Early light-induced proteins protect Arabidopsis from photooxidative stress (original) (raw)
Abstract
The early light-induced proteins (ELIPs) belong to the multigenic family of light-harvesting complexes, which bind chlorophyll and absorb solar energy in green plants. ELIPs accumulate transiently in plants exposed to high light intensities. By using an Arabidopsis thaliana mutant (chaos) affected in the posttranslational targeting of light-harvesting complex-type proteins to the thylakoids, we succeeded in suppressing the rapid accumulation of ELIPs during high-light stress, resulting in leaf bleaching and extensive photooxidative damage. Constitutive expression of ELIP genes in chaos before light stress resulted in ELIP accumulation and restored the phototolerance of the plants to the wild-type level. Free chlorophyll, a generator of singlet oxygen in the light, was detected by chlorophyll fluorescence lifetime measurements in chaos leaves before the symptoms of oxidative stress appeared. Our findings indicate that ELIPs fulfill a photoprotective function that could involve either the binding of chlorophylls released during turnover of pigment-binding proteins or the stabilization of the proper assembly of those proteins during high-light stress.
Light is essential for plants through photosynthetic carbon assimilation. However, when absorbed light exceeds the photosynthetic capacities, reactive O2 species are generated in the chloroplasts, causing oxidative damage to proteins, lipids, and photosynthetic pigments (1, 2). This effect is amplified by environmental stresses such as low temperature or drought, for example, that inhibit the photosynthetic activity, leading to strong yield reduction in crops. In green plants, solar energy is collected by chlorophyll- and carotenoid-binding light-harvesting complexes (LHCs), which are encoded by a multigene family of LHC genes. The expression of these genes is tightly regulated by light (2–4). High light intensities inhibit transcription of LHC genes and activate synthesis of the early light-induced proteins (ELIPs), a class of proteins structurally related to the LHCs (5). The ELIPs are predicted to have three transmembrane helices, and they have sequence similarity to the LHCs in the central pair of helices (6, 7). The similarity is not only at the sequence level, however, because both LHCs and ELIPs bind chlorophyll and carotenoids (8). The ELIPs differ from the LHCs by their transient expression under high-light stress (5). Recently, a number of ELIP-type polypeptides, containing LHC motifs and inducible by high light, have been discovered in vascular plants: the one-helix high-light-induced proteins (9) and the two-helix stress-enhanced proteins (10).
The physiological role of the ELIPs in vascular plants has not yet been elucidated, although there have been several suggestions (11–14). The induction of ELIPs by high light intensities suggests a role in the acclimation to light stress rather than a light-harvesting function, but this has not yet been demonstrated. ELIP antisense transgenic tobacco plants did not exhibit any phenotype of sensitivity to high light levels (15). An obstacle in the analysis of the ELIP function in vivo is the multigenicity of these proteins. In this study, we have circumvented this difficulty by using chaos, an Arabidopsis mutant of the chloroplast signal recognition particle (cpSRP) system that is involved in the rapid targeting of LHC-type proteins, including ELIPs, to thylakoid membranes (16–18). The results presented in this study demonstrate that ELIPs protect plant leaves from photooxidation and suggest that this photoprotective function involves the maintenance of a low level of free chlorophyll under high-light stress conditions.
Materials and Methods
Plant Material and Growth Conditions.
Wild-type Arabidopsis thaliana (L.) Heynh. (ecotype Columbia) and the chaos mutant were grown in a phytotron under controlled conditions of light [300 μmol (photon)⋅m−2⋅s−1, 8-h photoperiod], temperature (22°C day and 18°C night), and relative air humidity (70%). Light stress was imposed by transferring plants aged 5 weeks to a growth chamber at 7°C (day)/6°C (night) and under a photon flux density of 1,000 μmol (photon)⋅m−2⋅s−1. Leaf temperature, measured with an infrared thermometer, was ≈10°C for both WT and chaos.
Spectroscopy.
A PAM-2000 fluorometer (Walz, Effeltrich, Germany) was used to measure modulated chlorophyll fluorescence. The maximal quantum yield of photosystem II (PSII) photochemistry (Fv/Fm), the quantum yield of PSII-mediated electron transport, and the extent of nonphotochemical energy dissipation (NPQ) were measured as described in ref. 19. Thermoluminescence (TL) measurements were performed on leaf discs with a custom-made thermoluminometer, as previously described (19). Each TL measurement was performed on four discs of 6-mm diameter punched out from four different leaves. Picosecond chlorophyll fluorescence measurements under Fo fluorescence conditions (Fo, initial fluorescence of oxidized PSII centers) were performed with a time-correlated single-photon counting system that has been described in detail (20).
ELIP Constructs and _Agrobacterium_-Mediated Transformation of the chaos Mutant.
The cDNAs encoding ELIP1 (At4g14690) and ELIP2 (At3g22840) were amplified by RT-PCR with RNA extracted from Arabidopsis (Col-0) leaves as described elsewhere (18). Primers used were 5′-ATAAGAATGCGGCCGCCATGCAGTCAGTTTTCGCTGCT-3′ and 5′-CGGGATCCTTTAGCTTTAGACTAGAGTCCC-3′) for ELIP1 and 5′-ATAAGAATGCGGCCGCAATGGCAACAGCATCGTTCAAC-3′ and 5′-CGGGATCCATTCATGGGCAAATCGTATTAA-3′ for ELIP2. These primers introduced a _Not_I and a _Bam_HI restriction site, respectively, upstream of the ATG start codon and downstream of the stop codon. These restriction sites were used to clone the cDNA between the promoter (cauliflower mosaic virus 35S/Ω leader) and the polyadenylation signal of pRTΩ/Not intermediate vector. These expression cassettes were then subcloned in the binary vector pGPTV HPT (21). All cDNA constructs were verified by sequencing. The binary vectors were introduced into A. tumefaciens strain C58C1 and used to transform Arabidopsis chaos mutant by vacuum infiltration (22). Primary transformants were selected in vitro on Hoagland/2 medium containing 50 μg/ml of hygromycin and were transferred to soil. Genetic analyses were performed on the progeny to select transformants containing only one copy of ELIP in the transgenic plants.
RNA and Protein Analysis.
Total RNA and thylakoid membrane proteins from leaves of the rosettes were extracted and analyzed as described previously (18, 23). For RNA gel blot analysis, the previously described cDNA of ELIP was gel-purified and labeled by random priming to be used as probe to analyze the ELIP transcript levels in the various transformants.
Results and Discussion
WT Arabidopsis plants were exposed to excess light energy induced by high photon flux density [1,000 μmol (photon)⋅m−2⋅s−1] and low temperature (7°C), causing a strong accumulation of both ELIP transcripts (Fig. 1A) and proteins (Fig. 1B) in the leaves. The same treatment was imposed on the chaos mutant that lacks cpSRP43, a subunit of the cpSRP complex (16, 17). This mutant has a pale-green phenotype because of a substantial reduction of the LHC level (16, 18). The chaos mutation is specific to the LHC protein family, and, so far, no other protein has been reported to be targeted by the cpSRP43 subunit. Neither the level of the photosystem reaction center proteins nor the photochemical efficiency of the photosystems are affected in chaos (16–18). Accordingly, the photochemical activity of chaos leaves, estimated by chlorophyll fluorescence measurements, was not inhibited relative to WT leaves (Table 1). Also, the level and activity of antioxidative enzymes (peroxidase, superoxide dismutase) in the chloroplasts did not differ significantly between WT and chaos (data not shown). Although the ELIP mRNA level in chaos plants exposed to high light at low temperature increased to WT level (Fig. 1A), very little ELIP was found in the thylakoids after 4 days under stress conditions (Fig. 1B). Because ELIPs use the cpSRP pathway for their insertion into the thylakoid membranes in vivo (18), this posttranscriptional regulation of ELIP in chaos is attributable to the low efficiency of protein integration by the mutated cpSRP.
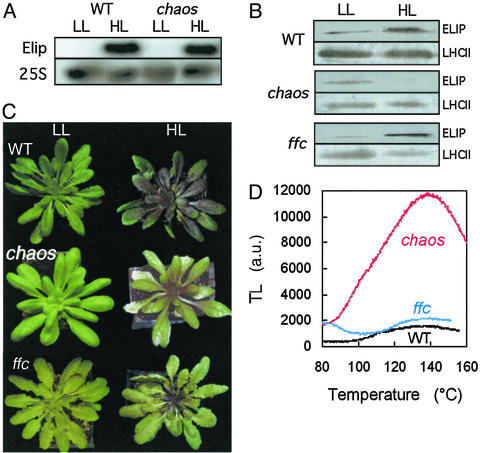
Figure 1.
ELIP synthesis and photooxidative stress in Arabidopsis plants exposed for 6 days to chilling stress in strong light [1,000 μmol (photon)⋅m−2⋅s−1 at 7°C]. (A) Northern blots of ELIP mRNA in WT Arabidopsis and in the chaos mutant before and after chilling stress in strong light. Equal loading of total RNA was checked with the 25S probe. (B) Western blots of ELIP in WT, chaos, and ffc plants. The level of the major LHCII (Lhcb1 + 2) is also shown, as a control of protein loading. (C) Picture of WT, chaos, and ffc plants before (LL) and after (HL) chilling stress in strong light. (D) Lipid-peroxidation-related TL band peaking at ≈135°C in leaves of WT, chaos, and ffc plants exposed to chilling stress in strong light. No TL peak was detected in leaves from WT, chaos, and ffc control plants.
Table 1.
Photosynthetic characteristics of leaves of WT Arabidopsis, chaos mutant, and chaos lines transformed with sense ELIP1 (T4–T6) or ELIP2 gene (T1–T3)
| Genotype | Photon flux density, μmol (photon)⋅m−2⋅s−1 | ||||
|---|---|---|---|---|---|
| 0 | 50 | 400 | |||
| Fv/Fm | φPSII | NPQ | φPSII | NPQ | |
| WT | 0.77 ± 0.01 | 0.57 ± 0.02 | 0.39 ± 0.06 | 0.34 ± 0.05 | 1.09 ± 0.14 |
| chaos | 0.81 ± 0.01 | 0.61 ± 0.02 | 0.34 ± 0.04 | 0.42 ± 0.02 | 1.13 ± 0.27 |
| ELIP2 overexpressors | |||||
| T1 | 0.80 ± 0.02 | 0.64 ± 0.04 | 0.31 ± 0.06 | 0.37 ± 0.04 | 1.28 ± 0.24 |
| T3 | 0.80 ± 0.01 | 0.64 ± 0.02 | 0.36 ± 0.05 | 0.41 ± 0.02 | 1.19 ± 0.10 |
| T2 | 0.80 ± 0.01 | 0.64 ± 0.01 | 0.26 ± 0.03 | 0.45 ± 0.02 | 0.99 ± 0.03 |
| ELIP1 overexpressors | |||||
| T4 | 0.81 ± 0.01 | 0.61 ± 0.01 | 0.32 ± 0.06 | 0.38 ± 0.04 | 1.24 ± 0.16 |
| T5 | 0.80 ± 0.01 | 0.65 ± 0.03 | 0.32 ± 0.07 | 0.48 ± 0.03 | 0.82 ± 0.14 |
| T6 | 0.79 ± 0.01 | 0.64 ± 0.01 | 0.31 ± 0.06 | 0.44 ± 0.02 | 1.16 ± 0.07 |
In contrast to WT plants, chaos plants exposed to chilling stress in high light suffered from extensive photooxidative damage, which manifested as leaf bleaching (Fig. 1C). Photooxidation was quantified by measuring leaf chemiluminescence, which originates from electronically excited states such as singlet O2 (1O2) and triplet carbonyl provoked by radical chain reactions in lipid peroxidation (24, 25). Luminescence was thermally stimulated by slowly warming the sample from 25°C to ≈160°C, leading to the appearance of an emission band peaking at ≈135°C (Fig. 1D). The amplitude of this TL band is a good index of the level of lipid hydroperoxides in the sample (25), which correlates with other assays of lipid peroxidation (26). A strong TL signal was measured in chaos leaves exposed to high-light stress, indicating the occurrence of lipid peroxidation. No such signal was found in WT leaves. When the high-light treatment at low temperature was imposed in an atmosphere with a reduced O2 level (2%), leaf bleaching did not occur and the TL signal was very low (data not shown), confirming the involvement of oxygen in the photoresponse of chaos.
The results presented in Fig. 1 raise the possibility that ELIPs are involved in the protection of plants against photooxidation. At the rosette stage, a mutant of another cpSRP subunit (ffc) exhibits the same lack of LHC as chaos (17, 18). However, under photooxidative stress conditions, ffc accumulated normal levels of ELIPs (Fig. 1B) and behaved like WT (Fig. 1C). This finding ruled out a link between photosensitivity and LHC deficit in chaos. The differential behavior of chaos and ffc under stress conditions also suggests that ELIPs selectively interact with the 43-kDa subunit of cpSRP, which is consistent with our recent finding that cpSRP43 has a much higher affinity for LHC proteins than cpSRP54 (unpublished results).
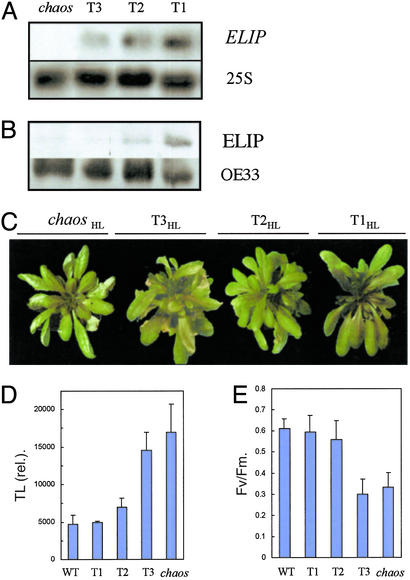
Confirmation of the ELIP role in photostress resistance was performed by overexpressing the ELIP gene in chaos. Even in the complete absence of the transit complex (cpSRP54−/cpSRP43−), a residual accumulation of antenna was still observed, resulting from the basal activity of an alternative targeting pathway (18). Moreover, ELIPs are known to be stable when integrated into the thylakoid membranes (12). Therefore, constitutive ELIP expression was tested to increase its level in chaos before stress application. Arabidopsis ELIP2 cDNA fused to a constitutive promoter was transformed into the chaos mutant. Selection of homozygous plants carrying one locus of the construct was performed. Three representative transformants (named T1, T2, and T3) showing different ELIP levels were selected. Transcript analysis reveals that different levels of transgene expression in the transformants (Fig. 2A) correlated with the protein accumulation in mature leaves of plants grown for 6 weeks in low light (Fig. 2B). No modification of pigment content was observed in those plants (data not shown). The plants were exposed to the high-light treatment that severely damaged chaos leaves, and we found various levels of photosensitivity. The highest ELIP overexpressor (T1; Fig. 2A) was found highly phototolerant and did not bleach in high light (Fig. 2C). The TL signal was low in T1 and comparable to that of the WT (Fig. 3D). The expression of the ELIP gene was very low in line T3, and this line was as photosensitive as chaos. The ELIP level in line T2 appeared to be intermediate between T1 and T3. This line was also intermediate with respect to its resistance to chilling stress in high light. The photochemical efficiency of PSII was also determined after 6 days of stress, and the ranking of the transformants with respect to their phototolerance was similar to that observed for TL (Fig. 2E). To summarize, the phototolerance of the chaos transformants was found to be proportional to the ELIP gene expression and the amount of ELIPs accumulated before the stress treatment, thus clearly establishing the photoprotective function of ELIPs in Arabidopsis. The Arabidopsis genome contains two ELIP genes. The ELIP1 gene was overexpressed in chaos with qualitatively similar results (data not shown); i.e., constitutive expression of ELIP1 also increased the phototolerance of the chaos plants.
Figure 2.
Overexpression of the ELIP2 gene in the chaos Arabidopsis mutant and its repercussion on the resistance to photooxidation. (A) Northern blots of ELIP mRNA in three transformed chaos plants (T1, T2, and T3). Equal loading of total RNA was checked with the 25S probe. (B) Western blot of ELIP in T1-,T2-, and T3-transformed chaos lines. The blots were repeated four times by using different protein preparations, with similar results. Equal protein loading was checked with a Western blot analysis of OE33 (33-kDa protein of the PSII oxygen-evolving complex). (C) Pictures of chaos and the transgenic chaos lines T1, T2, and T3 after chilling stress in strong light [HL, 4 days at 1,000 μmol (photon)⋅m−2⋅s−1 at 7°C]. (D) Lipid peroxidation, estimated by the amplitude of the 135°C TL band. (E) PSII photochemical efficiency, as measured by the chlorophyll fluorescence ratio Fv/Fm, in leaves of WT, chaos, and the chaos transformants T1,T2, and T3 after 6-day exposure to chilling stress in high light.
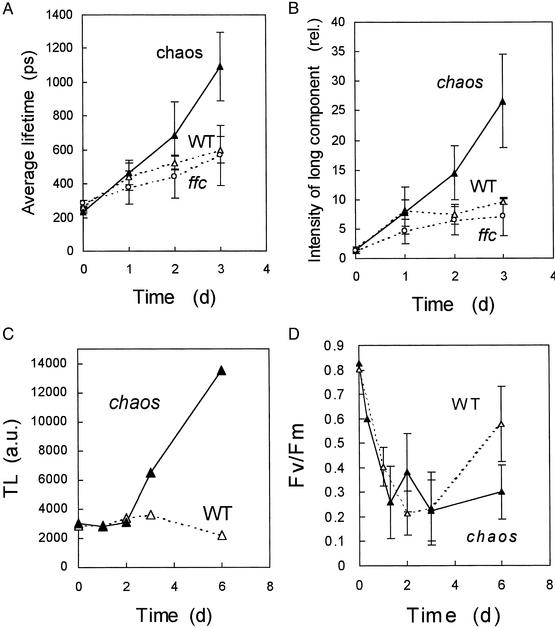
Figure 3.
Chlorophyll fluorescence lifetime measurements in Arabidopsis leaves (WT, chaos, ffc) during chilling stress in high light [1,100 μmol (photon)⋅m−2⋅s−1 at 6°C]. (A) Average lifetime of chlorophyll fluorescence. (B) Intensity of the long fluorescence component with a lifetime of ≈5 ns. Data are mean values of 25–50 separate experiments ± SD. (C and D) The time course of lipid peroxidation (measured by the amplitude of the 135°C TL emission band) (C) and of PSII photoinhibition (measured by the decrease in the Fv/Fm chlorophyll fluorescence ratio) (D) during the stress treatment.
Two functions have been hypothesized for ELIPs, namely a transient pigment carrier function (12) and an energy dissipation function (11, 13, 14). The latter function is not supported by the chlorophyll fluorescence measurements presented in Table 1. NPQ through increased heat emission (27) was similar in WT, chaos, and the chaos transformants. Also, the Fv/Fm of PSII was not influenced by ELIP accumulation. Thus, constitutive expression of ELIP1 or ELIP2 did not increase thermal energy dissipation.
ELIPs were initially discovered in etiolated developing plants during the first hours of greening when protochlorophyllide is massively converted to chlorophyll (28), and it has been suggested that the physiological function of ELIPs could involve chlorophyll binding (12). Possibly, ELIPs could bind the newly formed chlorophyll molecules and could participate in their integration into the photosynthetic complexes. Accordingly, purified ELIPs have been shown to contain chlorophyll a as well as the xanthophyll lutein (8). Uncoupled chlorophyll can be detected in vivo by the lifetime of its fluorescence, which is much longer than the fluorescence lifetime of chlorophyll bound to photosynthetic complexes (29, 30). The average chlorophyll fluorescence lifetime strongly increased in chaos leaves exposed to chilling stress in high light (Fig. 3A). This effect was because of the appearance of a slow component of chlorophyll fluorescence with a lifetime of ≈5 ns (Fig. 3B), which corresponds to the in vitro lifetime of diluted chlorophyll solutions. After 3 days of stress, this component represented >25% of the fluorescence intensity, whereas it represented <2% in unstressed leaves. In WT, the average fluorescence lifetime and the intensity of the long-lived component changed little. The appearance of the 5-ns lifetime component in chaos strongly supports the idea that disorganized chlorophyll accumulated in chaos under light stress. This accumulation preceded lipid peroxidation, which took place only after 3 days of stress (Fig. 3C), and was not associated with an increased photoinhibition of the photosystems (monitored by the Fv/Fm ratio) in chaos relative to WT, at least during the first days of stress (Fig. 3D). As shown previously (19), PSII recovered partially in WT leaves during prolonged stress. The T1 transformant behaved like WT (data not shown).
Our results suggest that a low level of ELIP allows free chlorophyll to accumulate during photoinhibition in high light. Because of its photodynamic properties, free chlorophyll is potentially hazardous and is believed to be involved in photoinhibition of the photosystems (31). By reacting with molecular O2, free chlorophyll in the excited triplet state can generate the toxic 1O2, which in turn can damage thylakoid membrane components, causing leaf bleaching and tissue necrosis in chaos leaves. The fact that this phenomenon did not occur in WT (and in the T1 transformant) can be interpreted in two ways. One possibility could be that ELIPs serve as scavengers of free chlorophyll molecules released during the rapid turnover of the photosynthetic complexes and the reorganization of the photosynthetic machinery in high light, as suggested previously (12). When bound to ELIP, excited triplet chlorophylls can be safely deactivated by triplet–triplet energy transfer to protein-bound xanthophylls. Alternatively, the effect of ELIPs on the free chlorophyll level could be indirect by stabilizing the pigment–protein complexes and/or favoring their proper assembly. Additional studies are necessary to discriminate between these two possibilities. However, one can exclude that the appearance of uncoupled chlorophyll in the chaos mutant was because of the lack of LHC proteins because the ffc mutant, which exhibits a similar LHC deficiency when compared with chaos, was not photosensitive (Fig. 1 C and D). Furthermore, ffc, like WT, showed no increase in the long-lived chlorophyll fluorescence component characterizing the chaos response to high-light stress (Fig. 3B).
From an evolutionary viewpoint, the LHCs of green plants are believed to derive from the one-helix high-light-inducible proteins of Cyanobacteria, after successive gene modifications (7, 9, 13). It is also believed that the ELIPs arose first in this succession before the LHCs (13). Consequently, from this point of view, our results support the suggestion (see refs. 9, 13, and 32) that the initial function of LHC-type proteins was photoprotection. Light harvesting, then, as performed by the LHC polypeptides, is a recent addition to these photoprotective functions. The functions of light harvesting and energy dissipation coexist within the family of LHC-type proteins (27), and this dual activity probably represented an important evolutionary step for photosynthesis in a terrestrial environment in which light can fluctuate considerably (33). The high photosensitivity of chaos indicates that thermal energy dissipation by the LHCs is not sufficient to face all the light stress conditions that vascular plants can experience in the field so that the ELIP gene and other genes coding for ancestral forms of LHCs have been preserved in the genome of green plants (8, 9).
Barley cultivars bred for growth in northern Europe have been shown to accumulate more ELIPs than cultivars grown in southern Europe (34). This observation is consistent with our results showing a link between tolerance to chilling-induced photooxidation and ELIPs. Such intraspecific analysis of ELIP synthesis should be extended to other plants of agronomic interest, especially crop plants of tropical origin (e.g., maize, cucumber, or tomato), which are very sensitive to photodamage at chilling temperature (35). ELIPs constitute a plant defense system in light stress conditions, which have the potential to become new selection markers for the identification and the development of crop plants more tolerant to photooxidative stress conditions.
Acknowledgments
C.H. was supported by a research grant from Biogemma (Aubière, France). We thank the members of the Groupe de Recherches Appliquées en Phytotechnologie (Commissariat à l'Energie Atomique-Cadarache) for skillful technical and logistic assistance.
Abbreviations
LHC
light-harvesting complex
ELIP
early light-induced protein
cpSRP
chloroplast signal recognition particle
PSII
photosystem II
Fv/Fm
maximal quantum yield of PSII photochemistry
NPQ
nonphotochemical energy dissipation
TL
thermoluminescence
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Barber J, Andersson B. Trends Biochem Sci. 1992;17:61–66. doi: 10.1016/0968-0004(92)90503-2. [DOI] [PubMed] [Google Scholar]
- 2.Niyogi K K. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:333–359. doi: 10.1146/annurev.arplant.50.1.333. [DOI] [PubMed] [Google Scholar]
- 3.Teramoto H, Nakamori A, Minagawa J, Ono T. Plant Physiol. 2002;130:325–333. doi: 10.1104/pp.004622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escoubas J-M, Lomas M, Laroche J, Falkowski P G. Proc Natl Acad Sci USA. 1995;92:10233–10241. doi: 10.1073/pnas.92.22.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pötter E, Kloppstech K. Eur J Biochem. 1993;214:779–786. doi: 10.1111/j.1432-1033.1993.tb17980.x. [DOI] [PubMed] [Google Scholar]
- 6.Jansson S A. Trends Plant Sci. 1999;4:236–240. doi: 10.1016/s1360-1385(99)01419-3. [DOI] [PubMed] [Google Scholar]
- 7.Green B R, Kühlbrandt W. Photosynth Res. 1995;44:139–148. doi: 10.1007/BF00018304. [DOI] [PubMed] [Google Scholar]
- 8.Adamska I, Roobol-Boza M, Lindahl M, Andersson B. Eur J Biochem. 1999;260:453–460. doi: 10.1046/j.1432-1327.1999.00178.x. [DOI] [PubMed] [Google Scholar]
- 9.Jansson S, Andersson J, Kim S J, Jackowski G. Plant Mol Biol. 2000;42:345–351. doi: 10.1023/a:1006365213954. [DOI] [PubMed] [Google Scholar]
- 10.Heddad M, Adamska I. Proc Natl Acad Sci USA. 2000;97:3741–3746. doi: 10.1073/pnas.050391397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krol M, Spangfort M D, Huner N P A, Öquist G, Gustafsson P, Jansson S. Plant Physiol. 1995;107:873–883. doi: 10.1104/pp.107.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamska I. Physiol Plant. 1997;100:794–805. [Google Scholar]
- 13.Montané M-H, Kloppstech K. Gene. 2000;258:1–8. doi: 10.1016/s0378-1119(00)00413-3. [DOI] [PubMed] [Google Scholar]
- 14.Braun P, Banet G, Tal T, Malkin S, Zamir A. Plant Physiol. 1996;110:1405–1411. doi: 10.1104/pp.110.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruber J. Ph.D. thesis. Hannover, Germany: University of Hannover; 1999. [Google Scholar]
- 16.Klimyuk V I, Persello-Cartieaux F, Havaux M, Contard P, Schuenemann D, Meiherhoff K, Gouet P, Jones J D G, Hoffman N E, Nussaume L A. Plant Cell. 1999;11:87–100. doi: 10.1105/tpc.11.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amin P, Sy D A, Pilgrim M L, Parry D H, Nussaume L, Hoffman N E. Plant Physiol. 1999;121:61–70. doi: 10.1104/pp.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutin C, Havaux M, Carde J-P, Kloppstech K, Meierhoff K, Hoffman N, Nussaume L. Plant J. 2002;29:531–543. doi: 10.1046/j.0960-7412.2001.01211.x. [DOI] [PubMed] [Google Scholar]
- 19.Havaux M, Kloppstech K. Planta. 2001;213:953–966. doi: 10.1007/s004250100572. [DOI] [PubMed] [Google Scholar]
- 20.Briantais J-M, Dacosta J, Goulas Y, Ducruet J-M, Moya I. Photosynth Res. 1996;48:189–196. doi: 10.1007/BF00041008. [DOI] [PubMed] [Google Scholar]
- 21.Uberlacker B, Werr W. Mol Breed. 1996;2:293–295. [Google Scholar]
- 22.Bechtold N, Helis J, Pelletier G. C R Acad Sci. 1993;316:1194–1199. [Google Scholar]
- 23.Verwoerd T C, Dekker B M M, Hoekema A. Nucleic Acids Res. 1989;17:2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hideg E, Vass I. Photochem Photobiol. 1993;58:280–283. [Google Scholar]
- 25.Vavilin D V, Ducruet J-M. Photochem Photobiol. 1998;68:191–198. [Google Scholar]
- 26.Havaux M, Niyogi K K. Proc Natl Acad Sci USA. 1999;96:8762–8767. doi: 10.1073/pnas.96.15.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horton P, Ruban A V, Walters R G. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- 28.Myer G, Kloppstech K. Eur J Biochem. 1984;138:201–207. doi: 10.1111/j.1432-1033.1984.tb07900.x. [DOI] [PubMed] [Google Scholar]
- 29.Karukstis K K, Sauer K. Biochim Biophys Acta. 1983;725:384–393. [Google Scholar]
- 30.Mysliwa-Kurdziel B, Barthelemy X, Strzalka K, Franck F. Plant Cell Physiol. 1997;38:1187–1196. [Google Scholar]
- 31.Santabarbera S, Neverov K V, Garlaschi F M, Zucchelli G, Jennings R C. FEBS Lett. 2001;491:109–113. doi: 10.1016/s0014-5793(01)02174-3. [DOI] [PubMed] [Google Scholar]
- 32.Li X-P, Björkman O, Shih C, Grossman A R, Rosenquist M, Jansson S, Niyogi K K. Nature. 2000;403:391–395. doi: 10.1038/35000131. [DOI] [PubMed] [Google Scholar]
- 33.Kulheim C, Agren J, Jansson S. Science. 2002;297:91–93. doi: 10.1126/science.1072359. [DOI] [PubMed] [Google Scholar]
- 34.Bei-Paraskevopoulou T, Kloppstech K. Physiol Plant. 1999;106:105–111. [Google Scholar]
- 35.Wise R R. Photosynth Res. 1995;45:79–97. doi: 10.1007/BF00032579. [DOI] [PubMed] [Google Scholar]