Regulation of LiaRS-Dependent Gene Expression in Bacillus subtilis: Identification of Inhibitor Proteins, Regulator Binding Sites, and Target Genes of a Conserved Cell Envelope Stress-Sensing Two-Component System (original) (raw)
Abstract
The regulatory network of the cell envelope stress response in Bacillus subtilis involves both extracytoplasmic function σ-factors and two-component signal transducing systems. One such system, LiaRS, responds to cell wall antibiotics that interfere with the undecaprenol cycle and to perturbation of the cytoplasmic membrane. It is encoded by the last two genes of the liaIHGFSR locus. Here, we analyzed the expression of two LiaR-dependent operons, liaIHGFSR and yhcYZ-yhdA, and characterized a palindromic sequence required for LiaR-dependent activation. Since induction of the strong liaI promoter leads to both liaIH and liaIHGFRS transcripts, LiaR is positively autoregulated. Systematic deletion analysis of the liaI operon revealed that LiaF is a potent negative regulator of LiaR-dependent gene expression: a nonpolar liaF deletion led to constitutive activation of both characterized LiaR-dependent promoters. The liaF gene is conserved in both sequence and genomic context in the Firmicutes group of gram-positive bacteria, located directly upstream of liaSR orthologs. LiaH, a homolog of Escherichia coli phage shock protein A, also plays a more subtle role in negatively modulating the bacitracin-inducible expression from LiaR-dependent promoters. Our results support a model in which the LiaFRS module integrates both positive and negative feedback loops to transduce cell envelope stress signals.
Soil is one of the most complex microbial habitats on earth. Nutrient supply varies greatly and on short notice, as do many physicochemical parameters, such as temperature, oxygen concentration, and moisture. The presence of toxic chemicals and the high population density add another layer of complexity (51). Soil bacteria have adapted to this environment in many ways. A broad range of transport systems together with flexible metabolic capabilities allow them to use a variety of nutrient sources. An extensive set of secondary metabolites is thought to suppress the growth of competitors. This trait is specifically pronounced in the actinomycete group of soil bacteria, the most prodigious producers of antimicrobial compounds: two-thirds of all antibiotics in clinical use are synthesized by these bacteria alone (4, 61). Production of and resistance against antibiotics is therefore an important aspect of life in soil (48).
The cell envelope is the first and major line of defense against threats from the environment. It gives the cell its shape and counteracts the high inner osmotic pressure (16). It also provides an important sensory interface and molecular sieve between a bacterial cell and its surroundings, mediating both information flow and controlled transport of solutes. Because of its crucial role, it is an attractive target for numerous antibiotics (7, 34, 39, 56, 63). Therefore, monitoring cell envelope integrity is critical for survival.
By applying genome-wide transcript profiling, the regulatory network of the cell envelope stress response in Bacillus subtilis was recently characterized: addition of cell wall inhibitors such as bacitracin (produced by Bacillus spp.) and vancomycin (a secondary metabolite of actinomycetes) induces several transmembrane signal transducing pathways, orchestrated by at least three alternative (extracytoplasmic function) σ-factors and four two-component systems (TCS) (10, 42). The use of different antibiotics allowed the differentiation between relatively antibiotic-specific (YvcPQ, BceRS in the case of bacitracin, or σW for vancomycin) and more general cell envelope stress responses, such as σM and the LiaRS (formerly YvqEC) TCS (42). Interestingly, the sensors of all cell envelope stress-sensing TCS (BceS, YvcQ, and LiaS) appear to define a new family of intramembrane sensing histidine kinases. These proteins share an unusually short sensing domain that is almost completely buried in the cytoplasmic membrane. It has been postulated that these kinases detect their signal with their transmembrane helices directly at the membrane interface (42).
The liaIHGFSR locus is expressed from a strictly LiaR-dependent σA-type promoter upstream of the liaI gene (P_liaI_). The LiaRS TCS senses vancomycin, bacitracin, ramoplanin, and nisin and mediates a 100- to 1,000-fold induction from P_liaI_ (43). All four drugs interfere with the lipid II cycle in the cytoplasmic membrane, the rate-limiting step of cell envelope polymer biosynthesis (hence the name; LiaRS stands for _l_ipid II-_i_nteracting _a_ntibiotics response _r_egulator and _s_ensor) (43). A strong stem-loop structure downstream of the second gene, liaH, results in two different transcripts: a major 1.1-kb mRNA consisting of liaIH and a ∼4-kb transcript, including the whole operon. A 74-nucleotide promoter region is fully sufficient for the strong antibiotic-inducible, LiaR-dependent activation of gene expression (43).
Here we identify LiaF, a putative membrane protein, as an integral part of the LiaRS TCS. Deletion of liaF leads to a completely derepressed, stimulus-independent expression from both characterized LiaR-dependent promoter regions. A key role for LiaF as part of a three-component signaling system (LiaFRS) is supported by genomic context clustering analysis: the liaFSR gene cluster is conserved in gram-positive bacteria with a low G+C content (Firmicutes). LiaH, a homolog of E. coli phage shock protein A (PspA), also negatively modulates induction of LiaR-dependent promoters. The minimal bacitracin-inducible promoter fragments controlling expression of both LiaR-dependent operons (liaIHGFSR and yhcYZ-yhdA) each harbor a putative LiaR-binding site, identified by comparative genomics and confirmed by mutational studies, that is essential for LiaR-dependent transcription.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. subtilis was routinely grown in LB medium at 37°C with aeration. All strains used in this study are derivatives of the laboratory wild-type strains W168 and CU1065 (W168 _trpC_2 _attSP_β) and are listed in Table 1. Kanamycin (10 μg/ml), chloramphenicol (5 μg/ml), spectinomycin (100 μg/ml), and tetracycline (10 μg/ml) were used for the selection of the B. subtilis mutants used in this study. Transformation was carried out as described elsewhere (31).
TABLE 1.
Strains used in this study
| Strain | Genotype or characteristic(s)a | Reference or source |
|---|---|---|
| E. coli strain | ||
| DH5αF′ | F′ endA1 hsdR17(rK− mK+) _gln_V44 thi-1 recA1 gyrA (Nalr) relA1 Δ(lacIZYA-argF)U169 deoR [Φ80 _dlac_Δ(lacZ)M15] | Lab stock |
| B. subtilis strains | ||
| W168 | Wild type, trpC2 | Lab stock |
| CU1065 | Wild type, _trpC2 att_SPβ | Lab stock |
| HB0920 | CU1065 liaH::kan | 42 |
| HB0933 | CU1065 liaR::kan | 42 |
| HB0950 | CU1065 att_SPβ2Δ2::P_liaI -cat-lacZ | 43 |
| TMB002 | CU1065 liaF::kan | This study |
| TMB003 | CU1065 liaG::kan | This study |
| TMB004 | CU1065 liaS::kan | This study |
| TMB016 | W168 amyE::(cat P_liaI_ -lacZ) | This study |
| TMB017 | W168 amyE::(cat P_liaI_ -lacZ) liaG::kan | This study |
| TMB018 | W168 amyE::(cat P_liaI_ -lacZ) liaF::kan | This study |
| TMB019 | W168 amyE::(cat P_liaI_ -lacZ) liaS::kan | This study |
| TMB020 | W168 amyE::(cat P_liaI_ -lacZ) liaR::kan | This study |
| TMB021 | W168 amyE::(cat P_liaI_ -lacZ) liaGF::kan | This study |
| TMB027 | HB0950 LiaFΔ(I151-D235)b | This study |
| TMB028 | HB0950 LiaFΔ(S189-V192)b | This study |
| TMB029 | HB0950 LiaFΔ(E126-D146)b | This study |
| TMB053 | W168 amyE::(cat P_liaG_[−68-914]-lacZ) | This study |
| TMB066 | W168 amyE::(cat _PyhcY_[−129-70]-lacZ) liaR::spec | This study |
| TMB069 | W168 amyE::(cat P_yhcY_[−129-70]-lacZ) liaHGF::kan liaR::spec | This study |
| TMB071 | W168 amyE::(cat P_yhcY_[−129-70]-lacZ) | This study |
| TMB072 | W168 amyE::(cat P_yhcY_[−129-70]-lacZ) liaH::kan | This study |
| TMB095 | W168 amyE::(cat P_yhcY_[−129-70]-lacZ) liaF::kan | This study |
| TMB096 | W168 amyE::(cat P_yhcY_[−71-70]-lacZ) liaHGF::kan | This study |
| TMB097 | W168 amyE::(cat P_yhcY_[−88-70]-lacZ) liaHGF::kan | This study |
| TMB098 | W168 amyE::(cat P_yhcY_[−97-70]-lacZ) liaHGF::kan | This study |
| TMB099 | W168 amyE::(cat P_yhcY_[−107-70]-lacZ) liaHGF::kan | This study |
| TMB100 | W168 amyE::(cat P_yhcY_[−117-70]-lacZ) liaHGF::kan | This study |
| TMB101 | W168 amyE::(cat P_yhcY_[−122-70]-lacZ) liaHGF::kan | This study |
| TMB102 | W168 amyE::(cat P_yhcY_[−129-70]-lacZ) liaHGF::kan | This study |
| TMB104 | W168 amyE::(cat P_yhcY_[−244-70]-lacZ) liaHGF::kan | This study |
| TMB107 | W168 amyE::(cat P_liaG_[−68-3]-lacZ) | This study |
| TMB108 | W168 amyE::(cat P_liaI_ -lacZ) liaH::kan | This study |
| TMB111 | W168 amyE::(cat P_liaI_[−102-72] [A−76T A−78T A−81T]-lacZ) | This study |
| TMB112 | W168 amyE::(cat P_liaI_[−102-72] [A−75T A−77T A−79T]-lacZ) | This study |
| TMB113 | W168 amyE::(cat P_liaI_[−102-72] [C−86A G−88A C−93A G−95A T−98A]-lacZ) | This study |
| TMB114 | W168 amyE::(cat P_liaI_[−102-72] [A−78C A−83C]-lacZ) | This study |
| TMB115 | W168 amyE::(cat P_liaI_[−102-72] [C−93A G−95A T−98A]-lacZ) | This study |
| TMB132 | W168 amyE::(cat P_liaG_[81-914]-lacZ) | This study |
| TMB133 | W168 amyE::(cat P_liaI_[−102-72] [C−86A T−87A G−88A]-lacZ) | This study |
| TMB182 | HB0950 LiaFΔ(I151-D235)bthrC::(spec P_xyl_-liaF) | This study |
| TMB183 | HB0950 LiaFΔ(S189-V192)bthrC::(spec P_xyl_-liaF) | This study |
| TMB184 | HB0950 LiaFΔ(E126-D146)bthrC::(spec P_xyl_-liaF) | This study |
Allelic replacement mutagenesis using LFH-PCR.
The long flanking homology PCR (LFH-PCR) technique is derived from a published procedure (62) and was performed as described previously (42). In brief, resistance cassettes were amplified from a suitable vector as template (27, 67). Two primer pairs were designed to amplify ∼1,000-bp DNA fragments flanking the region to be deleted at its 5′ and 3′ ends. The resulting fragments are here called the “up” and “do” fragments. The 3′ end of the up fragment as well as the 5′ end of the do fragment extended into the gene(s) to be deleted in a way that all expression signals of genes up- and downstream of the targeted genes remained intact. Extensions of ∼25 nucleotides were added to the 5′ end of the up-reverse and the do-forward primers that were complementary (opposite strand and inverted sequence) to the 5′ and 3′ ends of the amplified resistance cassette. All obtained fragments were purified using the PCR purification kit from QIAGEN. One hundred to 150 ng of the up and do fragments and 250 to 300 ng of the resistance cassette were used together with the specific up-forward and do-reverse primers at standard concentrations in a second PCR. In this reaction the three fragments were joined by the 25-nucleotide overlapping complementary ends and simultaneously amplified by normal primer annealing. The PCR products were directly used to transform B. subtilis. Transformants were screened by colony PCR, using the up-forward primer with a reverse check primer annealing inside the resistance cassette (see Table 3, below). The integrity of the regions flanking the integrated resistance cassettes was verified by sequencing PCR products of ∼1,000 bp amplified from chromosomal DNA of the resulting mutants. Sequencing was performed in-house by the GenoMIK Center. All PCRs were done in a total volume of 50 μl (10 μl for colony PCR) using the HotStar DNA polymerase Mastermix (QIAGEN) or TripleMaster polymerase mix (Eppendorf) according to the manufacturers' procedures. The primers used in this study are listed below in Table 3.
TABLE 3.
Oligonucleotides used in this study
| Primer no. or amplified fragment | Sequence |
|---|---|
| Oligonucleotides for LFH-PCRa | |
| kan cassette | fwd, CAGCGAACCATTTGAGGTGATAGG; rev, CGATACAAATTCCTCGTAGGCGCTCGG |
| kan-check | fwd, CATCCGCAACTGTCCATACTCTG; rev, CTGCCTCCTCATCCTCTTCATCC |
| spec cassette | fwd, CAGCGAACCATTTGAGGTGATAGGGACTGGCTCGCTAATAACGTAACGTGACTGGCAAGAG; rev, CGATACAAATTCCTCGTAGGCGCTCGGCGTAGCGAGGGCAAGGGTTTATTGTTTTCTAAAATCTG |
| spec-check | fwd, GTTATCTTGGAGAGAATATTGAATGGAC; rev, CGTATGTATTCAAATATATCCTCCTCAC |
| liaH-up | fwd, CTTGTTATTCGTCACTGCC; rev, CCTATCACCTCAAATGGTTCGCTGGTCCTTCATGAACTGACGC |
| liaH-do | fwd, CGAGCGCCTACGAGGAATTTGTATCGCAGACCAGACAAAAGCGGC; rev, CGCTAGATCCCCGCTGTCC |
| liaG-up | fwd, TTGTCGTCGGAATCGCATTGGC; rev, CCTATCACCTCAAATGGTTCGCTGCACATCTTTAACGACGACGGC |
| liaG-do | fwd, CGAGCGCCTACGAGGAATTTGTATCGCCAATCGACATCAAAACGGACA; rev, TTACCCGGCGTTTGACTCGC |
| liaF-up | fwd, AAGGATTTGCGGTCAAGTCC; rev, CCTATCACCTCAAATGGTTCGCTGGCAATGATCAATCCGAGAAGC |
| liaF-do | fwd, CGAGCGCCTACGAGGAATTTGTATCGGATGTGGATGTGAAGTACG; rev, TTCAAGCCGTATGAGGAGGC |
| liaS-up | fwd, GCTTTATCAGCAAGCGGTGACG; rev, CCTATCACCTCAAATGGTTCGCTGTCCCGTTGTCATGCGGATGGC |
| liaS-do | fwd, CGAGCGCCTACGAGGAATTTGTATCGGGCACTCAAATCGAAGTGA; rev, AACCGGGCTGGGAAACGAGGTC |
| liaR-up | fwd, GCTGTCATCAAGCTGGTTCGG; rev, CCTATCACCTCAAATGGTTCGCTGCGATGCTTCGCCGATGACTTC |
| liaR-do | fwd, CGAGCGCCTACGAGGAATTTGTATCGACGCACACCGAAATCATCTC; rev, CTCTTCATCTGATCCGACACAGC |
| Oligonucleotides for quantitative real-time RT-PCR, primer extension, Northern hybridization, and sequencing | |
| _liaR_-RT | fwd, ATTGAAGTCATCGGCGAAGC; rev, AAAGCTCCCGGCAAATTTGC |
| _rpsJ_-RT | fwd, GAAACGGCAAAACGTTCTGG; rev, GTGTTGGGTTCACAATGTCG |
| _rpsE_-RT | fwd, GCGTCGTATTGACCCAAGC; rev, TACCAGTACCGAATCCTACG |
| _yhcY_-PE | GGTTTCCGCAATCGTTTTCAGCG |
| yhcY probeb | fwd, GAGTTGCTGAGTCTGACAAACC; rev, CTAATACGACTCACTATAGGGAGATCGTGAAGCTCCTGAGCGAGGC |
| liaF sequencing | fwd, GCTTTATCAGCAAGCGGTGACG; rev, CCGAACCAGCTTGATGACAGC |
| Oligonucleotides for cloningc | |
| Cloning and site-directed mutagenesis of the liaI promoterd | |
| 0099 (fwd) | CCAT_GAATTC_CCGGTGCGAGATACGACTCC |
| 0100 (rev) | CGAT_GGATCC_TCCTCCAAAAAAGACGGAGATCCC |
| 0231 (fwd) | ACAT_GAATTC_GAGATACGACTCCGGTCTTATtTAtAtATCAATCTCTGATTCG |
| 0232 (fwd) | ACAT_GAATTC_GAGATACGACTCCGGTCTTATATtAtAtTCAATCTCTGATTCG |
| 0265 (fwd) | ACAT_GAATTC_GAGAaACaAaTCCGaTaTTATATAAAAATCAATCTCTGATTCG |
| 0266 (fwd) | ACAT_GAATTC_GAGATACGACTCCGGTCTTcTATAcAAATCAATCTCTGATTCG |
| 0267 (fwd) | ACAT_GAATTC_GAGAaACaAaTCCGGTCTTATATAAAAATCAATCTCTGATTCG |
| 0268 (fwd) | ACAT_GAATTC_GAGATACGACTCCGaaaTTATATAAAAATCAATCTCTGATTCG |
| Promoter deletion analysis of the liaG promoter | |
| 0204 (fwd) | CCATGAATTCTCCCTTCCGCACGTATCAATTCGC |
| 0205 (rev) | AGCCGGATCCTTTGTCATTCCTGGTG |
| 0222 (fwd) | CCATGAATTCGCAGGCCTAGGTTCATAAATGGC |
| 0296 (rev) | AGCCGGATCCCATTCGGTTTCATCCTTCTCATTC |
| Promoter deletion analysis of the yhcY promoter | |
| 0165 (rev) | CGAT_GGATCC_GTGTTGCTTTGATATCGTGCC |
| 0166 (fwd) | CGAT_GAATTC_GACAGTGAAAAGCGACTTGCC |
| 0168 (fwd) | CGAT_GAATTC_GCTTTTTCTTTTTCTCATCC |
| 0169 (fwd) | CGAT_GAATTC_CTTTTTCTCATCCAAAAGTCTG |
| 0259 (fwd) | CGAT_GAATTC_TCTCATCCAAAAGTCTGAAAG |
| 0260 (fwd) | CGAT_GAATTC_AAGTCTGAAAGAAAATCATCCTACAAGTG |
| 0170 (fwd) | CGAT_GAATTC_GAAAATCATCCTACAAGTG |
| 0171 (fwd) | CGAT_GAATTC_CCTACAAGTGAAGCAATGAA |
| 0172 (fwd) | CGAT_GAATTC_GAAATACAAAAAACTGGTATAATC |
| Complementation experiments with liaF | |
| 0035 (fwd) | AGG_AAGCTT_AGAAAGGAGGCGGACACCAGG |
| 0036 (rev) | TCC_GAATTC_TTTCTCATACGTACTTCACATCC |
Construction of transcriptional promoter-lacZ fusions.
An ectopic integration of a P_liaI_ -lacZ fusion was constructed based on the vector pAC6 (Table 2). This vector carries a chloramphenicol resistance cassette for selection in B. subtilis and integrates into the amyE locus by double crossing-over, resulting in a stable integration of P_liaI_-lacZ fusions (58). A promoter fragment similar to P_liaI-_83 described earlier for pSLZ83 (43) was amplified using the primers 99 and 100, thereby introducing EcoRI and BamHI sites, respectively (Table 3). Standard cloning techniques were applied (54). The insert was verified by DNA sequencing at the GenoMIK Center, Göttingen. The resulting pAC6-derived plasmid, pTM1 (Table 2), was linearized with ScaI and used to transform B. subtilis 168 with chloramphenicol selection, resulting in strain TMB016. Subsequently, individual genes of the lia locus were replaced with a kanamycin resistance cassette by transforming TMB016 with chromosomal DNA from strains HB0920, HB0933, and TMB002 to TMB004, resulting in strains TMB017 to TMB022 (see Table 1 for details). The pAC6 vector was also used to construct P_yhcY_- and P_liaG_-lacZ fusions, applying the same cloning strategy (Table 2). The primers used are listed in Table 3, and the resulting strains used in this study are given in Table 1.
TABLE 2.
Vectors and plasmids
| Plasmid | Genotype, sequence, or characteristic(s)a | Primers used for cloning | Reference or source |
|---|---|---|---|
| pAC6 | lacZ fusion vector, integrates at amyE, chloramphenicol resistance | 58 | |
| pXT | Vector for xylose-inducible gene expression, integrates at thrC, pDG1782 derivative, spectinomycin resistance | 18 | |
| pAJ601 | pAC6 P_yhcY_(−71-70)-lacZ | 0165/0172 | This study |
| pAJ602 | pAC6 P_yhcY_(−88-70)-lacZ | 0165/0171 | This study |
| pAJ603 | pAC6 P_yhcY_(−129-70)-lacZ | 0165/0168 | This study |
| pAJ604 | pAC6 P_yhcY_(−97-70)-lacZ | 0165/0170 | This study |
| pAJ606 | pAC6 P_yhcY_(−244-70)-lacZ | 0165/0166 | This study |
| pAJ607 | pAC6 P_yhcY_(−122-70)-lacZ | 0165/0169 | This study |
| pAJ608 | pAC6 P_yhcY_(−117-70)-lacZ | 0165/0259 | This study |
| pAJ609 | pAC6 P_yhcY_(−107-70)-lacZ | 0165/0260 | This study |
| pBD601 | pAC6 P_liaI_(−102-72) (A−76T A−78T A−81T)-lacZ | 0100/0231 | This study |
| pBD602 | pAC6 P_liaI_(−102-72) (A−75T A−77T A−79T)-lacZ | 0100/0232 | This study |
| pBD603 | pAC6 P_liaI_(−102-72) (C−86A G−88A C−93A G−95A T−98A)-lacZ | 0100/0265 | This study |
| pBD604 | pAC6 P_liaI_(−102-72) (A−78C A−83C)-lacZ | 0100/0266 | This study |
| pBD605 | pAC6 P_liaI_(−102-72) (C−93A G−95A T−98A)-lacZ | 0100/0267 | This study |
| pBD606 | pAC6 P_liaI_(−102-72) (C−86A T−87A G−88A)-lacZ | 0100/0268 | This study |
| pDH605 | pAC6 P_liaG_(81-914)-lacZ | 0204/0222 | This study |
| pSJ601 | pAC6 P_liaG_(−68-914)-lacZ | 0204/0205 | This study |
| pSJ607 | pAC6 P_liaG_(−68-3)-lacZ | 0204/0296 | This study |
| pSJ701 | pXT P_xyl_-liaF | 0035/0036 | This study |
| pTM1 | pAC6 P_liaI_(−83-72)-lacZ | 0099/0100 | This study |
Complementation of liaF in in-frame deletion mutants.
An ectopic integration of a P_xyl_ -liaF fusion was constructed based on the vector pXT (18). This vector is a pDG1782 derivative (26) that carries a spectinomycin resistance cassette for selection in B. subtilis and a xylose-inducible promoter for liaF expression and integrates into the thrC locus by double crossing-over, resulting in a stable integration of P_xyl_-liaF fusions. We amplified liaF with primers 35 and 36, thereby introducing EcoRI and HindIII sites, respectively (Table 3). Cloning and verification of DNA sequence were performed as described above. The resulting pXT-derived plasmid, pSJ701 (Table 2), was linearized with ScaI and used to transform B. subtilis TMB027 to TMB029 with spectinomycin selection, resulting in strains TMB182 to TMB184 (Table 1). For liaF expression, 0.2% xylose was added to the medium.
Measurement of induction by β-galactosidase assay.
Cells were inoculated from fresh overnight cultures and grown in LB medium at 37°C with aeration until they reached an optical density at 600 nm (OD600) of ≈0.4. The culture was split, adding bacitracin (50 μg/ml final concentration) to one-half (induced sample) and leaving the other half untreated (uninduced control). After incubation for an additional 30 min at 37°C with aeration, 2 ml of each culture was harvested and the cell pellets were frozen and kept at −20°C. The pellets were resuspended in 1 ml of working buffer and assayed for β-galactosidase activity as described elsewhere, with normalization to cell density (44).
Preparation of total RNA for quantitative real-time RT-PCR, Northern blotting, and primer extension analysis.
Total RNA was extracted from 10 ml of culture with and without bacitracin (50 μg/ml final concentration). Bacitracin was added to the culture at an OD600 of 0.4 (mid-log phase), and the cultures were incubated for 10 min at 37°C with aeration before the cells were harvested and rapidly frozen at −70°C. RNA was prepared using the RNeasy kit (QIAGEN) according to the manufacturer's protocol. The RNA was treated with DNase (using the on-column RNase-free DNase kit from QIAGEN) to remove remaining traces of chromosomal DNA that would interfere with the subsequent reaction. The success of this treatment was verified by a lack of product in a standard PCR, using the same primers as for the real-time reverse transcription-PCR (RT-PCR).
Quantitative real-time RT-PCR.
Measurement of transcript abundance was performed by quantitative real-time RT-PCR, using the QuantiTect SYBRgreen RT-PCR kit (QIAGEN) according to the manufacturer's procedure, with minor modifications. In brief, 100 ng of DNA-free total RNA was used in a total reaction volume of 25 μl with 0.5 μM of each primer (Table 3). The amplification reaction was carried out in an iCycler (Bio-Rad), using the following program: initial incubation of 50.0°C for 30 min, then a 95°C denaturing step for 15 min, followed by 40 cycles (94°C for 15 s, 60°C for 30 s, and 72°C for 30 s). After a subsequent incubation step (55°C for 1 min), the setpoint temperature was increased in 80 cycles (10 s each) by 0.5°C/cycle, starting at 55°C. Expression of rpsE and rpsJ, encoding ribosomal proteins, was monitored as a constitutive reference. Expression of liaR was calculated as the fold change based on the CT values for each gene, as described previously (60).
Primer extension mapping of the yhcY transcriptional start site.
For mapping of the yhcY promoter, HB0920 cells were grown in LB and total RNA was isolated from uninduced and bacitracin-induced (final concentration, 50 μg/ml) mid-logarithmic cultures as described above. Primer extension reactions for yhcY were set up as follows: 30 μg of heat-denatured RNA was hybridized at 65°C to ∼2 pmol of end-labeled primer yhcY-PE (Table 3) in buffer containing 60 mM NaCl, 50 mM Tris-HCl (pH 7.9), 10 mM dithiothreitol, and 40 U of RNasin (Promega) in a total volume of 30 μl. Following hybridization, 50 μl extension buffer (72 mM NaCl, 50 mM Tris-HCl [pH 7.9], 10 mM dithiothreitol, 20 mM MgCl2), deoxynucleoside triphosphates (10 mM), and 2 μl SuperScript II reverse transcriptase (Invitrogen) were added to the mixture and incubation continued at 37°C for 30 min. The primer extension products were precipitated with ethanol, resuspended in sequence loading buffer, and loaded onto 6% polyacrylamide sequencing gels. A PCR cycle sequencing kit (Epicenter) was used to generate sequencing ladders corresponding to the yhcY promoter region.
Comparative genomics analyses.
Multiple sequence alignments were performed using ClustalW, implemented in the BioEdit program package (29). Domain-based analysis of protein sequences was performed using the SMART database (55) at the website http://smart.embl-heidelberg.de/. The genomic context clustering analysis of the lia locus was performed using the ERGO database, which is available through Integrated Genomics, Inc. (http://www.integratedgenomics) and maintained by the GenoMIK Center, Göttingen. The initial identification of the putative LiaR-binding site was done by submitting promoter fragments of 200 nucleotides upstream of the ATG for all liaI homologs identified above to MEME at the website http://meme.sdsc.edu/meme/website/meme.html (2). The resulting weight matrix of the conserved sequence pattern was then used to screen the individual genomes harboring liaRS homologous genes for additional putative LiaR-binding sites with the help of the Virtual Footprint algorithm (46), implemented in the Prodoric database (45) at http://www.prodoric.de/vfp/. The graphical representation of the putative LiaR-binding site was generated using the Weblogo interface (13) at http://weblogo.berkeley.edu/.
RESULTS
LiaF is an integral part of the LiaRS-mediated signal transduction system.
With the exception of liaSR, encoding the sensor kinase and cognate response regulator of a classical bacterial TCS, the functions of the gene products of the liaIHGFSR locus are unknown. No homology can be found in the databases for liaI and liaG. Both genes encode hypothetical proteins, harboring two or one transmembrane helices, respectively, indicative of a membrane localization. The gene product of liaH belongs to the PspA/IM30 protein family (see below), while liaF encodes a putative membrane protein with homology to proteins of unknown function from other gram-positive bacteria.
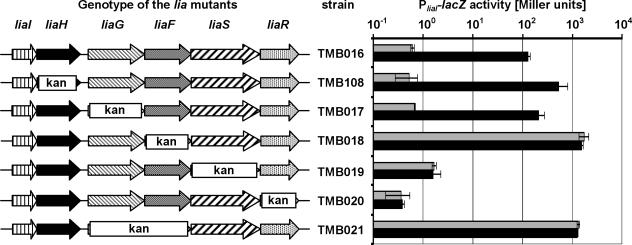
To investigate their possible roles in LiaRS-mediated signal transduction, we investigated the effects of various insertion-deletions in the lia locus on P_liaI_ activity as measured from a P_liaI_-lacZ reporter fusion integrated ectopically at the amyE locus (Fig. 1). The wild-type strain (TMB016) showed a strong response to the presence of bacitracin, resulting in a more-than-200-fold induction of P_liaI_ activity, while virtually no β-galactosidase activity could be detected in the liaR::kan strain (TMB020) under either inducing or noninducing conditions, consistent with the stringent LiaR dependence of P_liaI_ (43). The liaS::kan mutant TMB019, lacking the histidine kinase, no longer responded to bacitracin but did display a slightly increased basal expression level. Surprisingly, P_liaI_ was constitutively active in the liaF::kan mutant TMB018, even in the absence of the inducer. This activity was even 10-fold higher than that measured in the induced wild type.
FIG. 1.
P_liaI_ activity in response to deletions of lia genes. Cultures of strains TMB016 (wild type) and TMB017 to TMB020/TMB108 were grown in LB medium to mid-log phase (OD600, ∼0.4) and split. One half was induced by the addition of bacitracin (final concentration, 50 μg/ml; black bars), and the other half served as an uninduced control (gray bars). Cells were harvested 30 min postinduction and assayed as described previously (43). β-Galactosidase activity is expressed in Miller units (44). A log scale was applied for reasons of clarity. The genotype of the corresponding strains is shown on the left side (see Fig. 3A for labeling patterns of arrows). kan represents the area replaced by the kanamycin resistance cassette. The cassette itself has a size of approximately 1.5 kb in all mutants.
Resistance cassettes inserted into the chromosome can exhibit polar effects on downstream genes. This can either be a positive polar effect, due to readthrough from their own strong promoters (9), or a negative effect due to termination of transcription within the resistance cassette. To investigate possible polar effects, we used quantitative real-time RT-PCR to measure liaR transcription (in the insertion-deletion strains TMB017 to TMB019) relative to the uninduced wild type. In all strains, there was an ∼4-fold increase in liaR transcript. Since P_liaI_ transcription behaves identically in the wild type and a liaG::kan strain while it is constitutively active in the liaF::kan strain, there is no correlation between the weak positive polar effects from the kanamycin resistance cassettes and the observed effects on P_liaI_ activity. Instead, the results support a functional role for LiaF in inhibiting LiaRS-mediated signal transduction.
Spontaneous in-frame deletions in liaF lead to constitutive activity of P_liaI_.
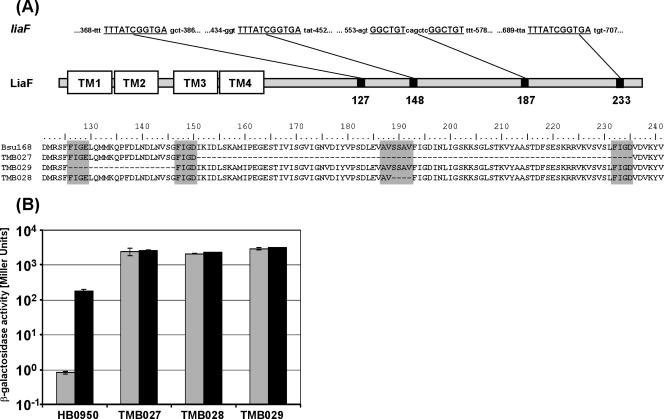
The strong effect of a liaF deletion on P_liaI_ prompted us to perform a genetic screen using strain HB0950, which carries an ectopic pJPM122-based P_liaI_ -cat-lacZ fusion integrated at att_SPβ (43). Direct plating of a mid-logarithmic culture of HB0950 on LB plates with chloramphenicol gave rise to numerous Cmr colonies. We reasoned that any mutation in liaF that renders its gene product dysfunctional would lead to such a spontaneous chloramphenicol resistance in the genetic background of HB0950. Indeed, PCR and sequence analyses indicated that alterations in liaF were associated with many of the spontaneous Cmr mutants. Three strains containing in-frame deletions in liaF were chosen for further analysis (Fig. 2A). These deletions likely arose from recombination between repeated nucleotide sequences (Fig. 2A, upper line)—as has been suggested for a comparable deletion in the Enterococcus faecium histidine kinase VanSB (17)—and result in the deletion of 84, 20, and 4 amino acids. All three in-frame deletion mutants showed a strong bacitracin-independent, constitutive upregulation of LacZ expression in β-galactosidase assays (Fig. 2B), a behavior similar to TMB018 (liaF::kan) (Fig. 1). The effects of these mutations could be complemented by reintroduction of a wild-type liaF allele, integrated as a single copy into the thrC locus and expressed from a xylose-inducible promoter (see Materials and Methods). In the resulting strains, TMB182 to -184 (Table 1), P_liaI is again switched off in the absence of bacitracin and inducible by addition of bacitracin (data not shown). Taken together, these data clearly demonstrate a negative role for the putative transmembrane protein LiaF in LiaRS-mediated signal transduction and identify regions in the hydrophilic C terminus that are crucial for LiaF function.
FIG. 2.
(A) Features and sequence of LiaF derivatives in spontaneous chloramphenicol-resistant mutants, based on in-frame deletions. A sequence alignment of the C-terminal half of LiaF is shown in the lower part, including the reference sequence of wild-type strain W168 in the first line. The amino acids corresponding to the 11- or 6-nucleotide repeat (underlined in the upper part for the liaF gene) are shaded in gray. A graphical representation of LiaF is shown in the middle. The four putative transmembrane helices (TM1 to -4) are located in the N-terminal half of LiaF. The black boxes indicate the positions of the four repeats. (B) P_liaI_ activity in HB0950 (wild type) and derived mutants harboring an in-frame deletion in liaF. Experimental conditions and labeling of the bars are as described for Fig. 1.
The genomic context of liaFSR is conserved in the Firmicutes group of gram-positive bacteria.
A functional connection between proteins is very often reflected by a clustering of their respective genes at the genomic level. For example, a functional connection has been demonstrated for some TCS involved in the cell envelope stress response in the Bacillus/Clostridium group of bacteria which cluster with operons encoding ABC transporters (33). In these detoxification units, the TCS senses the presence of a harmful compound and strongly induces the expression of the corresponding ABC transporter, which then facilitates removal of the compound (42, 47, 49).
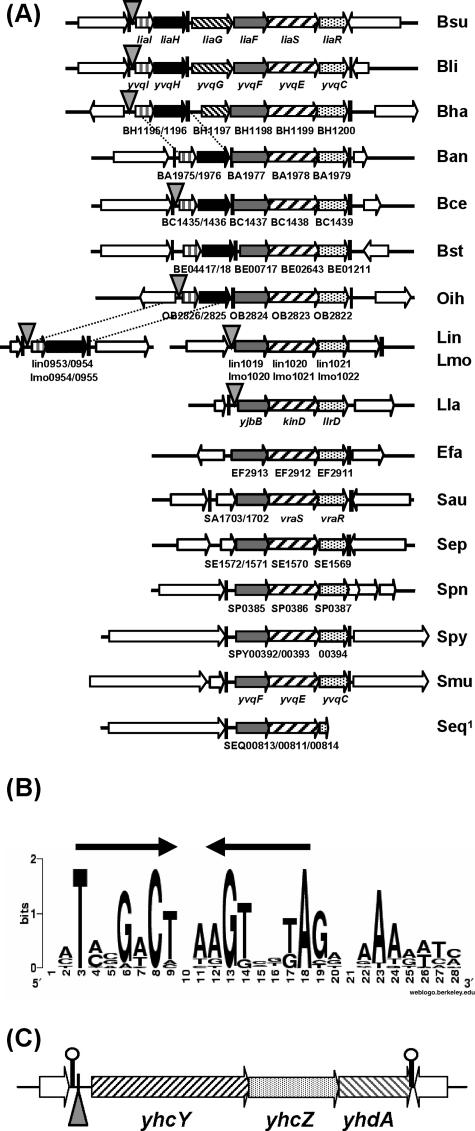
A genomic context clustering analysis of LiaS orthologs revealed a topological conservation of all three genes, liaFSR, in gram-positive bacteria with a low G+C content. Without exception, a liaF homolog is always located directly upstream of the TCS in all species harboring liaSR homologs (Fig. 3A). This finding further substantiates the functional link between LiaF and LiaRS. In contrast, homologs of liaIH are only present in the lia locus of bacilli. Both genes form a separate operon in Listeria species and are apparently lacking in the more distantly related cocci. The liaG gene is only found in Bacillus licheniformis and Bacillus halodurans, the bacteria most closely related to B. subtilis for which complete genome sequences are available (Fig. 3A).
FIG. 3.
Comparative genomics analysis of the lia locus and identification of the putative LiaR-binding site. (A) Conservation of the lia locus in other gram-positive bacteria with a low G+C content. The loci are drawn to scale, with the line of the B. subtilis lia locus corresponding to 7.5 kb. The genes of the lia locus are labeled differently for clarity. Hatched arrows represent genes coding for histidine kinases, and dotted arrows represent response regulators, homologous to LiaS and LiaR, respectively. The liaF homologs are shown in gray, liaH homologs are shown as black arrows, and liaI homologs are shown as white arrows with black vertical lines; genes flanking the lia locus are represented by white arrows. Putative terminators are marked as black vertical bars. The presence of putative LiaR-binding sites is indicated by the gray triangles (see Table 5, below, for details). Gene names are according to GenBank entries of the published genome sequences. Abbreviations of bacterial species: Bsu, B. subtilis; Bli, B. licheniformis; Bha, B. halodurans; Ban, B. anthracis; Bce, Bacillus cereus; Bst, B. stearothermophilus; Oih, Oceanobacillus iheyensis; Lin, Listeria innocua; Lmo, L. monocytogenes; Efa, Enterococcus faecalis; Sau, Staphylococcus aureus; Sep, Staphylococcus epidermidis; Spn, Streptococcus pneumoniae; Spy, Streptococcus pyogenes; Smu, Streptococcus mutans; Seq, Streptococcus equi; Lla, Lactococcus lactis. The genome of S. equi is not yet finished. The end of a contig lies inside the liaR homolog SEQ00814. (B) Graphical representation of the putative LiaR-binding site upstream of P_liaI_. The sequence is derived from a comparative genomics analysis (using the MEME algorithm) (2), based on the promoter regions upstream of liaI and its homologs in bacteria harboring homologous genes to both liaRS and liaI. This graphical representation was generated through the Weblogo page (13) at http://weblogo.berkeley.edu/logo.cgi. (C) Genomic context of the putative LiaR-binding site upstream of the yhcYZ-yhdA operon. The region is drawn to scale, representing 3,000 bp. The exact position of the LiaR-binding site is indicated by the gray triangle. Labeling of arrows is as for panel A.
Identification of a conserved promoter element as a putative binding site for LiaR homologous response regulators from the genus Bacillus.
Protein sequence comparisons (29) revealed an unusually high degree of sequence similarity in the C-terminal domain of all LiaR homologs (data not shown). This domain harbors a LuxR-like helix-turn-helix motif and defines the DNA-binding specificity of LiaR-like response regulators. We reasoned that this finding could be indicative of a conservation of the corresponding DNA-binding site within LiaR target promoters.
To test this hypothesis, we retrieved DNA sequences upstream of liaI homologous genes from the first nine bacterial species shown in Fig. 3A, assuming that these promoters are all subject to regulation by their corresponding LiaRS homologs. The sequences were submitted to the MEME website (2) to identify short stretches of high sequence similarity. One motif was present in all but two regions (Bacillus anthracis and Bacillus stearothermophilus) at a similar distance relative to the start codon. This motif includes an imperfect inverted repeat of 7 nucleotides, separated by 2 bp (Fig. 3B). The resulting weight matrix of this sequence pattern was subsequently submitted to the Virtual Footprint algorithm (46), implemented in the Prodoric database (45), in order to identify candidate LiaR target promoters in the B. subtilis genome. We retrieved three sequences within intergenic regions.
In addition to the P_liaI_ promoter region, two new potential LiaR-binding sites were identified. The first is associated with the yhcYZ-yhdA operon, a locus previously implicated as part of the LiaR regulon (42). The second putative LiaR box was located in a large noncoding region between the genes yozJ and rapK. Subsequent analyses with reporter fusions failed to reveal any transcriptional activity in either direction from this intergenic region (data not shown). Thus, this putative LiaR-binding site may be a remnant of a previously functional locus or a false positive generated by our search algorithm.
The yhcYZ-yhdA operon is expressed from a LiaR-dependent promoter.
A regulatory link between the yhcYZ-yhdA operon and the Lia system had been suggested previously: bacitracin treatment leads to increased expression of this operon in a liaH mutant relative to the wild type (42). Additionally, LiaR-dependent expression of the yhcYZ-yhdA operon was described in a comprehensive microarray study on TCS in B. subtilis: overexpression of LiaR, in the absence of its cognate histidine kinase LiaS, resulted in an induction of this operon (38). However, these experiments do not discriminate between direct and indirect effects of LiaR on the yhcYZ-yhdA operon.
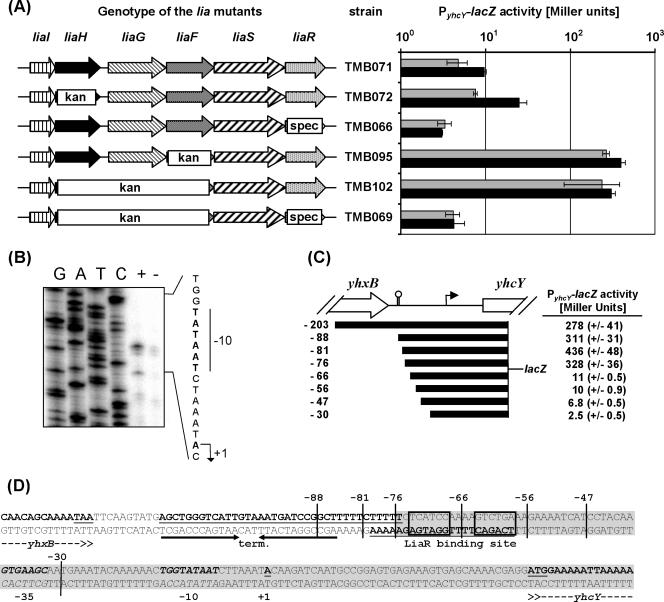
To determine if yhcYZ_-yhdA is regulated by the LiaFRS system, we constructed strains carrying an ectopically integrated P_yhcY_-lacZ fusion. Consistent with previous transcriptome analyses (42), P_yhcY was only weakly inducible by bacitracin in the wild type and was derepressed in the liaH mutant TMB072 (Fig. 4A). While the magnitude of induction was relatively weak compared to that observed with P_liaI_ (Fig. 1), induction was completely LiaR dependent and expression was constitutive in the liaF mutant (TMB095). Since the gene products of both liaH and liaF exhibited a negative effect on P_yhcY_ activity, we also introduced a liaHGF::kan insertion-deletion into TMB071 (resulting in strain TMB102). The mutant behaved like the liaF mutant, consistent with the idea that LiaR is already completely activated in a liaF mutant. As expected, both the liaH and liaF effects are completely LiaR dependent (Fig. 4A). The behavior of P_yhcY_ is comparable to P_liaI_, but the activity as well as the range of inducibility is lower. This weaker activity may be due to a poorer match between the LiaR box preceding yhcY compared to that preceding liaI (see Table 5, below).
FIG. 4.
Characterization of the yhcY promoter and its LiaR-dependent expression. (A) P_yhcY_ -lacZ activity in response to deletions of lia genes, determined by β-galactosidase assay with and without the addition of bacitracin, essentially as described above. See the legend to Fig. 1 for further details. A logarithmic representation of the resulting β-galactosidase activity was chosen for reasons of clarity. (B) Mapping of the transcriptional start site by primer extension analysis indicates that transcription initiates with an A, as shown in the sequence to the right. A primer extension reaction was performed with RNA prepared from strain HB0920 with and without the addition of bacitracin, using the primer yhcY-PE (see Table 3). The sequencing ladder to the left was generated using the same primer, following standard procedures. (C) Promoter deletion analysis of P_yhcY_. A graphical representation of the intergenic region and outline of the fragments used for the promoter dissection are shown on the left. The end of yhxB and the beginning of yhcY are labeled. The putative _rho_-independent terminator downstream of yhxB is indicated by the black stem-loop symbol. The arrow indicates the transcriptional start site. The distance of the 5′ end of the cloned fragments from the transcriptional start is indicated. The activity of these promoters was measured in a liaHGF::kan mutant. The corresponding strains TMB096 to TMB104 were inoculated in LB medium to mid-log phase (OD600, ∼0.4), and the cells from 2 ml were harvested and used for a β-galactosidase assay as described above (without the addition of bacitracin). The resulting β-galactosidase activities, expressed in Miller units (44), are shown. (D) Sequence of the promoter region upstream of yhcY. The transcriptional start site is highlighted in bold, underlined, and the promoter is in bold italics. The putative _rho_-independent yhxB terminator is indicated by arrows and highlighted in bold and underlined, and coding regions are given in bold, with the start and stop codon underlined. The palindrome of the putative LiaR-binding site is underlined and boxed. The 5′ end of the promoter fragments used for the promoter deletion analysis are marked. The minimal LiaR-dependent promoter fragment is shaded in gray. Note that the labeling of the 5′ end of the fragments for the promoter deletion analysis in this figure (C and D) is given relative to the transcription start (+1) for reasons of clarity. Thereby, this nomenclature differs from the labeling of the fragments for cloning (Tables 1 and 2), which are normalized relative to the A of the start codon of yhcY.
TABLE 5.
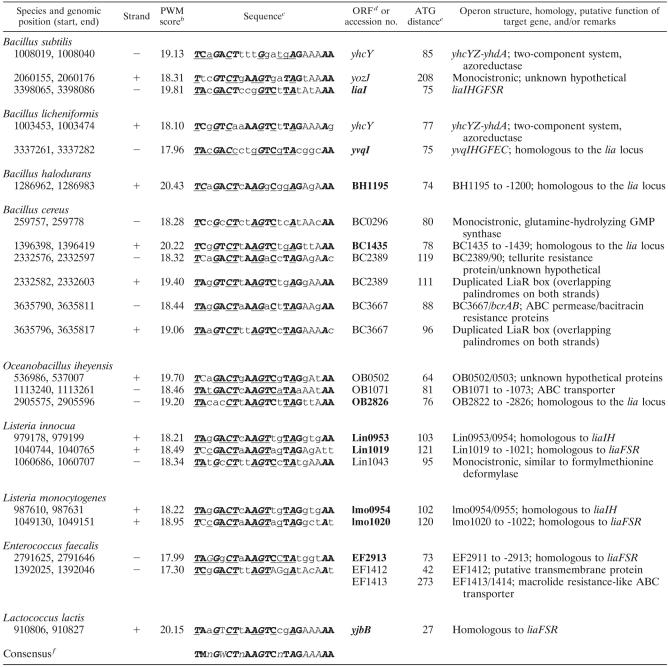
Conserved sequences of putative binding sites for LiaR homologous response regulatorsa and their corresponding target genes
We mapped the 5′ end of the yhcY transcript by primer extension to an A 41 nucleotides upstream of the start codon, thereby also verifying the induction of transcription by bacitracin in a liaH mutant (Fig. 4B). The yhcY promoter has a well-conserved extended −10 region (TGgTATAAT) but a poorly conserved −35 region (GTGAAG) (Fig. 4D). Northern analysis verified that the yhcYZ-yhdA genes constitute an operon of 2.2 kb in size (data not shown).
To further characterize the LiaR-dependent activity of P_yhcY_ and the role of the putative LiaR-binding site, a promoter deletion analysis was performed. Promoter fragments of decreasing length were cloned into pAC6 and verified by DNA sequencing. Integration of the resulting plasmids generated strains TMB096 through TMB104 (the smallest to largest P_yhcY_ fragments, respectively; see Table 1 and Fig. 4C for details). The results demonstrated that a complete LiaR box adjacent to the promoter region is necessary and sufficient for LiaR-dependent promoter activity (Fig. 4C and D).
Site-directed mutagenesis of the putative LiaR-binding site in P_liaI_.
To experimentally test the importance of individual residues within the conserved LiaR-binding motif, we generated a series of LiaR box mutants by site-directed mutagenesis (Table 4). Exchanging the five most highly conserved nucleotides of the LiaR box by an A led to a complete loss of promoter activity in strain TMB113. The same effect was observed with mutations affecting only the promoter-proximal half-side (TMB114 and TMB133). Interestingly, a mutant with the three highly conserved residues in the 5′ side of the palindrome replaced by A (strain TMB115) retained a very low level of bacitracin-inducible activity. All three LiaR boxes of B. subtilis harbor an A-stretch directly 3′ of the inverted repeat, with two residues also being conserved in the putative binding sites of LiaR homologs (Fig. 3B). Therefore, we constructed two additional mutants (TMB111 and -112), each harboring a different set of three A→T exchanges. These mutants each showed a significantly decreased, albeit less severely affected, P_liaI_ activity in the presence of bacitracin. These results support a functional role of this A-rich region, perhaps serving as a DNA-binding site (UP element) that interacts with the α-C-terminal domain of RNA polymerase (23).
TABLE 4.
Mutagenesis of the putative LiaR-binding site upstream of P_liaI_
DISCUSSION
A complex regulatory network, consisting of TCS and alternative σ-factors, orchestrates the cell envelope stress response in B. subtilis (42, 53). One such TCS, LiaRS, mediates the strong induction of its corresponding liaIHGFSR locus in the presence of antibiotics that interfere with the lipid II cycle, such as bacitracin, vancomycin, nisin, and ramoplanin (43). The LiaRS TCS also responds strongly to the presence of cationic antimicrobial peptides known to affect the cell envelope (53) and is weakly induced in response to secretion stress, alkaline shock, and the presence of detergents, organic solvents, ethanol, and some surfactants (32, 43, 52, 66). Here, we investigated the role of individual genes of the lia locus in the LiaRS-mediated cell envelope stress response. We found that LiaF and LiaH act as a strong and weak inhibitor of this signal transduction process, respectively (Fig. 1 and 2). Applying comparative genomics, we identified a putative LiaR-binding site that was verified by mutational analysis (Fig. 3 and Table 4). This LiaR box was also found in the promoter region of a second LiaR target locus, the yhcYZ-yhdA operon, the expression of which we studied in detail (Fig. 4).
LiaRS-mediated signal transduction is conserved in Firmicutes bacteria.
Using comparative genomics, we recognized LiaF-LiaRS as a cell envelope stress response system conserved in the phylum Firmicutes (Fig. 3A). We extended our comparative genomics studies to identify putative LiaR-binding sites and corresponding target genes in other Firmicutes bacteria (Table 5). LiaR boxes were primarily identified in organisms of the order Bacillales, harboring both liaFSR and liaIH homologs. Overall, the number of putative LiaR-binding sites per genome was low (one to four). In B. licheniformis, the closest sequenced relative to B. subtilis, the two putative LiaR-binding sites are also located upstream of homologous loci, i.e., yvqI (liaI) and yhcY. For the two Listeria species, the homologs of liaIH (first gene lin0953/Lmo0954) and liaFSR (lin1019/Lmo1020) are organized in two independent transcriptional units, as noted above (Fig. 3A). Interestingly, a putative LiaR-binding site was identified in the promoter regions of both loci. A LiaR box was also identified upstream of the liaF homologs in Enterococcus faecalis and Lactococcus lactis (Table 5 and Fig. 3A). Remarkably, three of the candidate target loci encode ABC transporters (including a putative bacitracin efflux pump), supporting the role of LiaRS homologs in mediating a cell envelope stress response (Table 5).
The sequence alignment of putative LiaR boxes identified a core motif of 16 nucleotides, followed by an A-rich 3′ extension that could function as an UP element (23). The core motif consists of an inverted repeat of 7 nucleotides, with a spacing of 2 or 4 nucleotides. LiaR belongs to the NarL/FixJ family of response regulators (50). The recognition of an inverted repeat is well established for this family of transcriptional regulators (12, 14, 20) as well as for other response regulators, such as Listeria monocytogenes VirR and Bradyrhizobium japonicum RegR (21, 41).
The conservation of the LiaF-LiaRS signal transduction system and several of its target operons in the Firmicutes (with the noteworthy exception of the genus Clostridium) (Fig. 3A) suggests that this is a conserved cell envelope stress system. Preliminary transcriptome analyses revealed that the lia homologous yvqIHGFEC locus in B. licheniformis is also strongly induced by bacitracin (T. Wecke and T. Mascher, unpublished observation). Other LiaRS homologs, such as Staphylococcus aureus VraSR, Listeria monocytogenes Lmo1021/1022, and Streptococcus pneumoniae HK03/RR03, also respond to cell envelope stress elicited by cell wall antibiotics and membrane perturbations (25, 28, 40). In the case of S. aureus, the VraSR system controls a large regulon of about 50 genes (40). It seems reasonable to postulate that all liaFSR homologs depicted in Fig. 3A encode cell envelope stress-sensing three-component systems. Although _liaFSR_-like operons are present in the streptococci and staphylococci, we were unable to identify LiaR boxes upstream of these loci. We speculate that the corresponding LiaR-like proteins have a distinct DNA-binding selectivity, a hypothesis supported by the divergence of their DNA-binding domains (data not shown).
Taken together, the data seem to indicate the existence of two subgroups within LiaRS-like cell envelope stress-sensing TCS. The genera Bacillus and Listeria harbor the complete lia locus (organized as two independent, but LiaR-dependent, transcriptional units for the latter). In B. subtilis, one prominent effect of activation is a strong overexpression of LiaH and, due to the operon organization, presumably also of the small membrane protein LiaI. In general, these systems have recognizable LiaR-binding sites (exceptions are B. anthracis and B. stearothermophilus) and seem to control only a small number of target genes (Table 5). Members of the second group (VraSR-like TCS) lack a recognizable LiaR box and homologs of liaIH but seem to control a larger regulon.
LiaF is a strong inhibitor of LiaRS-mediated signal transduction.
We identified LiaF as an essential part of the LiaRS signaling system that maintains the system in an inactive state (Fig. 1, 2, and 4A). It is presently unclear whether LiaF senses cell envelope stress directly or indirectly through LiaS. Most characterized proteins inhibiting TCS-mediated signal transduction—such as Sda and KipI in the sporulation phosphorelay of B. subtilis and FixT in FixLJ-mediated nitrogen fixation in _Sinorhizobium meliloti_—interfere with histidine kinase autophosphorylation, thereby suppressing activation of the cognate response regulators (8, 24, 64). Recently, a TCS inhibitor protein was described that shares many features with LiaF: deletion of yycH, encoding a transmembrane protein, results in uncoupled activity of YycFG (59), an essential TCS in B. subtilis and other gram-positive bacteria (11, 22). The three corresponding genes are cotranscribed and conserved by genomic context. It has been postulated that YycH affects the sensor domain of its corresponding histidine kinase, YycG (59). A similar role could be envisioned for LiaF.
LiaH, a PspA homolog, acts a negative modulator of LiaRS-mediated signal transduction.
We also presented evidence that LiaH acts as a weak—and presumably indirect—negative modulator for LiaR-dependent gene expression. The liaIH genes are expressed at a much higher level relative to the downstream genes liaGFSR, due to termination of transcription at a stem-loop structure downstream of liaH (43). The strong induction of liaIH is also apparent at the protein level: LiaH was described as a marker protein for bacitracin treatment in a proteomic study (3). Furthermore, LiaH is a highly abundant protein visible in one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis of cell lysates from cultures of the liaF mutant, even in the absence of bacitracin (data not shown).
LiaH belongs to the family of phage shock proteins. So far, only two members of this protein family have been investigated in detail, PspAs of Yersina enterocolitica and E. coli. The latter is induced by filamentous phage infection (6), hence the name phage shock protein. It is also induced by various other stress conditions, such as heat shock, osmotic shock, exposure to organic solvents and proton ionophores, and long incubation under alkaline conditions (6, 37, 65). Furthermore, misincorporation of secretin pore proteins also induces the psp locus in both Y. enterocolitica and E. coli, as does blocking of the Sec pathway (15, 30, 35, 36). In comparison, LiaH expression is induced by cell envelope stress generated by lipid II-interacting antibiotics, such as bacitracin and ramoplanin, but also by cationic antimicrobial peptides, alkaline shock, exposure to organic solvents, detergents, ethanol, and secretion stress (32, 42, 52, 53, 66). While the mechanisms of their transcriptional regulation differ, the range of inducing conditions for B. subtilis LiaH and E. coli PspA is remarkably similar.
PspA exhibits a dual function that is linked to two different cellular locations (6, 36): in unstressed cells, it is a cytosolic protein that acts as a negative regulator by inhibiting the transcriptional activator PspF (1, 5, 19). Under conditions of cell envelope stress (leaky outer membrane), it is peripherally bound to the inner surface of the cytoplasmic membrane, contributing to the maintenance of the proton motive force and overall membrane integrity (35). This membrane anchoring is mediated by protein-protein interaction with two transmembrane proteins, PspB and PspC (1, 5, 19). Based on our findings, it is tempting to postulate a similar dual role for LiaH. It functions as a weak negative transcriptional regulator without having a DNA-binding domain (Fig. 1 and 4A). Its cotranscription with liaI, coding for a putative membrane protein, suggests that LiaI might serve as a membrane anchor for LiaH, thereby facilitating a second (so-far-unknown) activity, linked to the inner surface of the cytoplasmic membrane.
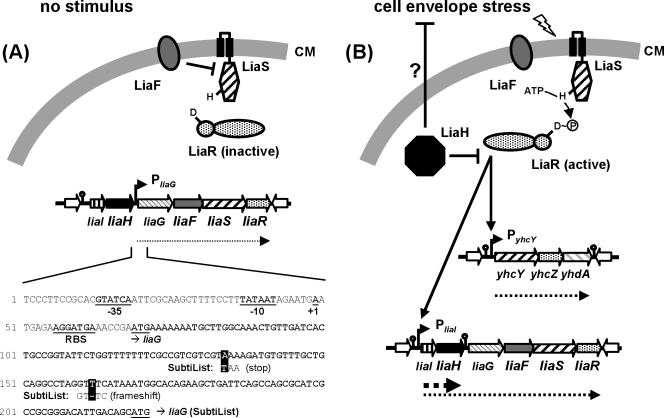
LiaFRS-mediated gene regulation: a working model.
Under normal growth conditions, the last four genes of the lia locus, liaGFSR, are expressed from a recently identified, weak constitutive promoter directly upstream of liaG (Fig. 5A and data not shown; note the corrected sequence for liaG) (see Materials and Methods). P_liaG_-dependent expression and the inhibitory activity of LiaF ensure that the system is present but switched off (Fig. 5A). In the presence of cell envelope stress, LiaS is released from LiaF-dependent inhibition and phosphorylated LiaR binds upstream of P_liaI_ and P_yhcY_ (Fig. 5B). P_liaI_ induction gives rise to two transcripts, resulting most notably in overexpression of LiaH. Due to significant readthrough transcription, this induction defines a positive autoregulatory feedback loop (stress-induced expression of liaGFSR) that allows the system to rapidly respond to envelope stress. The importance of this positive feedback loop is underscored by the identification of candidate LiaR boxes in the promoter regions of those liaFSR homologs that do not receive readthrough transcription from upstream liaIH expression (Fig. 3A), i.e., in Listeria species.
FIG. 5.
Model of LiaRS-dependent gene expression in B. subtilis. Transcripts are indicated by dotted lines. Activation is indicated by solid arrows, and inhibitions are shown by T-shaped lines. (A) P_liaG_-dependent expression of liaGFSR in the absence of cell envelope stress. LiaF inhibits the LiaRS TCS. The sequence of the corresponding promoter region is given below. Resequencing revealed two mistakes in the original genome sequence, which result in the generation of a stop codon and a frameshift within the 5′ end of liaG, respectively. Therefore, the corresponding LiaG protein is extended by another 50 amino acids at its N terminus. The added sequence harbors a signal peptide and a potential transmembrane helix, as identified using the SMART database (55). This indicates that LiaG is a putative membrane-anchored protein rather than of cytoplasmic localization, as was originally annotated. (B) LiaRS-dependent gene expression in the presence of cell envelope stress. LiaF repression is released, and activated LiaR binds to its target promoters, including its own operon (positive feedback loop). The strong overexpression of LiaH presumably counteracts cell envelope stress. Additionally, LiaH functions as an inhibitor of LiaR-dependent gene expression (negative feedback loop). See text for further details.
The physiological role of LiaH and the other LiaR targets is not yet clear. Based on its homology to PspA, we speculate that LiaH might be involved in counteracting envelope stress, possibly by securing membrane integrity. Additionally, it plays a role in a negative feedback loop, thereby counteracting continued LiaR activity. This allows the system to establish a level of LiaH appropriate for the stress condition (Fig. 5B). Two possible mechanisms for this LiaH-dependent feedback loop can be envisioned, both based on the analogy to E. coli PspA: first, LiaH could regulate LiaR activity through direct protein-protein interaction as suggested above. Second, LiaH could help to restore envelope integrity, thereby removing the stress signal that activated LiaRS in the first place. In the absence of envelope stress, LiaF would then again be able to inhibit LiaRS, thereby efficiently switching the system off. It is more and more recognized that such combined positive and negative feedback loops in a regulatory pathway play an important role for adaptive responses of a bacterial population to its environment (i.e., competence and sporulation in B. subtilis), allowing a fast and differentiated response (57).
Acknowledgments
We thank Sierd Bron for the gift of strains BFS1714 and BFS1715 and Tarek Msadek for the gift of plasmid pXT. Furthermore, we gratefully acknowledge the contributions of a number of students who were involved in various aspects of the results described herein during their lab rotation projects (in order of appearance): Sara L. Zimmer (Cornell University) for primer extension of P_yhcY_, Terry-Ann Smith (NYU) and Tina Wecke for contributing to the initial mutational analysis of the lia locus, Falk Kalamorz for SDS-PAGE analysis of LiaH overexpression, Diana Hoyer for taking part in the identification of P_liaG_, and Bastian Dörrbecker for the mutagenesis of the LiaR-binding site of P_liaI_. We also thank J. Stücke, in whose lab parts of this work were carried out, for his support.
This work was supported by grant MA 3269 from the Deutsche Forschungsgemeinschaft and grants from the Fonds der Chemischen Industrie (to T.M.) and by grant GM-47446 from the National Institutes of Health (to J.D.H.).
REFERENCES
- 1.Adams, H., W. Teertstra, J. Demmers, R. Boesten, and J. Tommassen. 2003. Interactions between phage-shock proteins in Escherichia coli. J. Bacteriol. 185**:**1174-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey, T. L., and C. Elkan. 1994. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 2**:**28-36. [PubMed] [Google Scholar]
- 3.Bandow, J. E., H. Brotz, L. I. Leichert, H. Labischinski, and M. Hecker. 2003. Proteomic approach to understanding antibiotic action. Antimicrob. Agents Chemother. 47**:**948-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentley, S. D., K. F. Chater, A. M. Cerdeno-Tarraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C. H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M. A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417**:**141-147. [DOI] [PubMed] [Google Scholar]
- 5.Bordes, P., S. R. Wigneshweraraj, J. Schumacher, X. Zhang, M. Chaney, and M. Buck. 2003. The ATP hydrolyzing transcription activator phage shock protein F of Escherichia coli: identifying a surface that binds σ54. Proc. Natl. Acad. Sci. USA 100**:**2278-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brissette, J. L., M. Russel, L. Weiner, and P. Model. 1990. Phage shock protein, a stress protein of Escherichia coli. Proc. Natl. Acad. Sci. USA 87**:**862-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bugg, T. D., and C. T. Walsh. 1992. Intracellular steps of bacterial cell wall peptidoglycan biosynthesis: enzymology, antibiotics, and antibiotic resistance. Nat. Prod. Rep. 9**:**199-215. [DOI] [PubMed] [Google Scholar]
- 8.Burkholder, W. F., I. Kurtser, and A. D. Grossman. 2001. Replication initiation proteins regulate a developmental checkpoint in Bacillus subtilis. Cell 104**:**269-279. [DOI] [PubMed] [Google Scholar]
- 9.Cao, M., L. Salzberg, C. S. Tsai, T. Mascher, C. Bonilla, T. Wang, R. W. Ye, L. Marquez-Magana, and J. D. Helmann. 2003. Regulation of the Bacillus subtilis extracytoplasmic function protein σY and its target promoters. J. Bacteriol. 185**:**4883-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao, M., T. Wang, R. Ye, and J. D. Helmann. 2002. Antibiotics that inhibit cell wall biosynthesis induce expression of the Bacillus subtilis σW and σM regulons. Mol. Microbiol. 45**:**1267-1276. [DOI] [PubMed] [Google Scholar]
- 11.Clausen, V. A., W. Bae, J. Throup, M. K. Burnham, M. Rosenberg, and N. G. Wallis. 2003. Biochemical characterization of the first essential two-component signal transduction system from Staphylococcus aureus and Streptococcus pneumoniae. J. Mol. Microbiol. Biotechnol. 5**:**252-260. [DOI] [PubMed] [Google Scholar]
- 12.Crater, D. L., and C. P. Moran, Jr. 2001. Identification of a DNA binding region in GerE from Bacillus subtilis. J. Bacteriol. 183**:**4183-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crooks, G. E., G. Hon, J. M. Chandonia, and S. E. Brenner. 2004. WebLogo: a sequence logo generator. Genome Res. 14**:**1188-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahl, J. L., B. Y. Wei, and R. J. Kadner. 1997. Protein phosphorylation affects binding of the Escherichia coli transcription activator UhpA to the uhpT promoter. J. Biol. Chem. 272**:**1910-1919. [DOI] [PubMed] [Google Scholar]
- 15.Darwin, A. J., and V. L. Miller. 2001. The psp locus of Yersinia enterocolitica is required for virulence and for growth in vitro when the Ysc type III secretion system is produced. Mol. Microbiol. 39**:**429-445. [DOI] [PubMed] [Google Scholar]
- 16.Delcour, J., T. Ferain, M. Deghorain, E. Palumbo, and P. Hols. 1999. The biosynthesis and functionality of the cell-wall of lactic acid bacteria. Antonie Leeuwenhoek 76**:**159-184. [PubMed] [Google Scholar]
- 17.Depardieu, F., P. Courvalin, and T. Msadek. 2003. A six amino acid deletion, partially overlapping the VanSB G2 ATP-binding motif, leads to constitutive glycopeptide resistance in VanB-type Enterococcus faecium. Mol. Microbiol. 50**:**1069-1083. [DOI] [PubMed] [Google Scholar]
- 18.Derre, I., G. Rapoport, and T. Msadek. 2000. The CtsR regulator of stress response is active as a dimer and specifically degraded in vivo at 37°C. Mol. Microbiol. 38**:**335-347. [DOI] [PubMed] [Google Scholar]
- 19.Dworkin, J., G. Jovanovic, and P. Model. 2000. The PspA protein of Escherichia coli is a negative regulator of σ54-dependent transcription. J. Bacteriol. 182**:**311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egland, K. A., and E. P. Greenberg. 2000. Conversion of the Vibrio fischeri transcriptional activator, LuxR, to a repressor. J. Bacteriol. 182**:**805-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emmerich, R., P. Strehler, H. Hennecke, and H.-M. Fischer. 2000. An imperfect inverted repeat is critical for DNA binding of the response regulator RegR of Bradyrhizobium japonicum. Nucleic Acids Res. 28**:**4166-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabret, C., and J. A. Hoch. 1998. A two-component signal transduction system essential for growth of Bacillus subtilis: implications for anti-infective therapy. J. Bacteriol. 180**:**6375-6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaal, T., W. Ross, E. E. Blatter, H. Tang, X. Jia, V. V. Krishnan, N. Assa-Munt, R. H. Ebright, and R. L. Gourse. 1996. DNA-binding determinants of the alpha subunit of RNA polymerase: novel DNA-binding domain architecture. Genes Dev. 10**:**16-26. [DOI] [PubMed] [Google Scholar]
- 24.Garnerone, A.-M., D. Cabanes, M. Foussard, P. Boistard, and J. Batut. 1999. Inhibition of the FixL sensor kinase by the FixT protein in Sinorhizobium meliloti. J. Biol. Chem. 274**:**32500-32506. [DOI] [PubMed] [Google Scholar]
- 25.Gravesen, A., B. Kallipolitis, K. Holmstrom, P. E. Hoiby, M. Ramnath, and S. Knochel. 2004. _pbp2229_-mediated nisin resistance mechanism in Listeria monocytogenes confers cross-protection to class IIa bacteriocins and affects virulence gene expression. Appl. Environ. Microbiol. 70**:**1669-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerout-Fleury, A. M., N. Frandsen, and P. Stragier. 1996. Plasmids for ectopic integration in Bacillus subtilis. Gene 180**:**57-61. [DOI] [PubMed] [Google Scholar]
- 27.Guerout-Fleury, A. M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic resistance cassettes for Bacillus subtilis. Gene 167**:**335-336. [DOI] [PubMed] [Google Scholar]
- 28.Haas, W., D. Kaushal, J. Sublett, C. Obert, and E. I. Tuomanen. 2005. Vancomycin stress response in a sensitive and a tolerant strain of Streptococcus pneumoniae. J. Bacteriol. 187**:**8205-8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41**:**95-98. [Google Scholar]
- 30.Hardie, K. R., S. Lory, and A. P. Pugsley. 1996. Insertion of an outer membrane protein in Escherichia coli requires a chaperone-like protein. EMBO J. 15**:**978-988. [PMC free article] [PubMed] [Google Scholar]
- 31.Harwood, C. R., and S. M. Cutting. 1990. Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, United Kingdom.
- 32.Hyyryläinen, H. L., M. Sarvas, and V. P. Kontinen. 2005. Transcriptome analysis of the secretion stress response of Bacillus subtilis. Appl. Microbiol. Biotechnol. 67**:**389-396. [DOI] [PubMed] [Google Scholar]
- 33.Joseph, P., G. Fichant, Y. Quentin, and F. Denizot. 2002. Regulatory relationship of two-component and ABC transport systems and clustering of their genes in the Bacillus/Clostridium group, suggest a functional link between them. J. Mol. Microbiol. Biotechnol. 4**:**503-513. [PubMed] [Google Scholar]
- 34.Kahne, D., C. Leimkuhler, W. Lu, and C. Walsh. 2005. Glycopeptide and lipoglycopeptide antibiotics. Chem. Rev. 105**:**425-448. [DOI] [PubMed] [Google Scholar]
- 35.Kleerebezem, M., W. Crielaard, and J. Tommassen. 1996. Involvement of stress protein PspA (phage shock protein A) of Escherichia coli in maintenance of the proton motive force under stress conditions. EMBO J. 15**:**162-171. [PMC free article] [PubMed] [Google Scholar]
- 36.Kleerebezem, M., and J. Tommassen. 1993. Expression of the pspA gene stimulates efficient protein export in Escherichia coli. Mol. Microbiol. 7**:**947-956. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi, H., M. Yamamoto, and R. Aono. 1998. Appearance of a stress-response protein, phage-shock protein A, in Escherichia coli exposed to hydrophobic organic solvents. Microbiology 144(Pt. 2)**:**353-359. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi, K., M. Ogura, H. Yamaguchi, K. Yoshida, N. Ogasawara, T. Tanaka, and Y. Fujita. 2001. Comprehensive DNA microarray analysis of Bacillus subtilis two-component regulatory systems. J. Bacteriol. 183**:**7365-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koch, A. L. 2003. Bacterial wall as target for attack: past, present, and future research. Clin. Microbiol. Rev. 16**:**673-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuroda, M., H. Kuroda, T. Oshima, F. Takeuchi, H. Mori, and K. Hiramatsu. 2003. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol. Microbiol. 49**:**807-821. [DOI] [PubMed] [Google Scholar]
- 41.Mandin, P., H. Fsihi, O. Dussurget, M. Vergassola, E. Milohanic, A. Toledo-Arana, I. Lasa, J. Johansson, and P. Cossart. 2005. VirR, a response regulator critical for Listeria monocytogenes virulence. Mol. Microbiol. 57**:**1367-1380. [DOI] [PubMed] [Google Scholar]
- 42.Mascher, T., N. G. Margulis, T. Wang, R. W. Ye, and J. D. Helmann. 2003. Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol. Microbiol. 50**:**1591-1604. [DOI] [PubMed] [Google Scholar]
- 43.Mascher, T., S. L. Zimmer, T. A. Smith, and J. D. Helmann. 2004. Antibiotic-inducible promoter regulated by the cell envelope stress-sensing two-component system LiaRS of Bacillus subtilis. Antimicrob. Agents Chemother. 48**:**2888-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 45.Münch, R., K. Hiller, H. Barg, D. Heldt, S. Linz, E. Wingender, and D. Jahn. 2003. PRODORIC: prokaryotic database of gene regulation. Nucleic Acids Res. 31**:**266-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Münch, R., K. Hiller, A. Grote, M. Scheer, J. Klein, M. Schobert, and D. Jahn. 2005. Virtual Footprint and PRODORIC: an integrative framework for regulon prediction in prokaryotes. Bioinformatics 21**:**4187-4189. [DOI] [PubMed] [Google Scholar]
- 47.Neumüller, A. M., D. Konz, and M. A. Marahiel. 2001. The two-component regulatory system BacRS is associated with bacitracin “self-resistance” of Bacillus licheniformis ATCC 10716. Eur. J. Biochem. 268**:**3180-3189. [DOI] [PubMed] [Google Scholar]
- 48.Nwosu, V. C. 2001. Antibiotic resistance with particular reference to soil microorganisms. Res. Microbiol. 152**:**421-430. [DOI] [PubMed] [Google Scholar]
- 49.Ohki, R., Giyanto, K. Tateno, W. Masuyama, S. Moriya, K. Kobayashi, and N. Ogasawara. 2003. The BceRS two-component regulatory system induces expression of the bacitracin transporter, BceAB, in Bacillus subtilis. Mol. Microbiol. 49**:**1135-1144. [DOI] [PubMed] [Google Scholar]
- 50.Pao, G. M., R. Tam, L. S. Lipschitz, and M. H. Saier, Jr. 1994. Response regulators: structure, function and evolution. Res. Microbiol. 145**:**356-362. [DOI] [PubMed] [Google Scholar]
- 51.Paul, E. A., and F. E. Clark. 1996. Soil microbiology and biochemistry, 2nd ed. Academic Press, San Diego, Calif.
- 52.Petersohn, A., M. Brigulla, S. Haas, J. D. Hoheisel, U. Volker, and M. Hecker. 2001. Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 183**:**5617-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pietiäinen, M., M. Gardemeister, M. Mecklin, S. Leskela, M. Sarvas, and V. P. Kontinen. 2005. Cationic antimicrobial peptides elicit a complex stress response in Bacillus subtilis that involves ECF-type sigma factors and two-component signal transduction systems. Microbiology 151**:**1577-1592. [DOI] [PubMed] [Google Scholar]
- 54.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 55.Schultz, J., F. Milpetz, P. Bork, and C. P. Ponting. 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 95**:**5857-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silver, L. L. 2003. Novel inhibitors of bacterial cell wall synthesis. Curr. Opin. Microbiol. 6**:**431-438. [DOI] [PubMed] [Google Scholar]
- 57.Smits, W. K., O. P. Kuipers, and J. W. Veening. 2006. Phenotypic variation in bacteria: the role of feedback regulation. Nat. Rev. Microbiol. 4**:**259-271. [DOI] [PubMed] [Google Scholar]
- 58.Stülke, J., I. Martin-Verstraete, M. Zagorec, M. Rose, A. Klier, and G. Rapoport. 1997. Induction of the Bacillus subtilis ptsGHI operon by glucose is controlled by a novel antiterminator, GlcT. Mol. Microbiol. 25**:**65-78. [DOI] [PubMed] [Google Scholar]
- 59.Szurmant, H., K. Nelson, E.-J. Kim, M. Perego, and J. A. Hoch. 2005. YycH regulates the activity of the essential YycFG two-component system in Bacillus subtilis. J. Bacteriol. 187**:**5419-5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Talaat, A. M., S. T. Howard, W. Hale IV, R. Lyons, H. Garner, and S. A. Johnston. 2002. Genomic DNA standards for gene expression profiling in Mycobacterium tuberculosis. Nucleic Acids Res. 30**:**e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thompson, C. J., D. Fink, and L. D. Nguyen. 2002. Principles of microbial alchemy: insights from the Streptomyces coelicolor genome sequence. Genome Biol. 3**:**REVIEWS1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wach, A. 1996. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast 12**:**259-265. [DOI] [PubMed] [Google Scholar]
- 63.Walsh, C. 2003. Antibiotics: actions, origins, resistance. ASM Press, Washington, D.C.
- 64.Wang, L., R. Grau, M. Perego, and J. A. Hoch. 1997. A novel histidine kinase inhibitor regulating development in Bacillus subtilis. Genes Dev. 11**:**2569-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiner, L., and P. Model. 1994. Role of an Escherichia coli stress-response operon in stationary-phase survival. Proc. Natl. Acad. Sci. USA 91**:**2191-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wiegert, T., G. Homuth, S. Versteeg, and W. Schumann. 2001. Alkaline shock induces the Bacillus subtilis σW regulon. Mol. Microbiol. 41**:**59-71. [DOI] [PubMed] [Google Scholar]
- 67.Youngman, P. 1990. Use of transposons and integrational vectors for mutagenesis and construction of gene fusions in Bacillus subtilis, p. 221-266. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & sons, Chichester, United Kingdom.