Glutamate and γ-aminobutyric acid mediate a heterosynaptic depression at mossy fiber synapses in the hippocampus (original) (raw)
Abstract
Mossy fiber synapses form the major excitatory input into the autoassociative network of pyramidal cells in the CA3 area of the hippocampus. Here we demonstrate that at the mossy fiber synapses, glutamate and γ-aminobutyric acid (GABA) act as autaptic and heterosynaptic presynaptic inhibitory transmitters through metabotropic glutamate receptors (mGluRs) and GABAB receptors, respectively. Both GABAB receptors and mGluRs are activated through spillover from adjacent synapses. We demonstrate that glutamate spillover caused by brief tetanic activation of mossy fiber terminals remains intact at physiological temperatures. The activation of GABAB receptors increased the threshold for mossy fiber long-term potentiation (LTP), whereas activation of mGluRs did not have such an effect. We speculate that this heterosynaptic depression provides the mossy fiber synapses with a mechanism to efficiently shape input patterns into CA3, increasing the sparseness of the mossy fiber signal and enhancing the capacity and performance of the CA3 associative network. The increase in LTP threshold through activation of presynaptic inhibitory receptors imparts a piesynoptic associative nature to mossy fiber LTP.
Pyramidal cells in the CA3 region of the hippocampus receive excitatory glutamatergic input mainly from dentate gyrus granule cells and from neighboring CA3 pyramidal cells. The axons of the dentate granule cells, called mossy fibers, form en passant synapses on a small segment of the proximal apical dendrites of the CA3 pyramidal cells. Mossy fiber synapses are large, and their presynaptic terminals engulf complex, branched dendritic spines (1) and contain up to 20 independent release sites (2, 3). They exhibit a strong activity-dependent, short-term plasticity and a presynaptic long-term potentiation (LTP) that is independent of _N_-methyl-d-aspartate (NMDA)-receptor activation (ref. 4, but see ref. 5). The smaller associational-commisural synapses from CA3 pyramidal cells show little activity-dependent, short-term plasticity and a NMDA-receptor-dependent LTP (6, 7).
The probability of release at the mossy fiber synapse is low under basal conditions, but increases dramatically during brief repetitive activation (7). Because of their location close to the soma of the CA3 pyramidal cells, their relatively large quantal size (3) and their facilitation, the mossy fiber synapse has been referred to as a “teacher” (8) input into the highly interconnected autoassociative CA3 network. It has been proposed that such networks can store patterns of inputs and retrieve them in a content addressable manner—–if presented with a part of a learned pattern, the full pattern is returned (9). Theoretical analysis has shown the importance of sparse coding for the storage capacity of autoassociative networks (10). For the CA3 region this requirement means that different patterns of activity in the dentate gyrus that represent different inputs into CA3 should activate as few common CA3 pyramidal cells as possible. This sparseness can be achieved at the level of the dentate gyrus, with different patterns sharing different dentate granule cells and therefore different mossy fibers. Because there is very little overlap in the innervation of CA3 pyramidal cells by mossy fibers this distribution results in few CA3 pyramidal cells being shared among different patterns. One mechanism that was suggested to achieve this sparseness of the signal from the dentate gyrus was competition at the level of dentate granule cells during learning (11).
Mossy fiber synapses possess inhibitory presynaptic G-protein coupled receptors for a number of transmitters, including glutamate (12–14), γ-aminobutyric acid (GABA) (15), adenosine (16), and dynorphin (17). Activation of these receptors by exogenous ligands inhibits or completely abolishes synaptic transmission. Here we report that endogenously released glutamate and GABA can exert a powerful depressant effect on mossy fiber synapses. Repetitive activation of the mossy fiber system leads to a spillover of glutamate from the synaptic cleft onto presynaptic metabotropic glutamate receptors (mGluRs) at neighboring synapses. In addition mossy fibers are shown to receive a powerful GABAergic presynaptic inhibition from nearby inhibitory interneurons. Mossy fibers themselves have been shown to contain GABA (18) and may contribute to the GABAergic inhibition. The heterosynaptic depression lasts at most a few seconds. Repetitive activation of the mossy fiber system therefore not only causes the necessary strong activation of CA3 pyramidal cells, it also provides the system with the necessary inhibitory signal that prevents the contamination of the input pattern with spurious signals.
METHODS
Brain slices of the hippocampal region of 5- to 15-day-old guinea pigs were obtained by using standard procedures. Slices were kept at least 1 hr at room temperature in a holding chamber containing artificial cerebrospinal fluid (ACSF) bubbled with 95% O2 and 5% CO2 and then transferred to a recording chamber that was continually perfused with ACSF at 1–1.5 ml/min. ACSF contained 120 mM NaCl, 10 mM glucose, 2.5 mM KCl, 1.3 mM MgSO4, 2.5 mM CaCl2, 1.3 mM NaH2PO4, and 26 mM NaHCO3. Glass recording electrodes with a tip opening of about 1 μm were filled with ACSF and inserted into stratum lucidum to record field excitatory postsynaptic potentials (fEPSPs). Bipolar tungsten microelectrodes were inserted into the dentate gyrus, and 100-μs pulses were used to elicit mossy fiber responses. Mossy fiber responses were identified through their characteristic short-term plasticity and their sensitivity to the group III mGluR agonist L(+)-2-amino-3-phosphopropionic acid (L-AP4) (13). fEPSPs were filtered at 2 kHz, digitized at 5 kHz, and analyzed online by using custom-made software (D. Selig, University of California, San Francisco). Data was further analyzed offline by using the idl data analysis environment (Research Systems, Boulder, CO). fEPSP amplitudes were measured as averages during a 1- to 2-ms window at the peak of the response. To prevent interference from other presynaptic inhibitory transmitters we used p-(3-aminopropyl)-p-diethoxymethyl-phosphinic acid (CGP35348) and naloxone in all of the glutamate spillover experiments. The GABA spillover experiments were performed in the presence of naloxone alone, unless otherwise noted. All experiments that involved long-term potentiation were done in D(−)-2-amino-5-phosphopentatonic acid (D-APV). Experiments were done at room temperature except where noted. Data is presented as mean ± SE and were compared by using two-tailed nonpaired Student’s t test unless otherwise noted. CGP35348 was a gift of Novartis AG, Basel. ((+)-(2S)-5,5-dimethyl-2-morpholineatic acid (SCH50911), (2S,3S,4S)-2-methyl-2-(carboxypropyl)glycine (MCCG), (S)-a-methyl-4-carboxyphenylglycine (MCPG), and D-APV were from Tocris (Ballwin, MO), and the rest of the chemicals were from Sigma.
RESULTS
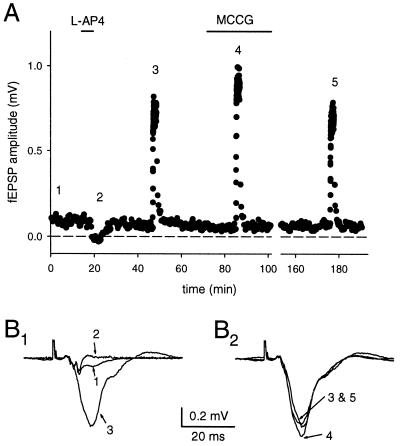
To confirm and extend previous findings of autaptic inhibition during prolonged increased mossy fiber activity (19), we conducted a series of experiments with the specific mGluR II antagonist MCCG. Mossy fiber fEPSPs were collected at 0.05 Hz during baseline and 100 pulses at 1 Hz were given to cause a large presynaptic facilitation of glutamate release. The amount of frequency facilitation was measured as the average of the fEPSP amplitude during the 1-Hz train (see Fig. 1). MCCG (500 μM) significantly increased the amplitude at 1 Hz by 15% ± 5% (P < 0.05, n = 7), but did not increase the amplitude of the fEPSP at low frequencies, suggesting that mGluRs are activated during 1-Hz stimulation, but not at low stimulus frequencies. This finding confirms the involvement of group II mGluRs in this phenomenon (19). MCCG can act as a partial agonist, and in a subset of our experiments the response at baseline was depressed. Only the absolute increase in the mossy fiber fEPSP can be compared under these circumstances, because the relative amount of frequency facilitation depends on the initial probability of release. The results with MCCG are very similar to those obtained with the broad spectrum antagonist MCPG, and because MCPG lacks partial agonistic effects, it was used for the remainder of the experiments.
Figure 1.
Group II mGluRs are responsible for autoinhibition during prolonged high-frequency stimulation of the mossy fiber synapses. An extracellular stimulating electrode was placed in the dentate gyrus, and field recordings were obtained from stratum lucidum. (A) The amplitude of the fEPSP is plotted over time. The selective mGluR agonist L-AP4 was used to confirm that the responses were mediated by mossy fibers. Increasing the stimulus frequency from 0.05 to 1 Hz caused pronounced frequency facilitation. This facilitation was significantly enhanced in the presence of the specific group II mGluR antagonist MCCG. (B) Superimposed averaged traces of responses obtained during the experiment plotted in A. The traces are numbered according to the times shown in A.
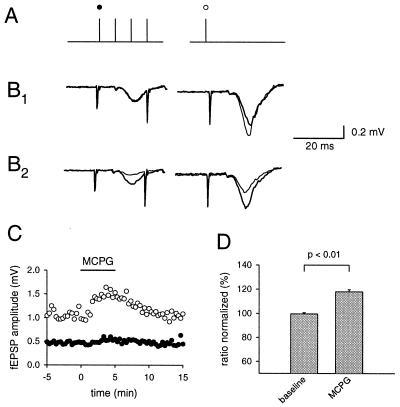
These prolonged trains of stimuli are unlikely to correspond to physiological activity patterns in dentate granule cells, because these cells have a low average firing frequency and give off short trains of action potentials, e.g., during sharp wave activity in the hippocampus (20–22). Furthermore there is evidence that spillover during prolonged trains has a substantial temperature dependence (23), a finding we were able to reproduce (data not shown). We therefore tested the ability of brief trains of action potentials to release enough glutamate to cause spillover. A mossy fiber pathway was stimulated with 3–5 pulses at a 40-ms interimpulse interval, and 200 ms later a test pulse was applied (Fig. 2A). Application of MCPG (1.5 mM) caused a significant increase in the amplitude of the fEPSP (118% ± 1.7%, n = 5, P < 0.01) after the brief burst of activity (Fig. 2D). The response to the initial stimulus in the short train was unaltered (Fig. 2 B1 and C). Application of the mGluR agonist L-AP4 affected both the responses to the first pulse in the train and the synaptic response 200 ms after the train (Fig. 2B2). To avoid possible contamination of the results presented above with other presynaptic inhibitory receptors, a GABAB receptor antagonist CGP35348 (500 μM) or SCH50911 (10 μM) and the opioid receptor antagonist naloxone (10 μM) were routinely present.
Figure 2.
Glutamate release by brief stimulus trains to mossy fibers activates presynaptic mGluRs. Mossy fibers were stimulated with a brief train and 200 ms later a test pulse was applied to the same pathway (A). To prevent interference from other presynaptic inhibitory transmitters we used CGP35348 and naloxone in all of the glutamate spillover experiments. MCPG (thin line) significantly increased the response to the test pulse, whereas the response to the first stimulus in the train remained unchanged (B 1). The first pulse is sensitive to mGluR activation (thin line), as revealed by the application of 1 μM of the exogenous mGluR agonist L-AP4 (1 μM) (B2). (C) The fEPSP amplitude for one example is plotted. (D) The ratio between test response and the first response is normalized and the increase in the ratio under MCPG (1.5 mM) is shown for five experiments.
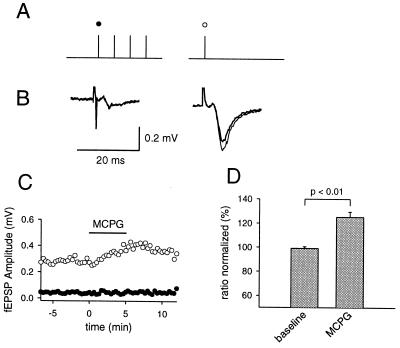
It has been suggested that glutamate uptake mechanisms play a major role in shaping spillover phenomena in the mossy fiber system (23) and elsewhere (24) in the hippocampus. We therefore conducted the same experiments with brief trains of stimuli at higher temperatures (34°–36°C). The response to MCPG was similar for the different temperatures (118% vs. 125%) (Fig. 3) and therefore the reminder of the experiments were done at room temperature by using the short trains of stimuli.
Figure 3.
Autaptic inhibition induced by brief tetani is still present at elevated temperatures. The same protocol (A) as used in Fig. 2 was applied during experiments at elevated temperatures (34°–36° C). A selective increase in the test response is apparent in the presence of MCPG (thin line, B), comparable to the effect at room temperature. (C) The fEPSP amplitude for one experiment is plotted. (D) The change in the ratio of test pulse to first pulse in the train is shown (n = 9).
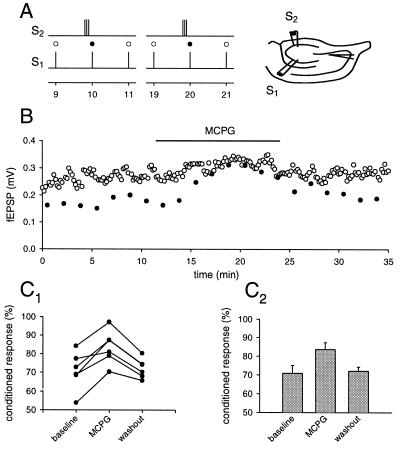
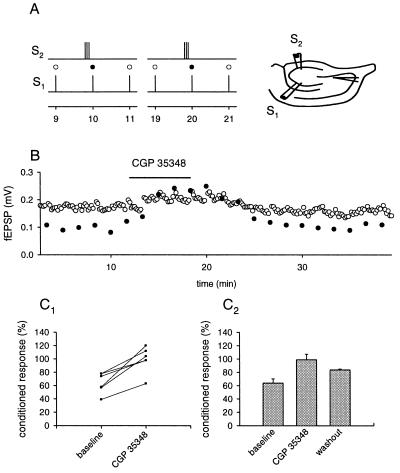
Using the same pathway to induce glutamate spillover and probe presynaptic inhibition is not absolutely conclusive for spillover phenomena. In the rat, group II mGluRs have been localized at some distance from the site of release, suggesting that their activation requires diffusion from the synaptic cleft. The same data is unfortunately not available for the guinea pig. One conclusive way to overcome this problem is to use two independent pathways (25, 26), one for the release of glutamate and the other to probe the effect of the activation of presynaptic mGluRs. Two pairs of stimulating electrodes were used, and their positions and the stimulus intensities were adjusted so that two independent mossy fiber paths could be stimulated (Fig. 4A). Pathway one was stimulated at a regular interval of 10 s. Every tenth stimulus was preceded by a conditioning train of 5–8 stimuli at 25–50 Hz on pathway two. The two pathways were stimulated 180–200 ms apart depending on the number of stimuli on pathway two. The response to the stimulation after a train on pathway two was depressed (29.2% ± 4%, n = 6) compared with stimuli without preceding activity. The application of MCPG (1.5 mM) significantly reduced this heterosynaptic depression in all cases (16.4% of unconditioned ±3.7%, n = 6).
Figure 4.
Brief tetanic stimulation causes glutamatergic heterosynaptic inhibition in the mossy fiber system. Two pairs of stimulating electrodes were inserted into the dentate gyrus, and the mossy fiber response was recorded with an extracellular field electrode in CA3 stratum lucidum. (A) Brief trains of stimuli to one pathway were used to produce glutamate spillover onto the other pathway. (B) The amplitude of the fEPSP plotted over time. The response to the conditioned stimulus (•) is depressed compared with the unconditioned stimulus (○). This depression disappears during the application of MCPG. The results of six experiments are summarized in C 1 and the average of these experiments is shown in C 2. Depression is calculated as percentage of unconditioned response.
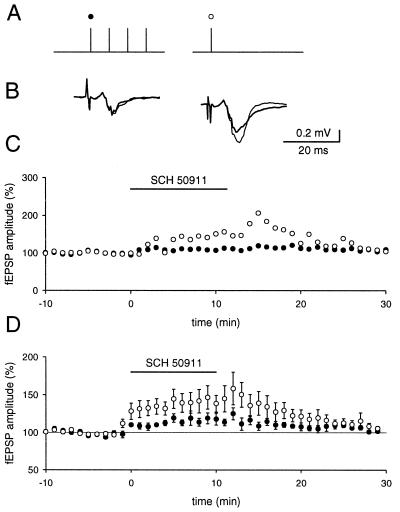
The presence of a class of GABAergic interneurons that receives a major input from mossy fibers and that is restricted to stratum lucidum (27–29) and the finding that GABA is present in mossy fiber synapses (18) suggests that GABA might play a similar role to that of glutamate. We therefore conducted a series of experiments to assess the influence of presynaptic GABAergic inhibition in the mossy fiber system. We were especially interested in knowing whether brief trains of activity in the mossy fiber system can evoke enough GABA release to influence GABAB receptors on mossy fiber terminals. Again, every 20 s a brief train of stimuli was applied to a mossy fiber pathway, followed 200 ms later by a test pulse to the same pathway (Fig. 5). The application of either CGP354348 (500 μM) or SCH50911 (10 μM) increased the response to both the first pulse in the train and the test pulse. The increase of the response to the test pulse was significantly larger than the increase of the response to the first stimulus (116% ± 3% vs. 147% ±4.7%, P < 0.01, n = 5). The same protocol, which was used to cause glutamatergic heterosynaptic effects, was used to probe for effects of GABAergic heterosynaptic inhibition. Substantial heterosynaptic inhibition was readily elicited, when GABAergic inhibition was still present (Fig. 6) and thus extensive readjustment of the position of the stimulus electrodes was not required in contrast to the experiments on glutamatergic synaptic inhibition. CGP35348 (500 μM) significantly reduced, or abolished the heterosynaptic inhibition (average 0.8% ± 8%, n = 6), sometimes revealing a slight facilitation, presumably the result of a small overlap of the two pathways. It also increased the baseline response of the unconditioned stimulus. MCPG was not present during these experiments. However, in the presence of CGP35348 some heterosynaptic inhibition often could be restored by repositioning the stimulating electrodes and/or increasing the stimulus strength, and this depression was susceptible to MCPG (1.5 mM). Thus GABAergic and glutamatergic presynaptic inhibition coexisted in this preparation under our experimental conditions.
Figure 5.
Brief stimulus trains cause an increase in GABA release. A brief stimulus train was given and 200 ms later a test pulse was applied to the same pathway (A). (B) The average response before and after the application of the GABAB receptor antagonist SCH50911 (thin line) is shown. (C) The time course of one experiment, in which the amplitude for the first response (•) and test response (○) are plotted. (D) Summary of five experiments is shown.
Figure 6.
GABAB receptors mediate heterosynaptic depression at mossy fiber synapses. (A) The protocol used for these experiments is shown. Two pairs of stimulating electrodes were placed in the dentate gyrus to stimulate two independent mossy fiber pathways. (B) The unconditioned fEPSP (○) and conditioned fEPSP (•) are plotted. (C1) Summary of six such experiments. (C2) The average of these experiments. Depression is calculated at percentage of unconditioned response.
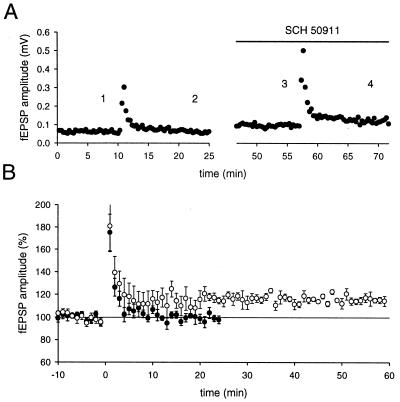
The effects described so far last for no more than a few seconds. It has been described earlier that synaptically released dynorphin, which can cause heterosynaptic inhibition lasting many minutes in the mossy fiber system, also raises the threshold for mossy fiber LTP (17). We therefore tested for possible effects of GABAergic and glutamatergic inhibition on the ability to induce mossy fiber LTP (Fig. 7). A train of stimuli that was subthreshold for the induction of LTP under normal conditions caused reliable induction of mossy fiber LTP when applied during inhibition of GABAB receptors with SCH50911 (10 μM) (n = 5). The same experimental protocol failed to reveal a shift in LTP threshold when using the mGluR antagonist MCPG (1.5 mM) (n = 4, data not shown), as has been reported (30).
Figure 7.
Activation of GABAB receptors increases the threshold for mossy fiber LTP. (A) The mossy fiber fEPSP amplitude for one experiment in which the identical stimulus (12 pulses at 25 Hz) was given, first under normal conditions, and a second time during the application of SCH50911. (B) The results of six experiments are summarized. The amplitude measurements were normalized to baseline before the tetanus. • indicate control conditions, and ○ were obtained after the addition of a GABAB receptor antagonist.
DISCUSSION
The mossy fiber synapses possess a number of G-protein coupled presynaptic receptors that, when activated, depress transmitter release. Because there are no axo-axonic synaptic contacts on the mossy fiber terminals themselves, these presynaptic receptors can be activated only by transmitter release from the mossy fiber synapses or through diffusion from other synapses. In this paper we provide evidence that mGluRs and GABAB receptors are involved in heterosynaptic signaling in the mossy fiber system. This heterosynaptic inhibition relies on the fact that these transmitters can leave the immediate vicinity of their release site and act at a distance. The spread of glutamate out of the synaptic cleft at mossy fiber synapses was suggested earlier (19). Other groups also have reported spillover of glutamate (26, 31), but suggested that these phenomena only occur during decreased glutamate uptake, because spillover was undetected at physiological temperatures (24, 26). Here we have studied homosynaptic and heterosynaptic spillover of glutamate and have demonstrated that at least some forms of spillover persist at near physiological temperature. The stimulus pattern used in the present study to evoke spillover is more reminiscent of in vivo activity in the dentate gyrus (21, 22) than the prolonged repetitive activation of mossy fiber pathways used in previous studies (19) and appears to be less sensitive to temperature and glutamate uptake. We also demonstrate that GABA can spread from neighboring inhibitory synapses and inhibit glutamate release from mossy fiber synapses, as has been observed in other parts of the brain (32, 33).
Theoretical analysis of hippocampal circuitry indicates that one of the major mechanisms for associative memory formation in the hippocampus lies in the Hebbian plasticity of the autoassociative network formed by CA3 pyramidal cells (10, 34). The memory capacity of autoassociative networks strongly depends on the sparseness of the input signal (35). The number of different patterns that can be stored increases if their inputs share as few elements as possible. The mossy fiber system exhibits several features in accord with such sparse coding. First, the connectivity between dentate gyrus and CA3 region is extremely low; the average probability for a given CA3 pyramidal cell to be connected to a given dentate granule cell is only 0.008% (11). Second, the average firing frequency of dentate granule cells is very low (21) and the mossy fiber synapses show pronounced facilitation. Thus a repetitively firing input carries a greatly amplified signal compared with the low number of randomly firing inputs. Third, it has been proposed that competitive learning within the dentate gyrus, in which perforant path LTP is accompanied by a heterosynaptic inhibition, mediated by inhibitory interneurons, contributes to sparse representation (11) by segregating different, but initially overlapping, enthorhinal inputs onto different sets of dentate granule cells.
We propose that heterosynaptic inhibition in the mossy fiber system provides an additional mechanism that further increases the sparseness of the input signal and thereby enhances the storage capacity of the CA3 network. The mossy fiber system exhibits striking morphological features that make it an ideal substrate for diffusion-mediated heterosynaptic inhibition. The mossy fibers all converge onto a very small area on the apical dendrite of CA3 pyramidal cells and lie in close proximity to one another (1). Mossy fiber boutons originating from one axon, however, are evenly and widely spaced throughout CA3 (29), with a considerable distance between the boutons from a single granule cell.
A strong input from the dentate gyrus, consisting of the repetitive firing of a number of mossy fibers, will lead to the inhibition of neighboring mossy fiber terminals through mGluR activation and through activation of GABAB receptors by CA3 interneurons. The amount of inhibition observed experimentally is a low estimate for the amount of inhibition at individual boutons. It is likely that the response is made up of boutons that are inhibited to a large extent and some that are not subject to inhibition at all. The inhibition of neighboring boutons leads to a selective activation and firing of only those CA3 pyramidal cells that are receiving the strong repetitive input and thereby sharpens the input pattern.
A further element of this competitive mechanism is realized in the increased threshold for mossy fiber LTP under GABAergic inhibition. A strong input that reaches the threshold for mossy fiber LTP will produce enough heterosynaptic inhibition to significantly raise the threshold for mossy fiber LTP in neighboring synapses for a few hundred milliseconds. It thereby provides a presynaptic associative mechanism for mossy fiber LTP. The heterosynaptic interaction of the mossy fiber system at the presynaptic level provides the CA3 network with a mechanism that optimizes the sparseness of the input signal. Synaptic responses to weakly firing fibers of a specific input pattern will be depressed as a function of their distance from a strongly firing fiber. This depression leads to a suppression of spurious inputs and an efficient coding of the pattern. A strong mossy fiber input will inhibit neighboring synapses for several hundred milliseconds. During this time these synapses therefore are less likely to fire the postsynaptic pyramidal cell. Because of the increased threshold for LTP these boutons are also less likely to undergo LTP, which decreases their likelihood to fire the postsynaptic cell probably for hours. This form of presynaptic organization is likely to increase the storage capacity and performance of the CA3 network.
Acknowledgments
We thank Drs. R. C. Malenka and C. Petersen and the members of the Nicoll lab for their valuable input, Helen Czerwonka for secretarial assistance, and Dr. D. Selig for the use of the neurophy program. R.A.N. is a member of the Keck Center for Integrative Neuroscience and the Silvio Conte Center for Neuroscience Research and is supported by grants from the National Institutes of Health. K.E.V. was supported by a grant from the Swiss National Science Foundation.
ABBREVIATIONS
GABA
γ-aminobutyric acid
LTP
long-term potentiation
mGluR
metabotropic glutamate receptor
fEPSP
field excitatory postsynaptic potential
L-AP4
L(+)-2-amino-3-phosphopropionic acid
MCCG
(2S,3S,4S)-2-methyl-2-(carboxypropyl)glycine
MCPG
(S)-a-methyl-4-carboxyphenylglycine
((+)-(2S)-5,5-dimethyl-2-morpholineatic acid
p-(3-aminopropyl)-p-diethoxymethyl-phosphinic acid
References
- 1.Amaral D G, Dent J A. J Comp Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- 2.Chicurel M E, Harris K M. J Comp Neurol. 1992;323:169–182. doi: 10.1002/cne.903250204. [DOI] [PubMed] [Google Scholar]
- 3.Jonas P, Major G, Sakmann B. J Physiol. 1993;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicoll R A, Malenka R C. Nature (London) 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 5.Johnston D, Williams S, Jaffe D, Gray R. Annu Rev Physiol. 1992;54:489–505. doi: 10.1146/annurev.ph.54.030192.002421. [DOI] [PubMed] [Google Scholar]
- 6.Zalutsky R A, Nicoll R A. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- 7.Salin P A, Scanziani M, Malenka R C, Nicoll R A. Proc Natl Acad Sci USA. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treves A, Rolls E T. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- 9.Bennett M R, Gibson W G, Robinson J. Philos Trans R Soc London Ser B. 1994;343:167–187. doi: 10.1098/rstb.1994.0019. [DOI] [PubMed] [Google Scholar]
- 10.Rolls E T. Hippocampus. 1996;6:601–620. doi: 10.1002/(SICI)1098-1063(1996)6:6<601::AID-HIPO5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 11.Rolls E T. In: Models of Brain Function. Cotterill R M J, editor. Cambridge: Cambridge Univ. Press; 1989. pp. 15–33. [Google Scholar]
- 12.Lanthorn T H, Ganong A H, Cotman C W. Brain Res. 1984;290:174–178. doi: 10.1016/0006-8993(84)90750-9. [DOI] [PubMed] [Google Scholar]
- 13.Manzoni O J, Castillo P E, Nicoll R A. Neuropharmacology. 1995;34:965–971. doi: 10.1016/0028-3908(95)00060-j. [DOI] [PubMed] [Google Scholar]
- 14.Kamiya H, Shinozaki H, Yamamoto C. J Physiol. 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson S M, Gähwiler B H. J Physiol. 1992;451:329–345. doi: 10.1113/jphysiol.1992.sp019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson S M, Haas H L, Gahwiler B. J Physiol (London) 1992;451:347–363. doi: 10.1113/jphysiol.1992.sp019168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisskopf M G, Zalutsky R A, Nicoll R A. Nature (London) 1993;362:423–427. doi: 10.1038/362423a0. [DOI] [PubMed] [Google Scholar]
- 18.Sloviter R S, Dichter M A, Rachinsky T L, Dean E, Goodman J H, Sollas A L, Martin D L. J Comp Neurol. 1996;373:593–618. doi: 10.1002/(SICI)1096-9861(19960930)373:4<593::AID-CNE8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Scanziani M, Salin P A, Vogt K E, Malenka R C, Nicoll R A. Nature (London) 1997;385:630–634. doi: 10.1038/385630a0. [DOI] [PubMed] [Google Scholar]
- 20.Muñoz M D, Núñez A, Garcia-Austt E. Brain Res. 1990;509:91–98. doi: 10.1016/0006-8993(90)90313-z. [DOI] [PubMed] [Google Scholar]
- 21.Jung M W, McNaughton B L. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- 22.Ylinen A, Soltesz I, Bragin A, Penttonen M, Sik A, Buzsaki G. Hippocampus. 1995;5:78–90. doi: 10.1002/hipo.450050110. [DOI] [PubMed] [Google Scholar]
- 23.Min M Y, Asztely F, Kokaia M, Kullmann D M. Proc Natl Acad Sci USA. 1998;95:4702–4707. doi: 10.1073/pnas.95.8.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asztely F, Erdemli G, Kullmann D M. Neuron. 1997;18:281–293. doi: 10.1016/s0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 25.Vogt K, Malenka R C, Nicoll R A. Neurosci Abstr. 1997;23:9. [Google Scholar]
- 26.Min M-Y, Rusakov D A, Kullmann D M. Neuron. 1998;21:561–570. doi: 10.1016/s0896-6273(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 27.Miettinen R, Gulyás A I, Baimbridge K G, Jacobowitz D M, Freund T F. Neuroscience. 1992;48:29–43. doi: 10.1016/0306-4522(92)90335-y. [DOI] [PubMed] [Google Scholar]
- 28.Freund T F, Buzsaki G. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 29.Acsády L, Kamondi A, Sik A, Freund T, Buzsaki G. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsia A Y, Salin P A, Castillo P E, Aiba A, Abeliovich A, Tonegawa S, Nicoll R A. Neuropharmacology. 1995;34:1567–1572. doi: 10.1016/0028-3908(95)00115-m. [DOI] [PubMed] [Google Scholar]
- 31.Kullmann D M, Erdemli G, Asztely F. Neuron. 1996;17:461–474. doi: 10.1016/s0896-6273(00)80178-6. [DOI] [PubMed] [Google Scholar]
- 32.Isaacson J S, Solís J M, Nicoll R A. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- 33.Dittman J S, Regehr W G. J Neurosci. 1997;17:9048–9059. doi: 10.1523/JNEUROSCI.17-23-09048.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Treves A, Rolls E T. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- 35.Skaggs W E, McNaughton B L. Curr Opin Neurobiol. 1992;2:209–211. doi: 10.1016/0959-4388(92)90014-c. [DOI] [PubMed] [Google Scholar]