CC Chemokines Mediate Leukocyte Trafficking into the Central Nervous System during Murine Neurocysticercosis: Role of γδ T Cells in Amplification of the Host Immune Response (original) (raw)
Abstract
According to a previous report, the degree of the host immune response highly correlates with severity of the disease in the murine model for neurocysticercosis. In wild-type mice, Mesocestoides corti infection induced a rapid and extensive accumulation of γδ T cells and macrophages in the brain. NK cells, dendritic cells, αβ T cells, and B cells were also recruited to the brain but at lower levels. In contrast, γδ T-cell-deficient mice exhibited decreased cellular infiltration and reduced central nervous system (CNS) pathology. To understand the mechanisms of leukocyte recruitment into the CNS, chemokine expression was analyzed in infected brains in the present study. MCP-1 (CCL2), MIP-1α (CCL3), and MIP-1β (CCL4) were up-regulated within 2 days after M. corti infection. Protein expression of RANTES (CCL5), eotaxin (CCL11), and MIP-2 was detected later, at 1 week postinfection. Correlating with the decreased cellular infiltration, delta chain T-cell receptor-deficient (TCRδ−/−) mice exhibited substantially reduced levels of most of the chemokines analyzed (with the exception of eotaxin). The results suggest that γδ T cells play an important role in the CNS immune response by producing chemokines such as MCP-1 and MIP-1α, enhancing leukocyte trafficking into the brain during murine neurocysticercosis.
Chemokines represent members of a class of chemotactic cytokines that mediate their function by signaling through seven transmembrane G-protein-coupled receptors (reviewed in reference 46). Chemokines were initially defined as modulators of leukocyte trafficking and positioning within tissues, which are fundamental requirements for effective immunity. More recently it has been discovered that chemokines are involved in inflammatory responses including leukocyte degranulation and mediator release as well as angiogenesis or angiostasis (46). There are approximately 40 to 50 chemokines that have been described and classified into four families (CC, CXC, C, and CX3C4) on the basis of spacing of cysteine residues at the amino terminus (5, 6, 46, 58). The CXC chemokines predominantly target neutrophils and subsets of T cells, whereas the CC chemokines target a variety of cell types, including T cells, macrophages, eosinophils, and basophils (46, 58).
Chemokine expression has been demonstrated to correlate with inflammatory pathology in neurological diseases (54), autoimmune diseases (28, 41), and infectious diseases (15, 26, 37, 38, 50, 59). Neurocysticercosis (NCC) is the most common parasitic disease of the human central nervous system (CNS) and is caused by the presence of Taenia solium metacestodes in the brain (19, 57, 72, 74). Seizures are the most common clinical manifestation associated with NCC (20, 73), and less common symptoms include headache, increased intracranial pressure, and altered mental state (18, 20, 60, 65). CNS infection with Mesocestoides corti has been used as a model for NCC (12). The CNS immune response in mice was characterized by the induction of severe CNS pathology and a massive recruitment of γδ T cells and macrophages (12, 13). It was demonstrated that γδ T cells regulate the development of the inflammatory response in the brain by producing type 1 cytokines (13). Furthermore, γδ T-cell-deficient mice exhibited decreased cellular infiltration and reduced CNS pathology. Therefore, γδ T cells appear to play a crucial role in the immunopathogenesis of murine NCC.
To understand the mechanisms involved in leukocyte recruitment in the brain, we performed a kinetic study to determine the chemokines induced in infected wild-type and delta chain T-cell receptor-deficient (TCRδ−/−) mice. The results suggest that CC chemokines are key players in leukocyte infiltration into the CNS and that γδ T cells can contribute by producing requisite chemokines.
MATERIALS AND METHODS
Mice.
Female 3- to 5-week-old C57BL/6 and TCRδ−/− mice on the C57BL/6 background were purchased from the Jackson Laboratory (Bar Harbor, Maine). Animal experiments were conducted under the guidelines of the University of Texas System, the U.S. Department of Agriculture, and the National Institutes of Health.
Parasites and inoculations.
M. corti metacestodes were maintained by serial intraperitoneal (i.p.) inoculations. Intracranial inoculations were performed as described previously (12).
Tissue processing.
The brain was immediately removed from perfused animals, embedded in optimal cutting temperature medium (O.C.T.), and snap-frozen as described previously (12, 13). Serial horizontal cryosections 10 μm in thickness were placed on silane prep slides (Sigma Biosciences, St. Louis, Mo.). One in every four slides was fixed in formalin for 12 min at room temperature and stained with hematoxylin and eosin. The remainder of the slides were air dried over night and fixed in fresh acetone for 20 s at room temperature. Acetone-fixed sections were wrapped in aluminum foil and stored at −80°C or processed immediately for immunohistochemistry or immunofluorescence.
Antibodies.
Anti-mouse biotinylated antibodies included GL3 (pan anti-γδ), 4E2/MCP (anti-MCP-1) purchased from Pharmingen (San Diego, Calif.), and anti-mouse MIP-2 and CRG-2 obtained from R&D Systems. Polyclonal antibodies against mouse MIP-1α, MIP-1β, and RANTES were also purchased from R&D Systems. The anti-mouse polyclonal antibody against eotaxin was purchased from Serotec. All antibodies were titrated in spleen sections from mice infected i.p. to determine optimal dilutions. Spleen sections were used as positive controls in all experiments. Negative controls consisted of sections from mock-infected mice that were injected intracerebrally with Hanks balanced salt solution (HBSS) (the buffer used in the parasite inoculum). In addition, negative staining controls from which the primary antibody was excluded were always included.
Immunohistochemistry.
Staining was performed as described previously (12, 13). For the purified antibodies, indirect immunohistochemistry was performed using specific biotinylated secondary antibodies obtained from Kirkegaard & Perry Laboratories (Gaithersburg, Md.). Positive cells were counted at a magnification of ×600, and results were scored as 1 to 100, 100 to 300, 300 to 500, and >500 positive cells per section.
Double immunofluorescence.
Double-immunofluorescence assays were performed in brain cryosections to detect chemokines produced by γδ T cells. All incubations were carried out at room temperature, and the slides were washed six times for 5 min each time. Sections were incubated first with the phycoerythrin-conjugated red fluorescent GL3 antibody (Pharmingen) for 1 h and then washed and incubated with biotinylated anti-MCP-1 or purified anti-MIP-1α antibody. The biotinylated antibody treatment was followed by incubation with streptavidin Alexa Fluor 488 conjugate (green fluorescence) (Molecular Probes) for 30 min. The purified anti-MIP-1α antibody was detected using green fluorescent anti-goat immunoglobulin G-Cy2 conjugate (Jackson Laboratories, West Grove, Pa.). Sections were then washed in phosphate-buffered saline (PBS) and mounted using FluorSave reagent (Calbiochem, La Jolla, Calif.) containing 0.3 μM 4′,6′-diamidino-2-phenylindole (DAPI)-dilactate (Molecular Probes).
Lymphocyte preparation.
Brain lymphocytes were isolated from C57BL/6 and TCRδ−/− mice 3 weeks after M. corti infection. The brains were removed as described previously. Each perfused brain was minced gently through a fine-mesh nylon screen using a syringe plunger and collected into 10 ml of HBSS containing 0.05% collagenase D (Boehringer Mannheim), 0.1 μg of TLCK (_N_α-_p_-tosyl-l-lysine chloromethyl ketone) (Sigma)/ml, 10 μg of DNase I (Sigma)/ml, and 10 mM HEPES buffer (pH 7.4) (Invitrogen, Carlsbad, Calif.). The mixture was rocked at room temperature for 45 min and then allowed to settle for 20 min. The supernatant was collected and pelleted at 200 × g for 5 min and resuspended in 5 ml of HBSS without Ca2+ and Mg2+. The suspension was layered onto 10 ml of a density gradient containing three parts of Ficoll-Hypaque (Amersham Pharmacia Biotech, Piscataway, N.J.) and one part of RPMI medium (Invitrogen) supplemented with 10% fetal bovine serum (Sigma), 10 mM HEPES (Invitrogen), and 50 μg gentamicin (Invitrogen)/ml. Each gradient was centrifuged at 300 × g for 30 min at room temperature. The overlaying medium and interface of tissue debris were removed. The entire 10 ml of gradient medium was then diluted fivefold with HBSS and centrifuged at 300 × g for 10 min. Cells were then washed three times in 1 ml of 0.1% bovine serum albumin in PBS and counted.
Peritoneal lymphocytes were isolated from C57BL/6 mice infected i.p. at 3 to 5 weeks postinfection (p.i.). The peritoneal cavity was washed with 15 ml of HBSS, and the cellular preparation was filtered through a 150-μm-pore-size nylon screen to remove parasites. Cells were centrifuged at 300 × g for 10 min, washed three times in 0.3% bovine serum albumin in PBS, and counted. For positive selection of γδ T lymphocytes, cell suspensions were incubated with 10 μg of Dynabeads M-280 streptavidin (Dynal, Lake Success, N.J.)/ml previously coated with 500 μg of biotinylated GL3 antibody (Pharmingen)/ml for 20 min at 4°C. Labeled cells were then selected using the Dynal magnetic particle concentrator and washed eight times according to the manufacturer's instructions. Purified populations of γδ T cells were found to be >95 to 97% pure on the basis of subsequent immunocytochemistry analyses.
RNase protection assay (RPA).
Using Trizol reagent (Invitrogen) according to the manufacturer's instructions, total RNA was extracted from brain lymphocytes and from positively selected γδ T cells. [α-33P]UTP-labeled antisense RNA transcripts for the chemokines were generated using the template set mCK-5 (Pharmingen). Total RNA (5 to 10 mg) was allowed to hybridize to the labeled probe for 12 to 16 h at 56°C. An RNase T1 mixture was used to digest single-stranded RNA, and the hybrids were analyzed on denaturing urea-polyacrylamide gels. Protected fragments were visualized by autoradiography and analyzed by densitometry. The results were analyzed by normalizing the values of each chemokine to the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene. The ratio of infected cells/HBSS control cells was obtained, and the data are presented as severalfold increase for each chemokine.
RESULTS
M. corti infection of the CNS.
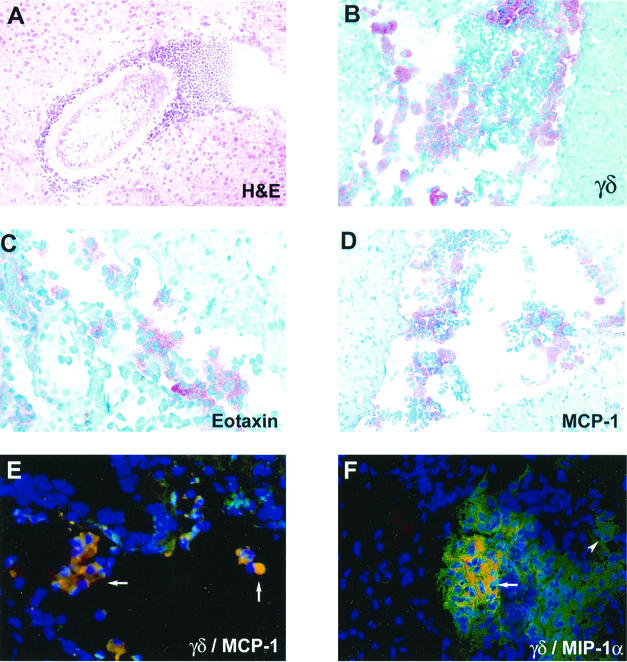
As reported previously (12, 13), infection of the CNS by M. corti leads to an infiltration of mononuclear cells by day 2 p.i. The parasites initially invade the extraparenchymal spaces of the brain such as the ventricles and subarachnoid spaces. However, by 3 weeks p.i. there are nearly equal numbers of parasites in the extraparenchymal and parenchymal regions of the brain. The cellular infiltrate either surrounds the parasite (Fig. 1A) or appears to be moving towards the parasite (12). Importantly, it has been shown that γδ T cells appear to be the dominant T-cell population in this infection and appear by day 2 to 3 p.i. (12). Figure 1B exhibits the results of an immunocytochemistry assay, showing an accumulation of γδ T cells into an extraparenchymal area of the brain.
FIG. 1.
Cellular infiltration and chemokine expression in the CNS during murine NCC. (A) Hematoxylin and eosin (H&E)-stained brain section from C57BL/6 mouse at 3 weeks p.i. (B) γδ T cells from C57BL/6 brain infected for 1 week as detected by immunohistochemistry. (C and D) Eotaxin (C)- and MCP-1 (D)-expressing cells from C57BL/6 brain cryosections from infected mice on day 3 as detected by immunocytochemistry. (E and F) Double-immunofluorescence assays were performed on brain cryosections of wild-type mice at 1 week p.i.; merged pictures are shown. MCP-1 (E) and MIP1-α (F) producing γδ T cells in a ventricular infiltrate are shown in yellow as double positive (arrows). γδ T cells (single positive) were detected with the GL3-phycoerythrin-conjugated antibody (red fluorescence), and chemokine-producing cells (single positive) were detected with a green fluorescent conjugate. The arrowhead points to single-positive cells. Cell nuclei visualized in blue (DAPI staining) are shown.
Up-regulation of CC chemokines in the brain after M. corti infection.
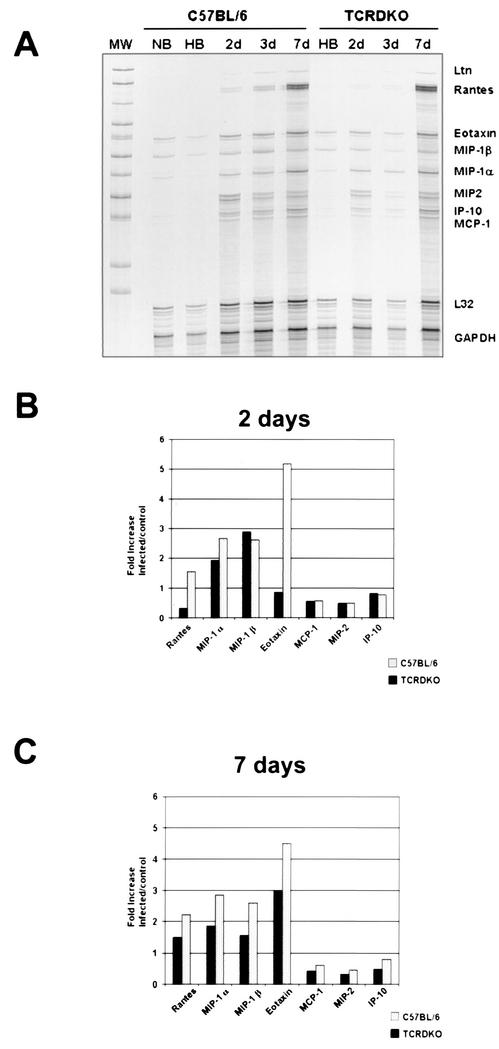
The expression of several chemokines was analyzed using a RPA. Normal mouse brains and HBSS-inoculated controls revealed the presence of low levels of eotaxin, MIP-1α, and MIP-1β (Fig. 2A). In wild-type mice, MIP-1α, MIP-1β, and eotaxin were the predominant transcripts at 2 days p.i. (Fig. 2B). Of the chemokines that are substantially up-regulated at 2 days p.i. in TCRδ−/− mice, mRNAs for MIP-1α and MIP-1β were found in levels similar to those for wild-type mice (Fig. 2B). By 1 week p.i., wild-type mice exhibited an increase in the levels of RANTES, in addition to the sustained predominance of MIP-1α, MIP-1β, and eotaxin (Fig. 2C). In TCRδ−/− mice, in contrast, eotaxin represented the predominant transcript at 1 week p.i. These results indicate that in wild-type mice, the CC chemokines RANTES, MIP-1α, MIP-1β, and eotaxin were the predominant mRNA species present in brain lymphocytes 1 week after M. corti infection. mRNAs for MCP-1, MIP-2, CRG-2, and lymphotactin (LTN) were detected at all of the early time points analyzed, although at less significant levels (severalfold increases of less than 1).
FIG. 2.
Predominance of CC chemokines in the brain after M. corti infection as assayed by RPA. Total RNA obtained from brain lymphocytes was used to detect the expression of several chemokines. (A) Autoradiograph of the RPA using the mCK5 template from Pharmingen at 2, 3, and 7 days (2d, 3d, and 7d, respectively) p.i. Normal brains (NB) and HBSS-inoculated brains (HB) were used as controls. MW, molecular weight markers. (B) Densitometry analysis of the chemokine expression at 2 days p.i. Samples were normalized to the GAPDH gene, and the infected sample cell/HBSS control cell ratios are shown. (C) Densitometry analysis at 7 days p.i. Data represent the values calculated for four mice at each time point.
Predominant expression of CC chemokines in the CNS.
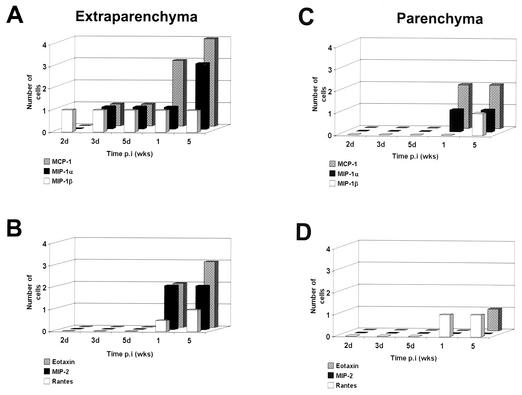
To further examine the chemokine profile present after M. corti infection, in situ immunohistochemistry was performed in brain cryosections. Specific antibodies against MIP-2, CRG-2, RANTES, MIP-1α, MIP-1β, MCP-1, and eotaxin were used. Examples are shown in Fig. 1C (eotaxin) and Fig. 1D (MCP-1); it is interesting that most of the staining is in the infiltrate and not the surrounding brain tissue. At the protein level, all of these chemokines, except CRG-2, were up-regulated in the brain after M. corti infection. CRG-2 protein was undetectable at all of the time points analyzed. In wild-type mice, MIP-1α-, MIP-1β-, and MCP-1-positive cells were detected within 2 to 3 days p.i. in extraparenchymal areas of the brain, and the amount of MIP-1α and MCP-1 increased dramatically after 1 week p.i. (Fig. 3A). Eotaxin, MIP-2, and RANTES were detected after 1 week p.i. (Fig. 3B), and MIP-1β, RANTES, and MIP-2 were consistently present in extraparenchymal areas in all of the wild-type mice analyzed during the course of the infection but at low levels.
FIG. 3.
Chemokine response in the brains of C57BL/6 mice. Mouse brains obtained after intracranial inoculation with M. corti metacestodes were analyzed by immunohistochemistry for the presence of several chemokines. (A and B) Positive cells in extraparenchymal infiltrates (subarachnoid spaces, meninges, and ventricles) were counted, and the number of positive cells was scored as follows: 1, 1 to 100 cells; 2, 100 to 300 cells; and 3, 300 to 500 cells. (C and D) Chemokine-producing cells associated with parenchymal infiltrates. The results indicate the average of results for at least two mice at each time point.
In parenchymal infiltrates, chemokine-producing cells were found with a predominance of MCP-1 expression after 1 week p.i. (Fig. 3C). MIP-1α and RANTES were also detected in the areas of cellular infiltration, and gradients of positive staining for MIP-1α were found in regions not directly associated with parasites. MIP-1β and eotaxin were detected in parenchymal infiltrates only after 5 weeks p.i. (Fig. 3D). MIP-2, however, was not detected in parenchymal infiltrates. HBSS-inoculated controls and normal mouse brains did not reveal the presence of any of the chemokines, as analyzed by immunohistochemistry (data not shown).
Decreased abundance of chemokine-producing cells in the brains of TCRδ−/− mice.
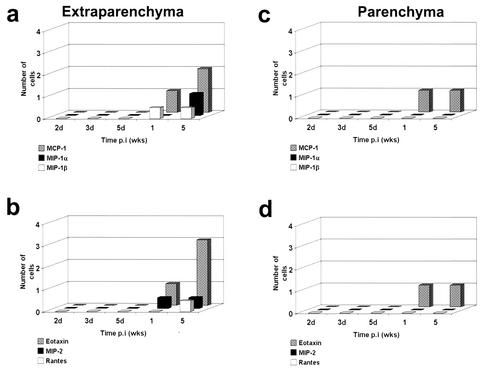
In TCRδ−/− mice, the frequency of MCP-1-, MIP-1α-, MIP-1β-, and RANTES-expressing cells (Fig. 4) was less after M. corti infection compared with that seen with infected normal mice. In all of the wild-type mice, MCP-1-, MIP-1α-, MIP-1β-, RANTES-, and eotaxin-positive cells were found at each of the time points analyzed. In contrast, MIP-1α was detected in only 40% (10 of 25) of the TCRδ−/− mice, MIP-1β was detected in about 1% (3 of 25) of the mice, and RANTES was present in only one mouse (1 of 25). Furthermore, γδ T-cell-deficient mice exhibited a delay in chemokine induction. In extraparenchymal areas of the brain, MIP-1α, MIP-1β, and MCP-1 were detected after 1 week p.i. (Fig. 4a) and RANTES-expressing cells were detected by 5 weeks p.i. Similar to the result for wild-type mice, MIP-2-positive cells were detected after 1 week p.i. However, in contrast to the decreased frequency of the previously described chemokines, in TCRδ−/− mice eotaxin was found in levels comparable to those seen with wild-type mice after 5 weeks p.i. (Fig. 3B). MCP-1 and eotaxin were the chemokines associated with parenchymal parasites in TCRδ−/− mice (Fig. 4c and d). MIP-1α-, MIP-1β-, and RANTES-positive cells were not detected in parenchymal infiltrates (Fig. 4c and d).
FIG. 4.
Chemokine response in the brain of γδ T-cell-deficient mice (TCRδ−/−) mice. Two mouse brains obtained after intracranial inoculation with M. corti metacestodes were analyzed by immunohistochemistry for the presence of several chemokines. (a and b) Positive cells in extra-parenchymal infiltrates (including subarachnoid spaces, meninges, and ventricles) were counted, and the number of positive cells was scored as follows: 1, 1 to 100 cells; 2, 100 to 300 cells; 3, 300 to 500 cells. (c and d) Chemokine-producing cells associated with parenchymal infiltrates.
γδ T cells produce MCP-1 and MIP-1α in the brain during murine NCC.
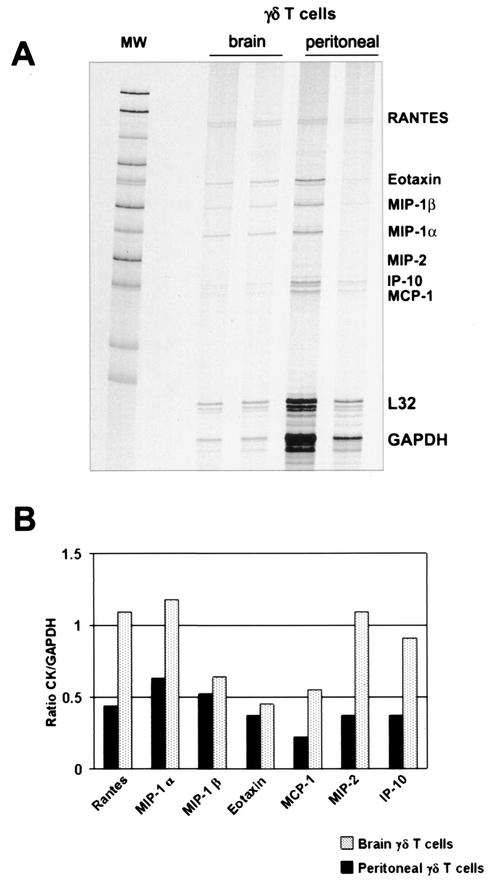
Due to the fact that γδ T cells are specifically recruited into the brain as soon as 2 days p.i. (12), the low abundance of chemokines detected in TCRδ−/− mice suggested that γδ T cells might participate in chemokine production early during infection. To determine the contribution of γδ T cells in chemokine production, a RPA was performed in positively selected γδ T cells isolated from infected brains and from the peritoneal cavity of mice infected i.p. (Fig. 5). Results indicate that both brain and peritoneal γδ T cells have detectable levels of most of the transcripts analyzed. These include RANTES, MIP-1α, MIP-1β, eotaxin, MCP-1, MIP-2, and CRG-2. Of interest is the presence of higher chemokine levels in brain γδ T cells compared to the levels seen with peritoneal γδ T cells (Fig. 5B). To further analyze the role of γδ T cells in chemokine expression, double-immunofluorescence assays for two chemokines in brain cryosections of _M. corti_-infected C57BL/6 mice were performed at 1 week p.i. The results indicate that in the brain, γδ T cells are associated with production of both MCP-1 (Fig. 1E) and MIP-1α (Fig. 1F). γδ T cells represented a predominant source of MCP-1 production, as most of the γδ T cells appeared as double positive. The present data suggest that γδ T cells contributed in the development of an inflammatory response by producing chemokines and enhancing lymphocyte recruitment into the CNS.
FIG. 5.
Chemokine analysis in isolated γδ T cells. Using an RPA, brain and peritoneal γδ T cells were analyzed for the expression of several chemokines. (A) Autoradiograph of total RNA from γδ T lymphocytes. MW, molecular weight markers. (B) Densitometry analysis of chemokine (CK) expression. Samples were normalized to the GAPDH to indicate relative mRNA abundance.
DISCUSSION
The immune responses detected in human NCC range from complete absence to a severe inflammatory reaction (52). In most cases, viable parasites have little surrounding inflammation (73), which correlates with an asymptomatic stage of NCC. In contrast, virtually all cases of symptomatic disease are characterized by prominent immunological responses in host nervous tissue (16, 31, 56).
In similarity to human NCC, the degree of the host response in mice highly correlates with the severity of the disease (12). By producing type 1 cytokines, γδ T cells appear to be critical players in the CNS immune response in murine infections (13). Furthermore, adoptive transfer experiments demonstrated that γδ T cells cause an increased accumulation of leukocytes in the brains of infected mice. γδ T cells were also found in the brains of NCC patients (J. I. Alvarez and J. M. Teale, unpublished data). Therefore, the murine model represents a valuable approach to further understand the immunopathogenesis of the human disease.
In the present study, the chemokine profile induced by M. corti infection indicates that chemokines such as MCP-1, MIP-1α, MIP-1β, and RANTES are involved in the infiltration of leukocytes into the brain. Although cells resident in the CNS are also involved in chemokine production, the vast majority of chemokine expression appears to be associated with the infiltration cells (Fig. 1C and D). This is a critical point that we are exploring further. In correlation with the early cellular infiltration observed in infected wild-type mice, MCP-1, MIP-1α, and MIP-1β were detected in the brain within 2 to 3 days p.i. (Fig. 3A). Given the fact that these chemokines were induced early, they are probably critical for the initiation of cellular infiltration in the CNS. MCP-1, MIP-1α, and MIP-1β target monocytes/macrophages, NK cells, T lymphocytes, and dendritic cells (6, 58). All of these cell types were found to infiltrate the CNS of wild-type infected mice. MIP-1α and MIP-1β also participate in the recruitment of B cells to the sites of inflammation (6, 58). Eotaxin, RANTES, and MIP-1α induce eosinophil cell migration (58). Even though immune responses against parasites are associated with abundant eosinophil infiltration, the cellular infiltrates in the brain displayed low numbers of eosinophils throughout the course of the infection in wild-type and TCRδ−/− mice.
MIP-2 and CRG-2 were the CXC chemokines analyzed during this infection. MIP-2 is a chemokine that does not have a human homologue, but it represents the interleukin-8 (IL-8) counterpart by its selective ability to influence the recruitment of neutrophils in mice (69). Even though MIP-2 was not a predominant chemokine expressed in the brain, it appears to influence the accumulation of neutrophils, as they were clearly detected during murine NCC, albeit in relatively small numbers, by GR1+ staining and morphology in the brain (13). Protein expression for CRG-2, the murine homologue of human interferon-inducible protein 10 (IP-10), was not detected. Absence of CRG-2 is puzzling, as large amounts of gamma interferon are present in this infection, a cytokine involved in up-regulation of IP-10. The absence of CRG-2 protein may be due to sensitivity issues when very low amounts were present. However, CRG-2 does not appear to play a major role in murine NCC.
In parenchymal infiltrates, the abundance of chemokine expression appears delayed. This correlates with the delayed appearance of both the parasite and inflammatory infiltrates in the parenchyma versus the nonparenchymal areas of the brain such as the ventricles and subarachnoid spaces (12). Presumably, this is the result of the additional barrier of the parenchyma itself. Although the chemokine response was less prominent in the parenchyma, MIP-1α, MIP-1β, RANTES, MCP-1, and eotaxin were associated with the cellular infiltrates closely associated with parenchymal parasites in wild-type mice. In contrast, low numbers of MCP-1- and eotaxin-positive cells were associated with parenchymal parasites in TCRδ−/− mice. Due to the fact that infiltration of inflammatory cells into the CNS in both extraparenchymal and parenchymal areas of the infected brain was a hallmark of the immune response in wild-type mice, CC chemokines appear fundamental for the leukocyte accumulation in _M. corti_-infected brains.
The results demonstrate a strong correlation between chemokine expression and the magnitude of the inflammatory response in the brain. TCRδ−/− mice, which exhibited little inflammatory response, also displayed a decreased abundance of chemokine expression. This finding, together with that of the predominance of γδ T cells in wild-type mice, suggested that γδ T cells might be important in the early production of the CC chemokines. Using double-immunofluorescence and mRNA analyses, it was demonstrated that γδ T cells participate in the production of MCP-1 and MIP-1α in the brain during murine NCC. This finding is of relevance, since monocytes (11), fibroblasts (36), and endothelial cells are the predominant cellular sources of MCP-1 (51, 66). Furthermore, astrocytes and perivascular microglia have been identified as the major cellular sites of MCP-1 and MIP-1α production in the brain (30, 41, 63). Of particular interest is the observation that during experimental autoimmune encephalomyelitis (EAE), SJL mice that have been depleted of γδ T cells exhibited reduced expression of MIP-1α and MCP-1 at disease onset (53). In EAE, therefore, γδ T cells might also function as a potent source of these chemokines that influence the trafficking of inflammatory cells into the CNS.
γδ T cells represent a minor population of circulating T cells; however, they are abundant in mucosal tissues (8, 34). The involvement of γδ T cells in a wide variety of infectious and autoimmune diseases has demonstrated the important role of this T-cell subset in innate and acquired immunity (32, 34, 75). In addition to the ability to produce various type 1 and type 2 cytokines (4, 10, 21, 23, 71), γδ T cells also play an immunomodulatory role through the secretion of chemokines such as MIP-1α, MIP-1β, and RANTES (9, 45). The present study supports a specialized immunoregulatory feature of γδ T cells. By producing MCP-1 and MIP-1α, γδ T cells presumably contribute to the development of an inflammatory response in the brain during a parasitic infection.
As murine NCC γδ T cells and chemokine-producing cells were detected at the same time point after infection, it is not clear whether chemokines initiate or amplify the CNS immune response. In EAE, expression of genes encoding chemokines follows the initial leukocyte entry into the CNS (28, 29, 40, 41). In this context, chemokines appear to amplify rather than initiate the inflammatory cellular accumulation in the brain. Chemokine expression can also precede CNS leukocyte infiltration, as shown by expression of IP-10 following lymphocytic choriomeningitis infection (1-3). Furthermore, many if not all of the cells that are intrinsic to the CNS, including those of neurons, the macroglia, and the microglia, have the ability to produce chemokines (17, 41). Astrocytes and microglia have been demonstrated to produce IP-10, RANTES, MIP-1α, and MCP-1 (28, 30, 41, 63). As naïve T cells do not cross the blood-brain barrier (35), the initiation of the immune responses in the brain might be attributable to the production of chemokines by cells intrinsic to the CNS in response to the presence of cytokines or parasite antigens. Subsequently, the effective recruitment of γδ T cells into the brain and the associated production of type 1 cytokines might lead to further chemokine induction and enhanced accumulation of leukocytes into the CNS. Therefore, CC chemokines appear to mediate the initial cellular infiltration. γδ T cells likely amplify such leukocyte infiltration into the CNS both indirectly, through type 1 cytokine-induced up-regulation of chemokines, and directly, by producing MCP-1 and MIP-1α early during infections.
Chemokine expression has been extensively studied in several neuroinflammatory disorders, including posttraumatic CNS inflammation (55), demyelinating diseases (27, 41, 44, 47, 49, 64), neurodegenerative diseases (7, 24, 25, 33, 39, 48, 68, 70), bacterial meningitis (43, 67), and viral encephalopathies (1, 3, 14, 42, 61, 62). IP-10, IL-8, MCP-1, MIP-1α, MIP-1β, and RANTES (among other chemokines) appeared commonly in these CNS diseases. However, little is known about the local chemokine response in the brain after parasitic infections. In human NCC, eotaxin and IL-5 levels were found to be elevated in serum samples of patients with symptomatic disease (22). Surprisingly, eotaxin was not detected in cerebrospinal fluid (22). It is possible that the high levels of eotaxin found systemically were induced by other parasitic infections, as areas of T. solium endemicity are often areas of endemicity for other parasites as well. The high expression of CC chemokines is a critical factor in the cellular infiltration detected in mouse brain. It is important to determine whether a similar profile is induced in human NCC.
Acknowledgments
This work was supported by grants NS35974 and AI19896.
REFERENCES
- 1.Asensio, V. C., and I. L. Campbell. 1997. Chemokine gene expression in the brains of mice with lymphocytic choriomeningitis. J. Virol. 71**:**7832-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asensio, V. C., and I. L. Campbell. 1999. Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci. 22**:**1453-1458. [DOI] [PubMed] [Google Scholar]
- 3.Asensio, V. C., C. Kincaid, and I. L. Campbell. 1999. Chemokines and the inflammatory response to viral infection in the central nervous system with a focus on lymphocytic choriomeningitis virus. J. Neurovirol. 5**:**65-75. [DOI] [PubMed] [Google Scholar]
- 4.Azuara, V., J. P. Levraud, M. P. Lembezat, and P. Pereira. 1997. A novel subset of adult γδ thymocytes that secretes a distinct pattern of cytokines and expresses a very restricted T cell repertoire. Eur. J. Immunol. 27**:**544-553. [DOI] [PubMed] [Google Scholar]
- 5.Baggiolini, M. 1998. Chemokines and leukocyte traffic. Nature 392**:**565-568. [DOI] [PubMed] [Google Scholar]
- 6.Baggiolini, M., B. Dewald, and B. Moser. 1997. Human chemokines: an update. Annu. Rev. Immunol. 15**:**675-705. [DOI] [PubMed] [Google Scholar]
- 7.Berman, J. W., M. P. Guida, J. Warren, J. Amat, and C. Brosnan. 1996. Localization of monocyte chemoattractant peptide-1 expression in the central nervous system in experimental autoimmune encephalomyelitis and trauma in the rat. J. Immunol. 156**:**3017-3023. [PubMed] [Google Scholar]
- 8.Bluestone, J. A., R. Khattri, R. Scianmmas, and A. I. Sperling. 1995. TCR γδ cells: a specialized T-cell subset in the immune system. Annu. Rev. Cell Dev. Biol. 11**:**307-353. [DOI] [PubMed] [Google Scholar]
- 9.Boismenu, R., L. Feng, Y. Xia, Y. Chang, and W. Havran. 1996. Chemokine expression by intraepithelial γδ T cells. J. Immunol. 157**:**985-992. [PubMed] [Google Scholar]
- 10.Born, W., C. Cady, J. Jones-Carson, A. Mukasa, M. Lahn, and R. O'Brien. 1999. Immunoregulatory functions of γδ T cells. Adv. Immunol. 71**:**77-144. [PubMed] [Google Scholar]
- 11.Calvo, C.-F., T. Yoshimura, M. Gelman, and M. Mallat. 1996. Production of monocyte chemotactic protein-1 by rat brain macrophages. Eur. J. Neurosci. 8**:**1725-1734. [DOI] [PubMed] [Google Scholar]
- 12.Cardona, A. E., B. I. Restrepo, J. M. Jaramillo, and J. M. Teale. 1999. Development of an animal model for neurocysticercosis: immune response in the central nervous system is characterized by a predominance of γδ T cells. J. Immunol. 162**:**995-1002. [PubMed] [Google Scholar]
- 13.Cardona, A. E., and J. M. Teale. 2002. γδ T cell-deficient mice exhibit reduced severity and decreased inflammatory response in the brain during murine cysticercosis. J. Immunol. 169**:**3163-3171. [DOI] [PubMed] [Google Scholar]
- 14.Conant, K., A. Garzino-Demo, A. Nath, J. C. McArthur, W. Halliday, C. Power, R. C. Gallo, and E. O. Major. 1998. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc. Natl. Acad. Sci. USA 95**:**3117-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook, D. N., M. A. Beck, T. M. Coffman, S. L. Kirby, J. F. Sheridan, I. B. Pragnell, and O. Smithies. 1995. Requirement of MIP-1α for an inflammatory response to viral infections. Science 269**:**1583-1585. [DOI] [PubMed] [Google Scholar]
- 16.Correa, D., D. Dalma, B. Espinoza, A. Plancarte, M. T. Rabiela, I. Madrazo, C. Gorodezky, and A. Flisser. 1985. Heterogeneity of humoral immune components in human cysticercosis. J. Parasitol. 71**:**535-541. [PubMed] [Google Scholar]
- 17.Coughlan, C. M., C. M. McManus, M. Sharron, Z.-Y. Gao, D. Murphy, S. Jaffer, W. Choe, W. Chen, J. Hesselgesser, H. Gaylord, A. Kalyuzhny, M.-Y. Lee, B. Wolf, R. W. Doms, and D. L. Kolson. 2000. Expression of multiple functional chemokine receptors and monocyte chemoattractant protein-1 in human neurons. Neuroscience 97**:**591-600. [DOI] [PubMed] [Google Scholar]
- 18.Cruz, M. E., I. Cruz, P.-M. Preux, et al. 1995. Headaches and cysticercosis in Ecuador, South America. Headache 35**:**93-97. [DOI] [PubMed] [Google Scholar]
- 19.Davis, L. E., and M. Kornfeld. 1991. Neurocysticercosis: neurologic, pathogenic, diagnostic and therapeutic aspects. Eur. Neurol. 31**:**229-240. [DOI] [PubMed] [Google Scholar]
- 20.Del Brutto, S. 1988. Neurocysticercosis: an update. Rev. Infect. Dis. 10**:**1075-1087. [DOI] [PubMed] [Google Scholar]
- 21.Duhindan, N., A. J. Farley, S. Humphreys, C. Parker, B. Rossiter, and C. G. Brooks. 1997. Patterns of lymphokine secretion among mouse γδ T cell clones. Eur. J. Immunol. 27**:**1704.. [DOI] [PubMed] [Google Scholar]
- 22.Evans, C., H. Garcia, A. Hartnell, R. Gilman, P. Jose, M. Martinez, D. Remick, T. Williams, and J. Friedland. 1998. Elevated concentrations of eotaxin and interleukin-5 in human neurocysticercosis. Infect. Immun. 66**:**4522-4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrick, D., M. Schrenzel, T. Mulvania, B. Hsieh, W. Ferlin, and H. Lepper. 1995. Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2-stimulating pathogens by γδ T cells in vivo. Nature 373**:**255-257. [DOI] [PubMed] [Google Scholar]
- 24.Fiala, M., L. Zhang, B. Sherry, D. D. Taub, M. C. Graves, S. Hama, D. Way, M. Weinand, M. Witte, D. Lorton, Y. M. Kuo, and A. E. Roher. 1998. Amyloid-beta induces chemokine secretion and monocyte migration across a human blood-brain barrier model. Mol. Med. 4**:**480-489. [PMC free article] [PubMed] [Google Scholar]
- 25.Gitter, B. D., L. M. Cox, R. E. Rydel, and P. C. May. 1995. Amyloid β peptide potentiates cytokine secretion by interleukin-1β-activated human astrocytoma cells. Proc. Natl. Acad. Sci. USA 92**:**10738-10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glabinski, A. R., and R. M. Ransohoff. 1999. Sentries at the gate: chemokines and the blood-brain barrier. J. Neurovirol. 5**:**623-634. [DOI] [PubMed] [Google Scholar]
- 27.Glabinski, A. R., M. Tani, S. Aras, H. Stoler, V. Tuohy, and R. Ransohoff. 1995. Regulation and function of central nervous system chemokines. Int. J. Dev. Neurosci. 13**:**153-165. [DOI] [PubMed] [Google Scholar]
- 28.Glabinski, A. R., M. Tani, R. Strieter, V. Tuohy, and R. Ransohoff. 1997. Synchronous synthesis of alpha- and beta-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of chronic experimental autoimmune encephalomyelitis. Am. J. Pathol. 150**:**617-630. [PMC free article] [PubMed] [Google Scholar]
- 29.Glabinski, A. R., M. Tani, V. Tuohy, R. Tuthill, and R. Ransohoff. 1995. Central nervous system mRNA accumulation follows initial leukocyte entry at the onset of acute murine experimental autoimmune encephalomyelitis. Brain Behav. Immun. 9**:**315-330. [DOI] [PubMed] [Google Scholar]
- 30.Gourmala, N. G., M. Buttini, S. Limonta, A. Sauter, and H. Boddeke. 1997. Differential and time-dependent expression of monocyte chemoattractant protein-1 mRNA by astrocytes and macrophages in rat brain: effects of ischemia and peripheral lipopolysaccharide administration. J. Neuroimmunol. 74**:**35-44. [DOI] [PubMed] [Google Scholar]
- 31.Grewal, J. S., S. Kaur, G. Bhatti, and N. Malla. 2000. Cellular immune responses in human neurocysticercosis. Parasitol. Res. 86**:**500-503. [DOI] [PubMed] [Google Scholar]
- 32.Haas, W., P. Pereira, and S. Tonegawa. 1993. Gamma/delta cells. Annu. Rev. Immunol. 11**:**637-685. [DOI] [PubMed] [Google Scholar]
- 33.Hausmann, E. H., N. E. Berman, Y. Y. Wang, J. B. Meara, G. W. Wood, and R. M. Klein. 1998. Selective chemokine mRNA expression following brain injury. Brain Res. 788**:**49-59. [DOI] [PubMed] [Google Scholar]
- 34.Hayday, A. C. 2000. γδ T cells: a right time and a right place for a conserved third way protection. Annu. Rev. Immunol. 18**:**975-1026. [DOI] [PubMed] [Google Scholar]
- 35.Hickey, W. F., B. L. Hsu, and H. Kimura. 1991. T-lymphocyte entry into the central nervous system. J. Neurosci. Res. 28**:**254-260. [DOI] [PubMed] [Google Scholar]
- 36.Hogaboam, C., N. Luckacs, W. Chensue, R. Strieter, and S. Kunkel. 1998. Monocyte chemoattractant protein-1 synthesis by murine lung fibroblasts modulates CD4+ T cell activation. J. Immunol. 160**:**4606-4614. [PubMed] [Google Scholar]
- 37.Huffnagle, G. B., and L. K. McNeil. 1999. Dissemination of C. neoformans to the central nervous system: role of chemokines, Th1 immunity and leukocyte recruitment. J. Neurovirol. 5**:**76-81. [DOI] [PubMed] [Google Scholar]
- 38.Huffnagle, G. B., L. K. McNeil, R. A. McDonald, J. W. Murphy, G. B. Toews, N. Maeda, and W. A. Kuziel. 1999. Cutting edge: the role of CCR5 in organ-specific and innate immunity to Cryptococcus neoformans. J. Immunol. 163**:**4642-4646. [PubMed] [Google Scholar]
- 39.Ishizuka, K. K., I. R. Katsugari, J. Takamatsu, and T. Miyakawa. 1997. Identification of monocyte chemoattractant protein-1 in senile and reactive microglia of Alzheimer's disease. Psychiatry Clin. Neurosci. 51**:**135-138. [DOI] [PubMed] [Google Scholar]
- 40.Karpus, W., N. Lukacs, B. L. McRae, R. M. Strieter, S. L. Kunkel, and S. D. Miller. 1995. An important role for the chemokine macrophage inflammatory protein-1α in the pathogenesis of the T cell-mediated autoimmune disease experimental autoimmune encephalomyelitis. J. Immunol. 155**:**5003-5010. [PubMed] [Google Scholar]
- 41.Karpus, W. J., and R. M. Ransohoff. 1998. Chemokine regulation of experimental autoimmune encephalomyelitis: temporal and spatial expression patterns govern disease pathogenesis. J. Immunol. 161**:**2667-2671. [PubMed] [Google Scholar]
- 42.Kelder, W., J. C. McArthur, T. Nance-Sproson, D. McClernon, and D. E. Griffin. 1998. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann. Neurol. 44**:**831-835. [DOI] [PubMed] [Google Scholar]
- 43.Lahrtz, F., L. Piali, K. S. Spanaus, J. Seebach, and A. Fontana. 1998. Chemokine and chemotaxis of leukocytes in infectious meningitis. J. Neuroimmunol. 85**:**33-43. [DOI] [PubMed] [Google Scholar]
- 44.Lane, E., V. C. Asensio, N. Yu, A. D. Paoletti, I. L. Campbell, and M. J. Buchmeier. 1998. Dynamic regulation of α- and β-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J. Immunol. 160**:**970-978. [PubMed] [Google Scholar]
- 45.Lehner, T., E. Mitchell, L. Bergmeier, M. Singh, R. Spallek, M. Cranage, G. Hall, M. Dennis, F. Villinger, and Y. Wang. 2000. The role of γδ T cells in generating antiviral factors and b-chemokines in protection against mucosal simian immunodeficiency virus infection. Eur. J. Immunol. 30**:**2245-2256. [DOI] [PubMed] [Google Scholar]
- 46.Mackay, C. R. 2001. Chemokines: immunology's high impact factors. Nat. Immunol. 2**:**95-99. [DOI] [PubMed] [Google Scholar]
- 47.McManus, C., J. W. Berman, F. M. Brett, H. Staunton, M. Farrell, and C. Brosnan. 1998. MCP-1, MCP-2 and MCP-3 expression in multiple sclerosis lesions: an immunohistochemical and in situ hybridization study. J. Neuroimmunol. 86**:**20-29. [DOI] [PubMed] [Google Scholar]
- 48.McTigue, D. M., M. Tani, K. Krivacic, A. Chernosky, G. Kelner, D. Maciejewski, R. Maki, R. M. Ransohoff, and B. T. Stokes. 1998. Selective chemokine mRNA accumulation in the rat spinal cord after contusion injury. J. Neurosci. Res. 53**:**368-376. [DOI] [PubMed] [Google Scholar]
- 49.Miyagishi, R., S. Kikuchi, T. Fukazawa, and K. Tashiro. 1995. Macrophage inflammatory protein-1α in the cerebrospinal fluid of patients with multiple sclerosis and other inflammatory neurological diseases. J. Neurol. Sci. 129**:**223-227. [DOI] [PubMed] [Google Scholar]
- 50.Murdoch, C., and A. Finn. 2000. Chemokine receptors and their role in inflammation and infectious diseases. Blood 95**:**3032-3043. [PubMed] [Google Scholar]
- 51.Nickel, R., L. A. Beck, C. Stellato, and R. P. Schleimer. 1999. Molecular mechanisms in allergy and clinical immunology. J. Allergy Clin. Immunol. 104**:**723-742. [DOI] [PubMed] [Google Scholar]
- 52.Ostrosky-Zeichner, P., E. Garcia-Mendoza, C. Rios, and J. Sotelo. 1996. Humoral and cellular immune response within the subarachnoid space of patients with neurocysticercosis. Arch. Med. Res. 27**:**513-517. [PubMed] [Google Scholar]
- 53.Rajan, A., V. C. Asensio, I. L. Campbell, and C. Brosnan. 2000. Experimental autoimmune encephalomyelitis on the SJL mouse: effect of γδ T cell depletion on chemokine and chemokine receptor expression in the central nervous system. J. Immunol. 164**:**2120-2130. [DOI] [PubMed] [Google Scholar]
- 54.Ransohoff, R. 1997. Chemokines in neurological disease models: correlation between chemokine expression patterns and inflammatory pathology. J. Leukoc. Biol. 62**:**645-651. [DOI] [PubMed] [Google Scholar]
- 55.Ransohoff, R. M., and M. Tani. 1998. Do chemokines mediate leukocyte recruitment in post-traumatic CNS inflammation? Trends Neurosci. 21**:**154-159. [DOI] [PubMed] [Google Scholar]
- 56.Restrepo, B. I., P. Llaguno, M. A. Sandoval, J. A. Enciso, and J. M. Teale. 1998. Analysis of immune lesions in neurocysticercosis patients: central nervous system response to helminth appears Th1-like instead of Th2. J. Neuroimmunol. 89**:**64-72. [DOI] [PubMed] [Google Scholar]
- 57.Roman, G., J. Sotelo, O. Del Brutto, A. Flisser, M. Dumas, N. Wadia, D. Botero, M. Cruz, H. H. Garcia, P. de Bittencourt, L. Trelles, P. Lorenzana, T. E. Nash, and A. Spina-Franca. 2000. A proposal to declare neurocysticercosis an international reportable disease. Bull. W. H. O. 78**:**399-406. [PMC free article] [PubMed] [Google Scholar]
- 58.Rothenberg, M. E., N. Zimmermann, A. Mishira, E. Brandt, L. Birkenberger, S. Hogan, and P. Foster. 1999. Chemokines and chemokine receptors: their role in allergic airway disease. J. Clin. Immunol. 19**:**250-265. [DOI] [PubMed] [Google Scholar]
- 59.Rottman, J. B., K. P. Granley, K. Williams, L. Wu, C. R. Mackay, and D. J. Ringler. 1997. Cellular localization of the chemokine receptor CCR5. Correlation to cellular targets of HIV-1 infection. Am. J. Pathol. 151**:**1341-1351. [PMC free article] [PubMed] [Google Scholar]
- 60.Rubalcava, M. A., and J. Sotelo. 1995. Differences between ventricular and lumbar cerebrospinal fluid in hydrocephalus secondary to cysticercosis. Neurosurgery 37**:**668-672. [DOI] [PubMed] [Google Scholar]
- 61.Sasseville, V. G., M. M. Smith, C. R. Mackay, D. R. Pauley, K. G. Mansfield, D. J. Ringler, and A. A. Lackner. 1996. Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am. J. Pathol. 149**:**1459-1467. [PMC free article] [PubMed] [Google Scholar]
- 62.Schmidtmayerova, H., H. S. Nottet, G. Nuovo, T. Raabe, C. R. Flanagan, L. Dubrovsky, H. E. Gendelman, A. Cerami, M. Bukrinsky, and B. Sherry. 1996. Human immunodeficiency virus type 1 infection alters chemokine β peptide expression in human monocytes: implications for recruitment of leukocytes into the brain and lymph nodes. Proc. Natl. Acad. Sci. USA 93**:**700-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simpson, J. E., J. Newcombe, M. L. Cuzner, and M. N. Woodroofe. 1998. Expression of monocyte chemoattractant protein-1 and other β-chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J. Neuroimmunol. 84**:**238-249. [DOI] [PubMed] [Google Scholar]
- 64.Sorensen, T. L., M. Tani, J. Jensen, V. Pierce, C. Lucchinetti, V. Folcik, S. Qin, J. Rottman, F. Sellebjerg, R. Strieter, J. L. Frederiksen, and R. M. Ransohoff. 1999. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Investig. 103**:**807-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sotelo, J., and C. Marin. 1987. Hydrocephalus secondary to cysticercosis arachnoiditis. A long-term follow-up review of 92 cases. J. Neurosurg. 66**:**686-689. [DOI] [PubMed] [Google Scholar]
- 66.Sozzani, S., P. Allavena, P. Proost, J. Damme, and A. Mantovani. 1996. Chemokines as targets for pharmacological intervention. Prog. Drug Res. 47**:**53-80. [DOI] [PubMed] [Google Scholar]
- 67.Sprenger, H., A. Rosler, P. Tonn, H. J. Braune, G. Huffman, and D. Gemsa. 1996. Chemokines in the cerebrospinal fluid of patients with meningitis. Clin. Immunol. Immunopathol. 80**:**155-161. [DOI] [PubMed] [Google Scholar]
- 68.Tarkowski, E., L. Rosengren, C. Blomstrand, C. Wikkelso, C. Jensen, S. Ekholm, and A. Tarkowski. 1997. Intrathecal release of pro- and anti-inflammatory cytokines during stroke. Clin. Exp. Immunol. 110**:**492-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tekamp-Olson, P., P. Gallegos, C. Bauer, J. McClain, B. Sherry, B. Fabre, M. Van Deventer, and A. Cerami. 1990. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J. Exp. Med. 172**:**911-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang, X., J. A. Ellison, A. L. Siren, P. G. Lysko, T. L. Yue, F. C. Barone, A. Shatzman, and G. Z. Feuerstein. 1998. Prolonged expression of interferon-inducible protein-10 in ischemic cortex after permanent occlusion of the middle cerebral artery in rat. J. Neurochem. 71**:**1194-1204. [DOI] [PubMed] [Google Scholar]
- 71.Wen, L., D. F. Barber, W. Pao, F. S. Wong, M. J. Owen, and A. Hayday. 1998. Primary γδ T cell clones can be defined phenotypically and functionally as Th1/Th2 cells and illustrate the association of CD4 with Th2 differentiation. J. Immunol. 160**:**1965-1974. [PubMed] [Google Scholar]
- 72.White, A. C., Jr. 1997. Neurocysticercosis: a major cause of neurological disease worldwide. Clin. Infect. Dis. 24**:**101-115. [DOI] [PubMed] [Google Scholar]
- 73.White, A. C., Jr. 2000. Neurocysticercosis: updates on epidemiology, pathogenesis, diagnosis, and management. Annu. Rev. Med. 51**:**187-206. [DOI] [PubMed] [Google Scholar]
- 74.White, A. C., Jr., P. Robinson, and R. Kuhn. 1997. Taenia solium cysticercosis: host-parasite interactions and the immune response. Chem. Immunol. 66**:**209-230. [PubMed] [Google Scholar]
- 75.Ziegler, H. K., M. J. Skeen, and K. M. Pearce. 1994. Role of α/β T and γ/δ T cells in innate and acquired immunity. Ann. N. Y. Acad. Sci. 730**:**53-70. [DOI] [PubMed] [Google Scholar]