Anti-IL-9 vaccination prevents worm expulsion and blood eosinophilia in Trichuris muris-infected mice (original) (raw)
Abstract
Production of neutralizing anti-IL-9 antibodies was induced in mice by immunization with mouse IL-9 coupled to ovalbumin. In the six mouse strains tested, a strong and long-lasting anti-IL-9 response developed with seric inhibitory titers of 10−3 to 10−5, as measured in an in vitro IL-9-dependent cell proliferation assay. In vivo, this immunization completely abrogated the increase in mast-cell protease-1 levels as well as the eosinophilia observed in mice after implantation of an IL-9-secreting tumor. We took advantage of this method to assess the role of IL-9 in infections with nematode Trichuris muris, where IL-9 production correlates with the resistant phenotype. C57BL/6 mice, which normally expel the parasite, became susceptible after anti-IL-9 immunization, demonstrating that IL-9 plays a critical role in this model. In addition, neutralization of IL-9 also inhibited parasite-induced blood eosinophilia. Taken together, the present data demonstrate the potency of our strategy to antagonize IL-9 in vivo and shows that this cytokine plays a major role in resistance against T. muris infection.
Since its discovery as a T and mast-cell growth factor produced by Th2 cells (1–3), IL-9 physiological roles have gradually expanded (4). Prominent features, disclosed by analysis of transgenic mice overexpressing IL-9, include increased susceptibility to lymphomagenesis (5), intestinal mastocytosis (6), expansion of the B-1 lymphocyte population (7), bronchial hyperresponsiveness (8, 9), and airway eosinophilia (10). In line with these observations, genetic analyses revealed a linkage between both IL9 and IL9R genes to human asthma (11, 12), a finding that was confirmed, with respect to IL-9, in murine models (13).
Although detrimental in asthma, elevated production of Th2 cytokines has been reported to correlate with resistance to certain parasite infections (14). IL-9, for example, was found to enhance mouse resistance to infection with the cecal dwelling nematode Trichuris muris (15). This resistance was associated with high IgE and IgG1 levels, as well as with pronounced intestinal mastocytosis.
On the basis of these observations, inhibiting IL-9 activity in vivo would probably be beneficial in asthma and deleterious in parasite infections. To test these predictions and evaluate the actual importance of IL-9 in these processes, we developed a method aimed at inducing anti-IL-9 autoantibodies in vivo.
The absence of T cell help has been suggested previously to be crucial for B cell tolerance toward self proteins (16). Therefore, by providing physically linked T cell help, it should be possible to overcome B cell nonresponsiveness toward self antigens. By using bovine luteinizing hormone (LH) as a self protein coupled to ovalbumin (OVA), Johnson et al. (17) were able to induce high titers of autoantibodies against LH, causing cows to become anestrous. Similarly, a vaccine that prevents pregnancy in women was developed by coupling human chorionic gonadotropin and ovine luteinizing hormone to tetanus and diphtheria toxoids (18). More recently, immunization with a fusion protein between an OVA epitope and mouse TNF-α was found to prevent experimental cachexia and collagen-induced arthritis in mice (19).
Here, we report that chemical linking of murine IL-9 to OVA results in the formation of a highly immunogenic complex that ensures production of high titers of neutralizing anti-IL-9 antibodies in mice. These autoantibodies were able to prevent IL-9-induced mast-cell activation and eosinophilia. In addition, they considerably increased mouse susceptibility to T. muris infection.
Materials and Methods
Mice and Parasites.
All mice used in this study were females bred at the Ludwig Institute's animal facility under specific pathogen-free conditions. The maintenance of T. muris and the method used for infection and evaluation of worm burden were as described by Wakelin (20). Mice were infected with approximately 200 eggs and bled or killed at various time points after infection, as described.
Cell Culture and Cytokines.
DMEM supplemented with 10% fetal calf serum/50 μM 2-mercaptoethanol/0.55 mM l-arginine/0.24 mM l-asparagine/1.25 mM l-glutamine was used for all experiments.
Recombinant murine IL-9 and IL-4 were purified from baculovirus-infected Sf9 insect cell cultures, as previously described (21). The supernatant of DBA/2 spleen cells cultured for 48 hr in the presence of 1 ng/ml of phorbol 12-myristate 13-acetate (Sigma) and 200 ng/ml of calcium ionophore A23187 (Sigma) was used as a source of natural mouse IL-9.
Preparation of IL-9-OVA Complexes and Immunization Protocol.
IL-9-OVA complexes were obtained by crosslinking mouse IL-9 and OVA (Sigma) with glutaraldehyde. The reaction was carried out under shaking in 0.1 M phosphate buffer pH 7, first at room temperature for 3 hr, then overnight at 4°C, by mixing equimolar amounts of purified recombinant murine IL-9 and OVA with glutaraldehyde (Merck) at a final concentration of 50 mM.
The complexed proteins were separated from the starting material by size exclusion chromatography on a Superose column (Pharmacia) equilibrated in PBS supplemented with Tween 20 (10−4; vol/vol) and 0.2 M NaCl. IL-9-OVA complexes were detected in column fractions by ELISA by using 2C12, a hamster monoclonal anti-IL-9 antibody produced in our laboratory for capture, and a rabbit anti-OVA antiserum followed by peroxidase-conjugated anti-rabbit antibody (Santa Cruz Biotechnology) for detection of complexes. The size of the conjugates ranged from 60 to greater than 1,000 kDa, as observed in SDS/PAGE. For immunization, all material containing IL-9 with a size exceeding 60 kDa was pooled.
Mice were primed subcutaneously in the tail with a 100-μl 1/1 mixture of complete Freund's adjuvant (CFA) (Difco) and complexed proteins in PBS [depending on the experiment (1, 2, 5) or 10 μg IL-9-OVA complex]. Two subcutaneous boosts were performed with the same quantity of antigen, mixed 1/1 with incomplete Freund's adjuvant (Difco), after 2 wk and 4 wk. In most experiments, mice were bled 15 days after the second boost. Control mice received either an equivalent amount of OVA in Freund's adjuvant or adjuvant alone.
Detection of Anti-IL-9 Antibodies.
Anti-IL-9 antibody titers were measured by testing the inhibitory activity of the sera on the proliferation of TS1 cells that respond to IL-9 and IL-4 (1). In this assay, one cytokine unit/ml, defined as the concentration required for half-maximal proliferation, corresponds to 25 pg/ml for IL-9 and 250 pg/ml for IL-4, respectively. Sera were serially diluted in 96-well plates containing culture medium and incubated in the presence of 2.5 units/ml mIL-9 or mIL-4 for 1 hr. TS1 cells were extensively washed and 2,500 cells added per well. Cells were incubated at 37°C, 8% CO2 for 3 days, and proliferation was measured by hexosaminidase activity determination (22).
Spontaneous and Specific Ig Production.
Baseline seric Ig levels were measured in groups of five 20-wk-old IL-9-OVA immunized or control C57BL/6 mice, as previously described (23). Antigen-specific responses were induced, 3 wk after the last boost with IL-9-OVA or CFA, in BALB/c mice by i.p. injection of Aspergillus protein (200 μg, Bayer, Wuppertal, Germany) in alum (2.25 mg, Pierce) twice a week for 4 wk. Anti-Aspergillus IgG1 and IgE antibodies, as well as total IgE, which is increased in response to Aspergillus, were measured by ELISA. For specific antibody determination, microtiter plates (Nunc, Immunoplates) were coated with Aspergillus antigen (10 μg/ml, Bayer) in 20 mM glycine buffer containing 30 mM NaCl, pH 9.2 and incubated overnight at 4°C. After washing in 0.1 M NaCl plus Tween 20 (5 × 10−4), serial dilutions of samples were added, and plates were incubated for 2–3 hr at 37°C. Plates were then washed as before and soaked for 7 min in 0.1 M NaCl containing Nonidet P-40 1% (Fluka) before further incubation. Bound Ig were detected by using rat anti-IgG1 or anti-IgE monoclonal antibodies coupled to peroxidase (IMEX, University of Louvain, Brussels). The assay was developed by adding 2,2′-azino-bis-(3-ethyl benzthiazoline 6 sulfonic acid) (Sigma), as described in PharMingen's ELISA protocol. The absorbance at 405 nm was measured, and nonsaturating serum dilutions were compared for analysis.
Blood Leukocyte Population Analysis and Eosinophil Counts.
Blood leukocyte populations were analyzed 10 mo after the last boost in C57BL/6 mice immunized with IL-9-OVA (four mice) or vehicle (four mice). Briefly, heparinized blood samples were centrifuged on a Ficoll layer (Ficoll/Paque, Pharmacia) and incubated for 5 min in 0.15M NH4Cl for red blood cell lysis. Cells were labeled with FITC-coupled anti-CD4 or anti-CD8 antibodies (H129.19 and 53–6.7, respectively; GIBCO/BRL), biotinylated rat anti-Mac-1 antibodies (M1/70, rat IgG1) followed by phycoerythrin-conjugated streptavidin (Becton Dickinson) and FITC-conjugated anti-IgM (LOMM9; IMEX). After staining, cells were fixed in paraformaldehyde (1.25%), and fluorescence intensity was measured on 10,000 cells/sample in a FACScan apparatus (Becton Dickinson).
Blood eosinophils were counted on slides prepared by centrifugation of 30,000 Ficoll-purified leukocyte cells in a Cytospin3 apparatus (Shandon, Pittsburgh, PA) and stained by using Diff-Quik Dade Behring (Deerfield, IL).
MMCP-1 ELISA.
Serum levels of MMCP-1 were measured by using an MMCP-1 ELISA kit from Moredun Animal Health (Penicuik, U.K.) as previously described (15). Briefly, rabbit anti-MMCP was used as capture antibody. Tenfold serial dilutions of serum were made from 1/10 to 1/10,000. A horseradish peroxidase-conjugated rabbit anti-mouse MMCP-1 was added and quantification made by reference to purified MMCP-1. Assays were developed as described above for Ig ELISAs.
Histology.
The cecum tip was removed at autopsy from _T. muris-_infected animals (21 days after infection) and fixed in Carnoy's fluid for 5 hr (for mast-cell counts) or in 4% neutral buffered formalin for 24 hr (for eosinophil counts), before processing by standard histological techniques. For mast-cell enumeration, sections were stained in 0.5% toluidine blue (pH 0.3) and for eosinophils, slides were incubated in 0.5% chromotrope 2R containing 1% phenol. The number of cells was determined in 20 cecal-crypt units per animal.
Statistical Analysis.
Statistical analysis was performed by using the Mann–Whitney U test, with P values below 0.05 considered significant.
Results
Induction of Anti-IL-9 Autoantibodies.
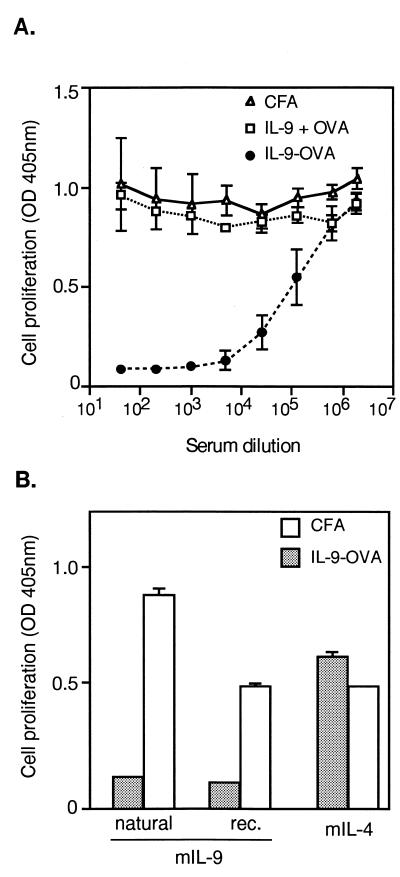
C57BL/6 mice were immunized with three injections of IL-9 crosslinked to OVA. Two weeks after the last injection, the anti-IL-9 response was evaluated by measuring seric inhibitory activities in a bioassay by using IL-9-dependent T cell line TS1. In a representative experiment shown in Fig. 1A, sera were found to strongly inhibit IL-9-induced proliferation, half-maximal inhibition of 2.5 units/ml mIL-9 being obtained at serum dilutions ranging from 10−4 to 10−5. Sera from mice immunized with noncomplexed IL-9 and OVA or with adjuvant only had no inhibitory activity. Also, IL-9–IL-9 complexes were unable to induce any anti-IL-9 response (data not shown). Because the immunization and the IL-9 assay were carried out with recombinant IL-9 produced in insect cells, it was important to verify that the sera also inhibited natural mouse IL-9. As shown in Fig. 1B, both forms of IL-9 were inhibited, whereas IL-4, which also stimulates TS1 cell proliferation, was not, thus providing clear specificity proof. Dose-response analyses showed that optimal antibody responses were obtained with three injections of 2-μg complexes. Increasing the amount of injected material to 10 μg did not increase inhibitory titers (data not shown).
Figure 1.
Induction of IL-9 specific autoantibodies. (A) Vaccination against IL-9. Groups of four C57BL/6 mice were injected subcutaneously with IL-9-OVA complexes in CFA (IL-9-OVA), with noncomplexed IL-9 and OVA (IL-9 + OVA) or with CFA alone (CFA). Boosts were carried out in IFA after 2 and 4 wk. Serially diluted sera, collected 2 wk after the last immunization, were tested for IL-9 inhibition in a TS1 cell proliferation assay. Cell growth was evaluated by measuring hexosaminidase activity. Means ± SD are indicated. (B) Specificity of anti-IL-9 activity. Pools of 1/320 diluted sera from immunized (IL-9-OVA) or control (CFA) mice were tested for growth inhibition of TS1 cells, in the presence of either natural mIL-9, baculovirus-derived mIL-9 (rec.), or mIL-4. Cell proliferation was measured after 3 days of culture. Results are given as means ± SD.
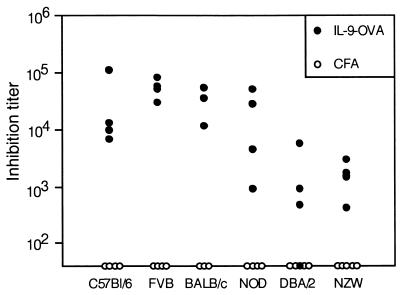
Because C57BL/6 mice have been reported to be low IL-9 producers (13), we sought to extend our observations to other mouse strains. Immunization of FVB, NOD, BALB/c, DBA/2, and NZW mice with IL-9-OVA complexes induced very significant anti-IL-9 responses in all strains tested, demonstrating the general applicability of our immunization protocol (Fig. 2). A striking feature of the observed anti-IL-9 response was its persistence (Fig. 3). In fact, titers remained elevated for more than a year after the last boost, suggesting that the vaccinated animals could be used to evaluate the consequences of long-term IL-9 blockade in vivo.
Figure 2.
Production of anti-IL-9 autoantibodies in different mouse strains. Eight-week-old C57BL/6, FVB, BALB/c, NOD, DBA/2, or NZW mice were injected with IL-9-OVA complexes (four mice) or adjuvant only (four mice), as described in Materials and Methods. Serially diluted sera, collected 2 wk after the last immunization, were tested in a TS1 cell proliferation assay. Inhibition titers, given for each mouse serum, correspond to serum dilutions inhibiting IL-9-induced cell proliferation by 50%.
Figure 3.
Persistence of the anti-IL-9 response in immunized mice. Three 8-wk-old C57BL/6 mice were injected s.c. with IL-9-OVA complexes in CFA. Mice were boosted with IFA after 2 and 4 wk. Serum was collected 2, 4, 6, 8, 12, 26, and 56 wk after priming and tested for anti-IL-9 activity in a TS1 cell proliferation assay. Results are given as the inhibition titer for individual mouse sera.
Inhibition of IL-9-Induced Mast-Cell Activation and Eosinophilia in IL-9-Vaccinated Mice.
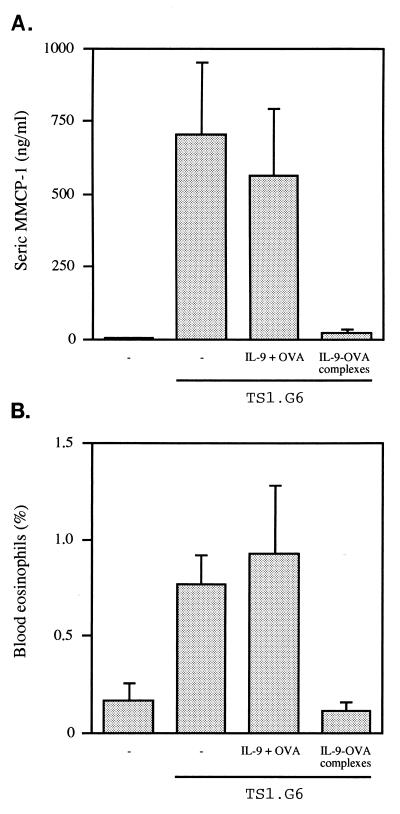
An IL-9-secreting T cell line, able to grow in C57BL/6 mice, was used to raise IL-9 levels in vivo. This cell line, TS1.G6, secretes ±1 ng IL-9/106 cells/48 hr in vitro (24) and has been shown previously to induce high serum levels of MMCP-1 (15). TS1.G6 cells were injected into the peritoneal cavity of untreated C57BL/6 mice or in mice immunized with either IL-9-OVA complexes or uncomplexed IL-9 and OVA. In both control groups, MMCP-1 concentrations measured 25 days after TS1.G6 inoculation rose from 6 ng/ml in nontumor-bearing mice to 700 ng/ml. By contrast, in mice immunized with IL-9-OVA complexes, MMCP-1 concentrations remained as low as 20 ng/ml (P = 0.03; Fig. 4A).
Figure 4.
Inhibition of IL-9-induced mast-cell activation and eosinophilia in immunized mice. Seven-week-old C57BL/6 mice subjected to the IL-9-OVA immunization protocol or immunized with noncoupled IL-9 and OVA or left untreated (five mice per group) were injected i.p. with 107 IL-9-producing TS1.G6 cells, 5 mo after the last boost. Five age-matched mice served as negative controls. All mice were tested 25 days later for MMCP-1 serum levels by ELISA (A), and blood leukocytes were isolated for eosinophil counting (B). Eosinophil percentages were determined by enumerating 20,000 cells per slide. Means ± SEM are indicated.
In addition to increasing MMCP-1 serum levels, TS1.G6 inoculation also raised the percentage of eosinophils in peripheral blood leukocytes from 0.16 ± 0.10 to 0.77 ± 0.15 (P = 0.016). This eosinophilia was completely abrogated in mice immunized with IL-9-OVA complexes (0.11 ± 0.05%; P = 0.02) but not in mice immunized with noncomplexed IL-9 and OVA (0.93 ± 0.35%) (Fig. 4B).
Analysis of body weight, basal Ig levels, and peripheral blood leukocyte composition showed no significant anomalies in unchallenged vaccinated animals (data not shown). The influence of anti-IL-9 vaccination on antibody responses was also evaluated. BALB/c mice, immunized with IL-9-OVA for 2 mo, were challenged with Aspergillus fumigatus antigen. Both anti-Aspergillus IgG1 and IgE responses developed similarly in vaccinated and control animals (P > 0.05; Table 1), indicating that anti-IL-9 immunization did not interfere with the development of normal antibody responses and did not prevent anti-Aspergillus IgE production.
Table 1.
Normal antibody responses against Aspergillus antigen in IL-9-OVA-vaccinated BALB/c mice
| Immunization | Plate | IgG1 | IgE |
|---|---|---|---|
| Vehicle | Aspergillus | 0.363 ± 0.002 | 0.428 ± 0.023 |
| BSA | 0.054 ± 0.001 | 0.109 ± 0.006 | |
| IL-9-OVA | Aspergillus | 0.227 ± 0.055 | 0.363 ± 0.083 |
| BSA | 0.046 ± 0.003 | 0.117 ± 0.028 |
Inhibition of T. muris Expulsion by Anti-IL-9 Vaccination.
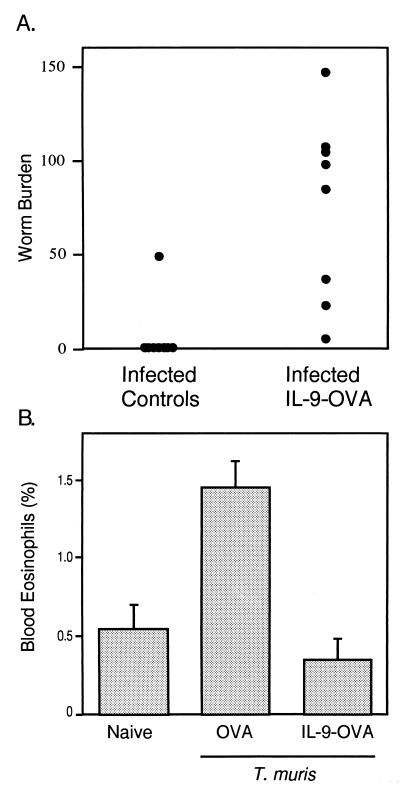
The preceding results demonstrated the ability of anti-IL-9 vaccination to interfere with IL-9 activities in vivo and suggested that this procedure could be used to unravel new IL-9 biological functions. TS1.G6 cells as well as IL-9 transgenic mice have previously been used to show that IL-9 can enhance resistance to the intestinal helminth T. muris (15). IL-9-vaccinated animals provided the opportunity to test the actual requirement for IL-9 in a resistant strain. C57BL/6 mice, which under normal circumstances rapidly expel the parasite, were vaccinated with IL-9-OVA and infected with T. muris eggs. Worms were counted in the cecum 34 days later. As shown in a representative experiment (Fig. 5A), anti-IL-9 vaccinated animals failed to expel the parasite by this time, whereas seven of the eight control mice were parasite free. Blood eosinophilia induced by the infection was also totally abrogated in the immunized mice (Fig. 5B). By contrast, cecal eosinophil and mast-cell accumulations were rather increased in vaccinated animals, although not to the point to reach statistical significance (P > 0.05; Table 2). Also, total IgE serum concentrations were similarly increased in OVA- or IL-9-OVA-vaccinated animals (data not shown).
Figure 5.
Altered response to T. muris in IL-9-OVA-vaccinated mice. (A) Failure of IL-9-OVA-immunized C57BL/6 mice to expel T. muris. C57BL/6 mice immunized with IL-9-OVA complexes or vehicle (eight mice per group) were infected by oral gavage of 200 T. muris eggs. Worms were counted in ceca on day 34 after infection, and results are given as worm burden in each individual. P value between groups is 0.001. On day 13, the worm burden, assessed to verify infectivity, was similar in all animals. (B) Inhibition of T. muris_-induced blood eosinophilia in IL-9-vaccinated mice._ Blood eosinophils were counted 23 days after infection from infected control or IL-9-vaccinated C57BL/6 mice or uninfected naive animals. Eosinophil percentages were determined by enumerating 500 cells per slide. Results are presented as the mean percentage of blood eosinophils ± SEM (four mice per group). P value is 0.029 for the difference between OVA and IL-9-OVA groups.
Table 2.
Normal intestinal mastocytosis and eosinophil infiltrates induced by T. muris infection in IL-9-OVA-immunized mice
| T. muris | Immunization | Mast cells | Eosinophils |
|---|---|---|---|
| − | − | 2 ± 1 | 8 ± 1 |
| + | OVA | 88 ± 63 | 56 ± 11 |
| + | IL-9-OVA | 201 ± 44 | 110 ± 11 |
Discussion
The present results show that immunization of mice with mouse IL-9 chemically complexed to OVA induces high titers of neutralizing anti-IL-9 antibodies. Anti-IL-9 vaccination was successful in the six mouse strains tested, irrespective of their IL-9 production levels. Proper covalent complex formation is essential because mice failed to produce neutralizing anti-IL-9 antibodies when IL-9 and OVA were simply mixed with adjuvant. Moreover, immunization with IL-9 crosslinked to OVA through carbodiimide or bisdiazobenzidine did not induce the production of neutralizing anti-IL-9 antibodies. Of note, these complexes failed to sustain IL-9-dependent cell growth in vitro, suggesting that modification of certain carboxyl and tyrosyl groups altered critical IL-9 epitopes. By contrast, IL-9-OVA complexes produced with glutaraldehyde still sustained cell proliferation (unpublished results).
Both T cell-dependent and -independent mechanisms have been proposed to explain the induction of self-reactive antibodies. The notion that potentially self-reactive B cells are nonresponsive because of a lack of autoreactive T cell help has been well established in many experimental settings. Several years ago, Stockinger (25) showed that nude mice reconstituted with T cells from complement factor C5-deficient mice, and not from normal donors, develop antibodies inhibiting complement activity, demonstrating the existence of anti-C5 specific B cells in C5-sufficient animals lacking functional T cells. This concept was confirmed with the advent of transgenic animals, expressing vesicular stomatitis virus glycoprotein (VSV-G), who were found to produce anti-VSV-G antibodies if immunized with VSV-G coupled to sperm-whale myoglobin, as a foreign carrier determinant (26). Still further refinement came from the demonstration that ubiquitin, modified by insertion of a single foreign immunodominant T helper epitope, was able to induce a strong and rapid ubiquitin-specific autoantibody response (16). Besides this T cell-controlled tolerance, an alternative possibility is that multimerization of antigen by itself triggers a T cell-independent antibody production. For example, mice transgenic for VSV-G can mount a T-independent IgM response against VSV-G when the protein is presented in a highly repetitive structure (on whole virions) but not in free form (soluble or at low concentration on cell surfaces) (27). In our model, mere polymerization of IL-9 with glutaraldehyde was unable to break B cell tolerance (unpublished results), indicating that IL-9 polymers, even very large in size (60–1,000 kDa), are not sufficient to induce anti-IL-9 responses. Moreover, the induction of anti-IL-9 autoantibodies is probably T cell dependent, because most of the anti-IL-9 autoantibodies are IgGs (unpublished results).
The anti-IL-9 antibodies induced by our vaccination procedure effectively suppressed IL-9 activities in vivo, as shown by the inhibition of mast-cell activation and eosinophilia consecutive to implantation of IL-9-secreting tumor cells. Anti-IL-9 vaccination thus provides a new tool to study IL-9 functions in vivo.
Immune responses to parasite infections represented an attractive system to evaluate the efficacy of anti-IL-9 vaccination, because IL-9 overproduction has been shown to promote rapid elimination of the cecal dwelling nematode T. muris (15). Our present observation that anti-IL-9 vaccination completely impairs worm expulsion provides the first formal demonstration of a strict requirement for this cytokine in this process. Experiments carried out with monoclonal anti-IL-9 antibodies derived from mice immunized with IL-9-OVA confirmed this conclusion (R.G., unpublished observations).
Many experiments have proven that resistance to T. muris requires a Th2 reaction. Administration of IL-12 (28) or of anti-IL-4 receptor antibodies prevents worm expulsion in otherwise resistant mice, whereas anti-IFN-γ has curative activity in susceptible strains (29). IL-13-deficient mice challenged with T. muris also fail to expel the parasite (30).
The impaired worm clearance observed in IL-9-vaccinated mice fits well with this notion. In addition, as IL-9 production in response to T. muris is markedly reduced in IL-4 knockout mice and transiently in IL-13-deficient animals (30), the possibility arises that IL-9 itself may be the limiting factor required for successful parasite expulsion. This possibility is supported by the fact that IL-4, IL-5, and IL-13 production was not reduced in IL-9-vaccinated mice (M.R., unpublished work). In this context, it is worth mentioning that IL-9 was the only Th2 cytokine significantly depressed in mice that fail to clear infection as a result of anti-TNF-α treatment (31). In addition, preliminary experiments indicate that anti-IL-9 antibodies, but not IL-13RIg, inhibit T. muris clearance in normal BALB/c mice (R.G., unpublished work). Whatever the relative contributions of the different Th2 cytokines, which are likely to depend on mouse genetic background, understanding the precise role of IL-9 in this model represents a major challenge. Indeed, inhibition of blood eosinophilia falls short of explaining the effect of IL-9 immunization, because anti-IL-5 antibodies also block eosinophilia but do not affect worm expulsion (32). In addition, the IgE response and intestinal mast-cell infiltration induced by the parasite were not impaired in IL-9-vaccinated mice.
Our results further extend the efforts made to generate autoantibodies capable of regulating biological processes. Potential benefits could be drawn from anti-IL-9-vaccination in asthma (8, 9, 13) or in pathologies involving eosinophil-mediated toxicity, like allograft rejection (33). Earlier attempts were successfully carried out mainly with hormones (17, 18), hormone receptors (34), or cellular components (35, 36), and recently extended to cytokines with reports of anti-IFN-α induction in AIDS patients (37) and of anti-TNF-α vaccination in mice (19). By allowing selective inhibition of particular cytokines in adult animals, our procedure opens new possibilities to study and manipulate cytokine functions in vivo. By using the experimental conditions described in this paper, we were recently able to generate autoantibodies against IL-4, IL-6, IL-12, and TNF-α, but so far _in vitro-_neutralizing antibodies were obtained only against IL-12 (Uyttenhove et al., personal communication). Elucidating the mechanisms responsible for these differences will be of prime importance for future applications.
Acknowledgments
We thank Mrs. B. De Lestré for expert technical assistance, Dr. C. Uyttenhove and Dr. A. Costesec (Ludwig Institute, Brussels) for gifts of reagents, and Ms. D. Markine and Dr. A. Vink for their kind help. We are grateful to Profs. A. Burny and D. Zagury for attracting our attention to the potency of anticytokine vaccination. This work was supported in part by the Belgian Federal Service for Scientific, Technical and Cultural Affairs, Opération Télévie, and Actions de Recherche Concertées, Communauté Française de Belgique, Direction de la Recherche Scientifique. M.R. is a scientific associate (Télévie) and J.-C. R. is a research associate with the Fonds National de la Recherche Scientifique, Belgium.
Abbreviations
CFA
complete Freund's adjuvant
OVA
ovalbumin
MMCP-1
mouse mast cell protease-1
Th
T helper cell
VSV-G
vesicular stomatitis virus glycoprotein
References
- 1.Uyttenhove C, Simpson R, Van Snick J. Proc Natl Acad Sci USA. 1988;85:6934–6938. doi: 10.1073/pnas.85.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hültner L, Druez C, Moeller J, Uyttenhove C, Schmitt E, Rüde E, Dörmer P, Van Snick J. Eur J Immunol. 1990;20:1413–1416. doi: 10.1002/eji.1830200632. [DOI] [PubMed] [Google Scholar]
- 3.Gessner A, Blum H, Röllinghoff M. Immunobiology. 1993;189:419–435. doi: 10.1016/S0171-2985(11)80414-6. [DOI] [PubMed] [Google Scholar]
- 4.Renauld J-C, Van Snick J. The Cytokine Handbook. 1998. pp. 313–331. [Google Scholar]
- 5.Renauld J-C, van der Lugt N, Vink A, van Roon M, Godfraind C, Warnier G, Merz H, Feller A, Berns A, Van Snick J. Oncogene. 1994;9:1327–1332. [PubMed] [Google Scholar]
- 6.Godfraind C, Louahed J, Faulkner H, Vink A, Warnier G, Grencis R, Renauld J-C. J Immunol. 1998;160:3989–3996. [PubMed] [Google Scholar]
- 7.Vink A, Warnier G, Brombacher F, Renauld J-C. J Exp Med. 1999;189:1413–1423. doi: 10.1084/jem.189.9.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Temann U A, Geba G P, Rankin J A, Flavell R A. J Exp Med. 1998;188:1307–1320. doi: 10.1084/jem.188.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLane M P, Haczku A H, van de Rijn M, Weiss C, Ferrante V, MacDonald D, Renauld J C, Nicolaides N C, Levitt R C. Am J Respir Cell Mol Biol. 1998;19:713–720. doi: 10.1165/ajrcmb.19.5.3457. [DOI] [PubMed] [Google Scholar]
- 10.Dong Q, Louahed J, Vink A, Sullivan C D, Messler C J, Zhou Y, Haczku A, Huaux F, Arras M, Holroyd K J, et al. Eur J Immunol. 1999;29:2130–2139. doi: 10.1002/(SICI)1521-4141(199907)29:07<2130::AID-IMMU2130>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 11.Holroyd K J, Martinati L C, Trabetti E, Scherpbier T, Eleff S M, Boner A L, Pignatti P F, Kiser M B, Dragwa C R, Hubbard F, et al. Genomics. 1998;52:233–235. doi: 10.1006/geno.1998.5445. [DOI] [PubMed] [Google Scholar]
- 12.Marsh D G, Neely J D, Breazeale D R, Ghosh B, Freidhoff L R, Ehrlich-Kautzky E, Schou C, Krishnaswamy G, Beaty T H. Science. 1994;264:1152–1156. doi: 10.1126/science.8178175. [DOI] [PubMed] [Google Scholar]
- 13.Nicolaides N, Holroyd K J, Ewart S L, Eleff S M, Kiser M B, Dragwa C R, Sullivan C D, Grasso L, Zhang L Y, Messler C J, et al. Proc Natl Acad Sci USA. 1997;94:13175–13180. doi: 10.1073/pnas.94.24.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkelman F D, Shea-Donohue T, Goldhill J, Sullivan C A, Morris S C, Madden K B, Gause W C, Urban J F J. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 15.Faulkner H, Renauld J-C, Van Snick J, Grencis R. Infect Immun. 1998;66:3832–3840. doi: 10.1128/iai.66.8.3832-3840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalum I, Jensen M R, Hindersson P, Elsner H I, Mouritsen I. J Immunol. 1996;157:4796–4804. [PubMed] [Google Scholar]
- 17.Johnson H E, DeAvila D M, Chang C F, Reeves J J. J Anim Sci. 1988;66:719–726. doi: 10.2527/jas1988.663719x. [DOI] [PubMed] [Google Scholar]
- 18.Talwar G P, Singh O, Pal R, Chatterjee N, Sahai P, Dhall K, Kaur J, Das S K, Suri S, Buckshee K, et al. Proc Natl Acad Sci USA. 1994;91:8532–8536. doi: 10.1073/pnas.91.18.8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalum I, Butler D M, Jensen M R, Hindersson P, Steinaa L, Waterston A M, Grell S M, Feldmann M, Elsner H I, Mouritsen S. Nat Biotechnol. 1999;17:666–669. doi: 10.1038/10878. [DOI] [PubMed] [Google Scholar]
- 20.Wakelin D. Parasitology. 1967;57:515–524. doi: 10.1017/s0031182000072395. [DOI] [PubMed] [Google Scholar]
- 21.Druez C, Coulie P, Uyttenhove C, Van Snick J. J Immunol. 1990;145:2494–2499. [PubMed] [Google Scholar]
- 22.Landegren U. J Immunol Methods. 1984;67:379–388. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- 23.Coutelier J-P, Coulie P G, Wauters P, Heremans H, van der Logt J. J Virol. 1990;64:5383–5388. doi: 10.1128/jvi.64.11.5383-5388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uyttenhove C, Druez C, Renauld J-C, Hérin M, Noël H, Van Snick J. J Exp Med. 1991;173:519–522. doi: 10.1084/jem.173.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stockinger B, Hausmann B. Eur J Immunol. 1988;18:249–253. doi: 10.1002/eji.1830180211. [DOI] [PubMed] [Google Scholar]
- 26.Steinhoff U, Burkhart C, Arnheiter H, Hengartner H, Zinkernagel R. Eur J Immunol. 1994;24:773–776. doi: 10.1002/eji.1830240343. [DOI] [PubMed] [Google Scholar]
- 27.Bachmann M, Hoffmann Rorher U, Kündig T M, Bürki K, Hengartner H, Zinkernagel R M. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 28.Bancroft A J, Else K J, Sypek J P, Grencis R K. Eur J Immunol. 1997;27:866–870. doi: 10.1002/eji.1830270410. [DOI] [PubMed] [Google Scholar]
- 29.Else K J, Finkelman F D, Maliszewski C R, Grencis R K. J Exp Med. 1994;179:347–351. doi: 10.1084/jem.179.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bancroft A J, McKenzie A N J, Grencis R K. J Immunol. 1998;160:3453–3461. [PubMed] [Google Scholar]
- 31.Artis D A, Humphreys N E, Bancroft A J, Rothwell N J, Potten C S, Grencis R K. J Exp Med. 1999;190:953–962. doi: 10.1084/jem.190.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Betts C J, Else K J. Parasite Immunol. 1999;21:45–52. doi: 10.1046/j.1365-3024.1999.00200.x. [DOI] [PubMed] [Google Scholar]
- 33.Le Moine A, Flamand V, Demoor F-X, Noël J-C, Surquin M, Kiss R, Nahori M-A, Petrolani M, Goldman M, Abramowicz D. J Clin Invest. 1999;103:1659–1667. doi: 10.1172/JCI5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chackerian B, Lowy D R, Schiller J T. Proc Natl Acad Sci USA. 1999;96:2773–2778. doi: 10.1073/pnas.96.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong X, Hamilton K J, Satoh M, Wang J, Reeves W H. J Exp Med. 1994;179:1243–1252. doi: 10.1084/jem.179.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalum I, Jensen M R, Gregorius K, Thomasen K M, Elsner H I, Mouritsen S. Mol Immunol. 1997;34:1113–1120. doi: 10.1016/s0161-5890(97)00147-8. [DOI] [PubMed] [Google Scholar]
- 37.Zagury D, Lecoq H, Gervi I, Le Buanec H, Zagury J F, Bizzini B, Burny A, Hermans P, Perja M, Santagostino E, et al. Biomed Pharmacother. 1999;53:90–92. doi: 10.1016/s0753-3322(99)80065-1. [DOI] [PubMed] [Google Scholar]