and metastasis by processes including extracellular matrix degradation and angiogenesis. These results suggest the existence of a tumor promoting role of a B-cell immune response induced by shed tumor associated antigens of solid, nonlymphoid tumors.
Keywords: tumor cell invasion, metastasis, B cells, granulocytes, macrophages
Introduction
Over the past few years, accumulating data indicate that tumor cell invasion and metastasis are regulated by mechanisms that alter peritumoral stroma, including extracellular matrix remodeling and angiogenesis [1,2]. Cancer cells are capable of producing enzymes and factors that degrade connective matrix and that stimulate angiogenesis [3,4]. Additionally, cancer cells can stimulate stromal cell to undergo a connective tissue remodeling process [5,6] that can enhance tumor growth [7–9]. Actually, an increasing number of studies suggest that stromal cells, influenced by cancer cells, play a significant role in the process of tumor invasion [10–15]. In fact, both connective matrix turnover and angiogenesis are normal events of connective tissue renovation, wound healing, and inflammatory responses. The conundrum is that stromal-mediated inflammatory cellular reactions can contribute either to the regression or progression of cancer. What triggers an inflammatory reaction to contribute to tumor progression, as opposed to a reaction that contributes to tumor regression, is not known.
In some inflammatory pathologic events, connective-tissue restoration is governed mainly by immune complexes (IC) that stimulate the stromal cells. In this scenario, IC are formed locally between shed antigens (Ag) and specific antibodies (Ab) generated by the immune response against these Ag. However, yet to be described is a specific role for IC, generated after humoral immune responses (HIR) against tumor antigens, in the processes of tumor growth, invasion, and metastasis (collectively, ‘tumor progression’). It is well known that tumor cells are capable of eliciting an HIR against tumor-associated antigens (TAA) [16,17]. Clinical studies have shown that Ab against shed, hyperexpressed, or mutated molecules of tumor cells (such as epithelial mucin, p53 and many others) occur in serum of cancer patients at a high frequency [18]. Although Ab against TAA have shown certain anti-tumor effects [19], many clinical oncology studies have reported, but not explained, potentially adverse effects of the humoral immune response against TAA [20,21]. In this paper, we explore the possibility that the HIR elicited against certain shed tumor antigens can generate IC that induce stromal cells to activate the remodeling process of the connective tissue matrix surrounding the tumor in a manner that favors tumor progression.
Methods
Cell Lines
Cell lines, shedding mucin with sTn epitope, were used: Met-129 (methylcholanthrene-induced mammary carcinoma in C3H mice), T-47D (human ductal breast carcinoma) and SW620 (human colon carcinoma). The sTn negative cell line B16F1 (C57BL murine melanoma) was also used. All these cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with fetal bovine serum (FBS, 10%). As source of Ab in the in vivo experiments, two hybridomas produced with P3X63Ag8 myeloma cells were used: B72.3 hybridoma (secreting an anti-sTn monoclonal IgG1); and 9D9 hybridoma (secreting an anti-rabbit LDL receptor monoclonal IgG1). All cell lines, except Met-129, were obtained from the American Type Culture Collection (Rockville, MD). Met 129 cell line was kindly provided by Dr. J. Vaage (Roswell Park Memorial Institute, Buffalo, NY).
Test of sTn Antigen Shedding by Tumor Cells
All tumor cell lines were tested for shedding sTn antigen. Briefly, tumor cells were cultured in DMEM supplemented with 10% fetal bovine serum. Samples of media were harvested after 3 days of culture, and centrifuged at 3000 rpm for 20 minutes at 4°C. The content of sTn antigen in the supernatants was tested by ELISA.
Mice
Five- to six-week-old female mice were used for all experiments. IgM heavy chain gene deleted (B-cell deficient) homozygous C57BL/6-Igh-6tm1Cgn mice (_µ_MT/_µ_MT) were purchased from Jackson Laboratories (Bar Harbor, ME). Normal, SCID-beige and athymic nude C3H mice (nu/nu) were purchased from Harlan Sprague-Dawley (Indianapolis, IN).
Metastasis Experiments
Five- to six-week-old female tumor-isogeneic mice were used for all in vivo experiments. Lung metastases were produced by injecting 2x105 tumor cells in 100 µ of PBS into the tail vein. Groups of six mice were used for all experiments. Two weeks after injection, the mice were sacrificed, the lungs dissected under microscope, dried and weighed. The metastases were counted under a microscope. The weight per lung metastasis was calculated as excess of lung weight divided by number of metastases. Liver metastases were produced by intra-splenic injection of tumor cells. Briefly, mice were anesthetized with ketamine/xylazine mixture, a 3-mm opening was made in the left flank, and 5x105 tumor cells (in 100 _µ_l of PBS) were injected into the lower pole of the spleen. Three weeks after injection, the mice were sacrificed, and the livers were dissected. The liver metastasis mass was represented in percent of the liver volume as described before [28]. All tumor cells used for injection had undergone the same number of passages in culture.
In Vivo B-Cell Depletion
On day 5, 7, and 9 post-tumor challenge, mice received intraperitoneal injections of 170 _µ_g of goat anti-mouse IgM (Pierce, Rockford, IL). Controls received 170 _µ_g polyclonal goat IgG or PBS alone. Samples of blood were obtained by tail incision at days 0, 1, 4, and 8 after the treatment began. B-cell concentration in peripheral blood was assessed by flow cytometry using anti-B220 labeled with phycoerythrin (Pharmingen, San Diego, CA).
Tumor-Infiltrating and Spleen B-Lymphocyte Separation
C3H mice were injected with 1.5x106 Met129 cells into each of two mammary pads. Tumors were grown for 4 weeks. Mice were sacrificed by CO2 asphyxiation, and tumors were harvested aseptically. Tumors were dispersed with DNAse/collagenase enzyme cocktail. The single-cell suspension was incubated with anti-B220 MAb. Cells were separated with magnetic beads coated with sheep-anti-rat IgG (Dyanal, Oslo, Norway). Cells were washed again and maintained in RPMI media supplemented with 10% FBS for 4 hours. Non-adherent cells were recovered, washed in PBS, and tested for viability by Trypan Blue exclusion (>95%). Cell purity was checked by flow cytometry using anti-B220 labeled with phycoerythrin, and was determined to be >90%. Spleens were obtained from normal five- to six-week-old female tumor-isogeneic mice. The spleens were dispersed, and the B cells were selected as described above.
In Vitro Invasion System
Granulocytes and monocytes were harvested from the peripheral blood of SCID-beige mice. Additional macrophages were harvested from peritoneal washes of the same mice. Platelets were eliminated by differential centrifugation. Red blood cells were lysed with ammonium chloride solution, and remaining cells from all samples were washed with PBS, and then pooled. Invasion studies were performed with MATRIGEL Basement Membrane Matrix (Becton-Dickinson, San Jose, CA). Stromal cells were resuspended in RPMI and mixed with Matrigel at a 2:1 ratio by volume. Matrigel-stromal cell mixture was layered into 6.5-mm, 3.0-_µ_m pore size Transwell polycarbonate membrane inserts (Corning Costar, Cambridge, MA) at a concentration of 2.5x105 stromal cells per well. Stromal mix was polymerized at 37°C for 2 hours, and then 2.5x104 T-47D cells in RPMI+1% FBS were layered on top of the polymerized stroma. Inserts were placed in wells (24-well plates) containing RPMI+10% FBS with or without the addition of B72.3 (obtained from B72.3 hybridoma culture) or HB-sTn (Dako, Carpinteria, CA) anti-sTn antibody (0.06 _µ_g/well) dialyzed in a 10 K dialysis cassette (Pierce, Rockford, IL) against PBS overnight to remove sodium azide. In experiments involving sodium azide, sodium azide was added at 0.001% per well. In experiments involving mannan, mannan (Sigma, St. Louis, MO) was added at 0.06 _µ_g/well. For studies to inhibit PMNE, methoxysuccinyl-alanyl-alanyl-prolyl-valine-chloromethylketone (MeOSAAPV-CMK; Sigma) was added at 0.1 _µ_g/well. Inserts were placed in fresh media either with or without relevant additives (e.g., B72.3 or HB-sTn Ab and/or inhibitors tested) every 24 hours to maintain the FBS gradient within the stroma. Tumor-cell migration was evaluated every 24 hours for a total of 72 hours by counting the number of colonies that had migrated through the stroma and to the bottom of the inserts. All experiments were repeated three times, and each experiment comprised four repetitions of each combination. The data were normalized by dividing all single data by the average of the control in each experiment; therefore, the control average in each histogram appears as 1.0±SEM.
In Vivo Tumor Promotion System
One million SW620 cells mixed with 5x104 hybridoma cells (B72.3 or 9D9) were injected subcutaneously in SCID-beige mice. Control mice received only 5x104 hybridoma cells. The growth of the tumor was monitored daily. When the tumors were 10 mm in one diameter in a mouse, that mouse was sacrificed. Samples of blood were obtained 7 days after tumor implant, and after sacrifice. The serum content of anti-sTn IgG1 and free sTn antigen was measured by ELISA. After sacrifice, the tumors were harvested and split in two. One half was fixed in formalin and embedded in paraffin. The other half was frozen. The tissue content of anti-sTn IgG1 and the free sTn antigen was analyzed in extracts of homogenized tissue by ELISA. The tissue distribution of capillary (CD31+), IgG1 and sTn antigen was assessed by immunohistochemistry. Briefly, frozen 5- to 10-_µ_m-thick sections were fixed in -20°C acetone-methanol solution for 10 seconds, washed in 0.1 M PBS (three times), and incubated in H2O2 for 20 minutes to eliminate tissue peroxidase for 30 minutes. After additional washes, the slides were incubated at 4°C with 4 _µ_g/ml PBS solution of peroxidase conjugated goat anti-mouse IgG1 (Sigma) or peroxidase conjugated (kit from Boehringer-Mannheim, Indianapolis, IN) B72.3 MAb, or fluorescein isothiocyanate (FITC)-conjugated CD31 (Pharmingen). After incubation in Ab solution, the slides were washed three times in PBS. The peroxidase activity was visualized with DAB, and FITC green fluorescence was visualized by fluorescence microscopy. The vascular density of the tumors was estimated by counting the number of fluorescent tubular structures per square millimeter.
Results
Reduced Metastasis in Host with an Innate or Induced Deficient Humoral Immunity
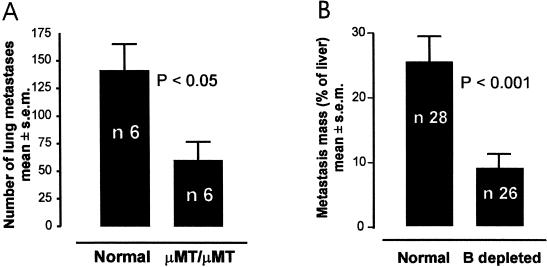
To preliminarily test whether the absence of an HIR against tumor cells may result in a reduction in the process of metastasis, we used a classic experimental mouse model of metastasis in which tumor cells (B16F10 melanoma) were injected in the tail veins of both immune-competent and innate B-cell-deficient (_µ_MT/_µ_MT) C57BL mice [27]. Two weeks after injection, the lungs of the humoral immune-competent mice showed significantly more metastases than the lungs of the B-cell-deficient mice (Figure 1_A_). The B-cell-deficient mice did not completely reject the tumor, but the growth of the metastatic colonies was confined by and encapsulated with connective tissue (not shown).
Figure 1.
Reduction of metastasis in conditions of B-cell deficiency or depletion. (A) Reduced lung metastasis in µMT/µMT B-cell deficient mice. (B) Reduction of liver metastasis in C3H mice receiving B-cell depletion treatment immediately after tumor-cell injection. Unpaired t test has been used to compare the mean of each column. The two-sided P value is shown on the graph. The error bars represent the SEM.
To further test whether a deficient HIR can slow tumor invasion, we induced a partial depletion of B lymphocytes in immune-competent mice previously injected with tumor cells. In this model, Met129 tumor cells (C3H mouse mammary carcinoma) were injected into the spleen. Splenic tumor develops and then rapidly metastasizes (in 7 days) to the liver, proving fatal in 3 weeks [28]. Seven days after tumor challenge, groups of mice were treated with anti-IgM Ab for depleting B cells in vivo [29]. One day after treatment, the number of peripheral B lymphocytes in the treated group was reduced from 20% to 50% of the control counts. Three weeks after tumor challenge, the mice undergoing B-cell depletion showed significantly less liver metastasis (Figure 1_B_).
Promotion of Metastasis by Tumor-Infiltrating B Lymphocytes in the Presence or Absence of T Cells
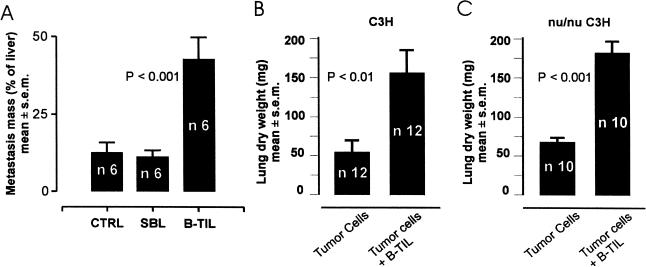
Next we tested whether B lymphocytes specifically involved in the anti-tumor immune response could actually promote metastasis. Tumor-infiltrating B lymphocytes (B-TIL) were isolated from Met129 tumors that were grown in the mammary pads of isogeneic C3H mice. In this experiment, C3H mice were injected in the spleen with 106 Met129 tumor cells. A group of these mice was additionally injected (IV) three times a week with 105 B-TIL (90% B220+ cells), or with B lymphocytes obtained from the spleen (SBL) of normal C3H mice. Three weeks after tumor-cell injection, the group infused with B-TIL showed significantly more liver metastases when compared to the controls which did not receive lymphocytes (Figure 2_A_). However, mice injected with SBL did not show any difference when compared with the controls (Figure 2_A_). To assess whether the observed B-cell involvement in the promotion of metastasis was T-cell independent, we used a model of lung metastasis in nu/nu mice. These are mice which are unable to mount a T-cell response against the tumor. Normal and nu/nu beige C3H mice received a tail-vein injection of Met129 cells, after which half of each group immediately received a supplement of B-TIL. Both normal and nu/nu beige mice treated with B-TIL developed significantly more metastases than the respective controls (Figure 2, B and C). The latter result suggests that the observed B-cell involvement in the promotion of metastasis was T-cell independent.
Figure 2.
Promotion of metastasis of tumor by tumor infiltrating B cells. (A) Increased liver metastasis of splenic implanted tumors in C3H mice after four injections (alternate days) of 104 B cells (B-TIL) obtained from the lymphocyte infiltration of the same tumor type, compared with non treated mice, and mice receiving the same number of B cells obtained from the spleen of normal mice (SBL). (B) Increased number of lung metastases obtained by tail vein injection of 105 tumor cells with or without B-TIL in immune competent C3H mice. (C) The same experiment repeated with nu/nu C3H mice also shows increased metastasis when the tumor cells are injected together with B-TIL. Unpaired t test has been used to compare the mean of each column. The two-side P value is shown on the graph.
Promotion of Tumor-Cell Invasion by Combined Action of TAA:Anti-TAA Ab Complexes, Granulocytes and Macrophages
To test whether Abs against TAA have a direct role in this observed promotion of metastasis, we used in vitro migration and invasion assays. T-47D human breast carcinoma cells were selected because they shed mucin with multiple sTn epitopes. The anti-sTn mouse monoclonal Abs HB-sTn and/or B72.3 (both IgG1; anti-sTn MAb) were used as anti-TAA Ab. Peripheral blood granulocytes (PMN) and peritoneal macrophages (MO) were obtained from C3H-SCID beige mice to avoid contaminating mouse NK cells, natural Abs, or any immune recognition by mouse T or B lymphocytes. T-47D cancer cells were plated on the surface of the Matrigel-coated Boyden chambers [32] that contained in the lower well either media alone, or media supplemented with Abs. All experiments were complement free.
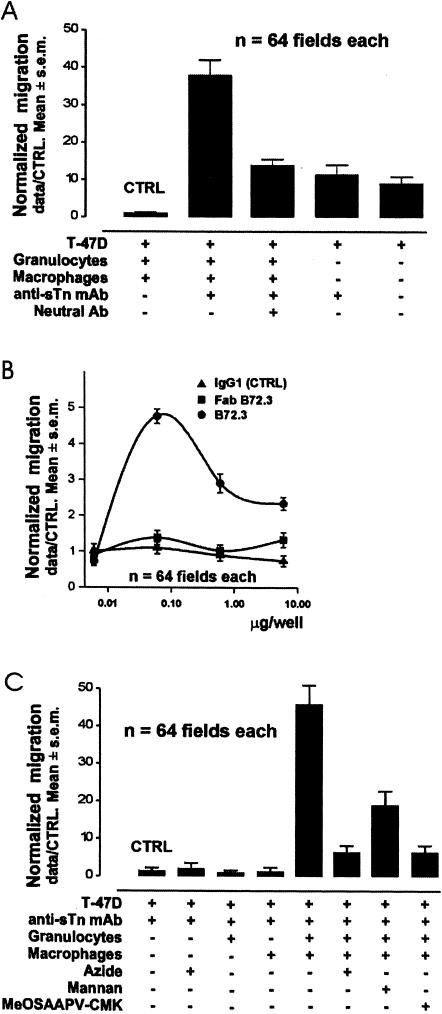
To test whether anti-tumor Ab alone can influence tumor-cell migration, chambers with Matrigel, but without PMN or MO, were supplemented with B72.3 MAb or HB-sTn MAb. No differences were observed in tumor-cell migration in 72 hours (Figure 3_A_). To test whether the stromal cells without Ab can influence tumor-cell migration, the Matrigel was embedded with PMN and MO. In all these chambers, tumor-cell migration in 72 hours was reduced (Figure 3_A_), showing that these cells inhibited tumor-cell invasion. To test whether the addition of any IgG1 Ab may influence the migration of tumor cells in the presence of a mixture of PMN and MO, we added neutral (of irrelevant specificity) IgG1 to the lower well. No difference of migration rates was observed after the addition of neutral Ab (Figure 3_B_). To test whether the addition of a specific Ab able to bind Ag shed by the tumor cells can influence tumor-cell migration, in the presence of stromal cells, we added B72.3 or HB-sTn to the lower well, and the gel was embedded with PMN and MO. In these experiments, the addition of anti-sTn MAb increased the migration rates of the tumor cells (Figure 3_A_). To test whether Ag shed by tumor cells was part of the mechanism inducing such increased tumor-cell migration, we repeated the above experiments using B16 melanoma cells which do not shed sTn antigens. The addition of anti-sTn Abs did not increase tumor-cell migration of B16 melanoma cells (data not shown).
Figure 3.
In vitro promotion of tumor invasion by stromal cells, TAA and anti-TAA Ab. Each individual migration rate is expressed as the number of migrated tumor cells in each field (data) divided by the average number of migrated tumor cells of the control combination (CTRL) (data/CTRL). Therefore, the CTRL mean appears as 1.0±SEM. (A) Stromal cells alone inhibit tumor-cell migration. However, a MAb binding sTn TAA in the presence of stromal cells increases tumor-cell migration. An excess of Ig of the same isotype, but nonspecific for tumor antigens (neutral Ab) inhibits the increase of tumor-cell migration induced by anti-sTn TAA MAb. Finally, MAb binding sTn TAA secreted by tumor cells, without stromal cells, does not change the natural ability of tumor cells to migrate. The mean of each column has been compared by unpaired t-test. (B) Comparative effects on tumor-cell migration of increasing amounts of the specific anti-tumor antigen MAb, the Fab segment of the same MAb and a neutral IgG of the same isotype. (C) Comparative effects on tumor-cell migration of the presence of anti-TAA Ab (anti-sTn-MAb). Each of azide, PMN, or MO, alone did not enhance tumor-cell migration in the Matrigel assay. The simultaneous addition of both PMN and MO increases tumor-cell migration. Peroxidase inhibition (azide), mannose receptor blocking (mannan) or elastase inhibition (MeOSAAPV-CMK) constrains the increase of tumor-cell migration induced by the combination of PMN, MO and anti-sTn TAA MAb.
Fc Receptor Involvement in IC Mediated Promotion of Tumor Invasion
Due to the absence of complement in these in vitro experiments, the signal induced by the complex of tumor-derived mucin and the sTn MAb may be conducted through the Fc receptors (FcR). To test this hypothesis, we repeated the above experiments using PMN-MO embedded matrix and the Fab fragment of B72.3. The migration rate of the tumor cells was not increased in these conditions (Figure 3_B_). To check whether monomeric IgG could compete with IC of mucin and B72.3 MAb, we added an excess of B72.3 MAb. An excess of B72.3 MAb produced a reduction in the increased tumor-cell migration (Figure 3_B_). Moreover, the increased tumor-cell migration was also reduced when an excess of neutral IgG1 (not recognizing sTn antigen) was added to the optimal dose of B72.3 (Figure 3_A_).
Requirement of Combined Action of PMN and MO for IC-Mediated Promotion of Tumor Invasion
We tested whether PMN alone, or MO alone, or the combination of PMN and MO were the active cells influencing tumor-cell migration in the presence of anti-TAA Ab and TAA. Matrigel was embedded with either PMN, or MO, or both together, and then B72.3 MAb was added to each of the lower wells. In these experiments, the addition of PMN+MO increased the migration rates of the tumor cells as expected. However, neither PMN nor MO alone enhanced the migration rates compared to the controls (Figure 3_C_).
Myeloperoxidase in the Cooperation between PMN and MO in IC-Mediated Promotion of Tumor Invasion
To assess whether PMN factors, such as myeloperoxidase (MyPo), are released after FcR-mediated activation by IC, and then activate MO to promote tumor-cell invasion, we treated the cultures in Matrigel with mannan as a mannose receptor blocker. In cultures with PMN+MO+mannan, tumor-cell invasion was substantially reduced, but not totally inhibited (Figure 3_C_).
Role of PMN Primary Granule Peptidases in the IC-Mediated Promotion of Tumor Invasion
Tumor-cell invasion is driven by tumor-cell motility and connective-matrix degradation. Matrix degradation is provoked by the cellular release of proteases in the milieu. Granulocyte Elastase (PMNE), and other peptidases are capable of degrading connective-tissue matrix. To assess whether primary granule peptidase release by PMN, after FcR-mediated activation by IC, could be a necessary factor in the observed promotion of tumor-cell invasion, we treated the cultures in Matrigel with methoxysuccinyl-alanyl-alanyl-prolyl-valine-chloromethylketone (MeOSAAPV-CMK) as a PMNE inhibitor, or with azide as a MyPo inhibitor. Tumor-cell invasion of the Matrigel layer was substantially reduced in cultures with PMN+MO+MeOSAAPV-CMK (Figure 3_C_), and in cultures with PMN+MO+azide (Figure 3_C_).
In Vivo Promotion of Tumor-Cell Invasion and Metastasis by Anti-TAA Antibodies
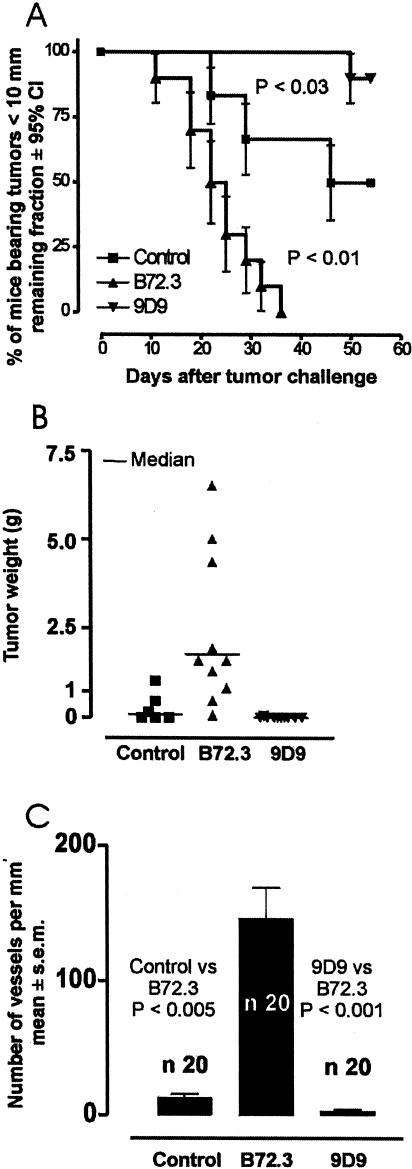
Our in vitro experiments only test tumor-cell invasion, and exclude in vivo variables such as complement and angiogenesis that may alter the effects observed in the Matrigel. To test whether an Ab recognizing a shed tumor antigen can accelerate in vivo tumor growth and invasion, we tested a model of subcutaneous tumor development in SCID-beige mice in which the natural host response against the tumor should be controlled by PMN and MO. To supply the anti-TAA Ab into the connective tissue matrix of the tumor, we implanted hybridoma cells producing anti-sTn Ab in the tumor. Groups of mice were injected subcutaneously with 106 SW620 cells, a human colon tumor which sheds mucin with sTn epitopes. One group (SW620) received SW620 cells alone. A second group (SW620+B72.3) received SW620 cells, mixed with 5x104 B72.3 hybridoma cells which produce anti-sTn MAb in simulating infiltrating plasma cells producing a specific MAb against a TAA. A third group (SW620+9D9) of mice received SW620 cells, mixed with 5x104 9D9 hybridoma cells which produce a MAb against rabbit LDL receptor that is unable to recognize any known human or mouse antigen. 9D9 hybridoma cells mimic infiltrating plasma cells producing a MAb which is unrelated to any TAA. Thirty-six days after tumor challenge, all mice of the SW620+B72.3 group developed tumors bigger than 1 cm Figure 4_A_) and metastases; and all died within 50 days. However, 60 days after tumor challenge, 50% of the SW620 group either failed to develop tumor and metastases, or developed tumors smaller than 1 cm (Figure 4, A and B). Twenty percent of the SW620 group survived more than 100 days. Similarly, all mice injected with SW620+9D9 developed significantly fewer and smaller tumors than the control (Figure 4, A and B). Eighty percent of the SW620+9D9 group survived more than 100 days. The histologic analysis of tumors from the SW620 group showed multiple necrotic areas, poor angiogenesis (Figure 4_C_), and a well-defined limit between the tumor cell tissue and the surrounding connective tissue. However, all tumors formed in the mice of the SW620+B72.3 group showed minimal necrosis, profuse angiogenesis (Figure 4_C_), IgG deposition in the matrix, and a poorly defined limit between the tumor and surrounding connective tissue. Histochemical analysis of the latter tumors and their metastases demonstrated that the majority of the tumor tissue was composed of SW620 cells, as recognized by their nuclear shape and the intra-cytoplasmic staining for cytokeratin and sTn antigen. Hybridoma cells, recognized by the nuclear shape and by cytoplasmic staining for mouse IgG, were always present in the tumors and metastases. The hybridoma cells typically formed clusters dispersed throughout the tumor structure, and mainly at the stroma-tumor cell interface.
Figure 4.
In vivo promotion of tumor growth by tumor antigens and anti-tumor antibodies. (A) One million SW620 human colon carcinoma cells shedding sTn TAA were injected: alone into eight mice (■); together with 5x 104 P3X63Ag8 hybridoma cells producing B72.3 anti-sTn MAb into eight mice (▲); or together with 5x104 P3X63Ag8 hybridoma cells producing the neutral 9D9 MAb into eight mice (▼). The survival curves represent the remaining fractions of mice with tumor smaller than 10 mm, following the method of Kaplan and Meier. Error bars show the 95% confidence interval (CI) for fractions at any particular time. (B) Representation of the individual tumor weight reached in 21 days of tumor progression in the same experimental groups. The horizontal bar represents the average. The two-tailed P value showed statistically significant differences between the media (control vs. B72.3 P<0.05; control vs. 9D9 P<0.01). (C) Average number of vessels per mm2 of tumor tissue section, identified by immune histochemistry using fluorescein conjugated anti-CD31 MAb. The t-test showed significant differences between the mean of each group.
Discussion
Apparent clinically adverse effects of the humoral anti-tumor immune response have been observed by several groups. To explain this paradox, different mechanisms have been proposed. Classically, it has been speculated that B lymphocytes could produce blocking antibodies that may provoke immunologic enhancement of tumor growth [23,24]. Recently, it has been described that B cells can produce IgG linked to latent transforming growth factor beta, which could prevent local cytolytic T lymphocyte action against tumor cells [22]. Additionally, it has been claimed that B-cell presentation of tumor Ag may alter the T-cell response against the tumor [25], or that B cells may mediate downregulation of interferon-gamma and interleukin-12 production [26]. Based on experimental and clinical data, it has been proposed that a humoral immune response can actually stimulate tumor growth depending on the activity of T-cell cytokines [33,34]. From our results of induction of metastasis in B-cell knockout mice and B-cell-depleted mice, it is evident that there is a reduction in both tumor growth and metastasis when B cells are absent or impaired. These results suggested that the impairment of a HIR did influence the metastatic process. Therefore, we believed that the HIR can influence mechanisms of tumor growth, invasion and metastasis. The results obtained by injecting additional B-TIL or SBL to tumor-bearing mice showed that such an influence is exerted by B lymphocytes which have been in contact with TAA. Altogether, this first set of results suggest that either the HIR impairs tumor-cell rejection [25] or promotes tumor progression.
Addition of B-TIL enhanced metastasis in nu/nu mice. This is an indication that the observed B-cell promotion of tumor progression is neither T-cell mediated, nor a consequence of T-cell inhibition (hence, is T-cell independent). In nu/nu mice, the metastasis-promoting effect caused by supplementation of B-TIL should therefore be explained as a direct consequence of specific B-cell products including antibodies against TAA.
The increase in metastases in this model could be attributed to two basic mechanisms: an increase of the tumor-cell extravasation (invasion) and/or an increase of the growth of metastases. The in vitro experiments presented here show the existence of a potential mechanism for promoting tumor progression involving an HIR induced by shed TAA, and involving a cooperative action between PMN and MO triggered by complexes of shed TAA and anti-TAA Ab. The absence of complement in the in vitro experimental system, and that Fab fragments of anti-sTn MAb were unable to reproduce that increase of tumor-cell invasion observed with intact anti-sTn MAb, suggest that the inductive effect of Ag-Ab complex on PMN and MO involves FcRs. Additionally, data from in vitro experiments showed that nonspecific IgG molecules inhibited that promotion of invasion mediated by MAb HBsTn and B72.3, suggesting that the action of immune complexes can be blocked by monomeric IgG. The fact that neither PMN nor MO alone are able to drive this enhancement of tumor-cell invasion shows that both cells are necessary, but not sufficient individually, to promote tumor-cell invasion. Blocking of MO mannose receptors reduced the cooperative effort of PMN and MO, strongly suggesting that PMN could be the cells receiving the first signal from the IC. IC-activated PMN may subsequently induce MO activation through MyPo/mannose receptor ligation [35]. The observed effect of azide on blocking the promotion of invasion suggests that PMN-MyPo, released by activated PMN, is crucial in this mechanism of the promotion of tumor-cell invasion. Inhibition of elastase (PMNE) veils the observed promotion of tumor-cell invasion, suggesting that matrix degradation by proteases released from PMN primary granules is an important part of the process. However, considering that other potent proteases may also be released by the PMN and MO, PMNE may have an additional role, such as activating MO-derived interleukin-1_β_ which could then act to promote tumor-cell invasiveness [36].
The in vivo experiments reinforced our in vitro observations, and allow extension of these findings to human tumor biology. In our in vivo model, mice host human tumor cells shedding a TAA which is recognized by a mouse Ab in forming IC that can bind mouse FcR on PMN and MO. In humans, shed TAA will be recognized by autologous Ab in forming IC that can bind to FcR on patient's stromal cells including PMN and MO. The data obtained from pure SW620 tumor injection showed that in SCID mice, without any anti-tumor T- or B-cell response, tumor can be rejected in at least 20% of the cases. However, aggressive and invasive tumor resulted from the combination of mucin-secreting SW620 carcinoma and B72.3 hybridoma secreting monoclonal anti-sTn antibody into the stroma. This is an indication that local Ab+shed TAA can trigger a mechanism that promotes tumor invasion. This observation is reinforced by the fact neither tumor growth nor invasion is promoted when SW620 tumor is infiltrated with the same myeloma cell type (P3X63Ag8 myeloma) but which secretes a MAb which does not bind to any tumor Ag (9D9 MAb). This mechanism of promotion of tumor-cell invasion does not involve T cells or NK cells, which are absent in the experiments with SCID-beige mice. That this mechanism of promotion is T-cell independent is further supported by the promotion of metastasis observed in nu/nu mice injected with B-TIL.
Both in vivo and in vitro experiments point to PMN and MO as effectors of this immune-complex-mediated tumor promotion. This stromal cell mediation involves the release of factors (i.e., PMNE, MyPo, metalloproteinases, and cytokines) which can directly promote matrix degradation (as observed in vitro), and angiogenesis (as observed in vivo) [33]. These factors may also indirectly promote tumor-cell motility and growth. The 9D9 hybridoma secretes a MAb unable to bind any TAA from SW620. Thus, that SCID mice injected with SW620 plus 9D9 hybridoma showed a significant reduction in tumor progression, suggests that ligation of monomeric IgG to the Fc receptors is able to block the IC-induced responses of PMN and MO that favor tumor-cell invasion. Altogether, these data suggest that different anti-tumor Abs may either favor or counteract tumor progression, depending on the nature of the tumor antigens. We could infer that shed TAA containing a single epitope may reduce tumor progression, but that polyvalent shed TAA may form complexes involving several Ig molecules which are then able to cross-link stromal cell FcR and induce reactions that favor tumor progression. This is the case with tumor-derived mucin which contains multiple tandemly repeated carbohydrate epitopes in each molecule, thereby being capable of binding several molecules of Ab to form immune complexes that can cross-link FcR. This mechanism could explain clinical observations reporting increased invasive behavior of tumors which secrete mucoprotein molecules common to many adenocarcinomas [30,31,37–39]. Also, this mechanism can explain the evident therapeutic effect on colon cancer by selective lymphadenectomy of lymph nodes involved in a massive B-cell response against sTn or Tn antigens [40].
In conclusion, these findings suggest the existence of a mechanism exclusively dependent on the HIR against TAA which can modulate tumor progression by involving a cooperative action of PMN and MO infiltrating the tumor tissue [41,42]. Antibodies and shed polyvalent TAA can form complexes in the connective-tissue matrix close to the tumor-cell front. These immune complexes can activate a cross talk between PMN and MO that ultimately favors matrix degradation, angiogenesis and tumor-cell invasiveness. Therefore, tumor progression can be induced by a mechanism triggered solely by a tumor-induced specific B-cell immune response that is T-cell independent. As a consequence, tumor cells not only are able to evade immune rejection, but have the additional capacity to take advantage of the immune system by inducing an HIR which can favor tumor progression.
Abbreviations
Ab
antibody
B-TIL
tumor-infiltrating B lymphocytes
FcR
Fc receptor
HIR
humoral immune response
IC
immune complexes
LDL
low-density lipoprotein
MeOSAAPV-CMK
methoxysuccinyl-alanyl-prolyl-valine-chloromethylketone
MAb
monoclonal antibody
MO
macrophages
MyPo
myeloperoxidase
PMN
peripheral blood granulocytes
PMNE
granulocyte elastase
SBL
splenic B lymphocytes
TAA
tumor associated antigens
References
- 1.Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE. Halting angiogenesis suppresses carcinoma cell invasion. Nat Med. 1997;3:1222–1227. doi: 10.1038/nm1197-1222. [DOI] [PubMed] [Google Scholar]
- 2.Boyd D. Invasion and metastasis. Cancer Metastasis Rev. 1996;15:77–89. doi: 10.1007/BF00049488. [DOI] [PubMed] [Google Scholar]
- 3.Sugiura Y, Shimada H, Seeger RC, Laug WE, DeClerck YA. Matrix metalloproteinases-2 and -9 are expressed in human neuroblastoma: contribution of stromal cells to their production and correlation with metastasis. Cancer Res. 1998;58:2209–2216. [PubMed] [Google Scholar]
- 4.Salven P, Lymboussaki A, Heikkila P, Jaaskela-Saari H, Enholm B, Aase K, von Euler G, Eriksson U, Alitalo K, Joensuu H. Vascular endothelial growth factors VEGF-B and VEGF-C are expressed in human tumors. Am J Pathol. 1998;153:103–108. doi: 10.1016/S0002-9440(10)65550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohtani H. Stromal reaction in cancer tissue: Pathophysiologic significance of the expression of matrix-degrading enzymes in relation to matrix turnover and immune/inflammatory reactions. Pathol Int. 1998;48:1–9. doi: 10.1111/j.1440-1827.1998.tb03820.x. [DOI] [PubMed] [Google Scholar]
- 6.Pyke C, Ralfkiaer E, Tryggvason K, Dano K. Messenger RNA for two type IV collagenases is located in stromal cells in human colon cancer. Am J Pathol. 1993;142:359–365. [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura T, Matsumoto K, Kiritoshi A, Tano Y, Nakamura T. Induction of hepatocyte growth factor in fibroblasts by tumor-derived factors affects invasive growth of tumor cells: in vitro analysis of tumor-stromal interactions. Cancer Res. 1997;57:3305–3313. [PubMed] [Google Scholar]
- 8.Janvier R, Sourla A, Koutsilieris M, Doillon CJ. Stromal fibroblasts are required for PC-3 human prostate cancer cells to produce capillary-like formation of endothelial cells in a three-dimensional co-culture system. Anticancer Res. 1997;17:1551–1557. [PubMed] [Google Scholar]
- 9.Rosen EM, Goldberg ID. Regulation of angiogenesis by scatter factor. EXS. 1997;79:193–208. doi: 10.1007/978-3-0348-9006-9_8. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen BS, Sehested M, Timshel S, Pyke C, Dano K. Messenger RNA for urokinase plasminogen activator is expressed in myofibroblasts adjacent to cancer cells in human breast cancer. Lab Invest. 1996;74:168–177. [PubMed] [Google Scholar]
- 11.Nielsen BS, Timshel S, Kjeldsen L, Sehested M, Pyke C, Borregaard N, Dano K. 92 kDa type IV coll agenase (MMP-9) is expressed in neutrophils and macrophages but not in malignant epithelial cells in human colon cancer. Int J Cancer. 1996;65:57–62. doi: 10.1002/(SICI)1097-0215(19960103)65:1<57::AID-IJC10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Swallow CJ, Murray MP, Guillem JG. Metastatic colorectal cancer cells induce matrix metalloproteinase release by human monocytes. Clin Exp Metastasis. 1996;14:3–11. doi: 10.1007/BF00157680. [DOI] [PubMed] [Google Scholar]
- 13.MacDougall JR, Matrisian LM. Contributions of tumor and stromal matrix metalloproteinases to tumor progression, invasion and metastasis. Cancer Metastasis Rev. 1995;14:351–362. doi: 10.1007/BF00690603. [DOI] [PubMed] [Google Scholar]
- 14.Segain JP, Harb J, Gregoire M, Meflah K, Menanteau J. Induction of fibroblast gelatinase B expression by direct contact with cell lines derived from primary tumor but not from metastases. Cancer Res. 1996;56:5506–5512. [PubMed] [Google Scholar]
- 15.Polette M, Gilles C, Marchand V, Seiki M, Tournier J-M, Birembaut P. Induction of membrane-type matrix metalloproteinase 1 (MT1-MMP) expression in human fibroblasts by breast adenocarcinoma cells. Clin Exp Metastasis. 1997;15:157–163. doi: 10.1023/a:1018404927753. [DOI] [PubMed] [Google Scholar]
- 16.Gorsky Y, Vanky F, Sulitzeanu D. Isolation from patients with breast cancer of antibodies specific for antigens associated with breast cancer and other malignant diseases. Proc Natl Acad Sci USA. 1976;73:2101–2105. doi: 10.1073/pnas.73.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Punt CJ, Barbuto JA, Zhang H, Grimes WJ, Hatch KD, Hersh EM. Anti-tumor antibody produced by human tumor-infiltrating and peripheral blood B lymphocytes. Cancer Immunol Immunother. 1994;38:225–232. doi: 10.1007/BF01533513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Disis ML, Cheever MA. Oncogenic proteins as tumor antigens. Curr Opin Immunol. 1996;8:637–642. doi: 10.1016/s0952-7915(96)80079-3. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg SA. Lysis of human normal and sarcoma cells in tissue culture by normal human serum: implications for experiments in human tumor immunology. J Natl Cancer Inst. 1977;58:1233–1238. doi: 10.1093/jnci/58.5.1233. [DOI] [PubMed] [Google Scholar]
- 20.Mikulski SM, Billing R, Terasaki PI. Inhibition of effector cell function in human antibody-dependent cellular cytotoxicity by sera from cancer patients. J Natl Cancer Inst. 1977;58:1485–1487. doi: 10.1093/jnci/58.5.1485. [DOI] [PubMed] [Google Scholar]
- 21.Bansal SC, Bansal BR, Thomas HL, Siegel PD, Rhoads JE, Jr, Cooper DR, Terman DS, Mark R. Ex vivo removal of serum IgG in a patient with colon carcinoma: Some biochemical, immunological and histological observations. Cancer. 1978;42:1–18. doi: 10.1002/1097-0142(197807)42:1<1::aid-cncr2820420102>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 22.Rowley DA, Stach RM. B lymphocytes secreting IgG linked to latent transforming growth factor-(beta) prevent primary cytolytic T lymphocyte responses. Int Immunol. 1998;10:355–363. doi: 10.1093/intimm/10.3.355. [DOI] [PubMed] [Google Scholar]
- 23.Sjögren HO, Hellström I, Bansal SC, Hellström KE. Suggestive evidence that the “blocking antibodies” of tumor-bearing individuals may be antigen-antibody complexes. Proc Natl Acad Sci USA. 1971;68:1372–1375. doi: 10.1073/pnas.68.6.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman ID. Immunological enhancement: a study of blocking antibodies. Adv Immunol. 1972;15:167–214. doi: 10.1016/s0065-2776(08)60685-9. [DOI] [PubMed] [Google Scholar]
- 25.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–630. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 26.Wijesuriya R, Maruo S, Zou J-P, Ogawa M, Umehara K, Yamashita M, Ono S, Fujiwara H, Hamaoka T. B cell-mediated downregulation of IFN-gamma and IL-12 production induced during anti-tumor immune responses in the tumor-bearing state. Int Immunol. 1998;10:1057–1065. doi: 10.1093/intimm/10.8.1057. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 28.Barberá-Guillem E, Alonso-Varona A, Vidal-Vanaclocha F. Selective implantation and growth in rats and mice of experimental liver metastasis in acinar zone one. Cancer Res. 1989;49:4003–4010. [PubMed] [Google Scholar]
- 29.Brodt P, Gordon J. Anti-tumor immunity in B lymphocyte-deprived mice: I. Immunity to a chemically induced tumor. J Immunol. 1978;121:359–362. [PubMed] [Google Scholar]
- 30.Cao Y, Schlag PM, Karsten U. Immunodetection of epithelial mucin (MUC1, MUC3) and mucin-associated glycotopes (TF, Tn, and sialosyl-Tn) in benign and malignant lesions of colonic epithelium: apolar localization corresponds to malignant transformation. Virchows Arch. 1997;431:159–166. doi: 10.1007/s004280050083. [DOI] [PubMed] [Google Scholar]
- 31.Kinney AY, Sahin A, Vernon SW, Frankowski RF, Annegers JF, Hortobagyi GN, Buzdar AU, Frye DK, Dhingra K. The prognostic significance of sialyl-Tn antigen in women with breast carcinoma treated with adjuvant chemotherapy. Cancer. 1997;80:2240–2249. doi: 10.1002/(sici)1097-0142(19971215)80:12<2240::aid-cncr4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 32.Shao ZM, Nguyen M, Alpaugh ML, O'Connell JT, Barsky SH. The human myoepithelial cell exerts antiproliferative effects on breast carcinoma cells characterized by p21WAF1/CIP1 induction, G2/M arrest, and apoptosis. Exp Cell Res. 1998;241:394–403. doi: 10.1006/excr.1998.4066. [DOI] [PubMed] [Google Scholar]
- 33.Prehn RT. Stimulatory effects of immune reaction upon growths of untransplanted tumors. Cancer Res. 1994;54:908–914. [PubMed] [Google Scholar]
- 34.Shearer WT, Parker CW. Antibody and complement modulation of tumor cell growth in vitro and in vivo. Fed Proc. 1978;37:2385–2389. [PubMed] [Google Scholar]
- 35.Lefkowitz DL, Mills K, Morgan D, Lefkowitz SS. Macrophage activation and immunomodulation by myeloperoxidase. Proc Soc Exp Biol Med. 1992;199:204–210. doi: 10.3181/00379727-199-43348. [DOI] [PubMed] [Google Scholar]
- 36.Bani MR, Garofalo A, Scanziani E, Giavazzi R. Effect of interleukin-1-beta on metastasis formation in different tumor systems. J Natl Cancer Inst. 1991;83:119–123. doi: 10.1093/jnci/83.2.119. [DOI] [PubMed] [Google Scholar]
- 37.Hanski C, Hofmeier M, Schmitt-Graff A, Riede E, Hanski ML, Borchard F, Sieber E, Niedobitek F, Foss HD, Stein H, Riecken EO. Overexpression or ectopic expression of MUC2 is the common property of mucinous carcinomas of the colon, pancreas, breast, and ovary. J Pathol. 1997;182:385–391. doi: 10.1002/(SICI)1096-9896(199708)182:4<385::AID-PATH861>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 38.Spicer AP, Rowse GJ, Lidner TK, Gendler SJ. Delayed mammary tumor progression in Muc-1 null mice. J Biol Chem. 1995;270:30093–30101. doi: 10.1074/jbc.270.50.30093. [DOI] [PubMed] [Google Scholar]
- 39.Joseph IBJK, Isaacs JT. Macrophage role in the anti-prostate cancer response to one class of antiangiogenic agents. J Natl Cancer Inst. 1998;90:1648–1653. doi: 10.1093/jnci/90.21.1648. [DOI] [PubMed] [Google Scholar]
- 40.Barberá-Guillem E, Arnold MW, Nelson MB, Martin EW., Jr First results for resetting the anti-tumor immune response by immune corrective surgery in colon cancer. Am J Surg. 1998;176:339–343. doi: 10.1016/s0002-9610(98)00192-5. [DOI] [PubMed] [Google Scholar]
- 41.Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh S, Ross SR, Acena M, Rowley DA, Schreiber H. Stroma is critical for preventing or permitting immunological destruction of antigenic cancer cells. J Exp Med. 1992;175:139–146. doi: 10.1084/jem.175.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]