Human Cytomegalovirus Activates Inflammatory Cytokine Responses via CD14 and Toll-Like Receptor 2 (original) (raw)
Abstract
Human cytomegalovirus (CMV) is a ubiquitous opportunistic pathogen that causes significant morbidity and mortality in immuncompromised people. An understanding of how CMV induces and circumvents host immunity is of critical importance in efforts to design effective therapeutics. It was recently discovered that mere cell contact by CMV particles leads to profound modulation of cellular gene expression, including induction of inflammatory cytokines and interferon-stimulated genes characteristic of innate immune detection. These findings suggest that a membrane receptor recognizes a CMV envelope protein(s), leading to innate immune activation. Here, we show that the pattern recognition receptors Toll-like receptor 2 (TLR2) and CD14 recognize CMV virions and trigger inflammatory cytokine production. Induction of inflammatory cytokines is mediated via TLR2-dependent activation of NF-κB. Since many of the pathological processes associated with CMV disease are facilitated or directly mediated by inflammatory cytokines, identification of the host membrane detection machinery may ultimately lead to improved therapeutics.
Human cytomegalovirus (CMV) is a ubiquitous opportunistic pathogen. Clinically, CMV disease correlates with immune suppression in which severe presentations are evident in neonates, persons with AIDS, and other immune-suppressed patient groups (38). CMV infection of neonates is associated with deafness, mental retardation, and mortality, whereas AIDS patients often suffer a blinding CMV retinitis, as well as pneumonia and gastrointestinal inflammation. In organ transplant recipients, a patient group hard hit by CMV infection, disease is associated with an increased frequency of graft rejection and is a major cause of posttransplant infection. The varied array of clinical disease correlates with the exceptionally broad tropism of this virus. Indeed, histological analysis of autopsy tissues obtained from patients with CMV disease has demonstrated infected cells in virtually all organs. At the cellular level, CMV can infect monocytes/macrophages, endothelial cells, epithelial cells, smooth muscle cells, fibroblasts, stromal cells, neuronal cells, neutrophils, and hepatocyes (13, 17, 35, 44, 48, 49, 61). In fact, CMV is a suspected pathogenetic agent in cardiovascular disease due to its ability to persist in large-vessel endothelial cells and to infect all cell types involved in cardiovascular lesions (24).
Cells exposed to CMV undergo a number of physiological changes that are rendered upon the cell with extremely rapid kinetics (15). These events include changes in Ca2+ homeostasis (1) and activation of phospholipase C and phospholipase A2, as well as increased release of arachidonic acid and its metabolites (1, 59). All of these changes can be triggered by UV-inactivated virions, suggesting that structural components of the virus are responsible for the alterations in cell physiology and intracellular signaling that occur during virus-cell contact and/or virus entry. Virus-cell contact also results in the activation of the transcription factors NF-κB and SP-1, as well as mitogen-activated protein (MAP) kinase, ERK1/2, and p38 (7, 26, 63).
Activation of transcription factors by CMV suggests that alterations in cellular transcription should occur in CMV-infected cells, and this is precisely the case. Several transcriptional-profiling studies reveal that cells infected with CMV exhibit profound reprogramming of gene expression (8, 47, 64, 65). Interestingly, the most strongly induced genes were indicators of innate immune activation. Antiviral genes belonging to the interferon-stimulated gene family (ISGs) and inflammatory genes, such as those for RANTES, interleukin 6 (IL-6), IL-7, IL-11, and cyclooxygenase 2 (COX-2), were all robustly induced in CMV-infected fibroblasts (8, 47, 64, 65). Induction of these innate immune markers did not require virus replication. Indeed, cells treated with only the primary ligand of CMV, glycoprotein B (gB) (6), exhibited a response very similar, but not wholly identical, to that of cells treated with intact virus (47). In particular, cells treated with gB strongly induced ISGs. Taken together, the findings suggest that a signal transduction pathway is activated by cell contact of CMV envelope proteins, resulting in numerous physiological changes that culminate, in part, with innate immune activation.
The innate immune system is an ancient, universal host defense system. A limited number of evolutionarily conserved germ line receptors found in plants, Drosophila, and humans mediate certain innate immune responses. Termed pattern recognition receptors, these molecules form the basis of the primary host alarm system in response to pathogen-associated molecular patterns (PAMPs) (23). Toll-like receptors (TLRs) are now understood to play a major role in pathogen recognition. Stimulation of TLRs by pathogens activates signal transduction pathways that lead to induction of a range of antimicrobial genes and inflammatory cytokines (2, 23, 29). In addition, key costimulatory molecules, such as CD80 and CD86, which are important for activation of adaptive immunity, are also induced as a consequence of TLR signaling. At present, 10 TLR molecules have been described in humans and mice. Ligands ranging from lipopolysaccharide (LPS) of gram-negative bacteria, peptidoglycan of gram-positive bacteria, flagellin, CpG DNA, and various components from mycobacteria, yeast, and parasitic pathogens, are all detected by TLRs. Although the precise molecular patterns are not completely characterized, PAMPs are hypothesized to be macromolecular modifications unique to these organisms.
Viruses have long been known to activate innate immune responses characterized by the induction of inflammatory cytokines and a comprehensive set of ISGs (45, 53). Until recently, the only identified molecular trigger of host innate responses was double-stranded RNA, a common replicative intermediate in the life cycle of many viruses. Double-stranded RNA can activate a key interferon transcription factor, interferon regulatory factor 3 (IRF-3), and induce synthesis of many antiviral genes. Generally, however, the mechanisms by which viruses activate innate immunity remain largely undefined. Since viruses are obligate intracellular parasites, virus-encoded proteins are synthesized by host machinery and ultimately bear protein modifications reflective of the host. Thus, it is not immediately obvious what PAMPs are displayed on viruses. Currently, however, data are clearly emerging showing that TLRs detect viruses and trigger inflammatory responses. Respiratory syncytcial virus (RSV) and mouse mammary tumor virus (MMTV) both signal through TLR4 (28, 42), the well-described LPS receptor. In the case of RSV, TLR4-deficient mice challenged with RSV exhibited a variety of impaired innate immune functions and an inability to clear the virus (20). These findings strongly suggest that Toll signaling pathways have an important role in the innate immunity to RSV. More recently, measles virus was reported to be detected by TLR2, a TLR with broad ligand recognition properties (5). The most striking common feature of these first three reports of virus detection by TLRs is that TLR responses were triggered by viral envelope glycoproteins. Specifically, the fusion protein of RSV, the envelope (env) protein of MMTV, and the hemagglutinin protein of measles virus were the identified triggers of the TLR responsiveness in their respective systems. Interestingly, all of these proteins play key roles in the virus entry pathway, such as attachment and fusion. These studies plainly point to a heretofore-unknown ability of the host to detect viruses during entry but prior to the onset of any replication events. Here, we report that TLR2 and CD14 form the central basis of the membrane detection machinery for CMV.
MATERIALS AND METHODS
Reagents and cells.
Human peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood of healthy volunteers. The cells were purified using Lymphocyte Separation Medium (MediaTech/CellGro, Herndon, Va.). TLR2-deficient mice were engineered as described previously (55) and were the gift of S. Akira (Osaka, Japan). TLR4-deficient C57BL10/ScNCR mice were obtained from the National Cancer Institute. C57BL/6 × 129 F2 (B6129F2) and C57BL10/SnJ mice were purchased from Jackson Laboratories (Bar Harbor, Maine). The mice were injected intraperitoneally with 1 ml of 3% thioglycolate, and cells were harvested 4 days later by peritoneal lavage. MY4 anti-CD14 antibody was purchased from Beckman Coulter (Miami, Fla.) and dialyzed against phosphate-buffered saline prior to use. Control immunoglobulin G was purchased from eBioscience (San Diego, Calif.). Phytohemagglutinin, phorbol myristate acetate, ionomycin, yeast zymosan, and LPS (Escherichiacoli 0111:B4) were obtained from Sigma (St. Louis, Mo.). The LPS was repurified by phenol extraction prior to use to remove lipopeptides, as described previously (21). IL-1β was purchased from R&D Systems (Minneapolis, Minn.). The AD169 strain of CMV was propagated in human diploid fibroblasts. UV inactivation of virions (UV-CMV) was performed in a UV Stratalinker 2400 (StrataGene) for 4 min at 9.9 × 105 μJ. UV inactivation of virions was confirmed by monitoring expression of the immediate-early (IE) gene products (IE1 and IE2) in infected human fibroblasts as previously described (7). The UV treatment described was sufficient to completely abolish IE gene expression. Dense bodies (DBs) and enveloped virions were purified by density gradient centrifugation (11, 22, 56). Cytokine-specific enzyme-linked immunosorbent assays (ELISAs) were performed using the OptEIA dual-antibody detection assay (BD Pharmingen, San Diego, Calif.) according to the manufacturer's directions.
Cytokine assays.
PBMC were plated in 24-well tissue culture dishes at 106/ml in RPMI 1640 medium (Gibco/BRL, Grand Island, N.Y.) supplemented with 10% fetal calf serum (Atlanta Biological, Norcross, Ga.) and antibiotics. The cells were incubated with no stimulus or with UV-irradiated CMV (1 × 103 to 2 × 104 PFU/ml). Control cultures were stimulated with LPS (10 to 100 ng/ml), zymosan (10 μg/ml), or IL-1β (100 ng/ml). The cultures were incubated for 18 h at 37°C, and the levels of IL-6 and IL-8 secretion were determined by ELISA analysis of culture supernatants. Human embryonic kidney HEK293 cells (American Type Culture Collection, Rockville, Md.) stably expressing CD14, TLR2, and TLR4 were cloned as previously described (27). HEK293 cells were plated in 24-well plates and allowed to grow to confluence, followed by stimulation with the appropriate treatment as described for PBMC. IL-8 secretion was measured in supernatants collected 18 h after stimulation. Peritoneal exudate cells (PECs) were isolated from wild-type and TLR2- or TLR4-deficient mice 4 days after intraperitoneal injection with thioglycolate. PECs were plated at 106 per well in 24-well plates and incubated with no stimulus or with CMV, as described for PBMC. The culture supernatants were harvested 18 h later, and IL-6 levels were determined by ELISA (OptEIA). The data shown are representative of three to five independent experiments.
NF-κB transcription assays.
For flow cytometry analysis of CHO transfectants, adherent monolayers of CHO/CD14 or CHO/CD14/TLR2 cells (14) were plated in six-well tissue culture dishes. After overnight incubation, the cells were washed twice with phosphate-buffered saline and mock treated, stimulated with zymosan A (10 μg/ml), or infected with CMV (multiplicity of infection, 0.1) in serum-free F12 medium. At 18 h posttreatment, the cells were detached from the surface with trypsin-EDTA, and CD25 surface expression was measured by flow cytometry as previously described (14).
NF-κB luciferase reporter gene assays in HEK293 cells.
HEK293 cells expressing TLR2, TLR4, and/or MD2 were transiently transfected with an NF-κB reporter construct using GeneJuice Transfection Reagent from Novagen (Madison, Wis.) according to the manufacturer's instructions. Briefly, the cells were plated on 96-well plates at 2.5 × 104 1 day before transfection. A total of 0.3 μg of DNA was used per well, containing 10 ng of human CD14 in pcDNA3 or empty vector (Invitrogen), 80 ng of NF-κB-driven firefly luciferase plasmid (pGL-3-Basic vector; Promega), 20 ng of herpes simplex virus thymidine kinase promoter containing Renilla luciferase plasmid (phRL-TK vector; Promega) and pUC18 plasmid DNAs. The cells were incubated overnight at 37°C in a 5% CO2 humidified incubator. The next day, the cells were stimulated for 6 to 8 h with UV-irradiated CMV. Control wells were stimulated with 100 ng of human IL-1β/ml. Then, the cells were lysed with 50 μl of Passive Lysis Buffer (Promega), and firefly and Renilla luciferase activities were measured using the Dual-Glo Luciferase Assay System (Promega) according to the manufacturer's instructions. Luciferase activity was calculated in relative light units as a ratio of NF-κB-dependent firefly luciferase activity to NF-κB-independent Renilla luciferase activity. The results are shown as the mean plus standard deviation of triplicate wells.
RESULTS
Inflammatory cytokines are induced by CMV-cell contact in human mononuclear cells.
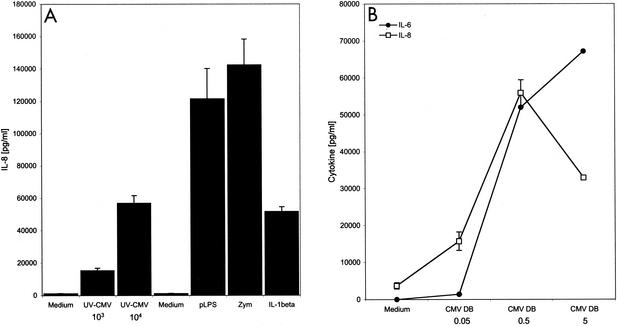
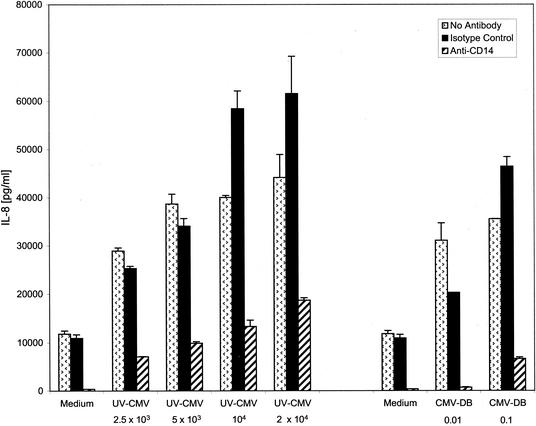
The majority of the studies of CMV effects on host cell gene expression have been conducted in human fibroblasts, the primary permissive cultured cell type. However, in vivo, CMV can infect many cell types, and it has a particularly critical relationship with monocytes. CD14+ monocytes and/or myeloid progenitor populations are sites of CMV latency and are capable of harboring quiescent viral genomes (30, 33). Virus replication can be reactivated from latently infected monocyte populations in a differentiation-dependent manner. Allogeneic stimulation or addition of interferon gamma to latently infected CD14+ monocytes results in dramatic onset of replication and release of infectious virus (50-52). Given the delicate balance between the host immune response and CMV's existence between the latent and active states, we asked if inflammatory responses were activated in monocytes exposed to CMV. When human PBMC were incubated with UV-inactivated CMV particles UV-CMV), IL-8 (Fig. 1) and IL-6 (data not shown) were induced. The response was similar regardless of whether the PBMC were obtained from CMV-immune or naïve donors (data not shown). These data support the notion that cellular contact with virus particles triggers signaling events that lead to inflammatory cytokine production. Although the virus stocks were negative for detectable interferon and LPS (data not shown), we wished to further rule out the possibility of a contaminating soluble factor. To that end, we tested the ability of purified defective particles known as DBs to illicit inflammatory cytokine production in PBMC. DBs are enveloped particles that arise during tissue culture growth. They contain virus-encoded envelope proteins and at least some tegument proteins but lack capsids and DNA (4, 46). Figure 1B shows that IL-8 and IL-6 were induced in monocytes by DBs in a dose-dependent manner. These data strongly support the conclusion that inflammatory cytokines are induced by CMV and that this induction does not require viral gene expression. The fact that DBs are primarily composed of envelope glycoproteins argues that envelope protein-cellular receptor interactions initiate the triggering event. Since CD14+ monocytes are targeted by CMV and CD14 is a known pattern recognition component and a facilitator of inflammatory responses to many bacterial ligands, we asked if CD14 was involved in CMV-activated inflammation. We observed that IL-8 secretion in response to UV-CMV and to DBs was inhibited in PBMC treated with an anti-CD14 antibody but not in PBMC treated with isotype-matched control antibody (Fig. 2). The modest increase in IL-8 in high concentrations of CMV treated with the isotype control was very reproducible in our studies and in other viral systems, such as RSV; however, the mechanism for this enhancement is not known. Taken together, these data suggest that CD14 formed at least part of the membrane detection machinery for CMV.
FIG. 1.
CMV activation of human PBMC. (A) CMV stimulates cytokine secretion from normal human PBMC. Human PBMC (5 × 105/well) were incubated with no stimulus (Medium) or with UV-inactivated CMV (2.5 × 103 to 2 × 104 PFU/well), phenol reextracted LPS (pLPS; 100 ng/ml; TLR4 ligand), yeast zymosan (Zym; 10 μg/ml; TLR2 ligand), or IL-1β (100 ng/ml; TLR- and CD14-independent positive control ligand). The supernatants were harvested 18 h later, and IL-8 levels were determined by ELISA. The error bars indicate standard deviations. (B) CMV DBs induce both IL-6 and IL-8 cytokine secretion from human PBMC. CMV DBs (0.01 to 5 μl/well) were added to human PBMC as described for panel A. The supernatants were harvested 18 h later, and IL-6 and IL-8 levels were determined by ELISA.
FIG. 2.
IL-8 secretion from human monocytes is CD14 dependent. Human PBMC (5 × 105/well) were incubated with no stimulus (Medium) or with UV-inactivated CMV (2.5 × 103 to 2 × 104 PFU/well) or DBs (0.01 to 5 μl/well). The supernatants were harvested 18 h later, and IL-8 levels were determined by ELISA. Anti-CD14 monoclonal antibody (MY4; 5 μg/ml) or isotype control antibody was added to the cultures 30 min prior to the addition of stimulants. The error bars indicate standard deviations.
CMV virions trigger inflammatory cytokine production in a CD14-, TLR2-dependent manner.
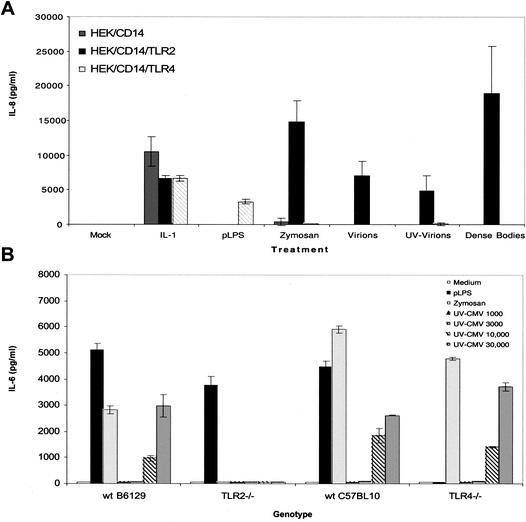
The fact that the CD14 pattern recognition receptor is involved in CMV inflammatory cytokine induction led us to consider the role of TLRs in mediating responses to CMV. Human embryonic kidney (HEK) cells or Chinese hamster ovary (CHO) cells exposed to CMV do not produce either inflammatory cytokines or ISGs (K. W. Boehme and T. Compton, unpublished results). Transcript analysis and fluorescence-activated cell sorting (FACS) assays suggest that HEK cells constitutively express TLR1, TLR3, TLR5, TLR6, TLR7, and TLR9 but do not express TLR2 and TLR4. HEK cells that stably express CD14, TLR2, TLR4, or combinations of these receptors were generated. Secretion of the inflammatory cytokine IL-8 in response to CMV was measured by ELISAs (27). In this experiment, we tested responses to purified banded virions, UV-inactivated virions, and DBs. As shown in Fig. 3A, all three CMV preparations stimulated IL-8 production in the TLR2-expressing cells but not in HEK cells or in HEK cells expressing TLR4. To further test the role of TLRs in CMV innate responses, the inflammatory cytokine responses of wild-type and TLR-deficient mice to CMV were determined. Thioglycolate-elicited peritoneal macrophages (PECs) were isolated from wild-type, TLR2−/−, or TLR4−/− mice and tested for the induction of IL-6 secretion after exposure to CMV. Control cultures were stimulated with known TLR2 or TLR4 ligands. PECs from wild-type and TLR4−/− mice secreted IL-6 in response to CMV (Fig. 3B). In contrast, cells from the TLR2−/− mice exhibited no detectable IL-6 induction when exposed to CMV. The TLR2−/− cells did exhibit normal responsiveness to LPS, a TLR4 ligand. As expected, TLR2−/− PECs were unresponsive to the TLR2 ligand zymosan. Taken together, these data suggest that TLR2 is capable of recognizing CMV particles regardless of replication competence. The result of this recognition is the activation of a signal transduction pathway that leads to IL-8 synthesis.
FIG. 3.
CMV-induced cytokine secretion is CD14 and TLR2 dependent. (A) HEK293 cells stably transfected with CD14 alone or in combination with TLR2 or TLR4 were incubated with no stimulus (Mock) or with IL-1β (100 ng/ml; TLR- and CD14-independent positive control ligand), phenol-reextracted LPS (pLPS; 10 ng/ml; TLR4 ligand), yeast zymosan (10 μg/ml; TLR2 ligand), or virions, UV-inactivated virions, or DBs. The supernatants were harvested 18 h later, and IL-8 levels were determined by ELISA. The error bars indicate standard deviations. (B) PECs (105/well) isolated from thioglycolate-injected wild-type (wt), TLR2−/−, or TLR4−/− mice were incubated with no stimulus (Medium) or with pLPS (10 ng/ml; TLR4 ligand), yeast zymosan (10 μg/ml; TLR2 ligand), or UV-inactivated CMV (1 × 103 to 3 × 104 PFU/well). The supernatants were harvested 18 h later, and IL-6 levels were determined by ELISA.
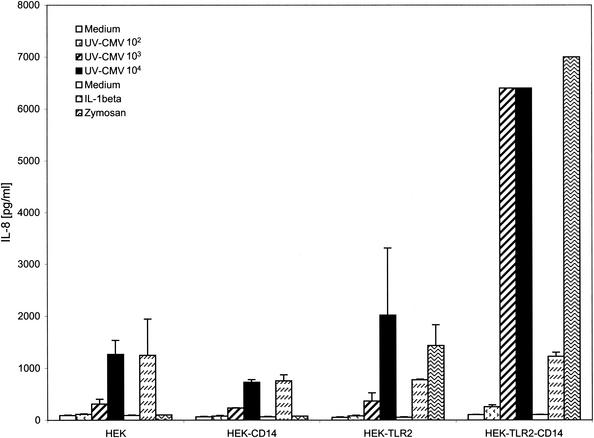
We next asked if CD14 was required for the TLR2-activated cytokine response. HEK TLR2 lines expressing or lacking CD14 were further characterized for CMV-induced inflammatory cytokine secretion. The data shown in Fig. 4 support an important enhancer role for CD14 in TLR2-dependent CMV responses. HEK cells expressing only TLR2 secreted modest but detectable levels of IL-8 in response to CMV. However, this response was significantly enhanced when CD14 was expressed on the same cells. These findings suggest that CD14 facilitates signaling in response to CMV, similar to its role as an important coreceptor in TLR-dependent responses to a diverse group of microbial components.
FIG. 4.
TLR2-dependent responses require CD14. HEK293 cell lines stably transfected with CD14, TLR2 alone, or a combination of TLR2 and CD14 were incubated with no stimulus (Medium) or with UV-inactivated HCMV (102 to 104 PFU/well), zymosan (10 μg/ml; TLR2 ligand), or IL-1β (100 ng/ml; TLR- and CD14-independent positive control ligand). The supernatants were harvested 18 h later, and IL-8 levels were determined by ELISA. The error bars indicate standard deviations.
TLR2 detection of CMV leads to NF-κB-dependent transcription.
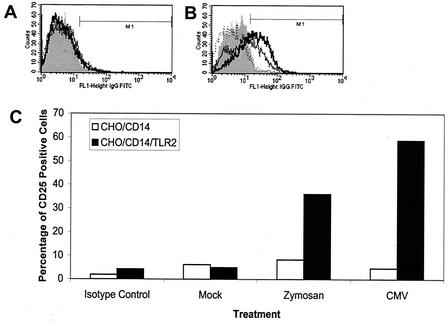
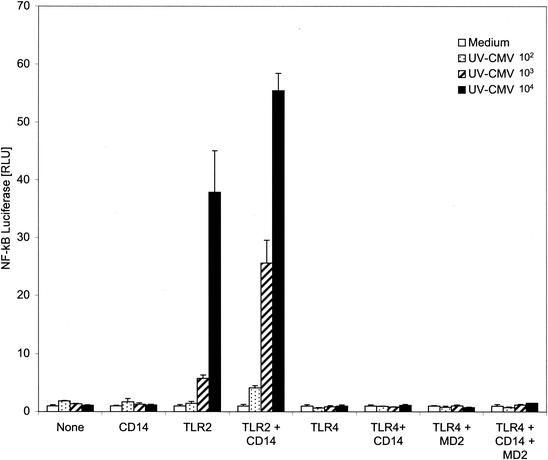
Fibroblasts and monocytes infected with CMV have been shown to exhibit activated NF-κB, as evidenced by nuclear translocation and increased DNA binding activity (62, 63). TLR signaling pathways trigger a series of interactions among specific intracellular mediators that ultimately result in the release of NF-κB from its endogenous inhibitor (2). Activated NF-κB undergoes nuclear translocation that leads to transcription of many genes containing NF-κB elements in their promoters, including many inflammatory cytokines. Two approaches were taken to test the hypothesis that CMV activation of TLR2 leads to an NF-κB-driven transcription response. First, CHO cells expressing CD14 and human TLR2 which had been engineered to contain an NF-κB-CD25 reporter construct (18) were analyzed. When NF-κB is activated in these cells, CD25 is transcribed, expressed, and displayed on the cell surfaces. FACS analysis was used to measure CD25 expression in cells exposed to CMV. CD25 expression was induced by zymosan and CMV in the TLR2-expressing cells, whereas little to no expression was detected in CHO cells expressing only CD14 (Fig. 5). Second, we characterized NF-κB responsiveness in HEK293 cells expressing combinations of TLR2, TLR4, MD2, and CD14 using an NF-κB-driven luciferase reporter gene assay (Fig. 6). These experiments demonstrate that CMV activates NF-κB in a TLR2-dependent manner, consistent with our observation of the TLR2-dependent induction of cytokine secretion by CMV. Also, as demonstrated for cytokine secretion from monocytes, CD14 serves an important enhancer function in modulating TLR-mediated NF-κB responses (Fig. 6).
FIG. 5.
TLR2 is required for NF-κB activation in response to CMV. (A and B) CHO/CD14 (A) or CHO/CD14/TLR2 (B) reporter cells were mock infected, treated with zymosan A (10 μg/ml), or infected with CMV (multiplicity of infection, 0.1), and NF-κB activity was measured using the NF-κB-driven CD25 reporter. At 18 h posttreatment-infection, cell surface expression of CD25 was measured by FACS. For each histogram, the vertical axis represents the relative cell numbers, and the horizontal axis represents the intensity of fluorescence staining. The shaded areas represent the isotype control, the dotted lines represent mock-infected cells, the thin solid lines represent zymosan A-treated cells, and the thick solid lines represent CMV-infected cells. IgG, immunoglobulin G; FITC, fluorescein isothiocyanate. (C) The percentages of CD25-positive cells from the data in panels A and B are presented graphically.
FIG. 6.
NF-κB transcriptional responses to CMV are mediated by TLR2 and enhanced by CD14. HEK293 cells stably transfected with TLR2, TLR4, or a combination of TLR4 and MD2 were transiently transfected with an NF-κB-driven firefly luciferase construct and a herpes simplex virus thymidine kinase-driven Renilla luciferase construct (for normalization). Where indicated, the cells were also transfected with CD14 or vector control DNA. Eighteen hours later, the cells were incubated with no stimulus (Medium) or with UV-inactivated HCMV (102 to 104 PFU/well). Following a 6-h stimulation, the cells were lysed and luciferase activity was determined using a Dual-Glo Luciferase Assay. The data are expressed as the mean (plus standard deviation) relative light units (RLU) of triplicate wells. The NF-κB luciferase activity of each well was normalized for transfection efficiency using the Renilla luciferase activity.
DISCUSSION
TLRs transmit signals that lead to innate immune activation in response to a wide range of microbial pathogens. Our findings show that TLR2 detects CMV, which results in activation of innate immune responses. This brings the number of viruses known to be sensed by TLRs to four. RSV and MMTV signal through TLR4 (28, 42), while measles virus, a member of the paramyxovirus family, like RSV, (5) and CMV, a herpesvirus, activate innate responses in a TLR2-dependent manner. All do so at the very earliest stages of the life cycle during the initial virus-cell contact. In addition, CD14 serves a critical function in eliciting inflammatory cytokine induction. The facilitating role of CD14 is an emerging paradigm in TLR signaling, although even in the well-studied LPS research, the precise role it serves is not known. In general, however, these findings suggest that the TLR system may be broadly capable of detecting viruses during the entry stage of infection. In the case of CMV, where the actual entry receptor is not known, we must further examine the relationship of CD14 and TLR2 in the virus life cycle itself, and these studies are under way. At present, however, there are no data to suggest that either of these proteins is an entry mediator, since CMV can bind, enter, and establish IE gene expression in the TLR2-negative, CD14-negative HEK cell line.
In the case of the simpler RNA viruses that have one or two envelope glycoproteins serving ligand and fusion functions, a single protein was the identified molecular trigger. Herpesviruses are considerably more structurally complex, and CMV contains at least 10 distinct envelope complexes organized into a variety of homooligomeric and heterooligomeric complexes. To date, we have been unable to achieve levels of inflammatory cytokine secretion with soluble gB comparable to those observed with CMV (data not shown). One possibility is that efficient activation is context specific and requires the higher valency of gB found in virions. It is also conceivable that the triggering mechanism for TLR2 activation may be more complex for herpesviruses than for the paramyxoviruses and MMTV. One possible molecular pattern for TLR activation pertains to structures formed during virus-cell fusion. While the RNA viruses mentioned above have single-component fusion systems, the herpesviruses employ multicomponent machines. At least three envelope glycoproteins conserved throughout the herpesviruses are needed to mediate membrane fusion in a number of human herpesviruses, including CMV, but the molecular basis of fusion structures has not been determined (9, 19, 39, 40; E. R. Kinzler and T. Compton, unpublished data). It is possible that fusion of a viral particle with the cell membrane leads to changes in the orientation of cell surface proteins that are critical to the signal transduction events. Further studies are required to decipher the triggering mechanism for CMV activation of inflammatory responses.
CMV has an intimate relationship with the host immune system. Individuals infected with CMV mount a robust immune response that suppresses persistent viral replication and facilitates a lifelong latency (34). Loss of immune control favors reactivation and the onset of disease. CMV devotes significant coding capacity to immune modulation. At least four gene products interfere with major histocompatibility complex class I antigen presentation (16, 58), and two CMV-encoded proteins interact with HLA-E, which leads to suppression of NK responses and protection from NK-mediated lysis (12, 54, 57). Although many immune functions are directly repressed during replication, here we have shown that CMV activates inflammatory cytokine responses via TLR2 during the prereplication phase of the life cycle. At first glance, activation of innate responses during virus entry would appear to be a strategic flaw in the anti-immune arsenal. However, data are emerging to suggest that inflammation induced by CMV may in fact facilitate its replication and dissemination. For example, the inflammatory mediator COX-2 is strongly induced by virion particles (8, 64). COX-2 induction leads to synthesis of prostaglandin (PG) E2. PGE2 then positively influences the expression of the critical viral transcriptional regulatory protein and overall infectious yields (66). In addition, NF-κB has well-described regulatory activity over the expression of the critical major IE proteins of CMV. The IE promoter contains consensus NF-κB elements that are crucial for their regulation (43). Later during the replication cycle, CMV encodes several chemokines and chemokine receptors that also provide potent inflammatory signals that are likely to contribute to pathogenesis as well (3, 34). Indeed, dissemination within an infected person is also aided by inflammation. Neutrophils and monocytes are recruited from the circulation to sites of infection by inflammatory cytokines. Neutrophils then greatly facilitate dissemination of infection by transporting infectious virions to alternate sites of infection, whereas monocytes harbor latent virus. Thus, the initial burst of inflammatory cytokines may facilitate mononuclear cell recruitment.
Interferon and ISGs are also robustly induced by CMV particles during entry. It is not yet clear what role, if any, CMV activation of TLR2 plays in these responses. TLR2 can activate the MAP kinase p38, which can lead to activation of IRF-3, a key transcriptional inducer of interferon, and a subset of ISGs (36, 60). CMV is known to activate p38 (10, 62) and IRF-3 (37, 41). The relationship of the virus with the interferon response, however, is different from that with inflammation. Replication-dependent shutoff of the interferon pathway and of ISG transcription is well documented (8, 31, 32). Further studies are required to determine the mechanism of CMV activation and modulation of these two powerful branches of the innate immune system. Many of the pathological processes associated with CMV reactivation (including retinitis, accelerated vascular disease, and graft rejection) appear to be mediated by the release of inflammatory cytokines. The definition of the host proteins involved in these events (TLR2 and CD14) should allow us to devise more specific ways to inhibit these responses. These studies also provide a mechanism for the association of polymorphisms in the TLR genes with the development of vascular disease (25).
Acknowledgments
This work was supported by NIH grants AI 34998 (to T.C.), AI 49309 and GM 63244 (to R.W.F.), and AI 51405 (to E.A.K.-J.). K.W.B. was supported by NIH training grant T32 GM 072 15.
REFERENCES
- 1.AbuBakar, S., I. Boldogh, and T. Albrecht. 1990. Human cytomegalovirus stimulates arachidonic acid metabolism through pathways that are affected by inhibitors of phospholipase A2 and protein kinase C. Biochem. Biophys. Res. Commun. 166**:**953-959. [DOI] [PubMed] [Google Scholar]
- 2.**Akira, S.2001. Toll-like receptors and innate immunity. Adv. Immunol. 78:**1-56. [DOI] [PubMed] [Google Scholar]
- 3.Alcami, A., and U. H. Koszinowski. 2000. Viral mechanisms of immune evasion. Trends Microbiol. 8**:**410-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldick, C. J., Jr., and T. Shenk. 1996. Proteins associated with purified human cytomegalovirus particles. J. Virol. 70**:**6097-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieback, K., E. Lien, I. M. Klagge, E. Avota, J. Schneider-Schaulies, W. P. Duprex, H. Wagner, C. J. Kirschning, V. Ter Meulen, and S. Schneider-Schaulies. 2002. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J. Virol. 76**:**8729-8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyle, K. A., and T. Compton. 1998. Receptor-binding properties of a soluble form of human cytomegalovirus glycoprotein B. J. Virol. 72**:**1826-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle, K. A., R. L. Pietropaolo, and T. Compton. 1999. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol. Cell. Biol. 19**:**3607-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browne, E. P., B. Wing, D. Coleman, and T. Shenk. 2001. Altered cellular mRNA levels in human cytomegalovirus-infected fibroblasts: viral block to the accumulation of antiviral mRNAs. J. Virol. 75**:**12319-12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browne, H., B. Bruun, and T. Minson. 2001. Plasma membrane requirements for cell fusion induced by herpes simplex virus type 1 glycoproteins gB, gD, gH and gL. J. Gen. Virol. 82**:**1419-1422. [DOI] [PubMed] [Google Scholar]
- 10.Chen, J., and M. F. Stinski. 2002. Role of regulatory elements and the MAPK/ERK or p38 MAPK pathways for activation of human cytomegalovirus gene expression. J. Virol. 76**:**4873-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Compton, T., R. R. Nepomuceno, and D. M. Nowlin. 1992. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191**:**387-395. [DOI] [PubMed] [Google Scholar]
- 12.Cosman, D., J. Mullberg, C. L. Sutherland, W. Chin, R. Armitage, W. Fanslow, M. Kubin, and N. J. Chalupny. 2001. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 14**:**123-133. [DOI] [PubMed] [Google Scholar]
- 13.Dankner, W. M., J. A. McCutchan, D. D. Richman, K. Hirata, and S. A. Spector. 1990. Localization of human cytomegalovirus in peripheral blood leukocytes by in situ hybridization. J. Infect. Dis. 161**:**31-36. [DOI] [PubMed] [Google Scholar]
- 14.Delude, R. L., A. Yoshimura, R. R. Ingalls, and D. T. Golenbock. 1998. Construction of a lipopolysaccharide reporter cell line and its use in identifying mutants defective in endotoxin, but not TNF-alpha, signal transduction. J. Immunol. 161**:**3001-3009. [PubMed] [Google Scholar]
- 15.Fortunato, E. A., A. K. McElroy, I. Sanchez, and D. H. Spector. 2000. Exploitation of cellular signaling and regulatory pathways by human cytomegalovirus. Trends Microbiol. 8**:**111-119. [DOI] [PubMed] [Google Scholar]
- 16.Gewurz, B. E., R. Gaudet, D. Tortorella, E. W. Wang, and H. L. Ploegh. 2001. Virus subversion of immunity: a structural perspective. Curr. Opin. Immunol. 13**:**442-450. [DOI] [PubMed] [Google Scholar]
- 17.Gnann, J. W., Jr., J. Ahlmen, C. Svalander, L. Olding, M. B. Oldstone, and J. A. Nelson. 1988. Inflammatory cells in transplanted kidneys are infected by human cytomegalovirus. Am. J. Pathol. 132**:**239-248. [PMC free article] [PubMed] [Google Scholar]
- 18.Golenbock, D. T., Y. Liu, F. H. Millham, M. W. Freeman, and R. A. Zoeller. 1993. Surface expression of human CD14 in Chinese hamster ovary fibroblasts imparts macrophage-like responsiveness to bacterial endotoxin. J. Biol. Chem. 268**:**22055-22059. [PubMed] [Google Scholar]
- 19.Haan, K. M., S. K. Lee, and R. Longnecker. 2001. Different functional domains in the cytoplasmic tail of glycoprotein B are involved in Epstein-Barr virus-induced membrane fusion. Virology 290**:**106-114. [DOI] [PubMed] [Google Scholar]
- 20.Haynes, L. M., D. D. Moore, E. A. Kurt-Jones, R. W. Finberg, L. J. Anderson, and R. A. Tripp. 2001. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J. Virol. 75**:**10730-10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165**:**618-622. [DOI] [PubMed] [Google Scholar]
- 22.Huber, M. T., and T. Compton. 1997. Characterization of a novel third member of the human cytomegalovirus glycoprotein H-glycoprotein L complex. J. Virol. 71**:**5391-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20**:**197-216. [DOI] [PubMed] [Google Scholar]
- 24.Jarvis, M. A., and J. A. Nelson. 2002. Mechanisms of human cytomegalovirus persistence and latency. Front. Biosci. 7**:**1575-1582. [DOI] [PubMed] [Google Scholar]
- 25.Kiechl, S., E. Lorenz, M. Reindl, C. J. Wiedermann, F. Oberhollenzer, E. Bonora, J. Willeit, and D. A. Schwartz. 2002. Toll-like receptor 4 polymorphisms and atherogenesis. N. Engl. J. Med. 347**:**185-192. [DOI] [PubMed] [Google Scholar]
- 26.Kowalik, T. F., B. Wing, J. S. Haskill, J. C. Azizkhan, A. S. Baldwin, Jr., and E. S. Huang. 1993. Multiple mechanisms are implicated in the regulation of NF-kappa B activity during human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 90**:**1107-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurt-Jones, E. A., L. Mandell, C. Whitney, A. Padgett, K. Gosselin, P. E. Newburger, and R. W. Finberg. 2002. Role of toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood 100**:**1860-1868. [PubMed] [Google Scholar]
- 28.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1**:**398-401. [DOI] [PubMed] [Google Scholar]
- 29.Medzhitov, R., and C. Janeway, Jr. 2000. The Toll receptor family and microbial recognition. Trends Microbiol. 8**:**452-456. [DOI] [PubMed] [Google Scholar]
- 30.Mendelson, M., S. Monard, P. Sissons, and J. Sinclair. 1996. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. J. Gen. Virol. 77**:**3099-3102. [DOI] [PubMed] [Google Scholar]
- 31.Miller, D. M., Y. Zhang, B. M. Rahill, K. Kazor, S. Rofagha, J. J. Eckel, and D. D. Sedmak. 2000. Human cytomegalovirus blocks interferon-gamma stimulated up-regulation of major histocompatibility complex class I expression and the class I antigen processing machinery. Transplantation 69**:**687-690. [DOI] [PubMed] [Google Scholar]
- 32.Miller, D. M., Y. Zhang, B. M. Rahill, W. J. Waldman, and D. D. Sedmak. 1999. Human cytomegalovirus inhibits IFN-α-stimulated antiviral and immunoregulatory responses by blocking multiple levels of IFN-α signal transduction. J. Immunol. 162**:**6107-6113. [PubMed] [Google Scholar]
- 33.Minton, E. J., C. Tysoe, J. H. Sinclair, and J. G. Sissons. 1994. Human cytomegalovirus infection of the monocyte/macrophage lineage in bone marrow. J. Virol. 68**:**4017-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mocarski, E. S., Jr. 2002. Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 10**:**332-339. [DOI] [PubMed] [Google Scholar]
- 35.Myerson, D., R. C. Hackman, J. A. Nelson, D. C. Ward, and J. K. McDougall. 1984. Widespread presence of histologically occult cytomegalovirus. Hum. Pathol. 15**:**430-439. [DOI] [PubMed] [Google Scholar]
- 36.Navarro, L., and M. David. 1999. p38-dependent activation of interferon regulatory factor 3 by lipopolysaccharide. J. Biol. Chem. 274**:**35535-35538. [DOI] [PubMed] [Google Scholar]
- 37.Navarro, L., K. Mowen, S. Rodems, B. Weaver, N. Reich, D. Spector, and M. David. 1998. Cytomegalovirus activates interferon immediate-early response gene expression and an interferon regulatory factor 3-containing interferon-stimulated response element-binding complex. Mol. Cell. Biol. 18**:**3796-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pass, R. F. 2001. Cytomegalovirus, p. 2675-2706. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Raven Press, New York, N.Y.
- 39.Pertel, P. E. 2002. Human herpesvirus 8 glycoprotein B (gB), gH, and gL can mediate cell fusion. J. Virol. 76**:**4390-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279**:**313-324. [DOI] [PubMed] [Google Scholar]
- 41.Preston, C. M., A. N. Harman, and M. J. Nicholl. 2001. Activation of interferon response factor-3 in human cells infected with herpes simplex virus type 1 or human cytomegalovirus. J. Virol. 75**:**890-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rassa, J. C., J. L. Meyers, Y. Zhang, R. Kudaravalli, and S. R. Ross. 2002. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc. Natl. Acad. Sci. USA 99**:**2281-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambucetti, L. C., J. M. Cherrington, G. W. Wilkinson, and E. S. Mocarski. 1989. NF-kappa B activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 8**:**4251-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schrier, R., P. Ghazal, C. Wiley, and J. Nelson. 1991. Human cytomegalovirus productively infects primary differentiated macrophages. J. Virol. 65**:**6581-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sen, G. C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55**:**255-281. [DOI] [PubMed] [Google Scholar]
- 46.Severi, B., M. P. Landini, G. Cenacchi, N. Zini, and N. M. Maraldi. 1992. Human cytomegalovirus nuclear and cytoplasmic dense bodies. Arch. Virol. 123**:**193-207. [DOI] [PubMed] [Google Scholar]
- 47.Simmen, K. A., J. Singh, B. G. Luukkonen, M. Lopper, A. Bittner, N. E. Miller, M. R. Jackson, T. Compton, and K. Fruh. 2001. Global modulation of cellular transcription by human cytomegalovirus is initiated by viral glycoprotein B. Proc. Natl. Acad. Sci. USA 98**:**7140-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinzger, C., M. Kahl, K. Laib, K. Klingel, P. Rieger, B. Plachter, and G. Jahn. 2000. Tropism of human cytomegalovirus for endothelial cells is determined by a post-entry step dependent on efficient translocation to the nucleus. J. Gen. Virol. 81**:**3021-3035. [DOI] [PubMed] [Google Scholar]
- 49.Sinzger, C., H. Muntefering, T. Loning, H. Stoss, B. Plachter, and G. Jahn. 1993. Cell types infected in human cytomegalovirus placentitis identified by immunohistochemical double staining. Virchows Arch. A 423**:**249-256. [DOI] [PubMed] [Google Scholar]
- 50.Soderberg-Naucler, C., K. N. Fish, and J. A. Nelson. 1997. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J. Clin. Investig. 100**:**3154-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soderberg-Naucler, C., and J. Y. Nelson. 1999. Human cytomegalovirus latency and reactivation—a delicate balance between the virus and its host's immune system. Intervirology 42**:**314-321. [DOI] [PubMed] [Google Scholar]
- 52.Soderberg-Naucler, C., D. N. Streblow, K. N. Fish, J. Allan-Yorke, P. P. Smith, and J. A. Nelson. 2001. Reactivation of latent human cytomegalovirus in CD14(+) monocytes is differentiation dependent. J. Virol. 75**:**7543-7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67**:**227-264. [DOI] [PubMed] [Google Scholar]
- 54.Sutherland, C. L., N. J. Chalupny, and D. Cosman. 2001. The UL16-binding proteins, a novel family of MHC class I-related ligands for NKG2D, activate natural killer cell functions. Immunol. Rev. 181**:**185-192. [DOI] [PubMed] [Google Scholar]
- 55.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol 165**:**5392-5396. [DOI] [PubMed] [Google Scholar]
- 56.Theiler, R. N., and T. Compton. 2002. Distinct glycoprotein o complexes arise in a post-Golgi compartment of cytomegalovirus-infected cells. J. Virol. 76**:**2890-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomasec, P., V. M. Braud, C. Rickards, M. B. Powell, B. P. McSharry, S. Gadola, V. Cerundolo, L. K. Borysiewicz, A. J. McMichael, and G. W. Wilkinson. 2000. Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science 287**:**1031.. [DOI] [PubMed] [Google Scholar]
- Tortorella, D., B. E. Gewurz, M. H. Furman, D. J. Schust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18**:**861-926. [DOI] [PubMed] [Google Scholar]
- 59.Valy-Nagy, T., Z. Bandi, I. Boldogh, and T. Albrecht. 1988. Hydrolysis of inositol lipids: an early signal of human cytomegalovirus infection. Arch. Virol. 101**:**199-207. [DOI] [PubMed] [Google Scholar]
- 60.Vasselon, T., W. A. Hanlon, S. D. Wright, and P. A. Detmers. 2002. Toll-like receptor 2 (TLR2) mediates activation of stress-activated MAP kinase p38. J. Leukoc. Biol. 71**:**503-510. [PubMed] [Google Scholar]
- 61.Waldman, W. J., P. W. Adams, D. A. Knight, and D. D. Sedmak. 1997. CMV as an exacerbating agent in transplant vascular sclerosis: potential immune-mediated mechanisms modelled in vitro. Transplant. Proc. 29**:**1545-1546. [DOI] [PubMed] [Google Scholar]
- 62.Yurochko, A. D., and E. S. Huang. 1999. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J. Immunol. 162**:**4806-4816. [PubMed] [Google Scholar]
- 63.Yurochko, A. D., T. F. Kowalik, S. M. Huong, and E. S. Huang. 1995. Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J. Virol. 69**:**5391-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu, H., J. P. Cong, G. Mamtora, T. Gingeras, and T. Shenk. 1998. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95**:**14470-14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu, H., J. P. Cong, and T. Shenk. 1997. Use of differential display analysis to assess the effect of human cytomegalovirus infection on the accumulation of cellular RNAs: induction of interferon-responsive RNAs. Proc. Natl. Acad. Sci. USA 94**:**13985-13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu, H., J. P. Cong, D. Yu, W. A. Bresnahan, and T. E. Shenk. 2002. Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. Proc. Natl. Acad. Sci. USA 99**:**3932-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]