ARC5, a cytosolic dynamin-like protein from plants, is part of the chloroplast division machinery (original) (raw)
Abstract
Chloroplast division in plant cells is orchestrated by a complex macromolecular machine with components positioned on both the inner and outer envelope surfaces. The only plastid division proteins identified to date are of endosymbiotic origin and are localized inside the organelle. Employing positional cloning methods in__Arabidopsis_ in conjunction with a novel strategy for pinpointing the mutant locus, we have identified a gene encoding a new chloroplast division protein, ARC5. Mutants of_ARC5_ exhibit defects in chloroplast constriction, have enlarged, dumbbell-shaped chloroplasts, and are rescued by a wild-type copy of ARC5. The ARC5 gene product shares similarity with the dynamin family of GTPases, which mediate endocytosis, mitochondrial division, and other organellar fission and fusion events in eukaryotes. Phylogenetic analysis showed that ARC5 is related to a group of dynamin-like proteins unique to plants. A GFP–ARC5 fusion protein localizes to a ring at the chloroplast division site. Chloroplast import and protease protection assays indicate that the ARC5 ring is positioned on the outer surface of the chloroplast. Thus, ARC5 is the first cytosolic component of the chloroplast division complex to be identified. ARC5 has no obvious counterparts in prokaryotes, suggesting that it evolved from a dynamin-related protein present in the eukaryotic ancestor of plants. These results indicate that the chloroplast division apparatus is of mixed evolutionary origin and that it shares structural and mechanistic similarities with both the cell division machinery of bacteria and the dynamin-mediated organellar fission machineries of eukaryotes.
The chloroplasts of plants and algae are widely believed to have evolved only once from a free-living cyanobacterial endosymbiont (1). Over evolutionary time, many of the genes once present in the endosymbiont have been transferred to the nuclear genome where they have acquired sequences encoding transit peptides that direct their gene products back to the chloroplast (1, 2). This scenario describes the origin of the five previously identified plastid division proteins in plants, all of which evolved from related cell division proteins in cyanobacteria, are encoded in the nucleus, and are localized inside the chloroplast. These include FtsZ1 and FtsZ2, tubulin-like proteins that localize to a ring at the site of plastid constriction (3–10), MinD and MinE, which regulate placement of the plastid division site (11–13), and ARTEMIS, which appears to mediate constriction of the envelope membranes (14).
Despite localization of the previously identified plastid division proteins inside the chloroplasts in plant cells, ultrastructural studies have shown that plastid division entails the coordinated activity of components localized outside as well as inside the organelle. In plants, the chloroplast division complex comprises electron-dense structures situated both on the stromal surface of the inner envelope membrane and on the cytosolic surface of the outer membrane (15). These structures have been termed the inner and outer plastid-dividing (PD) rings, respectively. A middle PD ring positioned in the intermembrane space has also been described in the red alga_Cyanidioschyzon merolae_ (16), and the dynamics of assembly and disassembly of the three PD rings have been investigated in detail in this organism (17, 18). Although it was previously hypothesized that the PD rings might contain FtsZ (4), recent evidence showing that the FtsZ ring assembles before and is separable from the PD rings in both_C. merolae_ and plants (19, 20) indicate that this is not the case. Thus, although it is assumed that the PD rings represent multiprotein complexes, their compositions remain unknown.
The Arabidopsis mutant arc5 contains an ethyl methanesulfonate (EMS)-induced mutation conferring a chloroplast division defect in which chloroplasts initiate but rarely complete constriction (21, 22). As a result, arc5 chloroplasts often exhibit a dumbbell shape (Fig.1B). This phenotype suggested that the ARC5 gene product might be a structural component of the chloroplast division complex. Here we show that _ARC5_is a member of the dynamin family of GTPases, which have not been shown previously to participate in chloroplast division, and that it localizes to the chloroplast division site in plants. However, in contrast with other chloroplast division proteins, ARC5 is positioned on the cytosolic surface of the organelle and has no obvious homologues in prokaryotes. Our findings reveal that the chloroplast division machinery is an evolutionary hybrid, combining structural and mechanistic features acquired from both the prokaryotic ancestor of chloroplasts and its eukaryotic host.
Figure 1.
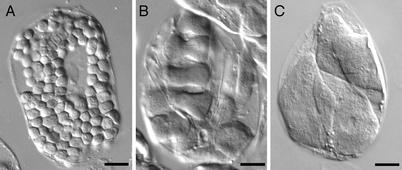
Comparison of chloroplasts in Arabidopsis leaf mesophyll cells. (A) Wild-type (L_er_). (B and C) arc5. Cells are from fixed tissue. (Bars, 10 μm.)
Materials and Methods
Plant Material.
Arabidopsis thaliana strains Columbia (Col-0) and Landsberg erecta (L_er_) were used for all experiments as indicated. The arc5 mutant was identified in the L_er_ background by Pyke and Leech (21). Plants were grown as described (4).
Microscopy.
Phenotypes were analyzed as previously described (4), except that the images were recorded with a Coolpix 995 digital camera (Nikon Corporation, Tokyo). For in vivo detection of GFP, fresh leaf tissue was mounted in water and viewed with an L5 filter set (excitation 455–495 nm, emission 512–575 nm) and a ×100 oil immersion objective of a Leica DMR A2 microscope (Leica, Wetzlar, Germany) equipped with epifluorescence illumination. Images were captured with a cooled charge-coupled device camera (Retiga 1350EX, Qimaging, Burnaby, British Columbia, Canada) and processed withphotoshop imaging software (Adobe Systems, San Jose, CA).
Fine Mapping of ARC5.
The arc5 mutation was previously mapped between markers nga162 (20.6 centiMorgan, cM) and AtDMC1 (32.6 cM) on chromosome 3 (23). To fine map ARC5, an F2population was generated from a cross between arc5 and Col-0 wild type. Of 7,000 F2 plants, 1,720 mutants were identified by microscopy. Markers used for PCR-based mapping (24) are provided in Table 1, which is published as supporting information on the PNAS web site, www.pnas.org.
Identification of a Candidate ARC5 Gene.
Bacterial artificial chromosome (BAC) clones MMB12 and MPN9 were double-digested with _Hinc_II and _Hind_III. The resulting fragments were inserted between the cauliflower mosaic virus 35S promoter and the octopine synthase terminator in the plasmid SN506 (4), which had been previously digested with Sma_I and_Hind_III to remove the insert. Ligation products were amplified in Escherichia coli strain DH5α, transferred to_Agrobacterium tumefaciens GV3101, and introduced into wild-type Arabidopsis (Col-0) by floral dipping as described (4). T1 transgenic plants were selected by growth on kanamycin and screened for an _arc5_-like phenotype by microscopy. The T-DNA inserts from the two _arc5_-like plants identified were amplified by PCR using vector primers flanking the inserts. The PCR products were sequenced to determine which fragments from the BAC clones were carried by these plants, as well as their orientation with respect to the promoter. Both plants carried the same_Hind_III-Hinc_II fragment from At3g19730 (see_Results). To confirm that this fragment was the source of the _arc5_-like phenotype in the T1transgenics, the fragment was subcloned into SN506 in the antisense orientation as described above and introduced into wild-type Col-0 plants. After selection on kanamycin, the phenotypes of the resulting T1 plants were determined by microscopy.
Amplification of ARC5 cDNA.
Primers used for RT-PCR were 5′-GAAAAAGGAACGGCGACGAAAAC-3′ and 5′-GCAAACATTGGACCAAAAAGCG-3′. Amplified cDNAs were subcloned into Bluescript KS+ (Stratagene) before sequencing.
Sequence Alignment and Phylogenetic Analysis.
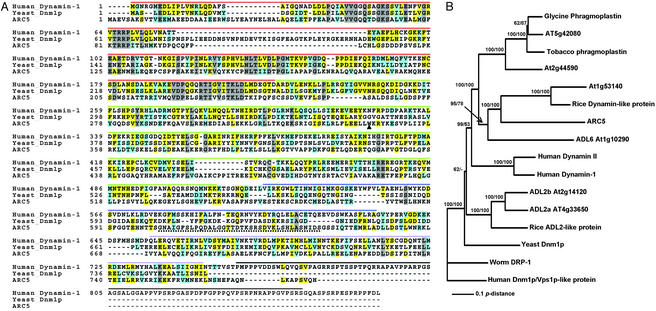
The amino acid sequence of ARC5 was deduced from the cDNA sequence. The sequence alignment shown in Fig. 3A was performed with theclustalw multiple alignment program (26) at the Biology Workbench 3.2 web site (http://biowb.sdsc.edu/). Protein sequences used for the phylogenetic analysis shown in Fig.3B were aligned with clustal x (27) using default settings. The alignment is available on request. Neighbor joining and maximum parsimony analyses were performed by usingpaup version 4.0b10 (28) with default settings except for ties being randomly broken. Neighbor-joining and maximum parsimony analyses produced topologically identical trees. Bootstrap analyses were performed on the neighbor-joining and maximum parsimony trees with one thousand replications. GenBank accession numbers for proteins aligned with ARC5 (longer form, accession no. AY212885) are as follows: human Dynamin-1 (NP_004399), yeast Dnm1p (NP_013100), At1g53140 (NP_175722), rice dynamin like protein (BAB56031), ADL6 (AAF22291), At5g42080 (NP_568602), Glycine phragmoplastin (AAB05992), tobacco phragmoplastin (CAB56619), At2g44590 (NP_181987), human Dynamin II (NP_004936), ADL2a (NP_567931), ADL2b (NP_565362), rice ADL2-like protein (BAB86118), worm Drp-1 (AAL56621), and human Dnm1p/Vps1p-like protein (JC5695).
Figure 3.
ARC5 is a dynamin-like protein. (A) Alignment of ARC5 with Dynamin-1 from Homo sapiens and Dnm1p from_Saccharomyces cerevisiae_. Gray boxes indicate completely conserved residues; yellow boxes are identical residues; cyan boxes are similar residues; dashes indicate gaps. The domain structure is indicated by the lines above the alignment. Red, GTPase domain; green, middle domain; blue, PH domain; lavender, GTPase effector domain; black, PR domain. The dotted underline indicates the sequence encoded by the alternatively spliced intron in ARC5. The triangle indicates the position of the arc5 mutation. (B) Phylogenetic analysis of ARC5 with an unrooted neighbor-joining tree. Bootstrap values are shown at selected nodes. The first and second bootstrap values are from the neighbor-joining and parsimony analyses, respectively. Nodes with <50% bootstrap support, or not present, are represented by a “−”. Accession numbers for the sequences aligned with ARC5 are listed in Materials and Methods.
Complementation Analysis.
The genomic fragment corresponding to ARC5 (At3g19730 and At3g19720 in the Arabidopsis database, see_Results_), including 1.9 kb and 1.1 kb of the 5′ and 3′ flanking DNA, respectively, was amplified from MMB12 by PCR using the primers 5′-GGAATTCCGAGTCGAGTTGCTTTGTTG-3′ and 5′-CGTCTAGAGCTTACCTCAAAGGTACATGGA-3′. The PCR product was digested with_Eco_RI and ligated into a derivative of the transformation vector pLH7000 (www.dna-cloning-service.com/binaries.htm) digested with _Eco_RI and _Sma_I. The construct was transferred to Agrobacterium tumefaciens GV3101 and introduced into arc5 plants by floral dipping. The phenotypes of the T1 plants were determined by microscopy.
GFP–ARC5 Localization.
The GFP sequence was amplified from plasmid smRS-GFP (29) with the primers 5′-CGGGATCCATGAGTAAAGGAGAAGAACT-3′ and 5′-GCTCTAGATAGTTCATCCATGCCATGT-3′. The PCR product was digested with_Bam_HI and _Xba_I. The ARC5 coding region and 1.1 kb of the 3′ flanking DNA were amplified from the MMB12 BAC clone with primers 5′-GGACTAGTACGATGGCGGAAGTATCAGC-3′ and 5′-CGGGATCCGCACCGAAGGAGCCTTTAGATT-3′. The PCR product was digested with_Spe_I and _Eco_RI. cDNA fragments encoding GFP and ARC5 were subcloned into Bluescript KS+ (Stratagene) that had been digested with _Eco_RI and Bam_HI to create a_GFP–ARC5 fusion construct. The ARC5 promoter was amplified from MMB12 with primers 5′-GACTAGTTGGCTCAACGCTTACCTCAA-3′ and 5′-CGGGATCCGCCATCGTCTCTTACGA-3′, and cloned into Bluescript KS+ (Stratagene) between the _Spe_I and _Bam_HI sites. The promoter fragment was then subcloned into the plasmid containing the GFP–ARC5 fusion construct at the 5′ end of the fusion. The resulting plasmid was digested with _Spe_I and_Eco_RI, and the promoter-GFP–ARC5 cassette was subcloned into a derivative of the transformation vector pLH7000 (www.dna-cloning-service.com/binaries.htm). The plasmid was transferred to Agrobacterium tumefaciens GV3101 and used to transform wild-type A. thaliana plants (Col-0) as described above. The GFP–ARC5 localization pattern was visualized by fluorescence microscopy in T1 plants.
In Vitro Chloroplast Import and Protease Protection Assays.
Transcription/translation reactions, chloroplast isolation, in vitro import reactions, proteolytic treatments, and postimport fractionation and analysis were performed as described (7). The longer_ARC5_ cDNA, after subcloning into Bluescript KS+ as described above, was used for these experiments.
Results
Phenotype of arc5.
Like other chloroplast division mutants, leaf mesophyll cells in_arc5_ (L_er_) mutants contain fewer and larger chloroplasts than do wild type cells, which have about 120 chloroplasts at maturity (21, 22) (Fig. 1A). The _arc5_mutant has been reported to contain an average of 13 chloroplasts in fully expanded mesophyll cells (21, 22), although the phenotype appears somewhat more variable in our hands, with arc5 cells typically containing between 3 and 15 chloroplasts (Fig. 1 B_and C). This difference may reflect differences in growth conditions. Chloroplast number is fairly uniform within an individual_arc5 plant, and, as shown for other arc mutants (30), chloroplast size in arc5 varies inversely with chloroplast number (not shown). As has been reported previously (21,22), arc5 chloroplasts frequently remain dumbbell shaped (Fig. 1B).
Fine Mapping of ARC5 and a Novel Antisense Strategy for Identification of an arc5 Candidate Gene.
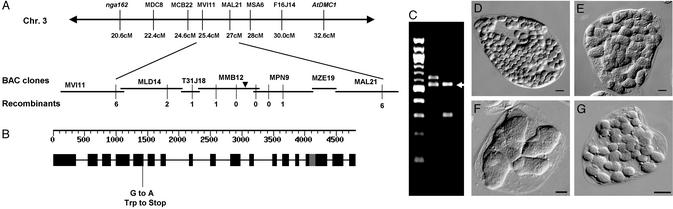
ARC5 was previously mapped to a region of chromosome 3 flanked by the markers nga162 (20.6 cM) and AtDMC1 (32.6 cM) (23) (Fig.2A Upper). We generated a new mapping population and used standard methods to fine map the arc5 locus to a 92-kb interval corresponding to two overlapping BAC clones, MMB12 and MPN9 (Fig. 2A Lower). This interval contained 24 predicted genes, but no clear_ARC5_ candidate. To identify the gene most likely to represent ARC5, we digested MMB12 and MPN9 with restriction enzymes, and subcloned the resulting fragments between the cauliflower mosaic virus 35S promoter and octopine synthase terminator in a derivative of the plant transformation vector pART27 (31), creating a library of fragments spanning the _arc5_-containing interval. The library was designed such that at least one fragment from each predicted gene in the interval would be ligated to the vector in the antisense orientation, with the expectation that an antisense fragment from ARC5 would yield an _arc5_-like phenotype. The library was used to transform wild-type Arabidopsis plants (Col-0), and transgenic plants were screened for chloroplast division defects by microscopy.
Figure 2.
Cloning of ARC5. (A) Fine mapping of_ARC5_. Triangle indicates the position of_ARC5_. (B) ORFs of ARC5. Black boxes represent exons; solid lines represent introns; gray box indicates the alternatively spliced intron. The mutation in_arc5_ and its position are indicated. (C) Amplification of the transgene inserts in two _arc5_-like T1 plants produced by transforming wild-type plants (Col-0) with a library of sense and antisense fragments derived from MMB12 and MPN9. A 100-bp DNA ladder (New England Biolabs) is shown at left. Arrow indicates a _Hind_III–_Hinc_II fragment from At3g19730 common to both arc5_-like transgenic lines. (D_–_G) Single leaf mesophyll cells from Col-0 wild-type (D), an arc5_-like T1 plant described in C (E), the arc5 (L_er) parent line used for complementation by ARC5 (F), and an_arc5 plant transformed with the ARC5 gene (G). (Bars in D_–_G, 10 μm.)
Among 120 independent T1 individuals examined, two had fewer and larger chloroplasts per cell than wild type (Fig.2E), and resembled arc5. The inserts carried by the transgenes in these two transgenic lines were amplified by PCR and sequenced. Two different inserts were detected in both lines (Fig. 2C). One line carried a fragment derived from the BAC backbone DNA and a second fragment derived from At3g19730 on MMB12 (Fig. 2C, lane 2). The other line carried a fragment from At3g19760 on MMB12 as well as the same fragment from At3g19730 (Fig.2C, arrow). In both plants, the At3g19730 fragment was present in the antisense orientation.
To confirm that the _arc5_-like plastid division phenotype in the two transgenic lines was caused by the shared At3g19730 fragment, we constructed a transgene containing this fragment in the antisense orientation, and introduced it into wild-type Arabidopsis(Col-0). Of 80 transgenic plants examined under the microscope, 20% had chloroplast division defects similar to those of _arc5_and the transgenic line shown in Fig. 2E. These findings indicated that the ARC5 gene corresponded to At3g19730.
Sequencing of arc5 and Mutant Complementation.
At3g19730 has homology to the dynamin family of GTPases (32). In the_Arabidopsis_ database, At3g19730 and the immediately adjacent sequence, At3g19720, were annotated as separate genes. However, sequence alignments to other dynamin-like proteins as well as EST data from Arabidopsis and other plants strongly suggested they were parts of a single gene, referred to hereafter as At3g19730/At3g19720, and that the annotated start codon for At3g19730 and stop codon for At3g19720 represented the true start and stop codons of this gene. This was confirmed by subsequent cDNA isolation and analysis (see below). To establish whether arc5 contained a mutation in this region of the genome, we sequenced the At3g19730/At3g19720 locus in both the arc5 mutant and in wild-type Landsberg erecta. The data showed the presence in_arc5_ of a G-to-A mutation_,_ which altered a tryptophan codon (TGG) to a stop codon (TAG) (Fig.2B). We then introduced a transgene containing the predicted At3g19730/At3g19720 locus plus 1.9 kb and 1.1 kb of the 5′ and 3′ flanking DNA, respectively, into arc5 mutants to determine whether the wild-type DNA could complement the_arc5_ mutation. Microscopic analysis of T1 transgenic plants indicated that the chloroplast division defect in the mutant was fully or partially rescued by the wild-type transgene (Fig. 2G). Differences in gene dosage resulting from position effects probably account for the partial complementation in some individuals (33). Taken together, the point mutation in At3g19730/At3g19720 in arc5, complementation of the mutant phenotype by the wild-type gene, and ability of a fragment from At3g19730/At3g19720 to confer an_arc5_-like phenotype in wild-type plants when expressed in the antisense orientation, indicate that the ARC5 locus and At3g19730/At3g19720 represent the same gene.
Analysis of ARC5 cDNAs and Gene Products.
To gain more information on the ARC5 transcription unit and encoded polypeptide, we used RT-PCR and primers spanning from 93 bp upstream to 152 bp downstream of the predicted At3g19730/At3g19720 start and stop codons, respectively, to amplify the corresponding cDNA. Sequence analysis revealed two distinct cDNA species. The longer cDNA contained a sequence that was spliced out of the shorter cDNA as the 15th intron (Fig. 2B); however, its presence in the longer cDNA did not interrupt the reading frame. More than half of the cDNA clones recovered were of this type. This cDNA encoded a protein of 777 aa (Fig. 3A) and 87.2 kDa. The protein can be aligned over its entire length with numerous members of the dynamin family and contains three motifs found in other dynamin-like proteins: a conserved N-terminal GTPase domain, a pleckstrin homology (PH) domain shown in some proteins to mediate membrane association, and a C-terminal GTPase effector domain thought to interact directly with the GTPase domain and to mediate self-assembly (32, 34) (Fig. 3A). The shorter cDNA encoded a protein of 741 aa and 83.5 kDa identical to that of the larger gene product except for the absence of 36 aa encoded by the sequence of the 15th intron (Figs. 2B and3A). These results suggest that the _ARC5_transcript is alternatively spliced. Alternative splicing of dynamin genes in several other organisms has also been documented (34). Because the shorter gene product lacks a portion of the PH domain (Fig.3A), the proteins encoded by the two splice variants may have different activities or localization patterns.
Phylogenetic analysis was performed to investigate the relationship between ARC5 and other members of the dynamin family of proteins. Only full-length sequences were used, although EST data indicate that related proteins are present in many plants and in green algae (not shown). ARC5 clustered with a group of proteins found in plants, but was in a distinct clade from other dynamin-like proteins in_Arabidopsis_ with functions in cell-plate formation and mitochondrial division (35, 36) (Fig. 3B). Surprisingly, the ARC5-like proteins clustered near ADL6, another _Arabidopsis_dynamin-like protein involved in vesicle trafficking from the transGolgi network to the vacuole in plants (37). This suggests that ARC5 and ADL6 could share a common dynamin-like ancestor.
Based on the similarity of ARC5 to dynamin and its relatives (Fig.3A), we conclude that ARC5 represents a new class of a dynamin-like proteins that functions specifically in chloroplast division.
Localization of ARC5.
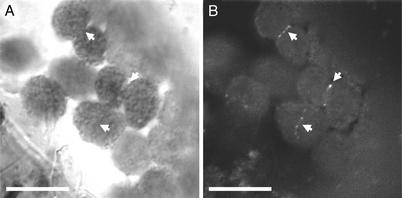
We expressed a GFP–ARC5 fusion protein in transgenic plants to investigate the subcellular localization of ARC5. Because overexpression of chloroplast FtsZ proteins can result in a dominant-negative phenotype (10), we used the native _ARC5_promoter to create the GFP–ARC5 transgene for expression in wild-type plants (Col-0). Fluorescence microscopy showed that the fusion protein was localized in a ring-like pattern at the site of the chloroplast constriction (Fig. 4). This ring could be faintly detected in unconstricted chloroplasts, suggesting that ARC5 may act at an earlier stage of division than previously hypothesized (21, 22). However, ARC5 is not required for FtsZ ring formation, the earliest known event in the assembly of the chloroplast division apparatus (18, 19, 49), because the FtsZ ring can be detected in the arc5 mutant (not shown). The GFP–ARC5 fusion protein was most obvious in visibly constricted chloroplasts, perhaps as a consequence of ring thickening during constriction. Similar localization patterns have been described for FtsZ1 and FtsZ2 (10). However, in contrast to the FtsZ rings, which are fairly uniform in fluorescence intensity, the GFP–ARC5 ring was more speckled in appearance (Fig. 4), suggesting that ARC5 localization at the division site may be discontinuous or that the ARC5-containing ring is not of uniform composition.
Figure 4.
GFP–ARC5 is localized to the constriction site of dividing chloroplasts. (A) Bright-field. (B) GFP fluorescence. Arrows indicate corresponding positions in the two images. (Bars, 10 μm.)
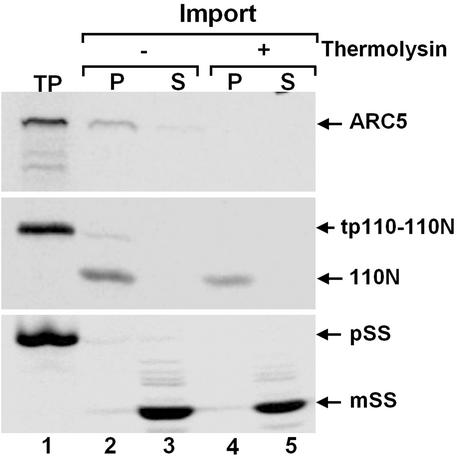
Even though ARC5 mediates chloroplast division, it is not predicted by subcellular targeting prediction programs to be imported to the chloroplast (results not shown). To further define the topology of the ARC5-containing ring with respect to the chloroplast envelope membranes, we used in vitro chloroplast import and protease protection assays. A radiolabeled translation product corresponding to the longer ARC5 cDNA was generated by coupled transcription/translation (Fig. 5 Top, lane 1), then incubated with isolated pea chloroplasts (Fig. 5 Top, lanes 2–5). Subsequent fractionation of the chloroplasts indicated that the translation product was associated with the membrane fraction, but was not processed (Fig. 5 Top, lanes 2 and 3). The binding of the ARC5 translation product to isolated chloroplasts may be effected in part by the PH domain, which has been shown to mediate lipid binding of other dynamin-like proteins (34, 38). In contrast, two chloroplast-targeted control proteins, one localized to the inner envelope (Fig. 5 Middle) and the other to the stroma (Fig. 5 Bottom), were processed on import, consistent with the presence of N-terminal transit peptides, and associated with the membrane and soluble chloroplast fractions, respectively (Fig. 5 Middle and Bottom, lanes 2 and 3). In addition, the two control proteins were both protected from proteolysis by thermolysin, which does not penetrate the outer envelope (39) (Fig. 5 Middle and Bottom, lanes 4 and 5), whereas the ARC5 translation product was fully degraded by this protease (Fig.5 Top, lanes 4 and 5). These data provide evidence that the ARC5-containing ring represented by the GFP–ARC5 fusion protein is situated on the cytosolic surface of the outer chloroplast envelope membrane. The position of ARC5 on the chloroplast surface is topologically equivalent to that of Dnm1p, a dynamin-like protein that mediates mitochondrial division in yeast (40).
Figure 5.
ARC5 is on the outside surface of chloroplast. Radiolabeled ARC5 (Top), tp110–110N, the precursor of the inner envelope marker protein 110N (Middle), and pSS, the precursor of the stromal marker protein mSS (Bottom), were produced by coupled in vitro transcription/translation and subsequently incubated with isolated pea chloroplasts (7). Chloroplasts were recovered by centrifugation and incubated with (+) or without (−) thermolysin. Intact chloroplasts were again recovered and fractionated into membrane (P) and soluble (S) fractions. TP, translation product.
Discussion
Although the arc5 mutation introduces a premature stop codon, the mutant phenotype probably results from a decreased rate of constriction rather than from a complete block in the process. This conjecture is based on the finding that_arc5_/arc1 double mutants have more and smaller dumbbell-shaped chloroplasts than does arc5, indicating that_arc5_ chloroplasts are capable of completing constriction (21, 23). The slow but continued chloroplast division in_arc5_ may be caused by the presence of a second_ARC5_ homologue (At1g53140) (Fig. 3B) in a duplicated region of the Arabidopsis genome (41) whose function might overlap to some extent with that of ARC5. Alternatively, it is conceivable that the arc5 gene product, although severely truncated, could retain partial activity because the mutation occurs just downstream of the GTPase domain. In either case, because total chloroplast volume is maintained as a constant proportion of cell size both in wild-type Arabidopsis plants and in mutants with reduced chloroplast numbers (30), the enlarged size of_arc5_ chloroplasts can probably be explained by sustained expansion of slowly constricting chloroplasts until the prescribed volume is reached.
Previous studies of the arc5 mutant phenotype have suggested that the gene product functions in the constriction of chloroplasts, but not of undifferentiated proplastids (22). This could be the result of reduced gene dosage in the mutant; expression of the _ARC5_homologue in arc5 mutants may be sufficient to maintain the division of the few, small proplastids in the meristem, whereas higher levels of ARC5 and its homologue may be required for division of the much larger chloroplasts during leaf expansion. The observation that all of the plastid division genes identified thus far are present in only one or two copies in the Arabidopsis genome suggests that the same gene products function in the division of all plastid types. Future analyses of protein levels and accumulation patterns for ARC5, its homologue, and other plastid division proteins will shed light on this issue.
Dynamin and its relatives are large GTPases that participate in a variety of organellar fission and fusion events in eukaryotes, including budding of endocytic and Golgi-derived vesicles, mitochondrial fission, mitochondrial fusion, and plant cell plate formation (reviewed in refs. 32 and 34). Dynamin has also been shown to regulate actin assembly and organization at membranes (42). ARC5 defines a new class of dynamin-like proteins that function specifically in plastid division, and its identification extends the range of cellular processes in which dynamin-like proteins participate. The molecular mechanisms by which this large group of GTPases function are still uncertain. Recent structural data (43, 44), along with studies showing that dynamin self-assembles into rings (45) and can tubulate and vesiculate liposomes in vitro (46, 47), support proposals that dynamin forms a collar around the neck of budding vesicles during endocytosis and acts as a GTP-stimulated, force-generating “constrictase.” However, other studies provide evidence that dynamin family members function as molecular switches, analogous to classical signaling GTPases (48). Functional investigations of dynamin and its relatives will clearly be relevant to future studies aimed at determining the biochemical mechanism underlying ARC5 activity during chloroplast division.
The localization of ARC5 on the outer envelope surface at the site of chloroplast constriction parallels that of the yeast dynamin-related protein Dnm1p and its orthologues in animals and plants, which localize to the mitochondrial division site on the cytosolic side of the outer mitochondrial membrane (32, 34, 36, 40). This observation suggests that ARC5 is the chloroplastic counterpart of these mitochondrial division proteins. However, mitochondrial and chloroplast division are not equivalent processes. Chloroplast division requires the activity of several prokaryotically derived proteins in the chloroplast stroma, including FtsZ1, FtsZ2, MinD, MinE, and ARTEMIS (6, 7, 11–14, 49), whereas related molecules are lacking in the mitochondria of animals, plants, and fungi, and no matrix-localized mitochondrial division proteins have been identified in these organisms.
Interestingly, the topology of the ARC5-containing ring is similar to that of the outer PD ring (15, 50). Like ARC5, which lacks homologues in cyanobacteria and was probably derived from the eukaryotic host cell, the outer PD ring has been proposed to be of eukaryotic origin (19). Thus, ARC5 could be an outer PD ring constituent. Furthermore, the outer PD ring is composed of filaments ≈5 nm in diameter (50), which is close to the diameter of previously observed dynamin strands (25). These similarities suggest that the outer PD ring filaments could be composed of ARC5. However, it has been suggested that the filament protein is an unidentified polypeptide of 56 kDa (50), which is considerably smaller than ARC5. Irrespective of the relationship between ARC5 and the outer PD ring, our finding that a dynamin-related protein functions in chloroplast division indicates that the chloroplast division machinery is partly of prokaryotic and partly of eukaryotic origin, and shares at least one component in common with the machineries regulating fission of other eukaryotic organelles. Identification of this new chloroplast division protein will facilitate studies aimed both at further dissection of the plastid division machinery and at understanding how the activities of protein complexes separated by the two envelope membranes are coordinated to achieve constriction of the organelle.
Supplementary Material
Supporting Table
Acknowledgments
We thank Kevin Pyke, University of Nottingham, and the_Arabidopsis_ Biological Resource Center, Ohio State University, for arc5 seeds, Stanislav Vitha for advice on microscopy, Kevin Stokes for help with phylogenetic analysis, Magali Tshiamala for technical assistance, and all of the members of the laboratory for helpful comments. This work was supported by a grant from the Michigan State University Intramural Research Grants Program.
Abbreviations
PD
plastid-dividing
PH
pleckstrin homology
Footnotes
Data deposition: The cDNA sequence reported in this paper has been deposited in the GenBank database (accession no. AY212885).
See commentary on page 3557.
References
- 1.Cavalier-Smith T. Trends Plant Sci. 2000;5:174–182. doi: 10.1016/s1360-1385(00)01598-3. [DOI] [PubMed] [Google Scholar]
- 2.Martin W, Stoebe B, Goremykin V, Hansmann S, Hasegawa M, Kowallik K V. Nature. 1998;393:162–165. doi: 10.1038/30234. [DOI] [PubMed] [Google Scholar]
- 3.Osteryoung K W, Vierling E. Nature. 1995;376:473–474. doi: 10.1038/376473b0. [DOI] [PubMed] [Google Scholar]
- 4.Osteryoung K W, Stokes K D, Rutherford S M, Percival A L, Lee W Y. Plant Cell. 1998;10:1991–2004. doi: 10.1105/tpc.10.12.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strepp R, Scholz S, Kruse S, Speth V, Reski R. Proc Natl Acad Sci USA. 1998;95:4368–4373. doi: 10.1073/pnas.95.8.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujiwara M, Yoshida S. Biochem Biophys Res Commun. 2001;287:462–467. doi: 10.1006/bbrc.2001.5588. [DOI] [PubMed] [Google Scholar]
- 7.McAndrew R S, Froehlich J E, Vitha S, Stokes K D, Osteryoung K W. Plant Physiol. 2001;127:1656–1666. [PMC free article] [PubMed] [Google Scholar]
- 8.Mori T, Kuroiwa H, Takahara M, Miyagishima S, Kuroiwa T. Plant Cell Physiol. 2001;42:555–559. doi: 10.1093/pcp/pce095. [DOI] [PubMed] [Google Scholar]
- 9.Osteryoung K W, McAndrew R S. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:315–333. doi: 10.1146/annurev.arplant.52.1.315. [DOI] [PubMed] [Google Scholar]
- 10.Vitha S, McAndrew R S, Osteryoung K W. J Cell Biol. 2001;153:111–119. doi: 10.1083/jcb.153.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colletti K S, Tattersall E A, Pyke K A, Froelich J E, Stokes K D, Osteryoung K W. Curr Biol. 2000;10:507–516. doi: 10.1016/s0960-9822(00)00466-8. [DOI] [PubMed] [Google Scholar]
- 12.Itoh R, Fujiwara M, Nagata N, Yoshida S. Plant Physiol. 2001;127:1644–1655. [PMC free article] [PubMed] [Google Scholar]
- 13.Maple J, Chua N H, Moller S G. Plant J. 2002;31:269–277. doi: 10.1046/j.1365-313x.2002.01358.x. [DOI] [PubMed] [Google Scholar]
- 14.Fulgosi H, Gerdes L, Westphal S, Glockmann C, Soll J. Proc Natl Acad Sci USA. 2002;99:11501–11506. doi: 10.1073/pnas.172032599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuroiwa T, Kuroiwa H, Sakai A, Takahashi H, Toda K, Itoh R. Int Rev Cytol. 1998;181:1–41. doi: 10.1016/s0074-7696(08)60415-5. [DOI] [PubMed] [Google Scholar]
- 16.Miyagishima S, Itoh R, Toda K, Takahashi H, Kuroiwa H, Kuroiwa T. J Electron Microsc. 1998;47:269–272. [Google Scholar]
- 17.Miyagishima S, Kuroiwa H, Kuroiwa T. Planta. 2001;212:517–528. doi: 10.1007/s004250000426. [DOI] [PubMed] [Google Scholar]
- 18.Miyagishima S, Itoh R, Toda K, Kuroiwa H, Kuroiwa T. Planta. 1999;207:343–353. doi: 10.1007/s004250050645. [DOI] [PubMed] [Google Scholar]
- 19.Miyagishima S, Takahara M, Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T. Plant Cell. 2001;13:2257–2268. doi: 10.1105/tpc.010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuroiwa H, Mori T, Takahara M, Miyagishima S Y, Kuroiwa T. Planta. 2002;215:185–190. doi: 10.1007/s00425-002-0734-4. [DOI] [PubMed] [Google Scholar]
- 21.Pyke K A, Leech R M. Plant Physiol. 1994;104:201–207. doi: 10.1104/pp.104.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson E J, Rutherford S M, Leech R M. Plant Physiol. 1996;112:149–159. doi: 10.1104/pp.112.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marrison J L, Rutherford S M, Robertson E J, Lister C, Dean C, Leech R M. Plant J. 1999;18:651–662. doi: 10.1046/j.1365-313x.1999.00500.x. [DOI] [PubMed] [Google Scholar]
- 24.Jander G, Norris S R, Rounsley S D, Bush D F, Levin I M, Last R L. Plant Physiol. 2002;129:440–450. doi: 10.1104/pp.003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klockow B, Tichelaar W, Madden D R, Niemann H H, Akiba T, Hirose K, Manstein D J. EMBO J. 2002;21:240–250. doi: 10.1093/emboj/21.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson J D, Higgins D G, Gibson T J. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swofford D L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods) Sunderland, MA: Sinauer Associates; 1998. , Version 4.0b10. [Google Scholar]
- 29.Davis S J, Vierstra R D. Plant Mol Biol. 1998;36:521–528. doi: 10.1023/a:1005991617182. [DOI] [PubMed] [Google Scholar]
- 30.Pyke K A. Plant Cell. 1999;11:549–556. doi: 10.1105/tpc.11.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gleave A P. Plant Mol Biol. 1992;20:1203–1207. doi: 10.1007/BF00028910. [DOI] [PubMed] [Google Scholar]
- 32.Danino D, Hinshaw J E. Curr Opin Cell Biol. 2001;13:454–460. doi: 10.1016/s0955-0674(00)00236-2. [DOI] [PubMed] [Google Scholar]
- 33.Hooykaas P J, Schilperoort R A. Plant Mol Biol. 1992;19:15–38. doi: 10.1007/BF00015604. [DOI] [PubMed] [Google Scholar]
- 34.Hinshaw J E. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu X, Verma D P. EMBO J. 1996;15:695–704. [PMC free article] [PubMed] [Google Scholar]
- 36.Arimura S-i, Tsutsumi N. Proc Natl Acad Sci USA. 2002;99:5727–5731. doi: 10.1073/pnas.082663299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin J B, Kim Y A, Kim S J, Lee S H, Kim D H, Cheong G W, Hwang I. Plant Cell. 2001;13:1511–1526. doi: 10.1105/TPC.000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S H, Jin J B, Song J, Min M K, Park D S, Kim Y W, Hwang I. J Biol Chem. 2002;277:31842–31849. doi: 10.1074/jbc.M204770200. [DOI] [PubMed] [Google Scholar]
- 39.Cline K, Werner-Washburne M, Andrews J, Keegstra K. Plant Physiol. 1984;75:675–678. doi: 10.1104/pp.75.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bleazard W, McCaffery J M, King E J, Bale S, Mozdy A, Tieu Q, Nunnari J, Shaw J M. Nat Cell Biol. 1999;1:298–304. doi: 10.1038/13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arabidopsis Genome Initiative. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- 42.Schafer D A, Weed S A, Binns D, Karginov A V, Parsons J T, Cooper J A. Curr Biol. 2002;12:1852–1857. doi: 10.1016/s0960-9822(02)01228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niemann H H, Knetsch M L, Scherer A, Manstein D J, Kull F J. EMBO J. 2001;20:5813–5821. doi: 10.1093/emboj/20.21.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang P, Hinshaw J E. Nat Cell Biol. 2001;3:922–926. doi: 10.1038/ncb1001-922. [DOI] [PubMed] [Google Scholar]
- 45.Hinshaw J E, Schmid S L. Nature. 1995;374:190–192. doi: 10.1038/374190a0. [DOI] [PubMed] [Google Scholar]
- 46.Takel K, McPherson P S, Schmid S L, De Camilli P. Nature. 1995;374:186–190. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- 47.Sweitzer S M, Hinshaw J E. Cell. 1998;93:1021–1029. doi: 10.1016/s0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- 48.Sever S. Curr Opin Cell Biol. 2002;14:463–467. doi: 10.1016/s0955-0674(02)00347-2. [DOI] [PubMed] [Google Scholar]
- 49.Osteryoung K W. Curr Opin Microbiol. 2001;4:639–646. doi: 10.1016/s1369-5274(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 50.Miyagishima S, Takahara M, Kuroiwa T. Plant Cell. 2001;13:707–721. doi: 10.1105/tpc.13.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Table