Structural basis by which alternative splicing confers specificity in fibroblast growth factor receptors (original) (raw)
Abstract
Binding specificity between fibroblast growth factors (FGFs) and their receptors (FGFRs) is essential for mammalian development and is regulated primarily by two alternatively spliced exons, IIIb (“b”) and IIIc (“c”), that encode the second half of Ig-like domain 3 (D3) of FGFRs. FGF7 and FGF10 activate only the b isoform of FGFR2 (FGFR2b). Here, we report the crystal structure of the ligand-binding portion of FGFR2b bound to FGF10. Unique contacts between divergent regions in FGF10 and two b-specific loops in D3 reveal the structural basis by which alternative splicing provides FGF10-FGFR2b specificity. Structure-based mutagenesis of FGF10 confirms the importance of the observed contacts for FGF10 biological activity. Interestingly, FGF10 binding induces a previously unobserved rotation of receptor Ig domain 2 (D2) to introduce specific contacts with FGF10. Hence, both D2 and D3 of FGFR2b contribute to the exceptional specificity between FGF10 and FGFR2b. We propose that ligand-induced conformational change in FGFRs may also play an important role in determining specificity for other FGF-FGFR complexes.
Fibroblast growth factors (FGF1–FGF23) control embryonic development and adult tissue homeostasis by binding and activating members of the FGF receptor family (FGFR1–FGFR4; refs. 1 and 2). FGFRs are composed of an extracellular ligand-binding portion consisting of three Ig-like domains (D1–D3), a single transmembrane helix, and a cytoplasmic portion with protein tyrosine kinase activity. An FGFR fragment encompassing D2 and D3 is the minimal unit sufficient for specific ligand binding (3). FGFR dimerization is an essential event in FGF signaling and requires heparin or heparan sulfate proteoglycans (HSPGs; refs. 4–7).
Binding specificity between FGFs and FGFRs is critical for the proper regulation of FGF signaling. The physiological significance of FGF-FGFR specificity is best demonstrated in Apert syndrome, a severe craniosynostosis syndrome, where point mutations in FGFR2 alter ligand binding affinity and specificity (8–10). Alternative splicing of FGFR mRNA is the main mechanism by which FGFRs with different ligand-binding profiles are generated. In FGFR1 to -3, D3 is encoded by the invariant exon IIIa followed by one of two alternative exons, IIIb (“b”) or IIIc (“c”). This alternative splicing event is regulated in a tissue-specific fashion with b expression restricted to epithelial lineages and c to mesenchymal lineages (11–14). Most FGFs activate more than one FGFR. The FGF7 subfamily is unique among FGFs because its members (FGF7, FGF10, and FGF22) are expressed exclusively by mesenchyme and interact specifically with the b splice variant of FGFR2 (FGFR2b) resident in overlying epithelium (15–17).
FGF7 and FGF10 bind FGFR2b with similar high affinity and compete with each other for this binding (18, 19). However, striking phenotypic similarities between the FGF10 and FGFR2b knockout mice have established FGF10 as the predominant ligand for FGFR2b in developmental patterning and organogenesis. Both FGFR2b-null and FGF10-null mice die at birth and show agenesis of the lungs and limbs, whereas FGF7-null mice are viable and have normal lungs and limbs (16, 20–22).
The exceptional specificity between the FGF7 subfamily and FGFR2b, together with the pivotal role of FGF10-FGFR2b signaling during development, makes the FGF10-FGFR2b complex an ideal model system for deciphering the structural determinants of FGF-FGFR binding specificity. In this report, we describe the crystal structure of the FGF10-FGFR2b complex and confirm the structural findings by mutational analysis. FGF10-FGFR2b specificity incorporates not only alternative splicing-dependent ligand-receptor interactions but also a ligand-induced conformational change in the receptor.
Methods
Crystallization and Data Collection.
The DNA fragment encoding residues 64–208 of human FGF10 was amplified by PCR and subcloned into the pET-9c bacterial expression vector (18). The construct was expressed in Escherichia coli BL21 (DE3) and found to be soluble. FGF10 was purified by heparin affinity, ion exchange, and size exclusion chromatography.
The DNA fragment encoding residues 140–369 of human FGFR2b (23) was amplified by PCR and subcloned into the pET-28a bacterial expression vector. Selenomethionine-substituted FGFR2b was produced by an auxotrophic protocol (24) and purified as previously described for FGFR2c (25). The purified protein was confirmed by mass spectroscopy to be the fragment of FGFR2b consisting of residues 140–369.
FGF10 and FGFR2b were mixed together and purified as described (25). Crystals were grown by vapor diffusion at 20°C by using the hanging drop method. Crystals of the FGF10-FGFR2b complex were obtained by mixing 2 μl of the crystallization buffer (3% PEG 400/2.0 M NH4SO4/0.1 M Tris⋅HCl, pH 8.5) with 2 μl of protein solution (7 mg/ml/25 mM Hepes, pH 7.5/150 mM NaCl). The FGF10-FGFR2b crystals belong to the hexagonal space group P6422 with unit cell dimensions a = b = 113.930 Å and c = 164.852 Å. The asymmetric unit contains a single FGF10-FGFR2b complex.
Crystals were frozen in a cryoprotectant composed by adding 9% sucrose (wt/vol), 2% glucose (wt/vol), 8% glycerol (vol/vol), and 8% ethylene glycol (vol/vol) to the reservoir buffer. Multiple anomalous dispersion data were measured at three wavelengths near the Se K edge by using a Q4R charge-coupled device detector system at beamline X4A of the National Synchrotron Light Source.
Structure Determination and Refinement.
All data were processed by using hkl (26). Attempts to determine the positions of FGF10 and D2 and D3 of FGFR2b by using amore (27) with the structures of FGF2 and FGFR2c (25) as search models were unsuccessful. cns (28) was used for phase evaluation from four Se sites with multiple anomalous dispersion data to 3.2 Å. An initial model was built in o (29) by manually placing the models of FGF2 and D2 and D3 of FGFR2c into a 3.2-Å electron density map calculated with experimental phases. The placement of the model was confirmed by rigid-body refinement in cns. Multiple cycles of simulated annealing, positional refinement, and B factor refinement were performed in cns, followed by model building into the 2Fo-Fc and Fo-Fc maps by using o. Final rounds of refinement were carried out against the 2.9-Å dataset from the peak wavelength (0.97880 Å), with the inclusion of water molecules, sulfate ions, and a PEG-400 molecule in the refined model. The final refined model consists of FGF10 residues 69–207, FGFR2b residues 151–359, 18 water molecules, 2 sulfate ions, and 1 PEG-400 molecule. The average B factor is 61.6 Å2 for all atoms, 66.9 Å2 for FGF10, 57.9 Å2 for FGFR2b, 47.0 Å2 for the water molecules, 112.4 Å2 for the sulfate ions, and 64.6 Å2 for the PEG-400 molecule. Representations of the structure were created by using molscript, raster3d, and grasp (30–32).
Structure-Function Analysis of FGF10.
D76A, R78A, and R155A point mutations were introduced into full-length human FGF10 (residues 38–208) by using the QuikChange site-directed mutagenesis kit (Stratagene). WT and mutant FGF10 proteins were expressed in E. coli and purified by heparin affinity, cation exchange, and size exclusion chromatography. The FGF10 mutants had similar chromatographic behaviors as the WT FGF10, indicating that the mutations did not compromise the tertiary structure of FGF10. DNA synthesis was measured by a [3H]thymidine incorporation assay by using serum-starved confluent cultures of Balb/MK cells (18).
Results and Discussion
Structure Determination.
A selenomethionine-labeled ligand-binding fragment of FGFR2b, encompassing D2 and D3 (residues 140–369), was expressed in E. coli and refolded in vitro from inclusion bodies. After purification, the labeled protein was mixed with excess FGF10 (residues 64–208), and the resulting 1:1 FGF10-FGFR2b complex was isolated by size-exclusion chromatography and crystallized.
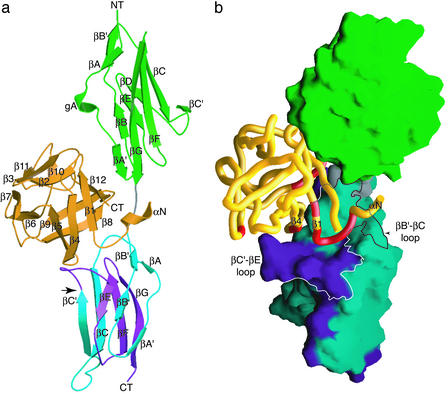
The crystal structure of the FGF10-FGFR2b complex was solved by using multiple anomalous dispersion and refined to 2.9-Å resolution. The refined model consists of 1 FGF10 molecule, 1 FGFR2b molecule, 18 water molecules, 2 sulfate ions, and 1 PEG-400 molecule. Data collection and refinement statistics are given in Table 1. The overall structure is shown in Fig. 1. FGF10 binds to a large and continuous bent surface on FGFR2b composed of D2, D3, and the D2-D3 linker of the receptor (Fig. 1b).
Table 1.
Data collection statistics
| λ1 | λ2 | λ3 | |
|---|---|---|---|
| Wavelength, Å | 0.97880 | 0.97933 | 0.97169 |
| Resolution, Å | 2.9 | 3.1 | 3.2 |
| Reflections (total/unique) | 279,164/14,520 | 271,083/14,612 | 207,935/11,488 |
| Completeness, % (outer shell) | 99.3 (100.0) | 99.1 (100.0) | 99.1 (100.0) |
| Overall I/σ | 25.8 | 26.3 | 22.3 |
| _R_sym*, % (outer shell) | 6.4 (27.9) | 6.4 (24.5) | 6.5 (29.3) |
| Refinement statistics | |||
| Resolution range, Å | 25–2.9 | ||
| _R_cryst/_R_free†, % | 23.9/28.8 | ||
| rms bond length deviation, Å | 0.008 | ||
| rms bond angle deviation, Å | 1.4 |
Figure 1.
Overall structure of the FGF10-FGFR2b complex. (a) Ribbon diagram of the FGF10-FGFR2b complex. D2 of FGFR2b is colored green. The first half of D3 is shown in blue. The alternatively spliced second half of D3 is colored purple. The linker connecting D2 and D3 is colored gray. FGF10 is shown in orange. The β-strands of FGF10 and FGFR2b are labeled according to published nomenclature (44). The N and C termini are labeled NT and CT, respectively. An arrow indicates the beginning of the alternatively spliced region of D3. (b) Molecular surface representation of FGFR2b. FGF10 is displayed as a Cα coil. αN, β1, and β4 of FGF10 are labeled accordingly. FGF10 regions that interact with D3 in the FGF10-FGFR2b structure are colored red. The ligand-binding cleft on D3 of FGFR2b, comprising the βB′-βC and βC′-βE loops, is outlined. Coloring is as in a.
Structure of FGF10.
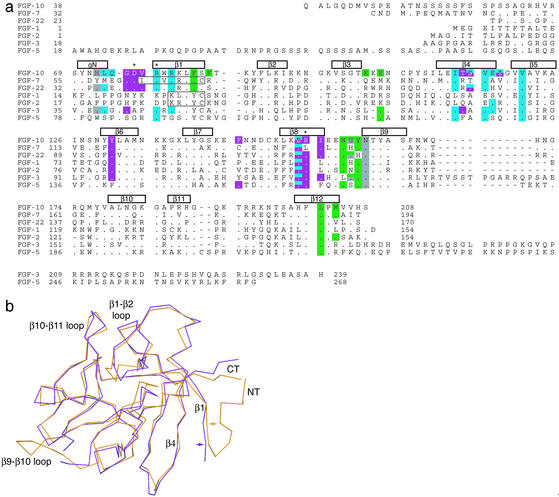
As anticipated on the basis of sequence homology, FGF10 adopts a β-trefoil fold consisting of 12 β-strands (β1 through β12; Figs. 1a and 2a). Superimposition of FGF10 onto FGF7, for which the crystal structure only in the absence of the receptor is available (33, 34), gives a rms deviation (rmsd) of 0.885 Å (Fig. 2b).
Figure 2.
Comparison of the FGF10 structure with other FGF structures. (a) Structure-based sequence alignment of human FGFs. Sequence alignment was performed by using the gcg wisconsin package. β-strand assignment is according to published nomenclature (44). The locations and lengths of the secondary structural elements are shown above the sequence alignment. The different lengths of β1 in the FGF1, FGF2, and FGF7 structures are indicated by boxes within the alignment. Both FGF1 and FGF2 have a proline N-terminal to β1 that restricts its length. The N-terminal 3/10 helix is labeled gN. FGF10 residues are colored with respect to the region of FGFR2b with which they interact: green for D2, gray for the linker region, blue for the first half D3, and purple for the alternatively spliced half of D3. Residues that interact with two regions are colored with both colors. A period indicates sequence identity with FGF10. A dash indicates a gap introduced to optimize the alignment. Asterisks denote residues in FGF10 that were mutated. The full sequences of the secreted ligands are shown. The numberings for FGF7, FGF10, and FGF22 start from the initiation methionine. (b) Superimposition of the FGF7 structure onto the FGF10 structure. FGF7 and FGF10 are colored purple and orange, respectively. The N and C termini are labeled NT and CT, respectively. Colored arrows mark the start of β1 in each ligand.
As in FGF7, β1 in FGF10 is longer than in FGF1 and FGF2 (Fig. 2a; refs. 33 and 34). Furthermore, the β10/β11 strand pair in FGF10 is defined by a single hydrogen bond between the two strands as in FGF7 (not shown). These structural distinctions represent hallmarks of the FGF7 subfamily and may also be present in FGF22, the most recently discovered member of this subfamily (35). The FGF7 subfamily is functionally distinct in that all its members interact specifically with FGFR2b. Thus, these structural features might play a role in determining the unique receptor specificity of the FGF7 subfamily. Indeed, in the FGF10-FGFR2b crystal structure, the extended portion of the β1 strand plays a critical role in FGFR2b binding (see below). As anticipated due to differing lengths, the β1-β2 and β9-β10 loops of FGF10 adopt different conformations than those of FGF1 and FGF2 (Fig. 2). These loops have the same length among the three FGF7 subfamily members (Fig. 2a).
Compared with receptor-bound FGF1 and FGF2, the N terminus of receptor-bound FGF10 is substantially more ordered and interacts extensively with the receptor D3. The first five ordered residues in FGF10 form a short helix (gN), which connects to β1 via a short loop (Fig. 1a).
The Alternatively Spliced D3 Plays a Decisive Role in FGF10-FGFR2b Specificity.
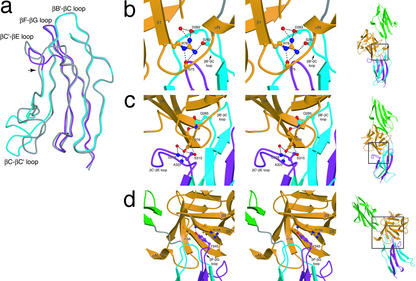
FGFR2b and its alternatively spliced counterpart, FGFR2c, differ only in the second half of D3. Superimposition of the D3s between the two isoforms gives a rmsd of 0.754 Å and reveals a conformational difference in the βC′-βE loop (Fig. 3a). The N terminus of this loop demarcates the beginning of the alternatively spliced half of FGFR2b. The βC′-βE loop is two residues shorter than its counterpart in FGFR2c and is in intimate contact with FGF10.
Figure 3.
Detailed interactions between FGF10 and FGFR2b at the FGF-D3 interface. (a) Superimposition of the D3 domains of the FGFR2b and FGFR2c isoforms. The βC-βC′ and βC′-βE loops were excluded from the superimposition. D3 of FGFR2b is colored blue, with the alternatively spliced second half colored purple. An arrow indicates the beginning of the alternatively spliced region of FGFR2b-D3. D3 of FGFR2c is colored gray. The difference in conformation of the βC-βC′ loops is a result of crystal lattice contacts (25, 44). (b) Stereo view of the interactions made by Arg-78 of FGF10 with the βB′-βC loop of D3. (c) Stereo view of the interactions made by Asp-76 of FGF10 with the βC′-βE loop of D3. (d) Stereo view of the interface between FGF10 and the βF-βG loop of D3. The side chains of interacting residues are displayed. (Right) A view of the FGF10-FGFR2b complex, with the region of interest indicated by a square. FGF10 and FGFR2b are colored as in Fig. 1. Oxygen atoms are colored red, nitrogen blue, and carbon atoms the same color as the molecules to which they belong.
FGF10 and FGFR2b engage in extensive and specific contacts at the FGF10-D3 interface (Table 2; Fig. 3 b_–_d), implicating this interface as the primary determinant of FGF10-FGFR2b affinity and specificity. This structural observation is consistent with previous data showing that an isolated FGFR2b D3 can bind FGF7 (23). Moreover, nearly all of the contacts at this interface are direct and water-mediated hydrogen bonds (Table 2), consistent with the discriminatory role of this interface in FGF10-FGFR2b specificity.
Table 2.
Interactions between FGF10 and the D3 cleft
| FGFR2b | FGF10 |
|---|---|
| Hydrogen bonds | |
| Lys-279 Nζ | Gln-74 Oɛ1 |
| Val-280 O | Gly-75 N (water-mediated) |
| Ser-282 O | Arg-78 NH2 |
| Ser-282 N | Leu-73 O |
| Asp-283 O | Arg-78 NH2 |
| Gln-285 N | Glu-154 Oɛ1 (conserved) |
| Gln-285 Oɛ1 | Glu-154 Oɛ2 (conserved, water-mediated) |
| Gln-285 Nɛ2 | Asp-76 O (conserved) |
| Ser-315 Oγ | Asp-76 Oδ1 |
| Ser-315 Oγ | Asp-76 Oδ2 |
| Gly-316 N | Asp-76 Oδ2 (water-mediated) |
| Gly-316 N | Thr-114 Oγ1 (water-mediated) |
| Gly-316 O | Thr-114 Oγ1 (water-mediated) |
| Ala-322 N | Asp-76 Oδ2 |
| Hydrophobic contacts | |
| Ile-317 | Phe-146 |
The majority of FGF10-D3 contacts occur within a wide cleft in D3 (Fig. 1b). One side of this cleft consists of the βB′ strand and the βB′-βC loop, both of which are in the first half of D3 and therefore are identical between the FGFR2b and FGFR2c isoforms. In contrast, the other side of the cleft is composed of the βC′-βE loop, which is in the second half of D3 and therefore unique to the FGFR2b isoform. The N terminus, β1 strand, β4 strand, and β7-β8 loop of FGF10 make specific contacts with both sides of the D3 cleft (Fig. 1b; Table 2). All these regions exhibit significant sequence diversity among FGFs (Fig. 2a). The β4 strand and N terminus of FGF7 have been shown to be required for FGFR2b binding by prior biochemical studies (33, 36–38). Similarly, the βC′-βE loop of the receptor has been shown to participate in FGF7 binding (39–41).
The only FGF10-D3 contact outside of the D3 cleft occurs between β8 of FGF10 and the βF-βG loop of D3 (Fig. 3d). Like the βC′-βE loop, the βF-βG loop is part of the second half of D3 and thus splice variant specific. Mutations in the βF-βG loop of FGFR2b have been demonstrated to reduce FGF7 binding (41). In summary, the FGF10-FGFR2b structure reveals numerous contacts between b splice isoform-specific loops of FGFR2b and divergent regions of FGF10, hence unveiling the molecular basis by which alternative splicing confers exceptional FGF-FGFR specificity.
Structure-Based Mutagenesis of FGF10 Confirms the Observed Mode of FGF10-FGFR2b Binding.
To assess the biological importance of the FGF10-FGFR2b contacts observed in the crystal structure, we selectively mutated Asp-76, Arg-78 and Arg-155 to alanine in full-length FGF10 (D76A, R78A, and R155A, respectively; Fig. 2a). Asp-76 and Arg-78, in the N terminus of FGF10, make important interactions with the D3 cleft (Fig. 3 b and c). Arg-78 is engaged in hydrogen bonds with the βB′-βC loop of the cleft (Table 2; Fig. 3b). In addition, Arg-78 makes intramolecular hydrogen bonds with the N terminus of FGF10 (Fig. 3b). These intramolecular hydrogen bonds stabilize the FGF10 N terminus, allowing it to make several specific contacts with the D3 cleft (Table 2; Fig. 3c). These contacts include the two highly specific hydrogen bonds involving Asp-76 of FGF10 and Ser-315 of FGFR2b (Fig. 3c). The involvement of these hydrogen bonds in conferring specificity between the FGF7 subfamily and FGFR2b is underscored by virtue of the fact that Asp-76 is unique to the FGF7 subfamily (Fig. 2a) and Ser-315 is located in the b splice isoform-specific βC′-βE loop (Fig. 3c). Indeed, substitution of Ser-315 in FGFR2b with alanine, the corresponding residue in FGFR2c, resulted in significant loss of FGF7 binding (40). Conversely, exchanging the C-terminal portion (residues 319–324) of the βC′-βE loop in FGFR2c with that of FGFR2b lowered binding of the resultant chimera to FGF2 without gaining FGF7 binding, suggesting an indispensable role for Ser-315 in conferring FGF7 binding specificity (41).
In contrast to Asp-76, Arg-78 of FGF10 is conserved in most FGFs with the notable exception of the prototypical FGF1 and FGF2 (Fig. 2a). Arg-155 of FGF10 also plays an important role in FGF10-FGFR2b specificity by making a hydrophobic contact with Tyr-345 of FGFR2b outside of the D3 cleft (Fig. 3d).
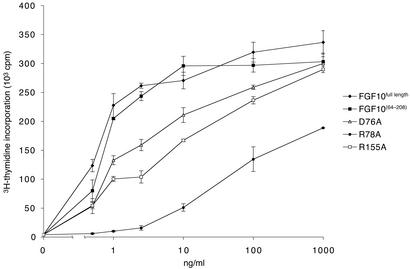
WT and mutant FGF10 proteins were expressed in E. coli and purified to homogeneity. The biological activity of the WT and mutant ligands was compared in a mitogenesis assay by using Balb/MK cells naturally expressing FGFR2b (Fig. 4). The activity of the FGF10 construct used for crystallization (residues 64 to 208) was similar to that of full-length FGF10. All three mutations reduced the capacity of FGF10 to induce DNA synthesis. The R78A mutant was severely compromised and achieved only 56% of the maximal mitogenic response produced by WT FGF10 (Fig. 4). The dramatic effect of the R78A mutation underscores the importance of Arg-78 and proper N-terminal conformation of FGF10 for FGFR2b binding.
Figure 4.
Structure-based mutagenesis of FGF10 confirms the importance of observed FGF10-FGFR2b contacts for FGF10 biological activity. Serum-starved Balb/MK cells were stimulated with increasing concentrations of WT or mutant FGF10. Sixteen hours later, [3H]thymidine was added for 6 h, and incorporation was determined as described (18). Each data point was performed in triplicate. WT FGF10 induced an 85-fold increase in DNA synthesis.
FGF10-Induced D2 Rotation Plays an Important Role in FGF10-FGFR2b Specificity.
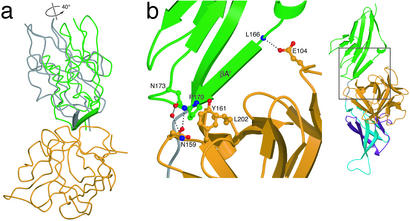
Superimposition of the receptor D2s between the FGF10-FGFR2b and FGF2-FGFR2c complexes (25) yields a rmsd of 0.684 Å with no notable secondary structure differences. Previous FGF-FGFR structures have revealed a generally hydrophobic and conserved FGF-D2 interface. Because the hydrophobic residues in FGF1 and FGF2 that contact D2 of FGFR2c are conserved in FGF10, we anticipated finding similar hydrophobic interactions at the FGF10-D2 interface. Interestingly, the FGF10-D2 interface is less hydrophobic and contains several unique contacts. Superimposition of the ligands reveals that D2 of FGF10-FGFR2b is rotated by 40° relative to its position in the FGF2-FGFR2c complex (Fig. 5a). A similar D2 rotation (25°) is also observed relative to the FGF1-FGFR2c structure (ref. 42; data not shown). The position of D3 relative to FGF is essentially unaltered among the three structures.
Figure 5.
FGF10-induced D2 rotation contributes to FGF10-FGFR2b specificity. (a) Relationship between the D2 domains after superimposition of FGF10 and FGF2 from the FGF10-FGFR2b and FGF2-FGFR2c structures. For the sake of clarity, D3 and the linker region are not shown. The direction and degree of rotation between the two domains is shown. D2 of FGFR2b is colored blue. D2 of FGFR2c is colored gray. The βA′ strands of both domains are shown as β-strand arrows. The remainders of the domains are displayed as Cα coils. FGF10 is shown in orange. (b) Interactions at the FGF10-D2 interface. The side chains of interacting residues are displayed. (Right) A view of the FGF10-FGFR2b complex, with the region of interest indicated by a square. FGF10 and FGFR2b are colored as in Fig. 1. Oxygen atoms are colored red, nitrogen blue, and carbon atoms the same color as the molecules to which they belong.
Comparison of the FGF-D2 interface between the FGF10-FGFR2b, FGF1-FGFR2c, and FGF2-FGFR2c structures suggests that the D2 rotation in the FGF10-FGFR2b structure is a ligand-induced event. At the center of the predominantly hydrophobic FGF-D2 interface in the FGF1-FGFR2c and FGF2-FGFR2c complexes is a tyrosine residue (Tyr-29 in FGF1 and Tyr-32 in FGF2; refs. 25 and 42). This tyrosine residue engages in hydrophobic contact with the FGFR-invariant Ala-167 located at the center of βA′ in FGFR2 D2. In addition, the hydroxyl group of this tyrosine makes two hydrogen bonds with the backbone of βA′. The presence of these hydrogen bonds in the midst of the highly hydrophobic FGF-D2 interface seems to constrain the position of D2 in the FGF1-FGFR2c and FGF2-FGFR2c structures.
In all three FGF7 subfamily members, this tyrosine is replaced by phenylalanine (Phe-83 in FGF10; Fig. 2a), which cannot hydrogen bond with βA′. Instead, the position of D2 in the FGF10-FGFR2b structure is determined by three specific hydrogen bonds between FGF10 and D2, involving Glu-104 and Asn-159 of FGF10 and Leu-166 and Asn-173 of FGFR2b (Fig. 5b). Asn-173 also engages in oxygen-aromatic contacts with Phe-85 and Tyr-161 of FGF10 (not shown). Because Asn-173 is conserved only in FGFR2, the D2 rotation and accompanying unique interactions most likely contribute to FGF10-FGFR2b specificity. Interestingly, it has been demonstrated that D2 of FGFR1 cannot substitute for D2 of FGFR2 in providing the full binding specificity of FGFR2b to FGF7 (43). Thus, based on the FGF10-FGFR2b structure and the available literature, we propose that D2 of FGFR2b plays an important role in conferring FGF10-FGFR2b specificity.
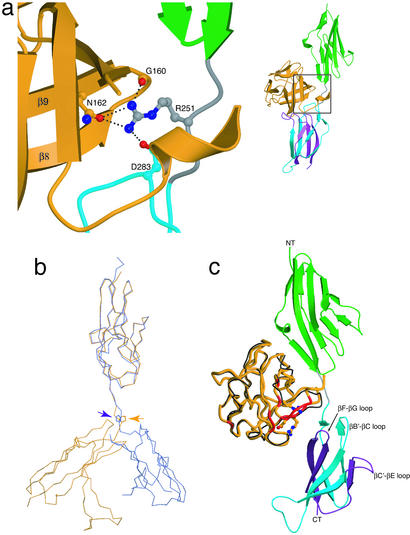
The FGF10-Linker Interface and D3 Orientation.
The FGFR-invariant Arg-251, situated in the D2-D3 linker, makes three highly conserved hydrogen bonds with the FGF10 ligand (Fig. 6a). These hydrogen bonds have already been described in the previously determined FGF-FGFR structures (25, 42, 44). Hence, the FGF10-FGFR2b structure reemphasizes the importance of the FGF-linker interface in providing general FGF-FGFR affinity. Significantly, the FGFR-invariant Pro-253 in the FGF10-FGFR2b structure adopts a trans configuration as in all previously reported FGF-FGFR complexes formed in the absence of heparin (9, 25, 42, 44, 45). This differs from the FGF1-FGFR2c-heparin structure, where Pro-253 is in a cis configuration (ref. 46; Fig. 6b). As a consequence of this cis isomerization, D3 in the FGF1-FGFR2c-heparin ternary complex is in a different orientation and interacts differently with the ligand than in the binary FGF-FGFR complexes (Fig. 6b; refs. 42 and 46). Based on this conformational disparity, it has been postulated that heparin catalyzes a trans to cis isomerization of Pro-253 and thereby converts a biologically inactive binary complex to a biologically active ternary complex (46). To explore this hypothesis, we constructed an FGF10-FGFR2b model containing Pro-253 in a cis conformation (Fig. 6c). In this model, D3 of FGFR2b cannot interact with D76, R78, and R155 of FGF10, all of which are confirmed to interact with FGFR2b by mutagenesis analysis (Fig. 4). Because the D76A, R78A, and R155A FGF10 mutants exhibit reduced cell proliferative activity in the presence of heparin (Fig. 4), the observed FGF10-FGFR2b contacts must also occur in the presence of heparin. Thus, it is unlikely that heparin induces a trans to cis isomerization of Pro-253.
Figure 6.
Conformation of the FGFR2b linker region. (a) Interactions of the FGFR-invariant linker Arg-251 with FGF10. The side chains of interacting residues are displayed. (Right) A view of the whole FGF10-FGFR2b structure, with the region of interest indicated by a square. FGF10 and FGFR2b are colored as in Fig. 1. Oxygen atoms are colored red, nitrogen blue, and carbon atoms the same color as the molecules to which they belong. (b) Configuration of the FGFR-invariant linker Pro-253 in the FGF10-FGFR2b and FGF1-FGFR2c-heparin structures. The equivalent D2s from the FGF10-FGFR2b and FGF1-FGFR2c-heparin structures are superimposed (rmsd = 0.652 Å). FGFR2b is colored yellow, and FGFR2c is colored blue. The location of the linker prolines are indicated by arrows. Note the dramatic difference in the position of D3 between the two structures. (c) A FGF10-FGFR2b model with Pro-253 in the cis conformation. This model was generated by separately superimposing FGF10 and FGFR2b-D3 from the FGF10-FGFR2b structure onto FGF1 and FGFR2c-D3 in the FGF1-FGFR2c-heparin structure (rmsd = 0.791 Å and 0.668 Å, respectively). D2 and D3 are shown in green and blue, respectively. The alternatively spliced half of D3 is colored purple. FGF1 is displayed as a black Cα coil, and FGF10 is displayed as a thicker orange coil. FGF10 regions that interact with D3 in the FGF10-FGFR2b structure are colored red. In addition, FGF10 residues whose mutations reduce the ability of FGF10 to activate FGFR2b are rendered in ball-and-stick. The N and C termini of the receptor are labeled NT and CT, respectively.
Concluding Remarks.
Deciphering the molecular mechanisms governing FGF-FGFR specificity is an essential task, given the multitude of fundamental and diverse physiological processes involving FGF signaling, such as organ patterning, angiogenesis, wound healing, and cell growth and differentiation. Alternative splicing is the main determinant of ligand-binding specificity in FGFRs. The FGF10-FGFR2b structure reveals numerous contacts between receptor loops that are specific to the b splice isoform and divergent regions of FGF10. In addition, FGF10-induced D2 rotation results in unique contacts between D2 and FGF10. Based on these results, we propose that specificity between FGF10 and FGFR2b involves both D2 and D3 of the receptor.
FGF10 and FGF7 are known to play unique and important roles in physiological tissue repair processes. Increased FGF7 expression is an important response mechanism to damaged epithelial tissues in the skin, bladder, and kidney (47). Conversely, overexpression of FGF7 is thought to contribute to psoriasis and inflammatory bowel disease (48, 49). FGF10/7-FGFR2b signaling is also involved in the pathogenesis of bladder, colon, and prostate cancers (50–52). Hence, the FGF10-FGFR2b structure should prove useful in the rational design of both FGF7 subfamily agonists and antagonists for the treatment of human diseases.
Acknowledgments
We thank Craig Ogata and Randy Abramowitz for synchrotron beamline assistance. We are grateful to Omar Ibrahimi for critical reading of the manuscript. Beamline X4A at the National Synchrotron Light Source, a Department of Energy facility, is supported by the Howard Hughes Medical Institute. This work was supported by grants from the National Institutes of Health (DE-13686 to M.M.; CA-80058 and CA-85214 to S.A.A.) and from the Peter Sharp Foundation (to S.A.A.).
Abbreviations
FGF
fibroblast growth factor
FGFR
FGF receptor
D2 and D3
Ig-like domains 2 and 3
HSPGs
heparan sulfate proteoglycans
rmsd
rms deviation
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID code 1NUN).
References
- 1.Ornitz D M, Itoh N. Genome Biol. 2001;2:1–12. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin G R. Genes Dev. 1998;12:1571–1586. doi: 10.1101/gad.12.11.1571. [DOI] [PubMed] [Google Scholar]
- 3.Johnson D E, Williams L T, Gritli-Linde A, Lewis P, McMahon A P, Linde A. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- 4.Rapraeger A C, Guimond S, Krufka A, Olwin B B. Methods Enzymol. 1994;245:219–240. doi: 10.1016/0076-6879(94)45013-7. [DOI] [PubMed] [Google Scholar]
- 5.Yayon A, Klagsbrun M, Esko J D, Leder P, Ornitz D M. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 6.Ornitz D M. BioEssays. 2000;22:108–112. doi: 10.1002/(SICI)1521-1878(200002)22:2<108::AID-BIES2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 7.Schlessinger J. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 8.Anderson J, Burns H D, Enriquez-Harris P, Wilkie A O, Heath J K. Hum Mol Genet. 1998;7:1475–1483. doi: 10.1093/hmg/7.9.1475. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahimi O A, Eliseenkova A V, Plotnikov A N, Yu K, Ornitz D M, Mohammadi M. Proc Natl Acad Sci USA. 2001;98:7182–7187. doi: 10.1073/pnas.121183798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu K, Herr A B, Waksman G, Ornitz D M. Proc Natl Acad Sci USA. 2000;97:14536–14541. doi: 10.1073/pnas.97.26.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstrohm A C, Greenleaf A L, Garcia-Blanco M A. Gene. 2001;277:31–47. doi: 10.1016/s0378-1119(01)00695-3. [DOI] [PubMed] [Google Scholar]
- 12.Beer H D, Vindevoghel L, Gait M J, Revest J M, Duan D R, Mason I, Dickson C, Werner S. J Biol Chem. 2000;275:16091–16097. doi: 10.1074/jbc.275.21.16091. [DOI] [PubMed] [Google Scholar]
- 13.Orr-Urtreger A, Bedford M T, Burakova T, Arman E, Zimmer Y, Yayon A, Givol D, Lonai P. Dev Biol. 1993;158:475–486. doi: 10.1006/dbio.1993.1205. [DOI] [PubMed] [Google Scholar]
- 14.Wuechner C, Nordqvist A C, Winterpacht A, Zabel B, Schalling M. Int J Dev Biol. 1996;40:1185–1188. [PubMed] [Google Scholar]
- 15.Ron D, Reich R, Chedid M, Lengel C, Cohen O E, Chan A M, Neufeld G, Miki T, Tronick S R. J Biol Chem. 1993;268:5388–5394. [PubMed] [Google Scholar]
- 16.De Moerlooze L, Spencer-Dene B, Revest J, Hajihosseini M, Rosewell I, Dickson C. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- 17.Xu X, Weinstein M, Li C, Naski M, Cohen R I, Ornitz D M, Leder P, Deng C. Development. 1998;125:753–765. doi: 10.1242/dev.125.4.753. [DOI] [PubMed] [Google Scholar]
- 18.Igarashi M, Finch P W, Aaronson S A. J Biol Chem. 1998;273:13230–13235. doi: 10.1074/jbc.273.21.13230. [DOI] [PubMed] [Google Scholar]
- 19.Lu W, Luo Y, Kan M, McKeehan W L. J Biol Chem. 1999;274:12827–12834. doi: 10.1074/jbc.274.18.12827. [DOI] [PubMed] [Google Scholar]
- 20.Guo L, Degenstein L, Fuchs E. Genes Dev. 1996;10:165–175. doi: 10.1101/gad.10.2.165. [DOI] [PubMed] [Google Scholar]
- 21.Min H, Danilenko D M, Scully S A, Bolon B, Ring B D, Tarpley J E, DeRose M, Simonet W S. Genes Dev. 1998;12:3156–3161. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S. Nat Genet. 1999;21:138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- 23.Miki T, Bottaro D P, Fleming T P, Smith C L, Burgess W H, Chan A M, Aaronson S A. Proc Natl Acad Sci USA. 1992;89:246–250. doi: 10.1073/pnas.89.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendrickson W A, Horton J R, LeMaster D M. EMBO J. 1990;9:1665–1672. doi: 10.1002/j.1460-2075.1990.tb08287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plotnikov A N, Hubbard S R, Schlessinger J, Mohammadi M. Cell. 2000;101:413–424. doi: 10.1016/s0092-8674(00)80851-x. [DOI] [PubMed] [Google Scholar]
- 26.Otwinowski Z, Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 27.Navaza J. Acta Crystallogr A. 1994;50:157–163. [Google Scholar]
- 28.Brunger A T, Adams P D, Clore G M, DeLano W L, Gros P, Grosse-Kunstleve R W, Jiang J S, Kuszewski J, Nilges M, Pannu N S, et al. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 29.Jones T A, Zou J Y, Cowan S W, Kjeldgaard G. Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 30.Kraulis P J. J Appl Crystallogr. 1991;24:946–950. [Google Scholar]
- 31.Merritt E A, Bacon D J. Methods Enzymol. 1997;277:505–524. doi: 10.1016/s0076-6879(97)77028-9. [DOI] [PubMed] [Google Scholar]
- 32.Nicholls A, Sharp K A, Honig B. Proteins. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- 33.Osslund T D, Syed R, Singer E, Hsu E W, Nybo R, Chen B L, Harvey T, Arakawa T, Narhi L O, Chirino A, Morris C F. Protein Sci. 1998;7:1681–1690. doi: 10.1002/pro.5560070803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye S, Luo Y, Lu W, Jones R B, Linhardt R J, Capila I, Toida T, Kan M, Pelletier H, McKeehan W L. Biochemistry. 2001;40:14429–14439. doi: 10.1021/bi011000u. [DOI] [PubMed] [Google Scholar]
- 35.Nakatake Y, Hoshikawa M, Asaki T, Kassai Y, Itoh N. Biochim Biophys Acta. 2001;1517:460–463. doi: 10.1016/s0167-4781(00)00302-x. [DOI] [PubMed] [Google Scholar]
- 36.Ron D, Bottaro D P, Finch P W, Morris D, Rubin J S, Aaronson S A. J Biol Chem. 1993;268:2984–2988. [PubMed] [Google Scholar]
- 37.Reich-Slotky R, Shaoul E, Berman B, Graziani G, Ron D. J Biol Chem. 1995;270:29813–29818. doi: 10.1074/jbc.270.50.29813. [DOI] [PubMed] [Google Scholar]
- 38.Sher I, Lang T, Lubinsky-Mink S, Kuhn J, Adir N, Chatterjee S, Schomburg D, Ron D. J Biol Chem. 2000;275:34881–34886. doi: 10.1074/jbc.M003293200. [DOI] [PubMed] [Google Scholar]
- 39.Bottaro D P, Fortney E, Rubin J S, Aaronson S A. J Biol Chem. 1993;268:9180–9183. [PubMed] [Google Scholar]
- 40.Wang F, Kan M, Xu J, Yan G, McKeehan W L. J Biol Chem. 1995;270:10222–10230. doi: 10.1074/jbc.270.17.10222. [DOI] [PubMed] [Google Scholar]
- 41.Gray T E, Eisenstein M, Shimon T, Givol D, Yayon A. Biochemistry. 1995;34:10325–10333. doi: 10.1021/bi00033a002. [DOI] [PubMed] [Google Scholar]
- 42.Stauber D J, DiGabriele A D, Hendrickson W A. Proc Natl Acad Sci USA. 2000;97:49–54. doi: 10.1073/pnas.97.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zimmer Y, Givol D, Yayon A. J Biol Chem. 1993;268:7899–7903. [PubMed] [Google Scholar]
- 44.Plotnikov A N, Schlessinger J, Hubbard S R, Mohammadi M. Cell. 1999;98:641–650. doi: 10.1016/s0092-8674(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 45.Schlessinger J, Plotnikov A N, Ibrahimi O A, Eliseenkova A V, Yeh B K, Yayon A, Linhardt R J, Mohammadi M. Mol Cell. 2000;6:743–750. doi: 10.1016/s1097-2765(00)00073-3. [DOI] [PubMed] [Google Scholar]
- 46.Pellegrini L, Burke D F, von Delft F, Mulloy B, Blundell T L. Nature. 2000;407:1029–1034. doi: 10.1038/35039551. [DOI] [PubMed] [Google Scholar]
- 47.Werner S. Cytokine Growth Factor Rev. 1998;9:153–165. doi: 10.1016/s1359-6101(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 48.Finch P W, Murphy F, Cardinale I, Krueger J G. Am J Pathol. 1997;151:1619–1628. [PMC free article] [PubMed] [Google Scholar]
- 49.MacDonald T T, Monteleone G, Pender S L. Scand J Immunol. 2000;51:2–9. doi: 10.1046/j.1365-3083.2000.00658.x. [DOI] [PubMed] [Google Scholar]
- 50.Ricol D, Cappellen D, El Marjou A, Gil-Diez-de-Medina S, Girault J M, Yoshida T, Ferry G, Tucker G, Poupon M F, Chopin D, et al. Oncogene. 1999;18:7234–7243. doi: 10.1038/sj.onc.1203186. [DOI] [PubMed] [Google Scholar]
- 51.Watanabe M, Ishiwata T, Nishigai K, Moriyama Y, Asano G. Pathol Int. 2000;50:363–372. doi: 10.1046/j.1440-1827.2000.01054.x. [DOI] [PubMed] [Google Scholar]
- 52.Yan G, Fukabori Y, McBride G, Nikolaropolous S, McKeehan W L. Mol Cell Biol. 1993;13:4513–4522. doi: 10.1128/mcb.13.8.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]