A nuclear export signal in the N-terminal regulatory domain of IκBα controls cytoplasmic localization of inactive NF-κB/IκBα complexes (original) (raw)
Abstract
Appropriate subcellular localization is crucial for regulation of NF-κB function. Herein, we show that latent NF-κB complexes can enter and exit the nucleus in preinduction states. The nuclear export inhibitor leptomycin B (LMB) sequestered NF-κB/IκBα complexes in the nucleus. Using deletion and site-directed mutagenesis, we identified a previously uncharacterized nuclear export sequence in residues 45–54 of IκBα that was required for cytoplasmic localization of inactive complexes. This nuclear export sequence also caused nuclear exclusion of heterologous proteins in a LMB-sensitive manner. Importantly, a LMB-insensitive CRM1 mutant (Crm1-K1) abolished LMB-induced nuclear accumulation of the inactive complexes. Moreover, a cell-permeable p50 NF-κB nuclear localization signal peptide also blocked these LMB effects. These results suggest that NF-κB/IκBα complexes shuttle between the cytoplasm and nucleus by a nuclear localization signal-dependent nuclear import and a CRM1-dependent nuclear export. The LMB-induced nuclear complexes could not bind DNA and were inaccessible to signaling events, because LMB inhibited NF-κB activation without affecting the subcellular localization of upstream kinases IKKβ and NIK. Our findings indicate that the dominant nuclear export over nuclear import contributes to the largely cytoplasmic localization of the inactive complexes to achieve efficient NF-κB activation by extracellular signals.
Regulatory pathways can be modulated by the subcellular compartmentalization of individual components. A prototypic example is signal-induced activation of the transcription factor NF-κB that plays an important role in immune and inflammatory responses and the regulation of apoptosis (1, 2). In unstimulated cells, inactive NF-κB preexists in the cytoplasm associated with its inhibitor IκB (3). On exposure to extracellular signals, a series of biochemical events targets the inhibitor protein for degradation, allowing the NF-κB to migrate into the nucleus to regulate gene expression.
Cis-acting elements of IκB govern its protein stability and subcellular localization (1, 2). IκBα, the most studied IκB family member, is composed minimally of three domains: an N-terminal regulatory domain that controls signal-dependent degradation, a central ankyrin repeat domain (ARD) that is necessary for NF-κB binding, and a C-terminal region rich in proline, glutamate/aspartate, serine, and threonine regulating basal turnover. An additional sequence, a leucine-rich nuclear export sequence (NES) within the last ankyrin repeat, is postulated to function during the termination of NF-κB activity (4). Activated NF-κB stimulates the synthesis of IκBα mRNA (5, 6), and newly synthesized IκBα proteins can enter the nucleus to bind to and remove NF-κB from gene promoters (7). It is believed that the C-terminal NES (C-NES) of IκBα can actively export these IκBα/NF-κB complexes out to the cytoplasm to restore the preinduction state of the complexes, a process known as postinduction repression (4).
The leucine-rich NES is a highly conserved sequence used by a variety of proteins to facilitate their delivery from the nucleus to the cytoplasm and is important in regulating protein function through subcellular localization (8). Nuclear export of proteins, such as HIV-1 Rev (9), cyclin B1 (10), and protein kinase A inhibitor (PKI; ref. 11), can be inhibited by leptomycin B (LMB), a Streptomyces metabolite (12). Several groups have reported that CRM1, related to the β-importin family of nuclear proteins, is the receptor for the leucine-rich NES and that LMB interferes with the interaction between CRM1 and NES by directly binding to CRM1 (13–17).
Based largely on mutagenesis and coexpression studies, it was suggested that cytoplasmic localization of the latent complexes is due to molecular masking of nuclear localization signals (NLS) on NF-κB by IκBα (18, 19). However, recent crystallographic analyses of NF-κB/IκBα complexes did not reveal clear evidence for complete masking of the NF-κB NLS sequences by IκBα ARD (20, 21). In addition, LMB-sensitive nuclear export was recently implicated in regulating the cytoplasmic localization of these complexes (22, 23). It was speculated that the observed LMB effects may be due to the presence of a continual postinduction repression process caused by a low level constitutive NF-κB activity in unstimulated cells (23). Herein, we provide evidence that presynthesized NF-κB/IκBα complexes accumulate in the nucleus with LMB. Moreover, unlike the postinduction repression mechanism, C-NES is disposable for this process. Instead, a previously undetermined NES motif in the N-terminal region (N-NES) of IκBα is essential for cytoplasmic localization of the latent complexes. We also provide evidence for a nucleocytoplasmic shuttling of NF-κB/IκBα complexes via NLS- and CRM1-dependent pathways. These results collectively provide deeper insights into the regulatory mechanisms governing the subcellular localization of the latent NF-κB/IκBα complexes.
Materials and Methods
Cell Culture.
HeLa, PC-3, and Cos-7 cells were maintained in DMEM (Cellgro, Mediatech) supplemented with 10% (vol/vol) FBS (HyClone), 1,000 units of penicillin G, and 0.5 mg/ml streptomycin sulfate (Sigma). HEK293 cells were maintained in DMEM with 10% (vol/vol) bovine calf serum and supplemented with antibiotics as described above on 0.1% (wt/vol) gelatin-coated dishes.
Reagents.
Acetyl-leucinyl-leucinyl-norleucinal (CI-I), DMSO, cycloheximide, and cytochalasin B were purchased from Sigma. Topotecan was a gift from SmithKline Beecham. Human recombinant tumor necrosis factor α (TNFα) and α-tubulin antibody were from Calbiochem. IgGs against IκBα (C21), p65 (A and C20), IKKβ (H-470), and NIK (H-248) were purchased from Santa Cruz Biotechnology. A monoclonal anti-Flag antibody was purchased from Kodak; horseradish peroxidase-conjugated anti-rabbit and anti-mouse antibodies and protein A were obtained from Amersham Pharmacia. FITC- and tetramethylrhodamine B isothiocyanate-conjugated anti-rabbit and anti-mouse antibodies were purchased from Sigma. NF-κB SN50, cell permeable inhibitory peptide, and NF-κB SN50M control peptide were purchased from Biomol (Plymouth Meeting, PA). LMB was generated as described (24).
Western Blotting, Immunoprecipitation, and Electrophoretic Mobility-Shift Assay (EMSA) Procedures.
Cell preparation, immunoprecipitation, and Western blotting were performed as described (25), and development was performed by using the enhanced chemiluminescent procedure according to the manufacturer's instructions (Amersham Pharmacia). Blots were exposed to x-ray film (Kodak). Nuclear extracts were made by hypotonic shock followed by salt extraction of the nuclei as described (25). The Igκ-κB probe and conditions for EMSA were as described (25).
Luciferase Reporter Assay.
HEK293 cells were seeded in six-well plates at the density of 5 × 104 cells per well and transfected with 10 ng of 3xκB-Luc or 3xMκB-Luc and 100 ng of CMV-β-gal vectors by using the standard calcium phosphate procedure (26) on the next day. At 36 h after the initial transfection, cells were treated with LMB (20 ng/ml) or 0.1% ethanol (a solvent control) for 30 min before addition of TNFα (10 ng/ml) for an additional 6 h. The cell extract preparation and the luciferase assay were performed according to the instructions in the luciferase kit from Promega. β-Galactosidase activity was measured by using the Galacton-plus kit purchased from Tropix (Bedford, MA). All transfection experiments were done in triplicate and repeated three times.
Enucleation Procedure.
Enucleation was performed as described (27) with the following modifications. HeLa cells were grown on 30-mm dishes and exposed to cytochalasin B (10 μg/ml) for 30 min at 37°C. Plates were secured upside down in 400-ml centrifuge bottles, bathed in growth medium containing cytochalasin B at the same concentration, and then centrifuged at 10,000 rpm for 15 min at 37°C in a Beckman Coulter JA-14 rotor. Plates with enucleated cells were gently rinsed with PBS and incubated with growth medium at 37°C for 30 min. Samples of enucleated cells were stained with Hoechst dye 33258 and photographed for determination of enucleation efficiency (not shown). Efficiency varied from ≈75%–95% for HeLa cells.
Generation of IκBα Mutants and Transfection Procedures.
N-terminally fused green fluorescent protein (GFP)-IκBα was generated by subcloning PCR-amplified human wild-type IκBα (MAD3) into _Hin_dIII–_Bam_HI sites of the pEGFP vector (CLONTECH). NES point mutations were introduced by two-step PCR mutagenesis, and the fusion constructs GFP-IκBα(1–55) and GFP-IκBα(1–55) N-NES mutant (Mut) were made with 5′ _Xho_I coding primer and 3′ IκBα oligonucleotide and subcloned into pEGFP with _Xho_I and _Bam_HI sites. All constructs were confirmed by nucleotide sequencing. Full-length human Flag-tagged IKKβ cDNA was cloned by screening a human kidney cDNA library with a PCR-amplified DNA fragment corresponding to the 463- to 665-amino acid region of IKKβ. The murine p65 cDNA (28) was blunt-end ligated into _Bam_HI/_Nhe_I Klenow blunt-ended pCMX vector. Similarly, the pCMX-p50 was generated by cleaving murine p105 cDNA (29) at _Nco_I sites, Klenow filled, and blunt-ligated into _Bam_HI/_Nhe_I Klenow blunt-ended pCMX. An amount of each plasmid vector was titrated to ensure the formation of IκBα and NF-κB complexes as measured by colocalization and coimmunoprecipitation. Cells were transfected in six-well dishes as described above. Stable HEK293 cell clones were generated by G418 selection and subsequent FACS sorting. Vectors for human wild-type CRM1 and the human equivalent of the yeast Crm1-K1 were constructed in pEGFP-N1 (CLONTECH) by replacing the EGFP open reading frame with the corresponding CRM1 open reading frame.
Analysis of NF-κB and IκBα Subcellular Localization.
After transfection of HEK293, PC-3, or Cos-7 cells as described above, cells were trypsinized and seeded on two-well chamber slides (Lab-Tek). After adhesion of cells, they were treated as described for each figure, washed twice with ice-cold PBS, and fixed with 3.7% (vol/vol) formaldehyde in PBS for several hours at 4°C. Fixed cells were then rinsed with PBS and permeabilized with 0.2% Triton X-100 in PBS. Cells were blocked with 2% (vol/vol) normal goat serum for 1 h and subsequently incubated with appropriate primary antibodies in PBS/0.2% Tween 20 at 37°C overnight. Staining was detected with either FITC- or tetramethylrhodamine B isothiocyanate-conjugated anti-mouse or anti-rabbit secondary antibodies. Cells were mounted with Prolong Antifade (Molecular Probes) and visualized and photographed with a Zeiss Axioplan epifluorescence microscope with the aid of fluorescein- or rhodamine-specific filters. GFP fusion proteins were visualized directly or under fixed conditions. Each experiment was repeated at least twice, and images representing greater than 90% of the total cell population are shown in figures.
Results
Enucleation Prevents LMB Inhibition of NF-κB Activation.
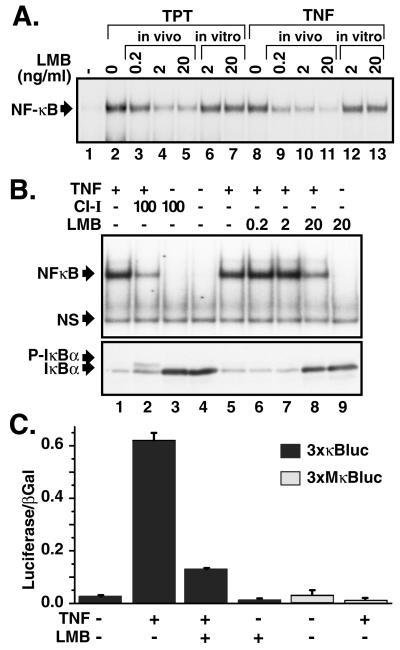
Consistent with a recent report (23), we found that pretreatment with LMB for 30 min inhibited NF-κB activation not only by cytokines such as TNFα (Fig. 1A, lanes 9–11) but also by the DNA-damaging agent topotecan (lanes 3–5). Addition of LMB directly to nuclear extracts did not affect NF-κB DNA binding (Fig. 1A, lanes 6 and 7), indicating that this chemical agent did not directly block NF-κB's ability to bind DNA. Consistent with EMSA, LMB also inhibited TNFα-induced NF-κB transcriptional activation (Fig. 1C). Unlike the proteasome inhibitor CI-I (Fig. 1B Lower, lane 2), LMB prevented IκBα degradation but did not cause accumulation of the phosphorylated IκBα form in TNFα-treated cells (Fig. 1B Lower, lanes 6–8). However, LMB failed to prevent IκBα degradation and NF-κB activation in enucleated cells (Fig. 2 A and B, lane 3). In enucleated cells, CI-I was able to prevent NF-κB activation by inhibiting IκBα degradation (Fig. 2B Upper, lane 2) causing accumulation of both phosphorylated (Fig. 2B Upper, lane 6) and multiubiquitinated IκBα forms (Fig. 2B Lower, lane 6). Consistent with the known action of LMB against CRM1 in the nucleus (12, 14–16, 30), these findings indicate that inhibition of NF-κB activation by LMB indeed requires an intact nucleus.
Figure 1.
LMB inhibits signal-inducible IκBα degradation and NF-κB activation. (A) PC-3 cells were treated with topotecan (TPT; 30 μM for 60 min; lanes 2–5) or TNFα (10 ng/ml for 15 min; lanes 8–11) in the presence or absence of various doses of LMB pretreatment for 30 min. Nuclear extracts were analyzed by EMSA by using an Igκ-κB probe. LMB was also added directly to nuclear extracts (as in lanes 2 and 8) and processed as described above (lanes 6, 7, 12, and 13). A section of the autoradiogram containing NF-κB complexes is shown. (B) HeLa cells were pretreated with CI-I (100 μM for 30 min; lanes 2 and 3) or LMB (ng/ml for 30 min; lanes 6–9) and then treated with TNFα (10 ng/ml for 15 min; lanes 1, 2, and 5–8). Nuclear extracts were analyzed by EMSA as described above (Upper). NS refers to a nonspecific band. Total cell extracts from parallel cell samples described in B were analyzed by Western blotting with IκBα antibody. P-IκBα refers to the position of the phosphorylated IκBα (Lower). (C) HEK293 cells were transiently transfected with a NF-κB-dependent reporter plasmid (3xκB-Luc or 3xMκB-Luc for control) and an internal control for transfection efficiency (CMV-β-Gal). At 36 h after transfection, cells were treated with or without TNFα in the presence or absence of LMB treatment. Cell extracts were analyzed for luciferase and β-galactosidase activities. Error bars are SD.
Figure 2.
LMB fails to inhibit TNFα-induced IκBα degradation and NF-κB activation in enucleated cells. (A) HeLa cells were enucleated as described in Materials and Methods, and the resulting cytoplasts were pretreated with LMB (20 ng/ml for 30 min; lanes 2 and 3) and then treated with TNFα (10 ng/ml for 15 min; lanes 3 and 4). Total cell extracts were prepared and analyzed by EMSA. (B Upper) Cytoplasts as described in A were pretreated for 30 min with either LMB (20 ng/ml, lanes 3 and 4) or CI-I (100 μM, lanes 5 and 6) and then treated with TNFα (lanes 2, 3, and 6). Samples were analyzed by Western blotting with IκBα antibody. (B Lower) A longer exposure of the Western blot used for B Upper is shown.
LMB Causes Nuclear Accumulation of Presynthesized NF-κB/IκBα Complexes Without Affecting IKKβ or NIK Localization.
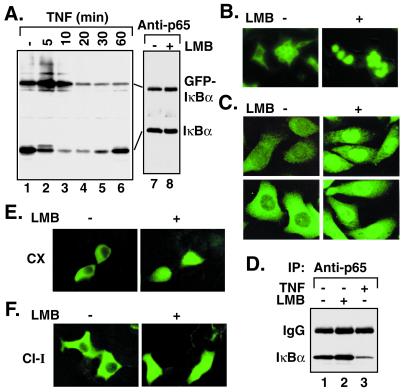
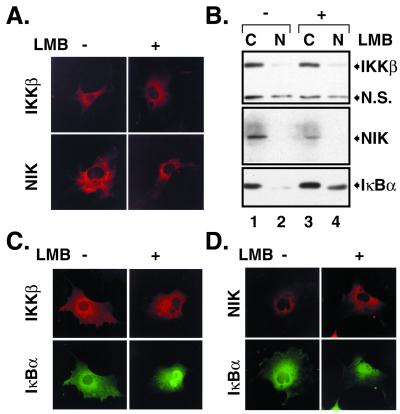
To visualize IκBα protein movement in live cells directly, we generated IκBα N-terminally tagged with the GFP and stably expressed it in HEK293 cells. Similar to the endogenous IκBα, GFP-tagged IκBα is associated with the endogenous p65 (Fig. 3A, lane 7) and degraded on exposure to TNFα (Fig. 3A, lanes 3–6). The amount of IκBα bound to p65 did not change with LMB treatments (Fig. 3A, lanes 7 and 8). Like the endogenous IκBα (Fig. 3C Left), the localization of GFP-IκBα was predominantly cytoplasmic (Fig. 3B Left). On treatment with LMB, a considerable fraction of GFP-IκBα or endogenous IκBα and p65 accumulated in the nucleus (Fig. 3 B and C Right). To determine directly whether the postinduction repression process is involved in nuclear accumulation of the complexes, de novo IκBα synthesis was blocked by the protein synthesis inhibitor cycloheximide for 1 h. LMB effects were still observed (Fig. 3E). Similarly, nuclear accumulation of IκBα after pretreatment with CI-I indicated that generation of free NF-κB via basal degradation of IκBα is also not required (Fig. 3F). LMB did not alter localization of transfected (Fig. 4A) or endogenous NIK and IKKβ (Fig. 4B), two kinases implicated in NF-κB signaling. Cotransfection studies showed that, although a considerable fraction of IκBα proteins accumulated in the nucleus, IKKβ remained in the cytoplasm within the same cell (Fig. 4C). Similar results were obtained with NIK (Fig. 4D). These results show that at lease one mechanism of NF-κB inhibition by LMB involves selective nuclear accumulation of presynthesized IκBα and NF-κB without affecting the localization of some of the key upstream kinases.
Figure 3.
LMB causes nuclear accumulation of IκBα without de novo protein synthesis or basal IκBα degradation. (A) A pool of HEK293 cells stably expressing GFP-IκBα was treated with TNFα (20 ng/ml) for the indicated times (lanes 2–6) and analyzed by Western blotting with IκBα antibody. Equal cell extracts prepared from untreated (lane 7) or LMB-treated (20 ng/ml for 60 min; lane 8) cells were processed for immunoprecipitation with p65 A antibody and analyzed by Western blotting with IκBα antibody. Horseradish peroxidase-conjugated protein A was used to detect bands. (B) HEK293 cells stably expressing the GFP-IκBα protein were untreated or treated with LMB (10 ng/ml for 30 min) and visualized under fluorescein-aided fluorescent microscopy. (C) PC-3 cells were untreated or treated with LMB as described for B, fixed, and stained with either IκBα (Upper) or p65 C20 (Lower) antibodies. (D) PC-3 cells were treated as described for C or with TNFα (10 ng/ml for 15 min), and the cell extracts were immunoprecipitated (IP) with p65 A antibody and analyzed by IκBα Western blotting with horseradish peroxidase-conjugated goat anti-rabbit antibody (lanes 1–3). (E) HEK293 cells stably expressing GFP-IκBα were pretreated with cycloheximide (CX; 20 μg/ml for 60 min), untreated or treated with LMB (20 ng/ml for 15 min), and then visualized as described above. (F) HEK293 cells stably expressing GFP-IκBα were pretreated with CI-I (100 μM for 60 min), untreated or treated with LMB, and then visualized.
Figure 4.
LMB does not cause nuclear accumulation of IKKβ and NIK. (A) Cos-7 cells were transfected with either Flag-tagged IKKβ (2 μg) or NIK (2 μg) expression vector and treated or left untreated with LMB (20 ng/ml for 60 min) 24 h after transfection. Cells were visualized with IKKβ and NIK antibodies, respectively. (B) PC-3 cells were untreated (lanes 1 and 2) or treated with LMB (20 ng/ml for 60 min; lanes 3 and 4), and crude cytoplasmic and nuclear extracts prepared as described (25) were analyzed by Western blotting with IKKβ-, NIK-, and IκBα-specific antibodies. N.S. refers to a nonspecific band. (C) Cos-7 cells were cotransfected with IκBα (1.0 μg), p50 (1.0 μg), and p65 (1.0 μg) constructs along with a Flag-tagged IKKβ (2 μg) construct, untreated or treated with LMB as described for A, and stained with IκBα and Flag antibodies. (D) Cos-7 cells were transfected and visualized as described for B, except that Flag-tagged NIK (2 μg) was used instead of Flag-tagged IKKβ.
A Functional NES Motif Resides Within the N-Terminal Region of IκBα.
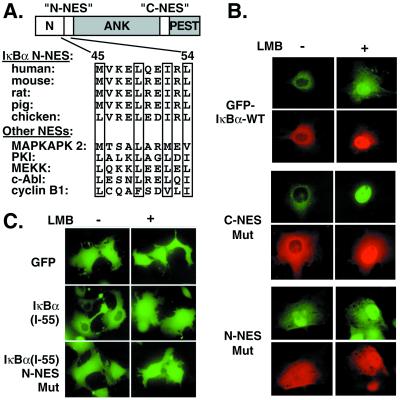
To address directly whether C-NES, implicated in postinduction repression, also regulates nuclear exclusion of inactive complexes, GFP-IκBα with C-NES mutations was tested for sensitivity to LMB as described above (Fig. 5B, C-NES Mut). Although both wild-type GFP-IκBα and p65 accumulated in the nucleus on LMB treatment (Fig. 5B, GFP–IκBα WT), we were unable to detect any obvious differences on the localization of GFP-IκBα caused by the C-NES mutation (Fig. 5B, C-NES Mut). However, we found that, although a deletion of N-terminal 36 amino acid residues of IκBα did not alter the LMB sensitivity, a N-terminal deletion of either 54 or 70 amino acid residues caused a lack of nuclear exclusion of IκBα in the absence of LMB treatments (data not shown). These studies suggested that the IκBα N-terminal region downstream of amino acid 36 might contain an export signal. Scanning the primary amino acid sequence revealed an evolutionarily conserved putative NES motif that aligns well with other well established NES sequences (Fig. 5A). To determine whether it represents a functional NES, the hydrophobic residues within this sequence were mutated to Ala in the context of wild-type GFP-IκBα (N-NES Mut). Significantly, the mutations in N-NES destroyed nuclear exclusion of GFP-IκBα transfected with p50 and p65 without LMB treatments (Fig. 5B, N-NES Mut). Immunoprecipitation experiments showed that this mutant IκBα efficiently complexed with NF-κB (not shown). LMB treatment did not alter the localization of N-NES Mut GFP-IκBα or their corresponding p65 proteins (Fig. 5B Right, N-NES Mut). These results indicate that N-NES is necessary for cytoplasmic localization of IκBα/NF-κB complexes.
Figure 5.
Identification of N-NES of IκBα. (A) N-NESs in different species are aligned with those of previously characterized shuttling proteins. The critical hydrophobic residues are boxed. ANK, ankyrin repeats; PEST, region rich in proline (P), glutamate (E), serine (S), and threonine (T). (B) GFP-IκBα wild type (WT) (1.0 μg) and GFP-IκBα mutants (1.0 μg) were transfected with p50 and p65 (1.0 μg each) expression vectors into Cos-7 cells and left untreated or treated with LMB (20 ng/ml for 60 min). GFP-IκBα and p65 were covisualized with GFP fluorescence (green) and by staining with p65 antibody (red). In the GFP-IκBα mutants, critical hydrophobic residues (bold) in C-NES (IQQQLGQLTLENLQML) and N-NES (MVKELQEIRLEP) were substituted with Ala. (C) GFP alone, IκBα N-terminal 55, and 55 residues with NES mutations as described above were fused C-terminally to GFP and transfected into Cos-7 cells. GFP and GFP fusion proteins were visualized directly in living cells with GFP fluorescence with or without LMB treatment.
To evaluate whether N-NES is sufficient for nuclear export, we engineered GFP fusion constructs attaching either amino acids 1–55 or 1–55 with NES mutations. Although GFP alone is distributed throughout the cell with or without LMB treatment (Fig. 5C, GFP), the addition of N-terminal 55 amino acid sequence causes exclusion of GFP from the nucleus [Fig. 5C, IκBα(1–55)]. LMB treatment abolishes nuclear exclusion of the latter protein. However, both nuclear export and LMB sensitivity are abrogated in GFP-IκBα(1–55) with mutated N-NES [Fig. 5C, IκBα(1–55) N-NES Mut]. These results show that N-NES is both necessary and sufficient for active nuclear exclusion. In addition, we found that the N-NES mutant of IκBα blocked the TNFα-induced NF-κB activation as efficiently as the Ser-to-Ala mutations on residues 32 and 36 of IκBα as measured by a κB-dependent luciferase assay (not shown). Thus, N-NES represents a previously uncharacterized functional motif of IκBα that is critical for regulation of NF-κB subcellular distribution.
Export of Inactive NF-κB/IκBα Complexes Depends on CRM1, Whereas Their Nuclear Import Is NLS-Mediated.
Kudo et al. (24) recently described a Schizosaccharomyces pombe mutant of the CRM1 nuclear export receptor (Crm1-K1) that behaves normally as an exporter but is completely resistant to LMB. To determine whether LMB effects are due to inhibition of CRM1 function, we transiently expressed a human Crm1-K1 equivalent in HEK293 cells stably expressing the GFP-IκBα. Although expression of empty vector or CRM1 wild type showed no effects, the Crm1-K1-transfected cells were markedly resistant to the LMB effects (Fig. 6A). These results show that CRM1 is involved in the nuclear export of the inactive NF-κB/IκBα complexes.
Figure 6.
Inhibition of NF-κB/IκBα nucleocytoplasmic shuttling by a p50 NLS peptide and a LMB-insensitive CRM1 mutant. (A) Empty vector, CRM1 wild type (WT), or LMB-insensitive Crm1-K1 mutant was transfected into HEK293 cells stably expressing GFP-IκBα. Cells were then treated or untreated with LMB (20 ng/ml for 15 min) and visualized with GFP fluorescence. (B) HeLa cells were pretreated with either LMB (20 ng/ml for 30 min; lane 4) or cell-permeable p50 NLS peptide (NF-κB SN50) for 1 h (100 μg/ml; lane 3) and stimulated with TNFα (10 ng/ml for 15 min; lanes 2 and 3). Nuclear extracts were analyzed for NF-κB activity with EMSA (Upper). Cytoplasmic extracts from the same samples were analyzed by Western blotting for IκBα and α-tubulin (loading control). (C) HeLa cells were untreated or treated with SN50 (100 μg/ml for 1 h) and then either untreated (Top) or treated with LMB (20 ng/ml for 30 min; Middle) and TNFα (10 ng/ml for 30 min; Lower). Cells were then stained with p65 antibody as described above. (D) Model of nucleocytoplasmic shuttling of the inactive NF-κB/IκBα complexes. See the text for details.
Although export is CRM1-dependent, it is unknown how NF-κB/IκBα complexes could enter the nucleus. Because crystal structures did not reveal clear masking of NF-κB NLS motifs by IκBα, in particular that of p50 NLS (20, 21), a cell-permeable p50 NF-κB NLS peptide was added to determine whether it could prevent nuclear import of the complexes (31). Importantly, immunostaining of the endogenous p65 (Fig. 6C) and IκBα (not shown) indicated that p50 NLS peptide was able to block LMB-induced nuclear accumulation of the latent complexes efficiently (Fig. 6C Middle Right; IκBα not shown). The equivalent amounts of the mutant version of the p50 NLS peptide failed to block the LMB effects (not shown). Control experiments with TNFα stimulation showed that NLS peptide inhibited TNFα-induced NF-κB activation as measured by EMSA (Fig. 6B Upper, lanes 2 and 3) without affecting IκBα degradation (Fig. 6B Lower, lanes 2 and 3). These results suggest that nuclear import of the latent NF-κB/IκBα complexes may depend on transient exposure of NF-κB NLS, likely from p50.
Discussion
The biological activity of NF-κB is regulated by subcellular localization. Inactive NF-κB is predominantly localized in the cytoplasm because of associated inhibitor proteins, including IκBα. However, the detailed biochemical basis for cytoplasmic localization of the inactive complexes is not well understood. Previous transfection studies have implicated a “cytoplasmic sequestration” model in which IκBα masks NLS of NF-κB dimers, thus rendering them incapable of entering the nucleus (18, 19). The data presented in this study revise that model to include a component of active nuclear export. Although free NF-κB accumulates in the nucleus because of exposed NLS motifs, free IκBα is distributed in the cytoplasm as well as the nucleus via a putative nonclassical NLS within the second ankyrin repeat (32). Despite the presence of at least three NLS motifs on NF-κB and IκBα proteins, their coexpression leads to mostly cytoplasmic localization of the complexes (18, 19). Recent analyses of NF-κB/IκBα cocrystals indicated that NLS on p50 and p65 may not be completely covered by IκBα ARD, whereas IκBα NLS seems to be masked (20, 21). In particular, Jacobs and Harrison (21) showed that NLSs on p50 and p65 are asymmetrically masked by IκBα ARD; the p65 NLS is masked by the IκBα ARD, but the NLS of p50 is exposed. The flexible N-terminal domain of IκBα (which was not in the cocrystals) was suggested to occlude the p50 NLS, thereby explaining how deletion of this region may allow the nuclear entry of the NF-κB/IκBα complexes (33, 34).
In the present study, we identified a canonical NES motif within the N-terminal 45–54 residues of IκBα that regulates the localization of IκBα/NF-κB complexes. We conclude that this sequence is a functional NES motif based on the following observations. First, it closely resembles the evolutionarily conserved leucine-rich NES from other well characterized nucleocytoplasmic shuttling proteins. Second, mutating the critical hydrophobic residues to Ala destroys efficient nuclear exclusion of the NF-κB/IκBα complexes. Third, this NES motif can cause LMB-sensitive nuclear exclusion of heterologous proteins that are normally distributed throughout the cytoplasm and the nucleus. Although LMB induces nuclear accumulation of the latent complexes via N-NES, the human equivalent of the S. pombe Crm1-K1 mutant (24) can render the complexes insensitive to LMB. We have demonstrated that mutating the human Crm1 at the conserved Cys-528 residue to a serine (Crm1-K1) can functionally compensate for the endogenous LMB-sensitive Crm1 in a mammalian system. Thus, nuclear exclusion of the latent complexes depends on IκBα N-NES and is mediated by a CRM1-dependent pathway.
We therefore suggest that an IκBα N-terminal sequence may possess dual functions in regulating the subcellular localization of latent NF-κB/IκBα complexes (Fig. 6D). One of its roles may be to mask one of the NLS motifs of NF-κB (likely p50 NLS; ref. 21) to keep the complexes in the cytoplasm. The second function is to allow continuous export of the complexes from the nucleus if they should enter the nucleus. Because the domain N-terminal to the ARD of IκBα is predicted to be flexible (21), it is possible that masking of the p50 NLS may be transient or leaky, leading to some nuclear import of the complexes. This transient or leaky masking explains why LMB effects may be partial. Alternatively, the masking and unmasking of the NLS may be regulated by an active mechanism. Importantly, we were able to block the nuclear import of these complexes efficiently by adding a cell-permeable p50 NLS peptide; this efficient blocking strongly implies that the import process depends on unmasking of a NLS motif.
This model of NF-κB/IκBα regulation (Fig. 6D) contrasts with that involved in nuclear export of NF-κB/IκBα complexes during postinduction repression, which was postulated to depend on C-NES (4). In our experimental settings, mutating C-NES did not affect subcellular localization of these complexes, nor was it refractory to LMB treatments. Moreover, nuclear accumulation of the complexes took place in the absence of de novo protein synthesis, a condition that would destroy the postinduction repression process. Finally, proteasome-dependent basal IκBα degradation, a process that was predicted to be necessary for generation of free NF-κB and nuclear accumulation of inactive complexes after LMB treatment (23), also did not affect this process. Taking these observations together, we suggest that C-NES is unlikely to be essential for the regulation of the inactive NF-κB/IκBα complexes in the preinduction state.
In summary, our findings are consistent with the hypothesis that cytoplasmic localization of inactive NF-κB/IκBα complexes is established by a potent CRM1-dependent nuclear export process that is continually counteracting a relatively weaker (or leaky) NLS-dependent nuclear import process. Nuclear import of the complexes is likely due to either passive or regulated unmasking of one of the NLS motifs on NF-κB by the flexible IκBα N-terminal region [(a) in Fig. 6_D_]. Once in the nucleus, the N-terminal NES allows efficient export of the complex in a CRM1-dependent fashion to achieve dominantly cytoplasmic localization of the complexes [(b) in Fig. 6_D_]. Thus, this model predicts that the inactive NF-κB/IκBα complexes are not static in their localization but dynamically shuttling between nucleus and cytoplasm. Moreover, the nuclear NF-κB/IκBα complexes are inactive, because they are incapable of binding DNA. These nuclear complexes are also inaccessible to signal transduction by upstream kinases, because at least IKKβ and NIK do not shuttle, as evidenced by the lack of LMB effects on their localization. Thus, LMB-induced nuclear sequestration of these inactive complexes blocks NF-κB activation by extracellular stimuli. In this context, LMB may act as a prototypical antiinflammatory agent by disrupting the regulation of NF-κB subcellular compartmentalization. It is also tempting to speculate that these nuclear import/export mechanisms may be affected under some physiological or pathological conditions to attenuate NF-κB inducibility. Further definition of the mechanisms involved in subcellular localization of latent NF-κB complexes would not only expand our understanding of NF-κB regulation but also likely provide a new class of drug targets to attenuate NF-κB functions selectively.
Acknowledgments
We are thankful to S. Ghosh for p105 cDNA, G. Nolan for p65 cDNA, K. Umesono for pCMX vector, T. Kurama for NIK cDNA, E. Alarid, S. Shumway, and J. Dahlberg for critical reading of the manuscript, and B. True for preparation of the figures. This work was supported by a Cremer Scholar Award (to T.T.H.) and by a Howard Hughes Medical Institute grant through the University of Wisconsin Medical School, by the Shaw Scientist Award from the Milwaukee Foundation, and by grants from the University of Wisconsin Comprehensive Cancer Center and the University of Wisconsin National Institute on Environmental Health Sciences Center (to S.M.).
Abbreviations
NES
nuclear export sequence
C-NES
C-terminal NES
N-NES
N-terminal NES
LMB
leptomycin B
ARD
ankyrin repeat domain
CI-I
acetyl-leucinyl-leucinyl-norleucinal
TNFα
tumor necrosis factor α
EMSA
electrophoretic mobility-shift assay
GFP
green fluorescent protein
Mut
mutant
NLS
nuclear localization signal
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Baeuerle P A, Baltimore D. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 2.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle P A, Baltimore D. Cell. 1988;53:211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- 4.Arenzana-Seisdedos F, Turpin T, Rodriguez M, Thomas D, Hay R T, Virelizier J-L, Dargemont C. J Cell Sci. 1997;110:369–378. doi: 10.1242/jcs.110.3.369. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Q, Cant C A, Moll T, Hofer-Warbinek R, Wagner E, Birnstiel M L, Bach F H, de Martin R. J Biol Chem. 1994;269:13551–13557. [PubMed] [Google Scholar]
- 6.Chiao P J, Miyamoto S, Verma I M. Proc Natl Acad Sci USA. 1994;91:28–32. doi: 10.1073/pnas.91.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arenzana-Seisdedos F, Thompson J, Rodriguez M S, Bachelerie F, Thomas D, Hay R T. Mol Cell Biol. 1995;15:2689–2696. doi: 10.1128/mcb.15.5.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattaj I W, Englmeier L. Annu Rev Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. [DOI] [PubMed] [Google Scholar]
- 9.Fritz C C, Green M R. Curr Biol. 1996;6:848–854. doi: 10.1016/s0960-9822(02)00608-5. [DOI] [PubMed] [Google Scholar]
- 10.Toyoshima F, Moriguchi T, Wada A, Fukuda M, Nishida E. EMBO J. 1998;17:2728–2735. doi: 10.1093/emboj/17.10.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen W, Meinkoth J L, Tsien R Y, Taylor S S. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 12.Nishi K, Yoshida M, Fujiwara D, Nishikawa M, Horinouchi S, Beppu T. J Biol Chem. 1994;269:6320–6324. [PubMed] [Google Scholar]
- 13.Stade K, Ford C S, Guthrie C, Weis K. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- 14.Kudo N, Khochbin S, Nishi K, Kitano K, Yanagida M, Yoshida M, Horinouchi S. J Biol Chem. 1997;272:29742–29751. doi: 10.1074/jbc.272.47.29742. [DOI] [PubMed] [Google Scholar]
- 15.Fornerod M, Ohno M, Yoshida M, Mattaj I W. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. Nature (London) 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 17.Kudo N, Wolff B, Sekimoto T, Schreiner E P, Yoneda Y, Yanagida M, Horinouchi S, Yoshida M. Exp Cell Res. 1998;242:540–547. doi: 10.1006/excr.1998.4136. [DOI] [PubMed] [Google Scholar]
- 18.Beg A A, Ruben S M, Scheinman R I, Haskill S, Rosen C A, Baldwin A S J. Genes Dev. 1992;6:1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- 19.Ganchi P A, Sun S C, Greene W C, Ballard D W. Mol Biol Cell. 1992;3:1339–1352. doi: 10.1091/mbc.3.12.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huxford T, Huang D-B, Malek S, Ghosh G. Cell. 1998;95:759–770. doi: 10.1016/s0092-8674(00)81699-2. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs M D, Harrison S C. Cell. 1998;95:749–758. doi: 10.1016/s0092-8674(00)81698-0. [DOI] [PubMed] [Google Scholar]
- 22.Sachdev S, Hannink M. Mol Cell Biol. 1998;18:5445–5456. doi: 10.1128/mcb.18.9.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez M S, Thompson J, Hay R T, Dargemont C. J Biol Chem. 1999;274:9108–9115. doi: 10.1074/jbc.274.13.9108. [DOI] [PubMed] [Google Scholar]
- 24.Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner E P, Wolff B, Yosida M, Horinouchi S. Proc Natl Acad Sci USA. 1999;96:9112–9117. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyamoto S, Seufzer B, Shumway S. Mol Cell Biol. 1998;18:19–29. doi: 10.1128/mcb.18.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao Z D, Xiong J, Takeuchi M, Kurama T, Goeddel D V. Nature (London) 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 27.Poste G. Exp Cell Res. 1972;73:273–286. doi: 10.1016/0014-4827(72)90049-3. [DOI] [PubMed] [Google Scholar]
- 28.Nolan G P, Ghosh S, Liou H C, Tempst P, Baltimore D. Cell. 1991;64:961–969. doi: 10.1016/0092-8674(91)90320-x. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh S, Gifford A M, Riviere L R, Tempst P, Nolan G P, Baltimore D. Cell. 1990;62:1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- 30.Ossareh-Nazari B, Bachelerie F, Dargemont C. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y-Z, Yao S, Veach R A, Torgerson T R, Hawiger J. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 32.Sachdev S, Hoffmann A, Hannink M. Mol Cell Biol. 1998;18:2524–2534. doi: 10.1128/mcb.18.5.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luque I, Gelinas C. Mol Cell Biol. 1998;18:1213–1224. doi: 10.1128/mcb.18.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latimer M, Ernst M K, Dunn L L, Drutskaya M, Rice N R. Mol Cell Biol. 1998;18:2640–2649. doi: 10.1128/mcb.18.5.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]